Abstract
Background
There are limited data on health status instruments in patients with peripheral artery disease and cardiovascular and limb events. We evaluated the relationship between health status changes and cardiovascular and limb events.
Methods and Results
In an analysis of the EUCLID (Examining Use of Ticagrelor in Symptomatic Peripheral Artery Disease) trial, we examined the characteristics of 13 801 patients by tertile of health status instrument scores collected in the trial (EuroQol 5‐Dimensions [EQ‐5D], EQ visual analog scale [VAS], and peripheral artery questionnaire). We assessed the association between the baseline health status measurements and major adverse cardiovascular events, major adverse limb events, and lower‐extremity revascularization procedures during trial follow‐up and the association between 12‐month health status change scores and subsequent end points during follow‐up. There were 13 217 (95%) patients with EQ‐5D scores, 13 533 (98%) with VAS scores, and 4431 (32%) with peripheral artery questionnaire scores. Patients in the lowest baseline EQ‐5D tertile (0 to <0.69) were more likely to be female with severe claudication compared with the highest tertile (0.79–1.0; P<0.01). Patients in the lowest VAS (0–60) and peripheral artery questionnaire (0–49) tertiles had lower ankle–brachial indices compared with the highest tertiles (80–100 and 76–108, respectively; P<0.01). There was a significant association between baseline EQ‐5D, VAS, and peripheral artery questionnaire scores and adjusted major adverse cardiovascular events, major adverse limb events, and lower‐extremity revascularization (P<0.05). Improved EQ‐5D and VAS scores over 12 months were associated with reduced risk of subsequent major adverse cardiovascular events or lower‐extremity revascularization (all P<0.01).
Conclusions
Although health status instruments are rarely used in clinical practice, these measures are associated with outcomes, including major adverse cardiovascular events, major adverse limb events, and lower‐extremity revascularization. Further research is needed to determine the relationship between changes in these instruments, revascularization, and outcomes.
Keywords: health status instruments, peripheral artery disease, quality of life
Subject Categories: Health Services, Peripheral Vascular Disease
Nonstandard Abbreviations and Acronyms
- ALI
acute limb ischemia
- ABI
ankle–brachial index
- EQ‐5D
EuroQol 5‐Dimensions
- EUCLID
Examining Use of Ticagrelor in Symptomatic Peripheral Artery Disease
- LER
lower‐extremity revascularization
- MACE
major adverse cardiovascular events
- MALE
major adverse limb events
- PAQ
peripheral artery questionnaire
- SF‐36
Short Form‐36
- VAS
visual analog scale
Clinical Perspective
What Is New?
The baseline scores on health status instruments, such as the EuroQol 5‐Dimensions, visual analog scale, and peripheral artery questionnaire, were associated with clinical outcomes, including major adverse cardiovascular events, major adverse limb events, and lower‐extremity revascularization.
Improvement in the EuroQol 5‐Dimensions and visual analog scales cores over 12 months was associated with a reduced risk in subsequent major adverse cardiovascular events or lower‐extremity revascularization events.
What Are the Clinical Implications?
Performance on several readily available health status instruments is associated with important clinical end points, and consideration should be given to using these instruments to care for patients with peripheral artery disease.
Wide variation exists in the use of health status instruments, or patient‐reported outcomes measures, in quantifying outcomes for patients with peripheral artery disease (PAD). The options also vary widely from non–disease‐specific health status instruments, such as the EuroQoL 5‐Dimensions (EQ‐5D) and Short Form‐36 (SF‐36), to disease‐specific health status instruments, like the peripheral artery questionnaire (PAQ), the Intermittent Claudication Questionnaire (ICQ), and the Peripheral Artery Disease Quality of Life (PADQOL) questionnaires. 1 , 2 , 3 , 4 , 5 The primary utility has been to characterize the functional limitations of PAD and how that can be improved with exercise training, lower extremity revascularization (LER), and pharmacotherapy. 6 , 7 , 8 , 9 Many of these instruments have been validated only in patients with intermittent claudication and may not be appropriate to be used in more advanced PAD, such as in critical limb ischemia. Furthermore, the performance of these health status instruments is inconsistent across metrics, such as reliability, internal consistency, and content and construct validity. 10 Perhaps most importantly, there is scant literature regarding how these health status instrument scores change over time in the PAD population, and if the scores (and changes in scores) are associated with subsequent cardiovascular or limb events. Understanding the association between these health status scores and clinical outcomes would potentially allow for increased risk stratification of patients with PAD.
The EUCLID (Examining Use of Ticagrelor in Symptomatic Peripheral Artery Disease) trial was a multicenter, double‐blind, randomized controlled trial that examined the effect of ticagrelor versus clopidogrel monotherapy and collected the EQ‐5D index score and EQ visual analog scale (VAS), as well as the PAQ, to examine patient‐reported quality of life over the course of the trial. 11 Because the trial provides follow‐up data on a large cohort of patients with PAD over 30 months, we are able to examine the change in EQ‐5D and PAQ scores over the trial as well as association of the scores with clinical end points. The current analysis was conducted to determine: (1) the characteristics of patients with PAD stratified by health status instrument score tertile, (2) the change in health status instrument scores over the course of the trial follow‐up, (3) the association of the baseline health status instrument scores with subsequent cardiovascular events, and (4) the association of the change in health status instrument score over time with subsequent cardiovascular events.
