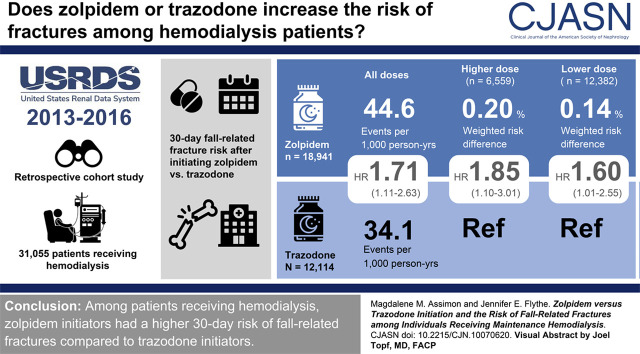
Visual Abstract
Keywords: fall, fracture, hemodialysis, insomnia, trazodone, zolpidem
Abstract
Background and objectives
Zolpidem, a nonbenzodiazepine hypnotic, and trazodone, a sedating antidepressant, are the most common medications used to treat insomnia in the United States. Both drugs have side effect profiles (e.g., drowsiness, dizziness, and cognitive and motor impairment) that can heighten the risk of falls and fractures. Despite widespread zolpidem and trazodone use, little is known about the comparative safety of these medications in patients receiving hemodialysis, a vulnerable population with an exceedingly high fracture rate.
Design, setting, participants, & measurements
Using data from the United States Renal Data System registry (2013–2016), we conducted a retrospective cohort study to investigate the association between the initiation of zolpidem versus trazodone therapy and the 30-day risk of hospitalized fall-related fractures among Medicare-enrolled patients receiving maintenance hemodialysis. We used an active comparator new-user design and estimated 30-day inverse probability of treatment-weighted hazard ratios and risk differences. We treated death as a competing event.
Results
A total of 31,055 patients were included: 18,941 zolpidem initiators (61%) and 12,114 trazodone initiators (39%). During the 30-day follow-up period, 101 fall-related fractures occurred. Zolpidem versus trazodone initiation was associated with a higher risk of hospitalized fall-related fracture (weighted hazard ratio, 1.71; 95% confidence interval, 1.11 to 2.63; weighted risk difference, 0.17%; 95% confidence interval, 0.07% to 0.29%). This association was more pronounced among individuals prescribed higher zolpidem doses (hazard ratio, 1.85; 95% confidence interval, 1.10 to 3.01; and risk difference, 0.20%; 95% confidence interval, 0.04% to 0.38% for higher-dose zolpidem versus trazodone; and hazard ratio, 1.60; 95% confidence interval, 1.01 to 2.55 and risk difference, 0.14%; 95% confidence interval, 0.03% to 0.27% for lower-dose zolpidem versus trazodone). Sensitivity analyses using longer follow-up durations yielded similar results.
Conclusions
Among individuals receiving maintenance hemodialysis, zolpidem initiators had a higher risk of hospitalized fall-related fracture compared with trazodone initiators.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_12_18_CJN10070620_final.mp3
Introduction
Patients receiving in-center maintenance hemodialysis have a substantial symptom burden that contributes to their high health care utilization rates, generally poor functional status, and compromised health-related quality of life (1–3). Most of these individuals suffer from some form of sleep disturbance, with the most common being insomnia, the inability to initiate, maintain, or achieve restful sleep that results in daytime impairment (4). Insomnia affects up to 50% of the hemodialysis population (5) and is associated with fatigue, impaired cognitive function, mood disturbances, lower quality of life, and higher hospitalization and mortality rates (6–8). Not surprisingly, people receiving hemodialysis rank insomnia alleviation as a top research priority (9).
The American Academy of Sleep Medicine and other guideline bodies recommend cognitive behavioral therapy (CBT) as the first-line therapeutic option for the treatment of chronic insomnia in adults (10–12). Even though small clinical trials have shown that CBT is effective for treating insomnia in the hemodialysis population (13,14), it remains inaccessible to many patients. As such, pharmacotherapy is often used to treat insomnia in dialysis-dependent kidney failure. Z-drugs (nonbenzodiazepine hypnotics) and trazodone (a sedating antidepressant) are the most commonly prescribed insomnia medications in the United States (15), with more than 20% of individuals receiving hemodialysis filling a prescription for one of these medications in 2016 (16).
Both z-drugs and trazodone have side effect profiles (e.g., drowsiness, dizziness, and cognitive and motor impairment) that can heighten fall risk (17,18), and general population studies have linked their use to falls and fractures (19–21). Such potential medication-related adverse events are of particular concern in individuals receiving hemodialysis, a patient population with fracture rates that exceed those of similarly aged people from the general population by two- to five-fold (22–24). Patients receiving hemodialysis may be particularly susceptible to the untoward effects of insomnia medications due to their frequent use of other sedating and/or disorienting drugs, recurrent exposure to dialysis-induced BP shifts, and high prevalence of autonomic dysfunction, peripheral neuropathy, cognitive impairment, and disease-related osteodystrophy. However, we lack population-specific comparative safety data to guide clinicians as they consider prescribing an insomnia medication to individuals receiving hemodialysis. Therefore, we undertook this study to investigate the comparative risk of fall-related fractures associated with z-drug versus trazodone therapy in the hemodialysis population.
Materials and Methods
This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board (18–0297). A waiver of consent was granted due to the study’s large size, data anonymity, and retrospective nature.
Data Source
We used data from the United States Renal Data System (USRDS), a national surveillance system that collects, analyzes, and distributes information on individuals with ESKD in the United States, (16) to conduct this study. The USRDS database includes the Medical Evidence and Death Notification forms and Medicare standard analytic files, including enrollment information and final action administrative claims (Medicare Part A, B, and D) (16).
