Abstract
Background
The prognostic significance of chronic kidney disease (CKD) in severe aortic stenosis is poorly understood and no studies have yet evaluated the effect of aortic‐valve replacement (AVR) versus conservative management on long‐term mortality by stage of CKD.
Methods and Results
We included 4119 patients with severe aortic stenosis. The population was divided into 4 groups according to the baseline estimated glomerular filtration rate: no CKD, mild CKD, moderate CKD, and severe CKD. The 5‐year survival rate was 71±1% for patients without CKD, 62±2% for those with mild CKD, 54±3% for those with moderate CKD, and 34±4% for those with severe CKD (P<0.001). By multivariable analysis, patients with moderate or severe CKD had a significantly higher risk of all‐cause (hazard ratio [HR] [95% CI]=1.36 [1.08–1.71]; P=0.009 and HR [95% CI]=2.16 [1.67–2.79]; P<0.001, respectively) and cardiovascular mortality (HR [95% CI]=1.39 [1.03–1.88]; P=0.031 and HR [95% CI]=1.69 [1.18–2.41]; P=0.004, respectively) than patients without CKD. Despite more symptoms, AVR was less frequent in moderate (P=0.002) and severe CKD (P<0.001). AVR was associated with a marked reduction in all‐cause and cardiovascular mortality versus conservative management for each CKD group (all P<0.001). The joint‐test showed no interaction between AVR and CKD stages (P=0.676) indicating a nondifferentialeffect of AVR across stages of CKD. After propensity matching, AVR was still associated with substantially better survival for each CKD stage relative to conservative management (all P<0.0017).
Conclusions
In severe aortic stenosis, moderate and severe CKD are associated with increased mortality and decreased referral to AVR. AVR markedly reduces all‐cause and cardiovascular mortality, regardless of the CKD stage. Therefore, CKD should not discourage physicians from considering AVR.
Keywords: aortic stenosis, aortic valve replacement, chronic kidney disease, kidney failure, outcome, survival
Subject Categories: Valvular Heart Disease, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- AVA
aortic valve area
- AVR
aortic valve replacement
- NYHA
New York Heart Association
- TAVR
transcatheter aortic valve replacement
Clinical Perspective
What Is New?
In severe aortic stenosis, moderate or severe chronic kidney disease (CKD) are associated with increased all‐cause and cardiovascular mortality.
Patients with severe aortic stenosis and moderate or severe CKD are less often referred for aortic valve replacement than those with no or mild CKD.
Aortic valve replacement is associated with a substantial reduction in all‐cause and cardiovascular mortality, regardless of the CKD stage, even after propensity matching, and in patients with severe CKD, transcatheter aortic valve replacement is associated with reduced cardiovascular mortality compared to surgical aortic valve replacement.
What Are the Clinical Implications?
Patients with severe aortic stenosis and moderate or severe CKD should be closely followed.
In severe aortic stenosis, moderate or severe CKD should not discourage physicians from considering aortic valve replacement.
Aortic stenosis (AS) is the most common valvular heart disease in Western countries, particularly affecting the elderly, and with a higher prevalence in patients with chronic kidney disease (CKD), including those with moderate to severe stage disease. 1 Aortic‐valve calcification also appears to progress more rapidly in patients with end‐stage renal disease than those with normal renal function. 2 , 3
CKD is one of the fastest‐growing non‐communicable diseases 4 that promotes cardiovascular calcification, increasing morbidity and mortality. 5 Indeed, patients with CKD are more likely to die from cardiovascular events than from renal failure. 5 Several studies have suggested that survival is poorer for patients with AS and concomitant CKD than for those with isolated CKD or AS. 6 , 7 CKD is also associated with poorer outcomes after surgical 8 or transcatheter 9 aortic‐valve replacement (AVR) compared with patients with normal kidney function, which may discourage physicians from considering AVR for some patients. 10 To date, there are no specific recommendations about the follow‐up and management of patients with severe AS and associated CKD. 11 , 12 Indeed, little is known about the prognostic significance of CKD stages in patients with severe AS, as previous studies mostly included patients with mild or moderate AS. 6 , 7 Furthermore, no studies have yet evaluated the effect of AVR on mortality compared to conservative management by stage of CKD.
Our objectives were therefore (1) to analyze the relationship between CKD stage and all‐cause and cardiovascular mortality, and (2) to evaluate the effect of AVR relative to conservative management according to kidney function in a large registry of patients with severe AS enrolled in 3 tertiary centers (Amiens, Brussels, and Lille).
