Abstract
Background
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) improves the discriminatory ability of risk‐prediction models in type 2 diabetes mellitus (T2DM) but is not yet used in clinical practice. We assessed the discriminatory strength of NT‐proBNP by itself for death and cardiovascular events in high‐risk patients with T2DM.
Methods and Results
Cox proportional hazards were used to create a base model formed by 20 variables. The discriminatory ability of the base model was compared with that of NT‐proBNP alone and with NT‐proBNP added, using C‐statistics. We studied 5509 patients (with complete data) of 8561 patients with T2DM and cardiovascular and/or chronic kidney disease who were enrolled in the ALTITUDE (Aliskiren in Type 2 Diabetes Using Cardiorenal Endpoints) trial. During a median 2.6‐year follow‐up period, 469 patients died and 768 had a cardiovascular composite outcome (cardiovascular death, resuscitated cardiac arrest, nonfatal myocardial infarction, stroke, or heart failure hospitalization). NT‐proBNP alone was as discriminatory as the base model for predicting death (C‐statistic, 0.745 versus 0.744, P=0.95) and the cardiovascular composite outcome (C‐statistic, 0.723 versus 0.731, P=0.37). When NT‐proBNP was added, it increased the predictive ability of the base model for death (C‐statistic, 0.779 versus 0.744, P<0.001) and for cardiovascular composite outcome (C‐statistic, 0.763 versus 0.731, P<0.001).
Conclusions
In high‐risk patients with T2DM, NT‐proBNP by itself demonstrated discriminatory ability similar to a multivariable model in predicting both death and cardiovascular events and should be considered for risk stratification.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00549757.
Keywords: cardiovascular diseases, diabetes complications, diabetes mellitus, type 2, pro‐B-type natriuretic peptide, proportional hazards models
Subject Categories: Biomarkers, Cardiovascular Disease, Risk Factors, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- CKD
chronic kidney disease
- CVCO
cardiovascular composite outcome
- CVD
cardiovascular disease
- HF
heart failure
- hs‐TnT
high‐sensitivity cardiac troponin
- MI
myocardial infarction
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- T2DM
type 2 diabetes mellitus
Clinical Perspective
What Is New?
In high‐risk patients with type 2 diabetes mellitus, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) was the major predictor of death and cardiovascular events and, by itself, demonstrated a discriminatory ability similar to a model formed by 20 important clinical variables.
When added to the multivariable model, NT‐proBNP significantly increased the model's ability to predict risk.
What Are the Clinical Implications?
Our findings underscore the ability of NT‐proBNP by itself to be as discriminatory as multiple variables combined, not as a suggestion to replace their use but rather to demonstrate the strength of the information encapsulated in this biomarker and its potential to improve risk‐stratification models in patients with type 2 diabetes mellitus and cardiovascular disease, chronic kidney disease, or both.
Individuals with type 2 diabetes mellitus (T2DM) have a higher risk of dying than people of comparable age and sex without diabetes mellitus. Cardiovascular disease (CVD) affects approximately one‐third of all people with T2DM and accounts for half of all deaths in this population despite major advances in the treatment of the disease. 1 , 2
Comorbidities associated with T2DM are important contributors to this increased risk. 3 Multivariable proportional hazards models to predict the risk of death and cardiovascular events incorporate factors known to influence survival such as demographic variables, cardiovascular conditions, and laboratory markers of disease severity and organ involvement. 4 Meanwhile, some existing risk‐prediction scores based on the use of these traditional variables were considered inaccurate in patients with T2DM. 5
BNPs (B‐type natriuretic peptides), biomarkers of myocardial stress, are well‐established predictors of outcomes in heart failure (HF). 6 , 7 They have also been shown to incrementally improve predictive discrimination of death and cardiovascular events when incorporated into multivariable models in the general population of individuals with T2DM, 8 , 9 , 10 , 11 especially in the presence of HF, 12 , 13 chronic kidney disease (CKD), 14 , 15 , 16 and recent acute coronary syndrome. 17 , 18 Despite the evidence, in clinical practice, the use of natriuretic peptides is not yet consolidated in the risk assessment of patients with T2DM.
A recent study quantitating the relative contributions of clinical variables and biomarkers in the ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome) trial found that the BNPs were the most important predictors of death and of having a nonfatal cardiovascular event. For death, natriuretic peptides levels alone provided predictive ability that was comparable to the use of all other conventional factors combined in a multivariable model. 17
In this study, we assessed the discriminatory ability provided by NT‐proBNP (N‐terminal pro‐BNP) by itself for the prediction of both death and a cardiovascular composite outcome (CVCO) compared with a multivariable model in patients with T2DM and CVD or/and CKD who were enrolled in the ALTITUDE (Aliskiren in Type 2 Diabetes Using Cardiorenal Points; NCT00549757) trial. 19
Methods
We performed an analysis of 5509 people (with complete data) of 8561 individuals screened at 838 centers in 36 countries and randomly enrolled in the ALTITUDE trial. 20
Male or female individuals ≥35 years of age were included if they used antidiabetic drugs or had documented fasting plasma glucose ≥126 mg/dL or 2‐hour plasma glucose ≥200 mg/dL; concomitant use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers without any adjustments to antihypertensive therapy for at least 4 weeks before randomization; and at least one of the following conditions: persistent macroalbuminuria (urine albumin‐to‐creatinine ratio ≥200 mg/g) and estimated glomerular filtration rate ≥30 mL/min per 1.73 m2; persistent microalbuminuria (urine albumin‐to‐creatinine ratio ≥20 mg/g) or/and a history of CVD (myocardial infarction, stroke, HF, or coronary heart disease, and a mean estimated glomerular filtration rate ≥30 mL/min per 1.73 m2). Patients were excluded if they had serum potassium >5.0 mmol/L directly preceding randomization, type 1 diabetes mellitus, unstable serum creatinine (≥20% difference between 2 consecutive serum creatinine measurements), New York Heart Association class III or IV HF, stroke, acute coronary syndrome, revascularization, HF hospitalization in the prior 3 months, history of cancer, renal transplant, uncontrolled hypertension, treatment with >2 agents blocking the renin–angiotensin aldosterone system, or use of potassium‐sparing diuretics. 20
The study was approved by the ethics committee or institutional review board at each participating center, and all participants signed informed consent before enrollment. 19 , 20
Participants were randomized to receive aliskiren or placebo. 20 The intervention had no effect on the primary and secondary end points but was associated with more adverse drug effects. 20
Demographic information and clinical data were recorded in an electronic case report form. All data pertaining to baseline variables including demographics, anthropometrics, clinical information, laboratory tests, and prior medical history were obtained at the time of randomization in the study. All events were reported to a centralized and independent adjudication committee at Brigham and Women's Hospital (Boston, MA) that classified events according to prespecified definitions (Data S1). 19 , 20
The study end points were defined as death from any cause and a CVCO (prespecified as a secondary cardiovascular end point in the ALTITUDE trial, as previously published, and defined as cardiovascular death, resuscitated cardiac arrest, nonfatal myocardial infarction, nonfatal stroke, or unplanned hospitalization for HF). 19 , 20
All laboratory variables were centrally measured. 20 NT‐proBNP and hs‐TnT (high‐sensitivity cardiac troponin) values <25 pg/mL and 13 ng/L were converted to 12.5 pg/mL and 6.5 ng/L, respectively (Data S2).
