Abstract
Adverse experiences in childhood and adolescence, defined as subjectively perceived threats to the safety or security of the child’s bodily integrity, family, or social structures, are known to be associated with cardiometabolic outcomes over the life course into adulthood. This American Heart Association scientific statement reviews the scientific literature on the influence of childhood adversity on cardiometabolic outcomes that constitute the greatest public health burden in the United States, including obesity, hypertension, type 2 diabetes mellitus, and cardiovascular disease. This statement also conceptually outlines pathways linking adversity to cardiometabolic health, identifies evidence gaps, and provides suggestions for future research to inform practice and policy. We note that, despite a lack of objective agreement on what subjectively qualifies as exposure to childhood adversity and a dearth of prospective studies, substantial evidence documents an association between childhood adversity and cardiometabolic outcomes across the life course. Future studies that focus on mechanisms, resiliency, and vulnerability factors would further strengthen the evidence and provide much-needed information on targets for effective interventions. Given that childhood adversities affect cardiometabolic health and multiple health domains across the life course, interventions that ameliorate these initial upstream exposures may be more appropriate than interventions remediating downstream cardiovascular disease risk factor effects later in life.
Keywords: AHA Scientific Statements, adolescent, cardiovascular diseases, child, diabetes mellitus, hypertension, obesity, stress, trauma
It is now well established that experiences in childhood and adolescence are associated with health over the life course.1 Adverse childhood experiences, which we define as experiences that threaten the child’s bodily, familial, or social safety or security, range from broad categories of maltreatment and household dysfunction2 to more targeted experiences of bullying,3 exposure to crime, victimization, and economic disadvantage3–5 (Table). Adverse experiences are highly prevalent; recent data from the BRFSS (Behavioral Risk Factor Surveillance System) demonstrate that 59% of the US adult population has experienced at least 1 adverse childhood event.6 Much of the existing literature on childhood adversities and health has focused on childhood maltreatment, including experiences of physical, sexual, and emotional abuse and neglect, and has shown that such adversities disrupt normative developmental processes and magnify risk for health consequences later in life.7 Cumulative measures have also been common ways of capturing exposure to childhood adversity, with many studies aggregating across the 10 items defined by the ACE study (Adverse Childhood Experiences)2: witnessing a parent being abused, living with a mentally ill person, living with a substance abuser, imprisonment of a household member, emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect, or parental separation or divorce. Additional research has expanded on the original set of ACE items to include adversities common in diverse, nonwhite, lower-socioeconomic-level communities, as well as adversities perpetrated by peers (eg, bullying).8–10 Given the interindividual variation in threat perception, there is a lack of consensus on precisely what constitutes childhood and adolescent adversity. Nevertheless, there is general agreement that the accumulation of these experiences, across either time or multiple domains, during childhood and adolescence detrimentally affects health. Moreover, cardiometabolic health outcomes and adverse experiences are strongly patterned by sex, race/ethnicity, socioeconomic status (SES), and nativity (place of birth, eg, whether born in the United States or elsewhere). For example, children living in lower-SES households and from racial/ethnic minorities are more likely to experience multiple adversities in childhood in addition to having a higher prevalence of cardiometabolic health outcomes.6,8,11 The relation between sex and immigration status and childhood adversity and cardiometabolic outcomes is more complex and is discussed in detail later.
Table.
Childhood Adversity Items Commonly Assessed for Association With Cardiometabolic Outcomes Individually or in Composite Scales
| Construct | Individual Item |
|---|---|
| Household dysfunction | Parental substance abuse |
| Violence at home | |
| Parental psychopathology | |
| Parental incarceration | |
| Parental death | |
| Parental separation or divorce | |
| Violence (not childhood maltreatment or domestic violence at home) | Witnessing violence or violence victimization |
| Dating violence | |
| Neighborhood safety | |
| Childhood maltreatment | Emotional abuse |
| Physical abuse | |
| Sexual abuse | |
| Physical neglect | |
| Other | Homelessness |
| Peer victimization (bullying) | |
| Discrimination | |
| Economic hardship | |
| Other traumatic event | |
| Death of close family friend or loved one |
The goal of this American Heart Association scientific statement is to review the scientific literature on the influence of childhood and adolescent adversity (hereafter referred to as childhood adversity) on cardiometabolic health outcomes, including obesity, hypertension, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD). Cardiometabolic diseases constitute the leading causes of morbidity and mortality for men and women in the United States12,13 and an escalating economic burden.14,15 Our assessment draws from several recent systematic reviews focused on various forms of childhood adversity, including childhood maltreatment,16 violence,5 and cardiometabolic outcomes, to identify evidence gaps and to provide suggestions for future research needed to inform policy and practice. Our broad goal is to summarize evidence necessary to guide and inform multilevel interventions designed to prevent and mitigate childhood adversity and associated risk for cardiometabolic disease.
Our review of the literature and proposed next steps are guided by a conceptual model (Figure) that recognizes 3 potential mechanisms of how childhood adversity could affect cardiometabolic health: behavioral, mental health, and biological mechanisms. As detailed later, behaviors such as smoking, sleep, activity, and caloric consumption all worsen as a result of stressful events and household dysfunction. Mental health problems may be induced or exacerbated by childhood adversity, predisposing children and youth to early manifestations of cardiometabolic disease risk. Lastly, because childhood adversity disrupts many of the regulatory processes of the body, biological processes can be directly affected. All of these mechanisms (behavioral, biological, and mental) interact, affect other cardiometabolic factors (eg, blood pressure, adiposity, glucose), and, in turn, affect cardiovascular morbidity and mortality. We also recognize several immutable factors, including sex, race/ethnicity, nativity status, and genetic predisposition, that exacerbate or buffer the effect of childhood adversity on cardiometabolic risk factors and ultimately cardiometabolic morbidity and mortality.
Figure.
