Abstract
BACKGROUND
Limited studies have evaluated population‐level temporal trends in mortality and cause of death in patients with contemporary managed atrial fibrillation. This study reports the temporal trends in 1‐year overall and cause‐specific mortality in patients with incident atrial fibrillation.
METHODS AND RESULTS
Patients with incident atrial fibrillation presenting to an emergency department or hospitalized in Ontario, Canada, were identified in population‐level linked administrative databases that included data on vital statistics and cause of death. Temporal trends in 1‐year all‐cause and cause‐specific mortality was determined for individuals identified between April 1, 2007 (fiscal year [FY] 2007) and March 31, 2016 (FY 2015). The study cohort consisted of 110 302 individuals, 69±15 years of age with a median congestive heart failure, hypertension, age (≥75 years), diabetes mellitus, stroke (2 points), vascular disease, age (≥65 years), sex category (female) score of 2.8. There was no significant decline in the adjusted 1‐year all‐cause mortality between the first and last years of the study period (adjusted mortality: FY 2007, 8.0%; FY 2015, 7.8%; P for trend=0.68). Noncardiovascular death accounted for 61% of all deaths; the adjusted 1‐year noncardiovascular mortality rate rose from 4.5% in FY 2007 to 5.2% in FY 2015 (P for trend=0.007). In contrast, the 1‐year cardiovascular mortality rate decreased from 3.5% in FY 2007 to 2.6% in FY 2015 (P for trend=0.01).
CONCLUSIONS
Overall 1‐year all‐cause mortality in individuals with incident atrial fibrillation has not improved despite a significant reduction in the rate of cardiovascular death. These findings highlight the importance of recognizing and managing concomitant noncardiovascular conditions in patients with atrial fibrillation.
Keywords: atrial fibrillation, cause of death, mortality
Subject Categories: Mortality/Survival
Nonstandard Abbreviations and Acronyms
- FY
fiscal year
Clinical Perspective
What Is New?
Despite improvements in the care of patients with atrial fibrillation, this retrospective population-level study of contemporary managed patients with new-onset atrial fibrillation in Ontario, Canada, between 2007 and 2015 demonstrated no change in the overall 1‐year all‐cause mortality.
The reduction in cardiovascular deaths at 1 year observed in the study population was accompanied with an increase in noncardiovascular death, thereby resulting in no change in overall 1‐year mortality.
What Are the Clinical Implications?
Noncardiovascular death is eroding the benefits of improvements in cardiovascular care of patients with atrial fibrillation.
Strategies to manage noncardiovascular comorbidities in patients with new-onset atrial fibrillation should be optimized to improve overall survival in this patient population.
The past 2 decades have witnessed an increase in use of evidence‐based pharmacological and nonpharmacological treatments for cardiovascular disease, which has resulted in declining mortality in patients who experience myocardial infarction 1 and congestive heart failure. 2 Improvements in the care of patients with atrial fibrillation (AF) have also occurred during this time, including the development of stroke and bleeding risk calculators 3 , 4 and confirmation of the benefits of oral anticoagulation on stroke and survival. 5 , 6 It is not known whether the evolution of AF care has also been associated with declining mortality as observed with other cardiac conditions.
Few population‐level studies have evaluated trends in mortality in unselected AF cohorts. The Framingham Heart Study reported a 25% reduction in all‐cause mortality in a community cohort of patients with new‐onset AF between 1958 and 2007. 7 The Danish National Patient Registry reported a 40% reduction in all‐cause mortality in hospital‐diagnosed patients with new‐onset AF between 1983 and 2012. 8 However, a paucity of data on trends in all‐cause mortality in patients with AF exists in the contemporary era, with available reports providing conflicting results. 9 , 10 , 11 Furthermore, changes in cause‐specific death in patients with AF has not been reported. At a population level, this knowledge is imperative as it provides insight into the possible influence of advances in medical care on survival in patients with AF and also suggests areas where the deployment of resources may improve the overall survival in this patient population. This is a critically important consideration as the prevalence of AF is expected to increase 250% by 2050. 12 At a patient level, this knowledge is also important as it will allow for informed shared decision‐making when planning individual care. Accordingly, the objective of this study was to describe changes in overall and cause‐specific mortality in a contemporary AF cohort.