Statistical Analysis
Baseline characteristics shown in Table 1 were selected to create the risk models. We examined all variables collected in the electronic case report form. The most statistically significant or clinically relevant baseline variables were added to the model. Nonsignificant variables were removed (P>0.05) unless considered clinically important. The distributions of baseline NT‐proBNP, hs‐TnT, and other variables that were found to be right‐skewed were log‐transformed before analysis. Continuous variables were included in the model unless there was clear evidence of nonlinearity. Between‐group differences were tested for statistical significance with Student t test or Wilcoxon rank sum test for continuous variables; the χ2 test was used for categorical variables.
Table 1.
Baseline Characteristics of Patients Classified by Outcome Status (N=5509)
| Death | CVCO | |||||
|---|---|---|---|---|---|---|
| No | Yes | P Value | No | Yes | P Value | |
| n=5040 | n=469 | n=4741 | n=768 | |||
| Age, y | 64.1±9.8 | 68.1±9.3 | <0.001 | 64.0±9.8 | 67.0±9.2 | <0.001 |
| Female sex | 1569 (31.1) | 129 (27.5) | 0.1 | 1466 (30.9) | 232 (30.2) | 0.69 |
| Race | 0.002 | 0.014 | ||||
| White | 2755 (54.7) | 267 (56.9) | 2565 (54.1) | 457 (59.5) | ||
| Black | 121 (2.4) | 12 (2.6) | 113 (2.4) | 20 (2.6) | ||
| Asian | 1876 (37.2) | 143 (30.5) | 1775 (37.4) | 244 (31.8) | ||
| Native American | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | ||
| Pacific Islander | 9 (0.2) | 2 (0.4) | 7 (0.1) | 4 (0.5) | ||
| Other | 278 (5.5) | 45 (9.6) | 280 (5.9) | 43 (5.6) | ||
| BMI, kg/m2 | 29.7±5.9 | 29.3±6.0 | 0.09 | 29.7±5.9 | 29.9±6.0 | 0.35 |
| SBP, mm Hg | 137.4±16.2 | 140.4±17.0 | <0.001 | 137.2±16.1 | 140.9±16.8 | <0.001 |
| DBP, mm Hg | 74.4±9.7 | 73.8±10.5 | 0.2 | 74.5±9.7 | 73.8±10.1 | 0.07 |
| Heart rate, bpm | 72.3±12.4 | 72.6±13.1 | 0.61 | 72.5±12.4 | 71.8±12.8 | 0.21 |
| Smoking status | 0.08 | 0.08 | ||||
| No smoker | 2498 (49.6) | 210 (44.8) | 2359 (49.8) | 349 (45.4) | ||
| Former | 1822 (36.2) | 193 (41.2) | 1715 (36.2) | 300 (39.1) | ||
| Current | 720 (14.3) | 66 (14.1) | 667 (14.1) | 119 (15.5) | ||
| Hemoglobin, g/dL | 13.2±1.7 | 12.8±1.8 | <0.001 | 13.2±1.7 | 13.0±1.8 | 0.004 |
| Serum albumin, mg/dL | 4.3±0.4 | 4.1±0.5 | <0.001 | 4.3±0.4 | 4.1±0.4 | <0.001 |
| HDL‐C, mg/dL | 46.2±12.7 | 46.9±13.9 | 0.25 | 46.2±12.7 | 46.6±13.3 | 0.43 |
| LDL‐C, mg/dL | 98.4±36.9 | 100.4±38.1 | 0.25 | 97.7±36.6 | 103.5±39.3 | <0.001 |
| Potassium, mEq/L | 4.5±0.5 | 4.5±0.5 | 0.65 | 4.5±0.5 | 4.5±0.5 | 0.26 |
| HbA1c, % | 7.7±1.5 | 7.9±1.7 | 0.015 | 7.7±1.5 | 7.9±1.7 | <0.001 |
| HbA1c, mmol/mol | 60.8±16.7 | 62.8±18.9 | 0.015 | 60.6±16.5 | 63.2±18.7 | <0.001 |
| eGFR, mL/min/1.73 m2 | 58.0±23.0 | 51.2±20.1 | <0.001 | 58.1±23.0 | 53.0±20.8 | <0.001 |
| eGFR category | <0.001 | <0.001 | ||||
| <30 | 111 (2.2) | 21 (4.5) | 108 (2.3) | 24 (3.1) | ||
| 30 to <45 | 1431 (28.4) | 192 (40.9) | 1331 (28.1) | 292 (38.0) | ||
| 45 to <60 | 1779 (35.3) | 152 (32.4) | 1682 (35.5) | 249 (32.4) | ||
| ≥60 | 1719 (34.1) | 104 (22.2) | 1620 (34.2) | 203 (26.4) | ||
| UACR geometric mean, mg/g | 209.2 (198.1–220.9) | 228.1 (188.1–276.5) | 0.37 | 206.4 (195.2–218.3) | 239.6 (205.9–278.9) | 0.05 |
| UACR, median (IQR) | 301.9 (62.8–894.9) | 284.6 (53.9–1272.1) | 0.31 | 297.9 (64.5–863.7) | 320.9 (52.8–1340.1) | 0.015 |
| UACR category | 0.59 | 0.67 | ||||
| <20 | 708 (14.0) | 69 (14.7) | 661 (13.9) | 116 (15.1) | ||
| 20 to <200 | 1245 (24.7) | 124 (26.4) | 1183 (25.0) | 186 (24.2) | ||
| ≥200 | 3087 (61.3) | 276 (58.8) | 2897 (61.1) | 466 (60.7) | ||
| BB on ECG | 480 (9.5) | 86 (18.3) | <0.001 | 438 (9.2) | 128 (16.7) | <0.001 |
| LVH on ECG | 340 (6.7) | 51 (10.9) | <0.001 | 319 (6.7) | 72 (9.4) | 0.008 |
| Q wave on ECG | 315 (6.3) | 53 (11.3) | <0.001 | 296 (6.2) | 72 (9.4) | 0.001 |
| T2DM diagnosis time, y | 0.29 | 0.16 | ||||
| >5 | 4124 (81.8) | 395 (84.2) | 3870 (81.6) | 649 (84.5) | ||
| 1–5 | 738 (14.6) | 63 (13.4) | 705 (14.9) | 96 (12.5) | ||
| <1 | 178 (3.5) | 11 (2.3) | 166 (3.5) | 23 (3.0) | ||
| Insulin use | 2912 (57.8) | 300 (64.0) | 0.009 | 2712 (57.2) | 500 (65.1) | 0.001 |
| Statin use | 3220 (63.9) | 293 (62.5) | 0.54 | 2996 (63.2) | 517 (67.3) | 0.027 |
| β‐Blocker use | 2453 (48.7) | 261 (55.7) | 0.004 | 2258 (47.6) | 456 (59.4) | <0.001 |
| ACEi use | 2143 (43.5) | 230 (50.1) | 0.006 | 1989 (43.0) | 384 (50.9) | <0.001 |
| ARB use | 2904 (58.6) | 241 (52.4) | 0.01 | 2757 (59.1) | 388 (51.5) | <0.001 |
| Aliskiren use | 2509 (49.8) | 237 (50.5) | 0.76 | 2346 (49.5) | 400 (52.1) | 0.18 |
| History of HF | 467 (9.3) | 113 (24.1) | <0.001 | 396 (8.4) | 184 (24.0) | <0.001 |
| History of CABG | 578 (11.5) | 64 (13.6) | 0.16 | 517 (10.9) | 125 (16.3) | <0.001 |
