Abstract
Background
In the PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation) Study, a simple, standardized, perioperative interruption strategy was provided for patients with nonvalvular atrial fibrillation taking direct oral anticoagulants (DOACs). Our objective was to define the factors associated with perioperative bleeding.
Methods and Results
We analyzed bleeding as the composite of major and clinically relevant nonmajor bleeding. Putative predictors of bleeding, and preoperative DOAC level were prospectively collected during recruitment. We used stratified logistic regression models for analysis. All statistical analyses were performed in R version 3.6.0. There were 3007 patients requiring perioperative DOAC interruption. More than one third of the included patients underwent a high bleeding risk procedure. The 30‐day rates of major and clinically relevant nonmajor bleeding were 3.02% in apixaban (n=1257), 2.84% in dabigatran (n=668), and 4.16% for rivaroxaban (n=1082). Multivariate analysis stratified by region found more bleeding for hypertension (odds ratio [OR], 1.79; 95% CI 1.07‐2.99; P=0.027), and prior bleeding (OR, 1.71; 95% CI, 1.08‐2.71; P=0.021). Surgical bleed risk classification (high‐ versus low‐risk) as a predictor of bleeding was only significant in the univariate analysis. The prediction model for major and clinically relevant nonmajor bleeding had an area under the curve of 0.71, and the preoperative DOAC level did not improve the area under the curve of the model.
Conclusions
In patients treated with DOACs who required an elective surgery/procedure and were managed with standardized DOAC interruption and resumption, there we did not find reversible risk factors for bleeding, suggesting that adjustment of the PAUSE management protocol to mitigate against bleeding is not needed.
Keywords: atrial fibrillation, bleeding, direct oral anticoagulant, surgery
Subject Categories: Thrombosis, Anticoagulants, Intracranial Hemorrhage, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- CrCl
creatinine clearance
- CRNM
clinically relevant nonmajor bleeding
- DOAC
direct oral anticoagulant
- MB
major bleeding
- PAUSE
Perioperative Anticoagulant Use for Surgery Evaluation
Clinical Perspective
What Is New?
A simplified, standardized, pharmacokinetic‐based perioperative interruption scheme clinically anchored on surgical bleeding risk results in low rates of bleeding.
Hypertension was the only potentially modifiable risk factor for bleeding.
In elective surgery, for patients following a standardized interruption schema, residual direct oral anticoagulant levels were not associated with bleeding outcomes.
What Are the Clinical Implications?
We do not advocate routine direct oral anticoagulant level measurement preoperatively.
Our results suggest that risk factors other than surgery type have only a modest impact on postinterventional bleeding that does not warrant modification of the PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation) Study protocol.
The perioperative management of patients with atrial fibrillation (AF) who are chronically anticoagulated, whether with warfarin or a direct oral anticoagulant (DOAC), exposes patients to potential risks for bleeding and thromboembolism. 1 The development of such complications may relate to the timing of anticoagulant interruption and resumption, the type of surgery/procedure undertaken, the use of heparin bridging anticoagulation, and patient‐related factors such as advanced age and comorbid conditions. Because patients with AF are typically older, often have multiple comorbidities, and frequently will require anticoagulant interruption (15% per year) for a surgery/procedure, optimizing perioperative anticoagulant management and identifying determinants of adverse outcomes is clinically important. 2 , 3 Moreover, with the advent of DOACs, more patients with AF are receiving anticoagulation, and a perioperative DOAC management strategy was recently published. 4
Among patients treated with warfarin, perioperative anticoagulant management is well‐described and determinants of adverse perioperative outcomes have been identified. 5 , 6 Studies assessing determinants of perioperative adverse outcomes in patients treated with warfarin found that advanced age, use of heparin bridging, renal insufficiency, aspirin co‐administration, and Charlson co‐morbidity score were predictive of major bleeding. 5 , 7
Increasingly, clinicians are having to manage patients treated with DOACs who require treatment interruption for an elective surgery/procedure, yet perioperative DOAC management has been only recently addressed. 6 In the PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation) Study, a simple, standardized, perioperative DOAC interruption (Figure 1) and resumption strategy was developed, and did not involve perioperative heparin bridging or the use of preoperative coagulation function testing. 4 , 8 , 9 , 10 , 11 This management strategy, anchored on surgery/procedure‐associated bleeding risk, was associated with low rates of arterial thromboembolism (≈0.3%) and major bleeding (≈1.5%) in 3007 enrolled patients. However, determinants of adverse perioperative outcomes associated with this management strategy have not been addressed. In addition, there is conflicting information regarding the value of measuring DOAC levels preoperatively. 12 , 13 Preoperative DOAC levels were obtained in 85% of patients in PAUSE, thus allowing us to also evaluate this variable as a putative predictor of bleeding. 11
Figure 1. The robust influence of surgical bleed risk is compensated by the perioperative bleeding protocol.
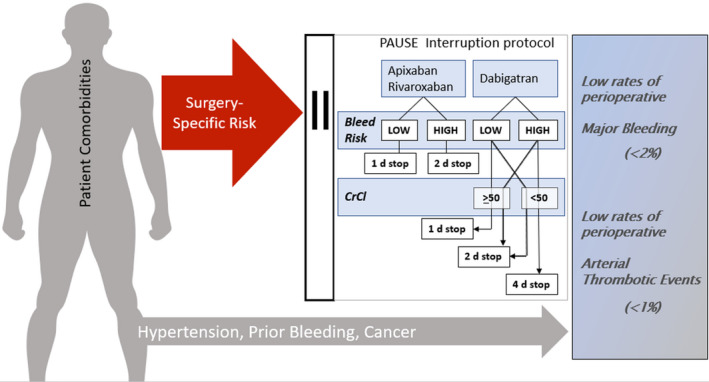
We measured a modest influence of patient‐specific risk factors towards the perioperative bleed risk, but the overall likelihood of bleeding and arterial thrombosis was low. CrCl indicates creatinine clearance; and PAUSE, Perioperative Anticoagulant Use for Surgery Evaluation study.
