Abstract
Bezlotoxumab is marketed for the prevention of recurrent Clostridioides difficile infection (rCDI). Its high cost could be determining its prescription to a different population than that represented in clinical trials. The objective of the study was to verify the effectiveness and safety of bezlotoxumab in preventing rCDI and to investigate factors related to bezlotoxumab failure in the real world. A retrospective, multicentre cohort study of patients treated with bezlotoxumab in Spain was conducted. We compared the characteristics of cohort patients with those of patients treated with bezlotoxumab in the pivotal MODIFY trials. We assessed recurrence rates 12 weeks after completion of treatment against C. difficile, and we analysed the factors associated with bezlotoxumab failure. Ninety-one patients were included in the study. The cohort presented with more risk factors for rCDI than the patients included in the MODIFY trials. Thirteen (14.2%) developed rCDI at 12 weeks of follow-up, and rCDI rates were numerically higher in patients with two or more previous episodes (25%) than in those who had fewer than two previous episodes of C. difficile infection (CDI) (10.4%); p = 0.09. There were no adverse effects attributable to bezlotoxumab. Despite being used in a more compromised population than that represented in clinical trials, we confirm the effectiveness of bezlotoxumab for the prevention of rCDI.
Keywords: Clostridium difficile, Clostridioides difficile, C. difficile infection, bezlotoxumab, recurrence
1. Introduction
Recurrences remain the main challenge in the clinical management of patients with Clostridioides difficile infection (CDI) [1]. Although CDI frequency varies significantly across different cohorts and surveillance studies [2,3,4], clinical trials have consistently shown a recurrence rate of approximately 25% [5,6,7]. Compared with vancomycin, treatment with fidaxomicin has been associated with a significant reduction in recurrent CDI (rCDI) during the first 4 weeks after the end of treatment [5,6]. More recently, bezlotoxumab (a monoclonal antibody targeted against toxin B) has been commercialised. The MODIFY trials showed a 40% reduction in the rate of rCDI at 12 weeks of follow-up when bezlotoxumab was added to the standard-of-care antimicrobial therapy for CDI [7].
However, real-world evidence from publications on bezlotoxumab is extremely limited [8,9]. Real-world studies with bezlotoxumab are essential because the drug is probably being used in a population different from that represented in the MODIFY trials and under conditions of use that are also different from those in the clinical trials. Moreover, the rigid evaluation criteria of the clinical trials limit the applicability of the results to real-world settings. For instance, patients who did not achieve “cure” of a CDI episode at the end-of-treatment visit in a clinical trial are not evaluated as a recurrence event [5,6,7]. Meanwhile, in real life, these patients would receive longer treatments or may be considered cured as, although intestinal rhythm might not be completely normalised, they have clearly improved with treatment and may suffer an rCDI.
Real-world studies also provide important information on safety surveillance. In the MODIFY trials, a higher rate of heart failure was reported from patients treated with bezlotoxumab than from those treated with placebo [10]. Last, real-world studies might show subpopulations in which the drug is less effective [11]. In summary, real-world studies are necessary to help clinical decision-making, especially in the case of drugs with restricted access due to their elevated costs, as is the case for bezlotoxumab.
2. Materials and Methods
This retrospective, multicentre cohort study included all patients receiving bezlotoxumab infusion during the duration of antimicrobial treatment for CDI between July 2018 and July 2019 in 13 Spanish hospitals. The primary endpoint of the study was to describe the rate of rCDI during the 12 weeks after the end of antimicrobial treatment for CDI.
Medical records were reviewed by local investigators, and data were introduced in an online database. The study coordinator sent queries to local investigators to address all inconsistencies or presumed mistakes in the data. CDI episodes were classified according to the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) criteria [12]. All patients receiving a haematopoietic progenitor transplant or a solid organ transplant, treatment with immunosuppressive agents, or chemotherapy or corticosteroids for more than 2 weeks or more than 20 mg per day for at least 1 week in the past 2 weeks, and patients with previous congenital or acquired humoural and/or cellular immunodeficiency were considered immunosuppressed. The severity of the episode was established according to the IDSA guidelines published in 2018 and to Zar et al. [12,13]. Recurrence was defined as a reappearance of the symptoms of the disease after symptom resolution from the previous episode, along with a positive test that demonstrated the presence of toxigenic C. difficile in the stool, during the follow-up [14]. Comorbidities and risk factors for recurrent CDI pre-established in the MODIFY studies were also recorded.
