Abstract
Background
Sudden cardiac death (SCD) is associated with severe coronary heart disease in the great majority of cases. Whether carotid intima‐media thickness (C‐IMT), a known surrogate marker of subclinical atherosclerosis, is associated with risk of SCD in a general population remains unknown. The objective of this study was to investigate the association between C‐IMT and risk of SCD.
Methods and Results
We examined a total of 20 862 participants: 15 307 participants of the ARIC (Atherosclerosis Risk in Communities) study and 5555 participants of the CHS (Cardiovascular Health Study). C‐IMT and common carotid artery intima‐media thickness was measured at baseline by ultrasound. Presence of plaque was judged by trained readers. Over a median of 23.5 years of follow‐up, 569 participants had SCD (1.81 cases per 1000 person‐years) in the ARIC study. Mean C‐IMT and common carotid artery intima‐media thickness were associated with risk of SCD after adjustment for traditional risk factors and time‐varying adjustors: hazard ratios (HRs) with 95% CIs for fourth versus first quartile were 1.64 (1.15–2.63) and 1.49 (1.05–2.11), respectively. In CHS, 302 participants developed SCD (4.64 cases per 1000 person‐years) over 13.1 years. Maximum C‐IMT was associated with risk of SCD after adjustment: HR (95% CI) for fourth versus first quartile was 1.75 (1.22–2.51). Presence of plaque was associated with 35% increased risk of SCD: HR (95% CI) of 1.37 (1.13–1.67) in the ARIC study and 1.32 (1.04–1.68) in CHS.
Conclusions
C‐IMT was associated with risk of SCD in 2 biracial community‐based cohorts. C‐IMT may be used as a marker of SCD risk and potentially to initiate early therapeutic interventions to mitigate the risk.
Keywords: Carotid Intima‐Media Thickness, Epidemiology, Sudden Cardiac Death
Subject Categories: Sudden Cardiac Death, Epidemiology
Nonstandard Abbreviations and Acronyms
- CCA‐IMT
common carotid artery intima‐media thickness
- C‐IMT
carotid intima‐media thickness
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
In 2 biracial community‐based cohorts, subclinical atherosclerosis, measured by carotid intima‐media thickness, was associated with risk of sudden cardiac death over a median follow‐up of >13 years.
What Are the Clinical Implications?
These results may suggest importance of subclinical atherosclerosis in sudden cardiac death risk and lead to early therapeutic interventions to prevent sudden cardiac death in the future.
Sudden cardiac death (SCD), defined as a sudden and unexpected pulseless condition with cardiac cause, has remained a public health issue in the United States and globally. 1 It has been estimated that 366 807 people experienced SCD in 2015 in the United States, 2 accounting for 15% of annual mortality. 3 Despite much effort, identifying individuals at high risk of SCD has been elusive. Thus, there is a need to investigate the risk factors for SCD and to improve risk stratification. 1
Atherosclerosis has been shown to be associated with coronary heart disease (CHD) and stroke. 4 , 5 Carotid intima‐media thickness (C‐IMT) is a known surrogate marker for atherosclerosis and has been shown to be associated with cardiovascular disease. 6 , 7 Early identification of subclinical atherosclerosis could lead to more aggressive lifestyle modifications and medical treatment. 8 Although SCD and CHD share many of the risk factors 9 and subjects who had SCD had severe CHD on autopsy, 10 whether subclinical atherosclerosis is associated with incidence of SCD in general population remains unknown. Thus, the objective of this study was to investigate the association between C‐IMT and risk of SCD in 2 community‐based cohorts: the ARIC (Atherosclerosis Risk in Communities) study and the CHS (Cardiovascular Health Study).
Methods
Subjects
The ARIC Study
The ARIC study enrolled 15792 individuals, aged 45 to 64 years at baseline (1987–1989), from four US communities in North Carolina, Mississippi, Minnesota, and Maryland. Details of the ARIC study have been described elsewhere. 11 The current study used all ARIC study subjects at baseline with C‐IMT data. Participants were excluded from the study if they did not have ultrasound data at baseline (n=430), or if they were Black participants in Minnesota or Washington field center or if they were of non‐Black and non‐White ethnicity (n=55).
The CHS Study
The CHS is a prospective population‐based observational cohort study of people ≥65 years old at baseline to evaluate risk factors for the development and progression of cardiovascular disease. The CHS recruited individuals from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. The initial 5201 participants were enrolled from January 1989 through June 1990; an additional 687 predominantly Black participants were recruited in 1992 to 1993. Details of the CHS have been previously described. 12 All participants in CHS were included in the present study, except for those with missing ultrasound data at the baseline clinic visit.
The study was approved by the institutional review boards at all institutions involved in the study, and informed consent was obtained from all participants. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC study and CHS policies.
Carotid Intima‐Media Thickness
C‐IMT was measured similarly, but slightly differently in the ARIC study and CHS. In both cohorts, carotid atherosclerosis was measured by ultrasound at baseline.
In the ARIC study, the ultrasound protocol to measure C‐IMT has been described previously. 6 , 7 , 13 Briefly, in the ARIC study, intima‐media thickness was measured in 3 carotid sites across the 2 carotid arteries: common carotid artery (1 cm proximal to the dilatation of the carotid bulb), the carotid bifurcation (1 cm proximal to the flow divider), and the internal carotid artery (1 cm distal to the flow divider). Mean C‐IMT was defined as mean of the 6 measurements. Maximum C‐IMT was defined as the maximum among the 6 measurements. Mean and maximum common carotid artery intima‐media thickness (CCA‐IMT) were defined as mean of the CCA‐IMT and maximum among the CCA‐IMT, respectively. Presence of plaque was judged by trained readers and defined as 2 of the following 3 criteria at any of the 6 segments: abnormal wall thickness (defined as C‐IMT >1.5 mm), abnormal shape (protrusion into the lumen and loss of alignment with adjacent arterial wall boundary), and abnormal wall texture (brighter echoes than adjacent boundaries). 13 Mean and maximum C‐IMT, mean and maximum CCA‐IMT, and presence of plaque were used for analyses.