METHODS
The data set from this study is held securely in coded form at ICES. Although data‐sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at http://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Data Sources
Ontario is Canada’s most populous province with 14.3 million individuals who have access to universal healthcare through a single‐payer healthcare system. Population‐level administrative databases at ICES capture details of hospital‐based care and physician services of all Ontarians. These databases were linked using unique coded identifiers and analyzed at ICES, thereby protecting patient confidentiality and allowing the creation of patient cohorts and long‐term follow‐up. The Canadian Institute for Health Information Discharge Abstract Database provided data on hospitalizations and patient comorbidities. The National Ambulatory Care Reporting Service database provided data on hospital‐based ambulatory care including emergency department (ED) visits. The Ontario Health Insurance Plan database was used to ascertain physician claims. The Registered Persons Database reported sex and birth and death dates. The Office of the Register General Deaths Registry provided information on cause of death determined from death certificates completed by treating physicians or the coroner. Statistics Canada postal code data were used to ascertain income quintile and rurality. The Ontario Drug Benefit prescription database was used to determine prescription drug use for patients ≥ 66 years of age. Information on patient demographics, clinical characteristics, and drug and other medical intervention use was available until March 31, 2018; mortality data until March 31, 2017; and cause of death until March 31, 2016. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require individual consent or research ethics board review.
Study Cohort
We identified Ontario residents between 18 and 105 years of age possessing a valid Ontario Health Insurance Plan number who presented to any Ontario ED or were hospitalized between April 1, 2007, and March 31, 2018, with a main diagnosis of AF (International Classification of Diseases, Tenth Revision [ICD‐10], diagnostic code I480.XX). This approach has previously been validated to identify the diagnosis of AF in the ED with a sensitivity of 96.6% and positive predictive value of 93%. 13 Based on expert recommendation, a look back period of 5 years before the index event was used to exclude individuals with a prior diagnosis of AF recorded with any prior ED visit or hospitalization to minimize misclassification of prevalent AF cases as incident AF cases, thereby allowing us to create a cohort of presumed new‐onset AF. 14 In addition, individuals residing within chronic care facilities were excluded to ensure the cohort consisted of community‐dwelling individuals with AF.
Baseline clinical characteristics of the cohort were determined using a 5‐year look back period and, where applicable, ICES‐derived validated disease‐specific registries (hypertension, diabetes mellitus, obstructive lung disease, and dementia). The use of cardiovascular procedures (echocardiography, angiography) within 1 year after the index event was determined. For the subset of the cohort ≥66 years of age, cardiovascular medications (statin, angiotensin converting enzyme inhibitor/angiotensinogen II receptor blocker, β‐blockers, oral anticoagulation, digoxin, and antiarrhythmic drugs [amiodarone, sotalol, dronaderone, flecainide, and propafenone]) were also determined.
Outcomes
The primary outcome was 1‐year mortality. Deaths were subsequently classified as attributed to a cardiovascular cause (all cardiovascular, ICD‐10: I00‐I78; ischemic heart disease, I20‐I25; heart failure, I50; sudden death, I46‐I47; ischemic stroke, I63‐I64; hemorrhagic stroke, I60‐I61; other) or noncardiovascular cause (cancer, C00‐D48; respiratory failure, J00‐J99; trauma, V01‐V98; infection, A00‐B99; other).
Statistical Analysis
Summary statistics were provided for the overall cohort and for each fiscal year (FY; April 1 to March 31) during the study period. Continuous variables were reported as mean±SD, and categorical variables were reported as frequencies and percentages. The Cochran–Armitage test for trend was used to determine the presence of linear temporal trends in patient characteristics, use of cardiovascular procedures and medications, and mortality (all‐cause, cardiovascular, noncardiovascular).
To account for the association of changing patient characteristics with mortality, risk‐adjusted mortality rates stratified by FY and adjusted for age, sex, prior stroke or transient ischemic attack, heart failure, hypertension, coronary artery disease, and peripheral vascular disease were determined by use of logistic regression models. The CIs of risk‐adjusted rates were computed based on the normal approximation to the binomial distribution. 15 Testing for time trends in adjusted mortality rates was conducted by computing Kendall’s τ‐b correlation coefficient and associated P value for trend. Statistical significance was defined by a 2‐tailed P‐value of<0.05. Statistical analysis was performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Cohort
The study cohort was composed of 144 668 Ontarians aged 18 to 105 who presented to an ED and were either discharged or hospitalized with a main diagnosis of AF from April 1, 2007 to March 31, 2018 (Figure 1). Exclusions because of a prior history of AF (n = 31 140) and residence within a long‐term care facility (n = 3226) resulted in an overall study cohort of 110 302 individuals with presumed new‐onset AF.
