Abstract
Background
Right bundle‐branch block (RBBB) occurs in 0.2% to 1.3% of people and is considered a benign finding. However, some studies have suggested increased risk of cardiovascular morbidity and mortality. We sought to evaluate risk attributable to incidental RBBB in patients without prior diagnosis of cardiovascular disease (CVD).
Methods and Results
We reviewed the Mayo Clinic Integrated Stress Center database for exercise stress tests performed from 1993 to 2010. Patients with no known CVD—defined as absence of coronary disease, structural heart disease, heart failure, or cerebrovascular disease—were selected. Only Minnesota residents were included, all of whom had full mortality and outcomes data. There were 22 806 patients without CVD identified; 220 of whom (0.96%) had RBBB, followed for 6 to 23 years (mean 12.4±5.1). There were 8256 women (36.2%), mean age was 52±11 years; and 1837 deaths (8.05%), including 645 cardiovascular‐related deaths (2.83%), occurred over follow‐up. RBBB was predictive of all‐cause (hazard ratio [HR], 1.5; 95% CI, 1.1–2.0; P=0.0058) and cardiovascular‐related mortality (HR,1.7; 95% CI, 1.1–2.8; P=0.0178) after adjusting for age, sex, diabetes mellitus, hypertension, obesity, current and past history of smoking, and use of a heart rate‐lowering drug. Patients with RBBB exhibited more hypertension (34.1% versus 23.7%, P<0.0003), decreased functional aerobic capacity (82±25% versus 90±24%; P<0.0001), slower heart rate recovery (13.5±11.5 versus 17.1±9.4 bpm; P<0.0001), and more dyspnea (28.2% versus 22.4%; P<0.0399) on exercise testing.
Conclusions
Patients with RBBB without CVD have increased risk of all‐cause mortality, cardiovascular‐related mortality, and lower exercise tolerance. These data suggest RBBB may be a marker of early CVD and merit further prospective evaluation.
Keywords: ECG, mortality, right bundle‐branch block, stress testing
Subject Categories: Cardiovascular Disease, Exercise, Risk Factors
Clinical Perspective
What Is New?
Right bundle‐branch block has been shown to be associated with an increased overall risk of mortality and cardiovascular‐related mortality in patients without known cardiovascular disease, even after adjusting for comorbidities.
Right bundle‐branch block is associated with a greater frequency of hypertension and more exercise‐associated limitations, including decreased aerobic capacity, slower heart rate recovery, and more dyspnea on exercise testing.
What Are the Clinical Implications?
Patients with incidentally discovered right bundle‐branch block may benefit from more intensive cardiovascular evaluation to rule out other subclinical conditions, though prospective studies are needed to define the precise pathophysiologic mechanisms to define the best tests to perform.
Right bundle‐branch block (RBBB) is defined by ECG findings suggestive of either significant delay or lack of electrical conduction through the RBB and distal Purkinje fibers, resulting in ventricular activation occurring primarily via the left bundle branch and fascicular system. 1 The prevalence of RBBB has been reported to be in the range of 0.2% to 1.3%. 1 Typically, it is an incidental finding noted on electrocardiography and is diagnosed using the American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society criteria. 2 These criteria consist of 3 parameters: (1) a QRS duration >120 ms, (2) a secondary R wave in V1 or V2, and (3) a wide, slurred S wave in leads I, V5, and V6. 2
In some cases, however, RBBB can be a signal of other, more sinister underlying conditions, including but not limited to ischemic, inflammatory, or infiltrative heart disease as well as pulmonary embolism. 1 , 3 , 4 , 5 In patients with underlying cardiovascular disease (CVD) such as heart failure (HF), RBBB is known to be a predictor of negative outcomes. 6 , 7 , 8 Multiple studies evaluating a range of acute coronary syndrome presentations, including unstable angina and ST‐elevation myocardial infarction, have demonstrated that RBBB is an independent predictor of in‐hospital and early (≈6 month) mortality. 6 , 7 , 9 Moreover, RBBB has been shown to be an independent predictor of decreased right ventricular ejection fraction (EF), which in itself is a predictor of adverse outcomes in patients with ischemic cardiomyopathy. 10
Whether the association between RBBB and mortality, as well as other adverse outcomes, remains in patients without prior CVD is controversial. Data from the Copenhagen City Heart Study, for example, which included 18 441 participants without prior myocardial infarction or HF, found that RBBB was a strong predictor of increased all‐cause mortality and cardiac death. 11 In contrast, a separate study conducted in women led by the Women's Health Initiative, did not find a significant relationship between RBBB and mortality in participants without CVD. 12
Thus, we sought to determine the prevalence and significance of RBBB on long‐term outcomes, including mortality, in patients without underlying CVD.
