Abstract
Background
The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines lowered the threshold of blood pressure (BP) for hypertension to 130/80 mm Hg. However, the clinical significance of isolated diastolic hypertension (IDH) according to the cutoff value of the 2017 ACC/AHA guidelines was uncertain.
Methods and Results
We analyzed the claims database of Japan Medical Data Center (a nationwide epidemiological database). We excluded individuals who were aged <20 years, had systolic hypertension, were taking antihypertensive medication, or had prevalent cardiovascular disease, and studied 1 746 493 individuals (mean age, 42.9±10.7 years; 961 097 men [55.0%]). The average observational period was 1107±855 days. Stage 1 IDH, defined as diastolic BP 80 to 89 mm Hg, and stage 2 IDH, defined as diastolic BP ≥90 mm Hg, were found in 230 513 (13.2%) and 16 159 (0.9%) individuals, respectively. Compared with individuals with normal diastolic BP, individuals with stage 1 and stage 2 IDH were older and more likely to be men. Prevalence of classic risk factors was higher in patients with IDH. Kaplan–Meier curves showed that stage 1 and stage 2 IDH were associated with a higher incidence of cardiovascular events, defined as myocardial infarction, angina pectoris, and stroke. Multivariable analysis showed that stage 1 (hazard ratio [HR], 1.17) and stage 2 (HR, 1.28) IDH were independently associated with a higher incidence of cardiovascular events. Subgroup analyses showed that the association of IDH with cardiovascular events was seen irrespective of age and sex.
Conclusions
The analysis of a nationwide epidemiological database showed that IDH based on the cutoff value in the 2017 ACC/AHA BP guidelines was associated with an elevated risk of subsequent cardiovascular events.
Keywords: cardiovascular disease, epidemiology, isolated diastolic hypertension, prevention
Subject Categories: Epidemiology, Hypertension
Nonstandard Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- BPLTTC
Blood Pressure Lowering Treatment Trialists' Collaboration
- CARDIA
Coronary Artery Risk Development in Young Adults
- DBP
diastolic blood pressure
- IDH
isolated diastolic hypertension
- JMDC
the Japan Medical Data Center
- JPHC
Japan Public Health Center‐based Prospective Study
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Clinical Perspective
What Is New?
Our comprehensive analysis of a nationwide epidemiological database including individuals without prevalent cardiovascular disease (CVD) showed that stage 1 and stage 2 isolated diastolic hypertension (IDH) based on the cutoff value of diastolic blood pressure in the 2017 American College of Cardiology/American Heart Association guidelines independently increased the risk of subsequent CVD.
The association of IDH and incident CVD was observed regardless of age and sex.
This is the first large‐scale epidemiological study demonstrating the association of IDH with incident CVD among the general population.
What Are the Clinical Implications?
Our study suggests the potential clinical significance of IDH according to the cutoff value of diastolic blood pressure in the 2017 American College of Cardiology/American Heart Association guidelines in the development of CVD.
Further studies are warranted to establish the optimal management strategy for IDH based on the latest American College of Cardiology/American Heart Association guidelines.
Hypertension is a major cause of cardiovascular disease (CVD) 1 , 2 , 3 and is diagnosed based on both systolic blood pressure (SBP) and diastolic blood pressure (DBP). The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for hypertension lowered the threshold of blood pressure (BP) from 140/90 mm Hg to 130/80 mm Hg. 4 However, the 2018 European Society of Cardiology/European Society of Hypertension guidelines for the management of arterial hypertension retained the cutoff value of BP for hypertension at 140/90 mm Hg. 5 Although several studies confirmed the validity of the updated ACC/AHA classification of BP, 6 , 7 lowering the threshold of diastolic BP to 80 mm Hg was based on expert opinion. 8 Further, there have been conflicting data regarding the influence of DBP on subsequent cardiovascular events. 9 , 10 , 11 , 12 , 13 , 14 Particularly, McEvoy et al 9 recently indicated that isolated diastolic hypertension (IDH) according to the cutoff value of DBP, which the 2017 ACC/AHA guidelines suggested was not significantly associated with an increased risk for cardiovascular events. Therefore, the cutoff value of DBP in the 2017 ACC/AHA guidelines is still under debate, and further investigation is warranted to verify the validity of these guidelines. In this study, we sought to explore the association of IDH based on the cutoff value of DBP, which the 2017 ACC/AHA guidelines indicated with the risk of subsequent cardiovascular events among the general population without a prevalent history of CVD using a nationwide epidemiological database.