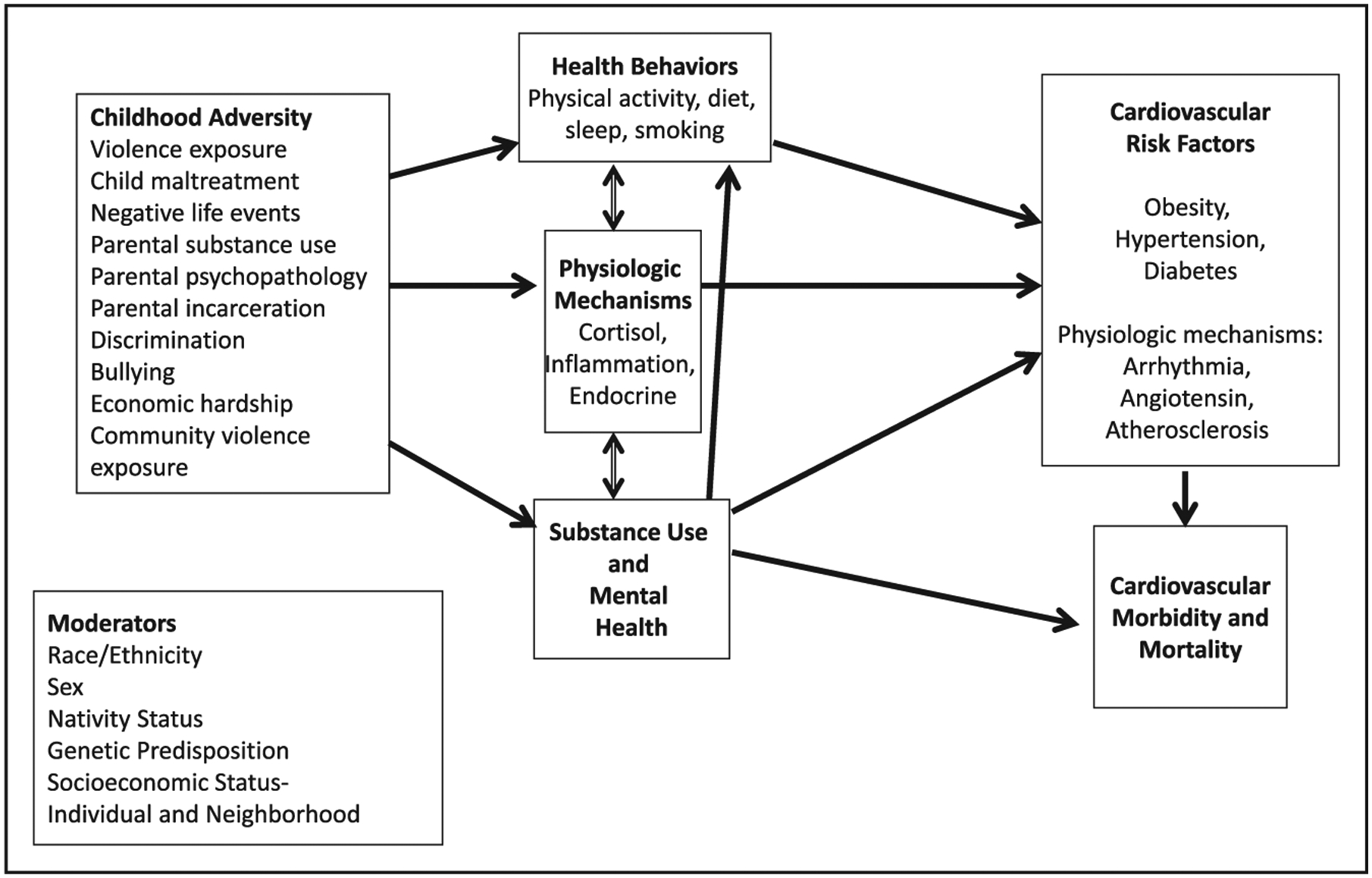
Conceptual model of the relation between childhood adversities and cardiometabolic health.
CHILDHOOD ADVERSITY AND CARDIOMETABOLIC OUTCOMES
Influence of Childhood Adversity on Cardiometabolic Outcomes
Several review articles suggest that childhood adversity is associated with increased risk of cardiometabolic disease, including CVD mortality and numerous CVD outcomes such as myocardial infarction, stroke, ischemic heart disease, and coronary heart disease.5,16,17 A recent systematic review found that childhood maltreatment was associated with CVD (myocardial infarction, stroke, ischemic heart disease, coronary heart disease) in 91.7% (22 of 24) of published studies.16 Research has also examined the relation between childhood adversity and risk factors for cardiometabolic disease. For example, several studies suggest that childhood adversity is associated with increased risk of hypertension and high blood pressure levels, although findings are mixed.5,16,18 Other studies also suggest that there may be an association between childhood adversity and obesity.3,19,20 A recent meta-analysis noted a positive association between childhood adversity and childhood-overweight measures in a pooled estimate of reviewed longitudinal studies.21 In addition, childhood adversity has been associated with increased risk of T2DM in adulthood.16,22,23
Severity, Timing, and Chronicity of Childhood Adversity on Cardiometabolic Outcomes
Childhood maltreatment (physical, sexual, or emotional abuse and neglect) is by far the most frequent operationalization of adversity, and this broad measure has been robustly associated with cardiometabolic risk.2,16 Studies have documented a more consistent association between physical and sexual abuse and cardiometabolic disease risk compared with other specific forms of adversity.23–29 Although some work has examined individual adverse experiences, other work has focused on the accumulation of these experiences and frequently demonstrates a dose-response relation with cardiometabolic risk. Several studies examining maltreatment as part of a composite measure of adversity have reported a dose-response relationship between number of childhood adversities and heightened risk for cardiometabolic diseases.2,27,30–34 This type of dose-response relationship suggests that reducing the number of co-occurring adversities could help to prevent the downstream cardiometabolic consequences of maltreatment. The ACE study, for example, noted a continuous dose-response relation between the number of childhood adverse experiences and the odds of ischemic heart disease.2 However, some research suggests more of a threshold effect.22 Data from the BRFSS note a relation between exposure to ≥4 childhood adversities and CVD.31 More recent research has expanded the potential domains of adverse experiences and incorporated severity and intensity into an adversity score,35 signaling a potential future direction in the assessment and modeling of adverse experiences and their relation with cardiometabolic risk.
In a recent review, all but 1 study considered adversities experienced before 18 years of age as 1 period without consideration for different developmental periods that may exist within childhood.5 For example, results from the Bucharest Early Intervention Project, which randomized children from extremely deprived institutional environments to therapeutic foster care, found that, for cortisol and parasympathetic nervous system reactivity, intervention effects were evident only among children placed in foster care before 24 months of age, suggesting that there may be sensitive periods in childhood during which the environment is particularly likely to alter stress response system development.36 Whether there are sensitive periods of exposure to adversity for the development of cardiometabolic outcomes remains unknown. Within the Nurses’ Health Study, Riley and colleagues37 noted that experiencing sexual abuse jointly in childhood and adolescence was associated with increased risk of hypertension in adulthood compared with not experiencing abuse at either time. A similar association was not noted among women who experienced abuse at only 1 time point, suggesting that chronicity of exposure, not timing, was relevant for the development of hypertension.37 However, childhood and adolescence, as defined by this study, are still quite broad periods in terms of development. More precise work on whether exposure during specific periods has differential effects on cardiometabolic risk is warranted.
Modifying Factors
Sex
There are sex differences in exposure to childhood maltreatment and in cardiometabolic outcomes. However, investigation into whether sex modifies the relation between childhood maltreatment and cardiometabolic outcomes has been limited. With regard to exposure to adversity, data from the National Comorbidity Survey Adolescent Supplement indicate that girls are more likely to experience sexual abuse and rape, for example, and boys are more likely to report experiences such as exposure to accidents or disasters.38 Maltreatment-specific data suggest that girls are more likely to experience sexual abuse and boys are more likely to experience physical abuse.39 With regard to cardiometabolic outcomes, many recent reviews have discussed sex differences, including an earlier age of onset of many cardiometabolic diseases in men, yet in adulthood, CVD mortality is higher in women.40,41 Recent studies have postulated that psychosocial stress may be a more important risk factor for cardiometabolic disease in women than in men, either because women are exposed to more psychosocial stress or because they are more vulnerable to its effects.42 For example, sex differences in T2DM in relation to depression and anxiety have been noted, with stronger associations observed in women.43,44 Furthermore, among young children, sex differences have also been noted in the relation between adversity and obesity, with girls being at increased risk of obesity in relation to adversity in early childhood.45 However, recent reviews have demonstrated that few studies have formally examined sex-related differences in the association between adversity and cardiometabolic outcomes. Among those that have, there was no consistent pattern of sex-related variations.16 Similarly, a review by Slopen et al46 of stress and cardiometabolic biomarkers in youth found that only 4 studies reported sex-stratified analyses with no consistent direction of effect. A recent meta-analysis also noted insufficient data to draw conclusions about sex-related differences based on adverse childhood events exposure and T2DM.47 Taken together, these data suggest large gaps in our knowledge and that a more rigorous examination of sex differences in the relation between childhood adversity and cardiometabolic outcomes is warranted.