We compared the presence of five pre-established risk factors for rCDI from the MODIFY studies (age over 65 years, previous CDI episode, immunosuppression, infection due to a hypervirulent strain, and severe episode) in the current cohort with that in bezlotoxumab-treated patients from the MODIFY trial. We also included three other important variables for comparison: renal impairment (the most consistent comorbidity associated with rCDI) [15,16], positive direct toxin detection in faeces (also related to both recurrence rate and severity, in contrast to toxin-negative cases in which diagnosis is made by nucleic acid amplification tests (NAAT)) [17,18], and treatment with fidaxomicin (although it remains unknown if fidaxomicin plus bezlotoxumab is superior to vancomycin plus bezlotoxumab, fidaxomicin treatment itself is associated with a decrease in rates of CDI recurrence) [5].
Categorical variables are described through absolute and relative frequencies, while quantitative variables are described using the mean and standard deviation (SD) for those variables with normal distributions and medians and interquartile ranges (IQR) for those with non-normal distributions. To identify the risk factors associated with CDI recurrence after receiving bezlotoxumab during the 12-week follow-up period, the chi-squared test was used to analyse quantitative variables, while Student’s t-test and ANOVA were used for the analysis of a qualitative variable versus a quantitative variable according to the number of categories. The statistical significance for failure was defined as p < 0.05. The Kaplan–Meier method was used to describe the cumulative probability of rCDI stratified by CDI history before bezlotoxumab and analysed using the log-rank chi-squared test. All statistical analyses were performed using the Stata 13 statistics program. The investigation was carried out following the rules of the Declaration of Helsinki of 1975, revised in 2013. The study was approved by the Clinical Research Ethics Committee from the coordinating centre.
3. Results
Ninety-one consecutive patients from 13 centres were registered in the database. The median age of the patients was 71 years, 46 (50.5%) were men, and the median Charlson index was 4. Thirty-nine (42.9%) patients received bezlotoxumab during the first CDI episode, 28 (30.8%) during the first recurrence, and 24 (26.4%) during the second or later recurrences. Patients were classified according to current definitions [19] as healthcare facility-onset, healthcare facility-associated (HO-HCFA) in 39 (42.9%) patients, community-onset, healthcare facility-associated (CO-HCFA) in 35 (38.5%), community-associated (CA) in 11 (12.1%), and indeterminate in 6 (6.6%).
Most of the patients (72) were treated with vancomycin, and 32 of them received a tapered regimen. Table 1 shows the antibiotic treatments against C. difficile, treatment duration, and duration of the follow-up period starting at the end of the treatment against C. difficile.
Table 1.
Duration of anti-C. difficile drug therapy.
| Anti-C. difficile Treatment | Patients | Duration of Treatment | Follow-up after the End of Anti-C. difficile Treatment | Bezlotoxumab Infusion Time from the Start of Treatment |
|---|---|---|---|---|
| Vancomycin + metronidazole | 5 | 10 (10–10) | 76 (75–79) | 2 (1–5) |
| Vancomycin | 40 | 11 (10–14) | 82 (77.5–86) | 6.5 (3–10) |
| Vancomycin (tapered) | 32 | 42 (35.5–55.5) | 62 (45–73) | 14 (3.5–29.5) |
| Fidaxomicin | 9 | 11 (10–13) | 79 (70.5–82) | 5 (2–8) |
| Fidaxomicin (extend regimen) | 4 | 24.5 (23–26.2) | 79 (71–88) | 12.5 (1.5–22) |
| FMT (after vancomycin) | 1 | 9 | 79 | 12 |
FMT: faecal microbiota transplant. Time is indicated in days; median (Q1–Q3).