Against this background, we accessed the database of the PAUSE study, and as part of a prespecified analysis, the aim of this study was to identify predictors of perioperative bleeding. Our specific objective was to identify clinically significant, especially modifiable, risk factors for perioperative bleeding within the context of the PAUSE management protocol.
Methods
Patients and Design
The PAUSE methodology has been published. 11 The authors do not wish additional data and methods used to conduct the analyses to be made available to other researchers for purposes of reproducing the results and conducting additional analyses.
In brief, adults age ≥18 years, chronically anticoagulated patients with AF on dabigatran, rivaroxaban, or apixaban were recruited into 3 cohorts. Patients with a creatinine clearance (CrCl) <30 mL/min (<25 mL/min for apixaban), based on the Cockcroft‐Gault formula, were excluded. Patients with cognitive impairment or psychiatric illness that could impair their ability to provide informed consent were also excluded. All included patients signed an informed consent form and patients were only allowed to be entered in the trial once. The study was managed by the McMaster Centre for Transfusion Research, and the institutional review board of each of the 23 participating clinical centers in Canada, the United States, and Europe approved PAUSE.
In the absence of a comparable interruption strategy, the 3 cohorts were managed according to a standardized interruption scheme based on estimated procedural bleeding risk and renal function for dabigatran. The procedure bleeding risk classification was similar to that which was used in the BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial, 6 and is concordant with the guidance by the International Society on Thrombosis and Haemostasis. 1 A complete list of procedures considered to be high bleed risk is shown in Table 1. Procedures with high bleeding risk had a longer interruption and resumption interval (2 days) versus those who underwent a low bleeding risk procedure (1 day). DOAC interruption was longer for patients on dabigatran if the CrCl was 30 to 50 mL/min: 2 days in patients having a low bleeding risk procedure; and 4 days in patients having a high bleeding risk procedure (Figure 1). The use of low‐molecular‐weight heparin bridging perioperatively was not adopted in PAUSE, given potentially worse outcomes on post hoc analyses of large AF trials. 14 Low‐dose low‐molecular‐weight heparin prophylaxis was permitted postoperatively in patients at risk for venous thromboembolism until the DOAC was resumed.
Table 1.
Bleeding Risk Classification According to Procedure
| High Bleeding Risk surgeries |
|---|
| 1) Any surgery requiring neuraxial anesthesia |
| Neuraxial anesthesia/injection; epidural anesthesia/injection |
| 2) Major intracranial or neuraxial surgery |
| Brain cancer resection; laminectomy or neuraxial tumor resection, intracranial (subdural, epidural) bleed evacuation |
| 3) Major thoracic surgery |
| Lobectomy; pneumonectomy; esophagectomy |
| 4) Major cardiac surgery |
| Coronary artery bypass; valve replacement or repair |
| 5) Major vascular surgery |
| Aortic aneurysm repair; aortobifemoral bypass; popliteal bypass; carotid endarterectomy |
| 6) Major abdominopelvic surgery |
| Hepatobiliary cancer resection; pancreatic cancer or pseudocyst resection; colorectal and gastric cancer resection; diverticular disease resection; inflammatory bowel disease resection renal cancer resection; bladder cancer resection; endometrial cancer resection; ovarian cancer resection; radical prostatectomy |
| 7) Major orthopedic surgery |
| Hip arthroplasty or hip fracture repair; knee arthroplasty or tibial osteotomy; shoulder arthroplasty; metatarsal osteotomy |
| 8) Other major cancer or reconstructive surgery |
| Head and neck cancer surgery; reconstructive facial, abdominal, limb surgery |
| Low bleeding risk surgery/procedures |
|---|
| 1) Gastrointestinal procedures |
| Colonoscopy; gastroscopy; sigmoidoscopy; endoscopic retrograde pancreaticocholangiography; capsule endoscopy; push enteroscopy; Barrett’s esophagus ablation |
| 2) Cardiac procedures |
| Permanent pacemaker implantation or battery change; internal cardiac defibrillator implantation or battery change; atrioventricular node ablation; coronary artery angiography (radial approach) |
| 3) Dental procedures |
| Tooth extraction (up to 2 extractions) |
| Endodontic (root canal) procedure |
| 4) Skin procedures |
| Skin biopsy |
| 5) Eye procedures |
| Phacoemulsification (cataract) |
Clinical Outcomes and Variable Definitions
The primary clinical outcome of this PAUSE substudy was bleeding, defined as a composite of clinically relevant nonmajor bleeding (CRNM) and major bleeding (MB) or MB alone; all these events were adjudicated by an independent events adjudication committee blinded to the DOAC cohort and based on the International Society on Thrombosis and Haemostasis criteria. 15 The preoperative DOAC levels were assessed using DOAC‐specific tests: a dilute thrombin time 16 was used for patients receiving dabigatran, whereas rivaroxaban and apixaban concentrations were assessed using DOAC‐calibrated anti‐factor Xa levels. 16 A cut point of 50 ng/mL was used to define a clinically acceptable low residual anticoagulant effect for a perioperative clinical setting. 11 Additional variables included as putative predictors of bleeding included age, sex, comorbidities, and medications. The clinical variables of the CHADS2 and CHA2DS2‐VASc were included; the scores were prospectively collected but did not affect the DOAC management strategy. Additionally, surgery‐specific bleed risk, renal disease (defined as CrCl <50 mL/min), prior history of major bleeding, and protocol interruption compliance were also considered potential predictors. The selection of the potential predictors was guided by biological plausibility and prior data on perioperative bleeding. 5 , 17 , 18 We did not assess determinants of arterial thromboembolism because there were too few events (n=10) to yield clinically meaningful analyses, even if we enriched this outcome to include any thromboembolic event, either venous or arterial (n=21).
