Cardiovascular disease is the dominant cause of death in patients on maintenance hemodialysis, with reports of 10 to 20 times higher than in the general population. 1 The association between chronic kidney disease and higher cardiovascular event rates following coronary intervention has been well documented. 2 Several studies have established that worse clinical outcomes following coronary revascularization track with the severity of renal dysfunction, with subjects on maintenance hemodialysis showing the worst clinical outcomes. 3 Patients on hemodialysis tend to have greater calcification of their coronary arteries, which hampers optimization of percutaneous coronary intervention (PCI) results 4 , 5 and leads to inappropriate vascular healing. 6 Both factors contribute to the poorer outcomes after coronary stent implantation in patients on hemodialysis. 7 , 8
As referenced above, the type of coronary lesions that develop both in native coronaries as well as in the neointima of stented lesions may influence outcomes in patients on maintenance hemodialysis. In an autopsy series of subjects dying of sudden coronary death, we described the most common cause of acute coronary thrombosis. Most frequently in occurrence was plaque rupture (65%), commonly found in men <50 years of age, followed by plaque erosion (30%), which was more commonly observed in young women (<50 years of age) smokers. The least frequent cause was calcified nodule (CN) (3%), which was found in older individuals 9 , 10 and was equally distributed in men and women. We defined CN as “a lesion with fibrous cap disruption that was associated with eruptive, dense, calcific nodules with the presence of luminal thrombus.” 2 Overall, because the incidence of CN is low and the number of cases collected by our institute far outnumbers by the other causes mentioned above, the precise mechanisms underlying the CN remain unknown. One possible hypothesis is that mechanical stress might fragment less calcified areas that are sandwiched between more rigid segments of sheet calcification. CN is most frequently observed in the right coronary artery, where hinge motion or excessive torsion stress is maximal during the cardiac cycle. 10 This hypothesis is supported by case reports and clinical studies. 11 , 12 , 13 One other point of distinction should also be made. It is important to recognize the difference between “eruptive CN” and “nodular calcification (NC)”; the latter occurs within the plaque and does not involve disruption of the fibrous cap or contact with the lumen, but is often associated with medial wall disruption with or without extension into the adventitia.
We described many years ago the phenomenon of neoatherosclerosis, in which lipid‐laden macrophage foam cells accumulate within the neointima of stented arteries, with or without necrotic core formation, or calcification or complications of thrombosis. Neoatherosclerosis as far as we know can encompass the full spectrum of atherosclerotic lesions, even the thrombotic lesions mentioned above. Thrombi in this setting are primarily associated with plaque rupture, whereas eruptive CNs have rarely been reported 11 , 14 and no systematic evaluation has ever been performed.
A study in this issue of the Journal of the American Heart Association (JAHA) by Nakamura et al15 adds interesting information about our knowledge of in‐stent restenosis (ISR) in the setting of hemodialysis and how this could potentially be in part responsible for the poor outcomes seen in these patients. The study was a histopathologic analysis of ISR lesions obtained by directional coronary atherectomy from 18 centers in Japan. 15 A total of 141 lesions were collected from the multicenter registry. However, a large number of cases were excluded (ie, 98 de novo and 14 recurrent restenosis lesions), leaving 29 ISR lesions from 29 patients for further evaluation. Eight lesions from 8 patients on dialysis and 21 lesions from 21 patients not on dialysis were collected, and histologic characteristics were compared between the 2 groups. Intravascular imaging evaluation (optical coherence tomography and/or intravascular ultrasound) demonstrated that CN/NC was observed at baseline (before first [ie, before stent placement] PCI) in most cases (4/7 cases; 57%) among patients on dialysis, whereas none was seen in the nondialysis cases (0/14). At follow‐up (ie, during PCI for the ISR lesion), the prevalence of in‐stent CN/NC was also significantly higher in the hemodialysis group compared with the nonhemodialysis group (75% versus 5%, respectively; P<0.01), despite the time to ISR being shorter in the hemodialysis group (median of 10.5 versus 23 months; P=0.13). On the other hand, the prevalence of in‐stent lipid‐rich plaque was significantly lower in the dialysis group (0% versus 43%, respectively; P=0.03). In all cases with an in‐stent CN/NC, the underlying calcification before stent implantation was moderate to severe. When tissue characteristics were stratified according to the duration of poststent implantation, most (4/6) of the in‐stent CN/NC in the dialysis group was observed within 1 year after stent implantation. The authors proposed the mechanisms of in‐stent CN/NC based on the duration after stent implantation. When CN/NC was observed early (in the first 3 months; n=2), the mechanism was related to the protrusion of a CN/NC (already present at the time of PCI) into the lumen through the stent struts. In‐stent CN/NC observed between 3 and 12 months (n=3) occurred from the underlying sheet calcification that fractured during stent implantation, and the least frequent cause was from in‐stent neoatherosclerosis (ie, neo‐CN or de novo CN) and occurred only late after stent implantation (N=2; >2 years after stenting).