Study Design and Population
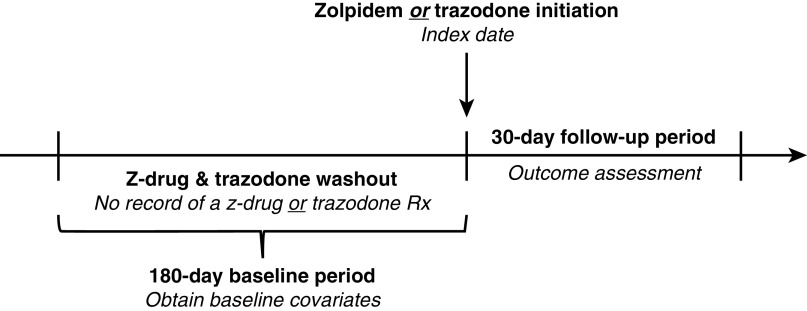
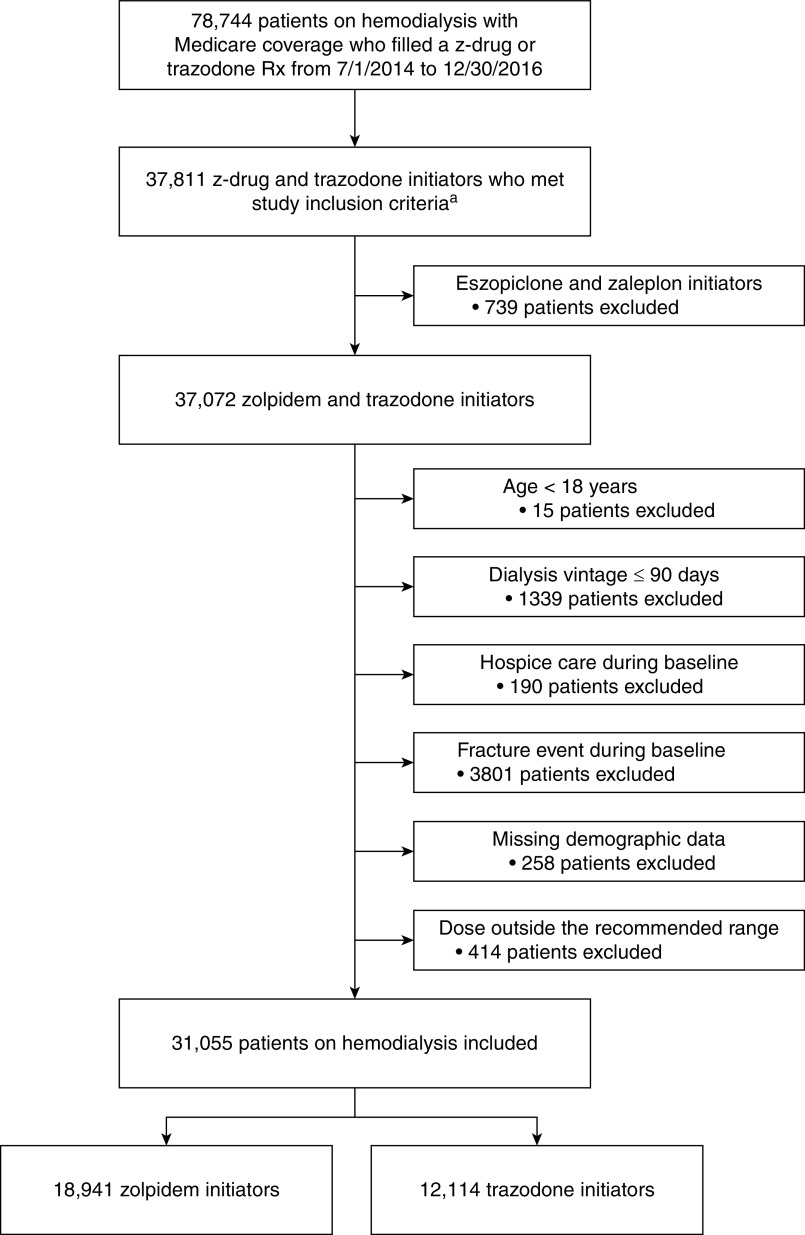
We conducted a retrospective cohort study using an active comparator new-user design (25) to investigate the association between zolpidem versus trazodone initiation and the 30-day risk of hospitalized fall-related fracture in the hemodialysis population (Figure 1). First, we identified patients on hemodialysis with Medicare coverage (Parts A, B, and D) who newly initiated z-drug (zolpidem, eszopiclone, zaleplon) or trazodone therapy from July 1, 2013 to December 30, 2016 after a 180-day washout period free of documented z-drug or trazodone prescription fills. Because zolpidem use comprised 97% of z-drug initiations (compared with 2% for eszopiclone and 1% for zaleplon), we elected to perform a head-to-head comparison of zolpidem and trazodone. Beyond zolpidem or trazodone new-use, additional inclusion criteria were receipt of in-center hemodialysis during the 180 days before study medication initiation (i.e., the baseline period) and continuous Medicare Part A, B, and D coverage during this period. We then applied the following exclusion criteria: (1) age <18 years at the beginning of baseline, (2) dialysis vintage ≤90 days at the beginning of baseline, (3) receipt of hospice care during baseline, (4) occurrence of a fracture event during baseline, (5) missing demographic data, and (6) initiation of zolpidem or trazodone at a starting dose outside of the recommended dosing range for insomnia management (Supplemental Table 1).
Figure 1.
Study design. Initiators of zolpidem and trazodone were defined as patients who had no record of a z-drug (zolpidem, eszopiclone, zaleplon) or trazodone prescription in the previous 180 days (i.e., the washout period). The index date was defined as the date of study medication initiation. Baseline covariates were identified in the 180-day period before the index date. Study follow-up began immediately after the index date. Rx, prescription.
Study Exposure
We used Medicare Part D prescription drug claims to identify new-users of zolpidem and trazodone and defined the index date as the date of the first zolpidem or trazodone prescription after the 180-day washout period.
Study Outcomes
We used Medicare Part A claims to ascertain our primary outcome of interest, 30-day fall-related fracture requiring hospitalization. We also evaluated a broader outcome, hospitalized fracture, in sensitivity analyses. Corresponding outcome definitions and discharge diagnosis codes are presented in Supplemental Tables 2 and 3.
Study Covariates
Baseline covariates included potential confounders and variables known to be strong risk factors for the study outcome (26). We identified covariates in the 180 days prior to the index date using Medicare Part A, B, and D claims. Covariates of interest included patient demographics, comorbid conditions, frailty indicators (27), prescription medication use, and metrics of health care utilization. We considered comorbid conditions and frailty indicators to be present if an applicable discharge diagnosis code (located in any position) or procedure code was associated with ≥1 institutional or physician supplier claim during the 180-day baseline period (Supplemental Table 4). We used Medicare Part D claims to capture relevant medication use (i.e., drugs associated with falls and/or fracture) on the last day of the baseline period (Supplemental Table 5).
