Abstract
Background
Dialysis is an independent risk factor for in‐stent restenosis (ISR) after stent implantation in coronary arteries. However, the characteristics of ISR in patients undergoing dialysis remain unclear, as there are no histological studies evaluating the causes of this condition. The aim of the present study was to investigate the causes of ISR between patients who are undergoing dialysis and those who are not by evaluating tissues obtained from ISR lesions using directional coronary atherectomy.
Methods and Results
A total of 29 ISR lesions from 29 patients included in a multicenter directional coronary atherectomy registry of 128 patients were selected for analysis and divided into a dialysis group (n=8) and a nondialysis group (n=21). Histopathological evaluation demonstrated that an in‐stent calcified nodule was a major histological characteristic of ISR lesions in the dialysis group and the prevalence of an in‐stent calcified nodule was significantly higher in the dialysis group compared with the nondialysis group (75% versus 5%, respectively; P<0.01). On the other hand, the prevalence of an in‐stent lipid‐rich plaque was significantly lower in the dialysis group compared with the nondialysis group (0% versus 43%, respectively; P=0.03). In all cases with an in‐stent calcified nodule, the underlying calcification before stent implantation was moderate to severe. When tissue characteristics were stratified according to duration post–stent implantation, an in‐stent calcified nodule in the dialysis group was mainly observed within 1 year after stent implantation.
Conclusions
In‐stent calcified nodules are a common cause of ISR in patients undergoing dialysis and are observed within 1 year after stent implantation, suggesting different causes of ISR between patients undergoing dialysis and those who are not.
Keywords: dialysis, in‐stent restenosis, calcified nodule, directional coronary atherectomy
Subject Categories: Percutaneous Coronary Intervention, Revascularization
Nonstandard Abbreviations and Acronyms
- DCA
directional coronary atherectomy
- ISR
in‐stent restenosis
- OUCH
Outcome of Cypher Stent in Hemodialysis Patients
- PCI
percutaneous coronary intervention
Clinical Perspective
What Is New?
We demonstrated that in‐stent calcified nodule was a major histological characteristic of in‐stent restenosis in patients undergoing dialysis and observed within 1 year after stent implantation, suggesting different causes of in‐stent restenosis between patients undergoing dialysis and those who are not.
What Are the Clinical Implications?
The current study is the first to provide a histopathological characteristic of in‐stent restenosis in patients undergoing dialysis.
Cardiovascular disease is the leading cause of morbidity and mortality in patients undergoing dialysis. 1 , 2 The mortality caused by cardiovascular disease in dialysis patients is 9 times higher than that reported in age‐/sex‐matched controls without renal failure. 3 A recent meta‐analysis demonstrated a reduced rate of target lesion revascularization in drug‐eluting stents compared with bare‐metal stents. 4 However, dialysis has been associated with target lesion revascularization even after implantation of a drug‐eluting stent. 5 The characteristics underlying in‐stent restenosis (ISR) in patients undergoing dialysis remain unclear owing to the lack of histological studies.
Directional coronary atherectomy (DCA) is a procedure for the removal of plaque mass to relieve obstruction of an atherosclerotic coronary artery. 6 It enables direct pathological evaluation of the collected tissue in de novo atherosclerotic or ISR lesions. 7 , 8 , 9 The aim of the present study was to elucidate the different causes of ISR among patients undergoing dialysis and those who are not using tissues obtained through DCA.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Population
Between June 2015 and January 2018, 128 consecutive patients who underwent percutaneous coronary intervention (PCI) with DCA at 18 centers were enrolled in a multicenter DCA registry. In the registry, a total of 141 lesions (collected using DCA) were reviewed. Of those, 98 and 14 lesions were excluded from this study because they were de novo lesions and lesions with recurrence of ISR (more than twice), respectively. Finally, a total of 29 lesions from 29 patients were examined: 8 and 21 lesions were collected from patients undergoing dialysis (dialysis group) and patients who are not (nondialysis group), respectively (Figure 1). ISR was defined as previously described: >50% angiographic stenosis at the stent segment or its edges (5‐mm segments adjacent to the stent). 10 The study was approved by the institutional ethics committee of each participating center, and all patients provided written informed consent.
Figure 1. Flowchart of inclusion and exclusion criteria.
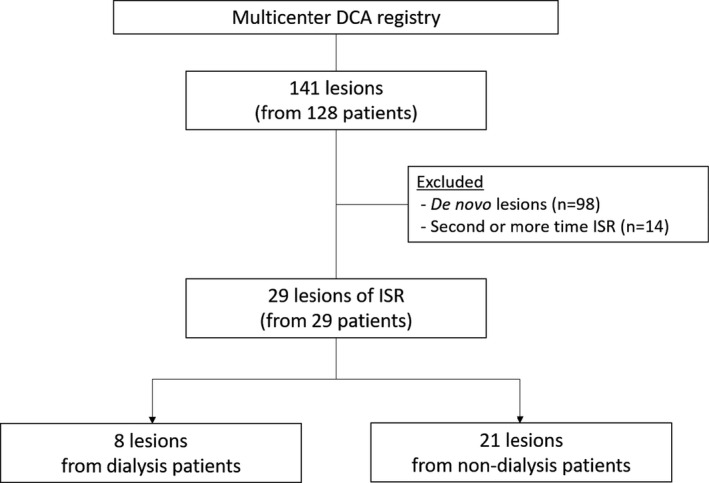
A total of 141 lesions collected using directional coronary atherectomy (DCA) from 18 institutions were reviewed. Of those, 98 and 14 lesions were excluded from this study because they were de novo lesions and lesions with recurrence of in‐stent restenosis (ISR; more than twice), respectively. The remaining 29 lesions from 29 patients (treated to ISR lesions with DCA) were included in this analysis: 8 and 21 lesions were included in the dialysis group and nondialysis group, respectively.