SES, Race, Ethnicity, and Immigration Status
Racial and ethnic minority children and children living in lower-SES households have a higher prevalence of childhood adversities; in addition, they experience a higher prevalence of cardiometabolic health outcomes across the life course.6,11 SES may be an upstream determinant of adversities because socioeconomic constraints may put children at higher risk for experiencing adversities (ie, exposure to violence, household dysfunction).17 Children living in lower-SES households may be more vulnerable to adverse experiences because potential economic, social, and emotional resources necessary to cope with and manage these adverse experiences may not be available to them as a result of their disadvantaged position. However, existing studies have rarely examined the potential modifying effect of race/ethnicity or lower SES on childhood adversities and cardiometabolic health relation. Immigration history has also been largely ignored as a potential modifying factor. A recent study of the National Survey of Children’s Health noted that regardless of SES, children of immigrant parents had, counterintuitively, lower levels of adversities compared with children of US-born parents.11 Similar associations were noted within participants of the Hispanic Community Health Study/Study of Latinos.48 Previous work has documented that despite higher poverty rates, children of immigrant parents have health outcomes that are similar to or better than those of children of US-born parents. Whether immigrant status modifies the association between childhood adversity and cardiometabolic health is unknown and warrants further research.
Mechanisms
As depicted in the Figure, at least 3 pathways are commonly identified to explain how childhood adversity may increase the risk of cardiometabolic (and other) diseases: behavioral, mental health, and biological.
Behavioral Factors
Evidence suggests that childhood adversity is associated with adverse health behaviors that increase the risk of cardiometabolic disease, including smoking, overeating, consumption of energy-dense foods, and inactivity.2,49,50 The association of childhood adversity with these behaviors was first tested by Felitti et al.2 In their retrospective ACE study, Felitti et al hypothesized that greater childhood adversity occurs in a setting of parental dysfunction in which children experience greater levels of distress and lack adequate support and “psychological nourishment.”51 Thus, in response to high levels of distress, children experiencing adversity are at higher risk of adopting adverse coping mechanisms (eg, smoking, overeating). For example, Anda and colleagues52 published data showing that exposure to ≥5 ACEs versus no exposure was associated with a 5-fold higher rate of early smoking initiation. Furthermore, the home environment is central to children’s daily experiences, particularly with regard to meal and activity patterns. The home environment may affect practices such as meal preparation, use of television (and other digital media), consumption of food outside the home, and participation in physical activity.8,27,33,34 Parental psychological distress is associated with lower consumption of fruits, vegetables, and high-calcium foods among children,33 plus fewer physical and more sedentary activities,25,35 each of which increases children’s risk of obesity.34,36,37 Childhood adversity has been associated with obesity in children as young as 5 years of age,45 and prospective research shows that childhood obesity and adolescent obesity have been associated with increased risk of cardiovascular risk factors and CVD among adults.53–55 Data also suggest that whereas an unhealthy diet and sedentary lifestyle adversely affect physical and mental health,56,57 a healthy diet and plentiful activity reduce inflammation, depression, and anxiety.58,59 Thus, evidence increasingly suggests that behavioral risk factors, in part, mediate the childhood adversity-cardiometabolic disease relationship. However, despite a strong retrospective association of trauma, stress, and adult obesity,60 little is known about the prospective impact of childhood adversity on behavioral mediators that predict cardiometabolic disease across the life course. In addition to behavioral factors, other unmeasured behaviors or biological processes likely are involved in the pathway from childhood adversity to adverse cardiometabolic outcomes.
Mental Health Factors
The association between early adversity and subsequent mental health problems in youth and adulthood is well known.61 It is thought to be moderated by genetic factors and mediated in part by neurobiological effects of trauma.62–67 A recent systematic review noted that mental disorders were a partial mediator of the association between childhood maltreatment and cardiometabolic disease16 but concluded that the findings should be interpreted with some caution because studies varied greatly in terms of how they modeled effects of mental disorders (eg, statistical models varied in their treatment of time, in combination with other disorders, or with health behaviors).24,27,28,30,68,69 Many childhood adversities, including childhood maltreatment and exposure to violence, are traumatic events that may result in posttraumatic stress disorder in some individuals. Although investigators have examined posttraumatic stress disorder in relation to a range of health outcomes (including rheumatoid arthritis, stroke, heart disease, and cancer), some of the strongest empirical research, in terms of methodology and findings, has been with cardiometabolic diseases.70,71 Numerous methodologically rigorous prospective population-based observational studies have found that posttraumatic stress disorder is associated with increased risk of incident CVD72–84 and T2DM.85–87 However, whether and how much of the relation between childhood adversity and cardiometabolic disease may be explained by post-traumatic stress disorder is unclear, especially because these studies have included traumatic events over the life course, with a substantial proportion of studies focused on veterans and military service-related trauma, not just adversity during childhood.
Childhood adversity increases the risk of mood and anxiety disorders,62,88 which are widely recognized as increasing the risk for cardiometabolic morbidity and mortality. A recent American Heart Association statement positioned major depressive disorder and bipolar disorder as conditions that predispose youth to accelerated atherosclerosis and early CVD.89 Although an extensive amount of research has focused on depression, a recent review called for equal attention to anxiety disorders as risk factors for CVD given the state of the current evidence.90 Childhood adversity converges with mental health problems in a number of ways that are relevant to cardiometabolic outcomes. For example, childhood adversity is an important indicator of a more persistent and treatment-refractory course of illness and affects response to both pharmacological and psychosocial treatment for youth and adults with mental health conditions.91–95 Among people with mental health conditions, childhood adversity, compared with no childhood adversity, results in greater cumulative exposure to mental health symptoms, associated stress, and biological perturbations.96–98
Beyond the direct effects of mental health problems on cardiometabolic outcomes, there are also indirect effects that further exacerbate cardiometabolic risk factors. For example, youth with mood disorders are less likely to achieve recommended levels of physical activity and sleep and more likely to have suboptimal dietary habits, all of which impart cardiometabolic disease risk.89 In addition, several pharmacological treatments used to treat mental health problems could contribute to cardiometabolic risk factor accumulation. Several second-generation antipsychotics and mood-stabilizing medications confer risk of weight gain and other cardiometabolic disturbances.99,100 It is unlikely, however, that the link between mental health and cardiometabolic outcomes is fully explained by psychotropic medications.77,101 In addition, contemporary medications used for the treatment of attention-deficit/hyperactivity disorder, anxiety, and depression in youth are not commonly associated with cardiometabolic risk factors.102–105
Biological Factors
Childhood adversities may disrupt many of the regulatory systems of the body, altering the immune, metabolic, neuroendocrine, and autonomic nervous systems.36,46,106 In the short term, altered stress responses likely help children function while living in high-risk households, for example, by increasing alertness. However, in the long term, these responses could trigger health problems, including chronic hypertension.107 Long-term hypothalamic-pituitary-adrenal axis activation, in response to prolonged experiences of stress, affects glucocorticoid metabolism and likely alters immune function. Studies document that childhood adversities predispose individuals to chronic inflammation,108–110 with elevation of interleukin-6, C-reactive protein, fibrinogen, and other biomarkers associated with cardiometabolic disease. Childhood adversities are also related to adverse trajectories in traditional cardiometabolic factors; the Georgia Stress and Heart Study, for example, showed that individuals who experience childhood adversity have faster increases in blood pressure from childhood to young adulthood.111 Similarly, a British cohort showed that childhood maltreatment led to accelerated increases in body mass index from childhood to adulthood.112 Several studies also demonstrated that childhood adversities increase markers of subclinical CVD, including markers of endothelial dysfunction (E-selectin, intercellular adhesion molecule-1),108 arterial stiffness, and carotid intima-media thickness progression.113,114 Preliminary data suggest that epigenetic changes may form part of the biological linkage between subjective experience of adversity and objective cardiometabolic derangement.115–118 Methylation of genes regulating pathways to obesity and metabolic disorders in adults exposed to child abuse also has been identified.119 However, research on DNA methylation is still an emerging field of study. Population-based studies are needed to clarify the role of epigenetics in the link between childhood adversities and CVD risk.
