Abstract
Revolutions in genetics, epigenetics, and bioinformatics are currently changing the outline of diagnostics and clinical medicine. From a nephrologist’s perspective, individuals with congenital anomalies of the kidney and urinary tract (CAKUT) are an important patient category: not only is CAKUT the predominant cause of kidney failure in children and young adults, but the strong phenotypic and genotypic heterogeneity of kidney and urinary tract malformations has hampered standardization of clinical decision making until now. However, patients with CAKUT may benefit from precision medicine, including an integrated diagnostics trajectory, genetic counseling, and personalized management to improve clinical outcomes of developmental kidney and urinary tract defects. In this review, we discuss the present understanding of the molecular etiology of CAKUT and the currently available genome diagnostic modalities in the clinical care of patients with CAKUT. Finally, we discuss how clinical integration of findings from large-scale genetic, epigenetic, and gene-environment interaction studies may improve the prognosis of all individuals with CAKUT.
Keywords: genetics and development, kidney development, clinical nephrology, molecular genetics, chronic kidney disease, gene-environment interaction, precision medicine, genetic counseling, clinical decision-making, computational biology, urogenital abnormalities, vesico-ureteral reflux, renal insufficiency, prognosis, epigenesis, genetic
Introduction
Aberrant embryonic kidney and urinary tract development results in a spectrum of different clinical phenotypes, which are collectively referred to as congenital anomalies of the kidney and urinary tract (CAKUT) (1–3). The spatiotemporal character of molecular disturbances is hypothesized to define the kidney and urinary tract defects of individuals (4) because early embryonic maldevelopment leads to kidney parenchyma malformations, whereas later interferences underlie ureteral anomalies (5). Mackie and Stephens (6) have proposed that the position of ureteric-bud formation on the nephric duct determines the CAKUT phenotype: insertion too low into the bladder results in vesicoureteral reflux, while insertion that is too high leads to obstructive uropathy (7). Although not consistently categorized within the CAKUT spectrum, lower urinary tract aberrations include dramatic phenotypes such as posterior urethral valves and bladder exstrophy (8). CAKUT is characterized by high phenotypic variability between cases and co-occurrence of different anomalies within the same individual (9,10). Genetic defects are known to underlie CAKUT, however, incomplete or nonpenetrance is often observed in affected families (11), and identical genetic mutations could result in different CAKUT subphenotypes between individuals and even within the same families, a phenomenon known as variable expressivity (12). In addition, epigenetic and environmental factors may directly affect the CAKUT phenotype and also display indirect effects on the long-term clinical outcome of patients (1).
From a clinical perspective, it is incredibly relevant to unravel the genetic, epigenetic, and environmental underpinnings of kidney and urinary tract malformations because CAKUT is the major cause of kidney failure in childhood. The European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association registry from 2016 reported CAKUT in approximately 30% of children with kidney failure, which was almost two-fold the incidence of the second most frequent cause (glomerulopathies) of childhood kidney failure (13). Notably, the median age to start RRT in patients with CAKUT compared with patients without CAKUT (31 versus 61 years, respectively) (14) emphasizes the essential role of pediatric and adult nephrologists in the prevention of kidney failure in affected individuals. Nevertheless, early-phase identification of patients who will develop CKD often remains problematic (15–17). Recent advancements of genetics and bioinformatics have completely revolutionized the field of medical genetics and now offer opportunities for development of clinical frameworks in genetic kidney diseases such as CAKUT (2,18). In this review, we discuss how these genetic diagnostic methods are currently integrated in the care for patients with CAKUT, and how they enable future development of precision-medicine approaches for developmental kidney and urinary tract defects.