METHODS
The authors cannot make data and study materials available to other investigators for purposes of reproducing the results because of licensing restrictions. Interested parties, however, could obtain and license the data by contacting the EUCLID steering committee and AstraZeneca, United Kingdom.
The EUCLID trial examined the effect of ticagrelor versus clopidogrel monotherapy in 13 885 patients with symptomatic PAD. The trial was conducted from December 2012 to March 2014. Participation in the trial was approved by the institutional review boards of participating sites. All patients gave written informed consent. The trial design and results have been previously described. 11 , 12 The trial did not demonstrate a benefit for ticagrelor over clopidogrel in patients with symptomatic PAD and did not meet the primary end point. Patients were enrolled if they were at least 50 years of age with symptomatic PAD and had either (1) previous revascularization of the lower limbs more than 30 days before randomization or (2) evidence of PAD as exhibited by an ankle–brachial index (ABI) ≤0.80. In cases where the ABI was ≥1.40, a toe–brachial index of ≤0.60 was used as proof of PAD. In the resulting population, 76.6% had claudication and 4.6% had critical limb ischemia (participants without claudication were mainly those with a prior history of LER). The EUCLID publications committee approved this secondary analysis with a waiver of informed consent.
Health‐Related Quality‐of‐Life Measures
We examined several health status instruments that were used to evaluate trial participant's quality of life at baseline and on follow‐up. The EuroQol‐5D (EQ‐5D) is a non–disease‐specific patient‐reported outcomes measure developed by the EuroQol Group. 13 It evaluates five dimensions of self‐reported quality of life, including mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. The EQ VAS is another component which asks respondents to indicate on a 20‐cm vertical scale “the best health you can imagine” and the “worst health you can imagine.” The Peripheral Artery Questionnaire (PAQ) is a 20‐item disease‐specific patient‐reported outcomes measure, which examines physical limitation, symptoms, quality of life, social function, and treatment satisfaction among patients with PAD. 1
Outcomes
The primary composite end points were major adverse cardiovascular events (MACE), which was defined as the composite of nonfatal myocardial infarction (MI), non‐fatal ischemic stroke, or cardiovascular death; major adverse limb events (MALE), which included major amputations and acute limb ischemia (ALI) requiring hospitalization; and LER. The individual components of MALE, as well as LER, were further analyzed.
To examine the baseline characteristics of patients by health status instrument score tertile, we analyzed the population of patients with available baseline scores for each of the health status instruments—the EQ‐5D index score, the EQ‐5D VAS, and the PAQ. We examined the scores for each of the health status instruments at months 12 and 24. Patients who did not have available or had negative scores were not included in the calculation of the overall mean and median scores at each of the trial follow‐up periods. For the analysis examining the association between change in EQ‐5D index score and VAS from baseline to 12 months and subsequent outcomes, we excluded patients who experienced a clinical event of interest prior to the 12‐month follow‐up and those patients who did not complete the EQ‐5D at 12 months.
Statistical Analysis
We examined the baseline characteristics of patients enrolled in the trial, stratified by tertile of health status instrument. Continuous variables were compared using an ANOVA or Kruskal–Wallis test and categorial variables were compared using chi‐square or Fisher's exact test. We reported continuous variables as median (25th–75th percentiles) and categorical variables as number (%). To examine the trends over time in EQ‐5D index and VAS, as well as PAQ scores, we reported the mean (standard deviation) and median (25th–75th percentiles) scores at baseline, 12 months, and 24 months.
To examine the association between baseline health status instrument scores and clinical events, we developed Cox proportional hazards models for each of the scores—the EQ‐5D index score, VAS, and PAQ. Adjustment variables are listed in Table S1. All adjustment variables were checked for nonlinearity using natural cubic splines, and the proportional hazards assumption was checked using weighted Schoenfeld residuals. A linear relationship can be assumed between health status scores and clinical outcomes. We determined which variables would be used for adjustment using stepwise selection with an inclusion criterion of P=0.05. Hazard ratios are presented for each 0.1‐ or 10‐unit increase in the health status instrument score. Hazard ratios with 95% confidence intervals and associated P‐values are presented for each health status instrument and each primary composite or individual end point.
Because of increased missingness in PAQ from baseline to 12 months (4431 patients completed the PAQ out of the 13 855), we only examined the association between change in EQ‐5D index and VAS from baseline to 12 months and subsequent outcomes. The PAQ is a longer, more involved instrument than the others, which may explain the increased missingness. We used a Cox proportional hazards model, landmarked at 12 months and controlled for the baseline scores in all unadjusted and adjusted models. For each outcome evaluated, patients had to be alive and event‐free at 12 months to be included in the analysis. We adjusted for the variables listed in Table S1. Finally, the linearity assumption was assessed for the change in EQ‐5D index score and VAS.
RESULTS
Among all patients enrolled in EUCLID, there were 13 217 patients with complete EQ‐5D scores (95.4%), 13 533 patients with EQ‐5D VAS scores (97.7%), and 4431 patients with PAQ scores (32.0%). For each health status instrument, baseline characteristics are demonstrated by tertile of instrument score (Tables S2 through S4). Patients in the highest EQ‐5D index score tertile (0.79–1.0) were less likely to be female; less likely to have severe claudication, rest pain, or tissue loss; and less likely to have a history of stroke, MI, or diabetes mellitus, compared with patients in the lowest score tertile (all P<0.001). Patients in the highest EQ‐5D VAS score tertile (80–100) were also less likely to have severe claudication or tissue loss, compared with patients in the lowest tertile (all P<0.001). Finally, patients in the highest PAQ score tertile (76–108) were less likely to have severe claudication or tissue loss and less likely to have a history of coronary artery disease and diabetes mellitus (all P<0.001).
