Abstract
Background
Elevated levels of serum homocysteine, via impaired nitric oxide production, and coronary microvascular dysfunction are associated with increased risk of major adverse cardiovascular events. However, whether serum homocysteine levels and coronary microvascular endothelial dysfunction (CMED) are linked remains unknown.
Methods and Results
This study included 1418 patients with chest pain or an abnormal functional stress test and with nonobstructive coronary artery disease (<40% angiographic stenosis), who underwent CMED evaluation with functional angiography and had serum homocysteine levels measured. Patients were classified as having normal microvascular function versus CMED. Patients in the CMED group (n=743; 52%) had higher mean age (52.1±12.2 versus 50.0±12.4 years; P<0.0001), higher body mass index (29.1 [25.0–32.8] versus 27.5 [24.2–32.4]; P=0.001), diabetes mellitus (12.5% versus 9.4%; P=0.03), and fewer women (63.5% versus 68.7%; P=0.04) compared with patients in the normal microvascular function group. However, they had lower rates of smoking history, and mildly lower low‐density lipoprotein cholesterol levels. Serum homocysteine levels were significantly higher in patients with CMED, and the highest quartile of serum homocysteine level (>9 µmol/L) was an independent predictor of CMED (odds ratio, 1.34 [95% CI, 1.03–1.75]; P=0.03) after adjustment for age; sex; body mass index; chronic kidney disease (CKD); diabetes mellitus; smoking exposure; low‐density lipoprotein cholesterol; high‐density lipoprotein cholesterol and triglycerides; and aspirin, statin, and B vitamin use.
Conclusions
Patients with CMED have significantly higher levels of serum homocysteine. Elevated serum homocysteine levels were associated with a significantly increased odds of an invasive diagnosis of CMED. The current study supports a potential role for homocysteine for diagnosis and target treatment in the patients with early coronary atherosclerosis.
Keywords: endothelial dysfunction, homocysteine, microvascular
Subject Categories: Endothelium/Vascular Type/Nitric Oxide
Nonstandard Abbreviations and Acronyms
- CBF
coronary blood flow
- CMED
coronary microvascular endothelial dysfunction
Clinical Perspective
What Is New?
Coronary endothelial microvascular dysfunction, a marker of early atherosclerosis, is associated with elevated levels of serum homocysteine.
High‐normal levels of homocysteine were associated with coronary endothelial microvascular dysfunction, as diagnosed invasively by acetylcholine provocative testing, even after adjusting for cardiovascular risk covariables.
In subpopulation analysis, there was no sex difference for this relationship; this association was also augmented in patients with elevated levels of serum homocysteine and taking B‐vitamin supplementation.
What Are the Clinical Implications?
The established link between serum homocysteine and adverse cardiovascular effects might be mediated through coronary microvascular endothelial dysfunction.
Further investigations are needed to establish a causal link and to assess if baseline serum homocysteine levels might help in risk stratification of patients at risk for future major adverse cardiovascular events.
Coronary endothelial dysfunction is the earliest clinically detectable form of coronary atherosclerosis. Sixty percent of patients presenting with angina and nonobstructive coronary artery disease (CAD) at clinically indicated coronary angiography have coronary endothelial dysfunction detected with pharmacologic provocation testing. 1 , 2 , 3 Coronary microvascular endothelial dysfunction (CMED) has been associated with increased mortality and a higher risk of major adverse cardiovascular events, including myocardial infarction, progressive congestive heart failure, and sudden cardiac death. 4 , 5 , 6 , 7 , 8 , 9 , 10 CMED was also shown to be associated with vulnerable plaque characteristics in epicardial vessel using optical coherence tomography 11 , 12 and virtual‐histology intravascular ultrasound studies. 13 Coronary endothelial dysfunction is a systemic disease 14 , 15 , 16 and is usually associated with elevated levels of systemic inflammatory markers like plasma‐soluble urokinase‐type plasminogen activator receptor, uric acid, and high‐sensitivity C‐reactive protein levels. 17 , 18 , 19
Elevated serum levels of homocysteine, which could be caused by an inherited deficiency of cystathionine synthase or other enzymes, have been linked to premature arteriosclerosis and increased risk of cardiovascular diseases such as CAD, peripheral arterial disease, stroke, venous thrombosis, and gestational hypertension. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Furthermore, in a study of patients with stable CAD, hyperhomocysteinemia was also found to be an independent predictor of cardiac death. 29
Several in vitro studies displayed a direct adverse effect of homocysteine on endothelial function, mainly by reducing nitric oxide activity and increasing local oxidative stress, leading to endothelial dysfunction. 30
We therefore hypothesize that elevated serum homocysteine levels could be associated with CMED. The aim of this study is to investigate the association between increased levels of serum homocysteine and CMED in patients with angina and nonobstructive coronary artery disease at angiography.