Methods
Inclusion and Exclusion Criteria
The data that support the findings of this study are available from the corresponding author upon reasonable request. Consecutive patients from 2000 to 2018, aged ≥18 years, diagnosed with AS (aortic leaflet calcification with a reduction of systolic movement and peak aortic jet velocity [Vmax]>2.5 m/s) in the echocardiography laboratories of 2 French (Amiens and Lille) and one Belgian (Brussels) tertiary center were identified and included in an electronic database. Exclusion criteria were: (1) aortic and/or mitral regurgitation of more than mild severity; (2) prosthetic valves, congenital heart disease (with the exception of the bicuspid valve), supravalvular or subvalvular AS, or dynamic left ventricular (LV) outflow tract obstruction; (3) mitral stenosis; and (4) refusal to participate in the study. The present analysis was based on a study of 4119 patients with severe AS (aortic valve area [AVA] <1 cm2 or indexed AVA <0.6 cm2/m2). Clinical and demographic baseline characteristics were collected. The Charlson comorbidity index was obtained for each patient. 13 Creatinine was measured (within 2 months around echocardiography) by isotope dilution mass spectrometry, standardized in each study center. The estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration equation based on serum creatinine values. 14 The study population was categorized based on the eGFR, according to Kidney Disease: Improving Global Outcomes staging. 15 CKD was defined as an eGFR <60 mL/min per 1.73 m2. Patients with CKD under maintenance dialysis were identified by the medical report. Patients were excluded if they had acute kidney injury, defined by a rapid increase in serum creatinine >50% or ≥0.3 mg/dL from the mean creatinine values or CKD status within 3 months before the echocardiogram. The study was approved by an independent ethics committee and conducted in accordance with institutional policies, national legal requirements, and the revised principles of the Declaration of Helsinki.
Echocardiography
All patients underwent comprehensive Doppler‐echocardiographic assessment with commercially available ultrasound systems. Aortic flow was systematically recorded by continuous‐wave Doppler, from several views. The view identifying the highest velocities was used to determine the Vmax. Three consecutive measurements in this view for patients in sinus rhythm or 5 for patients in atrial fibrillation (AF), were systematically averaged. AVA was calculated using the continuity equation. The left ventricular ejection fraction (LVEF) was calculated using Simpson's biplane method. 16
Follow‐Up and EndPoints
Median follow‐up was 3.3 (interquartile range: 1.5–5.8) years. Patients were followed by clinical consultations and echocardiography at the outpatient clinics of the 3 tertiary‐care centers. A few patients were followed at public hospitals or private practices by referring cardiologists working in collaboration with the tertiary centers. Given the retrospective nature of the study, informed consent was waived, and all the patients agreed to participate in the study when contacted for follow‐up. Events were ascertained by direct patient interview and clinical examination and/or repeated follow‐up letters, questionnaires, and telephone calls to physicians, patients, and (if necessary) next of kin. Clinical decisions on medical management and referral for AVR were made by the cardiology team, including cardiologists and cardiac surgeons, with the approval of the patient's physician and based on current practice guidelines and the operative risk. Early AVR was defined as AVR performed within the first 3 months of inclusion. Patients not undergoing AVR during the first 3 months after baseline echocardiography were considered as conservatively managed. Survival analysis in this conservatively managed group continued until the last follow‐up on medical management (censored at AVR). The primary end point was all‐cause mortality and the secondary end point cardiovascular mortality. Perioperative mortality was defined as death occurring within 30 days of AVR, or during the hospitalization if the patient was hospitalized for a longer period.
Statistical Analysis