Methods
This study was approved by the institutional review board of the Mayo Clinic in Rochester, Minnesota. The requirement for informed consent was waived. Data, analytical methods, and study materials will be made available upon reasonable request.
Study Participants
Patients without CVD who were referred for stress testing at the Mayo Clinic in Rochester, Minnesota, between 1993 and 2010 were included. There was no age limit to inclusion (the oldest patient was 97 years of age). If multiple tests were available for a patient, the first test chronologically was selected to maximize follow‐up. Over this time span, the Mayo Clinic integrated stress center database prospectively collected 374 different variables on all patients. Only Minnesota residents were included. Full mortality data was obtained from Mayo Clinic records or the Minnesota death index when available over follow‐up through February 2016. The absence of clinical evidence of CVD was defined as lack of coronary artery disease (CAD), structural heart disease, HF (EF <50% or HF with preserved EF), or cerebrovascular disease. We additionally excluded all patients with known arrhythmogenic substrate (Brugada pattern, long‐QT syndrome, catecholaminergic polymorphic ventricular tachycardia, arrhythmogenic right ventricular cardiomyopathy, hypertrophic obstructive cardiomyopathy, and idiopathic ventricular tachycardia). Exclusion of CVD, including CAD, HF, and structural heart disease was based on most‐recent physician notes, outside records, and results of any prior testing (echocardiogram, cardiac imaging tests, ECG, etc). Treadmill testing was carried out according to the standard Bruce protocol in the majority of patients (90.5%), with others tested based on various treadmill or cycle ergometer protocols. 13
Clinical Outcomes
The primary end point was all‐cause mortality. Secondary end points included cardiovascular death caused by coronary disease, myocardial infarction, HF, or incident arrhythmias; these were assessed over the lifetime of follow‐up. In addition, we evaluated differences in outcomes of stress testing (heart rate recovery when available, dyspnea as a reason for stopping, and functional aerobic capacity as measured using metabolic equivalent tasks) at the time of stress testing.
Statistical Analysis
All analyses were performed in the cohort described above using SAS Studio 5.0. Categorical variables were summarized by frequency and percentage and compared using chi‐square test of continuity. Continuous variables are reported as mean±SD and compared using a Student t test. Kaplan–Meier survival curves were calculated to determine survival segregated by the presence or absence of RBBB. HRs from a Cox proportional hazards model were computed to determine the effect of baseline characteristics on all‐cause mortality and cardiovascular mortality in patients with versus without RBBB. A stratification method was used to adjust for confounding variables and to determine an adjusted HR. Potential confounders included those variables distributed unequally among the groups and those that could be associated with the risk of RBBB and mortality outcomes. Statistical significance was set at 0.05.
Results
Study Participants
In total, 31 979 patients underwent exercise stress testing at the Mayo Clinic in Rochester, Minnesota between 1993 and 2010. Of the 31 979 patients, 22 806 (71.3%) did not have pre‐existing CVD and were included in our cohort. In patients without CVD, 8256 (36.2%) were women, and the mean age was 52±11 years. From this cohort, 220 (0.96%) patients had RBBB on electrocardiography. Overall, patients with RBBB were older, more commonly male, and more likely to have comorbidities, including hypertension and diabetes mellitus, compared with patients without RBBB (Table 1).
Table 1.