Statistical Analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). We described baseline characteristics across individuals who initiated zolpidem and trazodone as count (%) for categorical variables and as mean±SD or median (quartile 1, quartile 3) for continuous variables. We compared baseline covariate distributions using standardized differences. A standardized difference >0.10 represents an imbalance between exposure groups (28).
We used an intention-to-treat analytic approach to evaluate the association between zolpidem versus trazodone initiation and the 30-day risk of hospitalized fall-related fracture. We selected a 30-day follow-up period because zolpidem is indicated for the short-term treatment of insomnia, and according to its package insert, clinical trials supporting zolpidem’s efficacy were 4–5 weeks in duration (17). Individuals were followed forward in time from the index date to the first occurrence of an outcome, censoring, or competing event. Censoring events included: (1) change of modality to home hemodialysis or peritoneal dialysis; (2) kidney transplantation; (3) kidney function recovery; (4) loss of Medicare Part A, B, or D coverage; (5) being lost to follow-up; (6) completion of 30-days of follow-up; and (7) study end (December 31, 2016). Death was treated as a competing event.
In primary analyses, we estimated both relative and absolute effect measures to assess the association between zolpidem versus trazodone initiation and the 30-day risk of hospitalized fall-related fracture. We estimated substitution hazard ratios (HRs), a relative effect measure (null value = 1.00), and their 95% confidence intervals (95% CIs) using Fine and Gray proportional subdistribution hazard models (29). We used the Aalen-Johansen nonparametric estimator to estimate the cumulative incidence of fall-related fracture in each treatment group and computed risk differences (RDs), an absolute-effect measure (null value = 0.00%) (30). We obtained 95% CIs for RDs using a nonparametric bootstrap with 500 resamples.
We used inverse probability of treatment (IPT) weighting for confounding control. Briefly, we calculated the predicted probability (i.e., propensity score) of initiating zolpidem versus trazodone as a function of baseline covariates using logistic regression. We generated IPT weights from propensity scores using standard methods (31,32). We estimated weighted (i.e., adjusted) HRs by applying IPT weights in our regression models and estimated weighted (i.e., adjusted) RDs by applying IPT weights to the Aalen-Johansen estimator.
We conducted two secondary analyses using the same methods to estimate HRs and RDs. Because the US Food and Drug Administration (FDA) has issued Drug Safety Communications related to zolpidem dosing (33,34), we compared the new use of higher and lower zolpidem doses (Supplemental Table 6) to trazodone. Given that we considered three different treatments in this analysis, we estimated propensity scores using multinomial logistic regression and generated IPT weights using standard methods for multicategorical exposures (32,35). We also evaluated the association between zolpidem (any dose) versus trazodone initiation and fall-related fracture in clinically relevant subgroups, including individuals with and without relevant fall-related risk factors (advanced age, female sex, frailty, and use of central nervous system [CNS]-active medications) (36,37). We estimated interaction P values by including interaction terms between the study exposure and subgroups of interest in regression models and used IPT weights from the full study cohort for confounding control (38). Additionally, because the zolpidem package insert recommends starting zolpidem at lower doses in patients who are elderly, female, and debilitated (i.e., frail) and notes that dose adjustments may be necessary when using zolpidem with CNS-active medications (17), we determined the proportion of zolpidem initiators receiving higher doses in each of these groups.
We conducted several sensitivity analyses to evaluate the robustness of our primary study findings. First, we extended study follow-up in our intention-to-treat analyses and evaluated the 60- and 90-day risk of hospitalized fall-related fractures. Second, we used an on-treatment (i.e., as-treated) analytic approach to assess the association between zolpidem versus trazodone initiation and the risk of hospitalized fall-related fracture. In our on-treatment analyses, all available follow-up time was considered, and index study medication discontinuation (using a 7-day grace period) and switching to a non-index study medication were additional censoring events. Finally, we considered a broader outcome, hospitalized fracture, and repeated primary 30-day intention-to-treat analyses.
Results
Figure 2 displays the flow diagram of study cohort selection. A total of 31,055 individuals receiving in-center hemodialysis were included in the study: 18,941 zolpidem initiators (61%) and 12,114 trazodone initiators (39%). Overall, study patients had an average age of 60±15 years, 46% were women, 39% were Black, 19% were Hispanic, and the most common cause of dialysis-dependent kidney failure was diabetes (46%).
Figure 2.
Flow diagram depicting study cohort assembly. aTo be included in the study, patients had to receive in-center hemodialysis during the 180 days prior to study medication initiation (i.e., the baseline period) and have continuous Medicare Part A, B, and D coverage during this period. Rx, prescription.
The propensity score distributions of zolpidem and trazodone initiators exhibited substantial overlap (Supplemental Figure 1), indicating the study groups were highly comparable. Table 1 and Supplemental Table 7 show the baseline characteristics of study patients stratified by medication. Before IPT weighing, baseline covariates were generally well balanced between treatment groups (standardized differences ≤0.10), with some exceptions (e.g., Hispanic ethnicity, history of depression and anxiety, skilled nursing facility admissions). After IPT weighting, all baseline covariates were well balanced between treatment groups.
Table 1.