The study population was divided in into 4 groups according to the eGFR at the time of inclusion: normal, with an eGFR ≥60 mL/min per 1.73 m2; mild CKD, with an eGFR of 45 to 59 mL/min per 1.73 m2; moderate CKD, with an eGFR of 30 to 44 mL/min per 1.73 m2; and severe CKD, with an eGFR <30 mL/min per 1.73 m2. The normal group was used as the reference. Continuous variables are expressed as the means (±1 SD) or medians (25th and 75th percentiles), and categorical variables as frequency percentages and counts. The relationship between baseline continuous variables and those of the groups was explored using 1‐way ANOVA tests (for normally distributed variables) or Kruskal–Wallis tests (for non‐normally distributed variables). Pearson's χ2 statistic or Fisher's exact test were used to examine the association between baseline categorical variables and those of the groups. The significance of the differences between the referent group and the others was examined if they were significant across groups. Individual differences were compared using Mann–Whitney U tests (with a Bonferroni correction for multiple comparisons) and Tukey tests for normally distributed data. Five‐year survival rates (±1 standard error) were estimated using the Kaplan–Meier method and compared using 2‐sided log‐rank tests. Multivariable analyses of all‐cause and cardiovascular mortality were performed using Cox proportional hazards models. We entered covariates considered to have potential prognostic impact into the model on an epidemiological basis. They were age, sex, body surface area (BSA), Charlson comorbidity index (not including age and renal failure), New York Heart Association (NYHA) class, history of hypertension, coronary artery disease, AF, LVEF, and Vmax. The effect of AVR on outcome was analyzed as a time‐dependent covariate using the entire follow‐up period. 17 Pre‐selected baseline prognostic variables, which differed between patients depending on their management (i.e. AVR or conservative), were used in the construction of the propensity score built at each stage of CKD: Age, sex, AVA, Vmax, stroke volume, Charlson index, Euroscore II, LVEF, systolic pulmonary pressures, NYHA, AF, BSA, prior myocardial infarction, hypertension, and coronary artery disease. Quadric terms were added for age, pulmonary pressures, and Vmax to the propensity model to achieve a better balance after propensity score weighting. The probability of receiving AVR was assessed with a multivariable logistic regression model in each of 10 multiple imputations replicates for missing independent variables. The Markov chain Monte Carlo method was used for multiple imputations. The final propensity score was the average of these 10 probabilities. For average treatment effect on the treated analysis, weights were calculated to resemble the treated population (AVR), i.e. treated patients had a weight equal to one while for untreated patients, their weights corresponded to the odds of being treated their probability of being treated. 18 , 19 This weighting method permits the estimation of the treatment effect on the population who resemble the patients receiving AVR. Balance after weighting was defined as a standardized mean difference below 0.25. 20 Effect of AVR was assessed with a weighted time varying covariate Cox model with robust estimation of the variance. An interaction term between AVR and CKD stage was added to evaluate the difference in AVR effect between CKD stages. Survival curves were plotted with Kaplan–Meier in the weighted population. All tests were 2‐sided, with a level of significance set at P<0.05. Statistical analyses were performed using SPSS version 18.0 software (IBM, Armonk, New York), SAS® software (version 9.4, SAS Institute Inc., Cary, NC), and R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
The study population consisted of 4119 patients (1982 males [48.1%], mean age 78 years, mean eGFR 68 mL/min per 1.73 m2). The median eGFR was 67 (51–83) mL/min per 1.73 m2. Kidney function was normal (eGFR ≥60 mL/min per 1.73 m2) for 2552 patients (62%) and 1567 patients (38%) had CKD: 860 (55%) with mild CKD (eGFR 45–59 mL/min per 1.73 m2), 477 (30%) with moderate CKD (eGFR 30–44 mL/min per 1.73 m2), and 230 (15%) with severe CKD (eGFR<30 mL/min per 1.73 m2). Among patients with severe CKD, 53 (23%) were under maintenance dialysis.
The demographic, clinical, and echocardiographic characteristics of the study population, according to eGFR group, are presented in Table 1. Patients with CKD were older (all P<0.001), more often women (all P<0.05), and more symptomatic, with higher NYHA stages (all P<0.001) than patients with normal renal function (eGFR ≥60 mL/min per 1.73 m2). Patients with CKD were more often diagnosed with hypertension (all P<0.05) and AF (all P<0.001) than those with normal kidney function. Patients with severe CKD were more often diabetic (P<0.001), had more severe coronary artery disease (P<0.05), and more often had a history of myocardial infarction (P<0.001) than patients without CKD (Table 1).
Table 1.
Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Population With Severe Aortic Stenosis According to CKD Stage
| Variable | No CKD (n=2552) | Mild CKD (n=860) | Moderate CKD (n=477) | Severe CKD n=230) | P Value |
|---|---|---|---|---|---|
| Demographics, baseline data and symptoms | |||||
| Age, y | 76±10 | 80±7* | 81±7* | 80±8* | <0.001 |
