Abstract
Background
Epidemiological and animal studies have associated systemic inflammation with blood pressure (BP). However, the mechanistic factors linking inflammation and BP remain unknown. Fatty acid–derived eicosanoids serve as mediators of inflammation and have been suggested to regulate renal vascular tone, peripheral resistance, renin‐angiotensin system, and endothelial function. We hypothesize that specific proinflammatory and anti‐inflammatory eicosanoids are linked with BP.
Methods and Results
We studied a population sample of 8099 FINRISK 2002 participants randomly drawn from the Finnish population register (53% women; mean age, 48±13 years) and, for external validation, a sample of 2859 FHS (Framingham Heart Study) Offspring study participants (55% women; mean age, 66±9 years). Using nontargeted liquid chromatography–mass spectrometry, we profiled 545 distinct high‐quality eicosanoids and related oxylipin mediators in plasma. Adjusting for conventional hypertension risk factors, we observed 187 (34%) metabolites that were significantly associated with systolic BP (P<Bonferroni‐corrected threshold of 0.05/545). We used forward selection linear regression modeling in FINRISK to define a general formula for individual eicosanoid risk score. Individuals of the top risk score quartile in FINRISK had a 9.0 (95% CI, 8.0–10.1) mm Hg higher systolic BP compared with individuals in the lowest quartile in fully adjusted models. Observed metabolite associations were consistent across FINRISK and FHS.
Conclusions
Plasma eicosanoids demonstrate strong associations with BP in the general population. As eicosanoid compounds affect numerous physiological processes that are central to BP regulation, they may offer new insights about the pathogenesis of hypertension, as well as serve as potential targets for therapeutic intervention.
Keywords: blood pressure, eicosanoids, hypertension, liquid chromatography–mass spectrometry, metabolite
Subject Categories: Hypertension, Epidemiology, Inflammation
Nonstandard Abbreviations and Acronyms
- 12‐HHTrE
12‐hydroxyheptadecatrienoic acid
- FHS
Framingham Heart Study
- LC‐MS
liquid chromatography–mass spectrometry
- MS
mass spectrometry
- TXA2
thromboxane A2
- TXB2
thromboxane B2
Clinical Perspective
What Is New?
Fatty acid–derived eicosanoids serve as mediators of inflammation and have been suggested to regulate renal vascular tone, peripheral resistance, renin‐angiotensin system, and endothelial function.
We assayed a comprehensive panel of >500 distinct high‐quality eicosanoids and related oxylipin mediators in community‐based samples of >10 000 individuals using liquid chromatography–mass spectrometry and relate these eicosanoids and eicosanoid profiles to blood pressure traits.
What Are the Clinical Implications?
We observed that 187 (34%) eicosanoids and related oxylipin mediators were significantly associated with systolic blood pressure.
Individuals in the top quartile of a 6‐metabolite risk score had a 9.0 mm Hg higher systolic blood pressure and 2‐fold greater odds of hypertension compared with individuals in the bottom quartile.
In conclusion, as eicosanoid species affect numerous physiological processes that are central to blood pressure regulation, they may offer new insights about the pathogenesis of hypertension, as well as serve as potential new targets for therapeutic intervention.
A vast majority of patients with hypertension (>95%) are classified as having primary (essential) hypertension, a heterogeneous condition of hypertension that has no identifiable cause (by definition). Essential hypertension is most likely the consequence of an interaction between genetic factors and environmental factors (eg, obesity, insulin resistance, sedentary lifestyle, stress, and sodium intake). 1 Intriguingly, all of the aforementioned factors are also related to chronic low‐grade inflammation, underscoring the need to further investigate inflammation as a potential mainstay pathologic mechanism underlying hypertension. 2
The upstream initiation of inflammatory activity in humans is governed mainly by substrates and products of polyunsaturated fatty acids. 3 Termed eicosanoids, the small‐molecule derivatives of arachidonic acid and other polyunsaturated fatty acids serve as both activators and suppressors of systemic inflammatory activity. 3 Data derived mainly from animal studies suggest that eicosanoid compounds affect renal vascular tone, urine sodium excretion, peripheral resistance, kidney disease, renin‐angiotensin‐aldosterone system, and endothelial function, factors that are central to blood pressure (BP) regulation itself. 4 , 5 , 6 , 7 , 8 , 9 Published data have also demonstrated that a few, select eicosanoids, such as 20‐hydroxyeicosatetraenoic acid, and genetic polymorphisms that regulate the levels of these eicosanoids are altered in small samples of individuals and animals with hypertension. 10 , 11 , 12
Until recently, sensitive methods for detecting and quantifying eicosanoids in large sample sizes were lacking. However, mass spectrometry (MS) based analytics now allow for the rapid large‐scale quantification of several hundred upstream eicosanoids in human plasma. 13 , 14 Our goal was to gain a more detailed understanding of how upstream inflammatory mediators are related to an individual's prevalence for hypertension. We quantified a comprehensive panel of >500 distinct high‐quality upstream eicosanoids and related oxylipin mediators in FINRISK 2002 (n=8099) and FHS (Framingham Heart Study) Offspring (n=2859) cohort participants using liquid chromatography–MS (LC‐MS) and related these eicosanoids and eicosanoid profiles to BP traits.
Methods
Availability of Data and Materials
The data that support the findings of this study are available from Finnish Institute for Health and Welfare Biobank (https://thl.fi/en/web/thl‐biobank). The data are not publicly available because they contain information that could compromise research participant privacy/consent. The source code for the analyses is openly available at 10.5281/zenodo.3604123.