Select baseline characteristics of patients receiving hemodialysis in the United States who initiated zolpidem or trazodone
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Characteristic | Zolpidem, n=18,941 | Trazodone, n=12,114 | Std Diffa | Zolpidem, n=18,964 | Trazodone, n=12,100 | Std Diffa |
| Age, yr, mean±SD | 59±14 | 61±15 | 0.09 | 60±15 | 60±15 | 0.00 |
| Female, n (%) | 8611 (45) | 5740 (47) | 0.04 | 8794 (46) | 5620 (46) | 0.00 |
| Race, n (%) | ||||||
| Black | 7415 (39) | 4573 (38) | 0.03 | 7291 (38) | 4655 (38) | 0.00 |
| White | 10,420 (55) | 6962 (57) | 0.05 | 10,642 (56) | 6789 (56) | 0.00 |
| Other | 1106 (6) | 579 (5) | 0.05 | 1031 (5) | 656 (5) | 0.00 |
| Hispanic, n (%) | 4024 (21) | 1930 (16) | 0.15 | 3639 (19) | 2322 (19) | 0.00 |
| Low income subsidy, n (%) | 14,700 (78) | 9460 (78) | 0.01 | 14,768 (78) | 9427 (78) | 0.00 |
| Dialysis vintage, yr, median (quartile 1, quartile 3) | 3.6 (1.7, 6.7) | 3.4 (1.6, 6.3) | 0.06 | 3.5 (1.6, 6.5) | 3.5 (1.6, 6.5) | 0.00 |
| Cause of dialysis-dependent kidney failure, n (%) | ||||||
| Diabetes | 8589 (45) | 5821 (48) | 0.05 | 8814 (46) | 5637 (47) | 0.00 |
| Hypertension | 5746 (30) | 3382 (28) | 0.05 | 5567 (29) | 3544 (29) | 0.00 |
| Glomerular disease | 2220 (12) | 1287 (11) | 0.04 | 2135 (11) | 1365 (11) | 0.00 |
| Other | 2386 (13) | 1624 (13) | 0.02 | 2448 (13) | 1554 (13) | 0.00 |
| Anxiety, n (%) | 4019 (21) | 3559 (29) | 0.19 | 4654 (25) | 2984 (25) | 0.00 |
| Depression, n (%) | 4738 (25) | 4592 (38) | 0.28 | 5748 (30) | 3664 (30) | 0.00 |
| Arrhythmia, n (%) | 6370 (34) | 4262 (35) | 0.03 | 6506 (34) | 4146 (34) | 0.00 |
| Conduction disorder, n (%) | 2080 (11) | 1615 (13) | 0.07 | 2280 (12) | 1468 (12) | 0.00 |
| Heart failure, n (%) | 8907 (47) | 6128 (51) | 0.07 | 9204 (49) | 5882 (49) | 0.00 |
| Hypertension, n (%) | 17,076 (90) | 11,146 (92) | 0.07 | 17,243 (91) | 11,003 (91) | 0.00 |
| Ischemic heart disease, n (%) | 9137 (48) | 6102 (50) | 0.04 | 9302 (49) | 5940 (49) | 0.00 |
| Peripheral artery disease, n (%) | 6652 (35) | 4640 (38) | 0.07 | 6894 (36) | 4398 (36) | 0.00 |
| Stroke, n (%) | 4371 (23) | 3341 (28) | 0.10 | 4727 (25) | 3020 (25) | 0.00 |
| Diabetes, n (%) | 13,029 (67) | 8649 (71) | 0.06 | 13,249 (70) | 8456 (70) | 0.00 |
| Number of frailty indicators, n (%)b | ||||||
| 0 indicators | 9496 (50) | 5137 (42) | 0.16 | 8902 (47) | 5665 (47) | 0.00 |
| 1 indicator | 4153 (22) | 2528 (21) | 0.03 | 4091 (22) | 2622 (22) | 0.00 |
| 2 indicators | 2083 (11) | 1478 (129) | 0.03 | 2189 (12) | 1401 (12) | 0.00 |
| 3 indicators | 1350 (7) | 1048 (9) | 0.06 | 1459 (8) | 927 (8) | 0.00 |
| 4 indicators | 883 (5) | 885 (7) | 0.11 | 1078 (6) | 690 (6) | 0.00 |
| ≥5 indicators | 976 (5) | 1038 (9) | 0.14 | 1245 (7) | 795 (7) | 0.00 |
| Use of ≥1 other CNS-active med, n (%)c | 9022 (48) | 6301 (52) | 0.09 | 9429 (50) | 6040 (50) | 0.00 |
| Number of baseline hospital admissions, median (quartile 1, quartile 3) | 1 (0, 2) | 1 (0, 2) | 0.09 | 1 (0, 2) | 1 (0, 2) | 0.00 |
| SNF admission during baseline, n (%) | 2211 (12) | 2341 (19) | 0.21 | 2785 (15) | 1775 (15) | 0.00 |
| Psychotherapy visit during baseline, n (%) | 994 (5) | 1178 (10) | 0.17 | 1358 (7) | 857 (7) | 0.00 |
All covariates were measured during the 180-d baseline period. The weighted cohort is the pseudo-population generated by inverse probability of treatment weighting. Supplemental Table 7 displays the full list of baseline characteristics considered in our analyses stratified by study medication. All variables in Supplemental Table 7 were used to estimate propensity scores and inverse probability of treatment weights. CNS, central nervous system; SNF, skilled nursing facility; med, medication; std diff, standardized difference.
A std diff >0.10 represents meaningful imbalance between groups (28).
A total of 14 validated frailty indicators associated with mobility impairment and falls were considered. Frailty indicators included abnormal gait, abnormal loss of weight or underweight, cachexia, debility, difficulty walking, failure to thrive, fall history, malaise or fatigue, muscular wasting or disease atrophy, muscle weakness, pressure ulcers, senility, using durable medical equipment (cane, walker, bath equipment, or commode), and using nursing or health care services (27).
CNS-active medications included antidepressants (selective serotonin reuptake inhibitors, serotonin-NE reuptake inhibitors, tricyclic antidepressants), antipsychotics, benzodiazepines, antiepileptics, and opioids. These medications are associated with a heightened risk of fall according to the 2019 Beers Criteria (37).
Primary Analyses
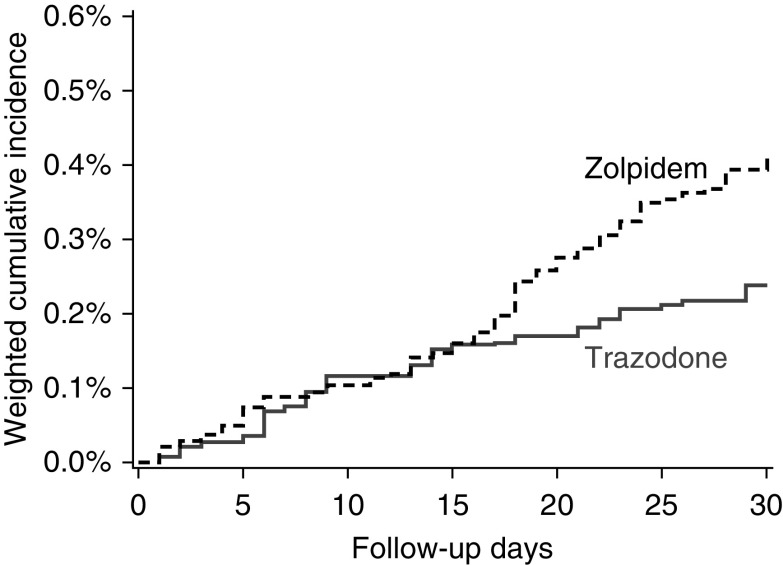
Under the intention-to-treat analytic paradigm, the study cohort was followed for a total of 2545 person-years (1576 person-years for zolpidem initiators and 969 person-years for trazodone initiators). The median follow-up was 30 days in both the zolpidem and trazodone groups and loss to follow-up was minimal (<11 patients). A total of 101 fall-related fracture events requiring hospitalization occurred during the 30-day follow-up period at an incidence rate of 40.5 events/1000 person-years (68 events at a rate of 44.6/1000 person-years among zolpidem initiators; and 33 events at a rate of 34.1 events/1000 person-years among trazodone initiators). The most common fracture types were hip/pelvis (50%), vertebral (17%), and arm (14%). Figure 3 and Supplemental Table 8 display the association between zolpidem versus trazodone initiation and fall-related fractures requiring hospitalization. Compared with individuals initiating trazodone, individuals initiating zolpidem had a higher 30-day risk of fall-related fracture (weighted HR, 1.71; 95% CI, 1.11 to 2.63 and weighted RD, 0.17%; 95% CI, 0.07% to 0.29%).