Methods
A total of 1991 consecutive patients with chest pain referred for clinically indicated coronary angiography and functional assessment, between 1992 and 2019, who were found to have angiographically normal coronary arteries or mild CAD (<40%) were enrolled in the Mayo Clinic Endothelial Database. Of these, 1418 patients with serum homocysteine levels taken up to 2 weeks before the index coronary angiogram were included in this study. Patients with acute coronary syndrome, myocardial infarction, or cerebrovascular accident within the preceding 6 months, use of radiographic contrast agents within 12 hours of the catheterization, and advanced chronic kidney disease (CKD) (glomerular filtration rate <30 mL/min per m2) were excluded. All patients fasted for at least 8 hours and withheld all prescription medications that could affect coronary vasoreactivity for at least 48 hours before the study procedure (calcium channel blockers, beta blockers, nitrates). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board. All patients provided written informed consent for participation in the current protocol. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Functional Angiography
The study protocol has been previously described in detail. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 In brief, patients underwent diagnostic coronary angiography using standard clinical protocols. Those with no CAD or nonobstructive CAD (angiographic stenosis <40%) went on to receive 5000 intravenous units of heparin, after which a Doppler guidewire (FloWire, Philips/Volcano Corp., San Diego, CA) was positioned in the mid–left anterior descending coronary artery. Intracoronary acetylcholine was infused at incremental doses for 3 minutes each with increasing concentrations of 10−6, 10−5, and 10−4 mol/L to assess endothelial function. Doppler measurements of mean peak velocity were performed after each acetylcholine infusion followed by repeat coronary angiography. Mid–left anterior descending coronary artery diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire using a quantitative coronary angiography program (Medis Corp, Leiden, The Netherlands). Coronary blood flow (CBF) was then calculated using the following formula, as previously described: 32 , 33 , 39 , 40 , 41 CBF=π×(mean peak velocity/2)×(coronary artery diameter/2)2. The maximal percent change in CBF in response to acetylcholine compared with the CBF at baseline (%ΔCBF) was then calculated, and CMED was defined as %ΔCBF <50%. This definition was predefined before analysis and is standardized as described in previous studies. 3
Clinical Assessment
Clinical history, laboratory data, and current medications were collected from a detailed chart review by an investigator blinded to functional angiography results. Data were collected on conventional cardiovascular risk factors including age, hypertension, diabetes mellitus, hyperlipidemia, smoking status, and body mass index; biochemical parameters including serum total cholesterol, low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein cholesterol, triglycerides, creatinine, and glycosylated hemoglobin. Smoking was defined as positive for exposure (current or former) or never. CKD was defined as estimated glomerular filtration rate <60 mL/min per 1.73m2. All blood levels documented had been drawn within 6 weeks of the index procedure. Dyslipidemia was defined by a documented history of hyperlipidemia, treatment with lipid‐lowering therapy, an LDL cholesterol level above the target (<130 mg/dL for low‐risk patients, <100 mg/dL for moderate‐high‐risk patients, <70 mg/dL for very‐high‐risk patients, and <55 mg/dL for extreme‐high‐risk patients on the basis of 10‐year atherosclerotic cardiovascular disease risk), high‐density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women, or triglycerides >150 mg/dL. Type 2 diabetes mellitus was defined as a documented history of or treatment for type 2 diabetes mellitus, or a glycosylated hemoglobin of >6.5%, if available. Hypertension was defined as a documented history of or treatment of the condition, a systolic blood pressure measurement of >130 mm Hg, or diastolic blood pressure measurement >90 mm Hg. 42
Statistical Analysis
Continuous variables distributed normally were expressed as the mean±standard deviation, and those with a skewed distribution were expressed as the median with interquartile range (median [Q25–Q75]). Categorical variables were expressed as frequency (percentage). For between‐group comparisons, unpaired t test was used for normally distributed continuous variables, Mann–Whitney U test for nonnormally distributed variables, and χ2 test (or Fisher’s exact test) for categorical variables. Univariable logistic regression analyses were performed to study the association between CMED and serum homocysteine levels. Multivariable logistic regression analyses were performed to estimate the independent association between serum homocysteine levels in the highest quartile and CMED. The covariables included were those that showed a trend of difference (P<0.25) in baseline characteristics between the CMED and non‐CMED group. We also decided a priori to include CKD 43 , 44 and B‐vitamin (B6, B9, and B12) 45 and statin use 28 , 46 in all adjusted models since they were previously shown to affect serum homocysteine levels and endothelial function, thus acting as potential confounders. We also examined the frequency of B‐vitamin consumption and statin use in our population and sought to compare the association of serum homocysteine and CMED in patients on these supplements/medications versus those who were not. For all tests, a P<0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro software (SAS Institute, Inc., Cary, NC).
Results
Baseline Characteristics
Baseline patients’ characteristics, categorized as CMED versus normal microvascular endothelial function, are summarized in Table 1. Of 1418 patients, 743 (52%) had CMED (%ΔCBF, −4 [−31 to −22]), while 675 had normal coronary microvascular function (%ΔCBF, 110 [76–161]) (P<0.0001). The distribution of homocysteine in our population was skewed with all but 6 patients having levels <30 μmol/L. Patients with CMED were more likely to have cardiovascular risk factors such as age, male sex, obesity, or diabetes mellitus. However, they had lower rates of smoking history, and mildly lower LDL cholesterol levels. Serum homocysteine levels were also higher in the CMED group as compared with the normal microvascular function group (8 [6–10] versus 8 [6–9]; P=0.006) (Figure 1).
Table 1.
