Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) accounts for half of heart failure hospitalizations, with limited data on predictors of mortality by sex and race. We evaluated for differences in predictors of all‐cause mortality by sex and race among hospitalized patients with HFpEF in the ARIC (Atherosclerosis Risk in Communities) Community Surveillance Study.
Methods and Results
Adjudicated HFpEF hospitalization events from 2005 to 2013 were analyzed from the ARIC Community Surveillance Study, comprising 4 US communities. Comparisons between clinical characteristics and mortality at 1 year were made by sex and race. Of 4335 adjudicated acute decompensated heart failure cases, 1892 cases (weighted n=8987) were categorized as HFpEF. Men had an increased risk of 1‐year mortality compared with women in adjusted analysis (hazard ratio [HR], 1.27; 95% CI, 1.06–1.52 [P=0.01]). Black participants had lower mortality compared with White participants in unadjusted and adjusted analyses (HR, 0.79; 95% CI, 0.64–0.97 [P=0.02]). Age, heart rate, worsening renal function, and low hemoglobin were associated with increased mortality in all subgroups. Higher body mass index was associated with improved survival in men, with borderline interaction by sex. Higher blood pressure was associated with improved survival among all groups, with significant interaction by race.
Conclusions
In a diverse HFpEF population, men had worse survival compared with women, and Black participants had improved survival compared with White participants. Age, heart rate, and worsening renal function were associated with increased mortality across all subgroups; high blood pressure was associated with decreased mortality with interaction by race. These insights into sex‐ and race‐based differences in predictors of mortality may help strategize targeted management of HFpEF.
Keywords: epidemiology, heart failure with preserved ejection fraction, outcomes
Subject Categories: Heart Failure, Cardiomyopathy, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- CKD‐EPI
Chronic Kidney Disease Epidemiology Collaboration
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision–Clinical Modification
- SBP
systolic blood pressure
Clinical Perspective
What is New?
In hospitalized patients with heart failure with preserved ejection fraction from the Atherosclerosis Risk in Communities Community Surveillance Study, White participants had higher 1‐year mortality than Black participants, and men had a higher risk of mortality than women in adjusted analysis.
Age, heart rate, and worsening renal function were associated with increased mortality across the subgroups; higher blood pressure was associated with decreased mortality, with significant interaction by race.
What are Clinical Implications?
The findings of the study have clinical implications for ongoing efforts to phenotype heart failure with preserved ejection fraction to identify the highest risk subgroups and target therapies.
Heart failure (HF) with preserved ejection fraction (HFpEF) constitutes half of HF cases today, and is predicted to be the predominant form of hospitalized HF over the next decade. 1 , 2 , 3 , 4 , 5 To date, there remain no proven pharmacologic therapies shown to affect survival in HFpEF, and its underlying pathophysiologic mechanisms remain unclear. 6 Population‐based studies have consistently demonstrated that HFpEF is more common in women compared with men; however, outcomes have been reported to be worse in men. 4 , 7 , 8 , 9 Of the limited studies that have reported HFpEF characteristics and outcomes by race, clinical outcomes have been either neutral or better in Black patients compared with White patients with HFpEF, 7 , 10 , 11 , 12 with limited data on predictors of mortality by either sex or race following hospitalization for HFpEF. 7
A major challenge in HFpEF is the heterogeneity of the patient population—from clinical characteristics to underlying mechanisms of disease implicated in disease pathogenesis. Understanding differences in sex and race are imperative as we refine phenotype‐based approaches to tackling this vastly unmet burden in cardiovascular disease today. 13 In this study we investigated differences in patient characteristics, 1‐year case fatality, and predictors of mortality by sex and race among hospitalized patients with HFpEF within the racially diverse ARIC (Atherosclerosis Risk in Communities) Community Surveillance Study.
Methods
Data Source
Anonymized data from the ARIC study are available through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. Interested researchers may additionally contact the ARIC study coordinating center to access the study data. All surveillance protocols were approved by local institutional review boards. Informed consent was not required because all data were anonymized by redacting personal identifiers. All supporting data are available within the article (and its online supplementary files).
Study Population
The ARIC study has conducted population‐based retrospective surveillance of HF hospitalization events in 4 communities in the United States since 2005: Forsyth County, NC; Washington County, MD; Jackson, MS; and 8 northwest suburbs of Minneapolis, MN. Details of the ARIC Community Surveillance Study have been provided elsewhere. 14 To summarize, HF surveillance eligibility is limited to those aged ≥55 years, with a hospitalization length of stay of at least 1 day. For the purposes of this study, those with a discharge date between January 1, 2005, and December 31, 2013, were included. Hospitalizations with International Classification of Diseases, Ninth Revision–Clinical Modification (ICD‐9‐CM) discharge diagnosis codes for congestive HF, hypertensive heart disease, chronic pulmonary heart disease, cardiomyopathy, acute pulmonary edema, dyspnea, or rheumatic heart disease were sampled using prespecified sampling fractions within the ARIC communities for age, sex, and race (Black participants or White).