In the CHS, carotid ultrasound scans were performed on 5178 original CHS cohort members in 1989 to 1990 and 683 Black cohort members at year 5 (1992–1993). Because of evidence of reader drift between the 2 ultrasound visits, the carotid ultrasound scans from the CHS baseline visit (1989–1990) were reread by year 5 readers. We used variables that adjusted for reader drift as recommended by the CHS. The maximal C‐IMT was defined as the mean of the maximal intima‐media thickness of the near and far wall on both the left and right sides. Absence of plaque was defined as a smooth intimal surface with no regional discrete plaque. 14 Intermediate‐ risk plaque was hyperdense, calcified, or homogeneous plaque or those with a mildly irregular surface. High‐risk plaques had an irregular or ulcerated surface or were hypodense or heterogeneous plaque occupying >50% of the total plaque volume. Intermediate‐ and high‐risk plaques were grouped and compared with no plaque.
Sudden Cardiac Death
SCD was defined as a sudden pulseless condition presumed to be caused by a ventricular tachyarrhythmia in a previously stable individual without evidence of a noncardiac cause of cardiac arrest. All cardiac arrest events occurred out of the hospital or in the emergency department. In the ARIC study, all events classified as having fatal CHD that occurred by December 31, 2012, were reviewed and adjudicated by a committee of cardiac electrophysiologists, cardiologists, and internists. After review of data available, cases were classified as definite sudden arrhythmic death, possible sudden arrhythmic death, not sudden arrhythmic death, or unclassifiable. For this analysis, SCD was defined as definite or possible sudden arrhythmic death. The administrative censoring date was December 31, 2012, based on the study’s adjudication schedule.
In CHS, the adjudication process was composed of multiple steps. A specialized committee in CHS adjudicated the cause of death. All CHD deaths that occurred through December 31, 2006, were reviewed by a cardiologist (N.S.) to classify SCD cases. A second physician reviewed a sample of the cases, with 88% interreviewer agreement and ҡ value 0.74 for SCD. 15
Covariates
At baseline, participants reported information on smoking and alcohol intake, underwent a physical examination, and provided blood pressure. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or treatment for hypertension in the ARIC study, and as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or hypertension medication use in participants with diagnosed hypertension in CHS. Body mass index was calculated as weight in kilograms divided by height in meters squared. Education was categorized as advanced (completed college or more), intermediate (high school to less than college), and no or basic (less than high school). Diabetes mellitus (DM) was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, treatment for DM, or a self‐reported physician diagnosis of DM. High‐density lipoprotein cholesterol level was determined using enzymatic methods, and low‐density lipoprotein cholesterol level was calculated using the Friedewald equation. In the ARIC study, prevalent CHD was defined as self‐reported CHD or the presence of a previous myocardial infarction by ECG at baseline. Prevalent heart failure (HF) was defined as self‐reported use of HF medications within 2 weeks (“Were any of the medications you took during the last 2 weeks for HF?) or “manifest” HF by Gothenburg criteria. 16 In CHS, baseline and incident CHD was defined as a history of myocardial infarction or a nonmyocardial infarction event (specifically, angina pectoris or a revascularization procedure [coronary artery bypass grafting or percutaneous coronary intervention]). 17 Methods used to assess prevalent and incident congestive HF events have been reported previously. 18 , 19 , 20 Cornell voltage for left ventricular hypertrophy was defined as a sum of S amplitude in V3 and R amplitude in aVL on ECG.
Statistical Analysis
Baseline characteristics were compared between those who developed SCD and those who did not develop SCD using χ2 tests for categorical variables and Kruskal‐Wallis rank test for continuous data. C‐IMT was divided into quartiles (first quartile the lowest). Kaplan‐Meier curves with the end point of SCD were constructed on the basis of C‐IMT quartiles. A log‐rank test was performed to examine differences among the 4 groups. Cox proportional hazards models were used to evaluate associations of incident SCD with baseline C‐IMT. C‐IMT was treated as a continuous variable with potential nonlinearities evaluated using smoothers, such as fractional polynomials (study‐specific quartiles or continuous). Categorical variables, such as presence versus absence of plaque for C‐IMT, were examined. Models were adjusted for 3 sets of adjustors: model 1 included age, sex, and race‐by‐center terms; model 2 additionally included education, hypertension, DM, Cornell voltage, CHD, HF, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking; and model 3 additionally included time‐varying adjustors (CHD and HF). Stratified analyses were performed on the basis of age (below versus above median), sex, race, CHD, HF, DM, hypertension, and obesity at baseline. As a secondary outcome, non‐SCD was defined as CHD death not meeting SCD criteria. Associations between C‐IMTs and non‐SCD were examined. As C‐IMT was obtained differently in the ARIC study and CHS, statistical analyses were performed separately in the ARIC study and CHS.
The 95% CIs were constructed; P<0.05 was considered significant. All statistical analysis was performed using Stata 14.0 (StataCorp, LP, College Station, TX).
Results
Basic Characteristics of the Cohorts
In the ARIC study, among 15 307 Black and White participants at baseline, 569 participants had SCD during a median of 23.5 years of follow‐up (1.81 cases per 1000 person‐years). In CHS, among 5555 Black and White participants at baseline, 302 participants developed SCD during a median of 13.1 years of follow‐up (4.64 cases per 1000 person‐years). Baseline characteristics of the cohorts by incident SCD status are shown in Table 1. Those who developed SCD were more likely to be older, men, Black, smokers, and obese and more likely to have history of DM, CHD, and HF compared with those without SCD during follow‐up. They had lower high‐density lipoprotein, higher low‐density lipoprotein, and higher Cornell voltage.
Table 1.