Baseline Characteristics of Patients With Normal Microvascular Function Versus CMED as Measured by Percent Change or Coronary Blood Flow With Successive Intracoronary Acetylcholine Infusion
| Coronary Microvascular Endothelial Function | P Value | ||
|---|---|---|---|
|
Normal N=675 |
Abnormal N=743 |
||
| Clinical parameters | |||
| Age, y | 50±12.4 | 52.1±12.2 | 0.0003 |
| Female sex, n (%) | 464 (68.7) | 472 (63.5) | 0.04 |
| Body mass index, kg/m2 | 27.5 (24.2; 32.4) | 29.1 (25.0; 32.8) | 0.001 |
| Hypertension, n (%) | 545 (80.5) | 606 (81.6) | 0.69 |
| Systolic BP, mm Hg | 124±17 | 124±17 | 0.56 |
| Diastolic BP, mm Hg | 75±10 | 75±10 | 0.88 |
| Diabetes mellitus, n (%) | 60 (8.9) | 98 (13.2) | 0.01 |
| HbA1c (%) | 5.3 (5.0 to 5.6) | 5.4 (5.1 to 5.7) | 0.01 |
| Dyslipidemia, n (%) | 392 (48.0) | 437 (58.8) | 0.77 |
| LDL cholesterol, mg/dL | 104±37 | 100±35 | 0.02 |
| HDL cholesterol, mg/dL | 55±18 | 53±17 | 0.08 |
| Triglycerides, mg/dL | 102 [70; 153] | 107 [76; 158] | 0.14 |
| Creatinine, mg/dL | 0.94±0.28 | 0.97±0.26 | 0.07 |
| CKD, n (%) | 74 (11.0) | 95 (12.8) | 0.3 |
| Smoking history, n (%) | 332 (49.3) | 323 (43.5) | 0.03 |
| ΔCBF (%) | 110 (76; 161) | −4 (−31 to 22) | <0.0001 |
| Homocysteine, μmol/L | 8 (6 to 9) | 8 (6 to 10) | 0.006 |
| Medications | |||
| Aspirin, n (%) | 344 (51.0) | 411 (55.3) | 0.1 |
| Statin, n (%) | 253 (37.5) | 336 (45.2) | 0.003 |
| Antihypertensives, n (%) | 431 (63.9) | 494 (66.5) | 0.3 |
| Antidiabetics, n (%) | 36 (5.3) | 69 (9.3) | 0.01 |
| Diuretics, n (%) | 108 (16.0) | 129 (17.4) | 0.5 |
| B‐vitamin use, n (%) | 160 (23.7) | 184 (24.8) | 0.64 |
BP indicates blood pressure; CKD, chronic kidney disease; CMED, coronary endothelial microvascular dysfunction; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and ΔCBF, blood flow change.
Figure 1. Difference in homocysteine levels between patients with normal coronary microvascular endothelial function and patients with CMED.
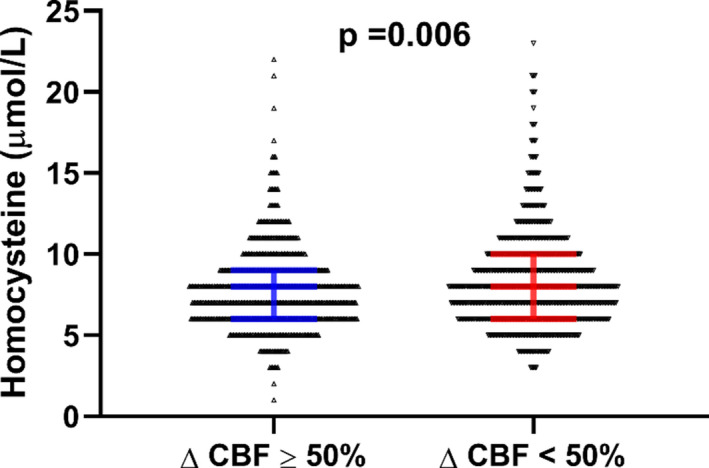
CBF indicates coronary blood flow; and CMED, coronary microvascular endothelial dysfunction.
Impact of Serum Homocysteine Levels on Coronary Microvascular Endothelial Function
There was a significant association between quartiles (first quartile ≤6; second quartile >6 to ≤8; third quartile >8 to ≤9; fourth quartile >9) of serum homocysteine levels and CMED (P=0.02) is shown in Figure 2. Patients in the highest quartile of serum homocysteine (>9 μmol/L) had a significantly higher prevalence of CMED as compared with those in the lowest quartile (<6 μmol/L), with an unadjusted odds ratio (OR) of 1.5 [1.2–2.1; P=0.0003). There were no significant differences in the frequency of CMED between the first, second, and third quartiles. Therefore, the first 3 quartiles were grouped together and were compared with the group with the highest quartile of serum homocysteine levels.
Figure 2. Association of homocysteine levels (by quartiles) and impaired blood flow reaction to acetylcholine infusion.
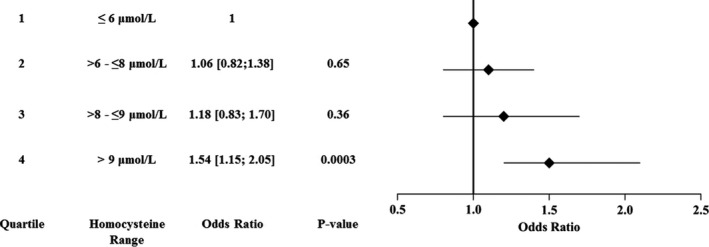
Differences between patients in the highest‐quartile group versus the lower‐3‐quartiles group are outlined in Table 2. Patients in the highest‐quartile group tended to be older, included more males, and had more hypertension, CKD, and smoking exposure. Furthermore, they had higher glycosylated hemoglobin levels and were on antidiabetics (insulin or oral hypoglycemics) more than those in other quartiles. However, they took fewer B vitamins and more diuretics than the group with serum homocysteine levels ≤9. Univariable logistic regression analysis (Table 3) showed that homocysteine levels in the highest quartile were significantly associated with CMED (OR, 1.46 [1.14–1.87]; P=0.003). In multivariable logistic regression analysis, elevated serum homocysteine levels remained a significant predictor of CMED (OR, 1.34 [1.03–1.75]; P=0.031) after adjusting for age, sex, body mass index, CKD, diabetes mellitus, smoking exposure, aspirin use, B‐vitamin use, statin use, LDL cholesterol, high‐density lipoprotein cholesterol, and triglycerides. Subgroup univariable regression analysis based on sex revealed that elevated serum homocysteine levels are a significant predictor of CMED in males (OR, 1.50 [1.02–2.20]; P=0.04) and borderline significant in females (OR, 1.36 [0.98–1.89]; P=0.06; P interaction=0.71). In multivariable regression analysis, elevated levels of serum homocysteine was predictive of CMED in men (OR 1.55 [1.01–2.39]; P=0.047) but not in women (OR, 1.21 [0.85–1.71]; P=0.29; P interaction=0.49) (Table 3). However, the P interaction is nonsignificant for both the unadjusted and adjusted models, which means that there is no difference in the association of homocysteine and CMED between the men and women.