Finally, although beyond the scope of this review, behavioral factors such as diet and physical activity as outlined earlier can reflect stress-induced physiological changes. For example, ghrelin is a neurohormone that is upregulated under stress conditions and has mild anxiolytic and antidepressant properties120; however, ghrelin also increases appetite, food-seeking behavior, and food-associated reward.121,122 In addition, maltreated children have been found to be deficient in leptin, a hormone that regulates energy balance.123 On the other hand, physical activity increases endorphins, postpran-dial satiety-hormone levels (peptide YY3-36124), and affective mood.125,126
LIMITATIONS
Although an increasing body of work has documented a relationship between childhood adversity and cardiometabolic outcomes and suggests potential underlying pathways, certain limitations of the literature should be considered.
Lack of Agreement on Definitions
As noted, existing literature in this area is based on a collection of heterogeneous measures and definitions of childhood adversity. Although the accumulation of these heterogeneous adverse experiences consistently predicts worse outcomes, little work has considered the differential effects that some of these experiences may have. In addition, limited research has carefully evaluated differential effects on the basis of the timing of when these experiences occur, with some studies suggesting that exposures in early childhood appear to have a more enduring impact. Furthermore, other prevalent adverse experiences not originally considered in the ACE study need to be evaluated for their potential influence on cardiometabolic risk. For example, although child victimization at the hands of adults has received more attention, recent studies have highlighted the important role of bullying or victimization by peers in predicting obesity and inflammation in later life.9,10 Other studies have highlighted the importance of experiences of racial discrimination in relation to health outcomes, which are more prevalent among racial/ethnic minorities.8 Given the high prevalence of individuals experiencing these and other stressors, it is important to consider the sociodemographic characteristics of the population being considered because its experiences of adversity will vary. Finally, adverse environmental conditions are nonrandomly distributed, are experienced to a disproportionate degree by subgroups, and are associated with risk for long-term familial and interpersonal adversities.
Few Truly Prospective Studies
Although longitudinal studies based on prospective measures have appeared more frequently in recent years, most of the research in the area is based on cross-sectional studies using retrospective reports by adults. Most research on childhood adversity and CVD, T2DM, hypertension, and obesity has been cross-sectional and has relied on retrospective reporting of childhood adversity.5,16 This is problematic because research shows that there is only moderate agreement between prospective and retrospective measures of childhood adversity, such that groups of individuals identified with these 2 different methods of assessment may show only limited overlap and different disease risk.127 Longitudinal designs are increasing in frequency, and a few longitudinal studies have assessed adversity in childhood and/or adolescence.4,9,10,29,68,109,111,128–131 As an example, the British National Child Development Study, a 50-year longitudinal study, assessed experiences of bullying when children were between 7 and 11 years of age. Bullying was associated with higher levels of C-reactive protein in midlife and, among women, with obesity in midlife.9 Nevertheless, retrospective reporting of adversity remains the predominant approach to measurement, even in longitudinal research. Longitudinal studies would enable the identification and tracking of behavioral, mental health, and biological mechanisms over time that then lead to cardiometabolic disease. In addition to strengthening the causal links, research that examines associations between childhood adversity and cardiometabolic risk over the life course is critical for elucidating when the deleterious effects of childhood adversity may begin to appear and thus could be targeted for prevention. To date, most investigations have focused on understanding how childhood adversity is associated with cardiometabolic risk in adulthood, although a growing number of studies have found detectable divergence resulting from adversity in trajectories of health risk in adolescence.106,128,131,132 However, other studies have not found clear evidence of divergence before adulthood111,112 or have found evidence to be stronger in adult samples compared with child/adolescent samples.20 Research explicitly investigating trajectories is limited, precluding firm conclusions on when excess risk resulting from adversity is reliably detectable. Prospective studies that begin during the prenatal period or preconception or follow cohorts from childhood through childbirth would also capture intergenerational adversity and perinatal programming.
Limited Identification of Mechanisms
As discussed, childhood adversity may provoke unhealthy behaviors and poor mental health or produce neurobiological alterations that initiate relevant pathophysiological processes. Few studies have explicitly tested the mechanisms linking childhood adversity and cardiometabolic disease with comprehensive mediation models.133 Moreover, no study of which we are aware has tested a range of mechanisms and attempted to quantify which mechanism may be most important. Such research is critical both to inform whether childhood adversity is causal in cardiometabolic disease and to identify targets for intervention.
FUTURE DIRECTIONS
Determinants of Resilience
Not all individuals with a history of childhood and adolescent adversity develop cardiometabolic health outcomes, which raises the question of the determinants of resilience and cardiometabolic health. Rather than being inherent to the child, resilience, that is, good mental and physical health despite the assails of early adversity, results from a complex interplay among the child’s genetics, natural temperament, knowledge and skills, past experiences, social supports, and cultural and societal resources.134 Better integration of our understanding of modifiable resilience factors into interventions and routine care settings could improve outcomes for children who may face adversity in the future or have had past adversities. Information on determinants of resilience may also offer important insights into the mechanisms underlying the relationships between childhood adversity and cardiometabolic health outcomes. Finally, information on determinants of resilience may improve the tailoring of interventions to those who can most benefit from them. Because no intervention is universally effective, understanding why certain interventions benefit some children and not others will enable the matching of interventions. Moreover, identification of modifiable factors that buffer the effects of adversity or improve resilience could provide targets for interventions.135 Few studies have focused on resilience factors such as positive coping, social support, and family dynamics that could modify the impact of childhood adversity on cardiometabolic health.136
Modifiers of Vulnerability
There has been limited examination of individual characteristics such as sex, race/ethnicity, and genetic factors in modifying the risks posed by childhood adversity. Among women, experiences of stress are most often associated with a higher prevalence and incidence of cardiometabolic outcomes compared with men, which may be attributed to differential behavioral or biological responses to stress. For example, some research suggests that the impact of childhood adversity on obesity3,20,137 is stronger or more consistent among women than men. However, consistent patterns of sex-related differences in the associations between childhood adversity and cardiometabolic diseases have not been observed.16 Similarly, there are important environmental factors, including individual, family, neighborhood, and community characteristics, that could modify the effects of childhood adversity on cardiometabolic outcomes and should be further characterized. For example, residential segregation, crime, and discrimination could be barriers to the adoption of healthy lifestyles (eg, physical activity and healthy dietary patterns), thereby contributing to poor cardiovascular health. Finally, several studies suggest that genes may influence how children interact with their environment, biasing their biological responses and affecting risk of clinical outcomes.138 Studies of gene-environment interplay, which includes genotype-environment and epigenetics, have, to date, focused largely on mental health outcomes. Future research should explore how childhood adversity may interact with genetic vulnerability in producing cardiometabolic health outcomes. Epigenetic approaches might examine how childhood adversity alters gene expression and whether such alterations influence cardiometabolic outcomes.