Figure 3.
The 30-day cumulative incidence of fall-related fractures among zolpidem and trazodone initiators. The plot depicts 30-day inverse probability of treatment weighted cumulative incidence of fall-related fractures among zolpidem initiators (black dashed line) and trazodone initiators (gray solid line). An intention-to-treat analytic approach was used. Death was treated as a competing event.
Secondary Analyses
Of the 18,941 zolpidem initiators, 12,382 (65%) were prescribed lower doses and 6559 (35%) were prescribed higher doses at first use. Analyses comparing lower and higher doses of zolpidem to trazodone and the 30-day risk of fall-related fracture produced results analogous to our primary study findings. Initiation of zolpidem (regardless of dose) was associated with higher risk of 30-day hospitalized fall-related fracture compared with trazodone (Table 2). However, the observed association was more pronounced among individuals initiating higher zolpidem doses (higher-dose zolpidem versus trazodone, weighted HR, 1.85; 95% CI, 1.10 to 3.01 and weighted RD, 0.20%; 95% CI, 0.04% to 0.38%; and lower-dose zolpidem versus trazodone, weighted HR, 1.60; 95% CI, 1.01 to 2.55 and weighted RD, 0.14%; 95% CI, 0.03 to 0.27%).
Table 2.
Association between lower- and higher-dose zolpidem initiation versus trazodone initiation and 30-d fall-related fracture risk
| Medication | n | No. of Events | Rate Per 1000 p-y | Crude HR (95% CI) | Weighted HR (95% CI) | Crude RD (95% CI) | Weighted RD (95% CI) |
|---|---|---|---|---|---|---|---|
| Trazodone | 12,114 | 33 | 34.1 | 1.00 (ref.) | 1.00 (ref.) | 0.00% (ref.) | 0.00% (ref.) |
| Lower-dose zolpidema | 12,382 | 46 | 46.2 | 1.36 (0.87 to 2.12) | 1.60 (1.01 to 2.55) | 0.10% (−0.01% to 0.23%) | 0.14% (0.03% to 0.27%) |
| Higher-dose zolpidemb | 6559 | 22 | 41.7 | 1.23 (0.72 to 2.01) | 1.85 (1.10 to 3.01) | 0.06% (−0.07% to 0.21%) | 0.20% (0.04% to 0.38%) |
An intention-to-treat analytic approach was used in all analyses. Fine and Gray proportional subdistribution hazards models were used to estimate HR, and the Aalen-Johansen estimator was used to estimate RDs. Death was treated as a competing event. Inverse probability of treatment weighting was used for confounding control. Crude HRs and RDs (i.e., unadjusted estimates) and weighed HRs and RDs (i.e., adjusted estimates) are presented. 95% CI, 95% confidence interval; HR, hazard ratio; No., number; p-y, person years; ref., referent; RD, risk difference.
Lower zolpidem doses were formulation dependent and defined as: ≤1.75 mg/d for sublingual tablets used to treat middle of the night awakening; ≤5 mg/d for regular release tablets, the oral spray formulation, and sublingual tablets used to treat sleep initiation; and ≤6.25 mg/d for controlled release tablets.
Higher zolpidem doses were formulation dependent and defined as: >1.75 mg/d for sublingual tablets used to treat middle of the night awakening; >5 mg/d for regular release tablets, the oral spray formulation, and sublingual tablets used to treat sleep initiation; and >6.25 mg/d for controlled release tablets.
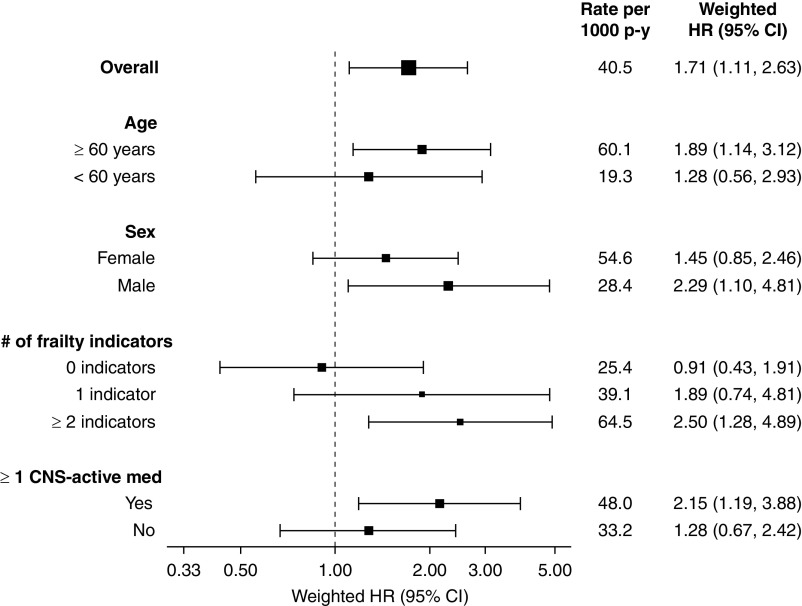
Subgroup analyses suggested the association between zolpidem versus trazodone initiation and 30-day hospitalized fall-related fracture may be more potent in patients aged ≥60 years (versus <60 years), males (versus females), individuals with (versus without) frailty indicators, and those using (versus not using) ≥1 other CNS-active medication (Figure 4, Supplemental Table 9). However, corresponding interaction P values did not reach the threshold for significance. Finally, because the zolpidem package insert recommends using lower initial doses in women, older adults, and people using other CNS-active medications, among other vulnerable groups, we characterized zolpidem-prescribing practices in these high-risk patients in our hemodialysis cohort (Supplemental Table 10). Despite recommendations to initiate zolpidem therapy at lower doses in these individuals, 30% of females, 30% of older adults, 32% of debilitated individuals (i.e., having ≥1 frailty indicator), and 38% of patients using another CNS-active medication were prescribed higher than recommended doses.