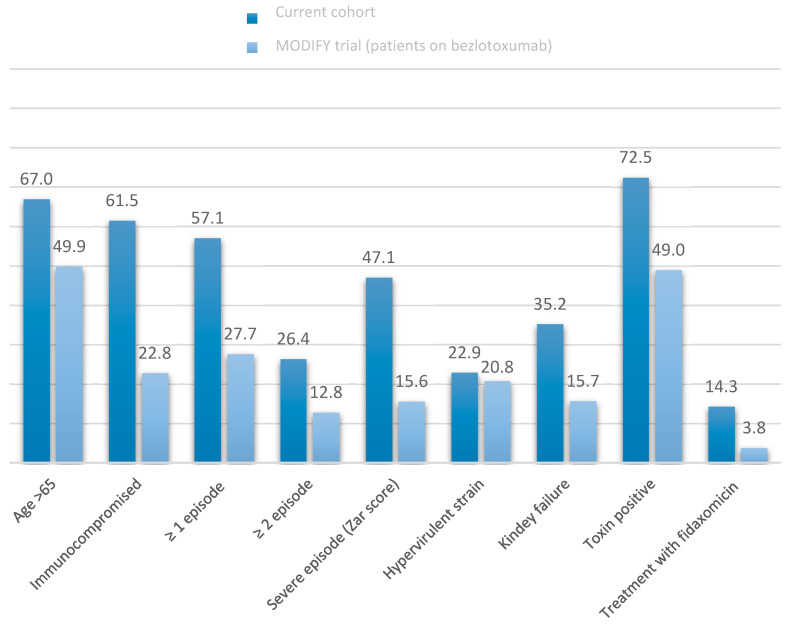
As shown in Figure 1, the current cohort included patients with a higher risk of rCDI than those treated with bezlotoxumab in the MODIFY trials. They were older (67.0% vs. 49.9% aged over 65 years), had a higher proportion of previous CDI episodes (57.1% vs. 27.7%) and immunosuppression (61.5% vs. 22.8%), suffered more severe disease (44.9% vs. 15.6%), and more frequently experienced kidney failure (35.2% vs. 15.7%). Furthermore, the proportion of patients diagnosed by direct toxin detection was higher (72.5% vs. 49.0%) in the current cohort. In contrast, a lower number of patients was treated with fidaxomicin (3.8%) in the MODIFY trial than in the present cohort (14.3%). The rate of CDI produced by the hypervirulent strain was similar in both groups.
Figure 1.
Comparison of variables in current cohort and bezlotoxumab-treated patients in MODIFY trial. Numbers show the percentage of patients.
After a median follow-up time after the end of treatment of 74 (49–81) days and 84 (81–89) days after the infusion of bezlotoxumab, 13 out of 91 (14.3%) patients developed rCDI. The median time from the end of antibiotic therapy to recurrence was 19 (8–36) days. All recurrences occurred during the first 8 weeks.
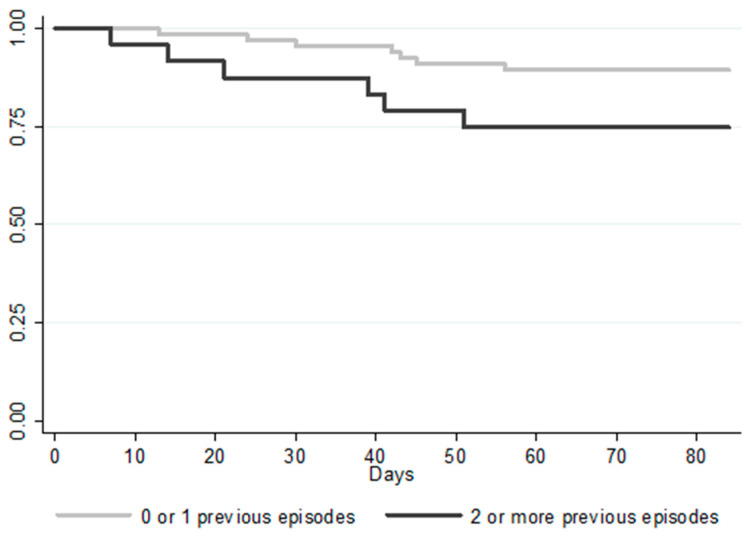
Table 2 shows the variables in patients with and without recurrence. Although a statistically significant difference was not reached, the rate of rCDI was numerically higher in patients who had suffered two or more previous CDI episodes (25.0% vs. 10.4%; p = 0.09). Figure 2 shows the Kaplan–Meier curves for the time to rCDI analysis for patients who had suffered two or more previous episodes. Similarly, the recurrence rate was also higher in patients with the 027 ribotype (4 out of 10; 40%), although in our population, the strain ribotype was only determined in 48 patients.
Table 2.