Definition of HF
Medical records of hospitalized patients from 20 hospitals indicating signs or symptoms of HF were abstracted and adjudicated by ARIC physicians, as has been previously described. 14 Hospitalizations were classified as those for definite acute decompensated HF, probable acute decompensated HF, stable chronic HF, not HF, or unclassifiable based on the medical record information available. Acute decompensated HF was defined as evidence of new‐onset or worsening of signs or symptoms of HF, compared with stable chronic HF. HF type was determined by the abstracted ejection fraction, from either the inpatient diagnostic tests or preadmission imaging studies. HF was classified as HFpEF in patients with left ventricular ejection fraction ≥50% and HF with reduced ejection fraction (HFrEF) in patients with an ejection fraction of <50%. Patients were excluded if they had any prior documented ejection fraction <50%. Hospitalizations that were not classified as definite or probable acute decompensated HF were excluded, and those with HFrEF or other primary cardiomyopathy (infiltrative, restrictive, or valvular) were excluded (Figure 1).
Figure 1. Patient inclusion flow chart.
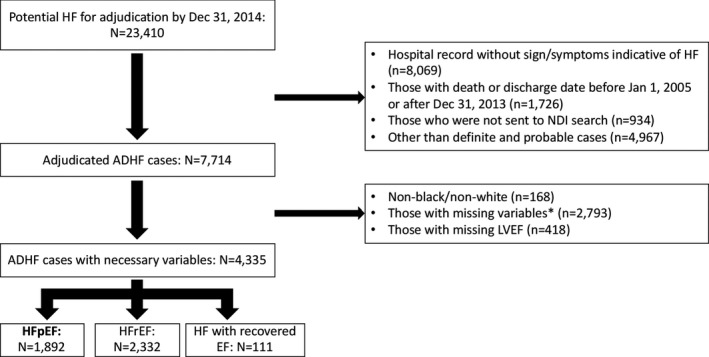
ADHF indicates acute decompensated heart failure; EF, ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; and NDI, National Death Index.
Baseline Characteristics, Comorbid Conditions, and Outcomes
All data on baseline characteristics and comorbidities were obtained from detailed abstraction of the medical record. For laboratory measurements, ARIC systematically recorded the worst (eg, highest value for serum creatinine and serum urea nitrogen and lowest value for hemoglobin and sodium) and last values during hospitalization. Kidney function was primarily evaluated by estimated glomerular filtration rate, which was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) creatinine‐based formula with the worst and last serum creatinine, respectively. 15 , 16 Covariates of interest were age, race, sex, history of HF, hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, pulmonary hypertension, chronic obstructive pulmonary disease (COPD), heart rate, systolic blood pressure (SBP), serum sodium (worst), estimated glomerular filtration rate (worst), and body mass index (BMI). Blood pressure (BP) and heart rate measured at admission were used in this analysis. Mortality outcomes were assessed for up to 1 year following admission by linking hospital records to the National Death Index.
Statistical Analysis
To account for the sampling design, all analyses were conducted using survey procedures and weighted by the inverse of the sampling probabilities to appropriately account for the stratified sampling design. Details are provided elsewhere but the sampling probabilities were created based on ICD‐9‐CM codes, field center, sex, and race (by race in Forsyth County and Jackson only) to optimize variance estimates. 17 Therefore, all results are presented (including in the Tables and Figures) as weighted number of hospitalizations. 18 Baseline patient characteristics were described according to sex and race subgroups. Means were compared using t tests, and proportions were compared using chi‐square tests. Survival probability was estimated by Kaplan‐Meier method. Adjusted hazard ratios (HR) of 1‐year mortality were compared by race and sex using Cox proportional hazards models (HR), 19 with adjustment for the following covariates: age, race, and sex (minimally adjusted model), and additionally history of HF hospitalization, hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, pulmonary hypertension, COPD, end‐stage renal disease, heart rate, SBP, serum sodium, estimated glomerular filtration rate, BMI, and hemoglobin (fully adjusted model). We selected variables that are established predictors of mortality in HF since the ARIC surveillance study has limited data available as it relies on data abstraction from medical records. 20 Each variable was tested for interaction with sex and race, respectively. All statistical tests were 2‐sided, with a P value <0.05 considered statistically significant. All statistical analyses were performed using STATA version 13 (StataCorp LLC).
