Abstract
Background
The coronavirus disease 2019 (COVID‐19) has developed into a global outbreak. Patients with cardiovascular disease (CVD) with COVID‐19 have different clinical characteristics and prognostic outcomes. This study aimed to summarize the clinical characteristics and laboratory indicators of patients with COVID‐19 with CVD, especially the critically ill patients.
Methods and Results
This study included 244 patients diagnosed with COVID‐19 and CVD (hypertension, coronary heart disease, or heart failure). The patients were categorized into critical (n=36) and noncritical (n=208) groups according to the interim guidance of China’s National Health Commission. Clinical, laboratory, and outcome data were collected from the patients’ medical records and compared between the 2 groups. The average body mass index of patients was significantly higher in the critical group than in the noncritical group. Neutrophil/lymphocyte ratio, and C‐reactive protein, procalcitonin, and fibrinogen, and d‐dimer levels at admission were significantly increased in the critical group. The all‐cause mortality rate among cases of COVID‐19 combined with CVD was 19.26%; the proportion of coronary heart disease and heart failure was significantly higher in deceased patients than in recovered patients. High body mass index, previous history of coronary heart disease, lactic acid accumulation, and a decrease in the partial pressure of oxygen were associated with death.
Conclusions
All‐cause mortality in patients with COVID‐19 with CVD in hospitals is high. The high neutrophil/lymphocyte ratio may be a predictor of critical patients. Overweight/obesity combined with coronary heart disease, severe hypoxia, and lactic acid accumulation resulting from respiratory failure are related to poor outcomes.
Registration
URL: https://www.chictr.org.cn; Unique identifier: ChiCTR2000029865.
Keywords: cardiovascular disease, coronavirus disease, COVID‐19, respiratory failure, SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Subject Categories: Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- COVID‐19
coronavirus disease 2019
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
Clinical Perspective
What Is New?
The study comprehensively describes the phenotypic and biochemical characteristics of those with a previous history of cardiovascular disease and were admitted with coronavirus disease 2019.
We found that neutrophil/lymphocyte ratio, C-reactive protein, procalcitonin, fibrinogen, and d-dimer levels at admission were significantly increased in those who develop critical disease; and those with higher body mass index, lactate levels, or previous history of coronary heart disease were more likely to die as compared with those who did not.
What Are the Clinical Implications?
There are distinct phenotypic and laboratory patterns that are associated with increase in mortality among those with previous cardiovascular disease and admitted with coronavirus disease 2019.
It is important to identify these patients early to improve their outcomes.
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in Wuhan, China. The disease caused by the virus was subsequently named coronavirus disease 2019 (COVID‐19) by the World Health Organization. 1 SARS‐CoV‐2 is mainly transmitted via respiratory droplets or physical contact, and humans have been proven susceptible. 2 , 3 In only a few months, it has spread to people in countries across the globe, including the United States. 4 The genetic characteristics of SARS‐CoV‐2 differ significantly from those of SARS‐CoV and Middle East respiratory syndrome coronavirus. 5 , 6 The main symptoms of COVID‐19 include fever, fatigue, and dry cough. Most patients have mild symptoms and good prognoses. Severe cases may quickly progress to acute respiratory distress syndrome, septic shock, or metabolic acidosis. These complications are difficult to treat and may lead to coagulopathy and death. 3
Patients with cardiovascular disease (CVD) who are infected by SARS‐CoV‐2, especially those who are critically ill, have different clinical characteristics and prognostic outcomes. According to current reports, the proportion of patients diagnosed with COVID‐19 comorbid with CVD is approximately 29.3% to 45.7%, 7 , 8 and the mortality rate is 15%. 8 Patients with CVD with COVID‐19 are more prone to severe pulmonary edema, acute respiratory distress syndrome, multiple organ failure, and death. 7 , 8 Nonetheless, the previous studies did not provide comprehensive and sufficient knowledge on the patients with CVD with COVID‐19. In the present study, we examined the clinical characteristics and outcomes of patients with CVD with confirmed COVID‐19 admitted to 3 hospitals in Wuhan, China. Our aim was to summarize the clinical characteristics and laboratory indicators of patients with COVID‐19 with CVD, especially the critically ill patients.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data and materials have been made publicly available at the Chinese Clinical Trial Registry and can be accessed at http://www.chictr.org.cn.