Statistical Analysis
Continuous variables were reported as means and SD; categorical variables were reported as frequencies and proportions. The main analysis of the PAUSE trial was anchored on the null hypothesis that the protocol could have unsafe bleeding outcomes higher than 2%; concordantly, we elected a single‐tail analysis. The proportion and 1‐sided 95% CI for arterial thromboembolism, CRNM+MB, and MB were reported. The association of each of the putative risk factors with CRNM+MB was measured using logistic regression for univariate analysis. A Forest plot was used to report the odds ratio (OR), 95% CI, and P value from the univariate analysis. Candidate variables were included in the multivariate logistic regression model, stratified by areas to control for geographical confounding by having a different constant term for each stratum. The ORs, 95% CIs, and P values, as well as the model area under the curve (AUC) from the multivariate logistic regression model were reported, where P‐value <0.05 was considered statistically significant. There were no added adjustments for multiplicity.
In addition, we used a machine learning model, known as extreme gradient boosting analysis, to predict the risk of CRNM+MB, which produced the relative importance rankings for the selected variables. Comparing with logistic regression model, the extreme gradient boosting analysis model is a nonparametric model, which does not have conflicts with multicollinearity, can manage missing values automatically, and can perform exhaustive interactions between variables. However, the disadvantages of an extreme gradient boosting analysis model are that it does not derive the significance of the predictors, it is hard to deploy the model because of its complexity, and efforts are needed to reduce the risk of overfitting. 19 , 20 , 21 A grid search on hyperparameters with 5‐fold cross‐validation was carried out to find the best model based on Gini index. The AUC of the extreme gradient boosting analysis model was also reported, but the methodology does not allow individual measures of association for the selected variables. All analyses were performed in R version 3.6.0.
Results
Patient Characteristics
The 3 DOAC cohorts comprised a total of 3007 DOAC interruptions analyzed in which there were 102 (3.39%; 1‐sided 95% CI, 0–3.98) patients with a bleeding event (Tables 2 and 3). Active cancer was present in 8.95% (n=269) of patients, and 7.96% (239) had a history of stroke. The mean (SD) CHA2DS2‐VASc score was 3.41±1.64.
Table 2.
Cohorts Demographics and Comorbidities Stratified by Major/Clinically Relevant Non‐Major Bleeding
|
No Bleeding (N=2905) |
Bleeding (N=102) |
|
|---|---|---|
| Age (SD) | 72.51 (9.40) | 73.26 (9.18) |
| Male, % | 1912 (65.82) | 76 (74.51) |
| BMI (SD) | 29.79 (6.50) | 28.85 (5.66) |
| Race | ||
|
2809 (97.40%) | 94 (94.95%) |
|
75 (2.60%) | 5 (5.05%) |
|
21 (0.72%) | 3 (2.94%) |
| Risk scores | ||
|
2.07 (1.30) | 2.21 (1.34) |
|
3.41 (1.64) | 3.49 (1.68) |
|
1.91 (0.88) | 2.15 (0.84) |
| Comorbidities | ||
|
476 (16.48%) | 18 (17.65%) |
|
2137 (73.74%) | 84 (83.17%) |
|
770 (26.52%) | 25 (24.51%) |
|
227 (7.82%) | 12 (11.76%) |
|
297 (10.23%) | 12 (11.76%) |
|
503 (17.36%) | 19 (18.63%) |
|
26 (0.90%) | 1 (0.98%) |
|
61 (2.10%) | 4 (3.92%) |
|
250 (8.61%) | 12 (11.76%) |
|
197 (6.80%) | 5 (4.90%) |
|
454 (15.62%) | 15 (14.71%) |
|
775 (26.68%) | 32 (31.37%) |
|
251 (8.64%) | 18 (17.65%) |
| Medications | ||
|
655 (22.55%) | 26 (25.49%) |
|
342 (11.77%) | 11 (10.78%) |
|
27 (0.93%) | 3 (2.94%) |
|
48 (1.65%) | 2 (1.96%) |
|
179 (6.16%) | 5 (4.90%) |
|
2754 (94.80%) | 94 (92.16%) |
| Surgery or procedure type | ||
|
952 (32.77%) | 55 (53.92%) |
|
1953 (67.23%) | 47 (46.08%) |
| Anesthesia type | ||
|
944 (32.50%) | 43 (42.16%) |
|
213 (7.36%) | 17 (16.67%) |
|
1603 (55.18%) | 39 (38.24%) |
| Residual DOAC level, ng/mL | ||
|
1951 (67.16%) | 69 (67.65%) |
|
349 (12.01%) | 14 (13.73%) |
|
154 (5.30%) | 4 (3.92%) |
|
451 (15.52%) | 15 (14.71%) |
| Surgery name | ||
|
1096 (37.73%) | 23 (22.55%) |
|
8 (0.28%) | 0 (0%) |
|
57 (1.96%) | 0 (0%) |
|
110 (3.79%) | 1 (0.98%) |
|
609 (20.96%) | 18 (17.65%) |
|
207 (7.13%) | 14 (13.73%) |
|
74 (2.55%) | 3 (2.94%) |
|
66 (2.27%) | 1 (0.98%) |
|
30 (1.03%) | 0 (0%) |
|
25 (0.86%) | 0 (0%) |
|
335 (11.53%) | 6 (5.88%) |
|
247 (8.50%) | 33 (32.35%) |
|
41 (1.41%) | 3 (2.94%) |
BMI indicates body mass index; CrCl, creatinine clearance in milliliters per minute; DOAC, direct oral anticoagulant; and N/A, not applicable.
Refers to HASBLED score without labile international normalized ratio component.
Refers to cancer that is being treated or is metastatic.
Refers to apixaban 2.5 mg twice‐daily, dabigatran 110 mg twice‐daily, or rivaroxaban 15 mg daily.