| History of PCI | 715 (14.2) | 72 (15.4) | 0.49 | 659 (13.9) | 128 (16.7) | 0.042 |
| History of MI | 735 (14.6) | 108 (23.0) | <0.001 | 662 (14.0) | 181 (23.6) | <0.001 |
| History of unstable angina | 443 (8.8) | 60 (12.8) | 0.004 | 394 (8.3) | 109 (14.2) | <0.001 |
| History of stroke | 476 (9.4) | 66 (14.1) | 0.001 | 434 (9.2) | 108 (14.1) | <0.001 |
| History of TIA | 211 (4.2) | 27 (5.8) | 0.11 | 183 (3.9) | 55 (7.2) | <0.001 |
| History of amputation | 160 (3.2) | 34 (7.2) | <0.001 | 158 (3.3) | 36 (4.7) | 0.06 |
| History of ulcer | 147 (2.9) | 29 (6.2) | <0.001 | 145 (3.1) | 31 (4.0) | 0.15 |
| History of AF | 381 (7.6) | 79 (16.8) | <0.001 | 336 (7.1) | 124 (16.1) | <0.001 |
| History of atrial flutter | 20 (0.4) | 3 (0.6) | 0.44 | 19 (0.4) | 4 (0.5) | 0.63 |
| Pacemaker | 114 (2.3) | 18 (3.8) | 0.033 | 99 (2.1) | 33 (4.3) | <0.001 |
| NT‐proBNP, pg/mL | 389.5±1091.9 | 1267.9±2611.8 | <0.001 | 357.1±1040.3 | 1126.1±2286.5 | <0.001 |
| hs‐TnT, ng/L | 18.2±17.9 | 39.1±124.9 | <0.001 | 17.9±17.7 | 32.9±98.6 | <0.001 |
Data are shown as mean±SD or n (%) except as noted. ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; BB, any bundle branch block; BMI, body mass index; CABG, coronary artery bypass grafting; CVCO, cardiovascular composite outcome; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; hs‐TnT, high‐sensitivity cardiac troponin; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; MI, myocardial infarction; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TIA, transient ischemic attack; and UACR, urine albumin‐to‐creatinine ratio.
Cox proportional hazards modeling was used to create the multivariable base risk model, which was formed using selected clinical and laboratory variables without NT‐proBNP. The discriminatory ability of the base model was compared with that of NT‐proBNP alone and with that of the base model after addition of NT‐pro BNP, using Harrell C‐statistics.
The base model was formed by 20 clinical variables: age (per 10 years), sex, smoking, history of coronary heart disease (previous hospitalizations due to percutaneous coronary intervention, coronary artery bypass grafting, angina, or myocardial infarction), history of stroke, history of prior HF, history of atrial fibrillation, insulin use, systolic blood pressure (per 10 mmHg), diastolic blood pressure (per 10 mmHg), heart rate (per 10 beats/min), left ventricular hypertrophy on ECG, Q wave on ECG, any bundle‐branch block on ECG, log‐transformed hs‐TnT (per 1 log unit), estimated glomerular filtration rate (per 10 mL/min per 1.73 m2), log‐transformed urine albumin‐to‐creatinine ratio (per 1 log unit), glycosylated hemoglobin (per 1%), low‐density lipoprotein cholesterol (per 1 mg/dL), and serum albumin (per 1 mg/dL).
We also performed an additional statistical analysis by dividing the study population into independent sets of training (patients randomized from 2007–2008, n=1969) and validation (patients randomized from 2009–2011, n=3540). We tested the base model of 20 clinical and laboratory variables, NT‐proBNP alone, and NT‐proBNP added to the base model in predicting death and the CVCO in the training data set. Then we evaluated the performance of these predictive models in the validation data set.
A significance level of 0.05 was considered statistically significant. Analyses were performed using Stata 14 (StataCorp).
Results
During median follow‐up of 2.6 years (interquartile range, 2.0–3.2), 469 patients (8.5%) died and 768 (13.9%) experienced a CVCO (cardiovascular death, 294 [5.3%]; myocardial infarction, 201 [3.6%]; HF unplanned hospitalization, 285 [5.2%]; stroke, 201 [3.6%]; resuscitated cardiac arrest, 21 [0.4%]) (Figure S1). Baseline characteristics of patients, classified by end points, death, and cardiovascular events, are presented in Table 1. In this analysis, 2763 patients were randomized to placebo and 2746 were randomized to aliskiren.
Compared with patients who survived, nonsurvivors were older, on average, with higher systolic blood pressure and glycosylated hemoglobin but lower levels of hemoglobin and albumin and lower estimated glomerular filtration rate, in addition to a higher previous load of diseases. Considering patients who had cardiovascular events, higher low‐density lipoprotein cholesterol and albuminuria were observed. Baseline levels of NT‐proBNP were higher in the nonsurvivor group and among those who developed cardiovascular events. There was no difference in aliskiren use between the groups.
Table 2 shows the composition of the base model, with 20 variables for the prediction of death, the univariable model of log‐transformed NT‐proBNP (log–NT‐proBNP), and the model containing the addition of log–NT‐proBNP to the model (21 variables). Table 3 shows the same models for the prediction of CVCO.
Table 2.