Methods
Study Design and Data Source
We conducted this retrospective observational analysis using the health claims database of the Japan Medical Data Center (JMDC; Tokyo, Japan), which has been described in detail in previous reports. 15 , 16 , 17 The JMDC collects data from >60 insurers and includes data for health insurance claims on insured individuals. More than 5 million individuals were registered in this database. Most individuals registered in the JMDC database are employees of relatively large companies in Japan. The JMDC database includes annual health checkup data including a questionnaire regarding medical history and status of medications and laboratory data. Data of clinical follow‐up from the first health checkup obtained by claim records are also included in the JMDC database. This database is available for anyone who purchases it from the JMDC (https://www.jmdc.co.jp/en/index).
Ethics
This study was approved by the institutional review board of the University of Tokyo (2018‐10862) in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived because of the anonymous nature of the JMDC database.
Definition
Incidence of CVD including myocardial infarction, angina pectoris, and stroke was evaluated using the International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis codes recorded in the claim records of each individual. 18 The primary end point was defined as a composite end point including myocardial infarction, angina pectoris, and stroke. Generally, healthcare professionals such as nurses measured the BP of resting individuals twice at health checkups according to the procedure recommended by the Ministry of Health, Labour and Welfare, and the Japanese Society of Cardiovascular Disease Prevention. The average of 2 measurements was recorded. We defined normal DBP as <80 mm Hg (and SBP <130 mmHg) stage 1 IDH as 80 mm Hg ≤ DBP <90 mm Hg (and SBP <130 mm Hg), and stage 2 IDH as DBP ≥90 mm Hg (and SBP <130 mm Hg). Obesity was defined as body mass index ≥25 kg/m2 according to the criteria of the Japan Society for the Study of Obesity (http://www.jasso.or.jp/contents/english/index.html#e1). 19 Abdominal obesity was defined as a waist circumference ≥85 cm for men and ≥90 cm for women. 20 Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL or ongoing antidiabetic therapy. Dyslipidemia was defined as low‐density lipoprotein cholesterol ≥140 mg/dL or high‐density lipoprotein cholesterol <40 mg/dL or triglycerides ≥150 mg/dL or ongoing lipid‐lowering therapy.
Statistical Analysis
We presented categorical and continuous data as number (percentage) and mean (SD). We compared categorical and continuous variables between groups using chi‐square test and 1‐way ANOVA. The long‐term event rate was estimated using Kaplan–Meier curves and log‐rank test to assess the differences in the event rate. We conducted multivariable Cox regression analysis including category of DBP, and established CVD risk factors at study entry (health checkup) including SBP, age, sex, obesity, high waist circumference, diabetes mellitus, dyslipidemia, and current cigarette smoking to identify the association of IDH with subsequent risk of composite end point. We additionally performed multivariable Cox regression analysis for heart failure, atrial fibrillation, and composite end point, defined as myocardial infarction, angina pectoris, and stroke. We performed multiple imputation for missing values (obesity, high waist circumference, diabetes mellitus, dyslipidemia, and current cigarette smoking), as previously described. 21 Multiple imputation is a statistical procedure to replace missing values in the original database with other plausible values by creating multiple filling‐in patterns to avoid bias caused by missing values in the original database. Multiple imputation is considered as an alternative procedure to analyze incomplete data as well. 22 Using multiple imputation by chained equation method, we replaced each missing value with a set of substituted plausible values by creating 20 filled‐in complete data sets. 23 We calculated hazard ratio and standard errors using Rubin rules. We also performed multivariable Cox regression analysis for the composite end point including population with all available measurements of confounding factors. The study population was divided into subgroups by age (≥50 years and 20–49 years) or sex. Incidence of composite end point was evaluated using Kaplan–Meier curves and log‐rank (Mantel–Cox) test in each subgroup. A P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (version 25, SPSS Inc) and STATA (version 16, StataCorp LLC).
Results
Study Population
We studied 2 943 563 individuals included in the JMDC database between January 2005 and August 2018. We excluded individuals with the following criteria: (1) age <20 years (n=36 788), (2) prior history of CVD and hemodialysis (obtained from the information including the patients’ questionnaire, recorded data on coronary artery revascularization, and recorded diagnosis of myocardial infarction, angina pectoris, and stroke before study enrollment) (n=101 934), (3) taking antihypertensive medication (n=202 303), (4) missing information about antihypertensive medication (n=391 975), (5) missing data on BP (n=3028), and (6) SBP ≥130 mm Hg (n=461 042). Finally, we included 1 746 493 individuals in this study. Of these, 1 499 821 individuals (85.9%) were classified as the normal DBP group, while 230 513 individuals (13.2%) and 16 159 individuals (0.9%) were classified as the stage 1 and stage 2 IDH groups, respectively. The average observational period was 1107±855 days.