Table 3.
Outcomes
| N | Bleeding: n, % (1‐sided 95% CI) | Major Bleeding: n, % (1‐sided 95% CI) | |
|---|---|---|---|
| All | 3007 | 102, 3.39 (0–3.98) | 43, 1.43 (0–1.83) |
| DOAC | |||
| Dabigatran | 668 | 19, 2.84 (0–4.11) | 6, 0.90 (0–1.73) |
| 110 mg | 248 | 8, 3.23 (0–5.63) | 2, 0.81 (0–2.41) |
| 150 mg | 420 | 11, 2.62 (0–4.24) | 4, 0.95 (0–2.1) |
| Rivaroxaban | 1082 | 45, 4.16 (0–5.28) | 20, 1.85 (0–2.65) |
| 15 mg | 181 | 8, 4.42 (0–7.67) | 6, 3.31 (0–6.28) |
| 20 mg | 901 | 37, 4.11 (0–5.34) | 14, 1.55 (0–2.39) |
| Apixaban | 1257 | 38, 3.02 (0–3.92) | 17, 1.35 (0–2) |
| 2.5 mg | 252 | 10, 3.97 (0–6.53) | 3, 1.19 (0–2.94) |
| 5 mg | 1005 | 28, 2.79 (0–3.77) | 14, 1.39 (0–2.14) |
| CrCl | |||
| <50 mL/min | 469 | 15, 3.20 (0–4.83) | 7, 1.49 (0–2.73) |
| ≥50 mL/min | 2538 | 87, 3.43 (0–4.07) | 36, 1.42 (0–1.86) |
| Surgery bleed risk | |||
| Low | 2000 | 30, 1.50 (0–2.02) | 18, 0.90 (0–1.32) |
| High | 1007 | 30, 2.98 (0–3.99) | 25, 2.48 (0–3.43) |
CrCl indicates creatinine clearance in milliliters per minute; and DOAC, direct oral anticoagulant.
Rates and Predictors of Any Bleeding (CRNM+MB)
The rates of MB+CRNM were 3.02% (1‐sided 95% CI, 0–3.92) in the apixaban cohort, 2.84% (0–4.11) in the dabigatran cohort, 4.16% (0–5.28) in the rivaroxaban cohort, and 3.39% (0–3.98) in the overall study population. In the univariate analysis (Figure 2), high‐bleed‐risk surgery (OR, 2.32; 95% CI, 1.57–3.43), hypertension, (OR, 1.73; 95% CI, 1.03–2.91), active cancer (OR, 2.18; CI, 1.31–3.63), prior bleeding or bleed predisposition (OR, 1.78; 95% CI, 1.15–2.74), use of postoperative low‐dose low‐molecular‐weight heparin (OR, 2.01; 95% CI, 1.27–3.20), and a platelet count <100 × 109/L (OR, 4.61; 95% CI, 1.14–18.7) were significantly associated with CRNM+MB. In the multivariate analysis stratified by region, hypertension (OR, 1.79; 95% CI, 1.07–2.99), and prior bleeding or bleed predisposition (OR, 1.71; 95% CI, 1.08–2.71) were significantly associated with CRNM/MB (Table 4). Specific surgery types, namely, general surgery (OR, 2.53; 95% CI, 1.29–4.95) and urologic procedures (OR, 4.70; CI, 2.73–8.08), were associated with an increased risk for CRNM/MB. The model for MB/CRNM had an AUC of 0.71 (SE 0.03). In contrast, the machine learning model had an AUC of 0.79; the relative importance of variables (Figure 3) again pushes into the model types of surgery as major predictors.
Figure 2. Forest plot of univariate logistic regression for CRNM/MB.
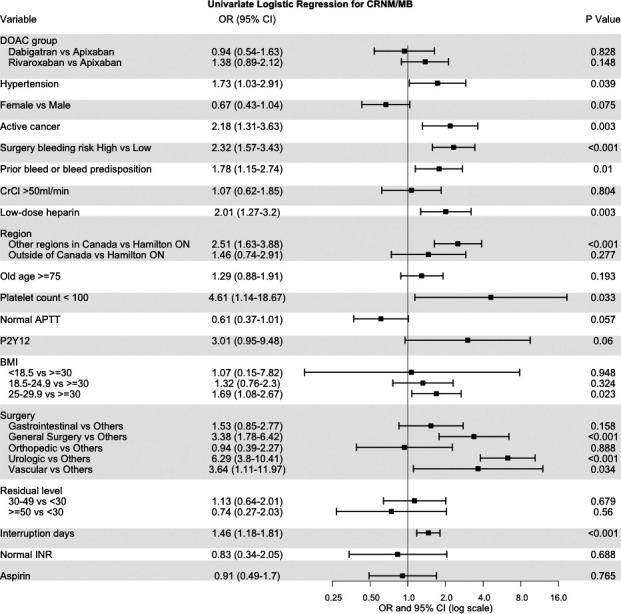
APTT indicates activated partial thromboplastin time; BMI, body mass index; CrCl, creatinine clearance; CRNM/MB, clinically nonmajor bleeding/major bleeding; DOAC, direct oral anticoagulant; INR, international normalized ratio; and OR, odds ratio.
Table 4.