| Male sex (n, %) | 1362 (53.4%) | 362 (42.1%)‡ | 162 (34.0%)‡ | 96 (41.7%)‡ | <0.001 |
| Body surface area, m2 | 1.83±0.2 | 1.82±0.2 | 1.80±0.2‡ | 1.81±0.2 | 0.015 |
| NYHA (n, %) | |||||
| 1–2 | 1810 (70.9%) | 549 (63.8%)* | 264 (55.3%)* | 107 (46.5%)* | <0.001 |
| 3–4 | 742 (29.1%) | 311 (36.2%)* | 213 (44.7%)* | 123 (53.5%)* | |
| Medical history and risk factors | |||||
| Hypertension (n, %) | 1738 (68.1%) | 640 (74.4%)‡ | 384 (80.5%)* | 191 (83.0%)* | <0.001 |
| Diabetes mellitus (n, %) | 613 (24.0%) | 237 (27.6%) | 139 (29.1%) | 96 (41.7%)* | <0.001 |
| Coronary artery disease (n, %) | 1156 (45.3%) | 422 (49.1%) | 239 (50.1%) | 120 (52.2%)‡ | 0.033 |
| Prior myocardial infarction (n, %) | 174 (6.8%) | 82 (9.5%) | 63 (13.2%)* | 33 (14.3%)* | <0.001 |
| Prior atrial fibrillation (n, %) | 543 (21.3%) | 266 (30.9%)* | 162 (34.0%)* | 95 (41.3%)* | <0.001 |
| Charlson comorbidity index (without age and renal failure) | 2.6±2.1 | 3.5±2.1* | 2.2±1.9* | 2.7±2.3 | <0.001 |
| Euroscore II§ | 2.7±2.8 | 3.9±3.7* | 4.4±3.9* | 5.0±5.1* | <0.001 |
| Echocardiographic parameters | |||||
| Aortic valve | |||||
| Aortic valve area, cm2 | 0.71 (0.57–0.85) | 0.70 (0.56–0.84) | 0.69 (0.56–0.84) | 0.73 (0.57–0.85) | 0.330 |
| Peak aortic jet velocity, m/s | 4.30 (3.80–4.79) | 4.26 (3.70–4.78)‡ | 4.10 (3.60–4.53)* | 4.03 (3.47–4.56)* | <0.001 |
| Transaortic mean pressure gradient, mm Hg | 46 (35–58) | 45 (33–58) | 41 (30–53)* | 40 (29–51)* | <0.001 |
| Indexed stroke volume, mL/m2 ‖ | 39 (32–45) | 37 (30–44) | 36 (30–44)‡ | 36 (29–46)‡ | 0.001 |
| Left and right ventricular function | |||||
| LV end‐diastolic diameter, mm | 47 (42–52) | 47 (42–53) | 47 (43–53) | 49 (43–53)‡ | 0.012 |
| LV end‐systolic diameter, mm | 30 (25–35) | 30 (24–35) | 31 (26–38)* | 31 (27–38)* | <0.001 |
| Ejection fraction (%) | 62 (56–67) | 62 (65–68) | 59 (51–65)* | 60 (50–65)* | <0.001 |
| sPAP, mm Hg¶ | 30 (25–38) | 33 (25–41)‡ | 35 (26–45)* | 38 (30–51)* | <0.001 |
Continuous normally distributed variables are expressed as mean±1 SD, non‐normally distributed continuous variables are expressed as median (25th and 75th percentiles), and categorical variables as percentages and counts. AS indicates aortic stenosis; CKD, chronic kidney disease; LV, left ventricular; NYHA New York Heart Association class; and sPAP, systolic pulmonary artery pressure.
P<0.001 individual category vs no CKD.
P<0.05 individual category vs no CKD.
Available for 3796 patients.
Available for 3939 patients.
Available for 3241 patients.
For echocardiographic variables, there were no differences between groups in terms of AVA (P=0.330), but Vmax decreased with decreasing renal function, whereas pulmonary pressure increased (all P<0.05). Patients with moderate and severe CKD had lower indexed stroke volumes (both P<0.05) and LVEFs and greater end‐systolic diameters (all P<0.001) than patients with normal kidney function (Table 1).
Outcome Impact of eGFR
During follow‐up, 1414 deaths (34.3%) were recorded, 930 of which were cardiovascular‐related (65.8%). Estimated 5‐year survival was 71±1% for patients without CKD, 62±2% for patients with mild CKD, 54±3% for patients with moderate CKD, and 34±4% for patients with severe CKD (P<0.001) (Figure 1A).
Figure 1. Kaplan–Meier (A) and adjusted (B) survival curves according to CKD stage.

CKD indicates chronic kidney disease.
By multivariable analysis, eGFR as a continuous variable, was independently associated with overall mortality (adjusted HR [95% CI]=0.98 [0.97–0.99] per 1‐mL decrement, P<0.001) and cardiovascular mortality (adjusted HR [95% CI]=0.99 [0.98–0.99] per 1‐mL decrement, P=0.003), after adjustments for age, sex, BSA, Charlson comorbidity index, NYHA class, history of hypertension, coronary artery disease, AF, LVEF, Vmax, and AVR treated as a time‐dependent variable. By multivariable analysis, patients with mild CKD had a similar risk of all‐cause death (P=0.56) and cardiovascular death (P=0.60) as patients without CKD, whereas patients with moderate or severe CKD had a significantly higher risk of all‐cause mortality (adjusted HR [95% CI] =1.36 [1.08–1.71], P=0.009 and HR [95% CI]=2.16 [1.67–2.79], P<0.001, respectively) (Figure 1B) and cardiovascular mortality (adjusted HR [95% CI]=1.39 [1.03–1.88], P=0.031 and adjusted HR [95% CI]=1.69 [1.18–2.41], P=0.004, respectively) (Table 2). Compared with patients with mild CKD, those with moderate CKD and severe CKD had a significantly higher risk of all‐cause mortality (adjusted HR [95% CI] =1.33 [1.01–1.74], P=0.040 and HR [95% CI]=1.96 [1.46–2.63], P<0.001, respectively) and cardiovascular mortality (adjusted HR [95% CI]=1.30 [1.01–2.01], P=0.047 and adjusted HR [95% CI]=1.51 [1.02–2.27], P=0.046, respectively). Compared with patients with moderate CKD, those with severe CKD had a significantly higher risk of all‐cause mortality (adjusted HR [95% CI] =1.57 [1.16–2.12], P=0.003).