Figure 4.
Association between zolpidem versus trazodone initiation and 30-day fall-related fracture risk within clinically relevant subgroups. An intention-to-treat analytic approach was used in all analyses. The figure displays the overall rate of fall-related fractures in each subgroup. Treatment-specific fall-related fracture rates for each subgroup are presented in Supplemental Table 9. Fine and Gray proportional subdistribution hazards models were used to estimate the association between the initiation of zolpidem versus trazodone and 30-day fall-related fracture risk in each subgroup. Inverse probability of treatment weighting was used for confounding control, and death was treated as a competing event. Weighed HRs (i.e., adjusted HRs) are presented. The square sizes of the HR point estimates are proportional to the size of the subgroup. The larger the square size the larger the subgroup. Interaction P values were ≥0.05 for all subgroups. 95% CI, 95% confidence interval; CNS, central nervous system; HR, hazard ratio; med, medication; p-y, person-years.
Sensitivity Analyses
Analyses expanding follow-up time to 60 and 90 days, employing an on-treatment analytic approach, and evaluating a broader study outcome, hospitalized fracture, produced results that were consistent with our primary analyses (Supplemental Tables 11–13).
Discussion
We evaluated the comparative risk of hospitalized fall-related fracture associated with zolpidem versus trazodone therapy among individuals receiving maintenance hemodialysis, finding that patients newly initiating zolpidem had a higher 30-day risk of fall-related fractures compared with patients newly initiating trazodone. This association was more pronounced among patients prescribed higher zolpidem doses.
Zolpidem is a positive allosteric modulator of the γ-aminobutyric acid (GABA) type A receptor complex (39). It enhances the activity of the inhibitory neurotransmitter GABA, which reduces neuronal excitability, causing sedative and hypnotic effects (39). Due to its GABAergic properties, zolpidem can also impair cognitive and motor function (39). FDA safety communications and a recently issued black box warning indicate that zolpidem, particularly at higher doses, can cause next-morning psychomotor impairment (e.g., dizziness, drowsiness) and complex sleep behaviors (e.g., sleep walking, sleep driving) that may result in falls and serious injuries, such as hip and vertebral fractures (33,34,40). Although individuals with dialysis-dependent kidney failure are not specifically mentioned as a high-risk group in the zolpidem package insert, it is well established that people with kidney disease experience higher rates of medication-related adverse events than those with normal kidney function (41,42).
Existing dialysis-specific evidence on the risks of zolpidem therapy is limited. Fall and fracture events were not observed in clinical trials evaluating the efficacy of zolpidem or other z-drugs in the hemodialysis population (43,44). However, it is likely that these trials were too small (10–23 patients) to detect safety signals (43,44). To date, only a few observational investigations evaluated the safety of insomnia medications in individuals receiving hemodialysis (45,46). One such study linked zolpidem to a higher risk of hip fracture (45). However, utilization of a non-user comparator group renders this study, and similarly designed investigations (38), susceptible to confounding by indication (47). Head-to-head comparisons of individual insomnia medications in dialysis-dependent kidney failure have not been conducted. Such inadequate safety data, coupled with the high-priority nature of insomnia alleviation for patients (9), has led diverse stakeholders to advocate for in-depth study of the risks and benefits of insomnia medications in the hemodialysis population (48).
Our study begins to fill this evidence gap by providing initial comparative safety data, showing that treatment with zolpidem (especially at higher doses) may elevate the already high underlying fall-related fracture risk in patients receiving hemodialysis more than trazodone. These findings plausibly stem from differences in the degree of drug-induced cognitive and motor impairment between the two medications. Zolpidem may be a stronger sedative than trazodone, potentially raising the risk of sedative-related falls and fractures to a greater extent. However, data comparing the sedative potency of zolpidem and trazodone and the incidence and severity of relevant side effects are needed to confirm this hypothesis.
One surprising finding was that our subgroup analyses suggested the association between zolpidem versus trazodone initiation and fall-related fractures was more pronounced in males (versus females). Pharmacokinetic studies conducted in healthy volunteers indicate that zolpidem clearance is lower in females than in males (33). At 8 hours after high-dose zolpidem administration, 15% of females (versus only 3% of males) had next-morning serum zolpidem concentrations capable of impairing alertness (33). In our cohort, males were prescribed higher zolpidem doses more often than females. It is plausible this dosing difference may have contributed to potential sex-based differences. However, further investigation is warranted, especially because the observed differences did not reach statistical significance.
Current prescribing guidance recommends starting zolpidem therapy at lower doses in patients who are elderly, female, and debilitated (i.e., frail), and considering dose adjustments when zolpidem and other CNS-active medications are used together (17). Despite these guidelines, 30% of older adults, 30% of females, 32% of frail individuals, and 38% of patients using other CNS-active medications in our study received higher than recommended zolpidem doses at therapy initiation. These findings highlight a potential lack of clinician awareness regarding zolpidem-dosing recommendations and underscore the importance of medication review and reconciliation. In addition, our findings suggest clinicians should exercise discretion when prescribing zolpidem to patients receiving hemodialysis. Trazodone may be a safer option, especially in patients with risk factors for falls and/or fractures. If zolpidem is unavoidable, lower doses should be used, and patients need to be monitored for signs of impaired motor function and/or cognitive performance (17). Clinicians should also be vigilant for potentially dangerous pharmacokinetic and pharmacodynamic drug interactions throughout the course of zolpidem therapy (17). Dose adjustments or cessation of concomitantly used medications may be required. As with all prescribing decisions, clinicians should explain potential risks and benefits of new medications to patients and use shared decision making to individualize prescription choices. In the case of zolpidem and trazodone, some patients may be willing to accept a higher risk of fall-related fracture with zolpidem in exchange for the possibility of enhanced sleep. Finally, adverse events can occur with all insomnia medications, including zolpidem and trazodone. As such, and consistent with current clinical practice guidelines (10–12), CBT should be used as the first-line therapeutic option for insomnia in patients receiving hemodialysis when feasible.