Death Prediction Models
| Variables | Base Model | Base Model+N‐TproBNP | NT‐proBNP by Itself | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (20 Variables; C‐Statistic, 0.744 [95% CI, 0.722–0.767]) | (21 Variables; C‐Statistic, 0.779 [95% CI, 0.758–0.800]) | (1 Variable; C‐Statistic, 0.745 [95% CI, 0.723–0.768]) | ||||||||||
| HR | 95% CI | P Value | χ2 | HR | 95% CI | P Value | χ2 | HR | 95% CI | P Value | χ2 | |
| Log NT‐proBNP, per 1 log unit | … | … | … | … | 1.62 | 1.49–1.77 | <0.001 | 118.6 | 1.94 | 1.81–2.07 | <0.001 | 383.4 |
| Log hs‐TnT, per 1 log unit | 1.85 | 1.63–2.11 | <0.001 | 85.0 | 1.49 | 1.29–1.71 | <0.001 | 30.8 | ||||
| Age, per 10 y | 1.57 | 1.39–1.77 | <0.001 | 54.0 | 1.43 | 1.26–1.61 | <0.001 | 32.8 | ||||
| Albumin, per 1 mg/dL | 0.55 | 0.43–0.69 | <0.001 | 25.2 | 0.77 | 0.6–0.98 | 0.035 | 4,.5 | ||||
| History of HF | 1.79 | 1.41–2.28 | <0.001 | 22.7 | 1.42 | 1.11–1.81 | 0.005 | 7.9 | ||||
| Heart rate, per 10 beats/min | 1.10 | 1.02–1.19 | 0.015 | 5.9 | 1.13 | 1.05–1.22 | 0.002 | 9.5 | ||||
| History of stroke | 1.38 | 1.06–1.80 | 0.02 | 5.8 | 1.43 | 1.10–1.87 | 0.008 | 7.1 | ||||
| HbA1c, per 1% | 1.08 | 1.01–1.14 | 0.02 | 5.7 | 1.09 | 1.02–1.15 | 0.007 | 7.2 | ||||
| Smoking | 1.17 | 1.02–1.35 | 0.03 | 4.9 | 1.17 | 1.01–1.34 | 0.03 | 4.6 | ||||
| LVH on ECG | 1.38 | 1.03–1.86 | 0.03 | 4.6 | 1.17 | 0.87–1.57 | 0.30 | 1.1 | ||||
| Q wave on ECG | 1.38 | 1.02–1.87 | 0.04 | 4.3 | 1.12 | 0.82–1.53 | 0.47 | 0.5 | ||||
| History of AF | 1.31 | 1.00–1.71 | 0.05 | 3.8 | 0.99 | 0.76–1.29 | 0.93 | 0.0 | ||||
| BB on ECG | 1.27 | 1.00–1.62 | 0.05 | 3.7 | 1.07 | 0.84–1.38 | 0.57 | 0.3 | ||||
| Log UACR, per 1 log unit | 1.05 | 0.99–1.11 | 0.10 | 2.7 | 1.03 | 0.98–1.10 | 0.24 | 1.4 | ||||
| SBP, per 10 mmHg | 1.05 | 0.98–1.12 | 0.15 | 2.0 | 1.03 | 0.97–1.10 | 0.33 | 0.9 | ||||
| Female sex | 1.16 | 0.92–1.46 | 0.22 | 1.5 | 0.95 | 0.75–1.21 | 0.67 | 0.2 | ||||
| History of CHD | 1.14 | 0.92–1.42 | 0.22 | 1.5 | 0.97 | 0.79–1.20 | 0.79 | 0.1 | ||||
| LDL‐C, 1 mg/dL | 1.00 | 1.00–1.00 | 0.24 | 1.4 | 1.00 | 1.00–1.01 | 0.02 | 5.9 | ||||
| eGFR, per 10 mL/min/1.73 m2 | 0.97 | 0.92–1.03 | 0.34 | 0.9 | 1.01 | 0.96–1.07 | 0.67 | 0.2 | ||||
| Insulin use | 1.05 | 0.85–1.28 | 0.66 | 0.2 | 1.13 | 0.92–1.39 | 0.26 | 1.3 | ||||
| DBP, per 10 mm Hg | 1.01 | 0.90–1.13 | 0.86 | 0.0 | 0.98 | 0.87–1.09 | 0.70 | 0.2 | ||||
AF indicates atrial fibrillation; BB, any bundle‐branch block; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HF, heart failure; HR, hazard ratio; hs‐TnT, high‐sensitivity cardiac troponin; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure; UACR, urine albumin‐to‐creatinine ratio.
Table 3.
CVCO Prediction Models
| Variables | Base Model | Base Model+NT‐proBNP | NT‐proBNP by Itself | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (20 Variables; C‐Statistic, 0.731 [95% CI, 0.714–0.749]) | (21 Variables; C‐Statistic, 0.763 [95% CI, 0.746–0.780]) | (1 Variable; C‐Statistic, 0.723 [95% CI, 0.704–0.741]) | ||||||||||
| HR | 95% CI | P Value | χ2 | HR | 95% CI | P Value | χ2 | HR | 95% CI | P Value | χ2 | |
| Log NT‐proBNP, per 1 log unit | … | … | … | … | 1.63 | 1.52–1.75 | <0.001 | 189.9 | 1.88 | 1.78–1.98 | <0.001 | 545.2 |
| Log hs‐TnT, per 1 log unit | 1.63 | 1.47–1.81 | <0.001 | 86.5 | 1.31 | 1.18–1.47 | <0.001 | 23.3 | ||||
| History of HF | 2.11 | 1.75–2.55 | <0.001 | 60.7 | 1.69 | 1.40–2.05 | <0.001 | 29.6 | ||||
| Age, per 10 y | 1.32 | 1.21–1.45 | <0.001 | 34.8 | 1.21 | 1.10–1.33 | <0.001 | 15.4 | ||||
| Albumin, per 1 mg/dL | 0.58 | 0.48–0.70 | <0.001 | 34.1 | 0.80 | 0.66–0.96 | 0.02 | 5.4 | ||||
| LDL‐C, 1 mg/dL | 1.00 | 1.00–1.01 | <0.001 | 17.3 | 1.01 | 1.00–1.01 | <0.001 | 30.1 | ||||
| History of AF | 1.49 | 1.21–1.85 | <0.001 | 13.5 | 1.12 | 0.9–1.38 | 0.32 | 1.0 | ||||
| History of stroke | 1.46 | 1.19–1.80 | <0.001 | 13.0 | 1.51 | 1.23–1.86 | <0.001 | 15.4 | ||||
| SBP, per 10 mmHg | 1.09 | 1.04–1.15 | 0.001 | 11.8 | 1.07 | 1.02–1.12 | 0.01 | 6.7 | ||||
| HbA1c, per 1% | 1.08 | 1.03–1.14 | 0.001 | 11.4 | 1.10 | 1.05–1.15 | <0.001 | 15.5 | ||||
| Smoking | 1.19 | 1.07–1.33 | 0.002 | 9.9 | 1.18 | 1.06–1.31 | 0.003 | 8.8 | ||||
| History of CHD | 1.29 | 1.09–1.53 | 0.003 | 8.8 | 1.10 | 0.93–1.30 | 0.25 | 1.3 | ||||
| Female sex | 1.26 | 1.05–1.51 | 0.01 | 6.5 | 1.04 | 0.87–1.25 | 0.65 | 0.2 | ||||
| Log UACR, per 1 log unit | 1.06 | 1.01–1.11 | 0.012 | 6.4 | 1.04 | 1.00–1.09 | 0.057 | 3.6 | ||||
| BB on ECG | 1.26 | 1.04–1.54 | 0.02 | 5.4 | 1.09 | 0.89–1.33 | 0.39 | 0.7 | ||||
| DBP, per 10 mm Hg | 0.94 | 0.86–1.03 | 0.16 | 2.0 | 0.92 | 0.85–1.01 | 0.07 | 3.3 | ||||
| Insulin use | 1.12 | 0.95–1.31 | 0.18 | 1.8 | 1.19 | 1.01–1.40 | 0.04 | 4.2 | ||||
| Q wave on ECG | 1.16 | 0.90–1.49 | 0.26 | 1.3 | 0.93 | 0.71–1.20 | 0.57 | 0.3 | ||||
| Heart rate, per 10 bpm | 1.03 | 0.97–1.10 | 0.32 | 1.0 | 1.06 | 1.00–1.13 | 0.06 | 3.6 | ||||
| LVH on ECG | 1.10 | 0.86–1.42 | 0.43 | 0.6 | 0.94 | 0.73–1.20 | 0.60 | 0.3 | ||||
| eGFR, per 10 mL/min/1.73 m2 | 0.99 | 0.95–1.03 | 0.76 | 0.1 | 1.03 | 0.99–1.08 | 0.10 | 2.7 | ||||
AF indicates atrial fibrillation; BB, any bundle‐branch block; CHD, coronary heart disease; CVCO, cardiovascular composite outcome; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HF, heart failure; HR, hazard ratio; hs‐TnT, high‐sensitivity cardiac troponin; LDL‐C, low‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure; and UACR, urine albumin‐to‐creatinine ratio.