Patients and Definitions
We recruited patients from January 10 to February 25, 2020, receiving medical intervention who were cured or who died (clinical end point) at the West District of Union Hospital, Union Wuhan Red Cross Hospital, and Union Jiangnan Hospital in Wuhan, China. This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology, and was registered in the Chinese Clinical Trial Registry (No. ChiCTR2000029865). Written informed consent was obtained from the patients involved or next of kin before enrollment. Adult patients (aged >18 years) were admitted to the 3 hospitals in Wuhan city. In total, 850 patients diagnosed with COVID‐19 according to the interim guidance of China’s National Health Commission were screened for inclusion in this study 9 ; of these, 278 patients also had CVD. CVD included previous diagnoses of hypertension, coronary heart disease (CHD), and heart failure (HF). The diagnosis and treatment of hypertension and HF were based on the corresponding guidelines. 10 , 11 CHD was diagnosed by coronary angiography or coronary computed tomography (CT) showing at least 1 epicardial main vessel stenosis of >50% in diameter or a clear history of old myocardial infarction. After excluding patients with systemic diseases or severe liver or kidney dysfunction, 244 patients were enrolled for further analysis. All diagnoses and treatments were conducted in strict accordance with the interim guidance of the National Health Commission. 9 Patients were categorized into the critical and noncritical groups according to the disease severity. Briefly, those with any of the following conditions were classified as critical (n=36): respiratory failure requiring mechanical ventilation, shock, and combined organ failure requiring intensive care unit monitoring and treatment. 9 In the noncritical group (n=208), patients with fever, respiratory symptoms, pneumonia confirmed by imaging, and other features were classified as having a mild disease. Those with any of the following conditions were classified as having a severe disease: respiratory distress with a respiratory rate of ≥30 beats/min; at rest, finger oxygen saturation ≤93%; and Pao 2/fraction of inspired oxygen ≤300 mm Hg (1 mm Hg=0.133 kPa). 9
Procedures
Initial investigations included a complete blood count and the following tests: C‐reactive protein, arterial blood gas analysis, markers of myocardial injury (creatine kinase [CK], creatine kinase–myocardial band [CK‐MB], troponin I, AST, lactate dehydrogenase [LDH]), coagulation profile (prothrombin time, activated partial thromboplastin time), serum biochemical test (including renal and liver function, blood lipids, and electrolytes), procalcitonin, brain natriuretic peptide, fibrinogen, d‐dimer, and body mass index. According to the criteria recommended by the Working Group on Obesity in China, a body mass index of 24 kg/m2 was used as the cutoff point for overweight and obesity. 12 Respiratory specimens, including nasal and pharyngeal swabs, bronchoalveolar lavage fluid, sputum, or bronchial aspirates, were tested for common viruses including influenza, avian influenza, respiratory syncytial virus, adenovirus, and parainfluenza virus. SARS‐CoV‐2, SARS‐CoV, and Middle East respiratory syndrome coronavirus testing using real‐time reverse transcription polymerase chain reaction assays was approved by the China Food and Drug Administration. These diagnostic criteria were based on the recommendation from the National Institute for Viral Disease Control and Prevention (China) (http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html). Routine bacterial and fungal examinations were also performed. All specimens were collected within 24 hours of admission. All patients underwent chest CT.
Outcomes
A patient was considered cured if there was a significant improvement in or disappearance of clinical symptoms and a significant improvement in lung CT images, and the patient showed negative nucleic acid tests for SARS‐CoV‐2 on 2 different days (simultaneously available). 9 Death was defined as all‐cause death in the hospital.
Data collection
We collected the clinical, laboratory, and outcome data from the patients’ medical records. The clinical outcomes were observed until February 25, 2020. Two researchers independently reviewed the medical records and collected the data.
Statistical Analysis
Values are presented as mean±SE, median (interquartile range [IQR]), or percentage in each group. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Differences were evaluated using 1‐way ANOVA and the Newman‐Keuls method. The means continuous variables were compared using independent group t‐tests when the data were normally distributed; otherwise, the Mann‐Whitney U test was used. Proportions for categorical variables were compared using the chi‐squared test. A P value of <0.05 was considered statistically significant. A multivariate logistic regression model was applied to analyze factors potentially related to the disease outcome.