Basic Characteristics of the Cohorts by Incident SCD Status
| Characteristic | ARIC Study | P value | CHS | P value | ||
|---|---|---|---|---|---|---|
| No SCD | SCD | No SCD | SCD | |||
| (n=14 738) | (n=569) | (n=5253) | (n=302) | |||
| Age, y | 54.1 (5.8) | 56.2 (5.6) | <0.001 | 72.8 (5.5) | 73.4 (5.7) | 0.06 |
| Men, n (%) | 6499 (44) | 360 (63) | <0.001 | 2181 (42) | 177 (59) | <0.001 |
| Black, n (%) | 3785 (26) | 234 (41) | <0.001 | 782 (15) | 57 (19) | 0.04 |
| Education, n (%) | ||||||
| Below high school | 3414 (23) | 218 (38) | 1502 (29) | 115 (38) | <0.001 | |
| High school | 6048 (41) | 197 (35) | <0.001 | 2657 (51) | 141 (47) | 0.10 |
| College or more | 5254 (36) | 153 (27) | 1094 (21) | 46 (15) | 0.10 | |
| Current drinking, n (%) | 8252 (56) | 273 (48) | <0.001 | 2589 (49) | 166 (55) | 0.03 |
| Current smoking, n (%) | 3783 (26) | 209 (37) | <0.001 | 616 (12) | 44 (15) | 0.08 |
| Diabetes mellitus, n (%) | 1357 (9) | 156 (28) | <0.001 | 797 (15) | 82 (27) | <0.001 |
| Hypertension, n (%) | 4979 (34) | 343 (61) | <0.001 | 3045 (58) | 201 (67) | 0.002 |
| Coronary heart disease, n (%) | 613 (4) | 133 (24) | <0.001 | 965 (18) | 106 (35) | <0.001 |
| Heart failure, n (%) | 663 (5) | 62 (11) | <0.001 | 220 (4) | 28 (9) | <0.001 |
| Systolic blood pressure, mm Hg | 120.9 (18.6) | 130.8 (22.5) | <0.001 | 136.1 (21.6) | 139.22 (22.7) | 0.01 |
| Diastolic blood pressure, mm Hg | 73.6 (11.19) | 76.7 (12.85) | <0.001 | 70.6 (11.3) | 71.2 (11.9) | 0.35 |
| Body mass index, kg/m2 | 27.6 (5.3) | 29.2 (6.0) | <0.001 | 26.6 (4.7) | 26.9 (4.2) | 0.35 |
| Cornell voltage, μV | 1216 (545) | 1488 (651) | <0.001 | 1388 (623) | 1556.8 (740) | <0.001 |
| LDL cholesterol, mg/dL | 137 (39) | 147 (39) | <0.001 | 130 (36) | 129 (37) | 0.59 |
| HDL cholesterol, mg/dL | 52 (17) | 46 (15) | <0.001 | 55 (16) | 50 (15) | <0.001 |
| IMT | ||||||
| Mean C‐IMT, mm | 0.74 (0.19) | 0.85 (0.23) | <0.001 | |||
| Maximum C‐IMT, mm | 0.99 (0.42) | 1.19 (0.55) | <0.001 | 1.43 (0.56) | 1.63 (0.65) | <0.001 |
| Mean CCA‐IMT, mm | 0.66 (0.15) | 0.74 (0.16) | <0.001 | |||
| Maximum CCA‐IMT, mm | 0.72 (0.18) | 0.81 (0.20) | <0.001 | 1.06 (0.21) | 1.12 (0.25) | <0.001 |
| Presence of plaque | 4768 (32) | 282 (50) | <0.001 | 2048 (39) | 154 (51) | <0.001 |
Data are presented as mean (SD) or number (percentage). ARIC indicates Atherosclerosis Risk in Communities; CCA‐IMT, common carotid artery IMT; CHS, Cardiovascular Health Study; C‐IMT, carotid IMT; HDL, high‐density lipoprotein; IMT, intima‐media thickness; LDL, low‐density lipoprotein; and SCD, sudden cardiac death.
C‐IMT and Risk of SCD
Figure 1 shows Kaplan‐Meier curves with the end point of SCD based on C‐IMT quartiles (Figure 1A in the ARIC study and Figure1B in CHS). Participants with thicker C‐IMT had a shorter time free from SCD compared with participants with thinner C‐IMT (P<0.001 for all C‐IMTs in the ARIC study and P<0.0001 in CHS).
Figure 1. Kaplan‐Meier curves with the end point of sudden cardiac death (SCD) based on carotid intima‐media thickness (C‐IMT) quartiles in the ARIC (Atherosclerosis Risk in Communities) study (A) and CHS (Cardiovascular Health Study) (B).
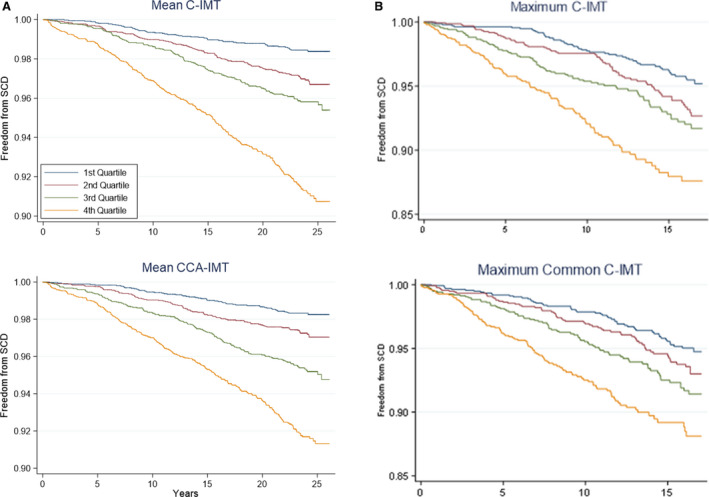
The x axis shows analysis time (years). P<0.001 for all C‐IMTs in A, and P<0.0001 in B. CCA‐IMT indicates common carotid artery intima‐media thickness.