The strengths of our study include using USRDS Medicare claims data to conduct a large-scale safety assessment, employing a new-user study design to mitigate biases common to observational studies of medications (e.g., selection bias, immortal time bias) (25), and performing a head-to-head comparison of insomnia medications to minimize the influence of confounding by indication (47,49). In addition, comparing zolpidem and trazodone reflects a clinically meaningful treatment decision frequently encountered by prescribers in clinical practice (47,50). Nonetheless, our results should be considered within the context of study limitations. First, because our study was observational, residual confounding may remain. Information on insomnia symptom severity and BP measurements are not available in the USRDS database. However, we accounted for numerous clinical and health care utilization metrics, including frailty indicators, to minimize confounding from difficult-to-measure factors. Second, despite using accurate zolpidem- and trazodone-dispensing information, we were unable to determine when patients started taking these medications and if they took zolpidem and trazodone as prescribed. Third, the study outcome, hospitalized fall-related fracture, was ascertained using discharge diagnosis codes, and misclassification may have occurred. Reassuringly, a sensitivity analysis considering a broader outcome, hospitalized fracture, yielded consistent results. Fourth, our results may not generalize to excluded populations such as individuals receiving home hemodialysis or peritoneal dialysis.
In conclusion, among individuals receiving maintenance in-center hemodialysis, zolpidem initiators had a higher 30-day risk of hospitalized fall-related fractures compared with trazodone initiators, suggesting trazodone may be a safer pharmacologic treatment option for the management of insomnia in this vulnerable population. Future studies are needed to further elucidate the risk-benefit profiles of insomnia medications in individuals with dialysis-dependent kidney failure.
Disclosures
In the last 3 years, M.M. Assimon reports receiving investigator-initiated research funding from the Renal Research Institute (a subsidiary of Fresenius Medical Care, North America), the Agency for Healthcare Research and Quality (AHRQ), and the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH); and reports recieving honoraria from the International Society of Nephrology (Kidney International Reports statistical reviewer). In the last 3 years, J.E. Flythe reports receiving speaking honoraria from the American Renal Associates, the American Society of Nephrology, Baxter, Dialysis Clinic, Inc., Fresenius Medical Care North America, the National Kidney Foundation, Renal Ventures, and multiple universities; reports receiving research funding from the NHLBI and NIDDK of the NIH, the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, and the Renal Research Institute (a subsidiary of Fresenius Medical Care, North America); reports serving on the medical advisory board of NxStage Medical, Inc.; reports serving as a scientific advisor or member of Kidney Disease Improving Global Outcomes (KDIGO) Executive Committee (since 2020), Kidney Health Initiative (KHI) Board of Directors (since 2019), KHI Patient Preferences Project Chairperson (since 2019), American Journal of Kidney Diseases Editorial Board (since 2017); CJASN Editorial Board (since 2017), Nephrology Dialysis and Transplantation Editorial Board, Hemodialysis Theme Editor (since 2018), Kidney Medicine Editorial Board (since 2019), and Kidney360 Associate Editor (since 2019); and reports receiving consulting fees from AstraZeneca and Fresenius Medical Care, North America.
Funding
M.M. Assimon and J.E. Flythe are supported by R01 HL152034 awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. J.E. Flythe is supported by grant K23 DK109401 awarded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Supplementary Material
Acknowledgments
The data reported here have been provided by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the United States government. The authors thank Mr. Matthew Tugman for his assistance with manuscript editing.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10070620/-/DCSupplemental.
Supplemental Figure 1. Propensity score distribution.
Supplemental Table 1. Starting doses of zolpidem and trazodone outside of the recommended range for insomnia management.
Supplemental Table 2. Outcome definitions.
Supplemental Table 3. ICD-9/10 diagnosis codes used to identify outcome events.
Supplemental Table 4. ICD-9/10 diagnosis codes and HCPCS procedure codes used to identify relevant comorbid conditions and frailty indicators.
Supplemental Table 5. Lists of relevant medications.
Supplemental Table 6. Definitions of lower and higher zolpidem doses.
Supplemental Table 7. Baseline characteristics of zolpidem and trazodone initiators.
Supplemental Table 8. Association between zolpidem vs. trazodone initiation and 30-day fall-related fracture risk using an intention-to-treat analytic approach.
Supplemental Table 9. Association between zolpidem vs. trazodone initiation and 30-day fall-related fracture risk within clinically relevant subgroups using an intention-to-treat analytic approach.
Supplemental Table 10. Proportion of patients in clinically relevant subgroups initiating zolpidem therapy at higher doses.
Supplemental Table 11. Association between zolpidem vs. trazodone initiation and 60- and 90-day fall-related fracture risk using an intention-to-treat analytic approach.
Supplemental Table 12. Association between zolpidem vs. trazodone initiation and fall-related fracture risk using an on-treatment analytic approach.
Supplemental Table 13. Association between zolpidem vs. trazodone initiation and 30-day fracture risk using an intention-to-treat analytic approach.