Results
Clinical Characteristics of All Patients
Basic patient information upon admission is shown in Table 1. The median age of all patients with COVID‐19 with CVD was 61 years (IQR, 53–67; range, 29–87 years), including 113 (46.31%) males and 131 females (53.69%). There were 208 (85.25%) and 36 (14.75%) patients in the noncritical and critical groups, respectively. The average ages of the patients in the noncritical and critical groups were 61.5 (IQR, 53.00–67.00) and 58.00 (IQR, 53.50–63.00) years, respectively (P=0.51). Moreover, the body mass index of patients in the critical group was significantly higher than that of patients in the non‐critical group (24.5 versus 22.0 kg/m2, P=0.006). The median time from symptom onset to admission was 10.00 days (IQR, 6.00–13.50) for all patients, and the out‐of‐hospital delays for patients in the critical and noncritical groups were 10.00 (IQR, 7.50–13.00) and 9.00 (IQR, 6.00–14.00) days, respectively (P=0.32). Among the patients, 21.72% had diabetes mellitus, the distribution of which was comparable in the critical and noncritical groups (25.00% versus 21.15%; P=0.60). Hypertension was the most common comorbidity in all patients (82.79%); the proportions of CHD and HF were 54.92% and 34.84%, respectively. No significant differences were observed between the groups for hypertension (77.78% versus 83.65%; P=0.39), CHD (55.56% versus 54.81%, P=0.93), or HF (38.89% versus 34.13%, P=0.58). Most patients had fever (197 cases [80.74%]); other symptoms included cough (164 cases [67.21%]), acratia or muscle ache (155 cases [63.52%]), chest pain or chest tightness (84 cases [34.43%]), diarrhea (30 cases [12.30%]), dyspnea (29 cases [11.89%]), rhinocleisis (23 cases [9.43%]), rhinorrhea (23 cases [9.43%]), and others (23 cases [9.43%]). Among the 244 patients, 47 (19.26%) died, and the mortality rate of critical patients (75.00%) was significantly higher than that of the noncritical patients (9.62%) (P<0.001). Twenty patients with a noncritical status became critically ill and died during treatment. Among them, 60% (12/20) had 3 types of CVD, and 80% (16/20) were overweight/obese (data not shown). Most patients with COVID‐19 with CVD died of respiratory failure (31 cases; 65.96%); the other causes of death included acute myocardial infarction (AMI) (6 cases; 12.77%), HF (4 cases, 8.51%), cerebrovascular accident (2 cases; 4.26%), and others (4 cases; 8.51%).
Table 1.
Baseline Characteristics of Patients at Admission and Death Cause
| Variables | All (n=244) | Critical (n=36) | Noncritical (n=208) | P Value* |
|---|---|---|---|---|
| Age, y, median (IQR) | 61.00 (53.00–67.00) | 58.00 (53.50–63.00) | 61.50 (53.00–67.00) | 0.51 |
| Sex, n (%) | 0.63 | |||
| Female | 131 (53.69) | 18 (50.00) | 113 (54.33) | |
| Male | 113 (46.31) | 18 (50.00) | 95 (45.67) | |
| BMI, kg/m2 | 22 (20.00–25.00) | 24.50 (21.50–26.00) | 22.00 (20.00–24.00) | 0.006 |
| Onset to admission, median (IQR), d | 10.00 (6.00–13.50) | 10.00 (7.50–13.00) | 9.00 (6.00–14.00) | 0.32 |
| Diabetes mellitus, n (%) | 53 (21.72) | 9 (25.00) | 44 (21.15) | 0.60 |
| CVD comorbidities, n (%) | ||||
| Hypertension | 202 (82.79) | 28 (77.78) | 174 (83.65) | 0.39 |
| CHD | 134 (54.92) | 20 (55.56) | 114 (54.81) | 0.93 |
| HF | 85 (34.84) | 14 (38.89) | 71 (34.13) | 0.58 |
| Signs and symptoms, n (%) | ||||
| Fever | 197 (80.74) | 28 (77.78) | 169 (81.25) | 0.63 |
| Cough | 164 (67.21) | 24 (66.67) | 140 (67.31) | 0.94 |
| Acratia or muscle ache | 155 (63.52) | 22 (61.11) | 133 (63.94) | 0.74 |
| Chest pain or chest tightness | 84 (34.43) | 12 (33.33) | 72 (34.62) | 0.88 |
| Diarrhea | 30 (12.30) | 2 (5.56) | 28 (13.46) | 0.27 |
| Dyspnea | 29 (11.89) | 6 (16.67) | 23 (11.06) | 0.40 |
| Rhinocleisis | 23 (9.43) | 5 (13.89) | 18 (8.65) | 0.35 |
| Rhinorrhea | 23 (9.43) | 5 (13.89) | 18 (8.65) | 0.35 |
| Others | 23 (9.43) | 5 (13.89) | 18 (8.65) | 0.35 |
| Death cause, n (%) | 47 (19.26) | 27 (75.00) | 20 (9.62) | <0.001 |
| Respiratory failure | 31 (65.96) | 20 (55.56) | 11 (5.29) | |
| AMI | 6 (12.77) | 3 (8.33) | 3 (1.44) | |
| HF | 4 (8.51) | 3 (8.33) | 1 (0.48) | |
| Cerebrovascular accident | 2 (4.26) | 0 (0.00) | 2 (0.96) | |
| Others | 4 (8.51) | 1 (2.78) | 3 (1.44) | |
AMI indicates acute myocardial infarction; BMI, body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; and IQR, interquartile range.
P values indicate differences between critical and non‐critical patients. P<0.05 was considered statistically significant.