The Clinical Spectrum of Phenotypes of Congenital Anomalies of the Kidney and Urinary Tract
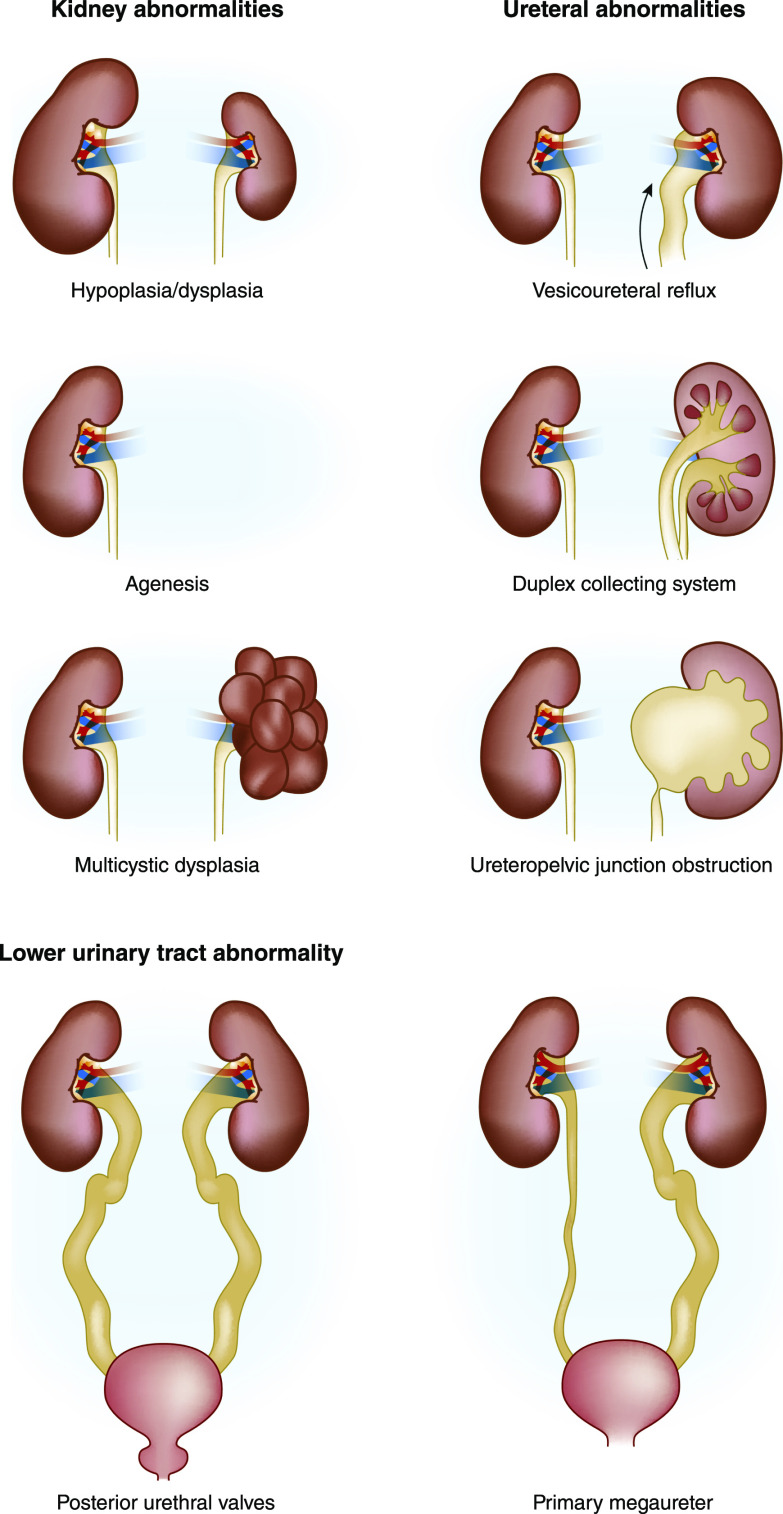
Overall, CAKUT affects 1:100–500 newborns and is the most frequent developmental defect in humans (19,20). The umbrella term CAKUT refers to the extensive spectrum of malformations of the kidney and urinary tract. Most phenotypes comprise permanent defects (e.g., malformations of the kidney parenchyma); however, mild antenatally detected hydronephrosis and low-grade vesicoureteral reflux may completely resolve during growth and development (21,22). The phenotypic complexity of CAKUT is often clinically subdivided into anatomic order (Figure 1): (1) parenchymal defects of the kidney such as agenesis, hypoplasia, dysplasia, or multicystic dysplastic kidney; (2) ureteral defects such as uretero-pelvic junction obstruction, ureterovesical junction obstruction, primary nonobstructive nonrefluxing megaureter, and vesicoureteral reflux; and (3) lower urinary tract malformations such as bladder exstrophy, bladder agenesis, and posterior urethral valves or urethral agenesis. In addition, spatial disturbances in urinary tract development are hypothesized to determine the presence of unilateral or bilateral defects (4), and may also result in duplex kidney (with or without a ureterocele), ectopic kidney tissue and/or ureteral orifice, and a horseshoe kidney. Moreover, co-occurrence of CAKUT is a regular finding within individual patients and is not intrinsically limited to anatomic boundaries. For example, approximately one in three cases with unilateral kidney agenesis or multicystic dysplastic kidney have vesicoureteral reflux or ureteropelvic junction obstruction on the contralateral side (23,24).
Figure 1.
Overview of CAKUT phenotypes, discerning parenchymal defects of the kidney, ureteral abnormalities, and posterior urethral valves as a lower urinary tract abnormality.
Although unique phenotypes often occur in affected individuals, the anatomic subdivision of CAKUT derived from imaging studies helps clinicians to determine follow-up strategies on diagnostics, prognostics, and management. Standardized guidelines to uniformize clinical decision making for individuals with different CAKUT phenotypes have not been developed due to the absence of systematically performed longitudinal studies. In part, the unavailability of such studies prevents clinicians from discriminating between patients with