In prediction of death, the C‐statistic of base model was 0.744 (95% CI, 0.722–0.767), and the mortality rates per 100 person‐years were 0.7 (95% CI, 0.4–1.2) in the 1st decile and 11.6 (9.9–13.7) in the 10th decile of predicted risk (Figure 1). The C‐statistic for NT‐proBNP as a single variable was 0.745 (95% CI, 0.723–0.768; P=0.95 versus model), and the mortality rates per 100 person‐years were 0.7 (95% CI, 0.4–1.2) in the 1st decile and 11.6 (95% CI, 9.9–13.6) in the 10th decile of NT‐proBNP (Figure 1).
Figure 1. Death prediction models by deciles of predicted risk/deciles of NT‐proBNP.
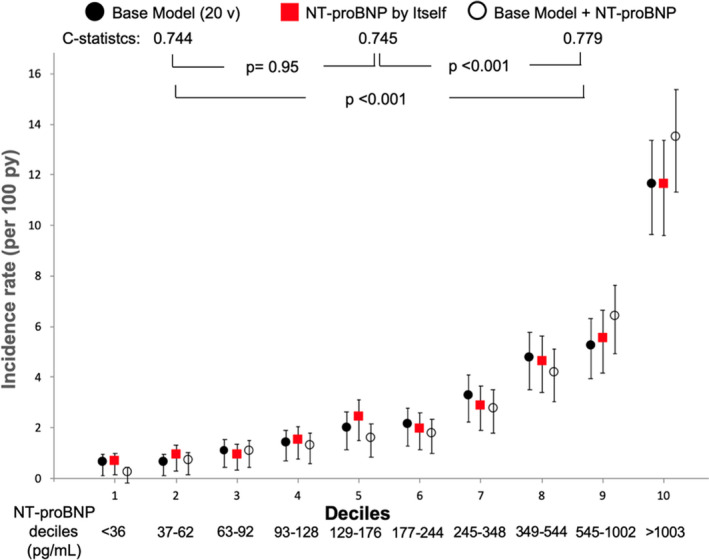
NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; and py, person/years. Base Model: formed by high sensitivity cardiac troponin, age, albumin, history of heart failure, heart rate, history of stroke, HbA1c, smoking, left ventricular hypertrophy on ECG, Q wave on ECG, history of atrial fibrillation, any bundle branch block on ECG, urine albumin‐to‐creatinine ratio, systolic blood pressure, sex, history of coronary heart disease, low density lipoprotein cholesterol, estimated glomerular filtration rate, insulin use, and diastolic blood pressure, in decreasing order of χ2; v=variables. Error bars represent 95% CIs.
In prediction of the CVCO, the C‐statistic for the 20‐variable model was 0.731 (95% CI, 0.714–0.749), and the incidence rates per 100 person‐years were 0.9 (95% CI, 0.5–1.5) in the 1st decile and 19.2 (95% CI, 16.8–22.0) in the 10th decile of predicted risk (Figure 2). The C‐statistic for NT‐proBNP alone was 0.723 (95% CI, 0.704–0.741; P=0.37 versus model), and the incidence rates per 100 person‐years were 1.3 (95% CI, 0.8–2.0) in the 1st decile and 19.4 (95% CI, 16.9–22.1) in the 10th decile of NT‐proBNP (Figure 2).
Figure 2. Cardiovascular composite outcome prediction models by deciles of predicted risk/deciles of NT‐proBNP.
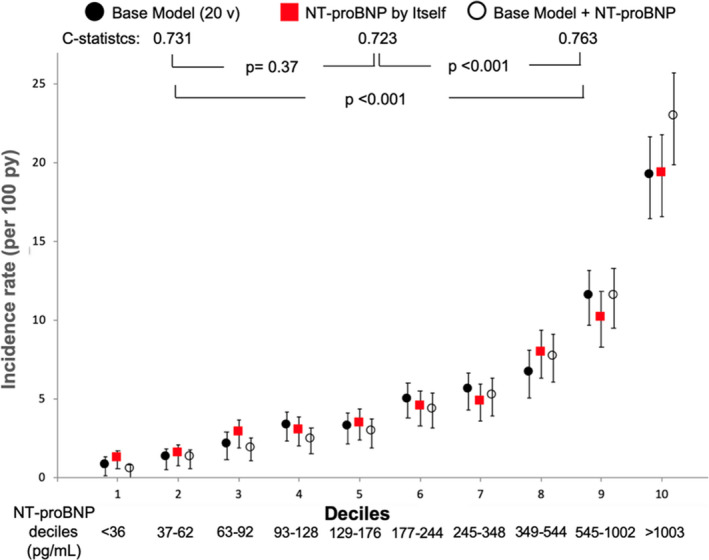
NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; and py, person/years. Base Model: formed by high sensitivity cardiac troponin, history of heart failure, age, albumin, low density lipoprotein cholesterol, history of atrial fibrillation, history of stroke, systolic blood pressure, HbA1c, smoking, history of coronary heart disease, sex, urine albumin‐to‐creatinine ratio, any bundle branch block on ECG, diastolic blood pressure, insulin use, Q wave on ECG, heart rate, left ventricular hypertrophy on ECG, and estimated glomerular filtration rate, in decreasing order of χ2; v=variables. Error bars represent 95% CIs.