Tables 2 and 3 show hazard ratios (HRs) of C‐IMT with SCD in the ARIC study and CHS. In the ARIC study (Table 2), the associations of C‐IMTs with SCD were significant and remained significant even after adjusting for other risk factors: C‐IMT was associated with risk of SCD after adjustment for traditional risk factors (HRs for fourth quartile versus first quartile [95% CIs]: 2.08 [1.48–2.93], 2.13 [1.51–3.00], 1.80 [1.29–2.51], and 2.03 [1.46–2.84] for mean C‐IMT, maximum C‐IMT, mean CCA‐IMT, and maximum CCA‐IMT, respectively). Presence of plaque was associated with risk of SCD (HR, 1.55; 95% CI, 1.28–1.86; P<0.001). When time‐dependent adjustors were added to model 2, these associations remained significant: HRs for fourth quartile versus first quartile (95% CIs): 1.64 (1.15–2.63), 1.71 (1.19–2.45), 1.49 (1.05–2.11), and 1.49 (1.05–2.11) for mean C‐IMT, maximum C‐IMT, mean CCA‐IMT, and maximum CCA‐IMT, respectively.
Table 2.
HRs and 95% CIs of SCD Based on C‐IMT Quartiles and Presence of Plaque in the ARIC Study
| Variable | First Quartile | Second Quartile | Third Quartile | Fourth Quartile | |
|---|---|---|---|---|---|
| Mean C‐IMT | Range, mm | 0.37–0.62 | 0.62–0.71 | 0.71–0.82 | 0.82–2.81 |
| Model 1 | Reference | 1.58 (1.13–2.21)* | 1.88 (1.36–2.60) † | 3.36 (2.46–4.59) † | |
| Model 2 | Reference | 1.39 (0.97–1.99) | 1.50 (1.06–2.13) ‡ | 2.08 (1.48–2.93) † | |
| Model 3 | Reference | 1.22 (0.84–1.77) | 1.16 (0.80–1.67) | 1.64 (1.15–2.63) ‡ | |
| Maximum C‐IMT | Range, mm | 0.44–0.76 | 0.76–0.87 | 0.87–1.07 | 1.07–5.70 |
| Model 1 | Reference | 1.51 (1.08–2.10) ‡ | 2.03 (1.48–2.80) † | 3.13 (2.28–4.28) † | |
| Model 2 | Reference | 1.41 (0.98–2.01) | 1.57 (1.11–2.23) ‡ | 2.13 (1.51–3.00) † | |
| Model 3 | Reference | 1.33 (0.92–1.94) | 1.35 (0.94–1.95) | 1.71 (1.19–2.45) § | |
| Mean CCA‐IMT | Range, mm | 0.30–0.57 | 0.57–0.64 | 0.64–0.74 | 0.74–2.57 |
| Model 1 | Reference | 1.41 (1.01–1.97) ‡ | 2.02 (1.48–2.76) † | 2.80 (2.06–3.80) † | |
| Model 2 | Reference | 1.23 (0.86–1.75) | 1.69 (1.21–2.35) § | 1.80 (1.29–2.51) † | |
| Model 3 | Reference | 1.13 (0.78–1.64) | 1.52 (1.07–2.15) ‡ | 1.49 (1.05–2.11) ‡ | |
| Maximum CCA‐IMT | Range, mm | 0.34–0.60 | 0.60–0.68 | 0.68–0.79 | 0.79–3.55 |
| Model 1 | Reference | 1.37 (0.97–1.92) | 2.02 (1.47–2.76) † | 3.00 (2.21–4.08) † | |
| Model 2 | Reference | 1.17 (0.81–1.69) | 1.62 (1.16–2.28)* | 2.03 (1.46–2.84) † | |
| Model 3 | Reference | 1.13 (0.78–1.64) | 1.52 (1.07–2.15) ‡ | 1.49 (1.05–2.11) ‡ | |
| Plaque | Absent | Present | |||
| Model 1 | Reference | 1.85 (1.56–2.19) † | |||
| Model 2 | Reference | 1.55 (1.28–1.86) † | |||
| Model 3 | Reference | 1.37 (1.13–1.67) § |
Model 1: adjusted for age, sex, and race‐by‐center terms.
Model 2: model 1 plus education, hypertension, diabetes mellitus, Cornell voltage, coronary heart disease, heart failure, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking.
Model 3: model 2 plus time‐varying adjustors from model 2.
ARIC indicates Atherosclerosis Risk in Communities; CCA‐IMT, common carotid artery intima‐media thickness; C‐IMT, carotid intima‐media thickness; HR, hazard ratio; and SCD, sudden cardiac death.
P<0.01.
P<0.001.
P<0.05.
P<0.005.
Table 3.
HRs and 95% CIs of SCD Based on C‐IMT Quartiles and Presence of Plaque in CHS
| Variable | First Quartile | Second Quartile | Third Quartile | Fourth Quartile | |
|---|---|---|---|---|---|
| Maximum C‐IMT | Range, mm | 0.57–0.99 | 0.99–1.31 | 1.31–1.77 | 1.77–4.26 |
| Model 1 | Reference | 1.33 (0.92–1.93) | 1.61 (1.12–2.31) ‡ | 2.51 (1.77–3.56) † | |
| Model 2 | Reference | 1.26 (0.87–1.83) | 1.39 (0.96–2.00) | 1.93 (1.35–2.77) † | |
| Model 3 | Reference | 1.20 (0.83–1.74) | 1.28 (0.89–1.85) | 1.75 (1.22–2.51) † | |
| Maximum CCA‐IMT | Range, mm | 0.61–0.92 | 0.92–1.03 | 1.03–1.16 | 1.16–2.59 |
| Model 1 | Reference | 1.16 (0.81–1.67) | 1.40 (0.98–1.99) | 1.93 (1.37–2.72) † | |
| Model 2 | Reference | 1.10 (0.76–1.58) | 1.23 (0.86–1.75) | 1.50 (1.06–2.14) ‡ | |
| Model 3 | Reference | 1.05 (0.73–1.51) | 1.15 (0.81–1.64) | 1.36 (0.95–1.93) | |
| Plaque | Absent | Present | |||
| Model 1 | Reference | 1.67 (1.32–2.11) † | |||
| Model 2 | Reference | 1.42 (1.12–1.80) § | |||
| Model 3 | Reference | 1.32 (1.04–1.68) ‡ |
Model 1: adjusted for age, sex, and race‐by‐center terms.
