Abstract
Background
After the coronavirus disease 2019 outbreak, social isolation measures were introduced to contain infection. Although there is currently a slowing down of the infection, a reduction of hospitalizations, especially for myocardial infarction, was observed. The aim of our study is to evaluate the impact of the infectious disease on ST‐segment–elevation myocardial infarction (STEMI) care during the coronavirus disease 2019 pandemic, through the analysis of recent cases of patients who underwent percutaneous coronary intervention.
Methods and Results
Consecutive patients affected by STEMI from March 1 to 31, 2020, during social restrictions of Italian government, were collected and compared with patients with STEMI treated during March 2019. During March 2020, we observed a 63% reduction of patients with STEMI who were admitted to our catheterization laboratory, when compared with the same period of 2019 (13 versus 35 patients). Changes in all time components of STEMI care were notably observed, particularly for longer median time in symptom‐to‐first medical contact, spoke‐to‐hub, and the cumulative symptom‐to‐wire delay. Procedural data and in‐hospital outcomes were similar between the 2 groups, whereas the length of hospitalization was longer in patients of 2020. In this group, we also observed higher levels of cardiac biomarkers and a worse left ventricular ejection fraction at baseline and discharge.
Conclusions
The coronavirus disease 2019 outbreak induced a reduction of hospital access for STEMI with an increase in treatment delay, longer hospitalization, higher levels of cardiac biomarkers, and worse left ventricular function.
Keywords: acute coronary syndrome, complications, coronavirus, interstitial pneumonia, percutaneous coronary intervention
Subject Categories: Cardiovascular Disease, Percutaneous Coronary Intervention, Health Services
Coronavirus disease (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2, was first identified in China and evolved into a global pandemic. 1 , 2 Patients displayed various clinical presentations, from asymptomatic to severe acute respiratory syndrome needing oral intubation and intensive care assistance.
Such condition reaches an extremely high mortality, especially in frail subjects experiencing cardiovascular diseases. 3 , 4 , 5 On February 21, 2020, the first Italian case of severe acute respiratory syndrome coronavirus‐2 infection was diagnosed in northern Italy, and later huge number of people were infected. On March 9, 2020, the Italian government declared a state of emergency, issuing severe restrictions. Hospitals started to institute infection emergency protocols, and imposed widespread restrictions on nonurgent hospital admissions and hospital‐based ambulatory care. 6 , 7 Adoption of these limitations has led to a gradual containment of epidemic, but on the other hand, a reduction of all admissions to the emergency departments (EDs), including acute coronary heart diseases, was observed. 8 , 9 , 10
The aim of our study is to evaluate the impact of the severe acute respiratory syndrome coronavirus‐2 infection on ST‐segment–elevation myocardial infarction (STEMI) care during the COVID‐19 pandemic, through the analysis of recent cases of patients who underwent percutaneous coronary intervention (PCI) at our department.
Methods
Patient Population
In this observational study, we included patients admitted for STEMI to the catheterization laboratory of Cardiology Department of "Policlinico Tor Vergata" of Rome, and in whom PCI was performed. We analyzed the period between March 1, 2020, when hospitals in our city started to institute protocols to contain COVID‐19, to March 31, 2020. Thirteen consecutive patients with STEMI arrived in our ED and were treated in our catheterization laboratory in March 2020. They were compared with 35 consecutive patients with STEMI who arrived in our catheterization laboratory in March of the previous year. None of the patients included in the study experienced COVID‐19 infection. No patient was excluded from the study.
When feasible, a complete revascularization has been achieved during index procedure or before discharge (staged PCI). All patients were informed about the procedure and provided written consent. Institutional review board approval was obtained.
Data Collection
The data that support the findings of this study are available from the corresponding author on reasonable request. Time intervals (minutes) until the reperfusion have been recorded as follows: symptom‐to‐first medical contact, obtained by interviewing each patient about the time from the onset of symptoms to call an ambulance or go to our ED (hub center) or ED of other hospital (spoke center); spoke‐to‐hub, defined as the time between admission to the ED of another center (spoke) to the ED of our center (hub); the ambulance transportation time was included in the spoke‐to‐hub delay; ED‐to‐catheterization laboratory, defined as the time from access to our ED to the entrance in the catheterization laboratory; catheterization laboratory‐to‐wire, defined as the time from patient arrival at the catheterization laboratory to the time of a successful wire crossing. In addition, we calculated the following cumulative times: symptom‐to‐wire and first medical contact‐to‐wire from patient‐reported symptom onset and first medical contact time to successful wire crossing time during PCI. Procedural data were systematically collected and reported. The final coronary flow was defined according to TIMI (Thrombolysis in Myocardial Infarction) flow grading system. In case of slow/no flow after stent implantation, an intracoronary glycoprotein IIb/IIIa inhibitor (eptifibatide) was administered.