Results
Overall Population
Of a total of 2847 hospitalizations, those with missing covariates for the fully adjusted model were excluded (n=955), leaving 1892 hospitalizations (weighted n=8987) included in this analysis (Figure 1). The baseline characteristics of patients hospitalized with acute HFpEF from 2005 to 2013 are summarized in Table 1. Characteristics of patients who were excluded compared with those included are summarized in Table S1. The mean age was 77.2 years (10.4 years); women constituted 64.9% of the overall study sample, and 23.4% were Black participants. There was a high prevalence of comorbidities including hypertension (88.7%), diabetes mellitus (47.2%), and coronary artery disease (44.4%), with a mean BMI of 31.3 kg/m2. Comparisons by sex and race are shown in Table 2. Mortality for the overall population was 33.3% at 365 days.
Table 1.
Characteristics of 8987 Hospitalized Patients With Acute HFpEF From the ARIC Community Surveillance Study (2005–2014)
| No. (%), Mean (SD), or Median [IQI] | Unweighted No. (Before Excluding Covariates Missing in the Fully Adjusted Model) | |
|---|---|---|
| N=8987 (weighted)* | 2847 | |
| Age, y | 77.2 (10.4) | 2847 |
| Men, % | 3153 (35.1) | 2847 |
| Black participants, % | 2101 (23.4) | 2847 |
| Admission to teaching hospital, % | 3084 (34.3) | 2847 |
| History of HF hospitalization, % | 2282 (25.4) | 2847 |
| Hypertension, % | 7968 (88.7) | 2847 |
| Diabetes mellitus, % | 4245 (47.2) | 2846 |
| Atrial fibrillation/flutter, % | 3602 (40.1) | 2847 |
| Coronary artery disease, % | 3989 (44.4) | 2591 |
| Pulmonary hypertension, % | 1859 (20.7) | 2845 |
| Sleep apnea, % | 1397 (15.5) | 2847 |
| COPD, % | 3414 (38.0) | 2847 |
| End‐stage renal disease, % | 467 (5.2) | 2847 |
| Heart rate, beats per min | 86.5 (23.6) | 2824 |
| SBP, mm Hg | 146.7 (32.3) | 2813 |
| BMI, kg/m2 | 31.3 (10.8) | 2440 |
| Sodium (worst), mmol/L | 136.1 (4.5) | 2825 |
| eGFR, mL/min per 1.73 m2 | 40.0 (22.3) | 2530 |
| BNP (worst), pg/mL | 550 [285–1041] | 1999 |
| NT‐proBNP (worst), pg/mL | 3640 [1611–8148] | 494 |
| Hemoglobin (last), g/dL | 11.2 (1.9) | 2821 |
ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IQI, interquartile interval; and SBP, systolic blood pressure.
The available unweighted number of patients was 1892 except for following laboratory results: B‐type natriuretic peptide (BNP; n=1378) and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; n=298).
Table 2.
Characteristics of Hospitalized Patients With Acute HFpEF From the ARIC Community Surveillance Study by Sex and Race
| Women (n=5834) | Men (n=3153) | P Value | White Participants (n=6886) | Black Participants (n=2101) | P Value | |
|---|---|---|---|---|---|---|
| Age (mean), y | 78.1 (10.1) | 75.6 (10.8) | <0.001 | 78.9 (9.3) | 71.9 (12.2) | <0.001 |
| Men, % | … | … | … | 35.3 | 34.4 | 0.38 |
| Black participants, % | 23.6 | 22.9 | 0.38 | … | … | … |
| Admission to teaching hospital, % | 33.1 | 36.6 | 0.01 | 35.7 | 29.8 | 0.01 |
| History of HF hospitalization, % | 26.2 | 23.8 | 0.26 | 22.0 | 36.4 | <0.001 |
| Hypertension, % | 90.0 | 86.3 | 0.02 | 87.4 | 92.8 | 0.001 |
| Diabetes mellitus, % | 47.8 | 46.2 | 0.50 | 42.1 | 64.1 | <0.001 |
| Atrial fibrillation/flutter, % | 39.5 | 41.1 | 0.51 | 44.8 | 24.5 | <0.001 |
| Coronary artery disease, % | 40.4 | 51.8 | <0.001 | 45.9 | 39.3 | 0.01 |
| Pulmonary hypertension, % | 22.8 | 16.7 | 0.003 | 20.4 | 21.7 | 0.52 |
| Sleep apnea, % | 12.6 | 21.0 | <0.001 | 14.9 | 17.7 | 0.13 |
| COPD, % | 38.0 | 38.0 | 0.98 | 38.6 | 36.2 | 0.35 |
| End‐stage renal disease, % | 4.3 | 6.9 | <0.001 | 3.6 | 10.3 | <0.001 |
| Heart rate, beats per min | 87.5 (23.3) | 84.7 (24.0) | 0.02 | 87.1 (22.8) | 84.7 (24.9) | 0.04 |
| SBP, mm Hg | 146.7 (32.0) | 146.7 (32.7) | 0.99 | 144.4 (29.1) | 154.1 (41.4) | <0.001 |
| BMI, kg/m2 | 31.6 (11.7) | 30.6 (8.6) | 0.03 | 30.3 (9.5) | 34.6 (14.4) | <0.001 |
| Sodium (worst), mmol/L | 135.8 (4.6) | 136.5 (4.4) | 0.002 | 135.9 (4.3) | 136.5 (5.0) | 0.02 |
| eGFR (worst), mL/min per 1.73 m2 | 38.8 (20.7) | 42.3 (24.9) | 0.002 | 40.2 (19.4) | 39.5 (31.7) | 0.56 |
| BNP (worst), pg/mL | 981.4 (1970.5) | 857.5 (1026.5) | 0.15 | 938.0 (1731.0) | 932.7 (1247.7) | 0.95 |
| NT‐proBNP (worst), pg/mL | 8055.4 (14615.8) | 8177.3 (12412.9) | 0.94 | 6726.6 (8139.2) | 9843.4 (19357.5) | 0.11 |
| Hemoglobin (last), g/dL | 11.0 (1.7) | 11.5 (2.1) | <0.001 | 11.3 (1.7) | 10.8 (2.3) | <0.001 |
Data are reported as number (percentage), mean (SD), or median [interquartile interval]. ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; BNP, B‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and SBP, systolic blood pressure.