Change in Health Status Instrument Scores Over the Trial Follow‐Up
There were 9979 patients with complete EQ‐5D index scores, 10 443 patients without missing EQ‐5D VAS scores, and 3225 patients without missing PAQ scores at baseline, the 12‐month follow‐up, and the 24‐month follow‐up. The EQ‐5D index score and EQ‐5D VAS scores remained stable across the 12‐ and 24‐month follow‐ups (Table S5). There was an increase in the mean and median PAQ scores at both the 12‐ and the 24‐month follow‐up periods. Figure S1A through S1C demonstrates the change in health status instrument scores at the various time points.
Association of Baseline Health Status Scores and Outcomes
There was a significant association between both baseline EQ‐5D index score and PAQ with MACE (EQ‐5D—adjusted HR, 0.96; 95% CI, 0.93–0.98; PAQ—adjusted HR, 0.92; 95% CI, 0.88–0.96), MALE (EQ‐5D—adjusted HR, 0.93; 95% CI, 0.88–0.98; PAQ—adjusted HR, 0.91; 95% CI, 0.84–1.00), major amputation (EQ‐5D—adjusted HR, 0.91; 95% CI, 0.85–0.98; PAQ—adjusted HR, 0.86; 95% CI, 0.75–0.98) and LER (EQ‐5D—adjusted HR, 0.95; 95% CI, 0.93–0.97; PAQ—adjusted HR, 0.90; 95% CI, 0.88–0.93), unadjusted and adjusted P<0.05 (Tables 1 and 2). For the EQ‐5D index score, the hazard ratio represents the change in hazard associated with 0.1‐point increase in the EQ‐5D scale; for the PAQ score, the hazard ratio represents the change in hazard associated with a 10‐point increase in the PAQ score. For the EQ‐5D VAS, there was a significant association between baseline EQ‐5D VAS and unadjusted and adjusted MACE (adjusted HR, 0.96; 95% CI, 0.93–0.98), MALE (adjusted HR, 0.93; 95% CI, 0.88–0.98), ALI requiring hospitalization (adjusted HR, 0.91; 95% CI, 0.85–0.98), and LER (adjusted HR, 0.94; 95% CI, 0.92–0.96; P<0.01 for all; Table 3). For the EQ‐5D VAS, the hazard ratio represents the change in hazard associated with a 10‐point increase in the EQ‐5D VAS score. Figures 1 and 2 demonstrate the association of EQ‐5D and PAQ scores with MACE and MALE, respectively. Figure 3 illustrates the association of EQ‐5D and PAQ scores with LER. Table S6 lists the number of clinical events or outcomes by tertile for each health status instrument.
Table 1.
Association Between EQ‐5D Index Score and Outcomes
| Clinical Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value* | HR (95% CI) | P Value* | |
| MACE† | 0.92 (0.90–0.94) | <0.0001 | 0.96 (0.93–0.98) | 0.0022 |
| MALE‡ | 0.90 (0.86–0.94) | <0.0001 | 0.93 (0.88–0.98) | 0.0112 |
| ALI requiring hospitalization§ | 0.97 (0.91–1.04) | 0.3766 | 0.96 (0.90–1.03) | 0.2827 |
| Major amputation‖ | 0.84 (0.79–0.89) | <0.0001 | 0.91 (0.85–0.98) | 0.0094 |
| LER¶ | 0.96 (0.94–0.98) | 0.0005 | 0.95 (0.93–0.97) | <0.0001 |
ABI indicates ankle–brachial index; ALI, acute limb ischemia; HR, hazard ratio; EQ‐5D, EuroQol 5‐Dimensions; LER, lower‐extremity revascularization; MACE, major adverse cardiovascular events; and MALE, major adverse limb events.
We used a Cox proportional hazards model, landmarked at 12 months and controlled for the baseline scores in all unadjusted and adjusted models.
Adjusted for Rutherford score, sex, inclusion criteria, prior carotid revascularization, prior carotid stenosis, diabetes mellitus, angiotensin II receptor blocker (ARB) use, prior minor amputation, prior major amputation, statin use and ABI, ticagrelor (study arm).
Adjusted for Rutherford score, inclusion criteria, region, prior carotid revascularization, diabetes mellitus, prior aspirin use, prior clopidogrel use, tobacco use, ABI, estimated glomerular filtration rate (eGFR), ticagrelor (study arm).
Adjusted for inclusion criteria, ABI, hypertension, prior carotid revascularization, ARB use, ticagrelor (study arm).
Adjusted for Rutherford score, inclusion criteria, prior coronary artery bypass graft (CABG), diabetes mellitus, statin use, prior major amputation, prior minor amputation, ABI, weight, ticagrelor (study arm).
Adjusted for inclusion criteria, region, ABI, tobacco use, diabetes mellitus, prior clopidogrel use, prior aspirin use, ticagrelor (study arm).
Table 2.