Standard color Doppler transthoracic echocardiography was performed, at the moment of hospital admission and discharge, recording left ventricle ejection fraction (LVEF) and possible mechanical complications of STEMI, as free wall rupture, ventricular septal rupture, infarct expansion with left ventricular aneurysm formation, intraventricular thrombus, and acute/ischemic mitral regurgitation.
The duration of in‐hospital stay and adverse events were reported as follows: cardiac death, cardiogenic shock, prolonged orotracheal intubation (>24 hours), use of mechanical circulatory support, acute pulmonary edema, acute renal failure, cerebral ischemic events, ventricular fibrillation/sustained ventricular tachycardia, nonsustained and ventricular tachycardia, new onset of atrial fibrillation, implantation of temporary or permanent pacemaker, acute stent thrombosis, and pericardial effusion.
Laboratory tests, including hemoglobin, fibrinogen, creatinine, creatine kinase–myocardial band, myoglobin, and ultrasensitive troponin I, were obtained before the PCI and during the hospitalization.
Statistical Analysis
Descriptive statistic was used to summarize the data; results are reported as means and SDs or medians and interquartile ranges (IQRs), as appropriate. They were compared using Student t test or the Mann‐Whitney rank sum test. Categorical variables are presented as counts and percentage values and were compared with the χ2 or Fisher exact test. Logistic and linear regressions were used to examine the relationship between demographic data and variables of outcomes. Results are reported as point estimates and 95% CIs. Both CI and P value are not adjusted for multiple testing. A 2‐sided P<0.05 was considered of statistical significance. Statistical analyses were performed using the Statistical Package for Social Sciences, version 26 (SPSS, Chicago, IL).
Results
In the period between March 1 and 31, 2020, we observed a 63% reduction of patients with STEMI who were admitted to our catheterization laboratory, when compared with the same period of 2019. Particularly, in March 2019, 35 consecutive patients with STEMI arrived in our catheterization laboratory, whereas 13 consecutive patients arrived in March 2020, during the first period of the COVID‐19 emergency in Italy. Demographic data are reported in Table 1. Changes in every time component of STEMI care were observed when compared with historical data from the prior year. Particularly, longer median times were observed in patients of 2020 (Table 1). All patients underwent primary PCI, except for one patient who underwent a rescue PCI in March 2019. Higher prevalence of patients with TIMI flow ≤2 at the end of the procedure and a greater use of intracoronary glycoprotein IIb/IIIa inhibitors were reported in patients of March 2020 (31% versus 3%; P=0.022). Complete procedural data are presented in Table 1.
Table 1.
Clinical Characteristics of Patients and Procedural Data
| Characteristic | March 2019 (n=35) | March 2020 (n=13) |
Difference (95% CI) |
P Value |
|---|---|---|---|---|
| Population | ||||
| Age, y | 62±10 | 65±12 | −2.1 (−9.2 to 5.0) | 0.559 |
| Male sex, n (%) | 31 (87) | 11 (85) | 0.713 | |
| Dyslipidemia, n (%) | 11 (31) | 8 (61.5) | 0.058 | |
| Smoke, n (%) | 19 (54) | 10 (77) | 0.180 | |
| Diabetes mellitus, n (%) | 8 (23) | 3 (23) | 0.571 | |
| Hypertension, n (%) | 23 (66) | 7 (54) | 0.450 | |
| Familiarity, n (%) | 14 (40) | 3 (23) | 0.276 | |
| Chronic renal failure, n (%) | 3 (9) | 2 (14) | 0.492 | |
| Chronic obstructive pulmonary disease, n (%) | 2 (6) | 2 (15) | 0.281 | |