Characteristics of the Study Population
Characteristics of the study population are shown in Table 1. Patients with IDH were older and more likely to be men. Patients with IDH had higher body mass index and waist circumference. SBP was higher in individuals with IDH. The prevalence of classic CVD risk factors such as dyslipidemia, diabetes mellitus, and current cigarette smoking were higher among those with high DBP.
Table 1.
Characteristics of the Study Population
| Missing | Normal DBP (1 499 821) | Stage 1 IDH (230 513) | Stage 2 IDH (16 159) | P Value | |
|---|---|---|---|---|---|
| Age, y | 0 (0.0) | 42.3±10.8 | 46.8±9.2 | 47.9±8.1 | <0.001 |
| 20–29 | 0 (0.0) | 222 284 (14.8) | 10 056 (4.4) | 322 (2.0) | |
| 30–39 | 0 (0.0) | 301 871 (20.1) | 28 899 (12.5) | 1453 (9.0) | |
| 40–49 | 0 (0.0) | 603 724 (40.3) | 103 223 (44.8) | 7533 (46.6) | |
| 50–59 | 0 (0.0) | 277 066 (18.5) | 67 678 (29.4) | 5565 (34.4) | |
| ≥60 | 0 (0.0) | 94 876 (6.3) | 20 657 (9.0) | 1286 (8.0) | |
| Men | 0 (0.0) | 781 285 (52.1) | 167 018 (72.5) | 12 794 (79.2) | <0.001 |
| Body mass index, kg/m2 | 785 (0.0) | 21.9±3.1 | 23.5±3.5 | 24.2±3.8 | <0.001 |
| Obesity | 785 (0.0) | 220 805 (14.7) | 69 007 (29.9) | 5944 (36.8) | <0.001 |
| Waist circumference, cm | 184 513 (10.6) | 78.3±8.8 | 83.1±9.4 | 84.9±9.6 | <0.001 |
| High waist circumference | 184 513 (10.6) | 236 623 (17.8) | 79 705 (36.4) | 6945 (44.4) | <0.001 |
| SBP, mm Hg | 0 (0.0) | 110.5±10.4 | 122.1±5.5 | 124.7±4.0 | <0.001 |
| Diabetes mellitus | 353 033 (20.2) | 26 517 (2.2) | 8381 (4.3) | 710 (5.1) | <0.001 |
| Dyslipidemia | 68 443 (3.9) | 452 217 (31.5) | 108 858 (48.0) | 8687 (54.3) | <0.001 |
| Current cigarette smoking | 12 966 (0.7) | 383 493 (25.8) | 67 471 (29.4) | 4691 (29.1) | <0.001 |
| Laboratory data | |||||
| Glucose, mg/dL | 355 924 (20.4) | 91.4±13.6 | 95.9±17.4 | 97.9±17.8 | <0.001 |
| HbA1c, % | 343 510 (19.7) | 5.4±0.5 | 5.5±0.6 | 5.6±0.7 | <0.001 |
| LDL‐C, mg/dL | 68 462 (3.9) | 116.5±30.8 | 125.6±31.8 | 128.5±31.9 | <0.001 |
| HDL‐C, mg/dL | 63 352 (3.6) | 64.9±16.4 | 61.6±16.6 | 60.3±16.4 | <0.001 |
| Triglycerides, mg/dL | 63 678 (3.6) | 92.3±68.6 | 121.8±96.4 | 137.6±113.6 | <0.001 |
Data are expressed as mean±SD or number (percentage). DBP indicates diastolic blood pressure; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; IDH, isolated diastolic hypertension; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
IDH and CVD Events
The number of CVD events is shown in Table 2. The actual rate of primary outcome was overall 0.57 (per 100 patient‐years). Actual rates of primary outcome of each group were 0.52 (per 100 patient‐years) in the normal DBP group, 0.81 (per 100 patient‐years) in the stage 1 IDH group, and 0.96 (per 100 patient‐years) in the stage 2 IDH group. Kaplan–Meier curves and the log‐rank test showed that the incidence of reaching the composite end point of myocardial infarction, angina pectoris, and stroke increased with higher DBP (Figure 1). Multivariable Cox regression analysis after multiple imputation showed that high DBP was independently associated with reaching the composite end point (Table 3). Multivariable Cox regression analysis after multiple imputation showed that high DBP was independently associated with elevated risk of heart failure, atrial fibrillation, and composite end point including myocardial infarction and stroke (Tables S1 through S3). Multivariable Cox regression analysis including population with all available measurements of confounding factors also showed that high DBP was independently associated with higher incidence of composite end point including myocardial infarction, angina pectors, and stroke (Table S4).