Mechanisms
To reverse or remediate biological risk for health outcomes among those exposed to childhood adversity (secondary prevention), it is important to better understand the mechanisms through which childhood adversity leads to cardiometabolic outcomes. On the one hand, childhood adversity begets psychosocial adversity and worse mental health in later life. Future research should test whether adult psychosocial adversity and mental health explain why individuals with a history of childhood adversity develop cardiometabolic health outcomes. These studies might highlight targets for interventions. On the other hand, we are only beginning to understand the biological mechanisms through which childhood adversity brings about cardiometabolic outcomes (biological embedding),139 including inflammation, abnormal neuroendocrine function, and others.139 Future research should test comprehensive biological mediation models to uncover intervention targets. Research clearly demonstrating that childhood adversity alters known mechanisms of cardiometabolic risk would provide compelling evidence that childhood adversity is indeed involved in the pathogenesis of cardiometabolic disease. However, rigorous evidence demonstrating these causal associations is limited.
Application of Modern Epidemiological Methods
Confounding bias, that the relation between childhood adversity and cardiometabolic disease is explained by a third variable, has not been fully accounted for in many studies beyond consideration of childhood SES, thus limiting our ability to make causal inferences on the adversity and cardiometabolic health relation. State-of-the-art epidemiological methods such as marginal structural models have transformed the epidemiological approach to time-varying confounding of time-varying exposures over the past 25 years, but to the best of our knowledge, these methods have not been applied to childhood adversity research. The possibility of time-varying confounding, that the behaviors and risk factors that are subsequently affected by exposure to childhood adversity may also increase the risk of adversity, is a major threat to causal inference. For example, findings in a genetically informative British cohort indicated that child characteristics such as intelligence quotient and adjustment problems predicted exposure to maltreatment by adults140 and chronic peer victimization (bullying).141 Others have shown the adverse effects of maltreatment and peer victimization on child adjustment. Childhood intelligence quotient and adjustment have been linked to cardiometabolic disease in adulthood.142,143 Marginal structural models can be used to account for the potential for dynamic feedback processes by which certain factors can act as either confounders or mediators of the effects of the childhood adversity-cardiometabolic disease relationship.144
Interventions
Another outstanding question is whether an effective reduction in childhood adversity or interventions aimed at buffering the effects of exposure to adversity can prevent or mitigate the likelihood of developing cardiometabolic disease.145 Only a few studies have examined the role of early intervention on the reduction of childhood adversities and cardiometabolic health.146,147 The Carolina Abecedarian Project noted lower levels of blood pressure among adults in their mid-30s who received early childhood intervention compared with a randomly allocated control group.146 Future research should also examine the potential benefit of preventive interventions targeting mental health, behavioral, and biological sequelae of childhood adversity that could buffer the effects on cardiometabolic health. The same questions can be raised about screening for childhood adversity. The National Council for Behavioral Health, in partnership with and sponsored by Kaiser Permanente National Community Benefit Fund, selected 14 organizations through a competitive process to pilot the Trauma Informed Primary Care Project with the goal of integrating the assessment of trauma into primary care to tailor patient care while addressing the consequences of past trauma.148 However, at this time, the benefits of such screening have not been quantified. As previously noted, SES is a potential antecedent to the experience of childhood adversities; thus, addressing social factors could reduce both childhood adverse experiences and the risk of cardiometabolic disease.147 Moreover, the benefit of screening for childhood adversities relies on an appropriate response to address the effects of any adversities identified. Addressing the effects of childhood adversity is complex and can pose challenges for providers.149,150 For adults, a retrospective assessment of childhood adversities and current trauma could be coupled with mental health assessments and screenings for substance use, although the validity of retrospective recall of childhood adversity remains debated.127 Newly identified mental health problems should lead to referrals and treatment for those conditions, in addition to already provided clinical care for physical health conditions. For minors, in addition to screening for adversity and behavioral and mental health issues, the safety and security of the child must be considered because the adversity identified may be ongoing. There are thus implications to screening, requiring proper training of providers not only on the assessment of the adversities but also on the necessary responses.
CONCLUSIONS
Childhood adversity is highly prevalent, with 59% of the US population reporting at least 1 adverse event experience.6 Substantial evidence links childhood adversity to cardiometabolic disease later in the life course, including heart disease, T2DM, and stroke, which are 3 of the top 10 causes of mortality in the United States. Given the high variability of cardiometabolic aberration and the multiplicity of pathways activated by childhood adversity, modification of the downstream cardiometabolic consequences is less desirable compared with addressing upstream adversity exposures. However, there are no national guidelines or recommendations on systematic surveillance for childhood adversity in the healthcare system, in part because of a limited understanding of how to prevent or mitigate adversity and to build resilience. Toward this goal, additional research, including longitudinal prospective studies, designed to guide and inform effective and timely individual/clinical and population-level preventive interventions is required. Areas for such research, as highlighted in this review, include defining exposure intensity, duration, and vulnerable periods during the life course and across generations; characterizing resiliency against progression toward cardiometabolic consequences; identifying biological factors that modify response to adversity and elucidating pathobiological pathways linking adversity to cardiometabolic outcomes; and demonstrating which interventions on upstream childhood adversity exposure prevent progression to cardiometabolic disease.
DISCLOSURES
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Shakira F. Suglia | Emory University Rollins School of Public Health | NHLBI† | None | None | None | None | None | None |
| Karestan C. Koenen | Harvard T.H. Chan School of Public Health | None | None | None | None | None | None | None |
| Renee Boynton-Jarrett | Boston University School of Medicine | None | None | None | None | None | None | None |
| Paul S. Chan | Mid America Heart Institute and the University of Missouri-Kansas City | NHLBI† | None | None | None | None | Optum Rxt | None |
| Cari J. Clark | Emory University Global Health | None | None | None | None | None | None | None |
| Andrea Danese | Institute of Psychiatry, King’s College London | UK MRC*; UK NSPCC/ESRC* | None | None | None | None | UK National Society for the Prevention of Cruelty to Children (NSPCC) Research Advisory Group* | None |
| Myles S. Faith | University at Buffalo School of Education and Psychology | None | None | None | None | None | None | None |
| Benjamin I. Goldstein | University of Toronto | None | None | None | None | None | None | None |
| Laura L. Hayman | University of Massachusetts Boston College of Nursing & Health Sciences | NIH* | None | None | None | None | None | None |
| Carmen R. Isasi | Albert Einstein College of Medicine Epidemiology & Population Health | None | None | None | None | None | None | None |
| Charlotte A. Pratt | National Heart, Lung, and Blood Institute NIH/NHLBI | None | None | None | None | None | None | None |
| Natalie Slopen | University of Maryland College Park | None | None | None | None | None | None | None |
| Jennifer A. Sumner | Center for Behavioral Cardiovascular Health, Columbia University Medical Center | NIH/NHLBI† | None | None | None | None | None | Columbia University Medical Center* |
| Aslan Turer | University of Texas-Southwestern | None | None | None | None | None | None | None |
| Christy B. Turer | University of Texas-Southwestern Medical Center | NIH† | None | None | None | None | None | None |
| Justin P. Zachariah | Baylor College of Medicine | NHLBI† | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Eric B. Loucks | Brown University | NIH 1R01AG048825-01 (This grant is focused on elucidating biological and behavioral mechanisms of how early life social adversity could influence later life obesity risk)† | None | None | None | None | None | None |
| Katie McLaughlin | University of Washington | None | None | None | None | None | None | None |
| Kathryn M. Rexrode | Brigham and Women’s Hospital | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Significant.