To the best of our knowledge, this is the first study to evaluate the histological features of ISR tissue in patients on hemodialysis. Although the number of cases in this study was relatively small, meaning patient selection bias cannot be dismissed in the clinical study design, and the evaluation was performed from tissue removed following directional coronary atherectomy, the authors showed that ISR tissue in patients on hemodialysis showed greater incidence of CN compared with the patient without hemodialysis in the real‐world clinical setting. These findings raise important questions about therapeutic approaches for ISR lesions in patients on hemodialysis.
However, before we discuss the result of this study further, we need to address the discrepancy between the histologic definition of CN and that used in intravascular imaging studies. By histology, fibrous cap disruption and attachment of luminal thrombus are used to define any lesion as CN. On the other hand, intravascular imaging modalities cannot detect fibrous cap disruption with the presence of thrombus over the protruding calcified nodular mass in the lumen because of their limited resolution and artifacts. 16 Therefore, it is likely that CN, as defined by intravascular imaging studies, includes some lesions of NC, which does not involve luminal thrombi. In the current imaging‐based study, the authors did not differentiate CN from NC as such a distinction is not possible with current technologies available.
The authors have proposed 3 types of possible mechanisms of in‐stent CN/NC based on the duration of poststent implantation: type 1, in‐stent CN/NC caused by the protrusion of NC from the underlying plaque (secondary CN/NC) (early phase; <3 months); type 2, in‐stent CN/NC caused by the protrusion of underlying NC, which was sheet calcification at the time of stent implantation and likely fragmented because of mechanical stress, with eventual transformation into NC (middle phase; 3–24 months); and type 3, de novo CN/NC that occurred from in‐stent neoatherosclerosis (late phase; >24 months). Over 2 decades, CVPath stent autopsy registry had received >1200 stented lesions from 700 sudden death victims. 6 In‐stent CN/NC caused by the protrusion of NC (ie, type 1) has been observed in limited cases (Figure 1). In these cases, NC protruded at the time of initial stent implantation or in the follow‐up phase and caused in‐stent CN/NC (Figure 2) in both the early phase and late phase. 14
Figure 1. Images of in‐stent nodular calcification.