Table 2.
Relative Risk of All‐Cause and Cardiovascular Death During Follow‐Up Associated With CKD
| Univariate Analysis HR (95% CI) | P Value | Multivariable Analysis* HR (95% CI) | P Value | |
|---|---|---|---|---|
| All cause death | ||||
| No CKD | Reference | Reference | ||
| Mild CKD | 1.44 (1.27–1.65) | <0.001 | 1.04 (0.85–1.29) | 0.56 |
| Moderate CKD | 1.95 (1.67–2.26) | <0.001 | 1.36 (1.08–1.71) | 0.009 |
| Severe CKD | 3.18 (2.65–3.81) | <0.001 | 2.16 (1.76–2.79) | <0.001 |
| Cardiovascular death | ||||
| No CKD | Reference | Reference | ||
| Mild CKD | 1.43 (1.22–1.68) | <0.001 | 1.08 (0.82–1.43) | 0.60 |
| Moderate CKD | 2.03 (1.69–2.43) | <0.001 | 1.39 (1.03–1.88) | 0.031 |
| Severe CKD | 2.87 (2.29–3.61) | <0.001 | 1.69 (1.18–2.41) | 0.004 |
Results of cox analyses. CI indicates confidence interval; CKD, chronic kidney disease; and HR, hazard ratio.
After adjustment for age, sex, body surface area, Charlson comorbidity index, New York Heart Association class, history of hypertension, coronary artery disease, atrial fibrillation, left ventricular ejection fraction, Vmax, and aortic valve replacement treated as a time‐dependent variable.
Impact of AVR According to CKD Stage
During follow‐up, 3080 patients (74.8%) underwent AVR (including 2531 [82.2%] surgical AVR and 549 [17.8%] by the transcatheter technique [TAVR]): 1970 (77.2%) in the normal kidney‐function group, 642 (74.7%) in the mild‐CKD group, 338 (70.9%) in the moderate‐CKD group, and 130 (56.5%) in the severe‐CKD group (P<0.001). Among these 3080 patients, 2510 (81.5%) underwent early AVR.
One hundred and one patients (3.3%) died during the perioperative period (after surgical AVR [89/2531:3.5%] or after TAVR [12/549:2.2%]): 48 patients (2.4%) in the normal kidney‐function group, 21 (3.3%) in the mild‐CKD group, 22 (6.5%) in the moderate‐CKD group, and 10 (7.7%) in the severe‐CKD group (P<0.001). On multivariate logistic regression analysis, after adjustments for age, sex, LVEF, and comorbidity, severe CKD was independently associated with perioperative mortality (adjusted odd ratio [95%]=2.25 [1.15–4.42]; P=0.018). During follow‐up, 10 patients (5.6% of patients with severe CKD at baseline not under maintenance dialysis) required definitive renal replacement therapy. Among these 10 patients, 9 experienced AVR (mean time between AVR and dialysis 9±15 months, median 3 [0.75–19.5] months) and 1 had been conservatively managed.
The baseline characteristics of the study population according to management (i.e. early AVR [surgical or transcatheter] or conservative management) are presented in Table S1. By univariate and multivariable analyses, early AVR (Table 3, Figure 2) was associated with a marked reduction in all‐cause and cardiovascular mortality in all groups, even for the severe‐CKD group (all P<0.001). After adjustment, there was no difference between surgical AVR and TAVR in terms of all‐cause or cardiovascular mortality for patients with no CKD (P=0.87 and P=0.70, respectively), mild CKD (P=0.84 and P=0.69, respectively), or moderate CKD (P=0.85 and P=0.84, respectively). In patients with severe CKD, TAVR was associated with reduced cardiovascular mortality compared with surgical AVR (P=0.040). The joint test showed that the interaction between AVR and CKD stages was not significant (P=0.676), indicating a non‐differential effect of AVR across stages of CKD.
Table 3.