Predictors of Perioperative Major and Clinically Relevant Non–Major Bleed. Multivariate Analysis Stratified By Regions (Grouped as Hamilton Area, Other Areas in Canada and Outside Canada)
| Predictor | OR (95% CI) | P value |
|---|---|---|
| DOAC group | ||
| Dabigatran vs apixaban | 0.86 (0.49–1.50) | 0.594 |
| Rivaroxaban vs apixaban | 1.21 (0.78–1.87) | 0.392 |
| Hypertension | 1.79 (1.07–2.99) | 0.027 |
| Female sex | 0.82 (0.52–1.31) | 0.415 |
| Active cancer | 1.21 (0.70–2.09) | 0.485 |
| Prior bleed or bleed predisposition | 1.71 (1.08–2.71) | 0.021 |
| CrCl >50 mL/min | 1.21 (0.68–2.17) | 0.515 |
| Low‐dose heparin | 1.51 (0.91–2.52) | 0.113 |
| Surgery | ||
| Gastrointestinal | 1.27 (0.69–2.35) | 0.443 |
| General surgery | 2.53 (1.29–4.95) | 0.007 |
| Orthopedic | 0.68 (0.27–1.72) | 0.415 |
| Urologic | 4.70 (2.73–8.08) | <0.001 |
| Vascular | 2.29 (0.67–7.78) | 0.185 |
Model AUC: 0.71.
AUC indicates area under the curve; CrCl, creatinine clearance in milliliters per minute; DOAC, direct oral anticoagulant; and OR, odds ratio.
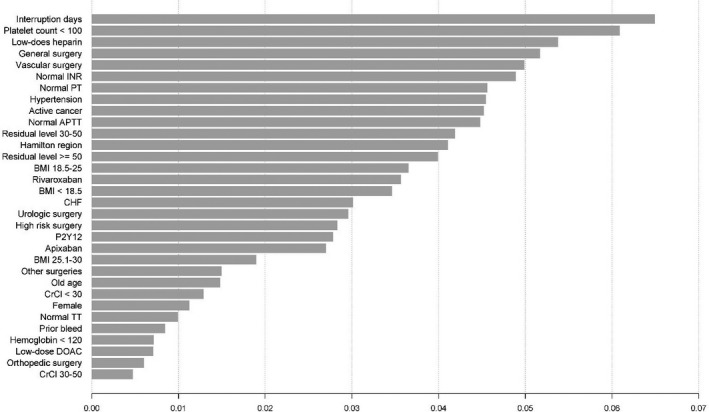
Figure 3. Influence of risk factors by extreme gradient boosting in clinically relevant nonmajor and major bleeding perioperatively.
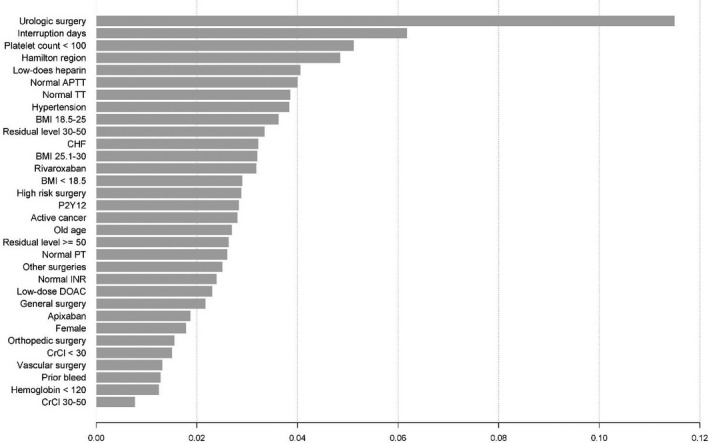
aPTT indicates activated partial thromboplastin time; BMI, body mass index; CHF, congestive heart failure; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; INR, international normalized ratio; OR, odds ratio; PT, prothrombin time; and TT, thrombin time.
Rates and Predictors of MB
MB was infrequent with 1.35% (1‐sided 95% CI, 0–2) in the apixaban cohort, 0.9% (0–1.73) in the dabigatran cohort, 1.85% (0–2.65) in the rivaroxaban cohort, and 1.43% (0–1.83) in the overall study population. In the univariate analysis (Figure 4), surgery bleeding risk (high versus low) was significantly associated with MB (OR, 2.76; 95% CI, 1.51–5.06), as were hypertension (OR, 3.33; 95% CI, 1.19–9.32) and high‐bleed‐risk surgery (OR, 2.76; 95% CI, 1.51–5.06). In the multivariate model, only hypertension (OR, 3.61; 95% CI, 1.39–9.37) was significantly associated with MB (Table 5). We saw a measurable change on AUC estimate in the machine learning, which increased to 0.81 from 0.74. In contrast to our initial estimates, body mass index had the highest relative importance for MB, with the lowest bleed rate among patients with body mass index >30 kg/m2 (Figure 4 and Figure 5).
Figure 4. Forest plot of univariate logistic regression for MB.
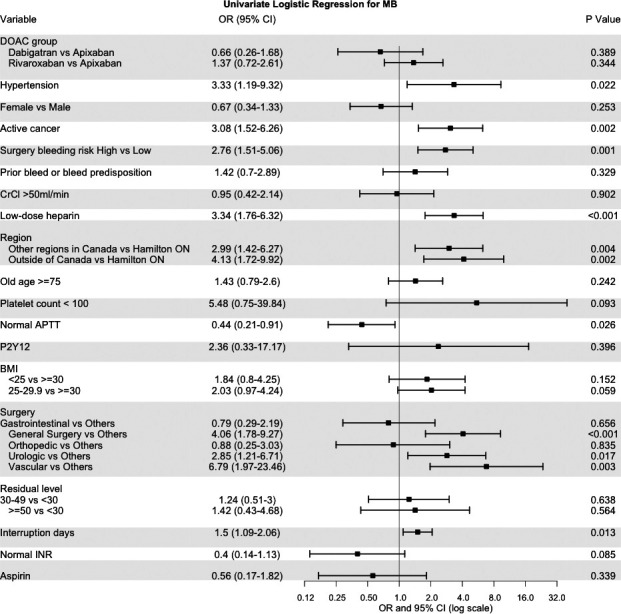
aPTT indicates activated partial thromboplastin time; BMI, body mass index; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; MB, major bleeding; INR, international normalized ratio; and OR, odds ratio.
Table 5.