Figure 1. Cohort creation.
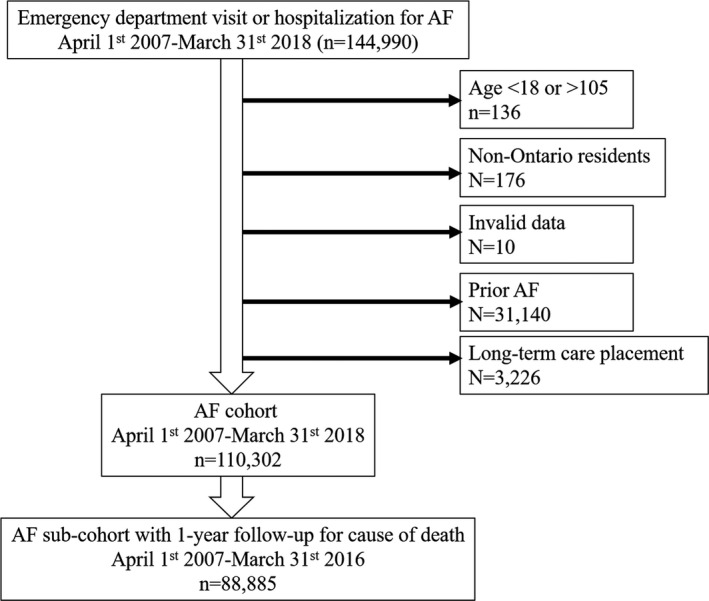
AF indicates atrial fibrillation.
Cohort Characteristics
The demographics and clinical characteristics of the cohort are summarized in Table 1. Between FY 2007 and FY 2017 the number of AF individuals increased from 8988/year to 10 713/year. The average age was 69±15 years, and the average congestive heart failure, hypertension, age (≥75 years), diabetes mellitus, stroke (2 points), vascular disease, age (≥65 years), sex category (female) score was 2.8. Hypertension was present in 69.7% of the cohort. A minority of individuals had other cardiovascular and stroke risk factors such as heart failure (19.9%), diabetes mellitus (24.2%), prior myocardial infarction (3.6%), and stroke (1.4%). Risks for bleeding including renal failure (2.1%) and a prior history of bleeding (3.2%) were also infrequent. Of the study cohort, 39.7% was admitted to the hospital with the index event.
Table 1.
Patient Characteristics
| Variable | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P for Trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of individuals | 8988 | 9067 | 9625 | 9895 | 10 110 | 10 369 | 9551 | 10 353 | 10 518 | 10 704 | 10 713 | … |
| Age, y (mean±SD) | 69±15 | 69±14 | 69±15 | 69±15 | 69±15 | 69±15 | 69±15 | 70±15 | 70±14 | 70±14 | 70±14 | <0.0001 |
| Male sex (%) | 51.4 | 50.9 | 51.5 | 50.7 | 51.4 | 51.2 | 50.7 | 49.9 | 50.4 | 51.8 | 51.0 | 0.53 |
| Rural primary address (%) | 16.8 | 17.0 | 16.6 | 17.0 | 16.4 | 16.4 | 16.2 | 16.9 | 16.2 | 17.5 | 16.8 | 0.82 |
| Income quintile | ||||||||||||
| 1 | 18.9 | 18.7 | 18.3 | 18.7 | 18.5 | 17.3 | 18.0 | 18.1 | 18.1 | 18.2 | 17.9 | 0.03 |
| 2 | 20.1 | 20.1 | 19.4 | 19.4 | 20.1 | 19.0 | 19.3 | 19.2 | 19.3 | 19.6 | 19.5 | |
| 3 | 19.8 | 19.0 | 19.4 | 20.0 | 19.3 | 20.1 | 19.5 | 19.5 | 20.1 | 19.7 | 19.9 | |
| 4 | 20.2 | 19.8 | 20.9 | 20.5 | 20.5 | 21.0 | 21.3 | 21.4 | 21.3 | 21.1 | 20.2 | |
| 5 | 20.7 | 22.0 | 21.5 | 21.1 | 21.1 | 22.2 | 21.6 | 21.4 | 20.9 | 21.0 | 22.0 | |
| Heart failure (%) | 21.2 | 21.3 | 19.8 | 20.3 | 19.6 | 20.2 | 20.7 | 20.0 | 18.9 | 18.9 | 18.1 | <0.0001 |
| Hypertension (%) | 68.5 | 69.8 | 69.2 | 69.7 | 70.8 | 70.2 | 70.0 | 70.7 | 70.0 | 69.4 | 68.8 | 0.67 |