Cohorts
The FINRISK 2002 study used a random population sample of individuals, aged 25 to 74 years, from 6 geographical areas of Finland. The sampling was stratified by sex, region, and 10‐year age group for a population sample of 13 500 individuals; the overall participation rate was 65.2% (n=8798). The sampling has been previously described in detail. 15 Plasma LC‐MS was performed successfully on n=8292 participants. After excluding 193 participants with missing covariate data, n=8099 individuals were included in the analyses as the discovery cohort for the present investigation.
The first‐generation (ie, the "original") cohort of the FHS included a random sample of two thirds of the adult population of Framingham, MA, who were enrolled in a longitudinal community‐based cohort study in 1948. The FHS Offspring includes 5124 participants, children of the first‐generation cohort and their spouses, who have been reexamined every 4 to 8 years since the first examination in 1971. The characteristics and study protocol of FHS Offspring cohort have been published. 16 For this study, we considered n=3002 individuals who participated in the 8 examination cycle of FHS Offspring in 2005 to 2008 and had assays for eicosanoids with LC‐MS. After excluding 143 participants with missing covariates, we included n=2859 participants as the replication cohort.
Ethical Approval
The FINRISK 2002 study was approved by Coordinating Ethics Committee of the Helsinki University Hospital District. FHS Offspring was approved by Boston University Medical Center's Institutional Review Board. All participants in both studies provided written informed consent. Participants' consent to publication of information was not required because the participants remain unidentifiable.
Clinical Evaluation and Definitions
Participants of both cohorts provided a medical history, including information on medication use, and underwent a physical examination and laboratory assessment of cardiovascular risk factors at baseline. The methods of these examinations have been described previously in detail. 15 , 16 At all examinations, a healthcare professional performed 2 (FHS Offspring) or 3 (FINRISK) sequential BP measurements using a mercury column sphygmomanometer on seated participants, according to a standardized protocol. We defined the BP at a given examination as the mean of all sequentially measured BP values. We defined hypertension as BP ≥140/90 mm Hg or use of antihypertensive medication. Antihypertensive medication use was based on self‐report in both studies. We defined pulse pressure as systolic minus diastolic BP and mean arterial pressure as [(2×diastolic BP)+systolic BP]/3. We defined body mass index (BMI) as weight (kg) divided by height (m) squared and current smoking as self‐reported daily use of tobacco products. In FINRISK 2002, prevalent diabetes mellitus was defined as self‐reported diabetes mellitus, a previous diagnostic code indicating diabetes mellitus in the nationwide Care Register for Health Care (International Classification of Diseases, Tenth Revision [ICD‐10], 17 codes E10–E14; International Classification of Diseases, Ninth Revision [ICD‐9], code 250; or International Classification of Diseases, Eighth Revision [ICD‐8], code 250), a previous diabetes mellitus medication purchase (Anatomical Therapeutic Chemical code A10*) in the nationwide Prescribed Drug Purchase register, or a diabetes mellitus medication code in the nationwide Reimbursed Medication Register. In FHS Offspring, prevalent diabetes mellitus was defined as a fasting plasma glucose ≥7.0 mmol/L or self‐reported use of glucose‐lowering medications. Using data from the Hospital Discharge and Drug Reimbursement Registers, we defined asthma in FINRISK using diagnostic codes indicating asthma (ICD‐10 codes J45‐J46 or ICD‐8/9 code 493), a previous asthma medication purchase (Anatomical Therapeutic Chemical codes R03BA, R03BC, R03DC, and R03AK), or having a special reimbursement for asthma medications. We estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula. 18 We defined NSAID use by a prescription purchase of medication under Anatomical Therapeutic Chemical group M01A (excluding subgroup M01AX and self‐care drug purchases).
Plasma Sampling
In FINRISK, blood samples were drawn after a minimum of 4 hours of fasting, the samples were kept at room temperature for 20 minutes before centrifugation, and the samples were stored at −70°C. In FHS, fasting samples were drawn, centrifuged for 22 minutes at 4°C, and separated plasma was stored at −80°C within 90 minutes of collection.
Eicosanoid Profiling
Using a directed nontargeted LC‐MS approach in conjunction with computational chemical networking of spectral fragmentation patterns, we identified 545 eicosanoids and related oxylipins in the FINRISK. The methods of plasma eicosanoid profiling using LC‐MS have been previously described in detail. 13 , 14 Metabolite data were adjusted for technical variation in off‐plate pooled plasma samples and in spike‐in internal standards. Missing values were replaced with minimum value for each eicosanoid abundance. The 6 eicosanoids and related oxylipin mediators included in the risk score were matched between FINRISK and FHS by comparing their LC‐MS profiles. These metabolites were also identified, if possible, through comparisons with reference standards and online databases.
Genotyping
The methods of single‐nucleotide polymorphism (SNP) genotyping and quality control have been previously described in detail. 19 In short, the participants of FINRISK were genotyped on Illumina CoreExome genotyping array. A reference panel of 1000 genomes was further used to impute genotypes.