Table 2.
Baseline Characteristics of Patients With Serum Homocysteine Level >9 μmol/L vs Those ≤9 μmol/L
| Homocysteine (μmol/L) | P Value | ||
|---|---|---|---|
|
≤9 N=1075 |
>9 N=343 |
||
| Clinical parameters | |||
| Age, y | 49.9±12.2 | 54.8±11.8 | <0.0001 |
| Female sex, n (%) | 757 (70.2) | 182 (52.9) | <0.0001 |
| Body mass index, kg/m2 | 27.9 (24.3 to 32.6) | 29.1 (25.3 to 32.9) | 0.02 |
| Hypertension, n (%) | 860 (80.0) | 291 (84.8) | 0.046 |
| Systolic BP, mm Hg | 123±17 | 127±17 | 0.0006 |
| Diastolic BP, mm Hg | 75±10 | 76±10 | 0.051 |
| Diabetes mellitus, n (%) | 110 (10.2) | 48 (14.0) | 0.054 |
| HbA1c (%) | 5.3 [5.0; 5.6] | 5.4 [5.2; 5.7] | <0.0001 |
| Dyslipidemia, n (%) | 621 (57.6) | 210 (61.1) | 0.26 |
| LDL cholesterol, mg/dL | 102±36 | 102±37 | 0.9 |
| HDL cholesterol, mg/dL | 55±16 | 53±18 | 0.08 |
| Triglycerides, mg/dL | 104 (68 to 154) | 109 (82 to 163) | 0.002 |
| Creatinine, mg/dL | 0.92±0.24 | 1.04±0.32 | <0.0001 |
| CKD, n (%) | 102 (9.5) | 67 (19.6) | <0.0001 |
| Smoking history, n (%) | 482 (44.8) | 176 (51.3) | 0.03 |
| ΔCBF (%) | 49 [−5 to 108) | 33 (−7 to 99) | 0.1 |
| Homocysteine, μmol/L | 7 (6 to 8) | 11 (10 to 13) | <0.0001 |
| Medications | |||
| Aspirin, n (%) | 560 (52.0) | 196 (57.0) | 0.1 |
| Statin, n (%) | 434 (40.3) | 157 (45.7) | 0.08 |
| Antihypertensive, n (%) | 683 (63.4) | 244 (70.9) | 0.01 |
| Antidiabetic, n (%) | 71 (6.6) | 34 (9.9) | 0.04 |
| Diuretics, n (%) | 148 (13.7) | 92 (26.7) | <0.0001 |
| B‐vitamin use, n (%) | 290 (26.9) | 56 (16.3) | <0.0001 |
BP indicates blood pressure; CKD, chronic kidney disease; HbA1c, glycosylated hemoglobin; HDL, high density lipoprotein; LDL, low‐density lipoprotein; and ΔCBF, blood flow change.
Table 3.
Association of Elevated Homocysteine Levels (>9) With Impaired CBF Reaction to Successive Acetylcholine Infusion
| All Patients | Males | Females | P interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | CI (95%) | P Value | ||
| Unadjusted model | ||||||||||
| Homocysteine >9 μmol/L | 1.46 | (1.14–1.87) | 0.003 | 1.50 | (1.02–2.20) | 0.04 | 1.36 | [0.98; 1.89] | 0.06 | 0.71 |
| Adjusted model | ||||||||||
| Homocysteine >9 μmol/L | 1.34 | (1.03–1.75) | 0.03 | 1.55 | (1.01–2.39) | 0.047 | 1.21 | [0.85; 1.71] | 0.29 | 0.49 |
Model adjusted for age, sex, body mass index, chronic kidney disease, diabetes mellitus, smoking exposure, aspirin use, statin use, B‐vitamin use, LDL cholesterol, HDL cholesterol, and triglycerides. CBF indicates coronary blood flow; HDL, high density lipoprotein; LDL, low‐density lipoprotein; and OR, odds ratio.
Stratification by Medication/Supplementation Use
Several medications, such as diuretics, statins, and B vitamins, affect the levels of homocysteine and thus the same relationship between serum homocysteine and CMED was investigated after stratification of medication use. The difference in baseline characteristics between patients taking B‐vitamin supplementation and those who were not are summarized in Table S1. Patients on B‐vitamin supplementation were generally older, included more women, and had more dyslipidemia. Patients on B‐vitamin supplementation had significantly lower levels of homocysteine (7 [6–9] versus 8 [6–10] μmol/L; P<0.0001) than those not on a supplementation. As shown in Table 4, in univariable logistic regression analysis, patients with serum levels of homocysteine in the highest quartile who were consuming B vitamins were more likely to have CMED (OR, 3.35 [1.72–6.50]; P=0.0004) (P interaction=0.005). After adjusting for age, sex, body mass index, CKD, diabetes mellitus, smoking exposure, aspirin use, statin use, LDL cholesterol, high‐density lipoprotein cholesterol, and triglycerides, the association remained significant (OR, 2.72 [1.35–5.47], P=0.005; P interaction=0.027). As for statin use, univariable and multivariable analyses in Table 4 showed that the association between serum homocysteine levels in the highest quartile and CMED was only present in patients not on any statin therapy at the time of testing. However, this difference is not significant as the P interaction was not significant between the 2 groups. The association between elevated serum homocysteine levels and CMED was not different in patients on diuretics versus those who are not, in both unadjusted (OR, 1.86 [1.09–3.18]; P=0.02 versus OR, 1.36 [1.02–1.80], P=0.03; P interaction=0.31) or adjusted models (OR, 1.89 [1.03–3.47]; P=0.04 versus OR, 1.27 [0.94–1.56]; P=0.13; P interaction=0.44).