Reduction in the Relative Risk of Events (All‐Cause and Cardiovascular Death) by Chronic Kidney Disease Stage According to Initial Management (Early AVR vs Conservative Management)
| Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI)* | P Value | |
|---|---|---|---|---|
| All cause death | ||||
| No CKD | 0.24 (0.21–0.29) | <0.001 | 0.21 (0.16–0.29) | <0.001 |
| Mild CKD | 0.35 (0.28–0.45) | <0.001 | 0.22 (0.13–0.35) | <0.001 |
| Moderate CKD | 0.51 (0.38–0.67) | <0.001 | 0.26 (0.15–0.46) | <0.001 |
| Severe CKD | 0.35 (0.24–0.51) | <0.001 | 0.24 (0.13–0.46) | <0.001 |
| Cardiovascular death | ||||
| No CKD | 0.28 (0.22–0.34) | <0.001 | 0.18 (0.12–0.27) | <0.001 |
| Mild CKD | 0.45 (0.34–0.60) | <0.001 | 0.13 (0.07–0.27) | <0.001 |
| Moderate CKD | 0.62 (0.44–0.87) | 0.007 | 0.21 (0.10–0.43) | <0.001 |
| Severe CKD | 0.44 (0.28–0.70) | 0.001 | 0.17 (0.06–0.46) | <0.001 |
AVR indicates aortic valve replacement; CI, confidence interval; CKD, chronic kidney disease; and HR, hazard ratio.
After adjustment for age, sex, body surface area, Charlson comorbidity index, Euroscore II, New York Heart Association class, history of hypertension, coronary artery disease, atrial fibrillation, left ventricular ejection fraction and Vmax.
Figure 2. Adjusted survival curves according to management (i.e. AVR or conservative) for each CKD stage.

AVR indicates aortic valve replacement; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; and RR, relative risk.
After propensity matching (after weighting by the odds: average treatment effect on the treated analysis), the AVR group and conservative management group were comparable in terms of baseline characteristics, including age, comorbidity, AS severity, and LV repercussions for each CKD stage (Tables S2 through S4). In this propensity analysis, AVR remained associated with substantially better survival for each CKD stage than conservative management (Cox time varying covariate P<0.001 for mild and moderate CKD and Cox time varying covariate P=0.0016 for severe CKD) (Figure 3). Results were similar without multiple imputations for missing variables (all P<0.001).
Figure 3. Kaplan–Meier survival curves according to management (i.e. AVR or conservative) for each CKD stage after propensity matching.
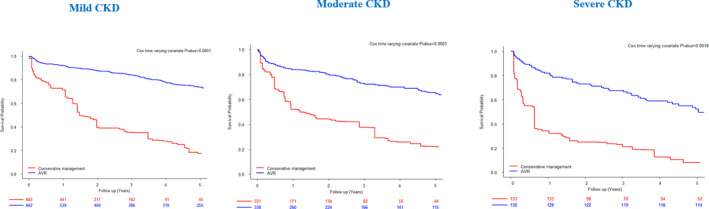
AVR indicates aortic valve replacement; and CKD, chronic kidney disease.
Discussion
This analysis, based on a large registry of patients with severe AS managed in routine clinical practice, shows that renal failure has a considerable impact on outcomes. Indeed, after adjusting for established outcome predictors, including age, symptoms, comorbidity, AS severity, LVEF, and AVR treatment as a time dependent variable, moderate and severe CKD were associated with a substantial increase in all‐cause and cardiovascular mortality. AVR was performed far less frequently in patients with moderate or severe CKD, although these patients had more symptoms and similar AVAs than patients without CKD. However, despite higher perioperative mortality, AVR was associated with a dramatic reduction of all‐cause and cardiovascular death at 5 years for all stages of CKD, even after propensity matching. Overall, these data show that CKD should not dissuade physicians from considering AVR in patients with severe AS.
CKD is a common condition in elderly patients, 4 leading to cardiovascular calcifications associated with increased morbidity and mortality. 5 In this context, AS is widespread and found in 9.5% of patients with CKD compared with 3.5% of the general elderly population. 7 The prevalence of AS increases as eGFR decreases, 1 especially in dialysis patients. In a retrospective series of 2408 patients undergoing surgical AVR, the prevalence of CKD was reported to be ≈33.7%, including 7.2% with severe CKD. 8 In our study, the prevalence of CKD was 38%, with 17% of patients having moderate or severe CKD, consistent with the Japanese registry CURRENT AS, 21 in which 15% of patients with severe AS had moderate or severe CKD. As expected, the prevalence of CKD was higher in the inoperable/high‐risk patient cohort of the PARTNER trial, in which 70% of patients had moderate or severe CKD. 9
In CKD, numerous metabolic contributors are involved in the development of degenerative valvular lesions and the exact contribution of each component and their synergy is yet to be understood. The clinical impact of hyperphosphatemia and elevated phosphate x calcium product has long been established. 22 Imbalance in calcium/phosphate homeostasis can induce the differentiation of valvular interstitial cells (VICs) to an osteoblastic phenotype, with increased expression of bone‐related mediators. 23 In addition, phosphate is also capable of activating apoptotic pathways in VICs and inducing the production of apoptotic bodies, 23 which promote the formation of amorphous calcium deposits.