Risk factors for recurrent Clostridioides difficile infection (CDI).
| Cohort | Recurrence | No Recurrence | p | 95% CI | |
|---|---|---|---|---|---|
| Number of patients | 91 | 13 | 78 | ||
| Men | 46 (50.5) | 5 (38.5) | 41 (52.6) | 0.35 | 0.53–5.9 |
| Age (years) * | 71 (59–82) | 68 (57–80) | 72 (60–82) | 0.96 | 0.96–1.04 |
| Age > 65 | 61 (66.3) | 8 (61.5) | 53 (68.0) | 0.65 | 0.22–2.54 |
| Age > 85 | 17 (18.7) | 3 (23.1) | 14 (18.0) | 0.66 | 0.33–5.64 |
| Charlson index * | 4 (2–6) | 3 (2–5) | 4 (2–6) | 0.22 | 0.64–1.11 |
| Kidney failure | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Cancer | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
| Leukaemia/Lymphoma | 17 (18.7) | 1 (7.7) | 16 (20.5) | 0.29 | 0.04–2.67 |
| Any neoplasm | 33 (36.3) | 3 (23.1) | 30 (38.5) | 0.29 | 0.12–1.89 |
| Liver disease | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.71 | 0.34–10.04 |
| Intestinal inflammatory disease | 6 (6.6) | 1 (7.7) | 5 (6.4) | 0.86 | 0.13–11.34 |
| Immunosuppression: | 56 (61.5) | 7 (53.9) | 48 (62.8) | 0.54 | 0.21–2.25 |
| Chemotherapy | 13 (14.3) | 2 (15.4) | 11 (14.1) | 0.90 | 0.22–5.68 |
| Steroids | 14 (15.4) | 1 (7.7) | 13 (16.7) | 0.42 | 0.05–3.49 |
| Immunosuppressive drugs (not steroids) | 16 (17.6) | 1 (7.7) | 15 (19.2) | 0.33 | 0.04–2.91 |
| Solid organ transplant | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
| Previous CDI episodes: | |||||
| 0 | 39 (42.9) | 5 (38.5) | 35 (44.9) | 0.73 | 0.24–2.70 |
| 1 | 28 (30.8) | 2 (15.4) | 26 (33.3) | 0.21 | 0.08–1.76 |
| ≥2 | 24 (26.4) | 6 (46.2) | 18 (23.1) | 0.09 | 0.85–9.59 |
| Proton pump inhibitor use | 59 (64.8) | 8 (61.5) | 51 (65.4) | 0.79 | 0.25–2.84 |
| Previous antibiotic treatment | 79 (86.8) | 10 (76.9) | 69 (88.5) | 0.27 | 0.10–1.88 |
| Classification of CDI episodes: | |||||
| CA | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| CO-HCFA | 35 (38.5) | 3 (23.1) | 32 (41.0) | 0.23 | 0.11–1.69 |
| HO-HCFA | 39 (42.9) | 7 (53.9) | 32 (41.0) | 0.39 | 0.52–5.46 |
| Indeterminate | 6 (6.6) | 2 (15.4) | 4 (5.1) | 0.19 | 0.55–20.59 |
| Toxin positive | 66 (72.5) | 8 (61.5) | 58 (74.4) | 0.34 | 0.16–1.88 |
| NAAT positive/toxin negative | 25 (27.5) | 5 (38.5)) | 20 (25.6) | 0.34 | 0.16–1.88 |
| IDSA severe or fulminant colitis | 35 (38.5) | 5 (38.5) | 30 (38.5) | 1.00 | 0.30–3.34 |
| Severe (Zar) | 41 (45.1) | 7 (53.9) | 34 (43.6) | 0.49 | 0.46–4.91 |
| Admitted to ICU | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| 027 ribotype (based on 48 patients) | 10 (20.8) | 4 (44.4) | 6 (15.4) | 0.07 | 0.91–21.29 |
| Concomitant antibiotics | 25 (27.5) | 1 (7.7) | 24 (30.8) | 0.12 | 0.02–1.52 |
| Anti-C. difficile treatment: | |||||
| Vancomycin | 40 (44.0) | 5 (38.5) | 35 (44.9) | 0.67 | 0.23–2.56 |
| Fidaxomicin | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.48 | 0.34–10.04 |
| Vancomycin/metronidazole | 5 (5.5) | 1 (7.7) | 4 (5.1) | 0.71 | 0.16–14.99 |
| Vancomycin (tapered) | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Fidaxomicin extended–pulsed | 4 (4.4) | 0 | 4 (5.1) | 0.40 | - |
| Faecal microbiota transplant | 1 (6.0) | 1 (7.7) | 0 | 0.01 | - |
| Extended/pulsed–tapered treatments | 36 (39.6) | 4 (30.8) | 32 (41.0) | 0.49 | 0.18–2.26 |
* Values are indicated with median and interquartile range (Q1–Q3). CDI: C. difficile infection; HO-HCFA: healthcare facility-onset, healthcare facility-associated; CO-HCFA: community-onset, healthcare facility-associated; CA: community-associated; NAAT: nucleic acid amplification tests; IDSA: Infectious Diseases Society of America; ICU: intensive care unit.