Statistical Analysis
We used R version 3.6.1 20 for all analyses. The source code for the analyses is openly available at 10.5281/zenodo.3604123. 21 Unless otherwise noted, we adjusted all analyses for age, sex, BMI, current smoking, diabetes mellitus, antihypertensive medication, and MS batch. We normalized eicosanoid abundances using median absolute deviation; we calculated the median of the absolute difference from the median, and used this value to scale all analyte values for a given assay plate. We used linear and logistic regression models to examine the associations between each eicosanoid molecule and BP traits (systolic BP, diastolic BP, pulse pressure, mean arterial pressure, and hypertension). We adjusted for multiple comparisons using Bonferroni correction to minimize the probability of type I error. 22 Relations between eicosanoids significantly associated with systolic blood arterial pressure were assessed using Spearman correlation and ordered using hierarchical cluster analysis with complete linkage method. We assessed the multivariable association between eicosanoids significantly related to systolic BP using stepwise linear regression modeling with forward selection and a Bonferroni‐corrected inclusion threshold of P=0.05/545. For the 6 eicosanoids that remained in the models, we calculated eicosanoid risk scores according to the formula β1X1+β2X2+...+βnXn, with Xn denoting the standardized value for the nth eicosanoid abundance, and βn denoting the regression coefficient from the regression model for systolic BP containing the statistically significant eicosanoids. 23 We assessed the odds of hypertension and increase in systolic BP by 1‐SD increases and by quartiles of the risk scores using unadjusted and multivariable adjusted logistic and linear regression models. We replicated these analyses in FHS Offspring using the eicosanoid abundances in FHS and the regression coefficients from FINRISK.
We also assessed the association between the eicosanoid risk score and systolic BP in subgroups by aspirin use, asthma status, age (younger than versus older than the median age of 49 years), BMI (<30 versus ≥30 kg/m2), glomerular filtration rate (<90 versus ≥90 mL/min), and NSAID use. We determined the association of the eicosanoid risk score with age, BMI, and estimated glomerular filtration rate using the Pearson correlation. We compared eicosanoid risk score levels between subgroups by aspirin use, asthma status, and NSAID use using the 2‐sample t test. To analyze the causative role of the eicosanoid risk score, we performed genome‐wide association study (GWAS) and 2‐sample mendelian randomization (MR) in the study sample. To account for ordered patterns in genetic data, we calculated multidimensional scaling based on raw Hamming distances using PLINK 24 version 1.9. We performed the GWAS for the continuous eicosanoid risk scores and the autosomes using SNPTEST 25 version 2.5.2, adjusted for age, sex, batch, and first 10 multidimensional scaling axes. We included in the mendelian randomization the SNPs that had Hardy‐Weinberg equilibrium >1E‐6, P<5E‐8, and minor allele frequencies >0.01 using TwoSampleMR. 26 For the outcome of the mendelian randomization, we used the GWAS results for automated systolic BP measurements in UK Biobank. 27 , 28 We estimated the causative roles using 5 distinct methods: inverse variance weighted, 29 weighted median, 30 weighted mode, 30 simple mode, 31 and MR‐Egger. 32
Results
The characteristics of FINRISK (n=8099; mean age, 48.0±13.1 years; 53.1% women) and FHS (n=2859; mean age, 66.3±8.9 years; 54.7% women) cohorts are shown in the Table.
Table 1.
Characteristics of the Discovery (FINRISK) and Replication (FHS) Samples
| Characteristics | FINRISK 2002 | FHS |
|---|---|---|
| No. of subjects | 8099 | 2859 |
| Age, mean (SD), y | 48.0 (13.1) | 66.3 (8.9) |
| Women, N (%) | 4300 (53.1) | 1564 (54.7) |
| BMI, mean (SD), kg/m2 | 26.9 (4.7) | 28.3 (5.4) |
| Systolic blood pressure, mean (SD), mm Hg | 135.1 (20.0) | 128.5 (17.2) |
| Diastolic blood pressure, mean (SD), mm Hg | 79.0 (11.3) | 73.4 (10.1) |
| Pulse pressure, mean (SD), mm Hg | 56.1 (16.1) | 55.1 (16.0) |
| Mean arterial pressure, mean (SD), mm Hg | 97.7 (12.7) | 91.8 (10.5) |
| Hypertension, N (%) | 3567 (44.0) | 1673 (58.5) |
| Antihypertensive medication, N (%) | 1177 (14.5) | 1389 (48.6) |
| Current smoker, N (%) | 2097 (25.9) | 256 (9.0) |
| Diabetes mellitus, N (%) | 446 (5.5) | 393 (13.7) |
Continuous variables are presented as mean (SD). Categorical variables reported as absolute and relative frequencies. BMI indicates body mass index; and FHS, Framingham Heart Study.
Association Between Eicosanoids and BP Traits
Of the eicosanoids and related oxylipin mediators, 187 (34.3%) were significantly associated with systolic BP, 124 (22.8%) with diastolic BP, 177 (32.5%) with mean arterial pressure, 161 (29.5%) with pulse pressure, and 155 (28.4%) with hypertension in FINRISK (Figure 1, Table S1). We selected systolic BP as our main outcome variable because of its strong association with cardiovascular diseases. We observed 175 (93.6%) positive and 12 (6.4%) negative associations for systolic BP (Figure 1, Table S1). The heat maps of pairwise correlations for the 187 metabolites related to systolic BP are shown in Figure 2 and Figure S1. This analysis revealed strong overall correlations, but only minor clustering of the eicosanoids.
Figure 1. Manhattan plot for associations between metabolites and systolic blood pressure in FINRISK 2002.