Procedure
All PCI procedures were performed via the femoral artery using an 8F sheath introducer, with administration of heparin to achieve an activated clotting time >250 seconds. After insertion of the guidewire to the target coronary artery, intravascular ultrasound and/or optical coherence tomography was performed to determine the window orientation of DCA (Atherocut, NIPRO) based on the plaque distribution. Tissue was obtained from the narrowest portion of the ISR lesion through DCA. The number of tissues debulked and the treatment strategy following DCA was at the discretion of the operators.
Histological Preparation and Assessment
Severely calcified tissues were decalcified in EDTA. All specimens were fixed in 10% neutral buffered formalin and subjected to paraffin embedding. Histological sections were cut (thickness: 6.5 μm) and stained with hematoxylin‐eosin and Movat pentachrome.
Pathological assessment was performed by 2 experienced investigators (G.N. and M.N.) who were blinded to the clinical status of patients. Any discrepancy between the findings of the 2 pathologists was resolved by discussion. Histological characteristics demonstrated various patterns. The cellular‐rich plaque was defined as a cell‐rich intimal lesion, predominantly composed of smooth muscle cells. 11 The proteoglycan or collagen appeared light green or yellow in Movat pentachrome stain, respectively. 12 The lipid‐rich plaque included lipid pool, macrophage foam cells, or necrotic core. The calcified nodule or nodular calcification were defined as small fragmented nodules of calcification surrounded by fibrin with or without thrombus, respectively. 13 The calcified plaque included punctate, sheet, or unclassified calcification. 14 , 15
Severity of Calcification
The degree of lesion calcification at initial PCI was classified according to angiography and intravascular ultrasound as follows: (1) none or mild; (2) moderate (radiopacities noted only during the cardiac cycle before contrast injection in angiography and/or calcification involving 1 or 2 quadrants of the vessel circumference in intravascular imaging devices); (3) severe (radiopacities noted without cardiac motion before contrast injection and/or calcification involving >2 quadrants in intravascular imaging devices); 16 and (4) calcified nodules were defined by expulsion of small calcific nodules into the lumen, and nodular calcification was defined by protruding calcific mass without eruptive nodules using optical coherence tomography. 17 , 18
Statistical Analysis
Continuous variables with normal distribution were expressed as mean±SD. Variables with non‐normal distribution were expressed as median (interquartile range). Comparisons of continuous variables with normal distribution were performed using Student t test. The nonparametric Mann–Whitney U test was used for comparisons of non‐normally distributed continuous variables. Categorical variables were expressed as counts and percentages, and the chi‐square test or Fisher exact test was used for comparison. A P value <0.05 denoted statistical significance. All analyses were performed using SPSS software version 24.0 (IBM).
RESULTS
Patient/lesion characteristics
Patient and lesion characteristics are summarized in Table 1. Age, sex, clinical presentation, and risk factors for coronary disease were similar in the dialysis and nondialysis groups. Bare‐metal stents were implanted in 2 of 8 lesions (25%) in the dialysis group, and 5 of 21 lesions (24%) in the nondialysis group. All of the patients undergoing dialysis were on hemodialysis, and the mean duration of dialysis treatment was 11.8±5.7 years. The serum phosphorus level (6.3±1.1 versus 3.5±0.7 mg/dL, P<0.01) and calcium‐phosphorus product (55.9±12.7 versus 32.0±3.8 mg2/dL2, P=0.02) in the dialysis group were significantly higher compared with that in the nondialysis group.
Table 1.