an impaired survival of kidney function progressing to kidney failure from those patients who have a more benign course. Furthermore, parents or family members of a child with CAKUT often inquire on recurrence risks and, in addition, the evolution of medical care implicates that the majority of patients with CAKUT now reach fertile age; both situations exemplify the need for individualized genetic counseling of patients with CAKUT and their family members. Thus, improved understanding of the molecular etiology of CAKUT seems the obvious starting point in the development of standardized clinical guidelines to improve the prognosis for patients.
Current Understanding of the Etiology of Congenital Anomalies of the Kidney and Urinary Tract
Implementation of next-generation sequencing–based genetic testing has significantly enhanced our knowledge of the etiologic basis of many diseases and has added to personalized patient care by contributing to patient treatment and genetic counseling, including recurrence-risk estimation and family planning. Thus, to improve the accuracy of precision-medicine strategies for CAKUT, better understanding of the molecular underpinnings of kidney and urinary tract maldevelopment is a top priority. Here, we highlight the essential aspects of the complex etiologic landscape of CAKUT.
Monogenic Congenital Anomalies of the Kidney and Urinary Tract
The assumption that CAKUT is a monogenic disorder in 10%–15% of the cases is based on several clinical observations (2,3,11). First, it is well described that CAKUT segregates within families (approximately 10%–25%) (25), frequently in an autosomal dominant mode of inheritance with incomplete penetrance and variable expressivity. Second, sporadic CAKUT can be part of a specific genetic syndrome affecting multiple organ systems of an individual. These syndromes are predominantly explained by heterozygous loss-of-function mutations or structural variants (see below) that occur de novo (e.g., loss-of-function mutations in HNF1B or large heterozygous deletions that disrupt multiple genes (26,27)), but also homozygous (e.g., in consanguineous families or by a founder effect in isolated populations) or compound heterozygous mutations leading to autosomal recessive disease have been reported (27–30). Finally, the observation that single-gene knockout mice show developmental kidney and urinary tract defects further supports the monogenic etiology of CAKUT (31).