Association Between PAQ Score and Outcomes
| Clinical Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value* | HR (95% CI) | P Value* | |
| MACE† | 0.88 (0.85–0.91) | <0.0001 | 0.92 (0.88–0.96) | <0.0001 |
| MALE‡ | 0.91 (0.84–0.98) | 0.0101 | 0.91 (0.83–1.00) | 0.0420 |
| ALI requiring hospitalization§ | 0.99 (0.90–1.08) | 0.8294 | 0.98 (0.89–1.08) | 0.6716 |
| Major amputation‖ | 0.83 (0.74–0.92) | 0.0006 | 0.86 (0.75–0.98) | 0.0242 |
| LER¶ | 0.90 (0.88–0.92) | <0.0001 | 0.90 (0.88–0.93) | <0.0001 |
ALI indicates acute limb ischemia; EQ‐5D, EuroQol 5‐Dimensions; HR, hazard ratio; LER, lower‐extremity revascularization; MACE, major adverse cardiovascular events; MALE, major adverse limb events; and PAQ, peripheral artery questionnaire.
We used a Cox proportional hazards model, landmarked at 12 months and controlled for the baseline scores in all unadjusted and adjusted models.
Adjusted for Rutherford score, sex, prior stroke, prior carotid revascularization, prior myocardial infarction (MI), prior percutaneous coronary intervention (PCI), prior coronary artery bypass graft (CABG), diabetes mellitus, statin use, tobacco use, prior minor amputation, age, ankle–brachial index (ABI), estimated glomerular filtration rate (eGFR), ticagrelor (study arm).
Adjusted for Rutherford score, inclusion criteria, prior carotid revascularization, diabetes mellitus, prior major amputation, statin use, ABI, region, and tobacco use, ticagrelor (study arm).
Adjusted for inclusion criteria, prior carotid revascularization, statin use, eGFR, ticagrelor (study arm).
Adjusted for Rutherford score, prior carotid revascularization, diabetes mellitus, prior major amputation, prior minor amputation, ABI, ticagrelor (study arm).
Adjusted for inclusion criteria, region, ABI, tobacco use, prior clopidogrel use, eGFR, ticagrelor (study arm).
Table 3.
Association Between EQ‐5D VAS Score and Outcomes
| Clinical Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value* | HR (95% CI) | P Value* | |
| MACE† | 0.92 (0.90–0.94) | <0.0001 | 0.96 (0.93–0.98) | 0.0018 |
| MALE‡ | 0.89 (0.85–0.93) | <0.0001 | 0.93 (0.88–0.98) | 0.0088 |
| ALI requiring hospitalization§ | 0.89 (0.84–0.95) | 0.0002 | 0.91 (0.85–0.98) | 0.0070 |
| Major amputation‖ | 0.88 (0.83–0.93) | <0.0001 | 0.93 (0.86–1.00) | 0.0535 |
| LER¶ | 0.95 (0.93–0.97) | <0.0001 | 0.94 (0.92–0.96) | <0.0001 |
ALI indicates acute limb ischemia; HR, hazard ratio; LER, lower‐extremity revascularization; MACE, major adverse cardiovascular event; MALE, major adverse limb event; and VAS, visual analog scale.
We used a Cox proportional hazards model, landmarked at 12 months and controlled for the baseline scores in all unadjusted and adjusted models.
Adjusted for Rutherford score, sex, inclusion criteria, region, prior stroke, prior carotid revascularization, prior myocardial infarction (MI), prior percutaneous coronary intervention (PCI), diabetes mellitus, statin use, tobacco use, prior minor amputation, age, ankle–brachial index (ABI), weight, estimated glomerular filtration rate (eGFR), ticagrelor (study arm).
Adjusted for Rutherford score, sex, inclusion criteria, prior carotid revascularization, prior carotid stenosis, diabetes mellitus, angiotensin II receptor blocker (ARB) use, prior minor amputation, prior major amputation, statin use and ABI, ticagrelor (study arm).
Adjusted for inclusion criteria, ABI, hypertension, prior carotid revascularization, ARB use, ticagrelor (study arm).
Adjusted for Rutherford score, inclusion criteria, prior carotid stenosis, prior carotid revascularization, diabetes mellitus, statin use, prior major amputation, prior minor amputation, ABI, weight, ticagrelor (study arm).
Adjusted for inclusion criteria, region, ABI, tobacco use, diabetes mellitus, prior clopidogrel use, prior aspirin use, ticagrelor (study arm).
Figure 1. Association of baseline EQ‐5D index score, EQ‐5D VAS, and PAQ scores and risk of MACE.