Sex Comparison of Characteristics and Prognosis in HFpEF
Acute HFpEF hospitalization comparisons by sex are summarized in Table 2. Compared with men, women were older (78.1 versus 75.6 years, P<0.001), with more hypertension (90.0% versus 86.3%, P=0.02), more pulmonary hypertension (22.8% versus 16.7%, P=0.003), and higher BMI (31.6 versus 30.6 kg/m2, P=0.03). Women had higher resting heart rate (87.5 versus 84.7 beats per minute, P=0.02), worse renal function (estimated glomerular filtration rate 38.8 versus 42.3 mL/min per 1.73 m2, P=0.002), and lower hemoglobin (11.0 versus 11.5 g/dL, P<0.001) compared with men. Men had higher rates of coronary artery disease (51.8% versus 40.4%, P<0.001) and sleep apnea (21.0% versus 12.6%, P<0.001). There was no difference in mortality by sex at 365 days: 34.4% in men versus 32.8% in women (Figure 2A, P=0.40). However, in the fully adjusted analysis (Table 3), men had increased risk of death (HR, 1.27; 95% CI, 1.06–1.52 [P=0.02]) compared with women.
Figure 2. Survival of hospitalized heart failure with preserved ejection fraction (HFpEF) by (A) sex and (B) race.
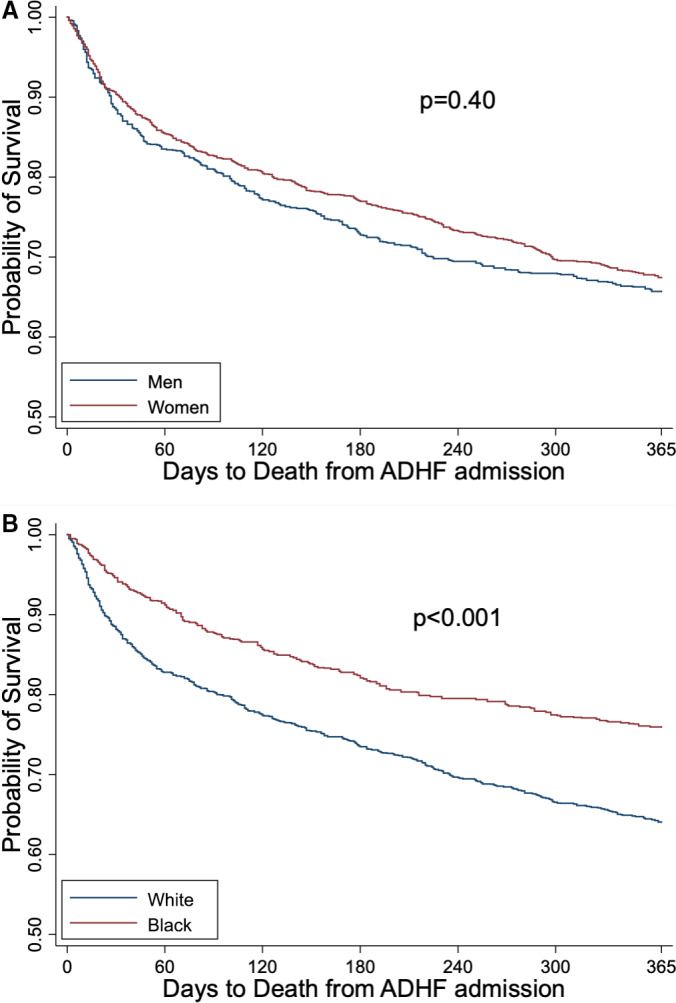
ADHF indicates acute decompensated heart failure.