Model 2: model 1 plus education, hypertension, diabetes mellitus, Cornell voltage, coronary heart disease, heart failure, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking.
Model 3: model 2 plus time‐varying adjustors from model 2. CCA‐IMT indicates common carotid artery intima‐media thickness; CHS, Cardiovascular Health Study; C‐IMT, carotid intima‐media thickness; HR, hazard ratio; and SCD, sudden cardiac death.
P<0.01.
P<0.001.
P<0.05.
P<0.005.
In CHS (Table 3), higher C‐IMT was associated with risk of SCD after adjustment for traditional risk factors: HRs for fourth quartile (95% CIs) were 1.93 (1.35–2.77) and 1.50 (1.06–2.14) for maximum C‐IMT and CCA‐IMT, respectively. Presence of plaque was associated with risk of SCD (HR, 1.42; 95% CI, 1.12–1.80). Maximum internal C‐IMT and presence of plaque remained significant after further adjustment by incident CHD and HF as time‐dependent variables.
The associations of C‐IMTs with SCD were qualitatively consistent across all subgroups in the ARIC study and CHS (Figure 2 and Tables 4 and 5). There was interaction between age and C‐IMT in the ARIC study (Table 4). In participants without CHD or HF at baseline, higher C‐IMT values were associated with higher SCD risk compared with those with lower C‐IMT values.
Figure 2. Stratified analyses based on age (below vs above median), sex, race, coronary heart disease (CHD), heart failure (HF), diabetes mellitus (DM), hypertension, and obesity at baseline.
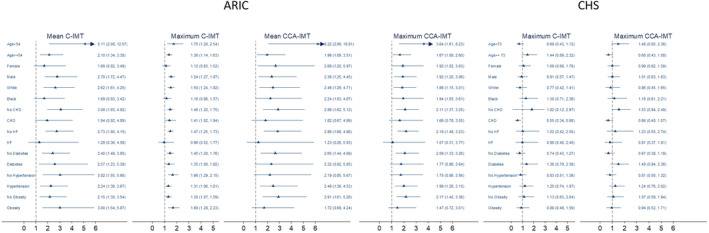
Adjusted for age, sex, race‐by‐center terms, education, hypertension, DM, Cornell voltage, CHD, heart failure, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking. ARIC indicates Atherosclerosis Risk in Communities; CCA‐IMT, common carotid artery intima‐media thickness; CHS, Cardiovascular Health Study; and C‐IMT, carotid intima‐media thickness.
Table 4.
Adjusted HRs of SCD by C‐IMTs (Above Versus Below Median) Across Demographic and Clinical Subgroups in the ARIC Study
| Variable | Mean C‐IMT | Maximum C‐IMT | Mean CCA‐IMT | Maximum CCA‐IMT |
|---|---|---|---|---|
| Age | 0.014 | 0.079 | 0.027 | 0.040 |
| Below median | 5.11 (2.08–12.57) | 1.75 (1.20–2.54) | 6.22 (2.06–18.81) | 3.64 (1.61–8.23) |
| Above median | 2.10 (1.34–3.30) | 1.36 (1.14–1.63) | 1.96 (1.09–3.51) | 1.67 (1.08–2.60) |
| Sex | 0.894 | 0.403 | 0.119 | 0.225 |
| Women | 1.69 (0.82–3.49) | 1.12 (0.83–1.52) | 2.68 (1.20–5.97) | 1.92 (1.02–3.63) |
| Men | 2.78 (1.72–4.47) | 1.54 (1.27–1.87) | 2.36 (1.25–4.45) | 1.92 (1.20–3.06) |
| Race | 0.382 | 0.319 | 0.703 | 0.998 |
| White | 2.62 (1.61–4.25) | 1.50 (1.24–1.82) | 2.46 (1.29–4.71) | 1.86 (1.15–3.01) |
| Black | 1.69 (0.83–3.42) | 1.16 (0.86–1.57) | 2.24 (1.03–4.87) | 1.94 (1.05–3.61) |
| CHD | 0.006 | 0.098 | 0.021 | 0.046 |
| No | 3.08 (1.93–4.92) | 1.46 (1.22–1.75) | 2.88 (1.62–5.12) | 2.11 (1.37–3.25) |
| Yes | 1.94 (0.92–4.09) | 1.41 (1.02–1.94) | 1.82 (0.67–4.99) | 1.66 (0.78–3.55) |
| HF | 0.021 | 0.027 | 0.056 | 0.047 |
| No | 2.73 (1.80–4.15) | 1.47 (1.25–1.73) | 2.86 (1.68–4.86) | 2.18 (1.48–3.23) |
| Yes | 1.28 (0.36–4.58) | 0.96 (0.52–1.77) | 1.23 (0.26–5.93) | 1.07 (0.31–3.77) |
| Diabetes mellitus | 0.075 | 0.083 | 0.080 | 0.082 |
| No | 2.40 (1.49–3.85) | 1.45 (1.20–1.76) | 2.65 (1.44–4.89) | 2.09 (1.33–3.26) |
| Yes | 2.57 (1.23–5.38) | 1.35 (1.00–1.82) | 2.32 (0.92–5.85) | 1.77 (0.86–3.64) |
| Hypertension | 0.024 | 0.012 | 0.265 | 0.358 |
| No | 3.02 (1.55–5.90) | 1.66 (1.29–2.15) | 2.19 (0.85–5.67) | 1.75 (0.86–3.56) |
| Yes | 2.24 (1.36–3.67) | 1.31 (1.06–1.61) | 2.48 (1.36–4.53) | 1.99 (1.26–3.15) |
| Obesity (BMI≥30 kg/m2) | 0.225 | 0.069 | 0.496 | 0.518 |
| No | 2.15 (1.30–3.54) | 1.30 (1.07–1.59) | 2.91 (1.61–5.28) | 2.17 (1.40–3.36) |
| Yes | 3.00 (1.54–5.87) | 1.69 (1.28–2.23) | 1.72 (0.69–4.24) | 1.47 (0.72–3.01) |
Age: median=54 years.