| Previous acute myocardial infarction, n (%) | 2 (6) | 1 (8) | 0.801 | |
| Previous percutaneous coronary intervention, n (%) | 3 (9) | 0 (0) | 0.276 | |
| Previous coronary artery bypass graft, n (%) | 0 (0) | 0 (0) | … | |
| STEMI localization, n (%) | ||||
| Anterior | 12 (34) | 6 (46) | 0.675 | |
| Inferoposterior | 16 (46) | 5 (38.5) | 0.902 | |
| Lateral | 7 (20) | 2 (15) | 0.958 | |
| Source, n (%) | ||||
| Our hospital | 12 (34) | 2 (15) | 0.356 | |
| Emergency ambulance | 8 (23) | 3 (23) | 0.711 | |
| Other hospital (spoke) | 15 (43) | 8 (61.5) | 0.409 | |
| Time components of STEMI care, median (IQR), min | ||||
| Symptom‐to‐FMC | 80 (60–207) | 120 (52.5–3600) | −60 (−738 to 23) | 0.284 |
| Spoke‐to‐hub* | 60 (37–122) | 132 (43–173) | −23 (−102 to 6) | 0.084 |
| ED‐to‐cathlab | 18 (10–42) | 10 (5–26) | 6 (−1 to 16) | 0.081 |
| Cathlab‐to‐wire | 24 (20–30) | 18 (8.5–40.5) | 4 (−8 to 13) | 0.472 |
| Symptom‐to‐wire | 200 (131–319) | 388 (177–3746) | −149 (−990 to −16) | 0.038 |
| FMC‐to‐wire | 89 (70–159) | 141 (65–196) | −22 (−81 to 18) | 0.251 |
| Procedure | ||||
| Primary percutaneous coronary intervention, n (%) | 34 (97) | 13 (100) | 0.538 | |
| Rescue percutaneous coronary intervention, n (%) | 1 (3) | 0 (0) | 0.538 | |
| Percutaneous coronary intervention duration, min | 44.2±19.1 | 49.6±28.6 | −5.4 (−19.8 to 8.9) | 0.450 |
| Contrast medium, mL | 142±58.8 | 172±136.9 | −30 (−87 to 27) | 0.295 |
| Staged percutaneous coronary intervention, n (%) | 15 (43) | 6 (46) | 0.838 | |
| No. of vessels, n (%) | ||||
| Single vessels | 14 (40) | 3 (23) | 0.546 | |
| Double vessels | 12 (34) | 6 (46) | 0.546 | |
| Triple vessels | 9 (26) | 4 (31) | 0.546 | |
| Culprit lesion, n (%) | ||||
| Left anterior descending artery | 12 (34) | 6 (46) | 0.759 | |
| Diagonal branch | 2 (6) | 0 (0) | 0.759 | |
| Left circumflex artery | 4 (11) | 1 (8) | 0.759 | |
| Left obtuse marginal artery | 1 (3) | 1 (8) | 0.759 | |
| Right coronary artery | 16 (46) | 5 (38.5) | 0.759 | |
| Outcome, n (%) | ||||
| TIMI grade flow ≤2 | 1 (3) | 4 (31) | 0.022 | |
| Eptifibatide | 1 (3) | 4 (31) | 0.022 | |
| Complete revascularization after PPCI | 19 (54) | 7 (54) | 0.978 | |
| Complete revascularization at discharge | 32 (91) | 9 (69) | 0.053 | |
Results presented as mean±SD, unless otherwise indicated. Percentages may not total 100 because of rounding. A 2‐sided P<0.05 was considered of statistical significance. Cathlab indicates catheterization laboratory; ED, emergency department; FMC, first medical contact; IQR, interquartile range; PPCI, primary percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and TIMI, Thrombolysis in Myocardial Infarction.
Patients who arrived directly to our ED were excluded. Spoke‐to‐hub time was calculated in 23 and 11 patients of 2019 and 2020, respectively.
Patients of March 2019 had higher values of LVEF at baseline when compared with patients of 2020 (42% [IQR, 37%–47%] versus 35% [IQR, 32.5%–42.5%]; difference, 5 [95% CI, 0–10]; P=0.014); this difference was also maintained at discharge, when patients of March 2019 improved significantly the LVEF to a median of 48% (IQR, 42%–55%), whereas patients of March 2020 did not show any significant variation (35% [IQR, 35%–40%]) (Figure). No mechanical complications were observed in the overall population, but functional mitral regurgitation grade more than moderate was 6% in 2019 and 15% in 2020 (P=0.03) (Table 2).
Figure 1. Left ventricular ejection fraction (LVEF) at baseline and discharge of patients with ST‐segment–elevation myocardial infarction of March 2019 and March 2020.