Table 3.
Adjusted HRs of 365‐Day Mortality in Patients With HFpEF by Sex and Race
| Minimally Adjusted Model* | Fully Adjusted Model** | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Men | 1.18 (0.99–1.41) | 0.06 | 1.27 (1.06–1.52) | 0.02 |
| Black participants | 0.76 (0.62–0.92) | 0.01 | 0.79 (0.64–0.97) | 0.02 |
Minimally adjusted model: adjusted for age, race, and sex, as appropriate.
Fully adjusted model: minimally adjusted model+history of heart failure hospitalization, hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, pulmonary hypertension, chronic obstructive pulmonary disease, end‐stage renal disease, heart rate, systolic blood pressure, serum sodium, estimated glomerular filtration rate, body mass index, and hemoglobin. HR indicates hazard ratio; and HFpEF, heart failure with preserved ejection fraction.
Predictors of Mortality by Sex in HFpEF
In multivariable analysis of predictors of mortality by sex (Figure 3), age, heart rate, and worse renal function were independent predictors of mortality in both men and women. In women, atrial fibrillation and COPD were associated with increased mortality; in men, pulmonary hypertension was associated with increased mortality; however, without a significant P value for interaction (Table S2). Higher SBP was associated with lower mortality for both men and women. Higher BMI was associated with lower mortality in men, with borderline significant interaction term by sex (odds ratio [OR] in men, 0.82 [95% CI, 0.73–0.93]; OR in women, 0.96 [95% CI, 0.90–1.02], P=0.06) (Table S2).
Figure 3. Adjusted odds ratios of 365‐day mortality by sex in heart failure (HF) with preserved ejection fraction.*.
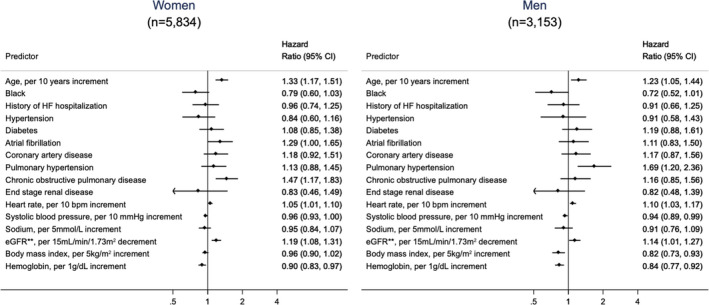
*Predictors in fully adjusted model: age, race, sex, history of HF hospitalization, hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, pulmonary hypertension, chronic obstructive pulmonary disease, end‐stage renal disease, heart rate, systolic blood pressure, serum sodium, and estimated glomerular filtration rate (eGFR).
Race Comparison of Characteristics and Prognosis in HFpEF
HFpEF comparisons by race are summarized in Table 2. Compared with White patients, Black patients were younger (71.9 versus 78.9 years, P<0.001), with higher rates of prior HF hospitalization (36.4% versus 22.0%, P<0.001), and lower rates of hospitalization at a teaching hospital (29.8% versus 35.7%, P=0.01). Black patients had more hypertension (92.8% versus 87.4%, P=0.001) and diabetes mellitus (64.1% versus 42.1%, P<0.001), along with higher BP (SBP 154 versus 144 mm Hg, P<0.001), and higher BMI (34.6 versus 30.3 kg/m2, P<0.001), compared with White patients. White patients had higher rates of coronary artery disease (45.9% versus 39.3%, P=0.01) and atrial fibrillation/flutter (44.8% versus 24.5%, P<0.001) compared with Black patients. White patients had significantly higher mortality at 1 year compared with Black patients (36.1% versus 24.4%; Figure 2B, P<0.001); this was seen in fully adjusted analysis (Table 3) as well, with Black participants having lower risk of death (HR, 0.79; 95% CI, 0.64–0.97 [P=0.02]).
Predictors of Mortality by Race in HFpEF
In multivariable analysis of predictors of mortality by race (Figure 4), age and heart rate were predictors of mortality in both races. Atrial fibrillation, COPD, and worse renal function were associated with worse survival in White participants and pulmonary hypertension was associated with worse survival in Black participants, but without a significant interaction by race (Table S3). Higher BMI was associated with improved survival in White participants only. Higher SBP was associated with improved survival in both races, with significant interaction by race (White: OR, 0.97 [95% CI, 0.94–1.00]; Black participants OR, 0.90 [95% CI, 0.85–0.95], P=0.02) (Table S3).