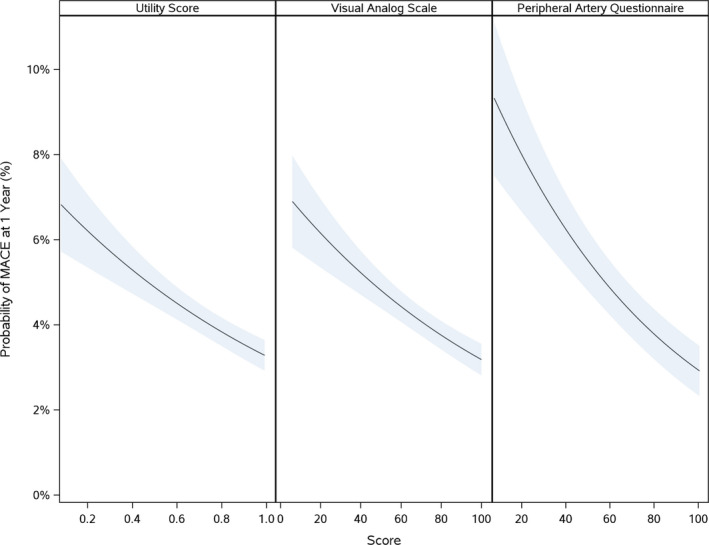
There is a significant association between the baseline EQ‐5D index score, EQ‐5D VAS, and the PAQ scores and the 1‐year probability of MACE. EQ‐5D indicates EuroQol 5‐Dimensions; MACE, major adverse cardiovascular events; PAQ, peripheral artery questionnaire; VAS, visual analog scale.
Figure 2. Association of baseline EQ‐5D index score, EQ‐5D VAS, and PAQ scores and risk of MALE.
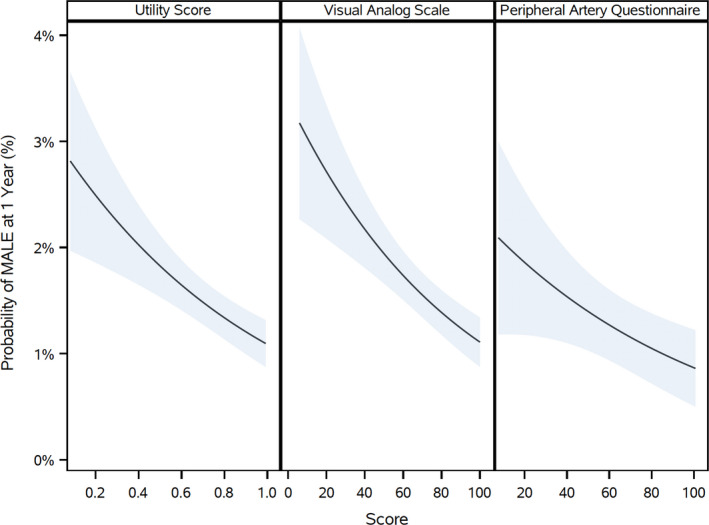
There is a significant association between the baseline EQ‐5D index score, EQ‐5D VAS, and the PAQ scores and the 1‐year probability of MALE. EQ‐5D indicates EuroQol 5‐Dimensions; MALE, major adverse limb events; PAQ, peripheral artery questionnaire; VAS, visual analog scale.
Figure 3. Association of baseline EQ‐5D index score, EQ‐5D VAS, and PAQ scores and risk of LER.
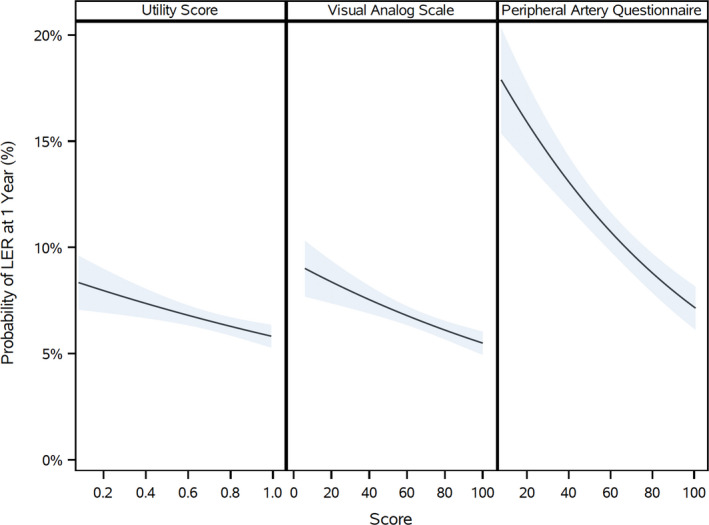
There is a significant association between the baseline EQ‐5D index score, EQ‐5D VAS, and the PAQ scores and the 1‐year probability of LER. EQ‐5D indicates EuroQol 5‐Dimensions; LER, lower‐extremity revascularization; PAQ, peripheral artery questionnaire; VAS, visual analog scale.
Change in EQ‐5D and Outcomes
There were 11 913 patients included in this analysis (1669 were excluded because of deaths or missing EQ‐5D instrument scores at the 12‐month follow‐up period). Table S7 illustrates the baseline characteristics of the included and excluded populations. Overall, patients excluded from this analysis were clinically similar to the patients included in the analysis. The association between change in PAQ from baseline to 12 months and outcomes could not be assessed due to the missingness of follow‐up PAQ scores. An improvement in EQ‐5D index scores and the EQ‐5D VAS was associated with a reduced unadjusted and adjusted risk of MACE (EQ‐5D index—adjusted HR, 0.90; 95% CI, 0.87–0.94; EQ‐5D VAS—adjusted HR, 0.88; 95% CI, 0.85–0.92) and LER events (EQ‐5D index—adjusted HR, 0.87; 95% CI, 0.84–0.90; EQ‐5D VAS—adjusted HR, 0.91; 95% CI, 0.87–0.94; all P<0.01; Table 4) but not with adjusted risk of MALE, ALI requiring hospitalization, or major amputation.
Table 4.