The introduction of next-generation sequencing has extensively defined this part of the genetic landscape of CAKUT. Currently >50 genes related to CAKUT etiology have been identified (for a recent list of genes implicated in CAKUT see [2]). The potential diagnostic value of genetic studies in kidney disease has recently been demonstrated in a large exome-based genetic screen of 3037 adults with CKD due to various causes (32). Interestingly, the authors found the highest diagnostic yield in patients with congenital or cystic kidney disease (24%; odds ratio, 24.4; 95% confidence interval, 10.6 to 56.4, versus a reference group of diabetic nephropathy). In a recent study of individuals with familial CAKUT, Van Der Ven et al. (28) identified pathogenic mutations in 13 known genes for isolated or syndromic CAKUT phenotypes in 29/232 (13%) families. Overall, the reported diagnostic yields of targeted sequencing screens range from 2% to 15% and appear heavily dependent on cohort characteristics (e.g., CAKUT phenotype, inclusion criteria, population of cases and controls) and the platform used for sequencing (10,28,33–35).
One important clinical benefit derived from recent large-scale, whole-exome sequencing (WES) studies is the identification of pleiotropy for genes implicated in syndromic forms of CAKUT (36). For example, PAX2 (heterozygous mutations in this gene were originally associated with papillorenal syndrome [Online Mendelian Inheritance in Man (OMIM) #120330] [37]) has been implicated in steroid-resistant nephrotic syndrome and FSGS in adults and children (38,39), whereas heterozygous mutations in HNF1B (known to cause the renal cysts and diabetes [RCAD] syndrome [OMIM #137920] [40]) also underlie isolated hyperuricemia, hypomagnesemia, and extrarenal features (41–43).
Copy-Number Variants in Congenital Anomalies of the Kidney and Urinary Tract
Structural aberrations in a considerable number of base pairs (i.e., copy-number variants) may represent “healthy” genetic variation or be the basis of human developmental diseases (44,45) such as neurodevelopmental delay, cardiac defects, autism spectrum disorder, and CAKUT. Several theoretic models propose how a particular copy-number variant leads to a developmental phenotype, ranging from a simplex model where dosage imbalance of one gene within the copy-number variant is responsible for the developmental phenotype to a model in which multiple modifier genes within and/or outside the copy-number variant ultimately determine the combination of developmental defects (46). Similar to syndromic CAKUT caused by single gene defects, these copy-number variant–based genomic disorders are characterized by incomplete penetrance and phenotypic variability (2).
The group of Sanna-Cherchi and Gharavi (47) first described the role of copy-number variants in the molecular etiology of CAKUT in a cohort of patients with kidney parenchymal defects. By performing genome-wide genotyping using single nucleotide polymorphism (SNP) arrays, the authors discovered that the burden of large and rare exonic copy-number variants in affected patients was much higher than in population-based controls. In addition, they identified disease-causing copy-number variants in 10% of patients and potentially pathogenic copy-number variants in 6% of patients (47). Next, the investigators replicated these findings in smaller cohorts of patients with CAKUT (48,49) before expanding copy-number variant analysis to 2824 patients, encompassing the entire CAKUT spectrum (29). This study confirmed the increased burden of large, rare, exonic copy-number variants in individuals with CAKUT by comparing them to >21,000 population-based controls (29). Interestingly, patients with kidney parenchymal defects were particularly enriched for exonic deletions, whereas patients with vesicoureteral reflux and posterior urethral valves showed a pronounced burden of exonic duplications (29), again emphasizing the genetic heterogeneity of CAKUT. Overall, a genomic disorder was diagnosed in 4% of patients and was predominantly identified in those with kidney parenchymal defects (e.g., deletion loci on chromosomes 17q12 [renal cysts and diabetes syndrome] and 22q11.2 [DiGeorge syndrome; OMIM #188400]). Only six pathogenic loci were implicated in 65% of CAKUT cases with a known genomic disorder, i.e., chromosomes 17q12, 22q11.2, 16p11.2, 1q21.1, 4p− (Wolf–Hirschhorn syndrome; OMIM #194190), and 16p13.11 (29). For the majority of these genomic disorders, a simplex copy-number variant model in which dosage imbalances of one particular gene driver lead to CAKUT has been proposed (29,40,50–52). However, complex models of pathogenic structural variants in the etiology of CAKUT still need to be studied.