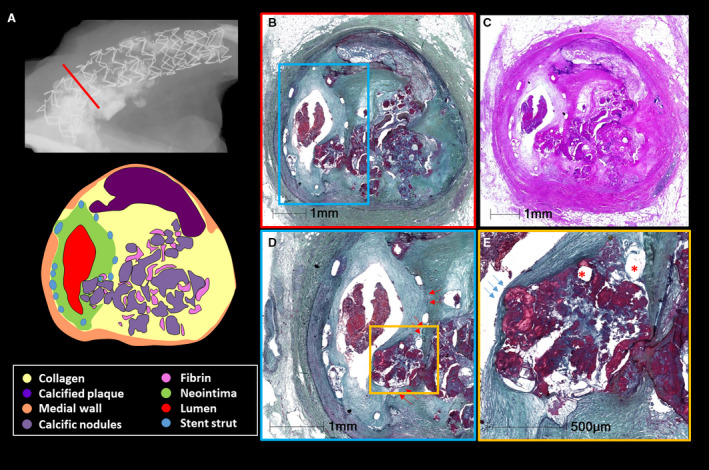
A 62‐year‐old woman with a history of coronary artery disease, breast cancer, and Hodgkin disease died because of sepsis. She underwent percutaneous coronary intervention with stenting in the right coronary artery 9 months before her death. A, Radiography image demonstrates severe calcification of the proximal right coronary artery with Xience (Santa Clara, CA) drug‐eluting stent in place. B and C, Images are corresponding cross‐section histologic images from the red line in image A, showing 50% in‐stent restenosis by neointimal thickening with underlying nodular calcification. D, Image is a high‐power image of blue boxed area in image B. Red arrows separate the border between neointimal tissue and underlying plaque tissue before stenting, suggesting that nodular calcification protruded into the lumen through the stent strut during stenting. E, Image shows the high‐power image of the boxed area in image D. Figure E is a high‐power image of the yellow boxed area in figure D. Note intact fibrous cap without luminal thrombus (blue arrows), which suggests that the lesion is nodular calcification and not a calcified nodule. Stent struts (asterisks) are located within the nodules (dark red stained area). B, D, and E, stained by Movat pentachrome; C, stained by hematoxylin and eosin.
Figure 2. Protrusion of nodular calcification into the lumen after stent implantation.
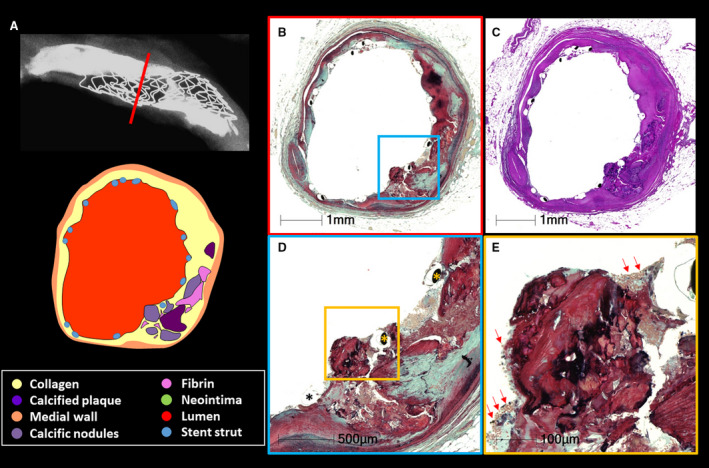
A 56‐year‐old man, a smoker with a history of hypertension and diabetes mellitus, presented with non–ST‐segment–elevation myocardial infarction and underwent percutaneous coronary intervention with a Resolute (Medtronic, Minneapolis, MN) drug‐eluting stent (DES) in the middle left anterior descending artery 1 month before death, from chronic heart failure. A, Radiographic image shows a DES implanted in a severely calcified coronary artery. B and C, Images that are corresponding cross‐section histologic images taken from the red line in image A. Essentially no neointimal growth is observed. D, Image is from the blue boxed area in image B. Nodular calcification protrudes into the lumen beyond the stent struts (asterisks). Image E is a high‐power image of the orange boxed area in D. Nodular calcification is not covered by a fibrous cap, but note attached thrombus. There is no fibrous cap identified in this section. B, D, and E, stained by Movat pentachrome; E, stained by hematoxylin and eosin.
However, the mechanisms of type 2 in‐stent CN/NC are questionable and difficult to prove. For the type 2 in‐stent CN/NC in the current study, poststenting intravascular imaging was available in only 1 case of 4 in the study. Therefore, one cannot be certain that there was no luminal protrusion after stenting. Also, even if underlying calcification did not protrude into the lumen and was not considered as NC at the time of stenting, intravascular imaging, especially intravascular ultrasound, can miss CN/NC because of acoustic shadowing. The number of individuals who underwent intravascular ultrasound/optical coherence tomography was not reported in this study. Therefore, assessments of plaque characteristics remain limited.