Chest CT Images
Characteristic chest CT images are shown in the Figure. These images revealed ground‐glass shadows in 1 or both lungs, and most lesions showed clumpy flocculent changes along the lung periphery. Mild cases (Figure A) showed focal flocculent changes in the unilateral/bilateral lungs, without pulmonary lobules or subsegmental fusion. Severe cases (Figure B) were characterized by bilateral multiple lobules and subsegmental integration. Critical cases (Figure C) showed large areas of bilateral lamellar and segmental fusion with almost complete consolidation of both lungs.
Figure 1.
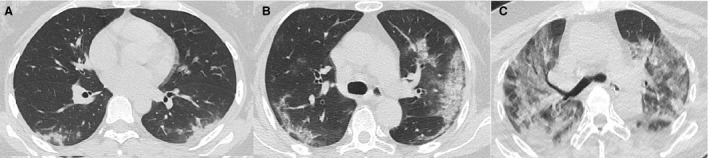
Chest computed tomography images of patients with coronavirus disease (COVID‐19).
A, Focal flocculent changes in the unilateral/bilateral lungs (mild case). B, Bilateral multiple lobules and subsegmental integration (severe case). C, Large areas of bilateral lamellar and segmental fusion with almost complete consolidation of both lungs (critical case).
Laboratory Findings Upon Admission
The differences in the laboratory parameters, including blood cell count and C‐reactive protein, procalcitonin, CK, CK‐MB, troponin I, AST, LDH, fibrinogen, and d‐dimer levels, between the 2 groups are shown in Table 2. The white blood cell count of most patients was normal at admission, with no significant difference between the critical and noncritical groups (7.23 [4.89–9.44] versus 5.86 [4.59–8.15] ×109/L; P=0.14). However, the neutrophil counts were higher (6.46 [3.74–8.41] versus 4.14 [3.08–6.47] ×109/L; P=0.01), and the lymphocyte (0.58 [0.35–0.88] versus 0.91 [0.64–1.29] ×109/L; P<0.001) and monocyte (0.25 [0.18–0.40] versus 0.38 [0.28–0.53] ×109/L; P<0.001) counts were significantly lower in the critical group than in the noncritical group. Moreover, the neutrophil/lymphocyte ratio was significantly higher in the critical group than in the noncritical group (critical, 9.82 [7.06–18.74] versus noncritical, 4.51 [2.70–8.90]; P<0.001). Compared with the noncritical group, the markers of myocardial injury such as CK (critical, 112.50 [51.50, 289.50] versus noncritical, 68.50 [45.00, 124.00] U/L; P=0.03), CK‐MB (critical 16.00 [11.50, 22.00] versus non‐critical 12.00 [9.00, 16.00] U/L, P=0.005), troponin I (critical 15.70 [8.10, 124.70] versus non‐critical 4.76 [2.00, 9.60] pg/mL, P<0.001), LDH (critical 435.00 [329.00, 641.50] versus non‐critical 291.00 [213.00, 398.00] U/L, P<0.001), and AST (critical 44.00 [28.00, 70.00] versus non‐critical 31.00 [22.50, 47.00] U/L, P=0.007) were higher in the critical group; moreover, C‐reactive protein (critical, 90.52 [68.08–126.45] versus noncritical, 34.29 [7.42–72.30] mg/L; P<0.001) and procalcitonin (critical, 0.41 [0.20, 1.61] versus noncritical, 0.09 [0.05–0.19] ng/mL; P<0.001) levels were significantly higher in the critical group. There were no significant differences in the liver and kidney function index, brain natriuretic peptide, blood lipids, or activated partial thromboplastin time between the 2 groups (all P> 0.05), although prothrombin time (13.95 [12.60–15.05] versus 13.20 [12.50, 14.10] s; P=0.03) was significantly longer, and blood urea nitrogen levels (6.50 [4.01–9.44] versus 4.55 [3.28–7.08] mmol/L; P=0.002) was slightly higher in the critical group. Further observation showed that the critical patients had higher fibrinogen (critical, 5.99 [4.58–9.63] versus noncritical, 4.37 [3.59–5.19] g/L; P<0.001) and d‐dimer (critical, 2.65 [1.19–8.00] versus noncritical, 0.74 [0.31–1.87] mg/L; P<0.001) levels at admission.
Table 2.