Table 2.
Cardiovascular Disease Events
| Missing | Normal DBP (1 499 821) | Stage 1 IDH (230 513) | Stage 2 IDH (16 159) | P Value | |
|---|---|---|---|---|---|
| Myocardial infarction | 0 (0.0) | 1564 (0.1) | 514 (0.2) | 45 (0.3) | <0.001 |
| Angina pectoris | 0 (0.0) | 16 826 (1.1) | 4133 (1.8) | 318 (2.0) | <0.001 |
| Stroke | 0 (0.0) | 6416 (0.4) | 1699 (0.7) | 163 (1.0) | <0.001 |
| Composite end point | 0 (0.0) | 23 563 (1.6) | 5903 (2.6) | 482 (3.0) | <0.001 |
Data are expressed as number (percentage). DBP indicates diastolic blood pressure; and IDH, isolated diastolic hypertension.
Figure 1. Crude cumulative incidences of composite end point including myocardial infarction, angina pectoris, and stroke (A); myocardial infarction (B); angina pectoris (C); and stroke (D).
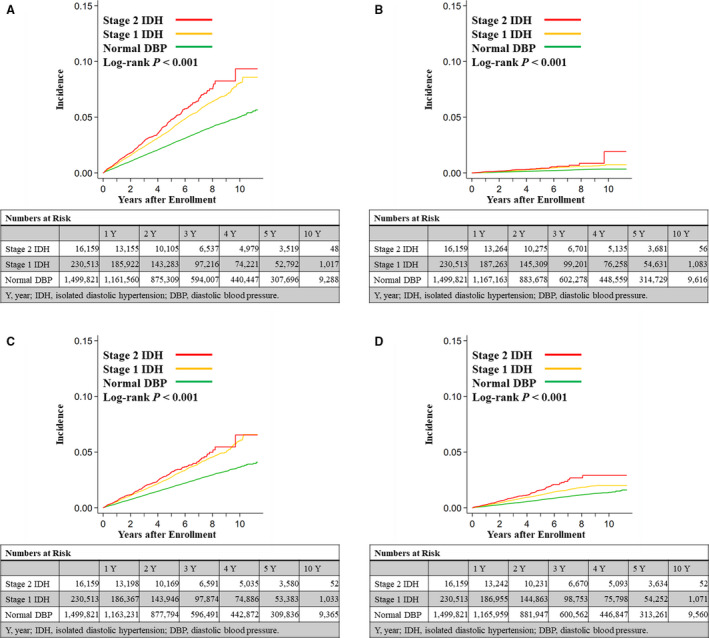
DBP indicates diastolic blood pressure; and IDH, isolated diastolic hypertension.
Table 3.
Multivariable Cox Regression Analysis for the Composite End Point After Multiple Imputation
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Category of DBP | |||
| Normal | Reference | ||
| Stage 1 IDH | 1.17 | 1.13–1.20 | <0.001 |
| Stage 2 IDH | 1.28 | 1.17–1.41 | <0.001 |
| SBP (per 10 mm Hg) | 1.04 | 1.03–1.05 | <0.001 |
| Age, y | 1.06 | 1.06–1.06 | <0.001 |
| Men | 0.99 | 0.96–1.01 | 0.348 |
| Obesity | 1.06 | 1.02–1.10 | 0.002 |
| High waist circumference | 1.14 | 1.10–1.18 | <0.001 |
| Diabetes mellitus | 1.45 | 1.38–1.53 | <0.001 |
| Dyslipidemia | 1.19 | 1.16–1.22 | <0.001 |
| Current cigarette smoking | 1.02 | 0.99–1.05 | 0.165 |
DBP indicates diastolic blood pressure; IDH, isolated diastolic hypertension; and SBP, systolic blood pressure.