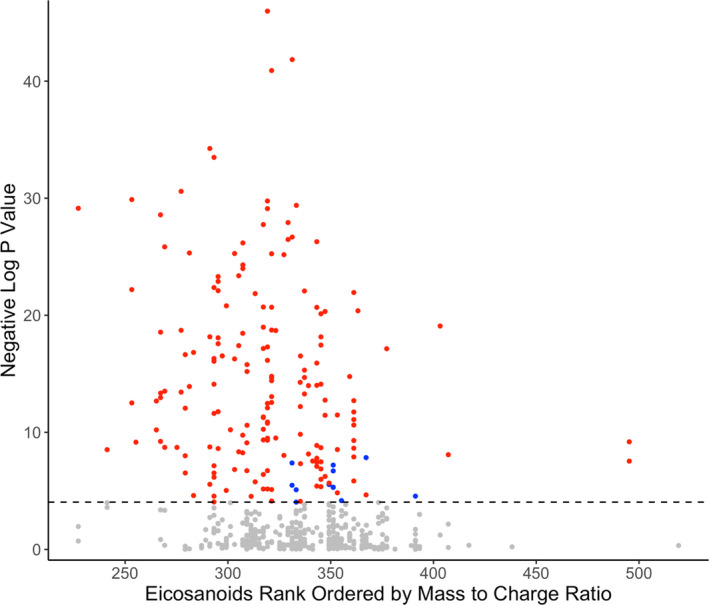
A significant association was observed for 187 of the 545 eicosanoids. Positive correlations are denoted in red, negative in blue, and insignificant in gray. Eicosanoids are ordered by the value of mass/charge ratio. Dashed line represents the Bonferroni‐corrected (P=0.05/545) level of significance. Analyses are adjusted for age, sex, body mass index, current smoking, diabetes mellitus, antihypertensive medication, and batch.
Figure 2. Correlation matrix for the 187 plasma metabolites related to systolic blood pressure in FINRISK 2002.
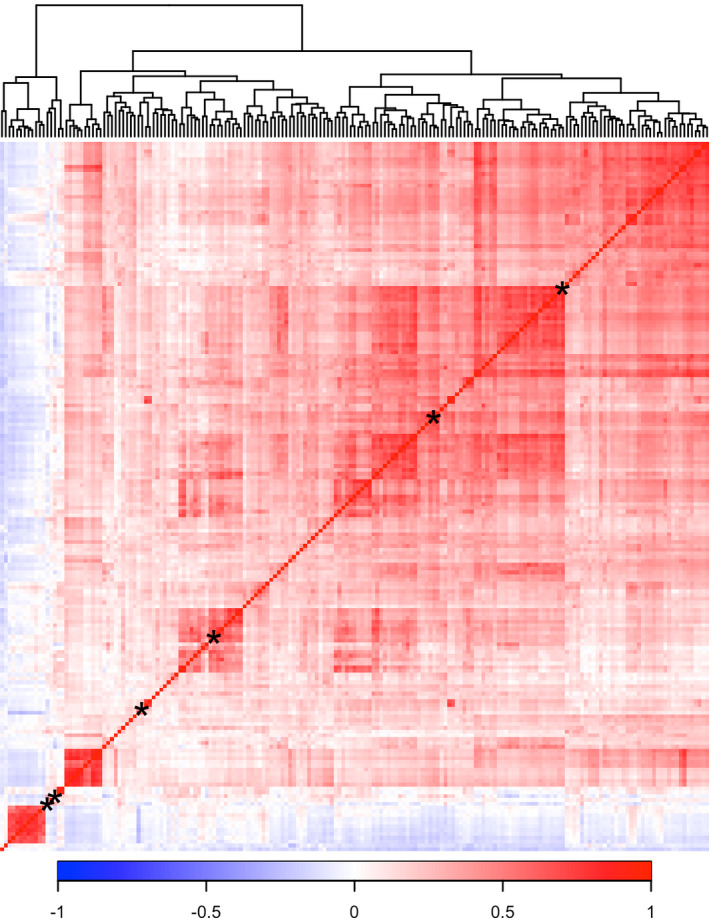
Relations between eicosanoids were calculated using Spearman correlation and ordered using hierarchical cluster analysis with complete linkage method. Only eicosanoids related to systolic blood pressure were included in the correlation matrix. Asterisk denotes position of the 6 metabolites in our eicosanoid risk score (Figure 3).
Independent Determinants of BP and Hypertension
We used forward selection linear regression modeling with a Bonferroni‐corrected inclusion threshold to define a set of metabolites that was independently associated with systolic BP. In FINRISK, these 6 metabolites were 11‐dehydro‐2,3‐dinor thromboxane B2 (TXB2), 12‐hydroxyheptadecatrienoic acid (12‐HHTrE), 265.1809/3.57 (putative eicosanoid), 295.2279/4.89 (putative eicosanoid), 319.2280/5.67 (unknown), and adrenic acid (Table S2). Of these 6 metabolites, 2 could not be detected in FHS plasma samples (11‐dehydro‐2,3‐dinor‐TXB2 and 295.2279/4.89). Comparing single‐metabolite associations, adjusted for relevant covariates, demonstrated that effect sizes between the metabolites were highly consistent across the 2 cohorts (Figure 3, Table S3).
Figure 3. The associations between a subset of 6 metabolites and systolic blood pressure (BP) in FINRISK and replication of results in FHS (Framingham Heart Study).
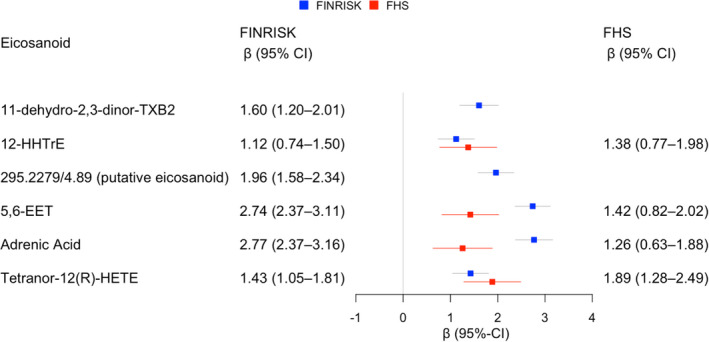
The β coefficients are for the association between 1‐SD increase in metabolite concentration and the absolute change of systolic BP (mm Hg) in the 2 study cohorts. All models were adjusted for age, sex, body mass index, current smoking, diabetes mellitus, antihypertensive medication, and batch. Of the 6 eicosanoids observed in FINRISK, 2 were not observed in FHS plasma samples. EET indicates epoxyeicosatrienoic acid; HETE, hexadecatrienoic acid; HHTrE, hydroxyheptadecatrenoic acid; and TXB2, thromboxane B2.
