Abstract
Interleukin (IL)-12 family member is a heterodimer glycoprotein, composed of two covalently linked subunits, α and β chains. The α subunit consists of IL-23p19, IL-27p28, and IL-12p35, and the β subunit includes IL-12p40 and Epstein-Barr virus-induced gene (Ebi3). IL-39 is a new heterodimeric IL-12 family member composed of IL-23p19 and Ebi3 subunits. IL-39 is secreted by lipopolysaccharide-stimulated B cells. Other immune cells, such as dendritic cells and macrophages, express IL-39 mRNA. In lupus-like mice, GL7+B cells and CD138+plasma cells are highly activated and widely expressed, promoting high expression of IL-39. IL-39 mediates inflammatory responses through binding to a heterodimer of IL-23R/gp130 receptor and activation of signal transducer and activator of transcription (STAT)1/STAT3 signal molecules. The serum levels of IL-39 were significantly increased in patients with acute coronary syndrome compared with patients with normal coronary arteries. This review discusses the biological characteristics, receptor, and signal pathway as well as biological activity of IL-39 and its potential role in inflammation and other diseases.
Keywords: interleukin 39, interleukin 12, B cells, systemic lupus erythematosus, acute coronary syndrome
Introduction
Each interleukin (IL)-12 family member is a heterodimer glycoprotein, composed of two covalently linked subunits, α and β chains. The α subunit consists of IL-23p19, IL-27p28, and IL-12p35, and the β subunit includes IL-12p40 and Epstein-Barr virus-induced gene (Ebi3). This predicts that six possible heterodimeric IL-12 family cytokines can be formed. To date, four heterodimeric molecules, IL-12, IL-23, IL-27, and IL-35 have been reported in nature with bioactivity [1-4]. Wang et al. [5, 6] reported that IL-23p19 and Ebi3 form a new IL-12 family heterodimer, IL-23p19/Ebi3, termed as IL-39. In addition, Ushach et al. [7] indicated that metrnl is a novel cytokine and suggests that it should be renamed as IL-39. Earlier studies revealed metrnl is a protein homologous to the neurotrophic factor metrn, also known as meteorin-like (metrnl), cometin, and subfatin [8-11]. However, it has no structural and functional similarities with the members of IL-12 family. In this review, we summarize the research progress on the structure, receptor, and signal pathway of IL-39 as well as its expression and biological function, to enable a better understanding of IL-39, and to develop tools and targeted molecules for novel therapeutic strategies in inflammation and other diseases.
Discovery of IL-39
IL-12 cytokine family members comprise of two distinct subunits (subunit a: IL-23p19, IL-27p28, and IL-12p35; subunit b: IL-12p40, Ebi3) forming a a/b heterodimer. IL-12, IL-23, IL-27, and IL-35 comprise of heterodimers p35/p40, p19/p40, p28/Ebi3, and p35/Ebi3, respectively [12]. Wang et al. [5, 6] reported that IL-12 family subunits p19 and Ebi3 form a new complex, named IL-39 (p19/Ebi3). Using Western blot, PCR, immune-coprecipitation, and sandwich ELISA methods, they found that p19 and Ebi3 bind to form a complex in different cell subsets. They verified that p19 and Ebi3 are co-expressed in murine RAW 264.7 macrophage cell line and dendritic cells (DC) from the bone marrow, and that the natural IL-39 (p19/Ebi3) complex is present in the supernatant of cultured RAW264.7 macrophage cells. They demonstrated that lipopolysaccharide (LPS)-activated B cells secrete a natural form of IL-39.
The IL-39 complex is a 54-kDa heterodimer. The two subunits, IL-23p19 and Ebi3, are shared with IL-23 and IL-27/IL-35, respectively. IL-23p19 is a four-helix bundle and has homology with IL-6 and granulocyte colony stimulating factor. Ebi3 was first identified in B lymphocytes infected with EB virus. The Ebi3 gene is located in human chromosome 17 and encodes a 34-kDa secretory glycoprotein with a signal peptide and 2 fibronectin type III domains [13]. The fibronectin type III repeat region is approximately 100 amino acid domain that includes binding sites for DNA, heparin, fibrin, and cell surface proteins [14]. Ebi3 has 27% of amino acid sequence homology with the IL-12p40 subunit [15, 16]. Like p40, it lacks a membrane-anchoring motif, corresponds to the extracellular portion of a type I cytokine receptor, and is associated with the p35 subunit of IL-12 to form heterodimeric hematopoietin, Ebi3/p35 [17].