References
- 1.Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, Switzer GE: Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol 16: 2487–2494, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Zhang JC, El-Majzoub S, Li M, Ahmed T, Wu J, Lipman ML, Moussaoui G, Looper KJ, Novak M, Rej S, Mucsi I: Could symptom burden predict subsequent healthcare use in patients with end stage kidney disease on hemodialysis care? A prospective, preliminary study. Ren Fail 42: 294–301, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amro A, Waldum B, Dammen T, Miaskowski C, Os I: Symptom clusters in patients on dialysis and their association with quality-of-life outcomes. J Ren Care 40: 23–33, 2014 [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association: Sleep-wake disorders. In: Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th Ed., Arlington, VA, American Psychiatric Publishing, 2013 [Google Scholar]
- 5.Anand S, Johansen KL, Grimes B, Kaysen GA, Dalrymple LS, Kutner NG, Chertow GM: Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: The comprehensive dialysis study. Hemodial Int 17: 50–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL: Consequences of insomnia. Sleep Med Clin 1: 351–358, 2006 [Google Scholar]
- 7.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS: Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep (Basel) 42: zsz007, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flythe JE, Hilliard T, Castillo G, Ikeler K, Orazi J, Abdel-Rahman E, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie C, Mehrotra R: Symptom prioritization among adults receiving in-center hemodialysis: A mixed methods study. Clin J Am Soc Nephrol 13: 735–745, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M: Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4: 487–504, 2008 [PMC free article] [PubMed] [Google Scholar]
- 11.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians : Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med 165: 125–133, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M, Hertenstein E, Jansson-Fröjmark M, Jennum PJ, Leger D, Nissen C, Parrino L, Paunio T, Pevernagie D, Verbraecken J, Weeß HG, Wichniak A, Zavalko I, Arnardottir ES, Deleanu OC, Strazisar B, Zoetmulder M, Spiegelhalder K: European guideline for the diagnosis and treatment of insomnia. J Sleep Res 26: 675–700, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Chen HY, Cheng IC, Pan YJ, Chiu YL, Hsu SP, Pai MF, Yang JY, Peng YS, Tsai TJ, Wu KD: Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int 80: 415–422, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Hou Y, Hu P, Liang Y, Mo Z: Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys 69: 531–537, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C: National use of prescription medications for insomnia: NHANES 1999-2010. Sleep (Basel) 37: 343–349, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Renal Data System : 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Volume 2: End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 17. Ambien® (zolpidem tartrate tablets) [package insert]. Bridgewater, NJ, Sanofi-Aventis U.S. LLC, 2019.
- 18. Desyrel® (trazodone hydrochloride tablets) [package insert]. Locust Valley, NY, Pragma Pharmaceuticals, LLC, 2018.
- 19.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA: Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 169: 1952–1960, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Donnelly K, Bracchi R, Hewitt J, Routledge PA, Carter B: Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis. PLoS One 12: e0174730, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J: Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ 343: d4551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA: Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol 24: 1461–1469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ 3rd: Trends in fracture incidence: A population-based study over 20 years. J Bone Miner Res 29: 581–589, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr JN, Melton LJ 3rd, Achenbach SJ, Atkinson EJ, Khosla S, Amin S: Fracture incidence and characteristics in young adults aged 18 to 49 years: A population-based study. J Bone Miner Res 32: 2347–2354, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray WA: Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol 158: 915–920, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T: Variable selection for propensity score models. Am J Epidemiol 163: 1149–1156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Schneeweiss S: Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: Evidence and recommendations. Pharmacoepidemiol Drug Saf 23: 891–901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 30.Cole SR, Lau B, Eron JJ, Brookhart MA, Kitahata MM, Martin JN, Mathews WC, Mugavero MJ; CNICS Research Network: Estimation of the standardized risk difference and ratio in a competing risks framework: Application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol 181: 238–245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole SR, Hernán MA: Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168: 656–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brookhart MA, Wyss R, Layton JB, Stürmer T: Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 6: 604–611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Adminstration: FDA Drug Safety Communication: Risk of next‐morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). Available at: http://wayback.archive-it.org/7993/20170112032712/http://www.fda.gov/downloads/Drugs/DrugSafety/UCM335007.pdf. Accessed May 15, 2020
- 34.US Food and Drug Adminstration: FDA Drug Safety Communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-new-label-changes-and-dosing-zolpidem-products-and. Accessed May 15, 2020
- 35.Imbens GW: The role of the propensity score in estimating dose-response functions. Biometrika 87: 706–710, 2000 [Google Scholar]
- 36.Nevitt MC, Cummings SR, Hudes ES: Risk factors for injurious falls: A prospective study. J Gerontol 46: M164–M170, 1991 [DOI] [PubMed] [Google Scholar]
- 37.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel: American Geriatrics Society 2019 updated AGS Beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 67: 674–694, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Rassen JA, Glynn RJ, Rothman KJ, Setoguchi S, Schneeweiss S: Applying propensity scores estimated in a full cohort to adjust for confounding in subgroup analyses. Pharmacoepidemiol Drug Saf 21: 697–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allain H, Bentué-Ferrer D, Polard E, Akwa Y, Patat A: Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: A comparative review. Drugs Aging 22: 749–765, 2005 [DOI] [PubMed] [Google Scholar]
- 40.US Food and Drug Adminstration: FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia. Accessed May 15, 2020
- 41.Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, Petersen LA, Small SD, Sweitzer BJ, Vander Vliet M, Leape LL; ADE Prevention Study Group: Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med 159: 2553–2560, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Evans RS, Lloyd JF, Stoddard GJ, Nebeker JR, Samore MH: Risk factors for adverse drug events: A 10-year analysis. Ann Pharmacother 39: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sabbatini M, Crispo A, Pisani A, Ragosta A, Cesaro A, Mirenghi F, Cianciaruso B, Federico S: Zaleplon improves sleep quality in maintenance hemodialysis patients. Nephron Clin Pract 94: c99–c103, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Dashti-Khavidaki S, Chamani N, Khalili H, Hajhossein Talasaz A, Ahmadi F, Lessan-Pezeshki M, Ghaeli P, Dalili S, Alimadadi A: Comparing effects of clonazepam and zolpidem on sleep quality of patients on maintenance hemodialysis. Iran J Kidney Dis 5: 404–409, 2011 [PubMed] [Google Scholar]
- 45.Winkelmayer WC, Mehta J, Wang PS: Benzodiazepine use and mortality of incident dialysis patients in the United States. Kidney Int 72: 1388–1393, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Psychoactive medications and adverse outcomes among older adults receiving hemodialysis. J Am Geriatr Soc 67: 449–454, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM; Agency for Health Care Research and Quality (U.S.): Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide, Rockville, MD, U.S. Department of Health and Human Services, 2013 [PubMed] [Google Scholar]
- 48.Flythe JE, Hilliard T, Lumby E, Castillo G, Orazi J, Abdel-Rahman EM, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie CM, Mehrotra R; Kidney Health Initiative Prioritizing Symptoms of ESRD Patients for Developing Therapeutic Interventions Stakeholder Meeting Participants: Fostering innovation in symptom management among hemodialysis patients: Paths forward for insomnia, muscle cramps, and fatigue. Clin J Am Soc Nephrol 14: 150–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund JL, Richardson DB, Stürmer T: The active comparator, new user study design in pharmacoepidemiology: Historical foundations and contemporary application. Curr Epidemiol Rep 2: 221–228, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brookhart MA: Counterpoint: The treatment decision design. Am J Epidemiol 182: 840–845, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.