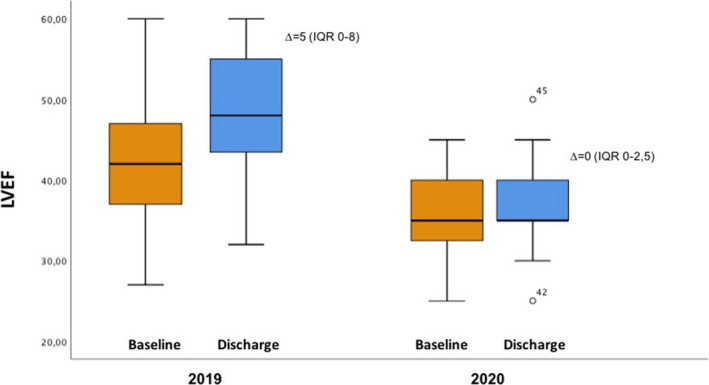
Data are expressed as median and interquartile range (IQR). The baseline and discharge median and IQR of LVEF in March 2019 was 42% (IQR, 37%–47%) and 48% (IQR, 42%–55%), respectively. The baseline and discharge median and IQR of LVEF in March 2020 was 35% (IQR, 32.5%–42.5%) and 35% (IQR, 35%–40%), respectively. The median of delta (∆) between baseline and discharge LVEF in patients of 2019 is 5 (IQR, 0–8); and in patients of 2020, 0 (IQR, 0–2.5). The difference was 3 (95% CI, 0–5; P=0.011).
Table 2.
In‐Hospital Data and Laboratory Blood Tests
| Characteristic | March 2019 (n=35) | March 2020 (n=13) |
Difference (95% CI) |
P Value |
|---|---|---|---|---|
| Complications, n (%) | ||||
| Cardiac death | 0 (0) | 0 (0) | … | |
| Cardiogenic shock | 0 (0) | 0 (0) | … | |
| Prolonged orotracheal intubation | 0 (0) | 0 (0) | … | |
| Mechanical circulation support | 0 (0) | 0 (0) | … | |
| Acute pulmonary edema | 3 (9) | 3 (23) | 0.390 | |
| Acute renal failure | 3 (9) | 1 (8) | 0.922 | |
| Cerebral ischemic events | 1 (3) | 0 (0) | 0.538 | |
| Ventricular fibrillation/sustained ventricular tachycardia | 1 (3) | 0 (0) | 0.538 | |
| Nonsustained ventricular tachycardia | 13 (37) | 2 (15) | 0.148 | |
| Atrial fibrillation (new onset) | 3 (9) | 2 (15) | 0.492 | |
| Temporary pacing | 2 (6) | 0 (0) | 0.379 | |
| Permanent pacemaker | 1 (3) | 0 (0) | 0.538 | |
| Acute stent thrombosis | 1 (3) | 0 (0) | 0.538 | |
| Free wall rupture | 0 (0) | 0 (0) | … | |
| Ventricular septal rupture | 0 (0) | 0 (0) | … | |
| Ventricular aneurysm | 0 (0) | 0 (0) | … | |
| Intraventricular thrombus | 0 (0) | 1 (8) | 0.097 | |
| Pericardial effusion | 1 (3) | 2 (15) | 0.111 | |
| Acute mitral regurgitation | 0 (0) | 0 (0) | … | |
| Ischemic MR >2+ | 2 (6) | 2 (15) | 0.03 | |
| Time of hospitalization, median (IQR), d | ||||
| CCU | 3.0 (2–4) | 5 (2.5–6) | −2 (−3 to 0) | 0.018 |
| Total | 6 (5–7) | 7 (6–10) | −2 (−4 to −1) | 0.008 |
| Laboratory blood tests | ||||
| Hemoglobin, g/dL | ||||
| Admission | 14.7±1.6 | 14.3±1.9 | 0.3 (−0.9 to 1.4) | 0.702 |
| 24 h | 13.9±1.6 | 13.2±2.2 | 0.4 (−1 to 2) | 0.608 |
| Discharge | 13.6±1.7 | 13.0±2.2 | 0.5 (−8 to 2.4) | 0.583 |
| Fibrinogen, median (IQR), mg/dL | ||||
| Admission | 354.5 (313–427.8) | 436 (403.5–626.5) | −101 (−197 to −30) | 0.007 |
| Serum creatinine, median (IQR), mg/dL | ||||
| Admission | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | −0.04 (−0.2 to 0.2) | 0.625 |
| 24 h | 0.9 (0.7–1.2) | 1.0 (0.8–1.6) | −0.09 (−0.4 to 0.1) | 0.272 |
| Discharge | 1.0 (0.8–1.1) | 1.1 (0.9–1.9) | −0.2 (−0.5 to 0.3) | 0.144 |
| Ultrasensitive troponin, median (IQR), ng/mL | ||||
| Admission | 317.6 (25–5675.7) | 1310 (58.4–18 394) | −668 (−3430 to 234) | 0.27 |
| Peak | 35 507 (14 462.8–86 726.0) | 57 098 (28 047.5–146 626.0) | −18 530 (−52 038 to 10 867) | 0.27 |
| Creatine kinase–myocardial band, median (IQR), ng/mL | ||||
| Admission | 6.7 (2.4–75.3) | 71.8 (11.6–83.5) | −31 (−70.7 to 6) | 0.128 |
| Peak | 119.0 (50.6–197.8) | 97.0 (53.3–323.0) | −16.6 (−89.4 to 55.9) | 0.617 |
| Myoglobin, median (IQR), ng/mL | ||||
| Admission | 134.5 (43–445.5) | 1710 (124.7–3409.2) | −127 (−3212 to 206) | 0.198 |
| Peak | 334 (111–1091) | 463 (250–3389.5) | −147 (−1615 to 147) | 0.270 |