Receptor and signaling pathway of IL-39
The receptors of IL-12 family include five different subunits: IL-12 receptor β1 (IL-12Rβ1), IL-12Rβ2, IL-23R, IL-27R (WSX-1), and gp130. The IL-12/IL-35/IL-23/IL-27 receptors are composed of IL-12Rβ1 and IL-12Rβ2, IL-12Rβ2 and gp130, IL-12Rβ1 and IL-23R, and IL-27R and gp130, respectively [18]. Because the subunits of IL-39 share subunits with IL-23, IL-27, and IL-35, it is concluded that the IL-39 receptor is composed of IL-23R, IL-27R, and gp130, which are the same as the formed homo- or hetero-dimer. Wang et al. [6] detected IL-39 receptors by receptor knock-down, Western blot, and FACS, and found that B cells expressed the IL-12 family receptors IL-23R and gp130, and that the expression of IL-23R and gp130 receptors time-dependently increased after LPS stimulation. IL-39 promotes the phosphorylation of signal transducer and activator of transcription (STAT)1 and STAT3 rather than other STAT family members such as STAT4 and STAT5 in B cells. When IL-23R or gp130 was knocked down, IL-39 did not induce STAT1 and STAT3 phosphorylation (Fig. 1). IL-39 was also suggested to signal via a heterodimer of IL-23R and gp130 [19]. Moreover, Floss et al. [20] recently demonstrated that the proposed IL-39 receptor complex is biologically active.
Figure 1.
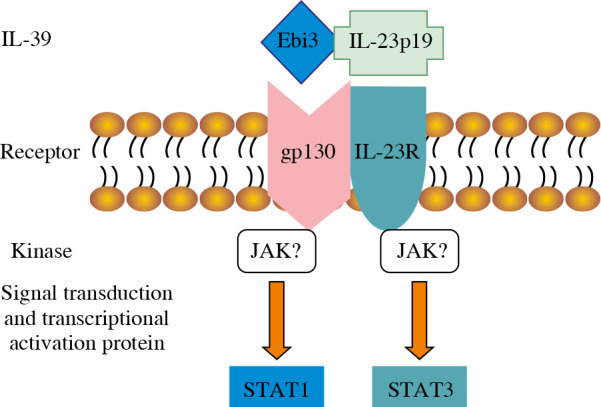
IL-39 and signaling pathway. IL-39 is a newly discovered member of the IL-12 family and comprises IL-23p19 and Ebi3 subunits. It binds to the IL-23R/gp130 receptor and promotes the phosphorylation of STAT1 and STAT3.
Secretion and expression of IL-39
IL-12, IL-23, and IL-27 are mature cytokines of the IL-12 family and are secreted by activated antigen presenting cells (APC) [2]. IL-35 is a member of the IL-12 family and is produced by regulatory T and B cells [21-24]. IL-39 is a new heterodimeric IL-12 family member composed of IL-23p19 and Ebi3 subunits and shares the Ebi3 subunit with IL-27 and IL-35. In vitro studies confirmed IL-23p19 and Ebi3 are secreted by LPS-stimulated B cells and have a positive correlation with duration of LPS-stimulation. When B-cell activation was inhibited by IFN-γ or Bcl-6 inhibitors, the IL-39 expression decreased significantly [6]. Other immune cells such as DC and macrophages express IL-23p19 and Ebi3 mRNA. IL-23p19 and Ebi3 mRNA expression levels are elevated in the presence of IL-4, and LPS inhibits the expression of IL-23p19 and Ebi3 mRNA in DC and macrophages. In lupus-like mice, GL7+B cells and CD138+plasma cells are highly activated and widely expressed, promoting high expression of IL-39. Moreover, the expression of IL-23p19 and Ebi3 was significantly higher than that of IL-27p28, IL-12p35, and IL-12p40 in GL7+B cells [6]. Ebi3 is expressed at very high level in B lymphocytes infected with EB virus. In vivo studies showed that Ebi3 is expressed in lymphoid organs, such as tonsils and spleen, and at a remarkably high level in in term placenta [15]. Ebi3 plays potential roles in the maintenance of pregnancy or the immunotolerance of human maternal body toward the fetus [15]. Previous studies reported the expression of Ebi3 in Hodgkin’s lymphoma and adult T-cell lymphoma/leukemia [25, 26].