Patient and Lesion Characteristics
| Dialysis | Nondialysis | ||
|---|---|---|---|
| n=8 | n=21 | P Value | |
| Age, y | 66.9±13.3 | 72.8±10.8 | 0.23 |
| Men, No. (%) | 6 (75) | 19 (91) | 0.28 |
| Clinical presentation | |||
| Stable angina pectoris, No. (%) | 4 (50) | 9 (43) | 0.25 |
| Acute coronary syndrome, No. (%) | 2 (25) | 2 (10) | |
| Silent myocardial ischemia, No. (%) | 2 (25) | 10 (47) | |
| Coronary risk factors | |||
| Hypertension, No. (%) | 6/7 (87)* | 17 (81) | 0.78 |
| Hyperlipidemia, No. (%) | 5/7 (71)* | 16 (76) | 1.0 |
| Diabetes mellitus, No. (%) | 4/7 (57)* | 6 (29) | 0.21 |
| Smoking history, No. (%) | 4/7 (57)* | 8 (38) | 0.42 |
| CKD, No. (%) | 8 (100) | 6 (29) | <0.01 |
| Duration of dialysis, y | 11.8±5.7 | – | – |
| Hemodialysis, No. (%) | 8 (100) | – | – |
| Calcium, mg/dL | 8.8±0.8 | 9.3±0.6 | 0.26 |
| Phosphorus, mg/dL | 6.3±1.1 | 3.5±0.7 | <0.01 |
| Calcium‐phosphorus product, mg2/dL2 | 55.9±12.7 | 32.0±3.8 | 0.02 |
| Medical history | |||
| Previous myocardial infarction, No. (%) | 2 (25) | 6 (29) | 1.0 |
| Previous CABG, No. (%) | 1 (13) | 1 (5) | 0.48 |
| Target artery, No. (%) | |||
| LMCA | 0 | 1 (5) | 0.35 |
| LAD | 4 (50) | 14 (66) | |
| LCX | 0 | 2 (10) | |
| RCA | 4 (50) | 4 (19) | |
| Stent type, No. (%) | |||
| BMS | 2 (25) | 5 (24) | 0.40 |
| First‐generation DES | 0 | 4 (19) | |
| Second‐generation DES | 6 (75) | 12 (57) | |
| Lesion calcification at initial PCI | |||
| None or mild, No. (%) | 0/7* | 11/14 (79)* | <0.01 |
| Moderate, No. (%) | 2/7 (29)* | 1/14 (7)* | |
| Severe, No. (%) | 2/7 (29)* | 3/14 (21)* | |
| Calcified nodule/nodular calcification, No. (%) | 4/7 (57)* | 0/14* | |
| Time to ISR, mo | 10.5 (6.0–19.0) | 23.0 (8.0–70.0) | 0.13 |
BMS indicates bare‐metal stent; CABG, coronary artery bypass graft; CKD, chronic kidney disease; DES, drug‐eluting stent; ISR, in‐stent restenosis; LAD, left anterior descending artery; LCX, left circumferential artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; and RCA, right coronary artery.
Cases without clinical or lesion history are excluded.
The severity of calcification at initial PCI procedure was greater in the dialysis group than the nondialysis group (P<0.01). All lesions in the dialysis group demonstrated more than moderate calcification, whereas 79% of the lesions in the nondialysis group showed none or mild calcification. Underlying calcified nodule/nodular calcification at initial PCI procedure was observed in 4 of 7 lesions in the dialysis group (57%). Time to ISR tended to be shorter in the dialysis group than the nondialysis group (median, 10.5 months [interquartile range, 6.0–19.0 months] versus 23.0 months [interquartile range, 8.0–70.0], respectively; P=0.13).
Neointimal Tissue of ISR Lesions Collected Through DCA
The characteristics of neointimal tissue collected from the dialysis and nondialysis groups are shown in Figure 2. The prevalence of cellular‐rich plaque, proteoglycan, collagen, and calcified plaque were similar among the groups. However, the prevalence of an in‐stent calcified nodule was significantly higher in the dialysis group than the nondialysis group (75% versus 5%, respectively; P<0.01). On the other hand, the prevalence of an in‐stent lipid‐rich plaque was significantly lower in the dialysis group compared with the nondialysis group (0% versus 43%, respectively; P=0.03). When tissue characteristics were stratified according to duration post–stent implantation (Figure 3), an in‐stent calcified nodule in the dialysis group was mainly observed within 1 year after stent implantation. In contrast, a lipid‐rich plaque in the nondialysis group was mainly observed beyond 1 year.
Figure 2. Pathological evaluation of tissue.
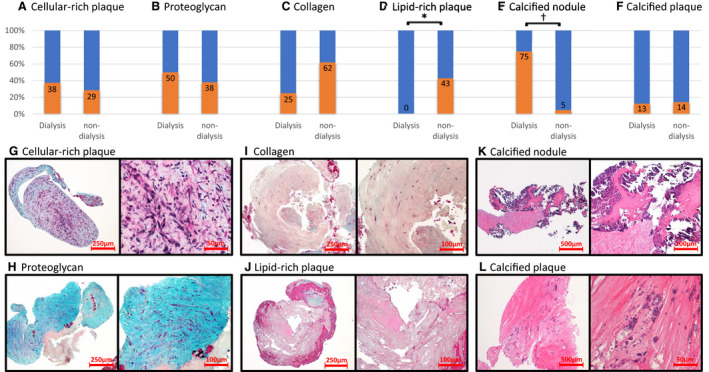
The comparison of the various types of tissues between the dialysis and nondialysis groups (A–F). All of the histological characteristics from 1 specimen were counted. The incidence of calcified nodules was significantly greater in the dialysis group than the nondialysis group (E). Lipid‐rich plaque was not observed in patients undergoing dialysis (D). Low‐ and high‐magnification images showing representative histological characteristics of the coronary plaque obtained by directional coronary atherectomy (DCA) (G–L). *P=0.03; † P<0.01.
Figure 3. Plaque characteristics of in‐stent restenosis (ISR) lesions stratified according to duration postimplantation.