ARIC indicates Atherosclerosis Risk in Communities; BMI, body mass index; CCA‐IMT, common carotid artery intima‐media thickness; CHD, coronary heart disease; C‐IMT, carotid intima‐media thickness; HF, heart failure; HR, hazard ratio; and SCD, sudden cardiac death.
Table 5.
Adjusted HRs of SCD by C‐IMTs (Above Versus Below Median) Across Demographic and Clinical Subgroups in CHS
| Variable | Maximum C‐IMT | Maximum CCA‐IMT |
|---|---|---|
| Age | 0.294 | 0.217 |
| Below median | 1.70 (1.18–2.46) | 1.13 (0.80–1.60) |
| Above median | 1.31 (0.96–1.81) | 1.51 (1.09–2.10) |
| Sex | 0.709 | 0.966 |
| Women | 1.38 (1.00–1.90) | 1.30 (0.94–1.79) |
| Men | 1.51 (1.06–2.17) | 1.28 (0.90–1.83) |
| Race | 0.397 | 0.655 |
| White | 1.77 (1.03–3.05) | 1.46 (0.810–2.64) |
| Black | 1.37 (1.05–1.79) | 1.26 (0.96–1.64) |
| CHD | 0.016 | 0.088 |
| No | 0.96 (0.64–1.43) | 0.98 (0.66–1.45) |
| Yes | 1.75 (1.30–2.35) | 1.49 (1.11–2.02) |
| HF | 0.966 | 0.612 |
| No | 1.41 (0.59–3.36) | 1.07 (0.50–2.30) |
| Yes | 1.44 (1.11–1.85) | 1.31 (1.02–1.70) |
| Diabetes mellitus | 0.271 | 0.174 |
| No | 1.81 (1.12–2.94) | 1.74 (1.05–2.90) |
| Yes | 1.33 (1.01–1.76) | 1.17 (0.89–1.55) |
| Hypertension | 0.457 | 0.394 |
| No | 1.54 (1.14–2.08) | 1.39 (1.03–1.88) |
| Yes | 1.28 (0.86, 1.90) | 1.12 (0.76, 1.68) |
| Obesity (BMI ≥ 30 kg/m2) | 0.678 | 0.838 |
| No | 1.30 (0.77–2.20) | 1.22 (0.71–2.10) |
| Yes | 1.48 (1.12–1.94) | 1.30 (0.99–1.70) |
Age: median=72 years in CHS.
BMI indicates body mass index; CCA‐IMT, common carotid artery intima‐media thickness; CHD, coronary heart disease; CHS, Cardiovascular Health Study; C‐IMT, carotid intima‐media thickness; HF, heart failure; HR, hazard ratio; and SCD, sudden cardiac death.
The association between C‐IMT and non‐SCD was examined. C‐IMT was associated with non‐SCD. The associations remained significant after time‐dependent adjustors were added (Tables 6 and 7).
Table 6.
HRs and 95% CIs of Non‐SCD Based on C‐IMT Quartiles and Presence of Plaque in the ARIC Study
| Variable | First Quartile | Second Quartile | Third Quartile | Fourth Quartile | |
|---|---|---|---|---|---|
| Mean C‐IMT | Range, mm | 0.37–0.62 | 0.62–0.71 | 0.71–0.82 | 0.82–2.81 |
| Model 1 | Reference | 1.17 (0.78–1.76) | 1.69 (1.16–2.47)* | 3.03 (2.12–4.35) † | |
| Model 2 | Reference | 1.06 (0.68–1.66) | 1.19 (0.78–1.81) | 1.71 (1.14–2.55)* | |
| Model 3 | Reference | 1.00 (0.62–1.62) | 1.17 (0.75–1.83) | 1.58 (1.03–2.42) ‡ | |
| Maximum C‐IMT | Range, mm | 0.44–0.76 | 0.76–0.87 | 0.87–1.07 | 1.07–5.70 |
| Model 1 | Reference | 0.91 (0.61–1.34) | 1.42 (1.00–2.03) | 2.54 (1.81–3.57) † | |
| Model 2 | Reference | 0.82 (0.53–1.26) | 1.09 (0.79–1.61) | 1.53 (1.04–2.25) ‡ | |
| Model 3 | Reference | 0.90 (0.56–1.43) | 1.09 (0.71–1.67) | 1.52 (1.01–2.30) ‡ | |
| Mean CCA‐IMT | Range, mm | 0.30–0.57 | 0.57–0.64 | 0.64–0.74 | 0.74–2.57 |
| Model 1 | Reference | 1.28 (0.85–1.92) | 1.85 (1.27–2.69) § | 2.90 (2.01–4.17) † | |
| Model 2 | Reference | 1.11 (0.71–1.75) | 1.50 (0.98–2.29) | 1.88 (1.25–2.84) § | |
| Model 3 | Reference | 1.03 (0.63–1.70) | 1.47 (0.93–2.32) | 1.85 (1.19–2.89)* | |
| Maximum CCA‐IMT | Range, mm | 0.34–0.60 | 0.60–0.68 | 0.68–0.79 | 0.79–3.55 |
| Model 1 | Reference | 1.19 (0.78–1.79) | 1.92 (1.32–2.80) § | 2.95 (2.05–4.25) † | |
| Model 2 | Reference | 0.99 (0.63–1.56) | 1.44 (0.95–2.19) | 1.85 (1.23–2.78) § | |
| Model 3 | Reference | 0.93 (0.56–1.53) | 1.44 (0.92–2.26) | 1.85 (1.19–2.86)* | |
| Plaque | Absent | Present | |||
| Model 1 | Reference | 1.83 (1.50–2.22) † | |||
| Model 2 | Reference | 1.40 (1.13–1.74) § | |||
| Model 3 | Reference | 1.31 (1.04–1.65) ‡ |
Model 1: adjusted for age, sex, and race‐by‐center terms.