Common Variants in Congenital Anomalies of the Kidney and Urinary Tract
From a population genetics viewpoint, common variants with small effects may provide an alternative molecular diagnosis in relatively common CAKUT phenotypes such as vesicoureteral reflux, antenatal hydronephrosis, horseshoe kidney, and duplex kidney. These common variants are identified by genome-wide association studies (GWAS) using genome-wide genotyping techniques. GWAS have contributed to our understanding of several kidney diseases such as IgA nephropathy, membranous nephropathy, and CKD (53–55). GWAS often require large cohorts of cases and population-matched controls to pick up significant signals, which is a major explanation for the current low number of GWAS studies in CAKUT. Darlow and coworkers (56) performed a genome-wide linkage and association study in 1147 European children with primary nonsyndromic vesicoureteral reflux and identified the chromosomal 10q26 locus as a major genetic contributor in vesicoureteral reflux, although the investigators were unable to identify a candidate gene in this region. Van Eerde et al. (57) conducted a two-stage, case-control study investigating 567 candidate SNPs in patients with vesicoureteral reflux with or without the presence of a duplex kidney. Common SNPs in four known CAKUT genes (GREM1, EYA1, ROBO2, and UPK3A) were associated with primary vesicoureteral reflux or duplex kidneys, however, none of these signals reached genome-wide significance (57).
One common limitation of GWAS results is that individual risk loci have such small effects that the clinical applicability of these findings for patients is limited. However, with the improvement of genetic methods, bioinformatic tools, and statistical genetic analyses, the next step is to generate oligo- or polygenic risk scores from GWAS data. Polygenic risk scores combine the sum of all known variants to calculate an overall risk of developing a particular disease (58). Recently, this approach was used for coronary artery disease, inflammatory bowel disease, and diabetes mellitus (59). In this study, generation and validation of genome-wide risk scores for the aforementioned traits identified a relative risk for disease development comparable to monogenic causes (59). Nevertheless, large-scale investigations including GWAS are needed before polygenic risk scores can be implemented in clinical medicine (58), and for CAKUT in particular.
Epigenetic Mechanisms in Congenital Anomalies of the Kidney and Urinary Tract
Epigenetics investigates causes of disease that result from gene expression modification. Given the tightly orchestrated molecular mechanisms that are active during kidney and urinary tract development, determination of epigenetic factors that regulate DNA transcription represent an important opportunity to understand the etiology of CAKUT (60). Among the best-studied epigenetic mechanisms in kidney disease are DNA cytosine methylation and histone tail modification (e.g., acetylation, phosphorylation, and methylation) (61). Both mechanisms alter the chromatin structure and may activate or, reciprocally, silence expression of key regulator genes leading to defective kidney and urinary tract development and differentiation (62). Li et al. (63) performed genome-wide methylome analyses in mice to identify functional epigenome-modifying enzymes and genome regions important in kidney development. Systematic targeting of DNA methyltransferases (Dnmt3a and Dnmt3b) and a hemimethylase responsible for methylation of newly synthesized DNA (Dnmt1) revealed that tissue-specific loss of Dnmt1 during kidney development in mice leads to hypodysplastic kidneys with undifferentiated nephrons and absence of fetal urine production (63). Depletion of Dnmt1 resulted in changes in cytosine methylation and expression levels in 415 genes. Among these candidate genes was Umod, a gene that encodes Tamm–Horsfall protein that has previously been implicated as major susceptibility gene in human CKD development (54).