Eicosanoid Risk Score
We defined an eicosanoid risk score using the effect sizes in FINRISK for the 6 previously mentioned metabolites (Table S2). The abundances of the 2 nondetected metabolites in FHS were treated as zero values. Individuals in the top risk quartile had 9.0 (95% CI, 8.0–10.1) mm Hg higher systolic BP in FINRISK and 6.8 (95% CI, 5.1–8.5) mm Hg higher systolic BP in FHS compared with individuals in the lowest quartile (Figures 4, 5 and Tables S4, S5, S6). The odds for hypertension were 2.3 (95% CI, 2.0–2.6) and 2.0 (95% CI, 1.6–2.5), respectively, for the top quartile compared with the lowest quartile (Figures 4 and 5). These associations were consistent across FINRISK and FHS. We observed no differences in these associations in subgroups by aspirin use, asthma status, age, BMI, kidney function, and NSAID use (Figure S2). The correlations of the eicosanoid risk score with age, BMI, and estimated glomerular filtration rate were 0.11 (P<0.001), 0.07 (P<0.001), and −0.02 (P=0.04), respectively. We observed no significant differences in the eicosanoid risk score levels in subgroups by aspirin use, asthma status, and NSAID use (P>0.5).
Figure 4. The association between the risk score and systolic blood pressure (BP) in FINRISK and FHS (Framingham Heart Study).

We calculated eicosanoid risk score for each participant according to the formula β1X1+β2X2+...+βnXn, with Xn denoting the standardized value for the nth eicosanoid abundance, and βn denoting the regression coefficient from the regression model containing the indicated eicosanoids. Analyses are adjusted for age, sex, body mass index, current smoking, diabetes mellitus, antihypertensive medication, and batch. Q indicates quartile.
Figure 5. The association between the risk score and hypertension in FINRISK and FHS (Framingham Heart Study).
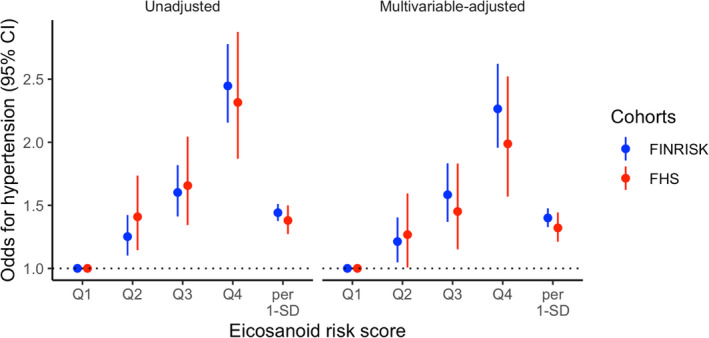
We calculated eicosanoid risk score for each participant according to the formula β1X1+β2X2+...+βnXn with Xn denoting the standardized value for the nth eicosanoid abundance, and βn denoting the regression coefficient from the regression model in FINRISK containing the indicated eicosanoids. Multivariable analyses are adjusted for age, sex, body mass index, current smoking, diabetes mellitus, antihypertensive medication, and batch. Q indicates quartile.
Two‐Sample Mendelian Randomization
We observed 222 SNPs in 2 chromosomes significantly associated with the eicosanoid risk score (Figure S3, Table S7). To account for linkage‐disequilibrium, in each 10‐kb window (r 2<0.001) only the SNP with lowest P value was retained (Table S8). We used the (n=436 419) GWAS results for systolic BP in UK Biobank as the outcome variables. 27 , 28 The 2‐sample mendelian randomization for the 3 SNPs and automated systolic BP measurement was nonsignificant (false discovery rate corrected P>0.26; Table S9).
Discussion
Using a directed nontargeted LC‐MS approach in well‐phenotyped, large community‐based cohorts, we identified 187 eicosanoids and related oxylipins that were associated with systolic BP. FINRISK 2002 participants in the top quartile of the eicosanoid risk score had 9.0 mm Hg greater systolic BP and a >2‐fold odds of hypertension, compared with individuals in the lowest quartile. These findings were replicated in the FHS Offspring participants.