Footnotes
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Heart, Lung, and Blood Institute.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on July 28, 2017, and the American Heart Association Executive Committee on December 11, 2017. A copy of the document is available at http://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
REFERENCES
- 1.Shonkoff JP, Garner AS; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 3.Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obes Rev. 2011;12:e159–e172. doi: 10.1111/j.1467-789X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slopen N, Koenen KC, Kubzansky LD. Cumulative adversity in childhood and emergent risk factors for long-term health. J Pediatr. 2014;164:631–638.e1. doi: 10.1016/j.jpeds.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Suglia SF, Sapra KJ, Koenen KC. Violence and cardiovascular health: a systematic review. Am J Prev Med. 2015;48:205–212. doi: 10.1016/j.amepre.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Adverse childhood experiences reported by adults: five states, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1609–1613. [PubMed] [Google Scholar]
- 7.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 8.Cronholm PF, Forke CM, Wade R, Bair-Merritt MH, Davis M, Harkins-Schwarz M, Pachter LM, Fein JA. Adverse childhood experiences: expanding the concept of adversity. Am J Prev Med. 2015;49:354–361. doi: 10.1016/j.amepre.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Takizawa R, Danese A, Maughan B, Arseneault L. Bullying victimization in childhood predicts inflammation and obesity at mid-life: a five-decade birth cohort study. Psychol Med. 2015;45:2705–2715. doi: 10.1017/S0033291715000653. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin JR, Arseneault L, Odgers C, Belsky DW, Matthews T, Ambler A, Caspi A, Moffitt TE, Danese A. Childhood bullying victimization and overweight in young adulthood: a cohort study. Psychosom Med. 2016;78:1094–1103. doi: 10.1097/PSY.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, Williams DR. Racial disparities in child adversity in the U.S.: interactions with family immigration history and income. Am J Prev Med. 2016;50:47–56. doi: 10.1016/j.amepre.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW; on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 14.Fox KM, Wang L, Gandra SR, Quek RG, Li L, Baser O. Clinical and economic burden associated with cardiovascular events among patients with hyperlipidemia: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:13. doi: 10.1186/s12872-016-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 16.Basu A, McLaughlin KA, Misra S, Koenen K. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol (New York). 2017;24:125–139. doi: 10.1111/cpsp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17:88. doi: 10.1007/s11886-015-0645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafson TB, Sarwer DB. Childhood sexual abuse and obesity. Obes Rev. 2004;5:129–135. doi: 10.1111/j.1467-789X.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 20.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19:544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 21.Elsenburg L, van Wijk K, Liefbroer A, Smidt N. Accumulation of adverse childhood events and overweight in children: a systematic review and meta-analysis. Obesity (Silver Spring). 2017;25:820–832. doi: 10.1002/oby.2179. [DOI] [PubMed] [Google Scholar]
- 22.Huffhines L, Noser A, Patton SR. The link between adverse childhood experiences and diabetes. Curr Diab Rep. 2016;16:54. doi: 10.1007/s11892-016-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, Wright RJ. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39:529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afifi TO, Mota N, MacMillan HL, Sareen J. Harsh physical punishment in childhood and adult physical health. Pediatrics. 2013;132:e333–e340. doi: 10.1542/peds.2012-4021. [DOI] [PubMed] [Google Scholar]
- 25.Duncan AE, Auslander WF, Bucholz KK, Hudson DL, Stein RI, White NH. Relationship between abuse and neglect in childhood and diabetes in adulthood: differential effects by sex, National Longitudinal Study of Adolescent Health. Prev Chronic Dis. 2015;12:E70. doi: 10.5888/pcd12.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126:920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott KM, Von Korff M, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, Haro JM, Lépine JP, Ormel J, Posada-Villa J, Tachimori H, Kessler RC. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry. 2011;68:838–844. doi: 10.1001/archgenpsychiatry.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suglia SF, Clark CJ, Boynton-Jarrett R, Kressin NR, Koenen KC. Child maltreatment and hypertension in young adulthood. BMC Public Health. 2014;14:1149. doi: 10.1186/1471-2458-14-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. 2008;121:e1240–e1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- 30.Stein DJ, Scott K, Haro Abad JM, Aguilar-Gaxiola S, Alonso J, Angermeyer M, Demytteneare K, de Girolamo G, Iwata N, Posada-Villa J, Kovess V, Lara C, Ormel J, Kessler RC, Von Korff M. Early childhood adversity and later hypertension: data from the World Mental Health Survey. Ann Clin Psychiatry. 2010;22:19–28. [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, Parks SE. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48:345–349. doi: 10.1016/j.amepre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med. 2016;50:344–352. doi: 10.1016/j.amepre.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman EM, Montez JK, Sheehan CM, Guenewald TL, Seeman TE. Childhood adversities and adult cardiometabolic health: does the quantity, timing, and type of adversity matter? J Aging Health. 2015;27:1311–1338. doi: 10.1177/0898264315580122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson RS, Boyle PA, Levine SR, Yu L, Anagnos SE, Buchman AS, Schneider JA, Bennett DA. Emotional neglect in childhood and cerebral infarction in older age. Neurology. 2012;79:1534–1539. doi: 10.1212/WNL.0b013e31826e25bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis CR, Dearing E, Usher N, Trifiletti S, Zaichenko L, Ollen E, Brinkoetter MT, Crowell-Doom C, Joung K, Park KH, Mantzoros CS, Crowell JA. Detailed assessments of childhood adversity enhance prediction of central obesity independent of gender, race, adult psychosocial risk and health behaviors. Metabolism. 2014;63:199–206. doi: 10.1016/j.metabol.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA 3rd. Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health. 2010;64:413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2013;52:815–830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Child Maltreatment: Facts at a Glance. 2013. https://www.cdc.gov/violenceprevention/pdf/childmaltreatment-facts-at-a-glance.pdf.