Role of IL-39 in inflammation and other disorders
IL-39 includes IL-23p19 and Ebi3 subunits, activates STAT1/ STAT3 signal molecules through binding to the IL-23R/ gp130 receptor, and mediates inflammatory responses. Pro-inflammatory cytokines promote the generation of IL-23p19 in endothelial cells [27]. Intercellular IL-23p19 increases the cell surface expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells, which enhances the attachment of leukocytes and increases their transendothelial migration [27]. Furthermore, high levels of Ebi3 expression were associated with a poor prognosis of lung cancer, and serum levels of Ebi3 in lung cancer patients were significantly higher than in healthy volunteers [16]. In addition, a reduction of Ebi3 expression by siRNA suppressed cancer cell proliferation and the induction of exogenous Ebi3 expression conferred growth-promoting activity [16]. Ebi3 down-regulated the expression of type I or type III collagen in the presence or absence of transforming growth factor-β (TGF-β) co-stimulation. Kudo et al. [28] found that collagen mRNA stability was reduced by Ebi3 via the induction of miR-4500. Ebi3 expression was decreased in keratinocytes of epidermis and regulatory T cells of dermis in systemic sclerosis (SSc) skin compared with normal skin, which may induce collagen synthesis in SSc dermal fibroblasts. They also found that gp130, the Ebi3 receptor, was expressed in both normal and SSc fibroblasts. Moreover, they revealed that the injection of Ebi3 alone into the skin improved mice skin fibrosis. Decreased Ebi3 in SSc skin may contribute to an increase in collagen accumulation and skin fibrosis [28]. Bacterial infection of peripheral blood leukocytes enhanced CsEbi3 (an Ebi3 homologue from tongue sole) expression and promoted an extracellular secretion of CsEbi3. Purified recombinant CsEbi3 (rCsEbi3) stimulated the respiratory burst activity of peripheral blood leukocytes and up-regulated the expression of IL-1β, IL-8, Myd88, interferon-induced gene 15, CD28, and chemokines [29]. These studies indicate that CsEbi3 is involved in the innate immune defense of peripheral blood leukocytes against microbial pathogens [27].
Wang et al. [6] found up-regulated proteinuria, enlarged spleen, and increased spleen weight, when IL-12p35 and IL-12p40 knocked-down GL7+B cells were injected into lupus-like mice. Although IL-23p19 and Ebi3 knocked-down GL7+B cells reduced the disease capacity in lupus-like mice. Moreover, when anti-mouse IL-39 polyclonal antibody was injected into lupus-like mice, reduced urinary protein, splenomegaly, abnormal activated B cells in spleen, antibody titers, and remission of kidney pathological inflammation were observed. Their study shows that IL-39 may play a pro-inflammatory role in lupus-like mice [6]. To explore a possible role of IL-39 in lupus-like mice, Wang et al. [5] performed experiments in vivo and in vitro. They found that IL-39 promoted the differentiation and development of neutrophils; IL-39 knock-down and anti-IL-39 antibody reduced the number of neutrophils in lupus-like mice; neutrophils in lupus-like mice secreted pathogenic cytokine B cell activating factor belonging to the TNF family (BAFF); and that IL-39 increased the number of neutrophils in systemic lupus erythematosus mice through a positive feedback loop of IL-39-BAFF [5]. Ramnath et al. [30] reported that interferon regulatory factor 6 (IRF6) regulated a subset of toll-like receptor 3 (TLR3) responses in human keratinocytes, including the production of a novel IL-12 family heterodimer (p19/Ebi3). They proposed that the TLR3-IRF6-p19/Ebi3 axis may regulate keratinocyte and immune cell functions in the context of cell damage and wound healing in the skin. Luo et al. [31] recently found that the serum levels of IL-39 were significantly increased in patients with acute coronary syndrome compared with patients with normal coronary arteries. Furthermore, IL-39 levels positively correlated with N-terminal of the prohormone brain natriuretic peptide, high-sensitivity C-reaction protein, and cardiac troponin I, and negatively correlated with left ventricular ejection fraction in acute coronary syndrome patients, suggests its potential role as biomarker for acute myocardial injury.
Conclusions
Some studies have reported that IL-39 is a new member of the IL-12 family and is composed of IL-23p19 and Ebi3 subunits. The combination of IL-23R and gp130 forms the IL-39 receptor. B cells stimulated by LPS and activated GL7+B cells produce IL-39 in lupus-like mice. Moreover, IL-39 mediates the inflammatory response through activation of STAT1/STAT3 signaling pathway in lupus-like mice. At present, a research on IL-39 is still in its initial stage, and many issues have not been solved, including whether other cells can produce IL-39 in addition to activated B cells, a relationship between IL-39 and other cytokines, biological effects and mechanisms of IL-39 in inflammatory diseases other than systemic lupus erythematosus and acute coronary syndrome. All these matters need to be further studied to provide novel treatments for various related diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81970735), the Natural Science Foundation of Zhejiang Province (LY17H010001, LY18H010003) and sponsored by K.C. Wong Magna Fund in Ningbo University.
Footnotes
The authors declare no conflict of interest.