Figure 2.
Kaplan–Meier plot of time to recurrent CDI.
We did not find any differences in the recurrence rates based on the anti-C. difficile drug received or whether standard or tapered–pulsed regimens had been used (Table 2). We also found no differences based on the microbiological technique used for the diagnosis, the age of the patients, or the severity of the episodes.
Thirteen patients (14.3%) had died by the time of the follow-up assessment point 12 weeks after the end of anti-C. difficile treatment. The median time to death after bezlotoxumab infusion and after the end of anti-C. difficile treatment was 28 (IQR 16–50) and 17 (IQR 2–46) days, respectively. Only in one patient was death directly related to CDI. Other causes of death were severe bacterial or fungal infections (five patients), progression of the underlying disease (two patients), progressive respiratory failure in a patient with COPD (one patient), massive haemoptysis (one patient), heart failure (one patient), and unknown (two patients). None of these 12 patients presented with rCDI before death. No adverse events apparently related to bezlotoxumab were reported by the investigators.
4. Discussion
Bezlotoxumab has been demonstrated to reduce the recurrence rate of CDI in clinical trials. However, there are only two published studies that have evaluated the effectiveness of bezlotoxumab in a real-world setting [8,9]. The present study confirms the effectiveness of bezlotoxumab in clinical practice. The observed rCDI rate (14.3%) was even lower than the rCDI rate reported in the pivotal clinical trial (17%). These results are remarkable considering the fact that bezlotoxumab is used (at least in Spain) in a much more compromised population than that represented in the MODIFY clinical trials. As shown in Figure 1, the patients included in our cohort presented a significantly higher risk of developing rCDI than those treated with bezlotoxumab in the MODIFY trials. The only factor that could have favoured a lower recurrence rate was the use of fidaxomicin for the treatment of CDI (somewhat more frequently observed in our cohort). However, only 13% of patients were treated with this drug; therefore, we cannot consider the influence of fidaxomicin in this study as a relevant factor. Additionally, the mortality during follow-up in our cohort (14%), higher than that described in the MODIFY trials (7%), indirectly shows a much more vulnerable population.
These results are comparable to those recently published from a North American cohort [9] and are apparently superior to those obtained in the only published European series [8]. The North American cohort (200 patients) consisted of nonhospitalised patients, with an age of 71 (median) similar to that of our cohort. However, it differs from the one presented here in that almost all patients (86.5%) had previous episodes of CDI and in a greater use of fidaxomicin as a treatment for the infection [9]. In contrast, the Finnish cohort (46 patients) comprised younger patients (mean age 66 years) that were frequently immunosuppressed and had numerous risk factors for recurrence (median, 4) [8].
Over one-third of our patients received tapered–pulsed antibiotic regimens against C. difficile. This approach is not standardised in clinical practice among participating centres. The most plausible explanation could be that the prescription of bezlotoxumab was typically (especially in the first months) dependent on approval by therapeutic committees or pharmacy services. In those cases, a prescription of longer regimens would ensure that the patient was still receiving treatment for C. difficile when approval was obtained. The use of tapered–pulsed regimens of vancomycin and fidaxomicin (not allowed in the MODIFY trials) could have influenced the results obtained in this study. However, we did not find any differences in the rate of rCDI when patients treated with conventional regimens (16.4%) were compared with those treated by the tapered or pulsed regimen (12.5%) (Table 2).
Since 13 patients died before the end of the 12-week follow-up, it could be said that the recurrence rate we presented could have been infraestimated. However, the median follow-up until death of these patients was approximately the same as the median time to rCDI in our cohort. Even if these patients were excluded from the analysis, the recurrence rate (16.7%) would be similar to the rate observed in the MODIFY trials.