Predictors of Perioperative Major Bleed. Multivariate Analysis Stratified by Regions (Grouped as Hamilton Area, Other Areas in Canada and Outside Canada)
| Predictor | OR (95% CI) | P Value |
|---|---|---|
| DOAC group | ||
| Dabigatran vs apixaban | 0.64 (0.25–1.64) | 0.353 |
| Rivaroxaban vs apixaban | 1.20 (0.62–2.33) | 0.583 |
| Hypertension | 3.61 (1.39–9.37) | 0.008 |
| Female sex | 0.60 (0.30–1.23) | 0.163 |
| Active cancer | 1.83 (0.85–3.96) | 0.125 |
| Low‐dose heparin | 1.83 (0.89–3.74) | 0.100 |
| Surgery | ||
| Gastrointestinal | 0.77 (0.27–2.20) | 0.626 |
| General surgery | 2.90 (1.20–6.98) | 0.018 |
| Orthopedic | 0.78 (0.21–2.94) | 0.713 |
| Urologic | 1.76 (0.71–4.38) | 0.226 |
| Vascular | 4.06 (1.09–15.16) | 0.037 |
| BMI | ||
| <25 vs ≥30 | 1.93 (0.83–4.52) | 0.129 |
| 25–29 vs ≥30 | 1.80 (0.85–3.79) | 0.123 |
| N/A vs ≥30 | 7.79 (0.93–65.23) | 0.058 |
Model AUC: 0.74.
AUC indicates area under the curve; BMI, body mass index; DOAC, direct oral anticoagulant; N/A, not applicable; and OR, odds ratio.
Figure 5. Influence of risk factors by extreme gradient boosting in major bleeding perioperatively.
aPTT indicates activated partial thromboplastin time; BMI, body mass index; CHF, congestive heart failure; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; INR, international normarlized ratio; PT, prothrombin time; and TT, thrombtin time.
Effect of Residual DOAC Level on Bleeding Outcomes
Drug level analysis was available for 2541 (84.5%) patients. In the apixaban cohort, there were 103 (9.48%) patients who had residual levels >50 ng/mL, 26 (4.86%) in the dabigatran cohort, and 29 (3.15%) in the rivaroxaban group. Among patients with elevated residual anticoagulant levels, MB+CRNM bleeding occurred in 1.33% (1‐sided 95% CI, 0−3.95) for the apixaban cohort, 2.78% (0–11.54) for dabigatran, and 5.08% (0–8.54) for the patients on rivaroxaban. There was no significant difference in bleedings between DOAC cohorts. In the stratified analysis with the DOAC level (>50 ng/mL versus ≤50 ng/mL) as a single predictor, there was no significant association with MB+CRNM observed (OR, 1.07; 95% CI, 0.38–2.96). In the multivariate model, adding DOAC level to the model did not improve the AUC.
Discussion
There are 2 main findings from this subanalysis of the PAUSE study data set that attempted to identify potentially modifiable risk factors for bleeding during DOAC interruption in the perioperative period. First, hypertension and a prior history of bleeding were independently associated with perioperative bleeding, and the addition of preoperative DOAC level measurement did not improve predictive utility. Second, the surgery/procedure‐associated bleed‐risk, as determined by the classification used in PAUSE, was not an independent predictor of bleeding, which suggests that adjustment of the PAUSE protocol for bleed‐risk by increasing the duration of perioperative DOAC interruption is effective in mitigating against perioperative bleeding. Note that these results are contingent on compliance with a standardized DOAC interruption protocol being followed.
In support of the precision and validity of our findings, the observed rate of MB+CRNM bleeding of 3.39% in PAUSE appeared comparable to the subanalyses of the AF trials for edoxaban and rivaroxaban. 22 , 23 For instance, in a subanalysis of the ENGAGE AF‐TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48), rates of MB+CRNM were 4.2% and 3.9% in patients who needed an interruption and were taking edoxaban 60 mg or warfarin, respectively. Moreover, the overall rate of MB (1.43%) also appeared comparable to the rate of MB (1.31%) among patients in the BRIDGE trial who had perioperative warfarin interruption and did not receive low molecular weight heparin bridging. 6
Other studies have reported similar determinants of perioperative bleeding risk. In the BIRDGE trial, hypertension was the only potentially modifiable predictor of perioperative MB+CRNM bleeding among patients treated with wafarin. 6 While acute perioperative hypertensive crises may lead to intracranial bleed, we were not able to correlate perioperative blood pressure management to bleeding events. We think that a more global effect was measured in our study and it is likely selecting for a frail population and additional comorbidities. Indeed, the Charlson score, as a graded measure of multiple coexisting comorbidities, is an independent predictor of bleeding among patients who need perioperative anticoagulation interruption. 7 Similarly, prior bleeding is a known independent risk factor for perioperative bleeding. 17 Active cancer was also associated with major bleeding events. The high bleeding risk among patients with active cancer has been well described among those who need chronic anticoagulation and surgery. 17 , 24 , 25 More recently, the understanding of bleeding risk among patients with cancer and AF is improving. In a post hoc analysis of the ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, patients with a history of cancer had a higher overall bleeding (hazard ratio, 1.30; 95% CI, 1.16–1.47) compared with those without cancer. 26
Residual preoperative DOAC levels did not appear to be determinants of bleed risk. This sheds light on the ongoing controversy about the value of preoperative DOAC level testing to guide clinical management. 27 , 28 , 29 Our findings do not support the routine use of DOAC level testing preoperatively when a standardized DOAC interruption protocol has been followed that intrinsically adjusts for interruption duration based on surgical bleed risk and renal function (for patients treated with dabigatran). It also possible, however, that since few patients had residual DOAC levels >50 ng/mL, there may have been insufficient power to assess the effect of different residual DOAC levels (<30 ng/mL or 30–49 ng/mL) as determinants of bleeding compared with higher DOAC levels, for example, as compared with 50–75 ng/mL or >75 ng/mL. The value of preoperative DOAC level testing is further questioned by a study that compared in vitro drug levels for apixaban, betrixaban, edoxaban, and rivaroxaban with tests of functional coagulation (including thromboelastogram, prothrombinase‐inducing clotting time, activated clotting time, prothrombin time, activated partial thromboplastin time) and found no definitive correlation. 30 Additional research is needed to explore the value of preoperative DOAC level testing, especially before urgent or emergency surgery or before administration of DOAC reversal agents.