Figure 4. Adjusted odds ratios of 365‐day mortality by race in heart failure (HF) with preserved ejection fraction.*.
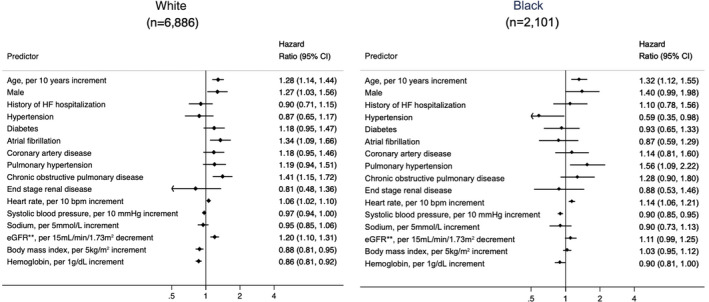
*Predictors in fully adjusted model: age, race, sex, history of HF hospitalization, hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, pulmonary hypertension, chronic obstructive pulmonary disease, end‐stage renal disease, heart rate, systolic blood pressure, serum sodium, and estimated glomerular filtration rate (eGFR).
Discussion
In the ARIC Community Surveillance Study, we compared clinical characteristics and outcomes in hospitalized patients with acute HFpEF by sex and race. The overall cohort was representative of clinical HFpEF today: female predominance, high rates of comorbidities, and a high overall mortality rate of 33.2% at 1 year following hospitalization. Men had an increased risk of mortality compared with women in adjusted analysis, and White participants had increased mortality compared with Black participants in unadjusted and adjusted analyses. Higher BP was associated with lower mortality among all subgroups, with a significant interaction by race. Age, elevated heart rate, renal disease, and anemia were associated with higher mortality among all subgroups. In a disease plagued by heterogeneity, the findings of this study highlight key prognostic factors associated with survival by sex and race.
Sex Differences in Mortality of HFpEF
We found that men had a higher risk of mortality than women in adjusted analysis, which is generally consistent with studies to date, 7 , 9 , 21 although a study of in‐hospital mortality in HFpEF suggested similar outcomes between men and women. 22 In multivariable analysis, atrial fibrillation and COPD were associated with higher mortality in women, while pulmonary hypertension was associated with higher mortality in men, but without significant interaction by sex. Atrial fibrillation is associated with adverse cardiovascular outcomes in HFpEF, and, when present in women with HFpEF, has been associated with a greater risk of adverse events compared with men. 23 Potential sex differences in underlying mechanisms of atrial fibrillation and implications for HFpEF are not well understood, however. Similarly, when present in the setting of HFpEF, pulmonary hypertension is associated with worse outcomes; however, sex differences in pulmonary hypertension are poorly understood. 24 Each of these comorbid conditions warrants further investigation as we try to better understand the role of sex in HFpEF phenotyping.
We found that BMI was inversely associated with mortality in men, with a signal for interaction by sex. Obesity is common in patients with HFpEF, and has been proposed as a unique phenotype of HFpEF associated with increased left ventricular remodeling, worse right ventricular function, increased ventricular volumes, and worse exercise capacity compared with nonobese patients with HFpEF. 25 However, there is a known inverse relationship between BMI and mortality in HF, often termed the obesity paradox. This has been previously described as either U‐shaped or J‐shaped in both HFpEF and HFrEF patient cohorts. 26 , 27 Therefore, while obesity may be implicated as a risk factor in the pathogenesis of HFpEF, 28 it appears to be protective to a certain extent after the development of HF, and our findings suggest that further study to understand the role sex interaction with BMI in HFpEF pathogenesis and prognosis is warranted.
Race Differences in Mortality in HFpEF
Our findings that Black patients with HFpEF have greater prevalence of hypertension, diabetes mellitus, and obesity, but less coronary artery disease when compared with White patients, is consistent with prior studies to date. 7 , 11 , 29 , 30 The comorbidity profiles of Black participants versus White participants with HFpEF likely bear mechanistic implications. Black participants have a greater incidence of hypertension and diastolic dysfunction at the population level when compared with White participants, and studies have shown increased left atrial volume, lower left ventricular diastolic function, and higher left ventricular mass indices in Black participants compared with White participants without HF, even after controlling for variables such as age, sex, BP, and common comorbidities. 31 , 32 The higher prevalence of obesity and diabetes mellitus in Black participants would suggest a predominantly metabolic syndrome phenotype of HFpEF, manifesting at an earlier age compared with White participants who are older with more coronary heart disease. We found that Black participants had lower mortality than White participants, even in fully adjusted analysis, suggesting that race may play a role in survival beyond comorbidities alone.