Table 4.
Association of Elevated Homocysteine Levels (>9) With Impaired CBF Reaction Stratified by Taking Certain Relevant Medications (B Vitamins, Statins, or Diuretics)
| On Medication/Supplement | Not on Medication/Supplement | P interaction | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Statins | |||||||
| Unadjusted model | 1.31 | (0.90–1.90) | 0.16 | 1.55 | (1.11–2.15) | 0.01 | 0.50 |
| Adjusted model* | 1.17 | (0.79–1.74) | 0.44 | 1.52 | (1.05–2.18) | 0.03 | 0.31 |
| B vitamins | |||||||
| Unadjusted model | 3.35 | (1.72–6.50) | 0.0004 | 1.26 | (0.96–1.65) | 0.1 | 0.005 |
| Adjusted model† | 2.72 | (1.35–5.47) | 0.005 | 1.20 | (0.89–1.61) | 0.22 | 0.027 |
| Diuretics | |||||||
| Unadjusted model | 1.86 | (1.09–3.18) | 0.02 | 1.36 | (1.02–1.80) | 0.03 | 0.31 |
| Adjusted model‡ | 1.89 | (1.03–3.47) | 0.04 | 1.27 | (0.94–1.56) | 0.13 | 0.44 |
CBF indicates coronary blood flow; HDL, high density lipoprotein; LDL, low‐density lipoprotein; and OR, odds ratio.
Model adjusted for age, sex, body mass index, chronic kidney disease, diabetes mellitus, smoking exposure, aspirin use, B‐vitamin use, LDL cholesterol, HDL cholesterol, and triglycerides.
Model adjusted for age, sex, body mass index, chronic kidney disease, diabetes mellitus, smoking exposure, aspirin use, statin use, LDL cholesterol, HDL cholesterol, and triglycerides.
Model adjusted for age, sex, body mass index, chronic kidney disease, diabetes mellitus, smoking exposure, aspirin use, statin use, B‐vitamin use, LDL cholesterol, HDL cholesterol, and triglycerides.
Discussion
In this study, we demonstrated that elevated serum homocysteine levels in patients with early coronary atherosclerosis are associated with endothelial dysfunction. The current study further supports a role for homocysteine in the mechanism, and potentially a therapeutic target, of early coronary atherosclerosis in humans. Interestingly, the association between elevated levels of serum homocysteine and CMED seemed to be augmented in patients on B‐vitamin supplementation, while this association was not statistically different between patients on statin or diuretic therapy as compared with those who are not.
Homocysteine is not obtained from diet but rather biosynthesized from essential amino acid methionine via a demethylation multistep process. Homocysteine plays an important mediator role in several basic metabolic processes. It could be reused in the methionine cycle, with the help of folate (B9) and cobalamin (B12), which is important for S‐adenosyl methionine production, an essential methylation agent used in several pathways. Alternatively, it could be irreversibly converted to cysteine, via transsulfuration assisted by pyridoxine (B6), to be used in protein synthesis and other biochemical processes. Through these mechanisms, homocysteine levels are maintained at a safe range with constant turnover and little accumulation. Therefore, the lack of B vitamins as well as other genetic variations affecting these enzymatic pathways could lead to hyperhomocysteinemia. In this study, we demonstrated a clear association between the highest quartile of serum homocysteine levels and coronary microvascular endothelial function, a feature of early atherosclerosis. The chronic effects of elevated homocysteine were previously shown in a case‐control study where a modest increase of homocysteine was associated with an increased risk of vascular diseases, including coronary, cerebrovascular, and peripheral artery disease. 47 This is the first study to demonstrate the association between modest elevations of homocysteine on CMED, a marker of early preobstructive atherosclerosis.
Serum homocysteine accumulation might also play a role in synaptic dysfunction and cell death, through oxidative‐stress generated by self‐looping mechanisms. 48 Some of the pathomechanisms of homocysteine accumulation’s effect on endothelial function have also been described in the literature. 49 For example, it has been hypothetically linked to increased levels of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, which is in turn a potent vasodilator vital for normal endothelial function. Another in vitro study using endothelial cells revealed that homocysteine treatment resulted in increased asymmetric dimethylarginine accumulation through suppression of dimethylarginine dimethylaminohydrolase activity. 50 In vitro studies also showed that homocysteine‐induced endothelial dysfunction was mainly due to increasing superoxide production and quenching nitric oxide, a potent vasodilator, production. Furthermore, tetrahydrobiopterin attenuated homocysteine‐induced damage to the endothelium by promoting nitric oxide synthase function. 51 Thus, our current study extends this previous observation and demonstrates that homocysteine is directly associated with endothelial dysfunction through dysregulation in the dilation/constriction pathways of coronary vasculature.
Serum homocysteine is usually kept below 15 μmol/L, with mild elevation defined as levels from 15 to 30 μmol/L. 27 , 52 We demonstrated a clear association between high‐normal to mild hyperhomocysteinemia and CMED. Moreover, another study also showed that a modest increase of homocysteine (>12.1 µmol/L) was associated with cardiovascular risk. 47 This trend was also seen with another inflammatory biomarker, uric acid, where high‐normal levels were associated with coronary endothelial dysfunction. 18 This suggests that currently established thresholds that define elevated serum homocysteine might not be reflective of the early pathophysiological effects of these normal‐range elevations.