Association Between Change in the EQ‐5D Index Score and VAS From Baseline to 12 Months and Subsequent Outcomes
| Clinical Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value* | |
| EQ‐5D index score | ||||
| MACE | 0.88 (0.85–0.91) | <0.01 | 0.90 (0.87–0.94) | <0.01 |
| MALE | 0.94 (0.86–1.02) | 0.13 | 0.94 (0.85–1.04) | 0.22 |
| ALI requiring hospitalization | 0.96 (0.86–1.08) | 0.48 | 0.92 (0.82–1.04) | 0.18 |
| Major amputation | 0.89 (0.79–1.00) | 0.06 | 0.92 (0.81–1.05) | 0.22 |
| LER | 0.85 (0.82–0.88) | <0.01 | 0.87 (0.84–0.90) | <0.01 |
| EQ‐5D VAS | ||||
| MACE | 0.87 (0.84–0.90) | <0.01 | 0.88 (0.85–0.92) | <0.01 |
| MALE | 0.94 (0.86–1.02) | 0.15 | 0.95 (0.86–1.04) | 0.27 |
| ALI requiring hospitalization | 0.97 (0.87–1.08) | 0.62 | 0.94 (0.83–1.06) | 0.30 |
| Major amputation | 0.89 (0.80–1.00) | 0.05 | 0.94 (0.82–1.08) | 0.41 |
| LER | 0.91 (0.87–0.94) | <0.01 | 0.91 (0.87–0.94) | <0.01 |
ALI indicate acute limb ischemia; EQ‐5D, EuroQol 5‐Dimensions; HR, hazard ratio; LER, lower‐extremity revascularization; MACE, major adverse cardiovascular event; MALE, major adverse limb event; PAQ, peripheral artery questionnaire; and VAS, visual analog scale.
We used a Cox proportional hazards model, landmarked at 12 months and controlled for the baseline scores in all unadjusted and adjusted models.
DISCUSSION
In this subanalysis of a large cohort of patients with PAD from the EUCLID trial, we examined the change in health status instrument scores over the trial follow‐up period as well as the association of health status scores with subsequent cardiovascular and limb events. Patients in the highest score tertile of the EQ‐5D or PAQ instruments at baseline were significantly more likely to be asymptomatic and less likely to have severe claudication or tissue loss. Additionally, the EQ‐5D index scores and VAS remained stable over the trial follow‐up, while PAQ scores increased. Furthermore, baseline EQ‐5D and PAQ scores were significantly associated with composite outcomes, including MACE, MALE, and LER. Finally, we demonstrated that an improvement in EQ‐5D over 12 months was associated with a reduced risk of MACE and LER subsequent to 12 months and up to 24 months.
Importantly, we demonstrated that patients in the highest score tertiles for both the PAQ and the EQ‐5D instruments were less likely to have severe claudication or tissue loss and less likely to have many of the comorbidities oftentimes associated with PAD. While many of the disease‐specific health status instruments, such as the PAQ and VascuQOL, have been validated in the PAD population, 1 , 3 , 10 , 14 , 15 , 16 other generic instruments, such as the EQ‐5D and SF‐36, 5 have primarily been used to validate many PAD‐specific health status instruments. It is critical for health status instruments to fully represent the intended disease state or behavior being measured. This is particularly challenging in PAD, as the perception of disease and burden of comorbidities often widely varies across patients with objectively similar clinical presentations. Indeed, health status instrument scores in PAD have also been shown to differ across patient populations. For example, despite having similar ABI values, an analysis of the PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry demonstrated that women were significantly more likely to have lower PAQ scores than men. 17 This is in line with our finding that women were much less likely to have PAQ scores in the highest tertile. Another analysis showed that while there was no significant difference in baseline and follow‐up scores between White and Black patients who had undergone a peripheral vascular intervention, the improvement in scores postintervention, was lower for Black patients. 18 Because there is increasing interest around trying to define which health status instruments are best used in various PAD populations (ie, intermittent claudication vs critical limb ischemia, clinical research vs clinical practice), further work is needed to understand how these instruments perform and differ in performance among various patient populations and if they accurately represent the breadth of the disease state of interest.
Perhaps most importantly though, we were able to demonstrate an association between the baseline score on the EQ‐5D and PAQ instruments and important clinical end points, such as MACE, MALE, and LER. There is little understanding for many of the PAD health status instruments about how performance is associated with clinical end points and objective measures. 19 A recent publication using data from the PORTRAIT registry demonstrated that the resting and exercise ABI were both associated with the PAQ limitations score, and the time to onset of claudication, pain‐free walking distance, and maximum walking distance were associated with the PAQ symptoms and overall summary score. 20 However, another analysis of the PAQ score showed it to be weakly associated with ABI, Rutherford classification, and hemodynamics. 21 An analysis of patients with PAD treated at an academic Veterans Administration Medical Center demonstrated a significant correlation between ABI and time to maximal claudication and the Walking Impairment Questionnaire (WIQ) distance and the SF‐36. 22 Our analysis provides increasing evidence that two commonly used health status instruments used in symptomatic PAD may help clinicians and investigators better understand the future risk of important clinical end points in this patient population.