Baseline Characteristics in Patients With and Without RBBB
|
No RBBB N=22 806 |
RBBB N=220 |
P Value | |
|---|---|---|---|
| Age, y | 52±11 | 59±12 | <0.0001 |
| Male | 14 596 (64.8%) | 178 (80.9%) | <0.0001 |
| Hypertension | 5245 (23.8%) | 75 (34.1%) | 0.0003 |
| Diabetes mellitus | 1368 (6.1%) | 20 (9.1%) | 0.06 |
| BMI | 29±5.6 | 29±5.9 | 0.61 |
| CKD | 388 (1.7%) | 3 (1.4%) | <0.0001 |
| Former smoker | 9452 (43.5%) | 95 (45.7%) | 0.89 |
| Active smoker | 2418 (11.2%) | 23 (11.1%) | 0.77 |
| HR‐limiting medications | 2942 (13%) | 37 (16.8%) | 0.09 |
Hypertension was defined as blood pressure >140/80. Diabetes mellitus was defined as A1C >6.5. CKD was defined as kidney disease ≥stage 3. HR‐limiting medications included β blockers, calcium channel blockers, and digoxin. BMI indicates body mass index; CKD, chronic kidney disease; HR, heart rate; and RBBB, right bundle‐branch block.
Figure 1 summarizes the indication for stress‐testing referral. There was no difference in frequency of referral. Symptoms for referral included syncope, dizziness, presyncope, fatigue, and chest pain. Arrhythmias included supraventricular tachycardia and premature beats.
Figure 1. Indications for referral for stress testing.
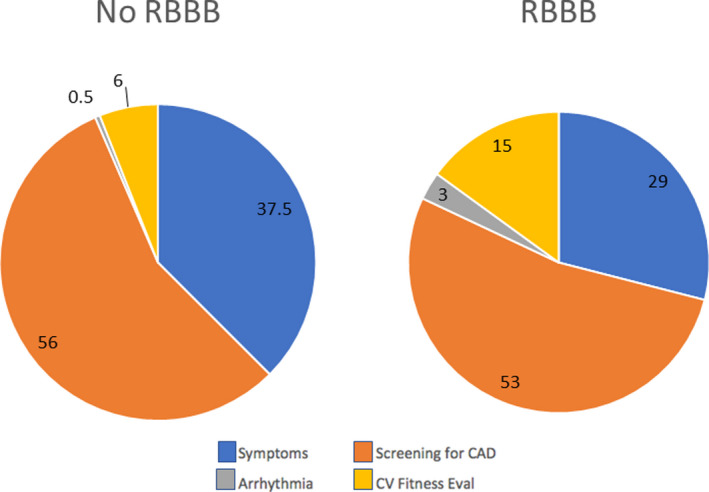
Shown are the indications for stress testing in patients with and without right bundle‐branch block (RBBB). Symptoms included dizziness, near‐syncope, syncope, fatigue, or chest pain. Arrhythmias included premature beats and supraventricular tachycardia. Numbers reported are percentage of the overall population. CAD indicates coronary artery disease; and CV, cardiovascular.
Clinical Outcomes
There were 1837 deaths (8.05%), including 645 cardiovascular‐related deaths (2.83%) over an average 12.4±5.1‐year follow‐up in the cohort. The presence of RBBB, detected on exercise stress testing, was associated with a significantly higher risk of cardiovascular death and all‐cause mortality (Table 2). Likelihood estimates revealed that age, sex, diabetes mellitus, hypertension, obesity, current and past smoking, and use of a heart rate‐lowering drug led to a higher risk of all‐cause mortality (Figure 2A) and cardiovascular mortality (Figure 2B). After adjusting for these confounders, RBBB remained independently associated with increased all‐cause mortality (HR, 1.5; 95% CI, 1.1–2.0; P=0.0058) (Figure 3A) and cardiovascular‐related death (HR, 1.7; 95% CI, 1.1–2.8; P=0.0178) (Figure 3B) in patients without CVD (Table 2). Kaplan–Meier curves are shown in Figure 3. Notably, separation between the curves for all‐cause mortality occurred at 1.5 to 2 years and for cardiovascular mortality at 5 years.