| Diabetes mellitus (%) | 21.7 | 22.3 | 23.3 | 22.9 | 23.7 | 24.4 | 25.1 | 25.3 | 25.6 | 25.8 | 26.0 | <0.0001 |
| Previous myocardial infarction (%) | 4.0 | 3.8 | 3.5 | 4.0 | 3.4 | 3.7 | 3.6 | 3.9 | 3.3 | 3.5 | 3.3 | 0.02 |
| Previous stroke syndrome (%) | 1.4 | 1.6 | 1.5 | 1.3 | 1.3 | 1.3 | 1.2 | 1.3 | 1.3 | 1.3 | 1.5 | 0.16 |
| Obstructive lung disease (%) | 28.8 | 28.7 | 29.3 | 29.6 | 29.6 | 29.9 | 30.3 | 31.0 | 30.9 | 31.7 | 30.4 | <0.0001 |
| Peripheral vascular disease (%) | 1.1 | 1.0 | 1.2 | 1.1 | 1.0 | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 | 1.0 | 0.06 |
| Previous cancer (%) | 4.6 | 4.2 | 4.6 | 4.7 | 4.6 | 4.6 | 4.5 | 4.2 | 4.5 | 4.7 | 4.1 | 0.29 |
| Dementia (%) | 3.4 | 4.5 | 4.6 | 4.3 | 4.4 | 4.8 | 4.8 | 4.6 | 4.6 | 4.7 | 4.3 | 0.008 |
| Renal disease (%) | 2.3 | 2.2 | 2.2 | 2.0 | 1.9 | 2.2 | 2.3 | 2.1 | 1.9 | 1.9 | 2.0 | 0.03 |
| Previous bleeding (%) | 3.7 | 3.5 | 3.2 | 3.1 | 3.1 | 3.3 | 3.1 | 3.0 | 3.3 | 3.0 | 3.3 | 0.03 |
| Charlson score | ||||||||||||
| 0–1 (%) | 80.1 | 79.3 | 79.7 | 78.9 | 79.1 | 79.2 | 78.2 | 79.0 | 79.1 | 79.3 | 79.8 | 0.42 |
| ≥2 (%) | 19.9 | 20.7 | 20.3 | 21.1 | 20.9 | 20.8 | 21.8 | 21.0 | 20.9 | 20.7 | 20.2 | |
| CHA2DS2VASc score (mean±SD) | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 2.8±1.7 | 0.007 |
| Hospital admission with index AF emergency department visit (%) | 46.6 | 44.9 | 42.4 | 41.5 | 40.6 | 39.0 | 40.7 | 38.1 | 36.8 | 35.0 | 32.8 | <0.0001 |
Year is fiscal year (April 1 to March 31). Income quintile 1 is the lowest income quintile. AF indicates atrial fibrillation; CHA2DS2VASc, congestive heart failure, hypertension, age (≥75 years), diabetes mellitus, stroke (2 points), vascular disease, age (≥65 years), sex category (female).
All‐Cause Mortality
The crude all‐cause mortality rate was 7.8% in FY 2007 and 7.9% in FY 2015 (P for trend = 0.44) (Figure 2). All‐cause mortality did not significantly change during the study period after adjustment for age, sex, prior stroke or transient ischemic attack, heart failure, hypertension, coronary artery disease, and peripheral vascular disease (adjusted mortality FY 2007, 8.0%; FY 2017, 7.8%; P for trend = 0.68).
Figure 2. One‐year all mortality fiscal years 2007 to 2015.