Subgroup Analyses
Results of the subgroup analyses are shown in Figure 2. Kaplan–Meier curves and the log‐rank test presented that the association of high DBP with the incidence of CVD was seen in individuals aged ≥50 years (Figure 2A), aged <50 years (Figure 2B), men (Figure 2C), and women (Figure 2D).
Figure 2. Crude cumulative incidences of composite end point including myocardial infarction, angina pectoris, and stroke in individuals aged ≥50 years (A), individuals aged <50 years (B), men (C), and women (D).
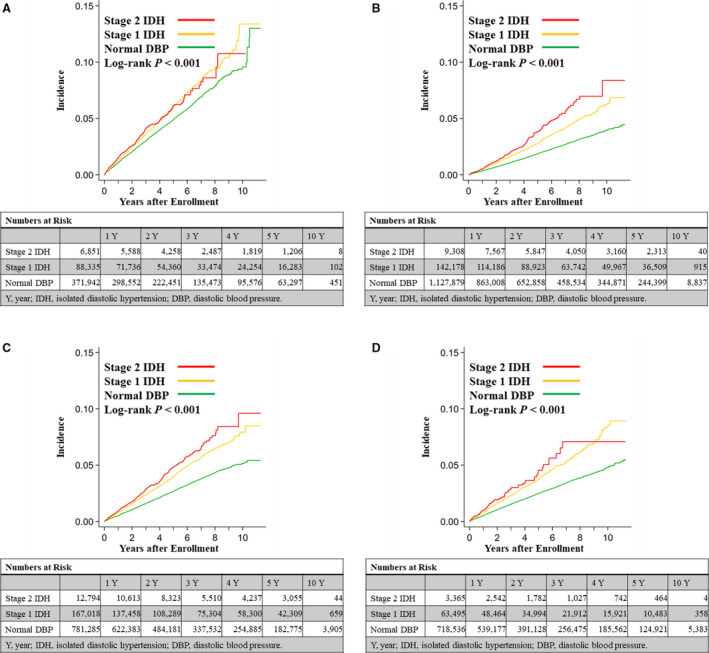
DBP indicates diastolic blood pressure; and IDH, isolated diastolic hypertension.
Discussion
Our analysis of a nationwide epidemiological database including 1 746 493 individuals who had normal SBP and no prior history of prevalent CVD demonstrated that the stage 1 and stage 2 IDH groups according to the cutoff value of DBP in the 2017 ACC/AHA guidelines were associated with an elevated risk of subsequent cardiovascular events. Individuals with stage 1 and stage 2 IDH had more compromised baseline parameters than individuals with normal DBP. However, even after adjustment for covariates, both stage 1 and stage 2 hypertension were associated with a higher incidence of subsequent cardiovascular events. The association of IDH and incident CVD was observed regardless of age and sex. To the best of our knowledge, this is the first large‐scale study uncovering the relationship between IDH based on the cutoff value of DBP in the 2017 ACC/AHA guidelines for BP and the development of CVD among the general population without prevalent CVD.
Reducing the threshold of BP for hypertension from 140/90 mm Hg to 130/80 mm Hg in the 2017 ACC/AHA guidelines 4 has attracted great clinical interest and has led to much debate. However, 2 large‐scale studies validated the 2017 ACC/AHA guidelines. 6 , 7 The analysis of the prospective cohort CARDIA (Coronary Artery Risk Development in Young Adults) study including 4851 young adults presented that patients with 1 stage hypertension and stage 2 hypertension as defined by the 2017 ACC/AHA guidelines had increased risk for subsequent CVD events compared with those with normal BP. 6 Similarly, the population‐based cohort study from the Korean National Health Insurance Service consisting of 2 488 101 young adults showed that individuals with baseline stage 1 and stage 2 hypertension compared with those with normal BP had a higher risk of cardiovascular events. 7
We earlier explored the relationship between stage 1/stage 2 hypertension and subclinical atherosclerosis. Our analysis including individuals undergoing voluntary health checkups showed that increased thickness of carotid intima‐media within the general population was seen in not only stage 2 but also stage 1 hypertension, suggesting the possible association between hypertension as defined by the 2017 ACC/AHA guidelines and subclinical atherosclerosis among the general population. 24 Further, we also reported that the prevalence of high cardio‐ankle vascular index increased in stage 1 hypertension, and further increased in stage 2 hypertension in men. 25 These studies imply the potential pathophysiological significance of stage 1 and stage 2 hypertension, which the 2017 ACC/AHA guidelines suggested among the general population.