Table 2.
Unadjusted and Adjusted HRs for Risk of Mortality From RBBB
| Condition | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All‐cause mortality | 1.5 (1.1–2.0] | 0.0090 | 1.5 (1.1–2.0) | 0.0058 |
| Cardiovascular‐related death | 1.6 (1.0–2.6] | 0.0112 | 1.7 (1.1–2.8) | 0.0178 |
HR indicates hazard ratio; and RBBB, right‐bundle branch block.
Adjusted for age, sex, diabetes mellitus, hypertension, obesity, current and past smoking, and use of a heart rate‐lowering drug
Figure 2. Hazard ratios for all‐cause mortality and cardiovascular related mortality in patients with vs without RBBB.

Shown are the hazard ratios for all‐cause mortality (A) and cardiovascular related mortality (B). Note fitness was defined as failure to reach 85% of the target heart rate (HR). Error bars indicate 95% CIs. Note error bars around age and BMI are small for the scale shown. HR‐lowering medications included β blockers, calcium channel blockers, and digoxin. BMI indicates body mass index; CV, cardiovascular; and RBBB, right bundle‐branch block.
Figure 3. Kaplan–Meier survival curve of (A) all‐cause mortality and (B) cardiovascular death in patients with and without right bundle‐branch block (RBBB).

Shown are the Kaplan–Meier survival curves for patients with RBBB (red curves) vs no RBBB (blue curves) over follow‐up.
Stress Test Results
The stress test was symptom‐limited in 95.5% of patients. With respect to exercise capacity, exercise stress‐testing data revealed that patients with RBBB had decreased metabolic performance as measured by metabolic equivalent tasks (7.4±2.7 versus 9.8±2.6, P<0.0001), slower heart rate recovery (12.3±12.4 versus 18.5±8.9 bpm, P<0.0001), and stopped more often because of dyspnea (62/220 versus 5108/22 806, 28.2% versus 22.4%; P<0.0399) on the exercise test compared with patients without RBBB (Table 3). Functional aerobic capacity was also significantly higher in patients without RBBB than those with RBBB (93.2±22.5 versus 77.5±24.4; P=0.01).
Table 3.
Exercise Stress Test Results in Patients With Versus Without RBBB
|
No RBBB N=22 806 |
RBBB N=220 |
P Value | |
|---|---|---|---|
| Resting heart rate | 76.8±13 | 73.1±14.3 | 0.05 |
| METS | 9.8±2.6 | 7.4±2.7 | <0.0001 |
| Peak heart rate | 162.9±19.9 | 134.4±28.4 | <0.0001 |
| Heart rate recovery | 18.5±8.9 | 12.3±12.4 | <0.0001 |
| Heart rate reserve | 86.1±20.8 | 87.0±12.1 | <0.0001 |
| Peak SBP | 176.6±25.4 | 161.9±31.7 | <0.0001 |
| Peak DBP | 76±16 | 74±17 | 0.003 |
| Rest SBP | 123.9±17.8 | 123.7±20.0 | 0.0003 |
| Rest DBP | 79.8±11.1 | 75±12.6 | 0.03 |
| Positive exercise ECG | 1095 (4.8%) | 7 (3.2%) | 0.26 |
DBP indicates diastolic blood pressure; HR, heart rate; METS, metabolic equivalent tasks; RBBB, right bundle‐branch block; and SBP, systolic blood pressure.
Discussion
Our study suggests that patients without underlying CVD, but who have RBBB on exercise stress testing, are at an increased risk for all‐cause mortality and cardiovascular mortality. In addition, our data support that these patients are more likely to have lower functional exercise capacity.