Results presented as mean±SD, unless otherwise indicated. Percentages may not total 100 because of rounding. A 2‐sided P<0.05 was considered of statistical significance. CCU indicates cardiology care unit; IQR, interquartile range; and MR, mitral regurgitation.
Longer duration of in‐hospital stay was observed in patients of March 2020. Nevertheless, no differences were observed in adverse events (Table 2). Blood test values showed numerically higher values of cardiac biomarkers and fibrinogen in patients of March 2020, as reported in Table 2. The regression analyses did not show any relationship between demographic characteristics and the following variables: number of patients experiencing STEMI, LVEF at baseline and discharge, length of hospitalization, and symptom‐to‐wire (Table S1).
Discussion
Similar to recent published studies in diverse geographic areas, we observed a reduction of 63% of STEMI‐related hospitalizations in our high‐volume center in Rome during the first month of COVID‐19 epidemic in Italy, when compared with the same period of 2019. 8 , 9 , 10 Also, relevant changes in time components of STEMI care were recorded. Our results confirmed numerically longer median times in most of components when compared with historical data from the prior year. The largest time difference was in the time from symptom onset to first medical contact and in the cumulative symptom onset to wire crossing during PCI. Most visibly, the huge delay was in the small number of patients with STEMI seeking medical help after institution of the control measures. Surely, the fear of patients to hospitalize and contract COVID‐19 plays an important role; this may also suggest why some patients with STEMI did not seek care at all. In addition, our results showed longer delays from the spoke center to the ED of our hub department. This could be explained by the implementation of precautionary measures with the aim of an early identification of the infection and its containment. Detailed medical and contact history, assessment of symptoms, and execution of radiological examinations, made before transferring patients to our catheterization laboratory, could be responsible for the longer spoke‐to‐hub time observed in patients with STEMI during the COVID‐19 outbreak. These are essential measures for containing infection, but this could significantly increase delays in diagnosis, staff activation, and transfer of patients with STEMI if healthcare systems are not prepared. Another aspect that has been highlighted is the numerical reduction of the patient transit time inside our hospital, from ED to the catheterization laboratory. This may have been influenced by the presence of an interventional cardiologist on site and the fact that most patients arrived from other hospitals and were directly transported inside the catheterization laboratory to avoid severe acute respiratory syndrome coronavirus‐2 intrahospital infection.
Once in catheterization laboratory, we observed shorter time in patients of 2020 for the catheterization laboratory‐to‐wire time. Nevertheless, we documented a higher prevalence of patients with TIMI flow ≤2 at the end of the procedures for the patients of March 2020, and greater use of glycoprotein IIb/IIIa inhibitor. These data are probably correlated with the late presentation after symptom onset and more complex lesions, despite the experience of the operators.