Laboratory Findings of Patients at Admission
| Variables (Normal Range) | Critical (n=36) | Noncritical (n=208) | P Value* |
|---|---|---|---|
| White blood cell count (3.5–9.5×109/L) | 7.23 (4.89–9.44) | 5.86 (4.59–8.15) | 0.14 |
| Neutrophil count (1.8–6.3×109/L) | 6.46 (3.74–8.41) | 4.14 (3.08–6.47) | 0.01 |
| Lymphocyte count (1.1–3.2×109/L) | 0.58 (0.35–0.88) | 0.91 (0.64–1.29) | <0.001 |
| Monocyte count (0.1–0.6×109/L) | 0.25 (0.18–0.40) | 0.38 (0.28–0.53) | <0.001 |
| Neutrophil/lymphocyte | 9.82 (7.06–18.74) | 4.51 (2.70–8.90) | <0.001 |
| Alanine aminotransferase (5–35 U/L) | 33.50 (23.00–56.50) | 33.00 (21.00–53.00) | 0.63 |
| Aspartate aminotransferase (8–40 U/L) | 44.00 (28.00–70.00) | 31.00 (22.50–47.00) | 0.007 |
| Blood urea nitrogen (2.9–8.2 mmol/L) | 6.50 (4.01–9.44) | 4.55 (3.28–7.08) | 0.002 |
| Creatinine (41–81 μmol/L) | 71.90 (59.60–95.85) | 65.50 (56.70–78.10) | 0.12 |
| Uric acid (155–357 μmol/L) | 240.80 (171.00–281.25) | 216.65 (164.20–275.40) | 0.62 |
| C‐reactive protein (0–8mg/L) | 90.52 (68.08–126.45) | 34.29 (7.42–72.30) | <0.001 |
| Procalcitonin (0–0.5 ng/mL) | 0.41 (0.20–1.61) | 0.09 (0.05–0.19) | <0.001 |
| Brain natriuretic peptide (0–100 pg/mL) | 76.70 (14.10–133.10) | 52.75 (25.00–73.15) | 0.21 |
| Troponin I (0–26.2 pg/mL) | 15.70 (8.10–124.70) | 4.76 (2.00–9.60) | <0.001 |
| Creatine kinase (26–140 U/L) | 112.50 (51.50–289.50) | 68.50 (45.00–124.00) | 0.03 |
| Creatine kinase–myocardial band (0–25 U/L) | 16.00 (11.50–22.00) | 12.00 (9.00–16.00) | 0.005 |
| Lactate dehydrogenase (0–245 U/L) | 435.00 (329.00–641.50) | 291.00 (213.00–398.00) | <0.001 |
| Total cholesterol (0–5.2mmol/L) | 3.68 (3.25–4.45) | 3.88 (3.45–4.44) | 0.36 |
| Triglycerides (0–1.7 mmol/L) | 1.31 (0.89–1.71) | 1.29 (1.04–1.68) | 0.90 |
| Low‐density lipoprotein cholesterol (2.7–3.1 mmol/L) | 2.10 (1.93–2.69) | 2.24 (1.91–2.72) | 0.52 |
| Prothrombin time (11.0–16 s) | 13.95 (12.60–15.05) | 13.20 (12.50–14.10) | 0.03 |
| Activated partial thromboplastin time (28–43.5 s) | 37.35 (32.10–42.25) | 36.20 (31.95–40.60) | 0.24 |
| Fibrinogen (2–4 g/L) | 5.99 (4.58–9.63) | 4.37 (3.59–5.19) | <0.001 |
| d‐dimer (<0.5 mg/L) | 2.65 (1.19–8.00) | 0.74 (0.31–1.87) | <0.001 |
P values indicate differences between critical and noncritical patients. P<0.05 was considered statistically significant.
Baseline Characteristics and Prognosis
We further analyzed the potential factors related to the clinical prognosis (Table 3). Overall, the mortality rate of patients with both COVID‐19 and CVD was 19.26% (47/244 cases). According to the admission times, 77 (39.09%) cured patients were treated early (≤7 days), whereas 57 (28.93%) patients were treated late (>11 days). Among the deceased patients, only 6 (12.77%) patients were treated early (≤7 days), and 22 (46.81%) patients were treated late (>11 days) (P=0.002). Patients who were overweight/obese were more likely to die (72.34% versus 27.66%; P<0.001). No significant difference was found in the proportion of patients with hypertension between the deceased and cured patients (P=0.18); however, the proportions of patients with CHD and HF were significantly higher (CHD, 74.47% versus 50.25%; P=0.003; HF, 57.45% versus 29.44%; P<0.001) among the deceased patients than among the cured patients. In addition, the critical group had a significantly higher proportion of deceased patients than the noncritical group (27 cases [57.45%] versus 20 cases [42.55%]; P<0.001). No significant differences were observed in the pH value, Paco 2, or oxygen saturation between the 2 groups (all P> 0.05). Nevertheless, the lactic acid accumulation was significantly higher and Pao 2 and Pao 2/fraction of inspired oxygen were significantly lower in the deceased patients than in the cured patients (P<0.01).
Table 3.