The C‐statistic for predicting death in the base model (0.744) was improved by adding NT‐proBNP (0.779, P<0.001 versus model). Similarly, the model ability for predicting the CVCO (0.731) was augmented by including NT‐proBNP in the model (0.763, P<0.001 versus model). C‐statistics for NT‐pro BNP alone were also improved by use of the base model plus NT‐proBNP in the prediction of both death (0.745 versus 0.779, P<0.001) and CVCO (0.723 versus 0.763, P<0.001) (Figures 1 and 2).
In the independent training and validation data sets, we reached the same conclusion—that NT‐pro‐BNP by itself had discriminatory capacity similar to the 20‐variable clinical model for death and the CVCO (Tables S1 through S3).
Sensitivity Analyses
We also performed a sensitivity analysis of 4929 individuals, excluding 580 patients with a previous history of HF. For the prediction of death, once again, NT‐proBNP alone was as good as the model (C‐statistic, 0.726 versus 0.733, P=0.68) and enhanced its ability when added to the model (0.733 versus 0.768, P<0.001).
The same type of sensitivity analysis, excluding individuals with a previous history of HF, was performed for the CVCO, for which NT‐proBNP as a single variable proved to be as discriminatory as the model (0.705 versus 0.714, P=0.42) and improved its strength when added to the model (0.714 versus 0.749, P<0.001).
Regardless of whether the inclusion criteria was CVD (n=2237) or CKD (n=3368), the finding of NT‐proBNP being as discriminatory as the model was confirmed for predicting death (C‐statistic, 0.711 versus 0.732 [P=0.18] among patients with CVD and 0.743 versus 0.746 [P=0.82] in those with CKD) and the CVCO (0.692 versus 0.711 [P=0.16] among patients with CVD and 0.722 versus 0.732 [P=0.40] in those with CKD). Only 96 patients (1.7%) met both criteria and were not assessed separately.
Sensitivity analyses considering body mass index and use of aliskiren are shown in Table S4.
Discussion
Our goal was to evaluate the discriminatory ability of NT‐proBNP by itself in high‐risk patients with T2DM and CVD, CKD, or both. We demonstrated that NT‐proBNP alone was able to predict both death and a CVCO as accurately as the multivariable model composed of the 20 most significant and relevant clinical variables.
Patients with T2DM are at 2 to 4 times greater risk of death and cardiovascular events than the general population. 2 Validated models, such as the Framingham risk score and the UKPDS (United Kingdom Prospective Diabetes Study) model, have shown limited ability to accurately estimate the cardiovascular risk of individuals with T2DM. 4 , 5 Several studies have proposed improvements for risk stratification of patients with T2DM, especially those in secondary prevention; suggested improvements include the incorporation of clinical information 21 and the addition of cardiac biomarkers. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
The importance of BNPs in improving the prediction of cardiovascular events has been well established when added to multivariable models. An analysis of 42 protein biomarkers in the SUMMIT (Surrogate Markers for Micro‐ and Macrovascular Hard Endpoints for Innovative Diabetes Tools) consortium involving individuals with T2DM and without apparent CVD and controls, NT‐proBNP, followed by hs‐TnT and 4 other proteins, revealed the ability to increase cardiovascular outcome prediction. 22 Abnormal NT‐proBNP and hs‐TnT levels were able to distinguish individuals with T2DM at high cardiovascular risk from those at low risk in the ARIC (Atherosclerosis Risk in Communities) study. 8 Another study that evaluated 237 biomarkers in 8401 individuals with dysglycemia (59% with previous CVD) who were enrolled in the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial also identified NT‐proBNP as the major predictor of cardiovascular events and death. 23 In patients with T2DM involved in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Evaluation) trial, the accuracy of 5‐year cardiovascular risk prediction was increased by 39% with NT‐proBNP and 46% with hs‐TnT in net reclassification index when added to the base model. 9 Among patients with T2DM and microalbuminuria enrolled in the Steno‐2 (Intensified Multifactorial Intervention in Patients With Type 2 Diabetes and Microalbuminuria) study, NT‐proBNP above the median was associated with an increased risk of CVD. 14 In the Sun‐MACRO (Sulodexide Macroalbuminuria) trial, the addition of NT‐proBNP to a multivariable model improved prediction of cardiovascular end points in patients with T2DM and macroalbuminuria. 15 Furthermore, in patients with T2DM and predialytic CKD and anemia who were evaluated in TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy), the addition of NT‐proBNP and TnT to the multivariable model was associated with net improvement of 17.8% in predicting cardiovascular outcome. 16 A previous analysis of the ALTITUDE trial showed that the response to treatment with aliskiren in cardiorenal outcomes was related to baseline levels of NT‐proBNP. 24
Life insurance companies have recognized the predictive strength of NT‐proBNP and use it to assess risk of death. 25 For death, some previous studies also demonstrated the ability of BNPs to improve prediction of the multivariable models in patients with T2DM with or without CVD. 9 , 10 , 11 , 15 , 16 , 17 Pfister et al showed that NT‐proBNP measured at discharge predicts death and cardiovascular events in patients with T2DM hospitalized for a broad spectrum of CVDs. 26 Studying older adults with T2DM, Bruno et al demonstrated that NT‐proBNP, adjusted for covariates, was the strongest predictor of cardiovascular mortality. 27
Notably, in an analysis of the ELIXA trial, BNPs alone were as predictive as the multivariable model for death but not for other outcomes in patients with T2DM ≤180 days after acute coronary syndrome. 17 We expanded knowledge about the discriminatory ability of NT‐proBNP by itself, demonstrating that it was as predictive as the base model not only for death but also for CVCO and in a clinical population of patients with T2DM and CVD, CKD, or both. In addition, we showed that these results were maintained even in sensitivity analyses, excluding patients with a history of HF or considering the 2 main inclusion criteria of the study, CVD or CKD. Furthermore, NT‐proBNP demonstrated incremental discriminatory strength when added to the model.
This study is a post hoc analysis of a large cohort of patients previously enrolled in a neutral clinical trial, with the possible limitations of secondary interpretations. In our data set, there was no information on left ventricular function, imaging exams, or social variables such as income and educational level, which could provide additional contribution to risk prediction.
The mechanisms by which NT‐proBNP as a single variable has been shown to be such a strong predictor of risk of death and cardiovascular events have not yet been fully elucidated. It is known that the concentrations of natriuretic peptides may change in relation to different variables such as race/ethnicity, 28 heart rate, 29 obesity, 30 volume overload, 24 left ventricular hypertrophy, 29 HF, 6 , 7 , 12 , 13 myocardial ischemia 17 , 18 , 31 atrial fibrillation, 32 CKD, 14 , 15 , 16 stroke, 33 and treatments. 7 , 34 BNPs are released from the heart as a counterregulatory response to increased stress on the wall, sympathetic tone, and vasoconstriction, but they are also associated with the regulation of numerous physiologic functions that control energy metabolism. 35 It is plausible that NT‐proBNP is sensitive to different influences that expand its potential discriminatory capacity when integrating cardiovascular and hemodynamic stress from several sources.