However, the validity of IDH defined by the 2017 ACC/AHA guidelines has not yet been well established. Further, contrary to the robust evidence supporting the clinical significance of SBP, there are conflicting data on the clinical importance of DBP and IDH. 9 , 10 , 11 , 12 , 13 , 14 McEvoy et al 9 reported that IDH based on the 2017 ACC/AHA guidelines was not related to incident cardiovascular events. Difference in study population, analyzed outcomes, treatment status (the study by McEvoy et al included patients taking antihypertensive medications), and races might contribute to the difference in results of our study and the study by McEvoy et al. Although further studies are required to confirm our results, we believe that our findings presenting the association between IDH and incident CVD among the general population without prevalent CVD are informative for the optimal management of BP and the primary prevention of subsequent CVD.
The clinical significance of DBP is influenced by multiple factors including race, age, baseline CVD risk, medication status, duration of the study, and clinical end points analyzed, which results in conflicting clinical outcomes. For example, Yano et al 26 reported the potential racial difference in the prognostic significance of DBP. Further, DBP usually decreases with age because of reduced compliance of blood vessels, which can complicate the association of DBP with the risk of CVD. However, our subgroup analyses showed that a higher incidence of subsequent CVD in stage 1 and stage 2 IDH was seen in young as well as old individuals. Further, a similar relationship was observed in both men and women. Therefore, the pathological significance of IDH did not seemingly depend on age and sex in our study population. Analyzed end point could also influence the study results. Preceding studies showed that lower DBP was associated with elevated risk of coronary artery disease. 27 , 28 Peri‐Okonny et al reported that DBP was significantly associated with angina with a J‐shaped relationship. 27 Therefore, both low DBP and high DBP could increase the risk of angina pectoris. Further investigations are needed to determine the optimal value of DBP for the prevention of coronary artery disease. Taking these into consideration, the clinical significance of DBP might be diverse, and the risk of IDH should be assessed from various perspectives such as genetic variation, racial difference, comparison between short‐ and long‐term observation, and targeted clinical outcomes.
Another important issue is whether pharmacological intervention could lower the risk of CVD among patients with stage 1 IDH. Regarding this critical point, Son et al 7 reported that patients with stage 1 hypertension not taking antihypertensive medications had an elevated incidence of CVD, whereas patients with stage 1 hypertension taking antihypertensive medications had a similar risk of CVD compared with those with normal BP, suggesting the efficacy of pharmacological intervention for patients with stage 1 hypertension. Further, the SPRINT (Systolic Blood Pressure Intervention Trial) strongly supports the importance of strict BP control in patients with hypertension. 29 Recent meta‐analyses of randomized trials showed that BP‐lowering treatment for individuals with SBP/DBP values in the ranges of 120 to 139/80 to 89 mm Hg was found to significantly reduce CVD risk. However, BP‐lowering treatment showed no significant benefits among individuals at low‐moderate risk. 30 Furthermore, the BPLTTC (Blood Pressure Lowering Treatment Trialists’ Collaboration) presented that protective effects of BP‐lowering treatments increased with baseline cardiovascular risk. In addition, BP‐lowering therapy according to estimated cardiovascular risk is more effective than that according to BP levels alone, supporting the use of cardiovascular risk assessment to guide BP management decision‐making in moderate‐ to high‐risk patients, particularly for primary prevention. 31 , 32 Therefore, well‐designed prospective studies or randomized controlled trials are needed to conclude the efficacy and the safety of pharmacological therapy for patients with stage 1 and stage 2 IDH. Body weight reduction is an alternative option for the management of BP. 33 , 34 , 35 , 36 , 37 We previously reported that body weight reduction (≥5%) could lower BP in the Japanese general population with body mass index ≥22 kg/m2 without any pharmacological intervention. 38 Therefore, it may be beneficial to recommend body weight reduction for individuals with elevated DBP and body mass index ≥22 kg/m2.