The upstream initiation of inflammatory activity in humans is governed mainly by substrates and products of polyunsaturated ω‐3 and ω‐6 20‐carbon fatty acids. 7 , 33 The small‐molecule derivatives of arachidonic acid and other polyunsaturated fatty acids, termed eicosanoids, serve as both activators and suppressors of systemic inflammatory activity. 34 , 35 Most research on systemic inflammation in humans has focused on downstream markers of inflammatory activity, such as cytokines and short‐term phase reactants. Recent work by us and others has shown that long‐term elevation in these downstream markers is associated with a variety of cardiovascular disease risk traits and outcomes. 36 , 37 In particular, several cross‐sectional and prospective studies in humans have found an association of plasma concentrations of downstream low‐grade inflammation markers, such as interleukin‐6, intercellular adhesion molecule‐1, CRP (C‐reactive protein), and tumor necrosis factor‐α, with arterial stiffness and hypertension. 38 , 39 , 40 , 41 , 42 , 43 , 44 Although downstream markers of inflammation are associated with hypertension and a variety of cardiovascular disease outcomes, evidence for a clinically important, causal role of these biomarkers has been mixed. 45 In addition, despite inflammation being pivotal in the development of atherosclerosis and certain medications with anti‐inflammatory properties clearly reduce cardiovascular disease risk, the extent to which any given inflammatory pathway warrants attention as a direct putative target for therapy is unknown. 46 Such results have now led experts to suggest that, where inflammation is concerned, causal factors may be upstream. 45
This study is the first to comprehensively examine the association between eicosanoids and BP in humans. Prior studies with study samples consisting of tens of hypertensive subjects with a panel of a few, mainly cytochrome P450 pathway eicosanoids have demonstrated that eicosanoids, in general, affect regulation of renal function, vascular tone, and the development of hypertension. 12 , 47 , 48 , 49 , 50 Our results from a large, population‐based sample demonstrate that a large number of eicosanoid species are related to BP in both a positive and a negative way. In addition, we demonstrate that a distinct eicosanoid score is related to a >2‐fold odds of hypertension. The subgroup analyses demonstrate that the association between our eicosanoid risk score and systolic BP was highly consistent, even in states that affect eicosanoid metabolism and excretion. Although the number of individuals in some subgroups was low, we observed no differences in the relation between the risk score and BP in subgroups by asthma status, age, aspirin use, BMI, and kidney function. Furthermore, the eicosanoid risk score demonstrated only weak correlations with these phenotypes, implying an independent role for eicosanoids in hypertension risk.
Eicosanoids are metabolized via 3 general pathways that involve cytochrome P450 monooxygenases, cyclooxygenases, and lipoxygenases. Several of the identified metabolites that remained in the 6‐eicosanoid risk score are members of these pathways. In addition, the key metabolites included in the eicosanoid risk score include both intermediate (eg, adrenic acid and 12‐HHTrE) and terminal (eg, 11‐dehydro‐2,3‐dinor‐TXB2), potentially reflecting key eicosanoid pathways and species related to BP regulation. The cytochrome P450 pathway metabolizes arachidonic acid to several eicosanoids, including 20‐hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. 8 These metabolites are critical in BP regulation and provide cardioprotective and renoprotective effects in chronic kidney disease. 8 Cyclooxygenase pathway produced prostanoids are involved in BP homeostasis and, in particular, short‐lived thromboxane A2 (TXA2; half‐life, 30 seconds) has important role in various cardiovascular diseases through action on platelet aggregation, vasoconstriction, and proliferation. 51 , 52 TXA2 is metabolized to inactive TXB2, which is degraded through 2 major pathways (dehydrogenation and β‐oxidation) and their combination, which results in the formation of 11‐dehydro‐2,3‐dinor‐TXB2. 53 , 54 Currently, factors affecting the relative production of 11‐dehydro‐2,3‐dinor‐TXB2 and analyte prognostic utility are not known. 52 However, 11‐dehydro‐2,3‐dinor‐TXB2 may have a role in atherothrombosis. 54 Another metabolite included in our 6‐eicosanoid risk score, 12‐HHTrE, is a nonenzymatic degradation product of TXA2 and prostaglandin H2 (an important precursor for eicosanoids). 55 12‐HHTrE is a natural ligand for leukotriene B(4) receptor 2 and is linked to synthesis of prostacyclin (prostaglandin I2), a potent vasodilator, and the main metabolite of 12‐HHTrE has antagonist effect to TXA2 receptor. 56 , 57 In addition to eicosanoid pathway products, adrenic acid was also included in our risk score. Adrenic acid is a polyunsaturated 22‐carbon fatty acid and mainly a substrate for eicosanoid production, and it has been associated with the regulation of adrenal blood flow. 58 Given the findings from our study and from previous experimental trials, these results provide a strong biological basis for how eicosanoids could affect human BP regulation through several different mechanisms. In particular, elevated 11‐dehydro‐2,3‐dinor‐TXB2 and 12‐HHTrE levels may be result of elevated TXA2 activity but their independent role and possible prognostic utility need to be evaluated in future research.
Our study has several strengths, such as unselected population sample, external replication of our results, and assays of a large number of eicosanoids. However, our results must be interpreted in the context of potential limitations. First, LC‐MS is a highly sensitive method for assessing circulating metabolites. Particularly, of the 6 metabolites that remained in the final forward‐selection regression model in FINRISK, only 4 were observed in FHS samples. This could in part be explained by the between‐cohort age and smoking disparities. Second, the unambiguous metabolite classification and identification is still a challenge in high‐throughput LC‐MS. However, we have previously demonstrated that these signals are highly consistent with known and putative eicosanoids and related oxylipins in human plasma. 14 Third, many eicosanoids have short half‐lives of <1 hour and the variance of the measured metabolites is expected to be highly affected by sample processing methods. However, our plasma samples were stored at −70 to 80°C on‐site following a strict protocol, and we studied standardized, rather than absolute, metabolite concentrations. Finally, our study demonstrates a strong proof‐of‐concept association between eicosanoids and BP. Although much is known of eicosanoid physiology, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 additional mechanistic and experimental studies are needed to assess the precise identities and functions of many of the observed metabolites. The eicosanoids included in the risk score are known to be produced in neuronal tissues, platelets, leukocytes, and smooth muscle cells, 59 which could be used as starting points for in vitro experiments.
Conclusions
Plasma eicosanoids demonstrate strong associations with BP in the general population, and differences between eicosanoid profiles are observed between normotensive versus hypertensive participants. Intriguingly, although most of the associations were positive (harmful species), we observed protective molecules as well. In our mendelian randomization analysis, however, we were unable to demonstrate a causal association between eicosanoids and BP. However, this analysis could be limited by the small GWAS sample size. Additional preclinical analyses are therefore needed to examine the causality between eicosanoids and BP and to clarify whether eicosanoids could serve as potential targets for therapeutic intervention.