Studies investigating effects of hyperhomocysteinemia treatment on coronary artery disease demonstrated conflicting results. Folate supplementation was shown to improve flow‐mediated dilatation of the brachial artery in human subjects with hyperhomocysteinemia. 53 In contrast, most randomized controlled trials have failed to show a beneficial effect of B‐vitamin supplementation on cardiovascular outcomes, despite reductions in serum homocysteine levels. 54 , 55 , 56 , 57 However, a substudy of the VISP (Vitamin Intervention for Stroke Prevention) trial demonstrated a beneficial effect of high‐dose B‐vitamin supplementation in patients >67 years of age by reducing risk of stroke, myocardial infarction, and death in the older cohort. 58
B‐vitamin use can decrease the levels of homocysteine in serum. 52 Yet trials have shown conflicting results regarding the effect of B‐vitamin supplementation on cardiac disease. 55 Further, statin treatment in elderly patients with high serum homocysteine showed overall reduction of CVD events 59 and was also associated with improvements in coronary endothelial function. 60 , 61 , 62 B‐vitamin supplementation was proven as a reliable method to decrease serum homocysteine level in most patients. However, the lack of efficacy of B‐vitamin supplementation on cardiovascular outcomes created controversy around this topic. As shown in our analysis, patients on B‐vitamin supplementation with elevated serum homocysteine levels (>9 μmol/L) are at even higher odds of having CMED, compared with those not taking supplementation. A possible explanation to this is that B vitamins may themselves play a role in atherogenesis. One study reported higher restenosis rates after angioplasty in patients taking B‐vitamin supplementation. 63 Another study suggested that despite decreasing the levels of homocysteine, S‐adenosyl methionine levels are increased, which alters normal methylation patterns, possibly leading to atherogenesis. 64 This effect was evident in the post‐hoc analysis of one trial, which showed that patients in B‐vitamin supplementation arm had more rapid progression of coronary diameter stenosis as evaluated by quantitative coronary angiography. 65 These patients may have also had higher levels of homocysteine before initiating vitamin treatment. Finally, B‐vitamin supplementation might unmask patients with mild forms of genetic hyperhomocysteinemia, which is not usually affected by B‐vitamin supplementation. However, no comparisons between genetic and other types of hyperhomocysteinemia effect on cardiovascular diseases were done.
In this study, statin therapy was not shown to affect the association between serum homocysteine and CMED. While some studies in the literature convey a beneficial effect of statins on endothelial function, 60 , 61 , 62 one study also reported that statin therapy showed the greatest benefit in individuals with increased baseline serum homocysteine levels. 59 No previous study evaluated the effect of statin on homocysteine‐induced CMED. The possible protective role of statins could be attributable to the anti‐inflammatory role of statins, which one in vitro study investigated and showed that statins have an attenuating effect on LDL cholesterol and homocysteine‐induced oxidation. 66 , 67 While statins may play a role in ameliorating the association between high‐normal levels of homocysteine and CMED the precise mechanism involved in this process, and its potential clinical implications will require further study.
Limitations
This study has several limitations. First, its retrospective and cross‐sectional design makes it challenging to derive causal associations, and the results should be considered as hypothesis generating. However, to our knowledge, this cohort is the largest database of patients undergoing an invasive assessment of endothelial function. Furthermore, some variables were not taken into account during clinical assessment. First, folate‐fortified food intake may directly affect folate levels in the blood, so B‐vitamin use is not the only factor that affects folate levels and therefore homocysteine levels. 68 We do not have the dose and frequency of B‐vitamin supplementation in our population. In one randomized controlled trial in a Chinese population, only medium (400 μg/day) or high‐dose (4000 μg/day) supplementation decreased homocysteine levels in patients in the quartile with the highest baseline homocysteine levels (>12 μmol/L). 69
Conclusions
Our current study demonstrates that early coronary atherosclerosis is associated with elevated homocysteine levels and even high‐normal levels of homocysteine (highest quartile, >9 μmol/L) are independently weakly yet significantly associated with CMED diagnosed invasively with pharmacologic provocation testing. Thus, the link between increased homocysteine levels and adverse cardiovascular events may potentially be mediated by coronary endothelial dysfunction; however no causal link can be established, requiring further investigation.
Sources of Funding
This work was funded by the Mayo Foundation.
Disclosures
Professor Amir Lerman declares consulting honoraria for Philips Volcano. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc 2020;9:e017746 DOI: 10.1161/JAHA.120.017746.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017746
For Sources of Funding and Disclosures, see page 8.