Previous studies have reported an increased risk of mortality for patients with CKD and AS. 6 , 24 , 25 In a report of 622 asymptomatic patients with severe AS, the outcome was poorer for the 27 patients with severe CKD. 24 In a population of 839 patients with AS, those with severe AS and CKD had similar risks of cardiac and all‐cause death, and both rates were significantly greater than those of patients with CKD or AS alone, although patients with mild/moderate AS and CKD had a greater proportion of noncardiac deaths during follow‐up. 6 Accordingly, in our study, which is the largest study of patients with severe AS focused on CKD, patients with moderate and severe CKD had a higher rate of all‐cause and cardiovascular mortality than those without CKD after adjustment for established outcome predictors. Conversely, patients with mild CKD had outcomes comparable to those of patients with normal kidney function. This finding can be explained by the fact that early‐stage CKD has a smaller impact on metabolic disorders and surgical decision making. Age is also known to have a significant impact on AS mortality, 24 and this may explain the higher mortality observed in the mild CKD group (81 years) than the non‐CKD group (76 years), which did not persist after adjustment.
According to current guidelines, AVR is a class I indication for severe symptomatic AS. 11 However, the potential benefit of AVR in patients with CKD is still uncertain and some patients may be recused from AVR because of CKD. 10 Indeed, Thourani et al. 8 reported poor long‐term survival for 59 patients with severe CKD undergoing surgical AVR and 114 requiring dialysis relative to that of patients with normal renal function. In a recent analysis of 28 716 patients undergoing TAVR in Germany, 31.5% had CKD ≥ stage 3 (eGFR <45 mL/min per 1.73 m2). 26 Despite being a less invasive procedure, TAVR for patients with severe CKD has still been reported to be associated with increased mortality, 9 especially for patients undergoing dialysis. 27 Therefore, physicians may not propose AVR to certain patients with CKD. 10 , 28 Indeed, in our study, AVR was performed much less frequently in patients with moderate or severe CKD, although these patients had more symptoms and similar AVAs than patients without CKD. No studies have yet compared the long‐term mortality of patients with severe AS at different stages of CKD according to management (i.e. AVR versus conservative management). We show that AVR is associated with a considerable reduction in all‐cause and cardiovascular mortality at 5 years for patients of all stages of CKD. AVR was consistently associated with improved outcomes, even for patients with severe CKD or those on dialysis, and even after adjustment or propensity matching. There was no difference between surgical AVR and TAVR in terms of survival for patients without CKD or those with mild or moderate CKD. However, for patients with severe CKD, TAVR was associated with reduced cardiovascular mortality relative to surgical AVR. Therefore, our results support AVR for patients with severe AS and CKD, even those with severe CKD, despite higher perioperative mortality of these patients.
Limitations
Our study was subject to the inherent limitations of those based on retrospective follow‐up data. The prognosis analysis was based on the eGFR at the time of enrolment and no data on renal events were collected during follow‐up. In addition, data on the etiology of the renal disease were not available in our database. The presence of frailty or other life‐threatening comorbidities not included in the Charlson index and the EuroSCORE II might have influenced the decision to perform surgery. Unfortunately, data on AS hemodynamic progression and on the occurrence of bioprosthetic dysfunction during follow‐up were not available in our database. The presence of sodium overload in patients with severe CKD may complicate assessment of the symptomatic status of AS. Our study could not estimate the prevalence of severe AS in the CKD patients because they were recruited in echocardiography laboratories. Finally, this study only concerned patients with severe AS and no significant valve regurgitation. Further studies are needed to evaluate the impact of AVR for other subsets of patients with AS and CKD.
Conclusions
In patients with severe AS, the presence of moderate or severe CKD is associated with increased all‐cause and cardiovascular mortality, decreased referral to AVR, and increased perioperative mortality. AVR is associated with a marked reduction in all‐cause and cardiovascular mortality, regardless of the CKD stage, even among dialysis patients. Therefore, moderate and severe CKD should not discourage physicians from considering AVR.
Sources of Funding
This work was supported by the French Government through the National Research Agency (ANR) Program Investissements d'Avenir (ANR‐16‐RHUS‐0003_STOP‐AS).