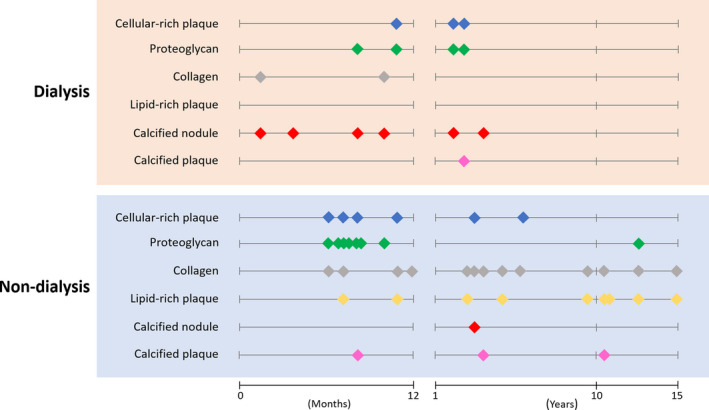
Each dot indicates the pathological characteristics of the tissues collected from ISR lesions (ie, cellular‐rich plaque, proteoglycan, collagen, lipid‐rich plaque, calcified nodule, and calcified plaque). The upper red box is the dialysis group, while the lower blue box is the nondialysis group. The plots stratified according to duration postimplantation (ie, ≤1 year and >1 year). In‐stent calcified nodule in the dialysis group was mainly observed within 1 year after stent implantation.
Detailed patient/lesion characteristics of all of the cases (ie, 7 cases) that showed calcified nodule in the neointimal tissues collected from ISR lesions are demonstrated in Table 2. Six of the 7 patients were undergoing dialysis and the severity of calcification before stent implantation was more than moderate in all cases. In the 2 cases, optical coherence tomography showed the protrusion of calcified nodules after stent implantation at initial PCI. The representative cases with an in‐stent calcified nodule are shown in Figures 4 and 5.
Table 2.
Details of Cases With an In‐stent Calcified Nodule
| Patient No. | Age, y/Sex | Dialysis | ISR Phase | Time to ISR, mo | Stent Type | Location | Underlying Calcification | Rotablator Before Stenting | Protrusion at Initial PCI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/male | + | Early | 2 | EES | RCA | Calcified nodule | − | + |
| 2 | 83/female | + | Early | 3 | BMS | RCA | Calcified nodule | − | + |
| 3 | 56/male | + | Mid | 7 | EES | LAD | Moderate | − | NA |
| 4 | 61/female | + | Mid | 10 | EES | RCA | Severe | + | NA |
| 5 | 70/male | + | Mid | 11 | BMS | RCA | Moderate | − | NA |
| 6 | 75/male | + | Late | 36 | BES | LAD | Severe/nodular calcification | + | − |
| 7 | 71/male | − | Late | 28 | EES | LAD | Severe | + | NA |
BES indicates biolimus‐eluting stent; BMS, bare‐metal stent; EES, everolimus‐eluting stent; ISR, in‐stent restenosis; LAD, left anterior descending artery; NA, not available; PCI, percutaneous coronary intervention; and RCA, right coronary artery.
Figure 4. Representative case of an early in‐stent calcified nodule in a patient undergoing dialysis.
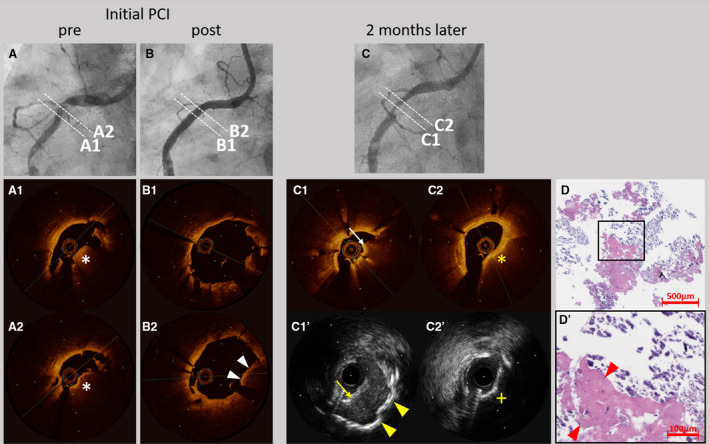
A 43‐year‐old man undergoing dialysis received everolimus‐eluting stents in the right coronary artery (RCA) for stable angina. Coronary angiography results before (A) and after (B) stent implantation are presented. Optical coherence tomography (OCT) showed a high‐backscattering protruding mass with signal attenuation suggesting the calcified nodule (white asterisks) (A1 and A2). In addition, OCT demonstrated the protrusion of the calcified nodule (white arrow heads) after stent implantation (B1 and B2). Two months later, the patient underwent coronary angiography for symptoms of unstable angina pectoris. Coronary angiography (C) revealed in‐stent restenosis in the RCA. OCT (C1) showed a red thrombus (white arrow), while intravascular ultrasound (C1’) showed a thrombus (yellow arrow) and sheet calcification at the bottom (yellow arrow heads). OCT (C2) demonstrated attenuations (yellow asterisk) and intravascular ultrasound (C2’) demonstrated acoustic shadow (yellow cross) suggesting the presence of the calcified nodule. Low‐ (D) and high‐ (D’) power images of the tissue collected through directional coronary atherectomy revealed the calcified nodule with fibrin deposition (red arrow heads). PCI indicates percutaneous coronary intervention.
Figure 5. Representative case of late in‐stent calcified nodule in a patient undergoing dialysis.