Baseline Characteristics at Admission and Prognosis
| Variables | Cured (n=197) | Died (n=47) | P Value* |
|---|---|---|---|
| Onset to admission, n (%) | 0.002 | ||
| ≤7 d | 77 (39.09) | 6 (12.77) | |
| ~11 d | 63 (31.98) | 19 (40.43) | |
| >11 d | 57 (28.93) | 22 (46.81) | |
| BMI (kg/m2) | <0.001 | ||
| <24 | 151 (76.65) | 13 (27.66) | |
| ≥24 | 46 (23.35) | 34 (72.34) | |
| CVD comorbidities, n (%) | |||
| Hypertension | 160 (81.22) | 42 (89.36) | 0.18 |
| CHD | 99 (50.25) | 35 (74.47) | 0.003 |
| HF | 58 (29.44) | 27 (57.45) | <0.001 |
| Clinical classification, n (%) | <0.001 | ||
| Noncritical | 188 (95.43) | 20 (42.55) | |
| Critical | 9 (4.57) | 27 (57.45) | |
| Blood gas analysis (normal range) | |||
| pH (7.35–7.45) | 7.42 (7.40–7.47) | 7.43 (7.38–7.47) | 0.74 |
| Lactic acid (0.5–1.7 mmol/L) | 1.20 (1.10–1.60) | 1.30 (1.30–2.10) | <0.001 |
| Pao 2 (80–100 mm Hg) | 94.00 (86.00–96.00) | 82.00 (67.00–95.00) | <0.001 |
| PaCO2 (35–45 mm Hg) | 43.00 (38.00–44.00) | 42.00 (38.00–44.00) | 0.97 |
| Oxygen saturation (93–100%) | 96.00 (95.00–98.00) | 96.00 (93.00–97.00) | 0.22 |
| PaO2/fraction of inspired oxygen (400–500 mm Hg) | 434.00 (407.00–444.00) | 156.00 (112.00–414.00) | <0.001 |
BMI indicates body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; and HF, heart failure.
P values indicate differences between cured and died patients. P<0.05 was considered statistically significant.
Potential Factors Related to Death
To investigate the potential factors related to the clinical outcome, we conducted further logistical regression. Baseline variables that were considered clinically relevant or that showed a univariate relationship with the outcome were introduced into the multivariate logistic regression model. 13 Considering the relatively small sample size in the present study, the results should be interpreted carefully. As shown in Table 4, patients who were overweight/obese (odds ratio [OR], 8.43; 95% CI, 3.81–18.64) or who had a history of CHD (OR, 2.83; 95% CI, 1.25–6.40), lactic acid accumulation (OR, 2.47; 95% CI, 1.27–4.81), or lower Pao 2 levels (OR, 1.04; 95% CI, 1.02–1.06) had an increased risk of death. We also performed propensity‐adjusted analysis to balance the baseline demographics between the 2 groups, and the results did not change (data not shown).
Table 4.
Potential Factors Related to Death in Patients With COVID‐19 Combined With CVD
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95%CI | |
| Age, y | 0.99 | 0.97–1.02 | … | … |
| Sex | … | … | ||
| Female | Ref | Ref | ||
| Male | 1.23 | 0.37–2.39 | ||
| BMI (kg/m2) | ||||
| <24 | Ref | Ref | Ref | Ref |
| ≥24 | 8.58 | 4.18–17.63 | 8.43 | 3.81–18.64 |
| Hypertension | … | … | ||
| No | Ref | Ref | ||
| Yes | 1.94 | 0.72–5.25 | ||
| CHD | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 2.89 | 1.42–5.89 | 2.83 | 1.25–6.40 |
| HF | … | … | ||
| No | Ref | Ref | ||
| Yes | 3.23 | 1.68–6.22 | ||
| Lactic acid, mmol/L | 3.20 | 1.81–5.65 | 2.47 | 1.27–4.81 |
| Pao 2 | 0.94 | 0.93–0.97 | 0.96 | 0.94–0.98 |
BMI indicates body mass index; CHD, coronary heart disease; COVID‐19, coronavirus disease 2019; HF, heart failure; and OR, odds ratio.
Discussion
Prognosis of COVID‐19 Combined With CVD
From January 10, 2020, to February 25, 2020, 850 patients with COVID‐19 were screened. The percentage of patients with COVID‐19 and CVD (32.70%; 278/850) was consistent with that reported previously, 7 , 8 without significant difference between sexes. The prognosis of COVID‐19 combined with CVD was significantly poorer than that of COVID‐19 alone. 8 , 14 Our results indicated that critical patients with both COVID‐19 and CVD had a significantly higher mortality risk than noncritical patients. In addition, patients with CVD who were overweight/obese tended to have a poor prognosis, which may be attributable to excessive inflammatory responses 8 and hypoxia, as well as inflammatory factors associated with snoring. 15 , 16 Hypertension, the most common complication among the 3 observed types of CVD, proved to be related to mild illness in these cases. Patients with CHD or HF tended to have more severe illness and had worse clinical outcomes. We observed that the shorter the time from onset to admission, the lower the severity of the disease and the better the prognosis. This, however, is merely because of the different prognoses, and not a predictor of prognosis. Respiratory failure was the leading cause of death, followed by AMI and HF. In addition, cerebrovascular accidents cannot be ignored.