The factors associated with bezlotoxumab failure in clinical practice should be investigated since they may help with using the drug in a more appropriate way. The results were numerically better in patients who had one or no previous episodes than in those who had two or more (Table 2). These results confirm those found by Hengel et al. [8] and suggest that bezlotoxumab should be used before multiple recurrence occurs, where otherwise the treatment of choice would be a faecal microbiota transplant. The rCDI rate was also numerically higher in patients associated with the 027 ribotype, but we only had this information for 48 patients, so we consider that the analysis of this variable is limited in our cohort. Due to the insufficient number of events, multivariate analysis could not be performed.
Bezlotoxumab has been demonstrated to be a safe drug. Heart failure was the cause of death for only one patient, and it occurred 12 weeks after the infusion of bezlotoxumab (day 86 postinfusion). Furthermore, none of the deaths occurred close in time to the infusion of the drug, and the researchers did not report any adverse events that seemed to be related to bezlotoxumab in their opinion.
Our study has certain limitations. This is a retrospective and multicentre cohort, which involves a risk of heterogeneity and loss of information. Since our definition of recurrence required microbiological confirmation of toxigenic C. difficile, some cases of rCDI might have been missed. However, all patients were managed by infectious diseases physicians skilled in the management and interpretation of the diagnostic tests for CDI.
5. Conclusions
Our study confirms the efficacy of bezlotoxumab for the prevention of rCDI in a real-world setting in Spain. The rCDI rate was comparable with that obtained in the MODIFY studies despite the presence of a much more compromised, at-risk population. The type of anti-C. difficile drug regimen did not influence the outcomes in our cohort. The results with bezlotoxumab were favourable regardless of age, severity, or comorbidities. However, the results appear to be worst in patients having two or more previous CDI episodes, which suggests that the use of bezlotoxumab should not be delayed.
Acknowledgments
Supported by Plan Nacional de I+D+i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0011) co-financed by European Development Regional Fund “A way to achieve Europe”, Operative program Intelligent Growth 2014–2020.
Author Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualisation, R.E.-S.; formal analysis, R.E.-S., M.R.-R., J.F.-F., S.G.F., M.O.S., A.C.Y., A.V.A., B.D.-P., M.J.R.H., E.M.D.L., O.M.S., C.S.B., C.A.C., B.G.-G., D.R.-P., A.R.-M., J.T.-C., F.L.-M. and J.C.R.; investigation, R.E.-S. and J.C.R.; methodology, R.E.-S. and J.C.R.; project administration, R.E.-S.; supervision, J.C.R.; validation, R.E.-S. and J.C.R.; writing—original draft, R.E.-S. and J.C.R.; writing—review and editing, M.R.-R., J.F.-F., S.G.F., M.O.S., A.C.Y., A.V.A., B.D.-P., M.J.R.H., E.M.D.L., O.M.S., C.S.B., C.A.C., B.G.-G., D.R.-P., A.R.-M., J.T.-C. and F.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee of University Hospital Ramón y Cajal (protocol code 199/19 and approved on 29/July/2019).
Informed Consent Statement
Patient consent was not obtained as the Ethics Committee approved. It was due it is a retrospective study without any intervention. Only cases treated previously to the date of the request for this study were included. Most of the patients were frail, elderly, and/or with difficulties to travel to the hospital. Furthermore, the disease does not require clinical reviews once resolved, so the patients are not followed by the doctors who treated them for the ICD.