References
- 1.Egwuagu CE, Yu CR, Sun L, et al. (2015): Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev 26: 587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, He C, Nair L, et al. (2015): Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease, Cytokine 75: 249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastelein RA, Hunter CA, Cua DJ (2007): Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 25: 221-242. [DOI] [PubMed] [Google Scholar]
- 4.Wang RX, Yu CR, Mahdi RM, et al. (2012): Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem 287: 36012-36021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Liu X, Zhang Y, et al. (2016): Interleukin (IL)-39 [IL-23p19/Epstein-Barr virus-induced 3 (Ebi3)] induces differentiation/expansion of neutrophils in lupus-prone mice. Clin Exp Immunol 186: 144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wei Y, Xiao H, et al. (2016): A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J Immunol 46: 1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ushach I, Burkhardt AM, Martinez C, et al. (2015): METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol 156: 119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen JR, Fransson A, Fjord-Larsen L, et al. (2012): Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp Neurol 233: 172-181. [DOI] [PubMed] [Google Scholar]
- 9.Li ZY, Zheng SL, Wang P, Xu TY, et al. (2014): Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther 20: 344-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao RR, Long JZ, White JP, et al. (2014): Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157: 1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng SL, Li ZY, Song J, et al. (2016): Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin 37: 571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringkowski S, Thomas PS, Herbert C (2014): Interleukin-12 family cytokines and sarcoidosis. Front Pharmacol 5: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devergne O, Hummel M, Koeppen H, et al. (1996): A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol 70: 1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skorstengaard K, Jensen MS, Sahl P, et al. (1986): Complete primary structure of bovine plasma fibronectin. Eur J Biochem 161: 441-453. [DOI] [PubMed] [Google Scholar]
- 15.Devergne O, Coulomb-L’Hermine A, Capel F, et al. (2001): Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol 159: 1763-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino R, Takano A, Oshita H, et al. (2011): Identification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer . Clin. Cancer Res 17: 6272-6286. [DOI] [PubMed] [Google Scholar]
- 17.Devergne O, Birkenbach M, Kieff E (1997): Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA 94: 12041-12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson BM, Sullivan JA, Burlingham WJ (2013): Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front Immunol 4: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa H, Mizoguchi I, Chiba Y, et al. (2016): Expanding diversity in molecular structures and functions of the IL-6/IL-12 heterodimeric cytokine family. Front Immunol 7: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floss DM, Schönberg M, Franke M, et al. (2017): IL-6/IL-12 cytokine receptor shuffling of extra-and intracellular domains reveals canonical STAT Activation via synthetic IL-35 and IL-39 signaling. Sci Rep 7:15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen P, Roch T, Lampropoulou V, et al. (2014): IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507: 366-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang RX, Yu CR, Dambuza IM, et al. (2014): Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20: 633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang XG, Xie Q (2015): IL-35: a potential therapeutic target for controlling hepatitis B virus infection. J Dig Dis 16: 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collison LW, Workman CJ, Kuo TT, et al. (2007): The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450: 566-569. [DOI] [PubMed] [Google Scholar]
- 25.Larousserie F, Bardel E, Pflanz S, et al. (2005): Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus-and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol 166: 1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedobitek G, Pazolt D, Teichmann M, et al. (2002): Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol 198: 310-316. [DOI] [PubMed] [Google Scholar]
- 27.Espigol-Frigole G, Planas-Rigol E, Ohnuki H, et al. (2016): Identification of IL-23p19 as an endothelial proinflammatory peptide that promotes gp130-STAT3 signaling. Sci Signal 9: ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo H, Wang Z, Jinnin M, et al. (2015): EBI3 Downregulation Contributes to Type I Collagen Overexpression in Scleroderma Skin. J Immunol 195: 3565-3573. [DOI] [PubMed] [Google Scholar]
- 29.Li MF, Sun BG, Xiao ZZ, et al. (2013): First characterization of a teleost Epstein-Barr virus-induced gene 3 (EBI3) reveals a regulatory effect of EBI3 on the innate immune response of peripheral blood leukocytes. Dev Comp Immunol 41: 514-522. [DOI] [PubMed] [Google Scholar]
- 30.Ramnath D, Tunny K, Hohenhaus DM, et al. (2015): TLR3 drives IRF6-dependent IL-23p19 expression and p19/EBI3 heterodimer formation in keratinocytes. Immunol Cell Biol 93: 771-779. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y, Liu F, Liu H, et al. (2017): Elevated serum IL-39 in patients with ST-segment elevation myocardial infarction was related with left ventricular systolic dysfunction. Biomark Med 11: 419-426. [DOI] [PubMed] [Google Scholar]