Sources of Funding
This work was supported by the Emil Aaltonen Foundation (Niiranen), the Paavo Nurmi Foundation (Niiranen), the Finnish Medical Foundation (Niiranen), the Finnish Foundation for Cardiovascular Research (Salomaa), the Academy of Finland (grant 321351 to Niiranen; grants 295741 and 307127 to Lahti; grant 321356 to Havulinna), Ellison Foundation (Cheng), the National Heart, Lung, and Blood Institute's FHS (Framingham Heart Study) (contracts N01HC25195, HHSN268201500001I, and 75N92019D00031), and the following National Institutes of Health grants: R01HL093328 (Vasan), R01HL107385 (Vasan), R01HL126136 (Vasan), R00HL107642 (Cheng), R01HL131532 (Cheng), R01HL134168 (Cheng and Jain), R01HL143227 (Cheng and Jain), R01ES027595 (Jain and Cheng), and K01DK116917 (Watrous). Dr Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. The funders play no role in the design of the study; the collection, analysis, and interpretation of the data; and the decision to approve publication of the finished manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
Salomaa has received honoraria from Novo Nordisk and Sanofi for consultations and travel support from Novo Nordisk. He also has ongoing research collaboration with Bayer Ltd (all unrelated to the present study). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S9
Figures S1–S3
Acknowledgments
We thank the participants and staff of the FINRISK 2002 and FHS (Framingham Heart Study). We thank Felix Vaura for the assistance with performing genome‐wide association study used in this article.
(J Am Heart Assoc. 2020;9:e017598 DOI: 10.1161/JAHA.120.017598.)
Supplementary materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017598
Preprint posted on MedRxiv March 30, 2020. doi: https://doi.org/10.1101/2020.02.08.20021022.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Carretero OA, Oparil S. Essential hypertension. Circulation. 2000;101:329–335. [DOI] [PubMed] [Google Scholar]
- 2. Cavalcante JL, Lima JAC, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 3. Harizi H, Corcuff J‐B, Gualde N. Arachidonic‐acid‐derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. [DOI] [PubMed] [Google Scholar]
- 4. Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberto N. The role of eicosanoids in angiotensin‐dependent hypertension. Hypertension. 1998;31:194–200. [DOI] [PubMed] [Google Scholar]
- 6. Capdevila J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens. 2013;22:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2019;176:1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roman RJ, Fan F. 20‐HETE: hypertension and beyond. Hypertension. 2018;72:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A. Soluble epoxide hydrolase‐dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol. 2014;306:H1146–H1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kujal P, Chábová VČ, Škaroupková P, Husková Z, Vernerová Z, Kramer HJ, Walkowska A, Kompanowska‐Jezierska E, Sadowski J, Kitada K, et al. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren‐2 transgenic hypertensive rats. Clin Exp Pharmacol Physiol. 2014;41:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward NC, Tsai I‐J, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20‐HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. [DOI] [PubMed] [Google Scholar]
- 13. Lagerborg KA, Watrous JD, Cheng S, Jain M. High‐throughput measure of bioactive lipids using non‐targeted mass spectrometry. Methods Mol Biol. 2019;1862:17–35. [DOI] [PubMed] [Google Scholar]
- 14. Watrous JD, Niiranen TJ, Lagerborg KA, Henglin M, Xu Y‐J, Rong J, Sharma S, Vasan RS, Larson MG, Armando A, et al. Directed non‐targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem Biol. 2019;26:433–442.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borodulin K, Tolonen H, Jousilahti P, Jula A, Juolevi A, Koskinen S, Kuulasmaa K, Laatikainen T, Männistö S, Peltonen M, et al. Cohort profile: the National FINRISK study. Int J Epidemiol. 2018;47:696–696i. [DOI] [PubMed] [Google Scholar]
- 16. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 17. International Statistical Classification of Diseases version 10 (in Finnish). Terveyden ja hyvinvoinnin laitos (THL); 2011. http://www.julkari.fi/handle/10024/80324. Accessed October 16, 2019. [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abraham G, Havulinna AS, Bhalala OG, Byars SG, De Livera AM, Yetukuri L, Tikkanen E, Perola M, Schunkert H, Sijbrands EJ, et al. Genomic prediction of coronary heart disease. Eur Heart J. 2016;37:3267–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R Core Team . R: A Language and Environment for Statistical Computing: Version 3.6.0. R Foundation for Statistical Computing; 2017. https://www.R‐project.org/. Accessed May 2, 2019. [Google Scholar]
- 21. Palmu J, Lahti L, Niiranen T. EicosanoidsBP: source code for the manuscript association between the gut microbiota and blood pressure in a population cohort of 6953 individuals: version 5. Zenodo; 2020. DOI: 10.5281/zenodo.3604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VanderWeele TJ, Mathur MB. Some desirable properties of the Bonferroni correction: is the Bonferroni correction really so bad?. Am J Epidemiol. 2019;188:617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second‐generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome‐wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. [DOI] [PubMed] [Google Scholar]
- 26. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR‐Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell R, Elsworth BL, Mitchell R, Raistrick CA, Paternoster L, Hemani G, Gaunt TR. MRC IEU UK Biobank GWAS pipeline version 2. data.bris. DOI: 10.5523/bris.pnoat8cxo0u52p6ynfaekeigi. [DOI]
- 28. Elsworth BL. MRC IEU UK Biobank GWAS pipeline version 1. data.bris. DOI: 10.5523/bris.2fahpksont1zi26xosyamqo8rr. [DOI]