Another epigenetic mechanism relevant to CAKUT development includes small noncoding RNAs (i.e., micro-RNAs [miRs]) that target mRNA and disturb protein synthesis by degradation of mRNA and/or inhibition of translation. By performing targeted sequencing, Kohl et al. (64) identified 73 kidney developmental miR genes in 1213 patients with isolated CAKUT. In addition, the authors found potentially pathogenic variants in two miRs (MIR19B1 and MIR99A) in two of 1213 (0.2%) individuals. Another ex vivo study using ureteral tissue discerned seven specific miRs that were upregulated in CAKUT, although their exact role in kidney development remains to be elucidated (65). Interestingly, the top miR (hsa-miR-144) is situated at the chromosomal 17q11.2 locus, which is rich in copy-number variants. Notwithstanding that this finding points toward a link between structural variations and epigenetic changes in the development of CAKUT, mechanistic epigenetic studies in CAKUT are still in an early phase.
Environmental Factors in the Development of Congenital Anomalies of the Kidney and Urinary Tract
Environmental factors can directly disturb kidney and urinary tract development, but the in utero environment may also play an indirect role in the long-term health of an individual (i.e., a phenomenon described as “fetal programming”) (66). For example, the developmental origins of health and disease hypothesis has associated the parental and intrauterine environment with permanent changes in the epigenome and, as such, the clinical phenotype (67). One striking example of fetal programming is the robust association between low birth weight, a proxy for intrauterine environmental circumstances, and a reduced nephron number (68). Other well-described environmental hazards in the etiology of CAKUT are maternal obesity and maternal diabetes (69–71). Because nephrogenesis takes place until the 34th–36th gestational week, clinicians should specifically be cautious not to prescribe medications that disturb kidney and urinary tract development in pregnant women and prematurely born infants (72,73).
To date, most studies have focused on the perinatal period to identify environmental factors that play a role in the molecular underpinnings of CAKUT. The gut microbiome is an important postnatal environmental factor recently implicated in the etiology and disease progression of CKD (74). The relationship between the gut microbiota composition and CKD appears to be bidirectional: toxins produced due to kidney failure affect the gut microbiome diversity, and the microbiome in turn may directly decrease kidney function (74). Patients with CAKUT have an increased risk for urinary tract infections often leading to frequent use of antibiotics (75). A recent study in children with different CAKUT phenotypes reported that gut microbiota-derived short chain fatty acids, such as propionate and butyrate, were indeed associated with CKD and hypertension in patients (76). As methodologies to determine the host-microbiota interactions are not yet fully standardized, more studies are needed to determine the exact role of the microbiome in CKD in general, and in CAKUT in particular.
Current Molecular Diagnostic Tools for Patients with Congenital Anomalies of the Kidney and Urinary Tract
Insight into the molecular background of kidney diseases has resulted in the development of genetic tests and improvement of genetic counseling for patients with kidney disease, whereas revolutionary advances in sequencing techniques and bioinformatic tools for big data analyses have led to the implementation of next-generation sequencing platforms in genome diagnostics laboratories for standardized use in health care (77,78). After having analyzed single genes one by one by Sanger sequencing for many years, the first next-generation sequencing–based efforts were focused on targeted gene panels that contain a specific set of genes within the context of a certain phenotype. Targeted gene panel sequencing facilitated faster and cheaper sequencing of multiple genes in parallel for many individuals at the same time, at reduced costs, and with a higher diagnostic yield (79). For kidney diseases specifically, gene panels were designed and implemented in genome diagnostics laboratories (80–82). Gene-panel sequencing involves capture of the DNA regions of interest, followed by sequencing. However, gene-panel enrichment is limited by its design. Consequently, the identification and implementation of additional genes requires redesign of the panel and repetitive optimization and validation of the approach, which is cost inefficient in a rapidly changing genetic environment. Because the costs for next-generation sequencing have decreased, WES has become the predominant approach in genome diagnostics. WES includes all of the coding exons of the genome, which facilitates gene-panel analysis without redesign of the experiment in case of updates. A WES-based test combined with variant analysis aimed at the genes of interest is known to have multiple advances over targeted gene-panel capture and sequencing (83): (1) newly identified genes can easily be added to the analysis without adjustment and validation of the laboratory flow—because the nephrogenetics field is evolving fast, with causal genes being identified on a regular basis, a flexible approach like WES facilitates rapid test updates; (2) gene-panel analysis from WES data prevents the identification of incidental findings; (3) data on the full exome is available and can be released for further investigation in case the molecular cause has not been identified, leading to a higher diagnostic yield (84); and (4) WES results in a considerable reduction of diagnostic time and health care costs, especially when applied early in the diagnostic process (80,85).