Our results underscore the ability of a single biomarker to be as discriminatory as multiple variables combined, not as a suggestion to replace their use but to demonstrate the strength of the information encapsulated in NT‐proBNP and its potential to improve models of risk stratification in high‐risk patients with T2DM.
Conclusions
In high‐risk patients with T2DM, NT‐proBNP by itself was as discriminatory as the model of 20 traditional clinical and laboratory variables in prediction of both death and cardiovascular events. This finding does not minimize the influence of multiple other factors in the prognosis but emphasizes the importance of this biomarker as a sensitive integrator of different variables and its potential role in risk stratification.
Data Sharing
The sponsor of this trial is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. Trial data are available according to the criteria and process described. 36
Sources of Funding
Novartis funded the ALTITUDE (Aliskiren in Type 2 Diabetes Using Cardiorenal Endpoints) trial. There was no sponsorship for this study.
Disclosures
Malachias serves on the advisory board and receives speaker fees from Biolab Sanus and Libbs, Brazil. Jhund receives speaker fees from Novartis and AstraZeneca; serves on the advisory boards of Novartis and Cytokinetics; receives research funding from Boehringer Ingelheim; and has been remunerated for time working on the DAPA‐HF, PARADIGM‐HF and PARAGON‐HF trials by the University of Glasgow. Wijkman is supported by grants from the Fulbright Commission, the Swedish Heart Association, the Swedish Society of Medicine, and Region Östergötland, Sweden; has served on advisory boards or lectured for MSD, Lilly, Novo Nordisk, and Sanofi; has organized a professional regional meeting sponsored by Lilly, Rubin Medical, Sanofi, Novartis and Novo Nordisk. Bentley‐Lewis is consultant to the TIMI (Thrombolysis in Myocardial Infarction) Study Group and Novo Nordisk. Chaturvedi serves as a data safety monitoring committee member for a trial sponsored by AstraZeneca. Desai receives research grants from Alnylam, AstraZeneca, and Novartis and consulting fees from Abbott, Alnylam, AstraZeneca, Amgen, Biofourmis, Boston Scientific, Boehringer‐Ingelheim, Corvidia, DalCor Pharma, Merck, Novartis, Relypsa, and Regeneron. Prescott is an employee of Novartis Pharmaceuticals. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, and Theracos and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Dinaqor, Tremeau. De Zeeuw serves on advisory boards and/or speaker for Bayer, Boehringer Ingelheim, Fresenius, Mundipharma, Mitsubishi‐Tanabe; steering committees and/or speaker for AbbVie and Janssen; and data safety and monitoring committees for Bayer. McMurray receives fees (all fees listed paid to Glasgow University) for serving on a steering committee from Bayer, fees for serving on a steering committee, fees for serving on an end point committee, and travel support from Cardiorentis, fees for serving on a steering committee and travel support from Amgen, fees for serving on a steering committee and travel support from Oxford University/Bayer, fees for serving as principal investigator of a trial and travel support from Theracos, fees for serving on a steering committee and travel support from AbbVie, fees for serving on a steering committee from DalCor, fees for serving on a data safety monitoring committee from Pfizer, fees for serving on a data safety monitoring committee from Merck, fees for serving on an executive committee, fees for serving as co‐principal investigator of a trial, fees for serving on a steering committee, fees for serving on an executive committee, travel support, and advisory board fees from Novartis, fees for serving as co‐principal investigator for a trial, fees for serving on a steering committee, and travel support from GlaxoSmithKline, fees for serving on a steering committee from Bristol‐Myers Squibb, fees for serving on a steering committee, fees for serving on an endpoint adjudication committee, and travel support from Vifor‐Fresenius. Pfeffer receives research support from Novartis; serves as a consultant for AstraZeneca, Corvidia, DalCor, GlaxoSmithKline, Jazz, MyoKardia, Novartis, Novo Nordisk, Pharmascience, Sanofi, and Takeda; and has equity in DalCor. The remaining authors have no disclosures to report.
Supporting information
Data S1–S2
Tables S1–S4
Figure S1
(J Am Heart Assoc. 2020;9:e017462 DOI: 10.1161/JAHA.120.017462.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rawshani A, Rawshani A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;300–301. [DOI] [PubMed] [Google Scholar]
- 3. Jhund PS, McMurray JJ, Chaturvedi N, Brunel P, Desai AS, Finn PV, Haffner SM, Solomon SD, Weinrauch LA, Claggett BL, et al. Mortality following a cardiovascular or renal event in patients with type 2 diabetes in the ALTITUDE trial. Eur Heart J. 2015;2463–2469. [DOI] [PubMed] [Google Scholar]
- 4. van Dieren S, Beulens JW, Kengne AP, Peelen LM, Rutten GE, Woodward M, van der Schouw YT, Moons KG. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart. 2012;360–369. [DOI] [PubMed] [Google Scholar]
- 5. Kengne AP, Patel A, Colagiuri S, Heller S, Hamet P, Marre M, Pan CY, Zoungas S, Grobbee DE, Neal B, et al. The Framingham and UK Prospective Diabetes Study (UKPDS) risk equations do not reliably estimate the probability of cardiovascular events in a large ethnically diverse sample of patients with diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron‐MR Controlled Evaluation (ADVANCE) Study. Diabetologia. 2010;821–831. [DOI] [PubMed] [Google Scholar]
- 6. Jhund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, Zile MR, Carson PE, McMurray JJV. Changes in N‐terminal pro‐B‐type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I‐Preserve study. Eur J Heart Fail. 2015;809–817. [DOI] [PubMed] [Google Scholar]
- 7. Myhre PL, Vaduganathan M, Claggett B, Packer M, Desai AS, Rouleau JL, Zile MR, Swedberg K, Lefkowitz M, Shi V, et al. B‐type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM‐HF Trial. J Am Coll Cardiol. 2019;1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gori M, Gupta DK, Claggett B, Selvin E, Folsom AR, Matsushita K, Bello NA, Cheng S, Shah A, Skali H, et al. Natriuretic peptide and high‐sensitivity troponin for cardiovascular risk prediction in diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2016;677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hillis GS, Welsh P, Chalmers J, Perkovic V, Chow CK, Li Q, Jun M, Neal B, Zoungas S, Poulter N, et al. The relative and combined ability of high‐sensitivity cardiac troponin T and N‐terminal pro‐B‐type natriuretic peptide to predict cardiovascular events and death in patients with type 2 diabetes. Diabetes Care. 2014;295–303. [DOI] [PubMed] [Google Scholar]
- 10. Bhalla MA, Chiang A, Epshteyn VA, Kazanegra R, Bhalla V, Clopton P, Krishnaswamy P, Morrison LK, Chiu A, Gardetto N, et al. Prognostic role of B‐type natriuretic peptide levels in patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2004;1047–1052. [DOI] [PubMed] [Google Scholar]