Model 2: model 1 plus education, hypertension, diabetes mellitus, Cornell voltage, coronary heart disease, heart failure, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking.
Model 3: model 2 plus time‐varying adjustors from model 2. ARIC indicates Atherosclerosis Risk in Communities; CCA‐IMT, common carotid artery intima‐media thickness; C‐IMT, carotid intima‐media thickness; HR, hazard ratio; and SCD, sudden cardiac death.
P<0.01.
P<0.001.
P<0.05.
P<0.005.
Table 7.
HRs and 95% CIs of Non‐SCD Based on C‐IMT Quartiles and Presence of Plaque in CHS
| Variable | First Quartile | Second Quartile | Third Quartile | Fourth Quartile | |
|---|---|---|---|---|---|
| Maximum C‐IMT | Range, mm | 0.57–0.99 | 0.99–1.31 | 1.31–1.77 | 1.77–4.26 |
| Model 1 | Reference | 1.07 (0.81–1.41) | 1.54 (1.20–1.99) § | 2.80 (2.20–3.56) † | |
| Model 2 | Reference | 1.04 (0.79–1.37) | 1.38 (1.06–1.78) ‡ | 2.12 (1.65–2.71) † | |
| Model 3 | Reference | 0.94 (0.71–1.24) | 1.18 (0.91–1.53) | 1.68 (1.31–2.15) † | |
| Maximum CCA‐IMT | Range, mm | 0.61–0.92 | 0.92–1.03 | 1.03–1.16 | 1.16–2.59 |
| Model 1 | Reference | 1.37 (1.04–1.80) ‡ | 1.81 (1.39–2.35) † | 2.59 (2.00–3.35) † | |
| Model 2 | Reference | 1.31 (0.99–1.72) | 1.58 (1.21–2.05)§ | 2.04 (1.57–2.64) † | |
| Model 3 | Reference | 1.16 (0.88–1.52) | 1.35 (1.04–1.76) ‡ | 1.61 (1.24–2.09) † | |
| Plaque | Absent | Present | |||
| Model 1 | Reference | 2.04 (1.72–2.42) | |||
| Model 2 | Reference | 1.69 (1.42–2.01) † | |||
| Model 3 | Reference | 1.47 (1.24–1.76) † |
Model 1: adjusted for age, sex, and race‐by‐center terms.
Model 2: model 1 plus education, hypertension, diabetes mellitus, Cornell voltage, coronary heart disease, heart failure, body mass index, high‐density lipoprotein and low‐density lipoprotein cholesterols, current drinking, and current smoking.
Model 3: model 2 plus time‐varying adjustors from model 2.
CCA‐IMT indicates common carotid artery intima‐media thickness; CHS, Cardiovascular Health Study; C‐IMT, carotid intima‐media thickness; HR, hazard ratio; and SCD, sudden cardiac death.
P<0.01.
P<0.001.
P<0.05.
P<0.005.
Discussion
In 2 biracial community‐based cohorts, one middle‐aged and the other elderly, subclinical atherosclerosis, measured by C‐IMT, was associated with risk of SCD over a median follow‐up of >13 years. The association remained significant after adjustment by traditional risk factors.
Previous studies have investigated and described associations between C‐IMT and CHD and all‐cause mortality. 5 , 21 C‐IMT is a known surrogate marker for atherosclerosis and has been shown to be associated with CHD 4 , 5 , 6 , 7 and stroke 14 , 22 in several epidemiological studies. On the other hand, for SCD as an outcome, many risk factors for SCD have been identified. It is known that SCD and CHD share many of the risk factors. 9 However, no previous study has prospectively looked at the association between C‐IMT and SCD. This is the first study to show that C‐IIMT is associated with risk of SCD.
There are several potential mechanisms linking subclinical atherosclerosis to SCD. As CHD remains the dominant cause of SCD in the United States and ≈50% of all deaths associated with heart disease are sudden, 23 it would be likely that atherosclerotic process is associated with SCD. In an autopsy study, >80% of the subjects with SCD had previously undiagnosed but anatomically severe CHD. 10 Subclinical atherosclerosis might cause ischemia, atherosclerotic plaque disruption, and thrombosis, leading to SCD. On the other hand, in absence of plaque rupture or thrombosis, the chronic subclinical atherosclerotic process might remodel left ventricular myocardium, leading to cardiomyopathy 24 , 25 through inflammation and sympathetic nervous surge. Cardiomyopathy has been a known and important risk factor of SCD. Inflammation has been shown to be associated with SCD. 26 , 27 Inflammatory status attributable to subclinical atherosclerosis might trigger ventricular arrhythmias, and then SCD. 28 Good cardiorespiratory fitness is associated with slow progression of carotid atherosclerosis. 29 Baseline cardiorespiratory fitness is associated with risk of SCD in a general population of middle‐aged men in Finland. 30 , 31 Although the link between subclinical atherosclerosis and SCD appears to be most likely mediated by atherosclerotic process, the association will be multifactorial.