- 40.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK; on behalf of the American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 41.Crea F, Battipaglia I, Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. 2015;241:157–168. doi: 10.1016/j.atherosclerosis.2015.04.802. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen LR, Frestad D, Michelsen MM, Mygind ND, Rasmusen H, Suhrs HE, Prescott E. Risk factors for myocardial infarction in women and men: a review of the current literature. Curr Pharm Des. 2016;22:3835–3852. [DOI] [PubMed] [Google Scholar]
- 43.Suglia SF, Demmer RT, Wahi R, Keyes KM, Koenen KC. Depressive symptoms during adolescence and young adulthood and the development of type 2 diabetes mellitus. Am J Epidemiol. 2016;183:269–276. doi: 10.1093/aje/kwv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demmer RT, Gelb S, Suglia SF, Keyes KM, Aiello AE, Colombo PC, Galea S, Uddin M, Koenen KC, Kubzansky LD. Sex differences in the association between depression, anxiety, and type 2 diabetes mellitus. Psychosom Med. 2015;77:467–477. doi: 10.1097/PSY.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R. Cumulative social risk and obesity in early childhood. Pediatrics. 2012;129:e1173–e1179. doi: 10.1542/peds.2011-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slopen N, Goodman E, Koenen KC, Kubzansky LD. Socioeconomic and other social stressors and biomarkers of cardiometabolic risk in youth: a systematic review of less studied risk factors. PLoS One. 2013;8:e64418. doi: 10.1371/journal.pone.0064418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, Gao H, Hao L, Liu L. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta-analysis. Metabolism. 2015;64:1408–1418. doi: 10.1016/j.metabol.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Llabre MM, Schneiderman N, Gallo LC, Arguelles W, Daviglus ML, Gonzalez F 2nd, Isasi CR, Perreira KM, Penedo FJ. Childhood trauma and adult risk factors and disease in Hispanics/Latinos in the US: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sociocultural Ancillary Study. Psychosom Med. 2017;79:172–180. doi: 10.1097/PSY.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr. 2000;20:21–44. doi: 10.1146/annurev.nutr.20.1.21. [DOI] [PubMed] [Google Scholar]
- 50.Hemmingsson E, Johansson K, Reynisdottir S. Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obes Rev. 2014;15:882–893. doi: 10.1111/obr.12216. [DOI] [PubMed] [Google Scholar]
- 51.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. [DOI] [PubMed] [Google Scholar]
- 53.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 54.Suglia SF, Clark CJ, Gary-Webb TL. Adolescent obesity, change in weight status, and hypertension: racial/ethnic variations. Hypertension. 2013;61:290–295. doi: 10.1161/HYPERTENSIONAHA.111.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 57.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 59.Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- 60.Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC. The weight of traumatic stress: a prospective study of post-traumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLaughlin KA. Future directions in childhood adversity and youth psychopathology. J Clin Child Adolesc Psychol. 2016;45:361–382. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. [DOI] [PubMed] [Google Scholar]
- 66.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 67.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–1119. [DOI] [PubMed] [Google Scholar]
- 68.Widom CS, Czaja SJ, Bentley T, Johnson MS. A prospective investigation of physical health outcomes in abused and neglected children: new findings from a 30-year follow-up. Am J Public Health. 2012;102:1135–1144. doi: 10.2105/AJPH.2011.300636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–520. [DOI] [PubMed] [Google Scholar]
- 70.Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, Thorp SR, Jeste DV. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23:709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koenen KC, Sumner JA, Gilsanz P, Glymour MM, Ratanatharathorn A, Rimm EB, Roberts AL, Winning A, Kubzansky LD. Post-traumatic stress disorder and cardiometabolic disease: improving causal inference to inform practice. Psychol Med. 2017;47:209–225. doi: 10.1017/S0033291716002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21:227–234. [DOI] [PubMed] [Google Scholar]
- 73.Jordan HT, Miller-Archie SA, Cone JE, Morabia A, Stellman SD. Heart disease among adults exposed to the September 11, 2001 World Trade Center disaster: results from the World Trade Center Health Registry. Prev Med. 2011;53:370–376. doi: 10.1016/j.ypmed.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 74.Scherrer JF, Chrusciel T, Zeringue A, Garfield LD, Hauptman PJ, Lustman PJ, Freedland KE, Carney RM, Bucholz KK, Owen R, True WR. Anxiety disorders increase risk for incident myocardial infarction in depressed and non-depressed Veterans Administration patients. Am Heart J. 2010;159:772–779. doi: 10.1016/j.ahj.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 75.Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62:970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gradus JL, Farkas DK, Svensson E, Ehrenstein V, Lash TL, Milstein A, Adler N, Sørensen HT. Associations between stress disorders and cardiovascular disease events in the Danish population. BMJ Open. 2015;5:e009334. doi: 10.1136/bmjopen-2015-009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, Cerdá M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of incident cardiovascular disease in aging veterans. Am J Geriatr Psychiatry. 2016;24:192–200. doi: 10.1016/j.jagp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health. 2015;105:757–763. doi: 10.2105/AJPH.2014.302342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen MH, Pan TL, Li CT, Lin WC, Chen YS, Lee YC, Tsai SJ, Hsu JW, Huang KL, Tsai CF, Chang WH, Chen TJ, Su TP, Bai YM. Risk of stroke among patients with post-traumatic stress disorder: nationwide longitudinal study. Br J Psychiatry. 2015;206:302–307. doi: 10.1192/bjp.bp.113.143610. [DOI] [PubMed] [Google Scholar]
- 81.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–256. doi: 10.1016/j.annepidem.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 83.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28:125–130. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kubzansky LD, Koenen KC, Spiro A 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- 85.Boyko EJ, Jacobson IG, Smith B, Ryan MA, Hooper TI, Amoroso PJ, Gackstetter GD, Barrett-Connor E, Smith TC; Millennium Cohort Study Team. Risk of diabetes in U.S. military service members in relation to combat deployment and mental health. Diabetes Care. 2010;33:1771–1777. doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaccarino V, Goldberg J, Magruder KM, Forsberg CW, Friedman MJ, Litz BT, Heagerty PJ, Huang GD, Gleason TC, Smith NL. Posttraumatic stress disorder and incidence of type-2 diabetes: a prospective twin study. J Psychiatr Res. 2014;56:158–164. doi: 10.1016/j.jpsychires.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72:203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW; on behalf of the American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 90.Tully PJ, Harrison NJ, Cheung P, Cosh S. Anxiety and cardiovascular disease risk: a review. Curr Cardiol Rep. 2016;18:120. doi: 10.1007/s11886-016-0800-3. [DOI] [PubMed] [Google Scholar]
- 91.Sala R, Goldstein BI, Wang S, Blanco C. Childhood maltreatment and the course of bipolar disorders among adults: epidemiologic evidence of dose-response effects. J Affect Disord. 2014;165:74–80. doi: 10.1016/j.jad.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shamseddeen W, Asarnow JR, Clarke G, Vitiello B, Wagner KD, Birmaher B, Keller MB, Emslie G, Iyengar S, Ryan ND, McCracken JT, Porta G, Mayes T, Brent DA. Impact of physical and sexual abuse on treatment response in the Treatment of Resistant Depression in Adolescent Study (TORDIA). J Am Acad Child Adolesc Psychiatry. 2011;50:293–301. doi: 10.1016/j.jaac.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 95.Agnew-Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:342–349. doi: 10.1016/S2215-0366(15)00544-1. [DOI] [PubMed] [Google Scholar]
- 96.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 97.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700. doi: 10.1097/chi.0b013e318040b25f. [DOI] [PubMed] [Google Scholar]
- 101.Sumner JA, Kubzansky LD, Roberts AL, Gilsanz P, Chen Q, Winning A, Forman JP, Rimm EB, Koenen KC. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol Med. 2016;46:3105–3116. doi: 10.1017/S0033291716001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reekie J, Hosking SP, Prakash C, Kao KT, Juonala M, Sabin MA. The effect of antidepressants and antipsychotics on weight gain in children and adolescents. Obes Rev. 2015;16:566–580. doi: 10.1111/obr.12284. [DOI] [PubMed] [Google Scholar]