Additionally, our analysis is novel in the field of PAD in that we assessed the association of the change in health status scores over time with various relevant clinical end points. We demonstrated that an improvement in the EQ‐5D and VAS at 12 months were both associated with a reduced risk of MACE and LER. However, as shown in Table 2, MALE and its individual components (ALI or major amputation) were not associated with health status. Patients who have better health status scores are less likely to undergo LER. Validation studies of the various health status instruments have examined the change in health status scores with some measure of clinical improvement, 1 , 10 , 23 , 24 but have not necessarily addressed how these changes might be associated with future risk of cardiovascular and limb events. Further work will be needed to determine what threshold of change on each instrument would be associated with a reduced risk of MACE or LER. This work is obviously complex on the individual patient level, because some patients may have a significant clinical improvement in PAD (improved ABI, walking distance, etc.), but may report continued poor quality of life secondary to concomitant and comorbid medical conditions.
There are several limitations of the current analysis. As this was a post hoc analysis, residual confounding likely remains. Because we could not analyze patients who had not completed the health status instruments at baseline or the various follow‐up periods, we may have not captured the results of these scores in perhaps a sicker patient population or among patients who may have not agreed to fill out the instruments for a variety of reasons. We excluded patients with a negative EQ‐5D score (284 patients of 13 885), which may have impacted the results of the analyses. There was significant missingness for the PAQ instrument, likely because it is a lengthy questionnaire and may be less likely to be completed at baseline and at follow‐up by study participants. Because of its increased length, it is possible that patients with worse PAD may be less likely to fill it out. We did not assess the association of the various instrument subdomains with clinical end points. Additionally, as with any clinical trial, the population of enrolled patients may not be entirely generalizable to a nontrial population of patients with PAD, because patients enrolled in clinical trials tend to be healthier and receive more guideline‐directed medical therapy. The study population in EUCLID was mostly comprised of individuals with either asymptomatic PAD (19%) or mild to moderate claudication (53%), thus likely to be clinically stable from a vascular standpoint at entry. Finally, the landmark analysis was based on patients alive and event free at 12 months, which may not represent a randomized population and as such may be biased.
CONCLUSIONS
Health‐related quality‐of‐life instruments are rarely used in clinical practice for the treatment of patients with PAD. Given the association of the scores of several of these instruments and outcomes such as MACE, MALE, and LER, consideration should be given to using them more frequently in clinical practice. Further work is needed to delineate the relationship between changes in health status scores, revascularization, and outcomes.
Sources of Funding
The EUCLID study was funded by AstraZeneca, United Kingdom.
Disclosures
Dr Rymer research grants from Boston Scientific and Abbott. Dr Smolderen is supported by an unrestricted research grant from Terumo, Boston Scientific, and Abbott Vascular. Dr Hiatt reports grant support to CPC Clinical Research from NIH, AstraZeneca, Amgen, Bayer, Janssen. Dr Berger reports advisory board fees from Janssen, Merck, and Takeda. Dr Norgren reports research grants from AnGes and Mitsubishi and steering committee honoraria from AnGes, AstraZeneca, Bayer, Cesca, Mitsubishi, and Pluristem. . Dr Baumgartner reports research grants from Abbott Vascular, Cook, Terumo, Amgen, and Boston Scientific and consulting fees from Amgen. Dr Fowkes reports serving on advisory boards for AstraZeneca, Bayer, and Merck. Dr Katona is an employee and shareholder of AstraZeneca. Dr Rockhold reports research funding from NIH, PCORI, Duke Clinical Research Institute, Astra Zeneca, ReNeuron, Luitpold, Alzheimer's Drug Discovery Foundation, Janssen, and BMS; consulting/honoraria detailed at https://dcri.org/about-us/conflict-of-interest/; and equity interest in Glaxosmithkline. Dr Jones reports research grants from Agency for Healthcare Research and Quality, AstraZeneca, American Heart Association, Bristol‐Myers Squibb, Doris Duke Charitable Foundation, and Patient‐Centered Outcomes Research Institute and honorarium/other from Bayer, Bristol‐Myers Squibb, and Janssen Pharmaceuticals. Dr Patel reports research grants from the Agency for Healthcare Research and Quality, AstraZeneca, Janssen, and Bayer and honoraria/other from Bayer and Janssen. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figure S1
(J Am Heart Assoc. 2020;9:e016573 DOI: 10.1161/JAHA.120.016573.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016573
For Sources of Funding and Disclosures, see page 9.