Type 3 in‐stent CN/NC (ie, de novo CN/NC) has only been reported once in an autopsy stent registry. 14 , 17 In accelerated atherosclerosis lesions, such as vein graft and in‐stent lesions, CN/NC can occur theoretically; however, we assume that such changes would take a far longer time than the authors report. CN/NC is mainly seen in older individuals, suggesting that the formation of CN is an event that takes decades to form. CNs/NCs in vein grafts have been reported only in 2 cases 15 and 33 years following coronary artery bypass grafting 11 , 18 ; one occurred at the hinge point where mechanical stress was the highest, whereas the cause of the other was unknown. In in‐stent lesions, because intravascular imaging devices cannot distinguish between de novo CN/NC and secondary CN/NC (ie, type 1), their relative prevalence remains unknown. However, we believe that in‐stent de novo CN/NC is extremely rare and difficult to prove as imaging must be performed before PCI and repeatedly after PCI. It is possible if stent struts are seen within the areas of calcification then the lesion likely occurred in a de novo manner. 14 Last, the time interval following stenting may have to be more than a decade or longer for CN/NC, to have had the time to form. We also need to recognize that stents form a rigid scaffold that makes the lesion less susceptible to mechanical stress and unlikely to lead to NC/CN unless there was a fracture of the stent that may also fracture the sheet calcium.
In summary, the work by Nakamura et al15 revealed that CNs/NCs were frequently seen in directional coronary atherectomy tissue removed from ISR lesions in patients on hemodialysis. The findings may help in the management of ISR lesions in patients on hemodialysis, which is one of the most challenging lesions to treat with PCI. However, directional coronary atherectomy–derived tissue samples provide only limited information and the mechanisms of in‐stent CN/NC remain controversial, especially the occurrence of de novo formation. Further studies are warranted to prove the mechanisms of in‐stent CN/NC.
Disclosures
CVPath Institute (Y.S., A.V.F., R.V.) has received institutional research support from R01 HL141425 Leducq Foundation Grant; 480 Biomedical; 4C Medical; 4Tech; Abbott; Accumedical; Amgen; Biosensors; Boston Scientific; Canon U.S.A.; Cardiac Implants; Celonova; Claret Medical; Concept Medical; Cook; CSI; DuNing, Inc; Edwards LifeSciences; Emboline; Endotronix; Envision Scientific; Lutonix/Bard; Gateway; Lifetech; Limflo; MedAlliance; Medtronic; Mercator; Merill; Microport Medical; Microvention; Mitraalign; Mitra assist; NAMSA; Nanova; Neovasc; NIPRO; Novogate; Occulotech; OrbusNeich Medical; Phenox; Profusa; Protembis; Qool; Recor; Senseonics; Shockwave; Sinomed; Spectranetics; Surmodics; Symic; Vesper; W.L. Gore; and Xeltis. A.V.F. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Sinomed; and Terumo Corporation; and is a consultant to Amgen; Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Lutonix Bard; and Sinomed. R.V. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; Cordis; CSI; Lutonix Bard; Medtronic; OrbusNeich Medical; CeloNova; SINO Medical Technology; ReCor; Terumo Corporation; W. L. Gore; and Spectranetics; and is a consultant to Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Cordis; CSI; Edwards Lifescience; Lutonix Bard; Medtronic; OrbusNeich Medical; ReCor; Sinomededical Technology; Spectranetics; Surmodics; Terumo Corporation; W. L. Gore; and Xeltis. Y.S. has no disclosures to report.
(J Am Heart Assoc. 2020;9:e018621 DOI: 10.1161/JAHA.120.018621.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 4.
See Article by Nakamura et al.