Disclosures
None.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2020;9:e017190 DOI: 10.1161/JAHA.120.017190.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Vavilis G, Bäck M, Occhino G, Trevisan M, Bellocco R, Evans M, Lindholm B, Szummer K, Carrero JJ. Kidney dysfunction and the risk of developing aortic stenosis. J Am Coll Cardiol. 2019;305–314. [DOI] [PubMed] [Google Scholar]
- 2. Kim D, Shim CY, Hong GR, Cho IJ, Chang HJ, Ha JW, Chung N. Effect of end‐stage renal disease on rate of progression of aortic stenosis. Am J Cardiol. 2016;1972–1977. [DOI] [PubMed] [Google Scholar]
- 3. Ohara T, Hashimoto Y, Matsumura A, Suzuki M, Isobe M. Accelerated progression and morbidity in patients with aortic stenosis on chronic dialysis. Circ J. 2005;1535–1539. [DOI] [PubMed] [Google Scholar]
- 4. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;2038–2047. [DOI] [PubMed] [Google Scholar]
- 5. Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;85–97. [DOI] [PubMed] [Google Scholar]
- 6. Patel KK, Shah SY, Arrigain S, Jolly S, Schold JD, Navaneethan SD, Griffin BP, Nally JV, Desai MY. Characteristics and outcomes of patients with aortic stenosis and chronic kidney disease. J Am Heart Assoc. 2019;9:e009980 DOI: 10.1161/JAHA.118.009980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samad Z, Sivak JA, Phelan M, Schulte PJ, Patel U, Velazquez EJ. Prevalence and outcomes of left‐sided valvular heart disease associated with chronic kidney disease. J Am Heart Assoc. 2017;9:e006044 DOI: 10.1161/JAHA.117.006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thourani VH, Keeling WB, Sarin EL, Guyton RA, Kilgo PD, Dara AB, Puskas JD, Chen EP, Cooper WA, Vega JD, et al. Impact of preoperative renal dysfunction on long‐term survival for patients undergoing aortic valve replacement. Ann Thorac Surg. 2011;1798–1807. [DOI] [PubMed] [Google Scholar]
- 9. Thourani VH, Forcillo J, Beohar N, Doshi D, Parvataneni R, Ayele GM, Kirtane AJ, Babaliaros V, Kodali S, Devireddy C, et al. Impact of preoperative chronic kidney disease in 2,531 high‐risk and inoperable patients undergoing transcatheter aortic valve replacement in the PARTNER trial. Ann Thorac Surg. 2016;1172–1180. [DOI] [PubMed] [Google Scholar]
- 10. Ishii M, Taniguchi T, Morimoto T, Ogawa H, Masunaga N, Abe M, Yoshikawa Y, Shiomi H, Ando K, Kanamori N, et al. Reasons for choosing conservative management in symptomatic patients with severe aortic stenosis—observations from the CURRENT AS Registry. Circ J. 2019;1944–1953. [DOI] [PubMed] [Google Scholar]
- 11. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;2739–2791. [DOI] [PubMed] [Google Scholar]
- 12. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;2440–2492. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;373–383. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;17–28. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;233–270. [DOI] [PubMed] [Google Scholar]
- 17. Bohbot Y, Meester DE, de Ravenstein C, Chadha G, Rusinaru D, Belkhir K, Trouillet C, Pasquet A, Marechaux S, Vanoverschelde JL, et al. Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients with severe aortic stenosis. JACC Cardiovasc Imaging. 2019;38–48. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. 2017;76–90. [DOI] [PubMed] [Google Scholar]
- 20. Stuart EA, Lee BK, Leacy FP. Prognostic score‐based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;S84–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Kato T, et al. Sudden death in patients with severe aortic stenosis: observations from the CURRENT AS Registry. J Am Heart Assoc. 2018;9:e008397 DOI: 10.1161/JAHA.117.008397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maher E. Aortic and mitral valve calcification in patients with end‐stage renal disease. Lancet. 1987;875–877. [DOI] [PubMed] [Google Scholar]
- 23. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;2181–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;3290–3295. [DOI] [PubMed] [Google Scholar]
- 25. Kawase Y, Taniguchi T, Morimoto T, Kadota K, Iwasaki K, Kuwayama A, Ohya M, Shimada T, Amano H, Maruo T, et al. severe aortic stenosis in dialysis patients. J Am Heart Assoc. 2017;9:e004961 DOI: 10.1161/JAHA.116.004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lüders F, Kaier K, Kaleschke G, Gebauer K, Meyborg M, Malyar NM, Freisinger E, Baumgartner H, Reinecke H, Reinöhl J. Association of CKD with outcomes among patients undergoing transcatheter aortic valve implantation. Clin J Am Soc Nephrol. 2017;718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szerlip M, Zajarias A, Vemalapalli S, Brennan M, Dai D, Maniar H, Lindman BR, Brindis R, Carroll JD, Hamandi M, et al. Transcatheter aortic valve replacement in patients with end‐stage renal disease. J Am Coll Cardiol. 2019;2806–2815. [DOI] [PubMed] [Google Scholar]
- 28. Hensey M, Murdoch DJ, Sathananthan J, Wood DA, Webb JG. Impact of chronic kidney disease on decision making and management in transcatheter aortic valve interventions. Can J Cardiol. 2019;1188–1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