- 29. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowden J, Smith GD, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–340. [DOI] [PubMed] [Google Scholar]
- 34. Elshenawy O, Shoieb S, Mohamed A, El‐Kadi A. Clinical implications of 20‐hydroxyeicosatetraenoic acid in the kidney, liver, lung and brain: an emerging therapeutic target. Pharmaceutics. 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roman RJ. P‐450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. [DOI] [PubMed] [Google Scholar]
- 36. Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C‐reactive protein, interleukin‐6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality: a population‐based, prospective study. Thromb Haemost. 2006;95:511–518. [DOI] [PubMed] [Google Scholar]
- 37. IL6R Genetics Consortium Emerging Risk Factors Collaboration , Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, et al. Interleukin‐6 receptor pathways in coronary heart disease: a collaborative meta‐analysis of 82 studies. Lancet Lond Engl. 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. [DOI] [PubMed] [Google Scholar]
- 39. Lakoski SG, Cushman M, Palmas W, Blumenthal R, D’Agostino RB, Herrington DM. The relationship between blood pressure and C‐reactive protein in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2005;46:1869–1874. [DOI] [PubMed] [Google Scholar]
- 40. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. [DOI] [PubMed] [Google Scholar]
- 41. Sesso HD, Jiménez MC, Wang L, Ridker PM, Buring JE, Gaziano JM. Plasma inflammatory markers and the risk of developing hypertension in men. J Am Heart Assoc. 2015;4:e001802 DOI: 10.1161/JAHA.115.001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C‐reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. [DOI] [PubMed] [Google Scholar]
- 43. McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, Gallacher J, Ben‐Shlomo Y, Cockcroft JR, Wilkinson IB. An analysis of prospective risk factors for aortic stiffness in men: 20‐year follow‐up from the Caerphilly prospective study. Hypertension. 2010;56:36–43. [DOI] [PubMed] [Google Scholar]
- 44. Chuang S‐Y, Hsu P‐F, Chang H‐Y, Bai C‐H, Yeh W‐T, Pan H‐W. C‐reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the Cardiovascular Disease Risk Factors Two‐Township Study. Am J Hypertens. 2013;26:657–664. [DOI] [PubMed] [Google Scholar]
- 45. Brunner EJ, Kivimäki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GDO, Rumley A, Casas JP, et al. Inflammation, insulin resistance, and diabetes–Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group . Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet Lond Engl. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 47. Laffer CL, Laniado‐Schwartzman M, Wang M‐H, Nasjletti A, Elijovich F. Differential regulation of natriuresis by 20‐hydroxyeicosatetraenoic acid in human salt‐sensitive versus salt‐resistant hypertension. Circulation. 2003;107:574–578. [DOI] [PubMed] [Google Scholar]
- 48. Ward NC, Puddey IB, Hodgson JM, Beilin LJ, Croft KD. Urinary 20‐hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med. 2005;38:1032–1036. [DOI] [PubMed] [Google Scholar]
- 49. Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9‐derived endothelium‐derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–515. [DOI] [PubMed] [Google Scholar]
- 50. Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008;51:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen H. Role of thromboxane A2 signaling in endothelium‐dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018;134:32–37. [DOI] [PubMed] [Google Scholar]
- 52. Olson MT, Kickler TS, Lawson JA, Mclean RC, Jani J, Fitzgerald GA, Rade JJ. Effect of assay specificity on the association of urine 11‐dehydro thromboxane B2 determination with cardiovascular risk. J Thromb Haemost. 2012;10:2462–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lingling W, Guixin C, Wei L, Hua S. Interaction between urinary 11 dehydrothromboxane B2 and some other risk factors in the occurrence of cerebral infarction. Open Med J. 2019;6:89–93. [Google Scholar]
- 54. DeFilippis AP, Oloyede OS, Andrikopoulou E, Saenger AK, Palachuvattil JM, Fasoro YA, Guallar E, Blumenthal RS, Kickler TS, Jaffe AS, et al. Thromboxane A(2) generation, in the absence of platelet COX‐1 activity, in patients with and without atherothrombotic myocardial infarction. Circ J. 2013;77:2786–2792. [DOI] [PubMed] [Google Scholar]
- 55. Maddipati KR, Romero R, Chaiworapongsa T, Zhou S‐L, Xu Z, Tarca AL, Kusanovic JP, Munoz H, Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014;28:4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Csanyi G, Lepran I, Flesch T, Telegdy G, Szabo G, Mezei Z. Lack of endothelium‐derived hyperpolarizing factor (EDHF) up‐regulation in endothelial dysfunction in aorta in diabetic rats. Pharmacol Rep. 2007;59:447–455. [PubMed] [Google Scholar]
- 57. Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T. 12(S)‐hydroxyheptadeca‐5Z, 8E, 10E‐trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med. 2008;205:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kopf PG, Zhang DX, Gauthier KM, Nithipatikom K, Yi X‐Y, Falck JR, Campbell WB. Adrenic acid metabolites as endogenous endothelium‐derived and zona glomerulosa‐derived hyperpolarizing factors. Hypertension. 2010;55:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez‐Fresno R, Sajed T, Johnson D, Li C, Karu N, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S3
Data Availability Statement
The data that support the findings of this study are available from Finnish Institute for Health and Welfare Biobank (https://thl.fi/en/web/thl‐biobank). The data are not publicly available because they contain information that could compromise research participant privacy/consent. The source code for the analyses is openly available at 10.5281/zenodo.3604123.