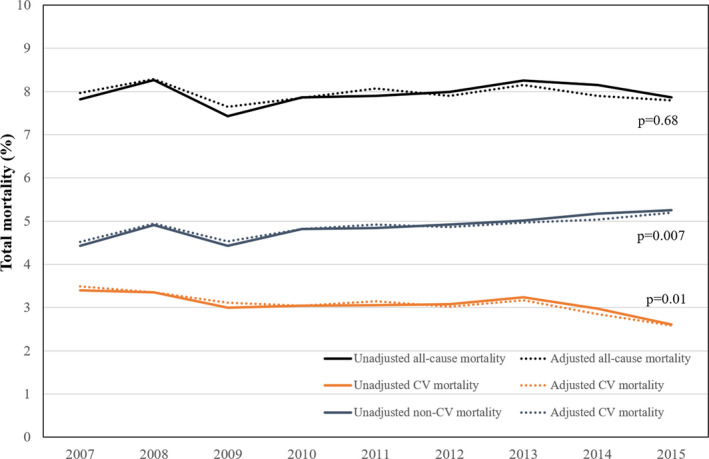
All‐cause mortality (black), cardiovascular mortality (red), and noncardiovascular mortality (blue) from fiscal years 2007 to 2015. Dashed line indicates unadjusted mortality, and solid lines indicate adjusted mortality. CV indicates cardiovascular.
Noncardiovascular Causes of Death
Of all deaths during the study period, 61% were noncardiovascular. The 1‐year noncardiovascular mortality rate increased during the study period (adjusted noncardiovascular mortality, FY 2007, 4.5%; FY 2017, 5.2%; P for trend = 0.007). Cancer was the most common cause of death overall, accounted for 30% of all deaths, and remained unchanged during the study period (FY 2007, 27.9%; FY 2015, 31.1%; P for trend = 0.99; Figure 3). Respiratory failure was the second most common noncardiovascular cause of death, contributed to 10% of all deaths, and increased during the study period (FY 2007, 7.7%; FY 2015, 11.8%; P for trend < 0.0001).
Figure 3. Causes of death fiscal years 2007 to 2015.

CV indicates cardiovascular.
Cardiovascular Causes of Death
Cardiovascular mortality comprised 39% of all deaths during the study period. Cardiovascular mortality declined during the study period (adjusted mortality, FY 2007, 3.5%; FY 2015, 2.6%; P for trend = 0.01). Ischemic heart disease was the most common cause of cardiovascular death, contributed to 16% of all deaths (Figure 3), and declined during the study period (FY 2007, 20.8%; FY 2015, 10.8%; P for trend < 0.001). Heart failure was the second most common cause of cardiovascular death, contributed to 3.8% of all deaths, and increased during the study period (FY 2007, 2.6%; FY 2015, 4.2%; P for trend < 0.001). Hemorrhagic and ischemic stroke deaths were infrequent (0.7% and 2.7% of all deaths, respectively).
Cardiovascular Drug and Test Use
Table 2 summarizes the use of cardiovascular medications and tests during the study period. Anticoagulation use increased in frequency during the study period (FY 2007, 65.2%; FY 2017, 72.7%; P for trend<0.001) with a progressive decline in warfarin when direct oral anticoagulants became clinically available in Ontario and subsequently covered by the provincial health insurance drug plan. β‐blocker and statin use also increased during the study period, whereas angiotensinogen converting enzyme inhibitor/angiotensin receptor blocker, antiarrhythmic drug, and digoxin use declined during the study period. Cardiac testing with echocardiography, stress testing, and coronary angiography was performed in 59%, 32%, and 7.0% of the cohort and increased during the study period.
Table 2.
Use of Cardiovascular Medications and Testing
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P for Trend | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug therapy | ||||||||||||
| Number of individuals* | 5681 | 5812 | 6032 | 6313 | 6406 | 6631 | 6368 | 6776 | 6867 | 6997 | 6957 | … |
| OAC† | ||||||||||||
| Any OAC (%) | 65.2 | 64.6 | 61.1 | 59.1 | 58.6 | 67.4 | 73.3 | 67.9 | 69.0 | 70.9 | 72.7 | <0.0001 |
| Warfarin (%) | 65.2 | 64.6 | 61.0 | 58.8 | 47.3 | 38.8 | 29.3 | 21.6 | 16.4 | 12.0 | 9.1 | <0.0001 |
| DOAC (%) | 0 | 0 | 0 | 0.3 | 18.6 | 38.6 | 52.0 | 51.6 | 56.7 | 62.2 | 66.4 | <0.0001 |
| β‐blocker (%) | 65.5 | 64.0 | 65.7 | 64.3 | 66.5 | 67.1 | 68.7 | 68.4 | 70.1 | 70.2 | 70.9 | <0.0001 |
| Anti‐arrhythmic drug‡ (%) | 19.8 | 18.9 | 17.7 | 17.4 | 17.7 | 16.6 | 15.5 | 17.4 | 15.8 | 15.5 | 15.4 | <0.0001 |
| Digoxin (%) | 25.7 | 25.1 | 23.4 | 22.4 | 21.3 | 19.3 | 19.1 | 18.2 | 16.4 | 14.0 | 13.3 | <0.0001 |
| Statin (%) | 49.3 | 49.1 | 53.0 | 53.1 | 54.7 | 55.0 | 53.6 | 52.7 | 53.8 | 54.1 | 54.5 | <0.0001 |
| ACEi/ARB (%) | 60.4 | 58.8 | 59.0 | 57.8 | 57.5 | 57.2 | 57.5 | 56.1 | 54.5 | 54.3 | 50.2 | <0.0001 |
| Cardiac testing | ||||||||||||
| Number of individuals§ | 8988 | 9076 | 9625 | 9895 | 10 110 | 10 369 | 9951 | 10 353 | 10 518 | 10 704 | 10 713 | |
| Echocardiography (%) | 52.2 | 54.1 | 56.9 | 57.6 | 58.5 | 57.2 | 57.6 | 61.3 | 61.7 | 63.2 | 64.3 | <0.0001 |
| Stress test (%) | 30.8 | 30.0 | 30.7 | 31.6 | 32.7 | 32.2 | 33.0 | 33.0 | 33.0 | 33.4 | 34.4 | <0.0001 |