References
- 1. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;734–744. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;1445–1453. [DOI] [PubMed] [Google Scholar]
- 4. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long‐term outcome. Coron Artery Dis. 2004;259–264. [DOI] [PubMed] [Google Scholar]
- 5. Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks DS, Gudapati S, Prisant LM, Weir B, diDonato‐Gonzalez C, Waller JL, Houghton JL. Mortality in patients with microvascular disease. J Clin Hypertens (Greenwich). 2004;304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serruys PW, di Mario C, Piek J, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short‐ and long‐term outcomes of coronary balloon angioplasty: the DEBATE study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation. 1997;3369–3377. [DOI] [PubMed] [Google Scholar]
- 10. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;363–368. [DOI] [PubMed] [Google Scholar]
- 11. Corban MT, Lerman LO, Lerman A. Coronary microvasculature: are the small and the mighty cross‐talking with the epicardial vessels? JACC Cardiovasc Interv. 2018;2069–2071. [DOI] [PubMed] [Google Scholar]
- 12. Usui E, Yonetsu T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Fukuda T, Sumino Y, Ohya H, Hamaya R, et al. Optical coherence tomography‐defined plaque vulnerability in relation to functional stenosis severity and microvascular dysfunction. JACC Cardiovasc Interv. 2018;2058–2068. [DOI] [PubMed] [Google Scholar]
- 13. Godo S, Corban MT, Toya T, Gulati R, Lerman LO, Lerman A. Association of coronary microvascular endothelial dysfunction with vulnerable plaque characteristics in early coronary atherosclerosis. EuroIntervention. 2019;387–394. [DOI] [PubMed] [Google Scholar]
- 14. Al‐Badri A, Kim JH, Liu C, Mehta PK, Quyyumi AA. Peripheral microvascular function reflects coronary vascular function. Arterioscler Thromb Vasc Biol. 2019;1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corban MT, Lerman LO, Lerman A. Endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2019;1272–1274. [DOI] [PubMed] [Google Scholar]
- 16. Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, Lennon RJ, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;2473–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mekonnen G, Corban MT, Hung OY, Eshtehardi P, Eapen DJ, Al‐Kassem H, Rasoul‐Arzrumly E, Gogas BD, McDaniel MC, Pielak T, et al. Plasma soluble urokinase‐type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non‐obstructive coronary artery disease. Atherosclerosis. 2015;55–60. [DOI] [PubMed] [Google Scholar]
- 18. Prasad M, Matteson EL, Herrmann J, Gulati R, Rihal CS, Lerman LO, Lerman A. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sara JDS, Prasad M, Zhang M, Lennon RJ, Herrmann J, Lerman LO, Lerman A. High‐sensitivity C‐reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non‐obstructive coronary artery disease. Am Heart J. 2017;1–11. [DOI] [PubMed] [Google Scholar]
- 20. Homocysteine SC. Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA. 2002;2015–2022. [DOI] [PubMed] [Google Scholar]
- 21. Anniwaer J, Liu MZ, Xue KD, Maimaiti A, Xiamixiding A. Homocysteine might increase the risk of recurrence in patients presenting with primary cerebral infarction. Int J Neurosci. 2019;654–659. [DOI] [PubMed] [Google Scholar]
- 22. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta‐analysis. Thromb Haemost. 1998;874–877. [PubMed] [Google Scholar]
- 23. Dymara‐Konopka W, Laskowska M. The role of nitric oxide, adma, and homocysteine in the etiopathogenesis of preeclampsia‐review. Int J Mol Sci. 2019;2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Green D, Tan J, Liao Y, Pearce WH, Schneider JR, et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;823–831. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Shih J, Cook TJ, Clearfield M, Downs JR, Pradhan AD, Weis SE, Gotto AM Jr; Air Force/Texas Coronary Atherosclerosis Prevention Study I . Plasma homocysteine concentration, statin therapy, and the risk of first acute coronary events. Circulation. 2002;1776–1779. [PubMed] [Google Scholar]
- 27. Cacciapuoti F. Hyper‐homocysteinemia: a novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J Thromb Thrombolysis. 2011;82–88. [DOI] [PubMed] [Google Scholar]
- 28. Toya T, Sara JD, Lerman B, Ahmad A, Taher R, Godo S, Corban MT, Lerman LO, Lerman A. Elevated plasma homocysteine levels are associated with impaired peripheral microvascular vasomotor response. Int J Cardiol Heart Vasc. 2020;100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rallidis LS, Kosmas N, Rallidi T, Pavlakis G, Kiouri E, Zolindaki M. Homocysteine is an independent predictor of long‐term cardiac mortality in patients with stable coronary artery disease in the era of statins. Coron Artery Dis. 2020;152–156.31609754 [Google Scholar]
- 30. Boger RH, Bode‐Boger SM, Sydow K, Heistad DD, Lentz SR. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;1557–1564. [DOI] [PubMed] [Google Scholar]
- 31. Corban MT, Prasad A, Nesbitt L, Loeffler D, Herrmann J, Lerman LO, Lerman A. Local production of soluble urokinase plasminogen activator receptor and plasminogen activator inhibitor‐1 in the coronary circulation is associated with coronary endothelial dysfunction in humans. J Am Heart Assoc. 2018;9:e009881 DOI: 10.1161/JAHA.118.009881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hasdai D, Cannan CR, Mathew V, Holmes DR Jr, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;203–208. [DOI] [PubMed] [Google Scholar]
- 33. Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;1133–1140. [DOI] [PubMed] [Google Scholar]
- 34. Widmer RJ, Flammer AJ, Herrmann J, Rodriguez‐Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;H393–H397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maniu CV, Higano ST, Lerman A. Assessing coronary endothelial dysfunction. Circulation. 2002;9:e48; discussion e48. [DOI] [PubMed] [Google Scholar]
- 37. Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI‐sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widmer RJ, Samuels B, Samady H, Price MJ, Jeremias A, Anderson RD, Jaffer FA, Escaned J, Davies J, Prasad M, et al. The functional assessment of patients with non‐obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;1694–1702. [DOI] [PubMed] [Google Scholar]