Conflicts of Interest
J.C.R. has received consulting and speaking fees from Astellas Pharma and MSD. R.E.-S. and M.O.S. have received support from Astellas and MSD to attend conferences and congresses. D.R.-P. declares having received honoraria from Pfizer, Angellini, and Astellas as payment for lectures; from MSD for consultancy tasks; and from Angellini and Astellas as payment for travel/accommodation for scientific purposes. A.C.Y. has received honoraria for the development of educational presentations for Pfizer. Other authors have not reported any conflicts of interest during the last year.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olsen M.A., Yan Y., Reske K.A., Zilberberg M.D., Dubberke E.R. Recurrent Clostridium difficile infection is associated with increased mortality. Clin. Microbiol. Infect. 2015;21:164–170. doi: 10.1016/j.cmi.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Eyre D.W., Walker A.S., Wyllie D., Dingle K.E., Griffiths D., Finney J., O’Connor L., Vaughan A., Crook D.W., Wilcox M.H., et al. Predictors of First Recurrence of Clostridium difficile Infection: Implications for Initial Management. Clin. Infect. Dis. 2012;55:S77–S87. doi: 10.1093/cid/cis356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheitoyan-Pesant C., Abou Chakra C.N., Pepin J., Marcil-Héguy A., Nault V., Valiquette L. Clinical and Healthcare Burden of Multiple Recurrences of Clostridium difficile Infection. Clin. Infect. Dis. 2016;62:574–580. doi: 10.1093/cid/civ958. [DOI] [PubMed] [Google Scholar]
- 4.Van Dorp S.M., Kinross P., Gastmeier P., Behnke M., Kola A., Delmée M., Pavelkovich A., Mentula S., Barbut F., Hajdu A., et al. Standardised surveillance of Clostridium difficile infection in European acute care hospitals: A pilot study, 2013. Eurosurveill. Eur. Cent. Dis. Prev. Control. 2016;21:20381. doi: 10.2807/1560-7917.ES.2016.21.29.30293. [DOI] [PubMed] [Google Scholar]
- 5.Louie T.J., Miller M.A., Mullane K.M., Weiss K., Lentnek A., Golan Y., Gorbach S., Sears P., Shue Y.-K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 6.Cornely O.A., Crook D.W., Esposito R., Poirier A., Somero M.S., Weiss K., Sears P., Gorbach S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox M.H., Gerding D.N., Poxton I.R., Kelly C., Nathan R., Birch T., Cornely O.A., Rahav G., Bouza E., Lee C., et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017;376:305–317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 8.Oksi J., Aalto A., Saila P., Partanen T., Anttila V.-J., Mattila E. Real-world efficacy of bezlotoxumab for prevention of recurrent Clostridium difficile infection: A retrospective study of 46 patients in five university hospitals in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1947–1952. doi: 10.1007/s10096-019-03630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengel R.L., Ritter T.E., Nathan R.V., Van Anglen L.J., Schroeder C.P., Dillon R.J., Marcella S.W., Garey K.W. Real-world Experience of Bezlotoxumab for Prevention of Clostridioides difficile Infection: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2020;7:ofaa097. doi: 10.1093/ofid/ofaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso C.D., Mahoney M.V. Bezlotoxumab for the prevention of Clostridium difficile infection: A review of current evidence and safety profile. IDR. 2019;12:1–9. doi: 10.2147/IDR.S159957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman R.E., Anderson S.A., Dal Pan G.J., Gray G.W., Gross T., Hunter N.L., LaVange L., Marinac-Dabic D., Marks P.W., Robb M.A., et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 12.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar F.A., Bakkanagari S.R., Moorthi K.M.L.S.T., Davis M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium difficile Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 14.Debast S.B., Bauer M.P., Kuijper E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014;20:1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 15.Phatharacharukul P., Thongprayoon C., Cheungpasitporn W., Edmonds P.J., Mahaparn P., Bruminhent J. The Risks of Incident and Recurrent Clostridium difficile Associated Diarrhea in Chronic Kidney Disease and End-Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2015;60:2913–2922. doi: 10.1007/s10620-015-3714-9. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande A., Pasupuleti V., Thota P., Pant C., Rolston D.D.K., Hernandez A.V., Donskey C.J., Fraser T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2015;36:452–460. doi: 10.1017/ice.2014.88. [DOI] [PubMed] [Google Scholar]
- 17.Origüen J., Corbella L., Orellana M.Á., Fernández-Ruiz M., Lopez-Medrano F., San Juan R., Lizasoain M., Ruiz-Merlo T., Morales-Cartagena A., Maestro1et G., et al. Comparison of the clinical course of Clostridium difficile infection in glutamate dehydrogenase-positive toxin-negative patients diagnosed by PCR to those with a positive toxin test. Clin. Microbiol. Infect. 2018;24:414–421. doi: 10.1016/j.cmi.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Guh A.Y., Hatfield K.M., Winston L.G., Martin B., Johnston H., Brousseau G., Farley M.M., Wilson L., Perlmutter R., Phipps E.C., et al. Toxin Enzyme Immunoassays Detect Clostridioides difficile Infection with Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. 2019;69:1667–1674. doi: 10.1093/cid/ciz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surawicz C.M., Brandt L.J., Binion D.G., Ananthakrishnan A.N., Curry S.R., Gilligan P.H., McFarland L.V., Mellow M., Zuckerbraun B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]