In addition, we observed that patients who arrived in March 2020 had higher values of serum fibrinogen and cardiac biomarkers at moment of hospital admission and as peak value. This suggests that a longer precoronary time induced greater myocardial damage, which depends on the interruption of the coronary flow during acute myocardial infarction. It is well known and widely demonstrated that ischemic time duration is the major determinant of infarct size; therefore, a prompt recognition and early management of STEMI is critical to reduce morbidity and mortality. 11 , 12 Coronary occlusion persisting for >90 minutes determines a degree of cell death involving 40% to 50% of myocardium at risk and, after 6 hours of continuous ischemia, myocardial recovery will be minimal, despite the fact that the collateral flow is good. 13 , 14 Many individuals who survive an acute myocardial infarction have residual infarct pathological features that predispose them to the subsequent development of left ventricular dysfunction and heart failure, which remain the major causes of death after myocardial infarction. 15 To confirm this, in our population of patients with STEMI who arrived later in March 2020, a worse left ventricular dysfunction at the echocardiography was assessed at baseline, when compared with patients of 2019. In this group, LVEF remains lower also at discharge, with an increased risk to develop heart failure.
As evidence of an early worse outcome, in March 2020, there was an extension of hospitalization time in the whole hospital stay, particularly influenced by cardiology care unit stay. This is related to the late presentation of these patients in the hospital, who have a greater reduction of ventricular function, requiring more intensive and targeted treatments.
Finally, we want to underline that the delay in treatment of STEMI during the COVID‐19 pandemic not only may entail a prognostic worsening of these patients, but also can weigh the economic burden of national healthcare system, because there is a threatening potential risk of evolving into heart failure.
Study Limitations
This is a preliminary observation report, and our study should be considered in the context of these limits. We present the experience of a single hospital in the treatment of STEMI, after the implementation of COVID‐19 pandemic protection protocols in a small cohort of patients. It is possible that the results obtained will improve over time as the experience of the medical staff and patients becomes more mature. In consideration of the small sample size and the study design, we cannot make meaningful statistical conclusions. In addition, the modest sample size of the study and its single‐center nature may not reflect the experience of other centers.
Conclusions
During the first phase of the COVID‐19 outbreak in Italy, a suggestive reduction in patients with STEMI who were admitted to our PCI center was observed, as was a longer time from symptom onset to first medical contact and to wire cross, influencing negatively the duration of hospitalization, cardiac biomarkers, and left ventricular function. Hospitals should not only consider the methods of containing and treating this infection, but also how the outbreaks of infection can affect the care systems of other conditions that endanger the lives of patients.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1
Acknowledgments
We acknowledge everyone involved in drafting and performing this study, in particular the catheterization laboratory unit, the cardiac care unit, and the cardiology ward.
(J Am Heart Assoc. 2020;9:e017126 DOI: 10.1161/JAHA.120.017126.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, et al. Identification of a novel coronavirus causing severe pneumonia in human. Chin Med J (Engl). 2020;133:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li L, Huang T, Wang Y, Wang Z, Liang Y, Huang T, Zhang H, Sun W, Wang Y. 2019 Novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garnier-Crussard A, Forestier E, Gilbert T, Krolak-Salmon P. Novel coronavirus (COVID-19) epidemic: what are the risks for older patients? J Am Geriatr Soc. 2020;68:939–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicastri E, Petrosillo N, Ippolito G, D’Offizi G, Marchioni L, Ascoli Bartoli T, Lepore L, Mondi A, Murachelli S, Antinori A. National Institute for the Infectious Diseases “L. Spallanzani” IRCCS: recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorbello M, El-Boghdadly K, Di Giacinto I, Cataldo R, Esposito C, Falcetta S, Merli G, Cortese G, Corso RM, Bressan F, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724–732. [DOI] [PubMed] [Google Scholar]
- 8. Tam CCF, Cheung KS, Lam S, Wong A, Yung A, Sze M, Lam YM, Chan C, Tsang T-C, Tsui M, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631 DOI: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ardati AK, Mena Lora AJ. Be prepared. Circ Cardiovasc Qual Outcomes. 2020;13:e006661 DOI: 10.1161/CIRCOUTCOMES.120.006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction. Circulation. 2004;109:1223–1225. [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, Bates ER, Jollis JG, Antman EM, Nichol G, O'Connor RE, Gregory T, Roettig ML, Peng SA, Ellrodt G, et al. Improving care of STEMI in the United States 2008 to 2012. J Am Heart Assoc. 2019;8:e008096 DOI: 10.1161/JAHA.118.008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao A, Kardouh Y, Darda S, Desai D, Devireddy L, Lalonde T, Rosman H, David S. Impact of the prehospital ECG on door-to-balloon time in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2010;75:174–178. [DOI] [PubMed] [Google Scholar]
- 14. Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992;86:81–90. [DOI] [PubMed] [Google Scholar]
- 15. Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. 2016;9:e002460 DOI: 10.1161/CIRCHEARTFAILURE.115.002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