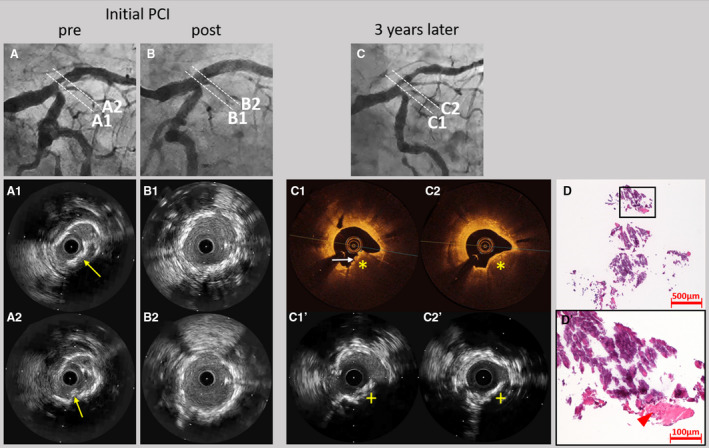
A 75‐year‐old man undergoing dialysis received a biolimus‐eluting stent in the left main coronary artery to left anterior descending artery (LAD) for stable angina. Coronary angiography before (A) and after (B) stent implantation are presented. Intravascular ultrasound (IVUS) showed a convex lesion with superficial hyperechoic signal accompanied by acoustic shadow suggesting the calcified nodule (yellow arrows) (A1 and A2). IVUS demonstrated no protrusions of the calcified nodule after stent implantation (B1 and B2). Three years later, the patient underwent coronary angiography for symptoms of stable angina. Coronary angiography (C) showed in‐stent restenosis in the ostium of the LAD. Optical coherence tomography showed high‐backscattering protruding mass with signal attenuation (yellow asterisks) and small eruptive calcified nodules on the surface (white arrow) (C1 and C2). IVUS demonstrated acoustic shadows (yellow crosses) suggesting the presence of the calcified nodule (C1’ and C2’). Low‐ (D) and high‐ (D’) power images of the tissue collected through directional coronary atherectomy revealed the calcified nodule with fibrin deposition (red arrow head). PCI indicates percutaneous coronary intervention.
DISCUSSION
The purpose of this study was to investigate the histopathological differences of ISR lesions in patients undergoing dialysis and those who are not. We demonstrated that the high prevalence of in‐stent calcified nodules was the major histological characteristic causing ISR in patients undergoing dialysis. In addition, in‐stent calcified nodules in patients undergoing dialysis were more prevalent within 1 year after stent implantation. A previous human autopsy study suggested that the tissue of ISR lesions was mainly characterized by smooth muscle cells, collagen/proteoglycan‐rich extracellular matrix, and neoatherosclerosis. 19 , 20 Thus, the use of drugs that inhibit smooth muscle cell proliferation in drug‐eluting stents or drug‐coated balloons is effective in reducing the occurrence of ISR.
On the contrary, clinical data for the effectiveness of drug‐eluting stents in patients undergoing dialysis have been disappointing. Registry studies using sirolimus‐eluting stents have shown that dialysis was the strongest predictor of ISR. 21 Prospective registry studies for patients undergoing dialysis, the OUCH (Outcome of Cypher Stent in Hemodialysis Patients) series, 22 , 23 , 24 have shown that the late lumen loss of sirolimus‐, paclitaxel‐, and everolimus‐eluting stents was 0.69±0.93 mm, 0.48±0.61 mm, and 0.37±0.63 mm, respectively. The rates of in‐segment binary restenosis were 29.6%, 14.5%, and 16%, respectively. Target vessel failure at 1 year was 24.5%, 20.2%, and 18%, respectively. These clinical data suggested the failure of drug‐eluting stents to prevent ISR in patients undergoing dialysis. The current study demonstrates that a calcified nodule is one of the major causes of ISR in patients undergoing dialysis, suggesting the presence of different characteristics of ISR in these patients that may cause excessive in‐stent lumen loss. To the best of our knowledge, the current histological study is the first to investigate the tissue characteristics of ISR lesions in patients undergoing dialysis.
One hypothesis of the mechanism of a calcified nodule states that “mechanical stress might fragment sheets of calcium, resulting in small nodules that become surrounded by fibrin, which might eventually erupt through the plaque surface.” 13 Although the mechanism of in‐stent calcified nodules remains poorly understood, there are several reports of early and late ISR caused by a calcified nodule. 25 , 26 , 27 , 28 In the current study, there might be 3 phases of in‐stent calcified nodule: early, mid, and late. In the early phase, an in‐stent calcified nodule was observed within 2 to 3 months after stent implantation (patients 1 and 2 in Table 2). In the 2 cases, the optical coherence tomography finding at initial PCI revealed the calcified nodules protruded through the stent struts. The protrusion of a calcified nodule followed by calcifying fibrin thrombus might cause the early in‐stent calcified nodules (Figure 6A). The previous report also suggested that the protrusion of calcified nodules may be responsible for in‐stent restenosis caused by calcified nodules. 25 , 28 In the mid phase, 3 cases demonstrated an in‐stent calcified nodule within 7 to 11 months after stent implantation (patients 3 through 5 in Table 2). Since there were no intravascular images in 3 cases, it was unclear whether the protrusion occurred. In these cases, underlying plaque characteristics were moderate to severe sheet calcification. Fragments of calcification as well as fibrin deposition caused by mechanical destruction of sheet calcification at the time of stent implantation might contribute to the formation of a calcified nodule on the stent surface (Figure 6B). In the late phase, 2 cases demonstrated in‐stent calcified nodule >2 years after stent implantation, suggesting the formation of a neocalcified nodule, a type of neoatherosclerosis (patients 6 and 7 in Table 2) (Figure 6C). Further clinical studies utilizing intravascular imaging are needed to prove this hypothesis.