Our finding that surgery/procedure‐related bleed‐risk classification did not predict perioperative bleeding may seem counterintuitive but may be explained by the fact that the PAUSE protocol intrinsically adjusted for bleed‐risk by increasing DOAC interruption from 2 days to at least 4 days in patients having low‐bleed‐risk and high‐bleed‐risk surgery. Thus, although there was, numerically, a higher proportion of major bleeds in patients having high‐ than low‐bleed‐risk surgery, other factors may have accounted for this difference in bleeding incidence, namely, the specific type of surgery/procedure. 31
Our study summarizes evidence on potential bleeding predictors from a protocol‐driven DOAC perioperative management. Despite major strengths, including availability of DOAC levels in 85% of patients, sample size, and objective outcome adjudication, this subanalysis has potential limitations. Creatinine clearance is a recognized determinant of perioperative bleeding risk, 32 and we were not able to analyze the full effect of this variable given that patients with severe renal insufficiency (CrCl <30 mL/min) were excluded from PAUSE. Given the low bleeding rates in each DOAC cohort, we were unable to perform adequately powered analyses for each DOAC, and it is uncertain whether the observed bleed risk factors are applicable to each DOAC type. To address this limitation, we used machine learning analysis to compensate for this limitation; however, our conclusions remained unchanged, thereby supporting our claim that the residual DOAC levels were not predictors of bleeding relative to surgical and patient‐specific determinants. Similarly, there was protocol‐mandated variability on the timing of DOAC re‐initiation, which we could not measure as a potential risk modifier. We did not analyze time to re‐initiation of DOAC after surgery, which per protocol was optionally extended in high‐risk intervention. Finally, the HASBLED score was created specifically for anticoagulated patients using vitamin K antagonist, and it is of limited value for perioperative bleeding risk stratification among warfarin‐anticoagulated patients 33 ; however, it has variables that do not apply to DOAC‐anticoagulated patients and given these limitations, it was not analyzed as a composite variable.
In summary, the PAUSE protocol for DOAC interruption incorporates surgery/procedure‐related bleed‐risk, DOAC half‐life, and renal function (for dabigatran) to guide the duration of DOAC interruption and timing of resumption around surgery and invasive procedures. Analysis of the PAUSE data set revealed hypertension and a prior history of bleeding as the only independent determinants of perioperative bleeding. Our findings also support the safety of the PAUSE protocol which, intrinsically through classification of surgery/procedure bleed‐risk and adjustment of DOAC interruption intervals, is designed to mitigate against perioperative bleeding.
Sources of Funding
This work was supported by Grant 313156 from the Canadian Institutes of Health Research and Grant G‐14‐0006136 from the Heart and Stroke Foundation of Canada. Additional support was provided by the CanVECTOR research network and Aniara‐Hyphen Biomed.
Disclosures
Dr Tafur is a consultant for Recovery Force and has received research or educational funding from: Janssen, Bristol‐Myers Squibb, Idorsia, Daichi Sanyo, Stago, and Doasesne. Dr Spyropoulos offers consultancy to Bayer, Ingelheim, Portola, Janssen; and has received research funding from Boehringer Ingelheim and Janssen. Dr. Schulman has grants and personal fees from Boehringer Ingelheim and Octapharma. In addition, he has personal fees from Bayer, Daichi Sankyo, Pfizer, Alnylam, and Sanofi. Dr. Caprini has served in a steering committee for Jansen R&D. In addition, he has served as consultant for Recovery Force, Alexion Pharmaceuticals, and has been in advisory boards for Pfizer and Sanofi. Dr Douketis has served in advisory committees for Pfizer, Sanofi, Leo Pharma, Bristol‐Myers Squibb, Portola, and Janssen. In addition, he has received a patent and royalties from The Merck Manual and Up‐to‐Date. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:017316 DOI: 10.1161/JAHA.120.017316.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Spyropoulos AC, Brohi K, Caprini J, Samama CM, Siegal D, Tafur A, Verhamme P, Douketis JD. Perioperative SSCSo, Critical Care T, Haemostasis of the International Society on T and Haemostasis. Scientific and Standardization Committee Communication: Guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: Recommendations for standardized reporting of procedural/surgical bleed risk and patient‐specific thromboembolic risk. J Thromb Haemost. 2019;1966–1972. [DOI] [PubMed] [Google Scholar]
- 2. Tafur A, Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart. 2018;1461–1467. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg BA, Peterson ED, Kim S, Thomas L, Gersh BJ, Fonarow GC, Kowey PR, Mahaffey KW, Sherwood MW, Chang P, et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, Vanassche T, Verhamme P, Shivakumar S, Gross PL, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark NP, Douketis JD, Hasselblad V, Schulman S, Kindzelski AL, Ortel TL, Investigators B. Predictors of perioperative major bleeding in patients who interrupt warfarin for an elective surgery or procedure: Analysis of the BRIDGE trial. Am Heart J. 2018;108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spyropoulos AC, Turpie AG, Dunn AS, Spandorfer J, Douketis J, Jacobson A, Frost FJ, Investigators R. Clinical outcomes with unfractionated heparin or low‐molecular‐weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;1246–1252. [DOI] [PubMed] [Google Scholar]