There are several limitations to this study. The category of BP was determined by BP measured at the initial health checkup, and measurements based on rigorous contemporary standards were not conducted. Therefore, misclassification could have occurred. Although we conducted a multivariable analysis, there could be unmeasured confounders and residual bias. Individuals registered in the JMDC database mainly comprised an employed, working‐age population, and there could be a “healthy worker” bias. Therefore, we need further investigations to generalize our results with other populations of different ethnicities, races, educational levels, and incomes. The incidence of CVD in our study is almost comparable to that in other nationwide epidemiological data in Japan (JPHC [Japan Public Health Center‐based Prospective Study] https://epi.ncc.go.jp/en/jphc/index.html). 39 Therefore, we believe that our data could have reflected real‐world clinical practice. However, recorded diagnoses are generally considered less well validated because of the nature of the retrospective design and administrative database. The data on CVD‐related deaths cannot be assessed in this database. We did not track the status of drug treatment during follow‐up. Although we excluded patients with a prior history of CVD as described in the Methods section, we are unable to eliminate the risk of misclassification, and, therefore, individuals with a prior history of CVD could be included in the analysis of this study. We also excluded patients taking antihypertensive medication. However, medications other than antihypertensives such as statins could have influenced BP values and affected the results.
Conclusions
IDH based on the cutoff value of DBP in the 2017 ACC/AHA BP guidelines was associated with a higher risk of CVD in the general population without prevalent CVD, suggesting the potential clinical significance of IDH in the development of CVD.
Sources of Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (19AA2007 and H30‐Policy‐Designated‐004), and the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04141).
Disclosures
Hidehiro Kaneko and Katsuhito Fujiu report research funding and scholarship funds from Medtronic Japan CO., LTD; Abbott Medical Japan CO., LTD; Boston Scientific Japan CO., LTD; and Fukuda Denshi, Central Tokyo CO., LTD. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2020;9:e017963 DOI: 10.1161/JAHA.120.017963.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017963
For Sources of Funding and Disclosures, see page 8.
References
- 1. Lawes CM, Vander Hoorn S, Rodgers A; International Society of H . Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3. Collaborators GBDRF . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 6. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, Gidding SS, Bress AP, Greenland P, Muntner P, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. 2018;320:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Son JS, Choi S, Kim K, Kim SM, Choi D, Lee G, Jeong SM, Park SY, Kim YY, Yun JM, et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association guidelines with subsequent cardiovascular disease events. JAMA. 2018;320:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 9. McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, Ballantyne CM, Coresh J, Selvin E. Association of Isolated Diastolic Hypertension As Defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA. 2020;323:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. [DOI] [PubMed] [Google Scholar]
- 11. Nielsen WB, Lindenstrom E, Vestbo J, Jensen GB. Is diastolic hypertension an independent risk factor for stroke in the presence of normal systolic blood pressure in the middle‐aged and elderly? Am J Hypertens. 1997;10:634–639. [DOI] [PubMed] [Google Scholar]
- 12. Strandberg TE, Salomaa VV, Vanhanen HT, Pitkala K, Miettinen TA. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow‐up of up to 32 years. J Hypertens. 2002;20:399–404. [DOI] [PubMed] [Google Scholar]
- 13. Sheriff HM, Tsimploulis A, Valentova M, Anker MS, Deedwania P, Banach M, Morgan CJ, Blackman MR, Fonarow GC, White M, et al. Isolated diastolic hypertension and incident heart failure in community‐dwelling older adults: insights from the Cardiovascular Health Study. Int J Cardiol. 2017;238:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies C . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 15. Goto A, Goto M, Terauchi Y, Yamaguchi N, Noda M. Association between severe hypoglycemia and cardiovascular disease risk in Japanese patients with type 2 diabetes. J Am Heart Assoc. 2016;5:e002875 DOI: 10.1161/JAHA.115.002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wake M, Onishi Y, Guelfucci F, Oh A, Hiroi S, Shimasaki Y, Teramoto T. Treatment patterns in hyperlipidaemia patients based on administrative claim databases in Japan. Atherosclerosis. 2018;272:145–152. [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki R, Konta T, Nishida K. Lipid‐lowering medication is associated with decreased risk of diabetic retinopathy and the need for treatment in patients with type 2 diabetes: a real‐world observational analysis of a health claims database. Diabetes Obes Metab. 2018;20:2351–2360. [DOI] [PubMed] [Google Scholar]