Figure 6. Mechanism of progression of in‐stent calcified nodule.
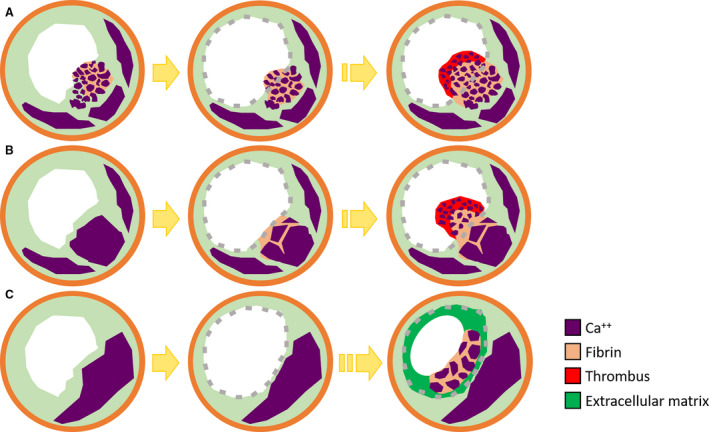
There might be 3 phases of in‐stent calcified nodules. In the early phase, protrusion of a calcified nodule followed by calcifying fibrin thrombus after stent implantation cause in‐stent calcified nodule (A). In the mid‐phase, fragments of calcification and fibrin deposition caused by mechanical destruction of sheet calcification at the time of stent implantation may contribute to the formation of calcified nodule (B). In the late phase, calcified nodule was formed in the stent over the years as a neocalcified nodule, a type of neoatherosclerosis (C).
Several possible factors may account for the higher prevalence of an in‐stent calcified nodule observed in patients undergoing dialysis. First, end‐stage renal disease and dialysis are established risk factors for severe coronary artery calcification 29 , 30 and a calcified nodule 31 in the native coronary artery. In addition, the previous studies revealed that high serum phosphorus promotes calcification and is associated with cardiac adverse events. 32 Second, high platelet reactivity in patients undergoing dialysis may lead to the formation of in‐stent calcified nodules. Some studies have revealed that dialysis is associated with high platelet reactivity 33 , 34 as a result of the exposure of blood to the membrane of the dialyzer 35 and the use of heparin. 36 Konishi et al 37 reported that patients undergoing dialysis had a significantly higher rate of thrombus formation after stent implantation, detected through optical coherence tomography, compared with patients who are not undergoing dialysis. We suggest that these 2 characteristics of patients undergoing dialysis, namely calcification in the coronary artery and high platelet reactivity, may be responsible for the higher prevalence of in‐stent calcified nodules.
The present study also demonstrated that lipid‐rich plaque was exclusively observed in patients who are not undergoing dialysis. Consistently, previous studies demonstrated that statins reduced the incidence of revascularization. 38 On the other hand, a randomized controlled trial showed that statins are less effective in patients undergoing dialysis. 39 The low incidence of lipid‐rich ISR in patients undergoing dialysis, shown in the current study, may partly explain the lower effectiveness of statins in patients undergoing dialysis.
Study Limitations
There were several limitations in this study. First, the indication of DCA depends on the operator and may be subject to selection bias. Second, the specimens obtained through DCA were part of ISR lesions and may not represent the entire plaque. Third, because many cases exhibited early restenosis, we were unable to investigate the plaque morphology of late‐stage ISR in patients undergoing dialysis. Finally, since the intravascular images at initial PCI were not available in several cases, some mechanistic hypotheses regarding in‐stent calcified nodules lacked their supportive data. Further studies are warranted to confirm the significance and application of the findings in a larger population.
CONCLUSIONS
In‐stent calcified nodules are a common cause of ISR in patients undergoing dialysis and are observed within 1 year after stent implantation. These findings suggest different characteristics of ISR between patients undergoing dialysis and those who are not.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
We thank S. Matsuno (The Cardiovascular Institute, Tokyo), T. Kobayashi (Kyoto‐Katsura Hospital, Kyoto), M. Taniwaki (Tokorozawa Heart Center, Saitama), N. Ogata (Ageo Chuo Hospital, Saitama), H. Oda (Niigata City General Hospital, Niigata), T. Kitamura (Suzuka General Hospital, Mie), K. Omi (Nihonkai General Hospital, Yamagata), A. Kozuki (Osaka Saiseikai Nakatsu Hospital, Osaka), K. Aihara, R. Funada (Gunma University Graduate School of Medicine, Gunma), H. Suzuki (Yokohama City Minato Red Cross Hospital, Kanagawa), T. Myojo (Hokkaido Medical Center, Hokkaido), F. Yoshimachi (Tokai University Hachioji Hospital, Tokyo), K. Mitsumata (Ayase Heart Hospital, Tokyo), Y. Kurumatani (Kofu‐Kyoritsu Hospital, Yamanashi), and R. Kubota (Toyota Kosei Hospital, Nagoya) for supplying the specimens. We also thank A. Yoshikawa (CV‐Hills, Tokai University), and Y. Ito (the Support Center for Medical Research and Education, Tokai University) for their valuable technical assistance.
(J Am Heart Assoc. 2020;9:e016595 DOI: 10.1161/JAHA.120.016595.)