References
- 1. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;S16–S23. [PubMed] [Google Scholar]
- 2. Lu R, Tang F, Zhang Y, Zhu X, Zhu S, Wang G, Jiang Y, Fan Z. Comparison of drug‐eluting and bare metal stents in patients with chronic kidney disease: an updated systematic review and meta‐analysis. J Am Heart Assoc. 2016;9:e003990 DOI: 10.1161/JAHA.116.003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aoki J, Ikari Y. Cardiovascular disease in patients with end‐stage renal disease on hemodialysis. Ann Vasc Dis. 2017;327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenvinkel P, Pecoits‐Filho R, Lindholm B. Coronary artery disease in end‐stage renal disease: no longer a simple plumbing problem. J Am Soc Nephrol. 2003;1927–1939. [DOI] [PubMed] [Google Scholar]
- 5. Kawamura Y, Nagaoka M, Ito D, Iseki H, Ikari Y. A case of percutaneous coronary intervention procedure successfully bailed out from multiple complications in hemodialysis patient. Cardiovasc Interv Ther. 2013;76–80. [DOI] [PubMed] [Google Scholar]
- 6. Torii S, Jinnouchi H, Sakamoto A, Mori H, Park J, Amoa FC, Sawan M, Sato Y, Cornelissen A, Kuntz SH, et al. Vascular responses to coronary calcification following implantation of newer‐generation drug‐eluting stents in humans: impact on healing. Eur Heart J. 2020;786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;911–919. [DOI] [PubMed] [Google Scholar]
- 8. Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;2129–2140. [DOI] [PubMed] [Google Scholar]
- 9. Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;260–267. [DOI] [PubMed] [Google Scholar]
- 10. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;1262–1275. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka T, Yahagi K, Ninomiya K, Tomii D, Nakanishi T, Koseki K, Okuno T, Sato Y, Sato K, Komiyama K, et al. ST‐segment elevation myocardial infarction related to variable calcified lesions in saphenous vein graft 33 years after coronary artery bypass grafting. JACC Cardiovasc Interv. 2018;e181–e183. [DOI] [PubMed] [Google Scholar]
- 12. Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, Kanaji Y, Murai T, Yonetsu T, Kakuta T, et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging. 2017;883–891. [DOI] [PubMed] [Google Scholar]
- 13. Torii S, Mustapha JA, Narula J, Mori H, Saab F, Jinnouchi H, Yahagi K, Sakamoto A, Romero ME, Narula N, et al. Histopathologic characterization of peripheral arteries in subjects with abundant risk factors: correlating imaging with pathology. JACC Cardiovasc Imaging. 2019;1501–1513. [DOI] [PubMed] [Google Scholar]
- 14. Mori H, Finn AV, Atkinson JB, Lutter C, Narula J, Virmani R. Calcified nodule: an early and late cause of in‐stent failure. JACC Cardiovasc Interv. 2016;e125–e126. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura N, Torii S, Tsuchiya H, Nakano A, Oikawa Y, Yajima J, Nakamura S, Nakano M, Masuda N, Ohta H, Yumoto K, et al. Formation of calcified nodule as a cause of early in‐stent restenosis in patients undergoing dialysis. J Am Heart Assoc. 2020;9:e016595 DOI: 10.1161/JAHA.120.016595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hao H, Fujii K, Shibuya M, Imanaka T, Kawakami R, Hatakeyama K, Asada Y, Masuyama T, Hirota S. Different findings in a calcified nodule between histology and intravascular imaging such as intravascular ultrasound, optical coherence tomography, and coronary angioscopy. JACC Cardiovasc Interv. 2014;937–938. [DOI] [PubMed] [Google Scholar]
- 17. Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in‐stent atherosclerosis. Nat Rev Cardiol. 2016;79–98. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto K, Natsuaki M, Serikawa T, Okabe M, Yamamoto Y. Nodular calcification in saphenous vein graft successfully treated by percutaneous coronary intervention. Case Rep Cardiol. 2018;5138705. [DOI] [PMC free article] [PubMed] [Google Scholar]