BP and Race Interaction in HFpEF
We found that higher SBP at the time of presentation with acute decompensated HF was protective among all 4 race and sex subgroups of HFpEF, with significant interaction by race. Hypertension is known to be more prevalent in patients with HFpEF compared with those with HFrEF, and is an established risk factor for incident HFpEF. 3 The paradoxical relationship between lower BP and poor prognosis is well established for patients with HFrEF, 33 , 34 and has been demonstrated in patients with HFpEF as well. 35 More recently, this relationship has been described as a reverse J curve for both patients with HFpEF and those with HFrEF. 36 Our finding of an interaction between BP and race in HFpEF is novel, and warrants further study.
Common Potent Predictors of Mortality in HFpEF
We found that age, heart rate, renal disease, and anemia were associated with increased mortality among all 4 subgroups of HFpEF. These are known markers of poor prognosis in HFrEF, and are increasingly being recognized to have prognostic implications in HFpEF. HFpEF has traditionally been considered a disease of an aging population; however, population studies and clinical trials are reporting an increasingly younger age of presentation or enrollment, suggesting an evolving demographic of younger patients with many comorbid conditions. Whether age in itself will remain a key underlying player in disease pathogenesis and prognosis remains to be seen. Heart rate has been known to be a marker of poor cardiac reserve and prognosis in HFrEF; however, there are limited data on heart rate and prognosis in patients with HFpEF. 37 , 38 Further investigation is needed to determine the implications of basal heart rate—whether elevated resting heart rate is a marker of disease severity and/or whether targeting heart rate in HFpEF would be associated with therapeutic success, recognizing that β‐blocker therapy has not proven successful to date in patients with HFpEF. Chronic kidney disease has long been known to be associated with poor prognosis in HF, of both phenotypes. 39 Chronic kidney disease in older patients has been proposed as an independent phenotype in HFpEF, 13 associated with worse prognosis compared with younger patients with relatively intact renal function. The underlying mechanisms of renal dysfunction in patients with HFpEF are many, including renal disease from long‐standing hypertension, diabetes mellitus, and the development of cardiorenal syndrome over time. Strategies to mitigate renal impairment both in the outpatient and inpatient settings are in need of further exploration.
Study Limitations
The findings of the present study should be interpreted in the context of several limitations. As this is the community surveillance component of the ARIC study, the data presented are from 4 US communities and may not represent the entire US population. Furthermore, the majority of Black patients are from the Jackson, MS, community, and therefore racial versus regional differences cannot entirely be separated. The ARIC community surveillance population includes patients >55 years, and may not be generalizable to younger patients with HFpEF. In addition, a significant number of HFpEF hospitalizations identified were excluded (33.2%) so that a complete case analysis could be completed, creating the potential for bias. Finally, residual confounding by socioeconomic status, education level, and income are potential additional limitations of this study, as these data are not available.
Conclusions
HFpEF represents a major unmet need in cardiovascular medicine today, with ongoing efforts to identify clinical and mechanistic subgroups, or phenotypes, within HFpEF to better target therapies. We sought to understand differences in sex and race in hospitalized patients with HFpEF from the community surveillance population of the ARIC study, a large sample that is unique in its geographic and racial diversity. We identified differences in patient characteristics and outcomes by sex and race in a diverse HFpEF population, which may help strategize targeted monitoring and management of HFpEF.
Sources of Funding
None.
Disclosures
K.S. receives funding from an American Heart Association Go Red for Women Network Grant (#16SFRN28780016), Dallas, TX, and a Johns Hopkins Clinician Scientist Award. K.S. is also a consultant and scientific advisory board member for Novartis and Janssen and receives honoraria. The remaining authors have no disclosures to report.