- 39. Bell MR, Britson PJ, Chu A, Holmes DR Jr, Bresnahan JF, Schwartz RS. Validation of a new UNIX‐based quantitative coronary angiographic system for the measurement of coronary artery lesions. Cathet Cardiovasc Diagn. 1997;66–74. [DOI] [PubMed] [Google Scholar]
- 40. Bove AA, Holmes DR Jr, Owen RM, Bresnahan JF, Reeder GS, Smith HC, Vlietstra RE. Estimation of the effects of angioplasty on coronary stenosis using quantitative video angiography. Cathet Cardiovasc Diagn. 1985;5–16. [DOI] [PubMed] [Google Scholar]
- 41. Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;3390–3395. [DOI] [PubMed] [Google Scholar]
- 42. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;1269–1324. [DOI] [PubMed] [Google Scholar]
- 43. Chao M‐C, Hu S‐L, Hsu H‐S, Davidson LE, Lin C‐H, Li C‐I, Liu C‐S, Li T‐C, Lin C‐C, Lin W‐Y. Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol. 2014;299–305. [DOI] [PubMed] [Google Scholar]
- 44. Xie D, Yuan Y, Guo J, Yang S, Xu X, Wang Q, Li Y, Qin X, Tang G, Huo Y, et al. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci Rep. 2015;16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chambers JC, Ueland PM, Obeid OA, Wrigley J, Refsum H, Kooner JS. Improved vascular endothelial function after oral B vitamins. Circulation. 2000;2479–2483. [DOI] [PubMed] [Google Scholar]
- 46. Reriani MK, Dunlay SM, Gupta B, West CP, Rihal CS, Lerman LO, Lerman A. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta‐analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011;704–716. [DOI] [PubMed] [Google Scholar]
- 47. O'Callaghan P, Meleady R, Fitzgerald T, Graham I; European Cg . Smoking and plasma homocysteine. Eur Heart J. 2002;1580–1586. [DOI] [PubMed] [Google Scholar]
- 48. Sibrian‐Vazquez M, Escobedo JO, Lim S, Samoei GK, Strongin RM. Homocystamides promote free‐radical and oxidative damage to proteins. Proc Natl Acad Sci USA. 2010;551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Esse R, Barroso M, Tavares de Almeida I, Castro R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: state‐of-the‐art. Int J Mol Sci. 2019;867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu LH, Guo Z, Feng M, Wu ZZ, He ZM, Xiong Y. Protection of DDAH2 overexpression against homocysteine‐induced impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells. Cell Physiol Biochem. 2012;1413–1422. [DOI] [PubMed] [Google Scholar]
- 51. Dhillon B, Badiwala MV, Maitland A, Rao V, Li SH, Verma S. Tetrahydrobiopterin attenuates homocysteine induced endothelial dysfunction. Mol Cell Biochem. 2003;223–227. [DOI] [PubMed] [Google Scholar]
- 52. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;2002–2006. [DOI] [PubMed] [Google Scholar]
- 54. Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and b vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygard O. Mortality and cardiovascular events in patients treated with homocysteine‐lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;795–804. [DOI] [PubMed] [Google Scholar]
- 56. Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM; Veterans Affairs Site I . Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end‐stage renal disease: a randomized controlled trial. JAMA. 2007;1163–1170. [DOI] [PubMed] [Google Scholar]
- 57. Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;565–575. [DOI] [PubMed] [Google Scholar]
- 58. Towfighi A, Arshi B, Markovic D, Ovbiagele B. Homocysteine‐lowering therapy and risk of recurrent stroke, myocardial infarction and death: the impact of age in the VISP trial. Cerebrovasc Dis. 2014;263–267. [DOI] [PubMed] [Google Scholar]
- 59. Drewes YM, Poortvliet RK, Blom JW, de Ruijter W, Westendorp RG, Stott DJ, Blom HJ, Ford I, Sattar N, Wouter Jukema J, et al. Homocysteine levels and treatment effect in the prospective study of pravastatin in the elderly at risk. J Am Geriatr Soc. 2014;213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Investigators E . Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (evaluation of nifedipine and cerivastatin on recovery of coronary endothelial function). Circulation. 2003;422–428. [DOI] [PubMed] [Google Scholar]
- 61. Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol‐lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;481–487. [DOI] [PubMed] [Google Scholar]
- 62. Wassmann S, Faul A, Hennen B, Scheller B, Bohm M, Nickenig G. Rapid effect of 3‐hydroxy‐3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res. 2003;e98–e103. [DOI] [PubMed] [Google Scholar]
- 63. Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K, Pasalary MN, Scherer E, Dambrink JH. Folate therapy and in‐stent restenosis after coronary stenting. N Engl J Med. 2004;2673–2681. [DOI] [PubMed] [Google Scholar]
- 64. Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;5–8. [DOI] [PubMed] [Google Scholar]
- 65. Loland KH, Bleie O, Blix AJ, Strand E, Ueland PM, Refsum H, Ebbing M, Nordrehaug JE, Nygard O. Effect of homocysteine‐lowering b vitamin treatment on angiographic progression of coronary artery disease: a Western Norway B Vitamin Intervention Trial (WENBIT) substudy. Am J Cardiol. 2010;1577–1584. [DOI] [PubMed] [Google Scholar]
- 66. Shi YF, Chi JF, Tang WL, Xu FK, Liu LB, Ji Z, Lv HT, Guo HY. Effects of rosuvastatin on the production and activation of matrix metalloproteinase‐2 and migration of cultured rat vascular smooth muscle cells induced by homocysteine. J Zhejiang Univ Sci B. 2013;696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. AnandBabu K, Sen P, Angayarkanni N. Oxidized ldl, homocysteine, homocysteine thiolactone and advanced glycation end products act as pro‐oxidant metabolites inducing cytokine release, macrophage infiltration and pro‐angiogenic effect in arpe‐19 cells. PLoS One. 2019;9:e0216899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palchetti CZ, Paniz C, de Carli E, Marchioni DM, Colli C, Steluti J, Pfeiffer CM, Fazili Z, Guerra‐Shinohara EM. Association between serum unmetabolized folic acid concentrations and folic acid from fortified foods. J Am Coll Nutr. 2017;572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Homocysteine Lowering Trialists C . Dose‐dependent effects of folic acid on blood concentrations of homocysteine: a meta‐analysis of the randomized trials. Am J Clin Nutr. 2005;806–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