For Sources of Funding and Disclosures, see page 9.
See Editorial by Sato et al.
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;2154–2169. [DOI] [PubMed] [Google Scholar]
- 2. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;276–284. [DOI] [PubMed] [Google Scholar]
- 3. de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;1782–1789. [DOI] [PubMed] [Google Scholar]
- 4. Palmerini T, Benedetto U, Biondi‐Zoccai G, Della Riva D, Bacchi‐Reggiani L, Smits PC, Vlachojannis GJ, Jensen LO, Christiansen EH, Berencsi K, et al. Long‐term safety of drug‐eluting and bare‐metal stents: evidence from a comprehensive network meta‐analysis. J Am Coll Cardiol. 2015;2496–2507. [DOI] [PubMed] [Google Scholar]
- 5. Otsuka Y, Ishiwata S, Inada T, Kanno H, Kyo E, Hayashi Y, Fujita H, Michishita I. Comparison of haemodialysis patients and non‐haemodialysis patients with respect to clinical characteristics and 3‐year clinical outcomes after sirolimus‐eluting stent implantation: insights from the Japan multi‐centre post‐marketing surveillance registry. Eur Heart J. 2011;829–837. [DOI] [PubMed] [Google Scholar]
- 6. Cubeddu RJ, Truong QA, Rengifo‐Moreno P, Garcia‐Camarero T, Okada DR, Kiernan TJ, Inglessis I, Palacios IF. Directional coronary atherectomy: a time for reflection. Should we let it go? EuroIntervention. 2009;485–493. [DOI] [PubMed] [Google Scholar]
- 7. Habara M, Otsuka F, Tsuchikane E, Terashima M, Nasu K, Kinoshita Y, Murata A, Suzuki Y, Kawase Y, Okubo M, et al. In vivo tissue characterization of human atherosclerotic plaques by optical coherence tomography: A directional coronary atherectomy study with histopathologic confirmation. Int J Cardiol. 2018;1–10. [DOI] [PubMed] [Google Scholar]
- 8. Chung IM, Gold HK, Schwartz SM, Ikari Y, Reidy MA, Wight TN. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002;2072–2081. [DOI] [PubMed] [Google Scholar]
- 9. Strauss BH, Umans VA, van Suylen RJ, de Feyter PJ, Marco J, Robertson GC, Renkin J, Heyndrickx G, Vuzevski VD, Bosman FT, et al. Directional atherectomy for treatment of restenosis within coronary stents: clinical, angiographic and histologic results. J Am Coll Cardiol. 1992;1465–1473. [DOI] [PubMed] [Google Scholar]
- 10. Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in‐stent restenosis: classification and implications for long‐term outcome. Circulation. 1999;1872–1878. [DOI] [PubMed] [Google Scholar]
- 11. Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, Komatsu R, Ikura Y, Ogami M, Shimada Y, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;2894–2900. [DOI] [PubMed] [Google Scholar]
- 12. Sakakura K, Nakano M, Otsuka F, Yahagi K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R. Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur Heart J. 2014;1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in‐stent atherosclerosis. Nat Rev Cardiol. 2016;79–98. [DOI] [PubMed] [Google Scholar]
- 14. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc Imaging. 2018;127–142. [DOI] [PubMed] [Google Scholar]
- 15. Torii S, Mustapha JA, Narula J, Mori H, Saab F, Jinnouchi H, Yahagi K, Sakamoto A, Romero ME, Narula N, et al. Histopathologic characterization of peripheral arteries in subjects with abundant risk factors: Correlating imaging with pathology. JACC Cardiovasc Imaging. 2019;1501–1513. [DOI] [PubMed] [Google Scholar]
- 16. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ, Leon MB. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;1959–1965. [DOI] [PubMed] [Google Scholar]
- 17. Ijichi T, Nakazawa G, Torii S, Nakano M, Yoshikawa A, Morino Y, Ikari Y. Evaluation of coronary arterial calcification ‐ Ex-vivo assessment by optical frequency domain imaging. Atherosclerosis. 2015;242–247. [DOI] [PubMed] [Google Scholar]
- 18. Sugiyama T, Yamamoto E, Fracassi F, Lee H, Yonetsu T, Kakuta T, Soeda T, Saito Y, Yan BP, Kurihara O, et al. Calcified plaques in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2019;531–540. [DOI] [PubMed] [Google Scholar]
- 19. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants bare‐metal and drug‐eluting stents. J Am Coll Cardiol. 2011;1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakano M, Otsuka F, Yahagi K, Sakakura K, Kutys R, Ladich ER, Finn AV, Kolodgie FD, Virmani R. Human autopsy study of drug‐eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikari Y, Kotani J, Kozuma K, Kyo E, Nakamura M, Yokoi H. Assessment of sirolimus‐eluting coronary stent implantation with aspirin plus low dose ticlopidine administration: one year results from CYPHER Stent Japan Post‐Marketing Surveillance Registry (J‐PMS). Circ J. 2009;1038–1044. [DOI] [PubMed] [Google Scholar]