Potential Mechanism Underlying Pathogenesis
The laboratory index of patients with COVID‐19 with CVD revealed certain characteristics. The white blood cell counts of most patients were normal at admission, and leukocyte and neutrophil counts were increased when patients with severe diseases had bacterial infections. However, because SARS‐CoV‐2 consumed a large number of CD4+ and CD8+ T lymphocytes, 8 , 17 the lymphocyte count was generally decreased, and the neutrophil/lymphocyte ratio may be used as an intuitive indicator of clinical severity. A recent study showed that the eosinophil count was also significantly decreased. 18 SARS‐CoV‐2 infection did not directly affect liver and kidney functions, lipid profile, or activated partial thromboplastin time in patients with CVD, but the fibrinogen and d‐dimer levels were significantly increased at admission in critical patients. This might be associated with vomiting and a poor appetite, reduced blood volume, and the need for invasive procedures, such as vascular puncture; extensive inflammatory damage to the vascular endothelium also activated the clotting system. 19
Recent research has confirmed that cardiac injury is common among hospitalized patients with COVID‐19 associated with a higher risk of in‐hospital mortality. However, in these early studies, observational indicators were limited to high‐sensitivity troponin or CK‐MB. 20 , 21 Troponin and CK‐MB had a high myocardial specificity, whereas CK, AST, and LDH had a low myocardial specificity. In terms of duration, CK, CK‐MB, and AST appeared in the early stage of myocardial injury and brevity, but troponin and LDH endured to later stages. 22 Among these indicators, we found that CK, CK‐MB, troponin I, AST, and LDH increased concomitantly in the critical group. This shows that the myocardium may have sustained damage. Although a recent autopsy performed by Xu et al 17 showed that the cardiomyocytes of patients with COVID‐19 were structurally intact, and they found only a small amount of inflammatory infiltration of mononuclear cells in the intercellular stroma. However, some case reports showed acute myocardial injury and cardiac dilatation in patients with confirmed COVID‐19. When following a therapeutic approach including intravenous immunoglobulin and steroids, ejection fraction, ventricular size, and cardiac biomarkers normalized within 2 to 3 weeks. 23 , 24 Thus, myocardial damage caused by SARS‐CoV‐2 infection varies greatly. Myocardial injury is significantly associated with fatal outcomes in COVID‐19 cases, although the prognosis of patients with underlying CVD but without myocardial injury is relatively favorable. 21 Inflammation may be a potential underlying mechanism for myocardial injury. Other common mechanisms may include viral myocarditis, cytokine‐driven myocardial damage, microangiopathy, and unmasked coronary artery disease. 25
In critical patients, C‐reactive protein and procalcitonin, which are inflammatory indicators, deserve particular attention. Similar to patients with SARS‐CoV and Middle East respiratory syndrome coronavirus infections, those infected with SARS‐CoV‐2 display an enhanced T‐helper 1 cell response and the release of corresponding inflammatory factors. At the same time, the expression of interleukin‐4 and interleukin‐10 is increased, indicating an enhancement in the T‐helper 2 cell response. 7 , 26 Inflammatory waterfalls highly proliferated proinflammatory CCR4+CCR6+ T‐helper 17 cells in CD4+ T cells, CD8+ T cells expressed high concentrations of cytotoxic particles, and excessive activation of T cells caused a stronger inflammatory storm. 17 Procalcitonin is a specific inflammatory indicator of bacterial infection, 27 , 28 and its significant increase in critical patients with COVID‐19 and CVD indicates that they are more susceptible to secondary bacterial infections.
In our study, the overall mortality rate was consistent with that reported in previous studies. 7 , 8 , 14 Respiratory failure type I was the leading cause of death in patients with COVID‐19, but some patients with both COVID‐19 and CVD were prone to acute cardiovascular events. Circulating cytokines released during a severe systemic inflammatory response could lead to atherosclerotic plaque instability and rupture. We found that AMI was secondary to respiratory failure, and an inflammatory storm was the most common cause of death in patients with COVID‐19 and CHD. Although evidence of substantial myocardial damage was inconclusive, 17 the accumulation of lactic acid and hypoxia caused by respiratory failure led to an abnormal vasoconstriction of the coronary arteries, and the occurrence of AMI was accelerated by myocardial hypoperfusion and hypercoagulability in the later stages of shock. According to the new international definition of AMI, 29 we considered that some patients with AMI should be categorized as having type 2 myocardial infarction. Because of the general lack of negative pressure cardiac catheterization, most patients did not receive optimal ischemia‐reperfusion therapy (in‐situ thrombolysis was preferred), and the mortality rate was extremely high.