- 8. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open‐label, parallel‐group, single‐centre study. Clin Pharmacokinet. 2010;259–268. [DOI] [PubMed] [Google Scholar]
- 10. Cui Y, Song Y, Wang J, Yu Z, Schuster A, Barrett YC, Frost C. Single‐ and multiple‐dose pharmacokinetics, pharmacodynamics, and safety of apixaban in healthy Chinese subjects. Clin Pharmacol. 2013;177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Douketis JD, Spyropoulos AC, Anderson JM, Arnold DM, Bates SM, Blostein M, Carrier M, Caprini JA, Clark NP, Coppens M, et al. The perioperative anticoagulant use for surgery evaluation (PAUSE) study for patients on a direct oral anticoagulant who need an elective surgery or procedure: design and rationale. Thromb Haemost. 2017;2415–2424. [DOI] [PubMed] [Google Scholar]
- 12. Testa S, Legnani C, Antonucci E, Paoletti O, Dellanoce C, Cosmi B, Pengo V, Poli D, Morandini R, Testa R, et al. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan NC, Coppens M, Hirsh J, Ginsberg JS, Weitz JI, Vanassche T, Douketis JD, Schulman S, Eikelboom JW. Real‐world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;353–359. [DOI] [PubMed] [Google Scholar]
- 14. Douketis JD, Healey JS, Brueckmann M, Eikelboom JW, Ezekowitz MD, Fraessdorf M, Noack H, Oldgren J, Reilly P, Spyropoulos AC, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE‐LY trial. Thromb Haemost. 2015;625–632. [DOI] [PubMed] [Google Scholar]
- 15. Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost. 2007;2211–2218. [DOI] [PubMed] [Google Scholar]
- 16. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tafur AJ, McBane R 2nd, Wysokinski WE, Litin S, Daniels P, Slusser J, Hodge D, Beckman MG, Heit JA. Predictors of major bleeding in peri‐procedural anticoagulation management. J Thromb Haemost. 2012;261–267. [DOI] [PubMed] [Google Scholar]
- 18. Cohen AT, Wagner MB, Mohamed MS. Risk factors for bleeding in major abdominal surgery using heparin thromboprophylaxis. Am J Surg. 1997;1–5. [DOI] [PubMed] [Google Scholar]
- 19. Torlay L, Perrone‐Bertolotti M, Thomas E, Baciu M. Machine learning‐XGBoost analysis of language networks to classify patients with epilepsy. Brain Inform. 2017;159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogunleye AA, Qing‐Guo W. XGBoost model for chronic kidney disease diagnosis. IEEE/ACM Trans Comput Biol Bioinform. 2019;1 DOI: 10.1109/TCBB.2019.2911071. [DOI] [PubMed] [Google Scholar]
- 21. Li W, Yin Y, Quan X, Zhang H. Gene expression value prediction based on XGBoost algorithm. Front Genet. 2019;1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, Spyropoulos AC, Hankey GJ, Singer DE, Nessel CC, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douketis JD, Murphy SA, Antman EM, Grip LT, Mercuri MF, Ruff CT, Weitz JI, Braunwald E, Giugliano RP. Peri‐operative adverse outcomes in patients with atrial fibrillation taking warfarin or Edoxaban: Analysis of the ENGAGE AF‐TIMI 48 Trial. Thromb Haemost. 2018;1001–1008. [DOI] [PubMed] [Google Scholar]
- 24. Shaw JR, Douketis J, Le Gal G, Carrier M. Periprocedural interruption of anticoagulation in patients with cancer‐associated venous thromboembolism: An analysis of thrombotic and bleeding outcomes. J Thromb Haemost. 2019;1171–1178. [DOI] [PubMed] [Google Scholar]
- 25. Tafur AJ, Wysokinski WE, McBane RD, Wolny E, Sutkowska E, Litin SC, Daniels PR, Slusser JP, Hodge DO, Heit JA. Cancer effect on periprocedural thromboembolism and bleeding in anticoagulated patients. Ann Oncol. 2012;1998–2005. [DOI] [PubMed] [Google Scholar]
- 26. Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Hacke W, Halperin JL, Hankey GJ, Mahaffey KW, et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019;145–152. [DOI] [PubMed] [Google Scholar]
- 27. Tripodi A, Chantarangkul V, Legnani C, Testa S, Tosetto A. Interlaboratory variability in the measurement of direct oral anticoagulants: results from the external quality assessment scheme. J Thromb Haemost. 2018;565–570. [DOI] [PubMed] [Google Scholar]
- 28. Ebner M, Birschmann I, Peter A, Hartig F, Spencer C, Kuhn J, Blumenstock G, Zuern CS, Ziemann U, Poli S. Emergency coagulation assessment during treatment with direct oral anticoagulants: limitations and solutions. Stroke. 2017;2457–2463. [DOI] [PubMed] [Google Scholar]
- 29. Tripodi A, Ageno W, Ciaccio M, Legnani C, Lippi G, Manotti C, Marcucci R, Moia M, Morelli B, Poli D, et al. Position Paper on laboratory testing for patients on direct oral anticoagulants. A Consensus Document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus. 2018;462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siddiqui F, Hoppensteadt D, Jeske W, Iqbal O, Tafur A, Fareed J. Factor Xa inhibitory profile of Apixaban, Betrixaban, Edoxaban, and Rivaroxaban does not fully reflect their biologic spectrum. Clin Appl Thromb Hemost. 2019;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost. 2010;884–890. [DOI] [PubMed] [Google Scholar]
- 32. Graves A, Yates P, Hofmann AO, Farmer S, Ferrari P. Predictors of perioperative blood transfusions in patients with chronic kidney disease undergoing elective knee and hip arthroplasty. Nephrology. 2014;404–409. [DOI] [PubMed] [Google Scholar]
- 33. Omran H, Bauersachs R, Rubenacker S, Goss F, Hammerstingl C. The HAS‐BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost. 2012;65–73. [DOI] [PubMed] [Google Scholar]