Based on prior studies, in patients with underlying CVD, RBBB is a predictor of negative outcomes, in particular mortality. 6 , 7 , 9 However, existing data on patients without underlying CVD are less clear, and results are contradictory with respect to the relationship between RBBB and mortality. The Copenhagen Heart Study found RBBB was a strong predictor of increased all‐cause mortality. 11 In contrast, the Women's Health Initiative Study did not find a relationship between RBBB and mortality. 12
Adjustments for sex may be a clear point of difference between the 3 studies. In our analysis, we did not perform sex‐specific analysis given the relatively smaller number of women than men represented. Our overall findings are more in line with those from the Copenhagen Heart Study given the increased risk of all‐cause mortality, cardiovascular‐related mortality, and reduced aerobic capacity found in patients with RBBB, but without prior known CVD.
One possible pathophysiological mechanism by which RBBB may contribute to mortality, specifically cardiovascular mortality, may include ventricular dyssynchrony. 14 This phenomenon may occur in patients with RBBB caused by delayed right ventricular systole. In turn, RBBB may also be caused by myocardial disease secondarily involving the right bundle, suggesting some subclinical myopathic process. 12 , 14 , 15 Thus, RBBB may signal the development of early myocardial disease, including idiopathic fibrosis, amyloidosis, sarcoidosis, and systemic sclerosis, which, as they progress, could lead to complete heart block, ventricular arrhythmias, HF, or death. 4 , 5 The heterogeneity of the causes of RBBB and the difficulty in distinguishing the cause of apparent RBBB on electrocardiography (ranging from subclinical myocardial injury to primary fascicular block or delay) may also account for the variability in outcomes seen between studies. 11 , 12 Of course, it may be possible to identify subtle variations in the RBBB morphology (such as QRS duration, precordial transition, etc), and glean markers for a specific cause (myopathic versus primarily fascicular), but this would likely require even larger data sets.
Heart rate recovery after exercise and functional exercise capacity as measured by metabolic equivalent tasks was significantly slower and lower, respectively, in patients with RBBB compared with those without the rhythm abnormality. This may be because of the hemodynamic effects of having a RBBB as described above, that is greater dyssynchrony in ventricular relaxation (because of depolarization dyssynchrony), ventricular remodeling, and resultant development of HF or arrhythmias. 12 Alternatively, it is possible that the RBBB suggests some element of premature conduction disease that may lead to subtle or subclinical chronotropic abnormalities prior to stress testing.
Limitations
Several limitations of this study should be noted. First, this was a retrospective analysis, and as such, our data were susceptible to inherent biases. Second, the data are from a large tertiary care center, which is subject to referral bias. Third, the majority of patients were male and White; thus, these results must be interpreted in such a context. However, we did adjust for sex specifically for this reason, as prior studies had contrasting results that could be sex‐based. Fourth, although multiple confounders were accounted for in the multivariable analysis performed, there may have been other residual confounders that influenced the ultimate results of the study. Fifth, although patients did not have any noted diagnosis of CVD, it does not mean that patients did not have some element of subclinical coronary disease, myocardial dysfunction, cerebrovascular disease, etc. Specifically, our study was not structured to provide further insights into the physiology underlying the differences in mortality seen with RBBB. It should be noted, however, that a scenario of testing only those patients for whom we systematically and completely excluded all pre‐existing CVD through a combination of invasive and noninvasive testing would not be generalizable to any patient population; thus, we see this as a positive aspect of our study. Finally, the patient population evaluated underwent stress testing presumably for a guideline‐recommended indication. Thus, some impetus for referral may have been based on concern for a not‐yet‐diagnosed cardiovascular condition, and the results must be interpreted in such a context. Although the current AHA/ACC guideline on risk assessment neither endorses nor does not endorse exercise testing for risk assessment, the practice at our institution often includes assessment of functional capacity more frequently than in other practices, which accounts for the frequency of the number of patients undergoing “routine” stress testing included in this population. 16 Thus, it is likely that the risk conferred by RBBB may be different, depending on the indication for which stress testing was performed (eg, presence of cardiac symptoms).
Conclusions
Our data suggest that patients with RBBB without prior diagnosis of CVD have an increased risk of cardiovascular‐related and all‐cause mortality. Our data also suggest that these patients have a higher likelihood of reduced exercise capacity compared with those without RBBB. Given the potential significance of these findings, these data merit further prospective and multicenter evaluation.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2020;9:e017430 DOI: 10.1161/JAHA.120.017430.)