Finally, we used a logistic regression model to explore the potential factors associated with patient death. The results showed that, among patients with COVID‐19 with CVD, overweight/obesity combined with CHD, severe hypoxia, and lactic acid accumulation resulting from respiratory failure, may increase the risk of death. However, because of the limited sample size, future studies are needed to confirm these findings.
Limitations
Our study had some limitations. First, it was a descriptive study. With a limited number of cases, it was difficult to assess host risk factors for disease severity and mortality with multivariable‐adjusted methods. Second, data from other centers indicate that individuals with diabetes mellitus accounted for a high proportion of critical patients. 7 , 8 However, in the present study, although the percentage of patients with diabetes mellitus was higher in the critical group than in the noncritical group, diabetes mellitus was not related to the clinical outcome. This may be attributable to the small sample size of this study. Third, this study focused only on the onset and outcome of the disease. It lacked dynamic observations of disease progression and complete epidemiological investigation. Finally, considering the relatively small sample size, some statistically significant results might be possible, and the results should be interpreted carefully.
Conclusions
SARS‐CoV‐2 is a completely novel virus, and our understanding of the pathogenic mechanism is poor. Patients with COVID‐19 with CVD comprise a special group because of their severe grade and poor prognosis. Critical patients have a high inflammatory response and hypercoagulability. The neutrophil/lymphocyte ratio may be a predictor of disease severity. Overweight/obesity combined with CHD, severe hypoxia, and lactic acid accumulation resulting from respiratory failure were related to poor outcomes.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e016796 DOI: 10.1161/JAHA.120.016796.)
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Kai Huang, Email: huangkai1@gmail.com.
Qiutang Zeng, Email: zengqt139@yeah.net.
References
- 1. Calisher C, Carroll D, Colwell R, Corley RB, Daszak P, Drosten C, Enjuanes L, Farrar J, Field H, Golding J, et al. Statement in support of the scientists, public health professionals, and medical professionals of China combatting COVID‐19. Lancet. 2020;395:e42–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS‐COV‐2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M, Ghinai I, Jarashow MC, Lo J, McPherson TD, et al. Active monitoring of persons exposed to patients with confirmed COVID‐19 - United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-NCOV) infected pneumonia (standard version). Military Med Res. 2020;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chinese Hypertension League, Chinese Society of Cardiology, Hypertension Committee of the Chinese Medical Doctor Association, Hypertension Branch of the China Association for the Promotion of International Exchanges of Health Care Hypertension Branch of the Chinese Geriatrics Society . 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol 2019;16:182–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 12. Yu S, Xing L, Du Z, Tian Y, Jing L, Yan H, Lin M, Zhang B, Liu S, Pan Y, et al. Prevalence of obesity and associated risk factors and cardiometabolic comorbidities in rural northeast China. Biomed Res Int. 2019;2019:6509083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Temirbekov D, Gunes S, Yazici ZM, Sayin I. The ignored parameter in the diagnosis of obstructive sleep apnea syndrome: the oxygen desaturation index. Turk Arch Otorhinolaryngol. 2018;56:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen VG, Fonseca V, Amaral JB, Camargo-Kosugi CM, Moreira G, Kosugi EM, Fujita RR. Inflammatory markers in palatine tonsils of children with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2020;86:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 19. Matijaca H, Gacina P, Rincic G, Matijaca A, Josipovic J, Stojsavljevic S. Effects of occupational stress on the activation of hemostatic and inflammatory system. Acta Clin Croat. 2019;58:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apple FS. The specificity of biochemical markers of cardiac damage: a problem solved. Clin Chem Lab Med. 1999;37:1085–1089. [DOI] [PubMed] [Google Scholar]
- 23. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. Mar 16 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, et al. First case of COVID‐19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019 (COVID‐19): possible mechanisms. J Cardiac Fail. 2020;26:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Velavan TP, Meyer CG. The COVID‐19 epidemic. Trop Med Int Health. 2020;25:278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid a in inflammation and other diseases. Adv Clin Chem. 2019;90:25–80. [DOI] [PubMed] [Google Scholar]
- 28. Dymicka-Piekarska V, Wasiluk A. procalcitonin (PCT), contemporary indicator of infection and inflammation. Postepy Hig Med Dosw (Online). 2015;69:723–728. [DOI] [PubMed] [Google Scholar]
- 29. Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, Rihal CS, Gersh BJ, Lewis B, Lennon RJ, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation. 2020;141:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]