- 103.Olfson M, Huang C, Gerhard T, Winterstein AG, Crystal S, Allison PD, Marcus SC. Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:147–156. doi: 10.1016/j.jaac.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994–1009. doi: 10.1097/CHI.ObO13e31817eOea7. [DOI] [PubMed] [Google Scholar]
- 106.Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun. 2012;26:239–250. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 107.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 108.Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: findings from the MIDUS study. Dev Psychol. 2015;51:1630–1644. doi: 10.1037/dev0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia Stress and Heart Study. Circulation. 2015;131:1674–1681. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Power C, Pinto Pereira SM, Li L. Childhood maltreatment and BMI trajectories to mid-adult life: follow-up to age 50 y in a British birth cohort. PLoS One. 2015;10:e0119985. doi: 10.1371/journal.pone.0119985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klassen SA, Chirico D, O’Leary DD, Cairney J, Wade TJ. Linking systemic arterial stiffness among adolescents to adverse childhood experiences. Child Abuse Negl. 2016;56:1–10. doi: 10.1016/j.chiabu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Hakulinen C, Pulkki-Råback L, Elovainio M, Kubzansky LD, Jokela M, Hintsanen M, Juonala M, Kivimäki M, Josefsson K, Hutri-Kähönen N, Kähönen M, Viikari J, Keltikangas-Järvinen L, Raitakari OT. Childhood psychosocial cumulative risks and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Psychosom Med. 2016;78:171–181. doi: 10.1097/PSY.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bick J, Naumova O, Hunter S, Barbot B, Lee M, Luthar SS, Raefski A, Grigorenko EL. Childhood adversity and DNA methylation of genes involved in the hypothalamus-pituitary-adrenal axis and immune system: whole-genome and candidate-gene associations. Dev Psychopathol. 2012;24:1417–1425. doi: 10.1017/S0954579412000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Demetriou CA, van Veldhoven K, Relton C, Stringhini S, Kyriacou K, Vineis P. Biological embedding of early-life exposures and disease risk in humans: a role for DNA methylation. Eur J Clin Invest. 2015;45:303–332. doi: 10.1111/eci.12406. [DOI] [PubMed] [Google Scholar]
- 117.Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Houtepen LC, Vinkers CH, Carrillo-Roa T, Hiemstra M, van Lier PA, Meeus W, Branje S, Heim CM, Nemeroff CB, Mill J, Schalkwyk LC, Creyghton MP, Kahn RS, Joëls M, Binder EB, Boks MP. Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nat Commun. 2016;7:10967. doi: 10.1038/ncomms10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suderman M, Borghol N, Pappas JJ, Pinto Pereira SM, Pembrey M, Hertzman C, Power C, Szyf M. Childhood abuse is associated with methylation of multiple loci in adult DNA. BMC Med Genomics. 2014;7:13. doi: 10.1186/1755-8794-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perelló M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychiatry. 2012;72:347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Danese A, Dove R, Belsky DW, Henchy J, Williams B, Ambler A, Arseneault L. Leptin deficiency in maltreated children. Transl Psychiatry. 2014;4:e446. doi: 10.1038/tp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 125.McMurray RG, Forsythe WA, Mar MH, Hardy CJ. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987;19:570–574. [PubMed] [Google Scholar]
- 126.Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J Psychosom Res. 1993;37:565–574. [DOI] [PubMed] [Google Scholar]
- 127.Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016;57:1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noll JG, Zeller MH, Trickett PK, Putnam FW. Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics. 2007;120:e61–e67. doi: 10.1542/peds.2006-3058. [DOI] [PubMed] [Google Scholar]
- 129.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doom JR, Mason SM, Suglia SF, Clark CJ. Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long-term cardiovascular disease risk in young adulthood. Soc Sci Med. 2017;188:166–175. doi: 10.1016/j.socscimed.2017.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wells NM, Evans GW, Beavis A, Ong AD. Early childhood poverty, cumulative risk exposure, and body mass index trajectories through young adulthood. Am J Public Health. 2010;100:2507–2512. doi: 10.2105/AJPH.2009.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin SH, Miller DP. A longitudinal examination of childhood maltreatment and adolescent obesity: results from the National Longitudinal Study of Adolescent Health (AddHealth) Study. Child Abuse Negl. 2012;36:84–94. doi: 10.1016/j.chiabu.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 133.Loucks EB, Almeida ND, Taylor SE, Matthews KA. Childhood family psychosocial environment and coronary heart disease risk. Psychosom Med. 2011;73:563–571. doi: 10.1097/PSY.0b013e318228c820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Traub F, Boynton-Jarrett R. Modifiable resilience factors to childhood adversity for clinical pediatric practice. Pediatrics 2017;139:e20162569. [DOI] [PubMed] [Google Scholar]
- 135.Gunnar MR, Fisher PA; Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18:651–677. [PubMed] [Google Scholar]
- 136.Appleton AA, Buka SL, Loucks EB, Rimm EB, Martin LT, Kubzansky LD. A prospective study of positive early-life psychosocial factors and favorable cardiovascular risk in adulthood. Circulation. 2013;127:905–912. doi: 10.1161/CIRCULATIONAHA.112.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Francis MM, Nikulina V, Widom CS. A prospective examination of the mechanisms linking childhood physical abuse to body mass index in adulthood. Child Maltreat. 2015;20:203–213. doi: 10.1177/1077559514568892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 140.Danese A, Moffitt TE, Arseneault L, Bleiberg BA, Dinardo PB, Gandelman SB, Houts R, Ambler A, Fisher HL, Poulton R, Caspi A. The origins of cognitive deficits in victimized children: implications for neuroscientists and clinicians. Am J Psychiatry. 2017;174:349–361. doi: 10.1176/appi.ajp.2016.16030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowes L, Maughan B, Ball H, Shakoor S, Ouellet-Morin I, Caspi A, Moffitt TE, Arseneault L. Chronic bullying victimization across school transitions: the role of genetic and environmental influences. Dev Psychopathol. 2013;25:333–346. doi: 10.1017/S0954579412001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dobson KG, Chow CH, Morrison KM, Van Lieshout RJ. Associations between childhood cognition and cardiovascular events in adulthood: a systematic review and meta-analysis. Can J Cardiol. 2017;33:232–242. doi: 10.1016/j.cjca.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 143.Winning A, Glymour MM, McCormick MC, Gilsanz P, Kubzansky LD. Childhood psychological distress as a mediator in the relationship between early-life social disadvantage and adult cardiometabolic risk: evidence from the 1958 British Birth Cohort. Psychosom Med. 2016;78:1019–1030. doi: 10.1097/PSY.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 144.VanderWeele TJ, Hawkley LC, Thisted RA, Cacioppo JT. A marginal structural model analysis for loneliness: implications for intervention trials and clinical practice. J Consult Clin Psychol. 2011;79:225–235. doi: 10.1037/a0022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dubowitz H, Thompson R, Proctor L, Metzger R, Black MM, English D, Poole G, Magder L. Adversity, maltreatment, and resilience in young children. Acad Pediatr. 2016;16:233–239. doi: 10.1016/j.acap.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;343:1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A. 2014;111:11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Trauma Informed Primary Care Initiative. 2016. https://www.thenational-council.org/trauma-informed-primary-care-initiative-learning-community/.
- 149.Flynn AB, Fothergill KE, Wilcox HC, Coleclough E, Horwitz R, Ruble A, Burkey MD, Wissow LS. Primary care interventions to prevent or treat traumatic stress in childhood: a systematic review. Acad Pediatr. 2015;15:480–492. doi: 10.1016/j.acap.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cohen JA, Kelleher KJ, Mannarino AP. Identifying, treating, and referring traumatized children: the role of pediatric providers. Arch Pediatr Adolesc Med. 2008;162:447–452. doi: 10.1001/archpedi.162.5.447. [DOI] [PubMed] [Google Scholar]