| Coronary angiography (%) | 6.5 | 5.9 | 6.7 | 6.7 | 6.9 | 7.2 | 7.7 | 7.2 | 7.3 | 7.7 | 7.4 | <0.0001 |
Year is fiscal year (April 1 to March 31). ACEi/ARB indicates angiotensin converting enzyme inhibitor/angiotensinogen II receptor blocker; DOAC, direct oral anticoagulant; and OAC, oral anticoagulant.
Drug use determined for the portion of the cohort ≥ 66 years of age.
Any OAC reflects any individual who received any OAC prescription during the 1‐year follow‐up period and may be less than the sum of the percentage of individuals who received warfarin and DOAC as some individuals may have received a prescription for both warfarin or DOAC during the follow‐up period.
Antiarrhythmic drugs included amiodarone, dronaderone, sotalol, flecainide, and propafenone.
Cardiac testing was determined using the entire cohort.
DISCUSSION
This observational study highlights a lack of improvement in 1‐year all‐cause mortality within the past decade in patients with contemporary managed incident AF who presented to the ED. Although the decline in cardiovascular mortality is encouraging, the overall prognosis after a new diagnosis of AF has not improved because of increasing noncardiovascular death. These findings highlight the importance of care beyond stroke reduction for patients with AF.
The 1‐year mortality in patients with incident AF observed in Ontario, Canada, is similar to that reported in other jurisdictions. Specifically, mortality 1 year after a new diagnosis of AF was 7.5% in the EURObservational Research Programme–Atrial Fibrillation General Registry, 16 16.1% the Geisenger Health System in Pennsylvania, 9 and 19.5% in Medicare beneficiaries >65 years of age. 17 Furthermore, our observation that noncardiovascular deaths comprise the majority of all deaths in patients with AF is consistent with the findings from recent contemporary real‐world cohorts of unselected patients with AF. For example, the XANTUS (Xarelto for Prevention of Stroke in Atrial Fibrillation) observational study 18 and the GARFIELD‐AF (Global Anticoagulant Registry in the FIELD–Atrial Fibrillation) observational study 19 reported primary noncardiac deaths in 58.5% and 59.5% of individuals, respectively, a proportion similar to that observed in our cohort from Ontario, Canada.
The causal pathway explaining the increasing rate of noncardiovascular death is not fully apparent, but may be related to the presence of multiple comorbidities in patients with AF. 20 Of the individuals in our cohort, 20% with new‐onset AF had at least 2 important comorbidities as reflected by a Charlson score≥2, a finding not surprising as AF typically occurs in the elderly. Prior work has highlighted that in the presence of a chronic condition, other chronic conditions are generally less likely to be treated. 21 Furthermore, in the setting of AF the prognosis with other medical conditions is generally worse. 22 , 23 , 24 , 25 Further work to understand the methods to reduce noncardiovascular death in patients with AF must be a priority given the anticipated epidemic of AF. 12
The clinically important reduction in the rate of cardiovascular death observed in this cohort of patients is laudable, however, primarily related to the management of coronary artery disease rather than AF‐specific care; the increasing rates of use of statins, stress testing, coronary angiography, and the 50% decline in death attributed to ischemic heart disease during the study period is supportive of this. Indeed, this finding is consistent with prior studies evaluating integrative care for patients with AF demonstrating a prominent reduction in cardiovascular death and resultant improvement in overall survival. 26 , 27 Integrated care has been identified as a priority for the care of patients with AF. 28 , 29 However, the current constructs of integrated care for patients with AF primarily focus on overall cardiovascular care and do not provide guidance on screening and managing concomitant noncardiovascular conditions, which if not optimally managed will erode gains achieved with the provision of optimal cardiovascular care.