For Sources of Funding and Disclosures, see page 7.
See Editorial by Birnbaum and Nikus
References
- 1. Stein R, Nguyen P, Abella J, Olson H, Myers J, Froelicher V. Prevalence and prognostic significance of exercise‐induced right bundle branch block. Am J Cardiol. 2010;105:677–680. [DOI] [PubMed] [Google Scholar]
- 2. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–e240. [DOI] [PubMed] [Google Scholar]
- 3. Nelson AJ, Wong GR, Roberts‐Thomson R, Parvar SL. Massive pulmonary embolism with acute cor pulmonale. Postgrad Med J. 2016;92:487–488. [DOI] [PubMed] [Google Scholar]
- 4. Draeger HT, Assassi S, Sharif R, Gonzalez EB, Harper BE, Arnett FC, Manzoor A, Lange RA, Mayes MD. Right bundle branch block: a predictor of mortality in early systemic sclerosis. PLoS One. 2013;8:e78808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol. 1997;30:1046–1051. [DOI] [PubMed] [Google Scholar]
- 6. Chan WK, Goodman SG, Brieger D, Fox KAA, Gale CP, Chew DP, Udell JA, Lopez‐Sendon J, Huynh T, Yan RT, et al. Clinical characteristics, management, and outcomes of acute coronary syndrome in patients with right bundle branch block on presentation. Am J Cardiol. 2016;117:754–759. [DOI] [PubMed] [Google Scholar]
- 7. Widimsky P, Rohac F, Stasek J, Kala P, Rokyta R, Kuzmanov B, Jakl M, Poloczek M, Kanovsky J, Bernat I, et al. Primary angioplasty in acute myocardial infarction with right bundle branch block: should new onset right bundle branch block be added to future guidelines as an indication for reperfusion therapy? Eur Heart J. 2012;33:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barsheshet A, Goldenberg I, Garty M, Gottlieb S, Sandach A, Laish‐Farkash A, Eldar M, Glikson M. Relation of bundle branch block to long‐term (four‐year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol. 2011;107:540–544. [DOI] [PubMed] [Google Scholar]
- 9. McCullough PA, Hassan SA, Pallekonda V, Sandberg KR, Nori DB, Soman SS, Bhatt S, Hudson MP, Weaver WD. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005;102:303–308. [DOI] [PubMed] [Google Scholar]
- 10. Sabe MA, Sabe SA, Kusunose K, Flamm SD, Griffin BP, Kwon DH. Predictors and prognostic significance of right ventricular ejection fraction in patients with ischemic cardiomyopathy. Circulation. 2016;134:656–665. [DOI] [PubMed] [Google Scholar]
- 11. Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J. 2013;34:138–146. [DOI] [PubMed] [Google Scholar]
- 12. Zhang ZM, Rautaharju PM, Soliman EZ, Manson JE, Cain ME, Martin LW, Bavry AA, Mehta L, Vitolins M, Prineas RJ. Mortality risk associated with bundle branch blocks and related repolarization abnormalities (from the Women's Health Initiative [WHI]). Am J Cardiol. 2012;110:1489–1495. [DOI] [PubMed] [Google Scholar]
- 13. Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971;3:323–332. [PubMed] [Google Scholar]
- 14. Miller BE, Rajsheker S, Lopez‐Candales A. Right bundle branch block and electromechanical coupling of the right ventricle: an echocardiographic study. Heart Views. 2015;16:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Xue M, Li Z, Wang H, Zhu L, Liu X, Meng H, Hou Y. Effects of an isolated complete right bundle branch block on mechanical ventricular function. J Ultrasound Med. 2015;34:2171–2177. [DOI] [PubMed] [Google Scholar]
- 16. Gibbons RJ, Baladay GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to update the 1997 exercise testing guidelines). Circulation. 2002;106:1883–1892. [DOI] [PubMed] [Google Scholar]