Our results have potential public health and clinical implications. As subclinical atherosclerosis seems to play a role in SCD, there may be public health benefit in primary prevention of SCD at a community level: by detecting the atherosclerotic process earlier and offering primary prevention measures, such as healthy lifestyle and risk factor modification, in a community, these primary prevention measures might be able to decrease incidence of SCD. Clinically, detecting subclinical atherosclerosis by C‐IMT could potentially help healthcare professionals provide patients with patient‐oriented care. As C‐IMT is shown to be associated with SCD, incorporating C‐IMT into SCD risk stratification scheme could also be considered. 1
The strengths of the study include its prospective population‐based design, biracial population, large sample size with a long follow‐up, and large number of incident SCD cases. Limitations of the study merit consideration. First, our definition of SCD was based on adjudicated fatal CHD. Other causes of SCD, such as inherited rhythm disorders, might not have been detected by our SCD definition. Second, we used single measurements of C‐IMT at baseline. There might have been serial changes in C‐IMT values over time. However, our focus was to see if these subclinical atherosclerosis measures were associated with incident SCD and not on serial changes of these variables over time. Last, there remains a possibility of residual confounding, although we adjusted for variables that were known to be associated with SCD.
In conclusion, subclinical atherosclerosis, measured by C‐IMT, was associated with risk of SCD in 2 biracial community‐based cohorts. These results may suggest importance of subclinical atherosclerosis in SCD risk and lead to early therapeutic interventions to prevent SCD in the future.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) study is performed as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The CHS (Cardiovascular Health Study) was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study and the CHS (Cardiovascular Health Study) for their important contributions.
(J Am Heart Assoc. 2020;9:e016981 DOI: 10.1161/JAHA.120.016981.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM Jr, Chen PS, Chugh SS, Costantini O, Exner DV, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–526. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 4. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. [DOI] [PubMed] [Google Scholar]
- 5. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr; Cardiovascular Health Study collaborative research group . Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 6. Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima‐media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the Aric (atherosclerosis risk in communities) study. J Am Coll Cardiol. 2010;55:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, Ballantyne CM. Common carotid artery intima‐media thickness is as good as carotid intima‐media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the atherosclerosis risk in communities (ARIC) study. Eur Heart J. 2012;33:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres J, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics‐2015 update: a report from the American Heart Association. Circulation. 2015131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 9. Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, Limacher MC, Johnson KC, Curb JD, Wassertheil‐Smoller S, et al. Risk factors for sudden cardiac death in post‐menopausal women. J Am Coll Cardiol. 2012;60:2674–2682. [DOI] [PubMed] [Google Scholar]
- 10. Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The ARIC investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 13. Li R, Duncan BB, Metcalf PA, Crouse JR 3rd, Sharrett AR, Tyroler HA, Barnes R, Heiss G; Atherosclerosis Risk in Communities (ARIC) study investigators . B‐mode‐detected carotid artery plaque in a general population. Stroke. 1994;25:2377–2383. [DOI] [PubMed] [Google Scholar]
- 14. Folsom AR, Yatsuya H, Psaty BM, Shahar E, Longstreth WT Jr. Carotid intima‐media thickness, electrocardiographic left ventricular hypertrophy, and incidence of intracerebral hemorrhage. Stroke. 2011;42:3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, Lemaitre RN, Rea TD, Durda JP, Chang JM, et al. Beta2‐adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006;113:1842–1848. [DOI] [PubMed] [Google Scholar]
- 16. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 17. Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, Savage PJ, Carlson M. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the cardiovascular health study. J Am Coll Cardiol. 2004;43:2236–2241. [DOI] [PubMed] [Google Scholar]
- 18. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 19. Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann Epidemiol. 1995;5:270–277. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki T, Katz R, Jenny NS, Zakai NA, LeWinter MM, Barzilay JI, Cushman M. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2008;1:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murakami S, Otsuka K, Hotta N, Yamanaka G, Kubo Y, Matsuoka O, Yamanaka T, Shinagawa M, Nunoda S, Nishimura Y, et al. Common carotid intima‐media thickness is predictive of all‐cause and cardiovascular mortality in elderly community‐dwelling people: Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005;1:S49–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V. Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the atherosclerosis risk in communities (ARIC) study. Stroke. 2012;43:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitrani RD, Myerburg RJ. Ten advances defining sudden cardiac death. Trends Cardiovasc Med. 2016;26:23–33. [DOI] [PubMed] [Google Scholar]
- 24. Badran HM, Mostafa A, Serage A, Fareed W, Abdelfatah E, Fathe A. Arterial mechanics in ischemic versus nonischemic cardiomyopathy: clinical and diagnostic impact. Echocardiography. 2009;26:785–800. [DOI] [PubMed] [Google Scholar]
- 25. Androulakis AE, Andrikopoulos GK, Richter DJ, Tentolouris CA, Avgeropoulou CC, Adamopoulos DA, Toutouzas PK, Trikas AG, Stefanadis CI, Gialafos JE. The role of carotid atherosclerosis in the distinction between ischaemic and non‐ischaemic cardiomyopathy. Eur Heart J. 2000;21:919–926. [DOI] [PubMed] [Google Scholar]
- 26. Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of c‐reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. [DOI] [PubMed] [Google Scholar]
- 27. Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, DeFilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd‐Jones D. Inflammation and sudden cardiac death in a community‐based population of older adults: the cardiovascular health study. Heart Rhythm. 2013;10:1425–1432. [DOI] [PubMed] [Google Scholar]
- 28. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. [DOI] [PubMed] [Google Scholar]
- 29. Lakka TA, Laukkanen JA, Rauramaa R, Salonen R, Lakka HM, Kaplan GA, Salonen JT. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle‐aged men. Ann Intern Med. 2001;134:12–20. [DOI] [PubMed] [Google Scholar]
- 30. Hagnas MJ, Lakka TA, Kurl S, Rauramaa R, Makikallio TH, Savonen K, Laukkanen JA. Cardiorespiratory fitness and exercise‐induced st segment depression in assessing the risk of sudden cardiac death in men. Heart. 2017;103:383–389. [DOI] [PubMed] [Google Scholar]
- 31. Laukkanen JA, Makikallio TH, Rauramaa R, Kiviniemi V, Ronkainen K, Kurl S. Cardiorespiratory fitness is related to the risk of sudden cardiac death: a population‐based follow‐up study. J Am Coll Cardiol. 2010;56:1476–1483. [DOI] [PubMed] [Google Scholar]