The increasing rate of noncardiovascular death observed in this study should encourage AF researchers to evaluate care pathways for managing a concomitant noncardiovascular condition in patients with AF and stimulate AF guidelines writers to highlight the importance of multimorbidity care in patients with AF beyond stroke and cardiovascular risk reduction. Our findings also have potential implications for AF trial design. Specifically, clinical trialists need to be mindful of the fact that therapies aimed at reducing cardiovascular events in patients with AF may not be associated with a long‐term reduction in all‐cause mortality as a result of competing noncardiac death. Careful patient selection and choice of appropriate trial end points may be necessary when assessing the utility of AF‐specific therapies.
Important limitations of this study must be acknowledged. First, as administrative databases have limited ability to identify patients with AF receiving care in an outpatient setting, this cohort was confined to patients with AF who presented to the ED or hospital. Although this approach has been validated and allows for the identification of patients with symptomatic AF, it may not be applicable to patients with AF solely receiving care in an outpatient setting. In addition, this approach may not identify all patients at the time of the first diagnosis of AF as some may patients may have been diagnosed as an outpatient before the initial ED visit. Second, ascertaining important clinical characteristics associated with mortality in patients with AF including type of AF (paroxysmal, persistent, or permanent), presence of left ventricular hypertrophy, and QRS duration is not possible in administrative databases. In addition, we are unable to exclude underreporting of comorbidities within administrative databases. Third, cause of death data were obtained from death certificate data, which may be prone to miscoding. Completion by physicians and classification of deaths in broader categories such as cardiovascular and noncardiovascular minimizes this misclassification error. Despite this, we suggest caution when interpreting specific cause of death data given the known discrepancies between cause of death reported on death certificates and that when adjudicated by a panel of physicians. 30 Fourth, it is possible that the logistic regression model used in this study may have inadequately adjusted for clinical factors associated with death during the study period. The strengths of this work include the decade‐long longitudinal population‐based design of a contemporary AF cohort with nearly complete availability of care and survival status. This approach eliminates selection biases associated with randomized clinical trials and single‐center and registry work.
In conclusion, despite a decline in cardiovascular mortality in individuals with incident AF, 1‐year overall survival has not improved as a result of increasing noncardiovascular death. Strategies to manage noncardiovascular comorbidities in patients with AF should be optimized.
Sources of Funding
Funding for this study was supported by the Cardiac Arrhythmia Network of Canada as part of the Networks of Centers of Excellence and by a Foundation grant (FDN‐154333) from the Canadian Institutes of Health Research. Dr Ko is supported by an Ontario Mid‐Career Investigator Award from the Heart and Stroke Foundation of Canada, Ontario Provincial Office. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long‐Term Care is intended or should be inferred. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and the decision to submit the article for publication were independent of the funding sources.
Disclosures
None.
Acknowledgments
We thank IMS Brogan Inc. for use of their Drug Information Database. Parts of this material are based on data and/or information provided by the Canadian Institutes for Health Information. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of Canadian Institutes for Health Information. Parts of this report are based on Ontario Registrar General information on deaths, the original source of which is ServiceOntario. The views expressed therein are those of the authors and do not necessarily reflect those of the Ontario Registrar General or Ministry of Government Services.