- 22. Ikari Y, Tanabe K, Koyama Y, Kozuma K, Sano K, Isshiki T, Katsuki T, Kimura K, Yamane M, Takahashi N, et al. Sirolimus eluting coronary stent implantation in patients on maintenance hemodialysis: the OUCH study (outcome of cypher stent in hemodialysis patients). Circ J. 2012;1856–1863. [DOI] [PubMed] [Google Scholar]
- 23. Ikari Y, Kyono H, Isshiki T, Ishizuka S, Nasu K, Sano K, Okada H, Sugano T, Uehara Y. Usefulness of everolimus‐eluting coronary stent implantation in patients on maintenance hemodialysis. Am J Cardiol. 2015;872–876. [DOI] [PubMed] [Google Scholar]
- 24. Kozuma K, Otsuka M, Ikari Y, Uehara Y, Yokoi H, Sano K, Tanabe K, Hibi K, Yamane M, Ishiwata S, et al. Clinical and angiographic outcomes of paclitaxel‐eluting coronary stent implantation in hemodialysis patients: A prospective multicenter registry: The OUCH‐TL study (outcome in hemodialysis of TAXUS Liberte). J Cardiol. 2015;502–508. [DOI] [PubMed] [Google Scholar]
- 25. Mori H, Finn AV, Atkinson JB, Lutter C, Narula J, Virmani R. Calcified nodule: An early and late cause of in‐stent failure. JACC Cardiovasc Interv. 2016;9:e125–e126. [DOI] [PubMed] [Google Scholar]
- 26. Alfonso F, Cuesta J, Bastante T, Aguilera MC, Benedicto A, Rivero F. In‐stent restenosis caused by a calcified nodule: A novel pattern of neoatherosclerosis. Can J Cardiol. 2016;302–303. [DOI] [PubMed] [Google Scholar]
- 27. Furuse E, Tanabe J, Tajiri M, Kawanaka H, Shimizu W. In-stent restenosis caused by calcified nodule 11 years after paclitaxel eluting stent implantation treated with drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. 2020;35:302–303. [DOI] [PubMed] [Google Scholar]
- 28. Kaihara T, Higuma T, Kotoku N, Kuwata S, Mitarai T, Koga M, Kamijima R, Izumo M, Ishibashi Y, Tanabe Y, et al. Calcified nodule protruding into the lumen through stent struts: An in‐vivo OCT analysis. [published online ahead of print, 2020 Mar 11]. Cardiovasc Revasc Med. 2020;S1553‐8389(20)30149‐4. 10.1016/j.carrev.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 29. Gruberg L, Rai P, Mintz GS, Canos D, Pinnow E, Satler LF, Pichard AD, Kent KM, Waksman R, Lindsay J, et al. Impact of renal function on coronary plaque morphology and morphometry in patients with chronic renal insufficiency as determined by intravascular ultrasound volumetric analysis. Am J Cardiol. 2005;892–896. [DOI] [PubMed] [Google Scholar]
- 30. Chin CY, Matsumura M, Maehara A, Zhang W, Lee CT, Yamamoto MH, Song L, Parviz Y, Jhalani NB, Mohan S, et al. Coronary plaque characteristics in hemodialysis‐dependent patients as assessed by optical coherence tomography. Am J Cardiol. 2017;1313–1319. [DOI] [PubMed] [Google Scholar]
- 31. Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, Kanaji Y, Murai T, Yonetsu T, Kakuta T, et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging. 2017;883–891. [DOI] [PubMed] [Google Scholar]
- 32. Sato T, Aoki J, Kozuma K, Maruyama Y, Nasu K, Otsuka M, Ando K, Hibi K, Uehara Y, Tanabe K, et al. Impact of serum phosphorus levels on outcomes after implantation of drug‐eluting stents in patients on hemodialysis. Circ J. 2018;388–395. [DOI] [PubMed] [Google Scholar]
- 33. Fu Q, Ishikawa S, Yokoyama N, Kozuma K, Takada K, Muraki A, Isshiki T. Enhanced platelet activation following coronary stent implantation in patients on hemodialysis. Cardiovasc Interv Ther. 2010;72–77. [DOI] [PubMed] [Google Scholar]
- 34. Oshima S, Noda K, Fukushima H, Nakamura S, Shono M, Kugimiya F, Higa K. Low responsiveness to thienopyridine in hemodialysis patients. Cardiovasc Interv Ther. 2010;18–23. [DOI] [PubMed] [Google Scholar]
- 35. Aggarwal A, Kabbani SS, Rimmer JM, Gennari FJ, Taatjes DJ, Sobel BE, Schneider DJ. Biphasic effects of hemodialysis on platelet reactivity in patients with end‐stage renal disease: a potential contributor to cardiovascular risk. Am J Kidney Dis. 2002;315–322. [DOI] [PubMed] [Google Scholar]
- 36. Gritters M, Borgdorff P, Grooteman MP, Schoorl M, Schoorl M, Bartels PC, Tangelder GJ, Nube MJ. Platelet activation in clinical haemodialysis: LMWH as a major contributor to bio‐incompatibility? Nephrol Dial Transplant. 2008;2911–2917. [DOI] [PubMed] [Google Scholar]
- 37. Konishi A, Shinke T, Otake H, Nakatani D, Nakagawa M, Inoue T, Hariki H, Osue T, Taniguchi Y, Iwasaki M, et al. Impact of hemodialysis on local vessel healing and thrombus formation after drug‐eluting stent implantation. J Cardiol. 2014;25–31. [DOI] [PubMed] [Google Scholar]
- 38. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;238–248. [DOI] [PubMed] [Google Scholar]