- 18. Davis KL, Meyers J, Zhao Z, McCollam PL, Murakami M. High‐risk atherosclerotic cardiovascular disease in a real‐world employed Japanese population: prevalence, cardiovascular event rates, and costs. J Atheroscler Thromb. 2015;22:1287–1304. [DOI] [PubMed] [Google Scholar]
- 19. Itoh H, Kaneko H, Kiriyama H, Yoshida Y, Nakanishi K, Mizuno Y, Daimon M, Morita H, Yatomi Y, Yamamichi N, et al. Effect of metabolically healthy obesity on the development of carotid plaque in the general population: a community‐based cohort study. J Atheroscler Thromb. 2020;27:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuzawa Y. Metabolic syndrome–definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12:301. [DOI] [PubMed] [Google Scholar]
- 21. Yagi M, Yasunaga H, Matsui H, Morita K, Fushimi K, Fujimoto M, Koyama T, Fujitani J. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke. 2017;48:740–746. [DOI] [PubMed] [Google Scholar]
- 22. Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Stat Med. 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 23. Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14:863–883. [PMC free article] [PubMed] [Google Scholar]
- 24. Itoh H, Kaneko H, Kiriyama H, Yoshida Y, Nakanishi K, Mizuno Y, Daimon M, Morita H, Yatomi Y, Komuro I. Relation between the updated blood pressure classification according to the American College of Cardiology/American Heart Association guidelines and carotid intima‐media thickness. Am J Cardiol. 2019;124:396–401. [DOI] [PubMed] [Google Scholar]
- 25. Kamon T, Kaneko H, Itoh H, Kiriyama H, Mizuno Y, Morita H, Yamamichi N, Komuro I. Gender‐specific association between the blood pressure category according to the updated ACC/AHA guidelines for hypertension and cardio‐ankle vascular index: a community‐based cohort study. J Cardiol. 2020;75:578–582. [DOI] [PubMed] [Google Scholar]
- 26. Yano Y, Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S, Ning H, Liu K, Greenland P, Lloyd‐Jones DM. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol. 2017;2:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peri‐Okonny PA, Patel KK, Jones PG, Breeding T, Gosch KL, Spertus JA, Arnold SV. Low diastolic blood pressure is associated with angina in patients with chronic coronary artery disease. J Am Coll Cardiol. 2018;72:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman F, Al Rifai M, Blaha MJ, Nasir K, Budoff MJ, Psaty BM, Post WS, Blumenthal RS, McEvoy JW. Relation of diastolic blood pressure and coronary artery calcium to coronary events and outcomes (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2017;120:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Group SR , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence. 12. Effects in individuals with high‐normal and normal blood pressure: overview and meta‐analyses of randomized trials. J Hypertens. 2017;35:2150–2160. [DOI] [PubMed] [Google Scholar]
- 31. Blood Pressure Lowering Treatment Trialists C . Blood pressure‐lowering treatment based on cardiovascular risk: a meta‐analysis of individual patient data. Lancet. 2014;384:591–598. [DOI] [PubMed] [Google Scholar]
- 32. Karmali KN, Lloyd‐Jones DM, van der Leeuw J, Goff DC Jr, Yusuf S, Zanchetti A, Glasziou P, Jackson R, Woodward M, Rodgers A, et al. Blood pressure‐lowering treatment strategies based on cardiovascular risk versus blood pressure: a meta‐analysis of individual participant data. PLoS Med. 2018;15:e1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, Mattfeldt‐Beman M, Oberman A, Sugars C, Dalcin AT, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849–858. [PubMed] [Google Scholar]
- 34. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, Phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 35. Horvath K, Jeitler K, Siering U, Stich AK, Skipka G, Gratzer TW, Siebenhofer A. Long‐term effects of weight‐reducing interventions in hypertensive patients: systematic review and meta‐analysis. Arch Intern Med. 2008;168:571–580. [DOI] [PubMed] [Google Scholar]
- 36. Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A. Long‐term effects of weight‐reducing diets in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fogari R, Zoppi A, Corradi L, Preti P, Mugellini A, Lazzari P, Derosa G. Effect of body weight loss and normalization on blood pressure in overweight non‐obese patients with stage 1 hypertension. Hypertens Res. 2010;33:236–242. [DOI] [PubMed] [Google Scholar]
- 38. Itoh H, Kaneko H, Kiriyama H, Nakanishi K, Mizuno Y, Daimon M, Morita H, Yamamichi N, Komuro I. Effect of Body Weight Change on Blood Pressure in a Japanese General Population with a Body Mass Index >/= 22 kg/m(2). Int Heart J. 2019;60:1381–1386. [DOI] [PubMed] [Google Scholar]
- 39. Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Association between mortality and incidence rates of coronary heart disease and stroke: the Japan Public Health Center‐based prospective (JPHC) study. Int J Cardiol. 2016;222:281–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