- 11. Scirica BM, Bhatt DL, Braunwald E, Raz I, Cavender MA, Im K, Mosenzon O, Udell JA, Hirshberg B, Pollack PS, et al. Prognostic implications of biomarker assessments in patients with type 2 diabetes at high cardiovascular risk: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;989–998. [DOI] [PubMed] [Google Scholar]
- 12. Rørth R, Jhund PS, Kristensen SL, Desai AS, Køber L, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, et al. The prognostic value of troponin T and N‐terminal pro B‐type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur J Heart Fail. 2019;40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonso N, Lupón J, Barallat J, de Antonio M, Domingo M, Zamora E, Moliner P, Galán A, Santesmases J, Pastor C, et al. Impact of diabetes on the predictive value of heart failure biomarkers. Cardiovasc Diabetol. 2016;151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaede P, Hildebrandt P, Hess G, Parving HH, Pedersen O. Plasma N‐terminal pro‐brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia. 2005;156–163. [DOI] [PubMed] [Google Scholar]
- 15. Bidadkosh A, Lambooy SPH, Heerspink HJ, Pena MJ, Henning RH, Buikema H, Deelman LE. Predictive properties of biomarkers GDF‐15, NTproBNP, and hs‐TnT for morbidity and mortality in patients with type 2 diabetes with nephropathy. Diabetes Care. 2017;784–792. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Uno H, Jarolim P, Desai AS, de Zeeuw D, Eckardt KU, Ivanovich P, Levey AS, Lewis EF, McGill JB, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin‐alfa) Therapy (TREAT). Am Heart J. 2011;748–755.e3. [DOI] [PubMed] [Google Scholar]
- 17. Wolsk E, Claggett B, Pfeffer MA, Diaz R, Dickstein K, Gerstein HC, Lawson FC, Lewis EF, Maggioni AP, McMurray JJV, et al. Role of B‐type natriuretic peptide and N‐terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and type 2 diabetes mellitus. J Am Heart Assoc. 2017;9:e004743 DOI: 10.1161/JAHA.116.004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Hall C, McCabe CH, Braunwald E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;1988–1995. [DOI] [PubMed] [Google Scholar]
- 19. Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, et al.; ALTITUDE Investigators . Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;2204–2213. [DOI] [PubMed] [Google Scholar]
- 20. Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, et al. Aliskiren trial in type 2 diabetes using cardio‐renal endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant. 2009;1663–1671. [DOI] [PubMed] [Google Scholar]
- 21. Seferovic JP, Pfeffer MA, Claggett B, Desai AS, de Zeeuw D, Haffner SM, McMurray JJV, Parving HH, Solomon SD, Chaturvedi N. Three‐question set from Michigan Neuropathy Screening Instrument adds independent prognostic information on cardiovascular outcomes: analysis of ALTITUDE trial. Diabetologia. 2018;581–588. [DOI] [PubMed] [Google Scholar]
- 22. Looker HC, Colombo M, Agakov F, Zeller T, Groop L, Thorand B, Palmer CN, Hamsten A, de Faire U, Nogoceke E, et al.; SUMMIT Investigators . Protein biomarkers for the prediction of cardiovascular disease in type 2 diabetes. Diabetologia. 2015;1363–1371. [DOI] [PubMed] [Google Scholar]
- 23. Gerstein HC, Paré G, McQueen MJ, Haenel H, Lee SF, Pogue J, Maggioni AP, Yusuf S, Hess S; Outcome Reduction With Initial Glargine Intervention Trial Investigators . Identifying novel biomarkers for cardiovascular events or death in people with dysglycemia. Circulation. 2015;2297–2304. [DOI] [PubMed] [Google Scholar]
- 24. Idzerda NMA, Persson F, Pena MJ, Brenner BM, Brunel P, Chaturvedi N, McMurray JJ, Parving HH, de Zeeuw D, Heerspink HJL. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) predicts the cardio‐renal response to aliskiren in patients with type 2 diabetes at high renal and cardiovascular risk. Diabetes Obes Metab. 2018;2899–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fulks M, Kaufman V, Clark M, Stout RL. NT‐proBNP predicts all‐cause mortality in a population of insurance applicants, follow‐up analysis and further observations. J Insur Med. 2017;107–113. [DOI] [PubMed] [Google Scholar]
- 26. Pfister R, Tan D, Thekkanal J, Hellmich M, Erdmann E, Schneider CA. NT‐pro‐BNP measured at discharge predicts outcome in multimorbid diabetic inpatients with a broad spectrum of cardiovascular disease. Acta Diabetol. 2007;91–97. [DOI] [PubMed] [Google Scholar]
- 27. Bruno G, Landi A, Barutta F, Ghezzo G, Baldin C, Spadafora L, Schimmenti A, Prinzis T, Cavallo Perin P, Gruden G. N‐terminal probrain natriuretic peptide is a stronger predictor of cardiovascular mortality than C‐reactive protein and albumin excretion rate in elderly patients with type 2 diabetes: the Casale Monferrato population‐based study. Diabetes Care. 2013;2677–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, Selvin E, Coresh J, Konety S, Butler KR, et al. Racial differences in circulating natriuretic peptide levels: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2015;9:e001831 DOI: 10.1161/JAHA.115.001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. York MK, Gupta DK, Reynolds CF, Farber‐Eger E, Wells QS, Bachmann KN, Xu M, Harrell FE Jr, Wang TJ. B‐type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol. 2018;2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;1590–1595. [DOI] [PubMed] [Google Scholar]
- 31. Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, Landaas S, Rouleau JL, Domanski MJ, Hall C, et al.; PEACE Investigators . Prognostic value of B‐Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;205–214. [DOI] [PubMed] [Google Scholar]
- 32. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, et al. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the CHARGE‐AF Consortium of community‐based cohort studies. Europace. 2014;1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maruyama K, Shiga T, Iijima M, Moriya S, Mizuno S, Toi S, Arai K, Ashihara K, Abe K, Uchiyama S. Brain natriuretic peptide in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;967–972. [DOI] [PubMed] [Google Scholar]
- 34. Luchner A, Burnett JC, Jougasaki M, Hense HW, Riegger GA, Schunkert H. Augmentation of the cardiac natriuretic peptides by beta‐receptor antagonism: evidence from a population‐based study. J Am Coll Cardiol. 1998;1839–1844. [DOI] [PubMed] [Google Scholar]
- 35. Kerkelä R, Ulvila J, Magga J. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J Am Heart Assoc. 2015;9:e002423 DOI: 10.1161/JAHA.115.002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novartis Global . Novartis Position on Clinical Study Transparency—Clinical Study Registration, Results Reporting and Data Sharing. Available at: https://www.novartis.com/our-science/clinical-trials/clinical-trial-information-disclosure. Accessed August 18, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S2
Tables S1–S4
Figure S1