(J Am Heart Assoc. 2020;9:e016810 DOI: 10.1161/JAHA.120.016810.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 2. Taylor CJ, Ordonez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: Population based cohort study. BMJ. 2019;364:I223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crinjs HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 4. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 5. Van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, Koudstaal PJ, Chang Y, Hellmons B. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–2448. [DOI] [PubMed] [Google Scholar]
- 6. Liew A, O’Donnell M, Douketis J. Comparing mortality in patients with atrial fibrillation who are receiving direct-acting oral anticoagulant or warfarin: a meta-analysis of randomized trials. J Thromb Haemost. 2014;12:1419–1424. [DOI] [PubMed] [Google Scholar]
- 7. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magani JW, Ellinor PT, et al. 50‐year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt M, Ulrichsen SP, Pedersen L, Botker HE, Nielsen JC, Sorensen HT. 30‐year nationwide trends in incidence of atrial fibrillation in Denmark and associated 5-year risk of heart failure, stroke, and death. Int J Cardiol. 2016;225:30–36. [DOI] [PubMed] [Google Scholar]
- 9. Williams BA, Honushefsky MS, Berger PB. Temporal trends in incidence, prevalence, and survival of patients with atrial fibrillation from 2004 to 2016. Am J Cardiol. 2017;120:1961–1965. [DOI] [PubMed] [Google Scholar]
- 10. Vasan S, Zuo Y, Kalesan B. Divergent temporal trends in morbidity and mortality related to heart failure and atrial fibrillation: age, sex, race, and geographic differences in the United States, 1991–2015. J Am Heart Assoc 2019;8(8):1991–2015. 10.1161/JAHA.118.010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freeman JV, Wang Y, Akar J, Desai N, Hrumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999–2013. Circulation. 2017;135:1227–1239. [DOI] [PubMed] [Google Scholar]
- 12. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. [DOI] [PubMed] [Google Scholar]
- 13. Atzema CL, Austin PC, Cong AS, Dorian P. Factors associated with 90-day death after emergency department discharge for atrial fibrillation. Ann Emerg Med. 2013;61:539–548. [DOI] [PubMed] [Google Scholar]
- 14. Hawkins NM, Daniele PR, Humphries KH, Ezekowitz JA, McAlister FA, Sandhu RK, Kaul P. Empirical insights defining the population burden of atrial fibrillation and oral anticoagulation using administrative data. Can J Cardiol. 2019;35:1412–1415. [DOI] [PubMed] [Google Scholar]
- 15. Hosmer DW, Lemeshow S. Confidence interval estimates of an index of quality performance based on logistic regression models. Stat Med. 1995;14:2161–2172. [DOI] [PubMed] [Google Scholar]
- 16. Lip GYH, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, Darabantiu D, Crijns HJGM, Kirchhof P, Vardas P, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: One year follow-up of the EURObservational research programme-atrial fibrillation general registry pilot phase (EORP-AF Pilot registry). Eur Heart J. 2014;35:3365–3376. [DOI] [PubMed] [Google Scholar]
- 17. Piccini JP, Hammill BG, Sinner BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camm AJ, Amarenco P, Haas S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, Goldhaber SZ, Goto S, Haas S, Hacke W, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proietti M, Marzona VT, Tettamanti M, Fortino I, Merlino L, Basili S, Mannucci PM, Boriani G, Lip GYH, Roncaglioni MC, et al. Long-term relationship between atrial fibrillation, multimorbidity and oral anticoagulant drug use. Mayo Clin Proc. 2019;94:2427–2436. [DOI] [PubMed] [Google Scholar]
- 21. Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. [DOI] [PubMed] [Google Scholar]
- 22. Jabre P, Roger VL, Murad MH, Chamberlain AM, ProkopL AF, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakley AJ, Weiner RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with sepsis. JAMA. 2011;306:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durheim MT, Holmes DN, Blanco RG, Allen LA, Chan PS, Freeman JV, Fonarow GC, Go AS, Hylek EM, Mahhafey KW, et al. Characteristics and outcomes of adults with chronic obstructive pulmonary disease and atrial fibrillation. Heart. 2018;104:1850–1858. [DOI] [PubMed] [Google Scholar]
- 25. Lau YC, Proietti M, Guiducci E, Blann AD, Lip GYH. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J Am Coll Cardiol. 2016;68:1452–1464. [DOI] [PubMed] [Google Scholar]
- 26. Hendriks JML, de Wit R, Crijns HJGM, Vrijhoef HJM, Prins MH, Pisters R, Pison LAFG, Blaauw Y, Tieleman RG. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–2699. [DOI] [PubMed] [Google Scholar]
- 27. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med. 2018;131:1359–1366. [DOI] [PubMed] [Google Scholar]
- 28. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A, Arnar D, Atar D, Auricchio A, Bax J, et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA consensus conference. Europace. 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 29. Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet. 2017;390:1873–1887. [DOI] [PubMed] [Google Scholar]
- 30. Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. [DOI] [PubMed] [Google Scholar]