References
- 1. Spertus J, Jones P, Poler S, Rocha‐Singh K. The peripheral artery questionnaire: a new disease‐specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;301–308. [DOI] [PubMed] [Google Scholar]
- 2. Chong PFS, Garratt AM, Golledge J, Greenhalgh RM, Davis AH. The intermittent claudication questionnaire: a patient‐assessed condition‐specific health outcome measure. J Vasc Surg. 2002;764–771. [PubMed] [Google Scholar]
- 3. Poku E, Duncan R, Keetharuth A, Essat M, Phillips P, Woods HB, Palfreyman S, Jones G, Kaltenthaler E, Michaels J. Patient‐reported outcome measures in patients with peripheral arterial disease: a systematic review of psychometric properties. Health Qual Life Outcomes. 2016;161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treat‐Jacobson D, Lindquist RA, Witt DR, Kirk LN, Schoor EN, Bronas UG, Davey CS, Regensteiner JG. The PADQOL: development and validation of a PAD‐specific quality of life questionnaire. Vasc Med. 2012;405–415. [DOI] [PubMed] [Google Scholar]
- 5. Vaidya A, Kleinegris MC, Severens JL, Ramaekers BL, Cate‐Hoek AJT, Cate HT, Joore MA. Comparison of EQ‐5D and SF‐36 in untreated patients with symptoms of intermittent claudication. J Comp Eff Res. 2018;535–548. [DOI] [PubMed] [Google Scholar]
- 6. Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the Claudication: Exercise versus Endoluminal Revascularization (CLEVER) study. Circulation. 2012;130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salisbury DL, Whipple MO, Burt M, Brown RJL, Hirsch A, Foley C, Treat‐Jacobson D. Translation of an evidence‐based therapeutic exercise program for patients with peripheral artery disease. J Vasc Nurs. 2018;23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treat‐Jacobson D, Bronas UG, Krause BJ, Robinson C, Santilli S, Leon A. Aerobic arm exercise to improve outcomes for patients with severe claudication and ischemic rest pain. J Vasc Med. 2012;204. [Google Scholar]
- 9. Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Cost‐effectiveness of endovascular revascularization compared to supervised hospital‐based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg. 2008;1472–1480. [DOI] [PubMed] [Google Scholar]
- 10. Conijn AP, Jens S, Terwee CB, Breek JC, Koelemay MJW. Assessing the quality of available patient reported outcome measures for intermittent claudication: a systematic review using the COSMIN checklist. Eur J Vasc Endovasc Surg. 2015;316–334. [DOI] [PubMed] [Google Scholar]
- 11. Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;32–40. [DOI] [PubMed] [Google Scholar]
- 12. Berger JS, Katona BG, Jones WS, Patel MR, Norgren L, Baumgartner I, Blomster J, Mahaffey KW, Held P, Millegard M, et al. Design and rationale for the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial. Am Heart J. 2016;86–93. [DOI] [PubMed] [Google Scholar]
- 13. The EQ‐5D Instruments . Available at: https://euroqol.org/. Accessed July 22, 2020.
- 14. Conijn AP, Santema TB, Bipat S, Koelemay MJ, de Haan RJ. Clinimetric evaluation of the vascular quality of life questionnaire in patients with lower limb ischemia. Eur J Vasc Endovasc Surg. 2017;412–418. [DOI] [PubMed] [Google Scholar]
- 15. Kumlien C, Nordanstig J, Lundstrom M, Pettersson M. Validity and test retest reliability of the vascular quality of life Questionnaire‐6: a short form of a disease‐specific health‐related quality of life instrument for patients with peripheral arterial disease. Health Qual Life Outcomes. 2017;187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoeks SE, Smolderen KG, Scholte Op Reimer WJ, Verhagen HJM, Spertus JA, Poldermans D. Clinical validity of a disease‐specific health status questionnaire: the peripheral artery questionnaire. J Vasc Surg. 2009;371–377. [DOI] [PubMed] [Google Scholar]
- 17. Roumia M, Aronow HD, Soukas P, Gosch K, Smolderen KG, Spertus JA, Abbott JD. Sex differences in disease‐specific health status measures in patients with symptomatic peripheral artery disease: data from the PORTRAIT study. Vasc Med. 2017;103–109. [DOI] [PubMed] [Google Scholar]
- 18. Zaitoun A, Al‐Najafi S, Musa T, Szpunar S, Light D, Lalonde T, Yamasaki H, Mehta RH, Rosman HS. The association of race with quality of health in peripheral artery disease following peripheral vascular intervention: the Q‐PAD study. Vasc Med. 2017;498–504. [DOI] [PubMed] [Google Scholar]
- 19. Issa SM, Hoeks SE, Scholte op Reimer WJM, Van Gestel YRBM, Lenzen MJ, Verhagen HJM, Pedersen SS, Poldermans D. Health‐related quality of life predicts long‐term survival in patients with peripheral artery disease. Vasc Med. 2010;163–169. [DOI] [PubMed] [Google Scholar]
- 20. Hammad T, Smolderen K, Spertus J, Jones PG, Shishehbor MH. Associations of exercise ankle–brachial index, pain‐free walking distance and maximum walking distance with the Peripheral Artery Questionnaire: finding from the PORTRAIT PAD Registry. Vasc Med. 2019;32–40. [DOI] [PubMed] [Google Scholar]
- 21. Johnston AL, Vemulapalli S, Gosch KL, Aronow HD, Abbott JD, Patel MR, Smolderen KG, Shishehbor M, Spertus JA, Jones WS. Ankle‐brachial index in patients with intermittent claudication is a poor indicator of patient‐centered and clinician‐based evaluations of functional status. J Vasc Surg. 2019;906–912. [DOI] [PubMed] [Google Scholar]
- 22. Izquierdo‐Porrera AM, Gardner AW, Bradham DD, Montgomery PS, Sorkin JD, Powell CC, Katzel LI. Relationship between objective measures of peripheral artery disease severity to self‐reported quality of life in older adults with intermittent claudication. J Vasc Surg. 2005;625–630. [DOI] [PubMed] [Google Scholar]
- 23. Larsen ASF, Reiersen AT, Jacobsen MB, Klow NE, Nordanstig J, Morgan M, Wesche J. Validation of the vascular quality of life questionnaire‐6 for clinical use in patients with lower limb peripheral arterial disease. Health Qual Life Outcomes. 2017;184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;1072–1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figure S1