Supporting information
Table S1–S3
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2020;9:e014669 DOI: 10.1161/JAHA.119.014669.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.014669.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 4. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the optimize‐HF registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 5. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal P, Paul T, Almarzooq ZI, Peterson JC, Krishnan U, Swaminathan RV, Feldman DN, Wells MT, Karas MG, Sobol I, et al. Sex‐ and race‐related differences in characteristics and outcomes of hospitalizations for heart failure with preserved ejection fraction. J Am Heart Assoc. 2017;6:e003330 DOI: 10.1161/JAHA.116.003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (adhere) database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 9. Deswal A, Bozkurt B. Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2006;97:1228–1231. [DOI] [PubMed] [Google Scholar]
- 10. Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol. 2004;94:1003–1007. [DOI] [PubMed] [Google Scholar]
- 11. Ziaeian B, Heidenreich PA, Xu H, DeVore AD, Matsouaka RA, Hernandez AF, Bhatt DL, Yancy CW, Fonarow GC. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. JACC: Heart Fail. 2017;5:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis EF, Claggett B, Shah AM, Liu J, Shah SJ, Anand I, O'Meara E, Sweitzer NK, Rouleau JL, Fang JC, et al. Racial differences in characteristics and outcomes of patients with heart failure and preserved ejection fraction in the treatment of preserved cardiac function heart failure trial. Circulation: Heart Fail. 2018;11:e004457. [DOI] [PubMed] [Google Scholar]
- 13. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, et al. Incidence and survival of hospitalized acute decompensated heart failure in four us communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, et al. Comparison of risk prediction using the CKD‐EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circulation: Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ, Rosamond WD. Trends in hospitalizations and survival of acute decompensated heart failure in four us communities (2005–2014): ARIC study community surveillance. Circulation. 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arora S, Stouffer GA, Kucharska‐Newton A, Vaduganathan M, Qamar A, Matsushita K, Kolte D, Reynolds HR, Bangalore S, Rosamond WD, et al. Fifteen‐year trends in management and outcomes of non‐ST‐segment‐elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203 DOI: 10.1161/JAHA.118.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bloom MW, Greenberg B, Jaarsma T, Januzzi JL, Lam CSP, Maggioni AP, Trochu JN, Butler J. Heart failure with reduced ejection fraction. Nat Rev Dis Primers. 2017;3:17058. [DOI] [PubMed] [Google Scholar]
- 21. Zsilinszka R, Shrader P, DeVore AD, Hardy NC, Mentz RJ, Pang PS, Peacock WF, Fonarow GC, Hernandez AF. Sex differences in the management and outcomes of heart failure with preserved ejection fraction in patients presenting to the emergency department with acute heart failure. J Cardiac Fail. 2016;22:781–788. [DOI] [PubMed] [Google Scholar]
- 22. Hsich EM, Grau‐Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, Fonarow GC. Sex differences in in‐hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163:430–437.e3. [DOI] [PubMed] [Google Scholar]
- 23. O'Neal WT, Sandesara P, Hammadah M, Venkatesh S, Samman‐Tahhan A, Kelli HM, Soliman EZ. Gender differences in the risk of adverse outcomes in patients with atrial fibrillation and heart failure with preserved ejection fraction. Am J Cardiol. 2017;119:1785–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guazzi M, Gomberg‐Maitland M, Arena R. Pulmonary hypertension in heart failure with preserved ejection fraction. J Heart Lung Transplant. 2015;34:273–281. [DOI] [PubMed] [Google Scholar]
- 25. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Padwal R, McAlister FA, McMurray JJ, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta‐analysis of individual patient data. Int J Obes. 2014;38:1110–1114. [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, Cleland JG. Body mass index and all‐cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose‐response meta‐analysis. Clin Res Cardiol. 2019;108:119–132. [DOI] [PubMed] [Google Scholar]
- 28. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 29. Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart failure with preserved ejection fraction in African Americans: The ARIC (atherosclerosis risk in communities) study. JACC: Heart Fail. 2013;1:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma K, Hill T, Grams M, Daya NR, Hays AG, Fine D, Thiemann DR, Weiss RG, Tedford RJ, Kass DA, et al. Outcomes and worsening renal function in patients hospitalized with heart failure with preserved ejection fraction. Am J Cardiol. 2015;116:1534–1540. [DOI] [PubMed] [Google Scholar]
- 31. Sharp A, Tapp R, Francis DP, Mc GTSA, Hughes AD, Stanton AV, Zambanini A, Chaturvedi N, Byrd S, Poulter NR, et al. Ethnicity and left ventricular diastolic function in hypertension an ascot (Anglo‐Scandinavian cardiac outcomes trial) substudy. J Am Coll Cardiol. 2008;52:1015–1021. [DOI] [PubMed] [Google Scholar]
- 32. Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR Jr, Sidney S, Wu CO, Cook NL, Lewis CE, et al. Race‐ethnic and sex differences in left ventricular structure and function: the coronary artery risk development in young adults (CARDIA) study. J Am Heart Assoc. 2015;4:e001264 DOI: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (optimize‐HF). Am Heart J. 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 34. Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. [DOI] [PubMed] [Google Scholar]
- 35. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, Jeon ES, Kim MS, Hwang KK, Chae SC, et al. Reverse j‐curve relationship between on‐treatment blood pressure and mortality in patients with heart failure. JACC. Heart Fail. 2017;5:810–819. [DOI] [PubMed] [Google Scholar]
- 37. Shang X, Lu R, Liu M, Xiao S, Dong N. Heart rate and outcomes in patients with heart failure with preserved ejection fraction: a dose‐response meta‐analysis. Medicine (Baltimore). 2017;96:e8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takada T, Sakata Y, Miyata S, Takahashi J, Nochioka K, Miura M, Tadaki S, Shimokawa H; Investigators C . Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the chart‐2 study. Eur J Heart Fail. 2014;16:309–316. [DOI] [PubMed] [Google Scholar]
- 39. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014;35:455–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