Although the application of next-generation sequencing has resulted in a rapid increase in the identification of disease-causing genes and the direct implementation in genome diagnostics, the translation to care for patients with CAKUT has been slow (80). Possible reasons could be: (1) because most of CAKUT etiology is still unexplained, the diagnostic value for WES in kidney disease and CAKUT specifically remains limited (32,35,86), (2) identification of variants of unknown clinical significance with limited options for follow-up experiments in diagnostics, (3) the risk of identification of medically actionable incidental findings, (4) financial issues and logistic impracticalities, and (5) nephrologists unaccustomed to using genetic tests as diagnostic and prognostic clinical modalities. In addition, although WES can be used to detect pathogenic copy-number variants, SNP microarrays currently remain the preferred modality for clinical genetic studies in syndromic CAKUT due to better accuracy and much lower costs.
We see great opportunities for improvement of CAKUT DNA testing by creating awareness among clinicians on the testing procedure and the concomitant options for counseling. We believe that the use of genetic testing in CAKUT in an early stage of the diagnostic trajectory will be beneficial to patients and their relatives because the test outcome can aid in disease classification and management. Recent investigations showed that approximately 50% of patients with CKD with disease onset at <25 years of age received a revision of the original clinical diagnosis based on genetic testing (32,86–88). Therefore, a genetics-first approach could result in a considerably shorter and less complicated diagnostic process, maximizing the opportunities to improve the clinical outcome of patients at an early stage (89). This might also be the case for CAKUT. For example, genetics-first approaches with rapid return of results could prevent invasive diagnostic procedures and the use of immunosuppressive agents in patients with CAKUT who have secondary proteinuria and hypertension, a late clinical phenotype that mirrors primary glomerulopathies. Furthermore, the ESCAPE study has shown that strict BP control in children with kidney parenchymal defects slows down CKD progression (90). Because mild kidney hypodysplasia can be missed by fetal ultrasound, genetic testing can shed light on the exact diagnosis, leading to better BP management and, as such, direct improvement of the clinical outcome.
The complexity of CAKUT prevents a one-size-fits-all genomic diagnostic approach for all patients with CAKUT. Before implementation of every form of personalized genetic testing, we should (besides taking the potential diagnostic yield into account) look at the test’s costs, the payer’s situation, and ethical considerations regarding the return of incidental findings. For example, performing SNP microarray testing in syndromic CAKUT shows a high diagnostic yield to detect pathogenic copy-number variants at relatively low costs (approximately $50–$200), whereas WES (approximately $1000) in familial CAKUT and sporadic cases with kidney parenchymal defects detects approximately one pathogenic variant in five to ten patients. Therefore, we feel that standard genetic testing of syndromic and severe CAKUT cases is reasonable and should be considered globally. However, in the case of isolated ureteral phenotypes or posterior urethral valves, our current understanding of the genetic architecture still is very limited and the diagnostic yield of genetic studies in patients with these particular kidney and urinary tract anomalies appears too low for standard clinical genetic screening at this moment.
Future Diagnostic Opportunities for Patients with Congenital Anomalies of the Kidney and Urinary Tract
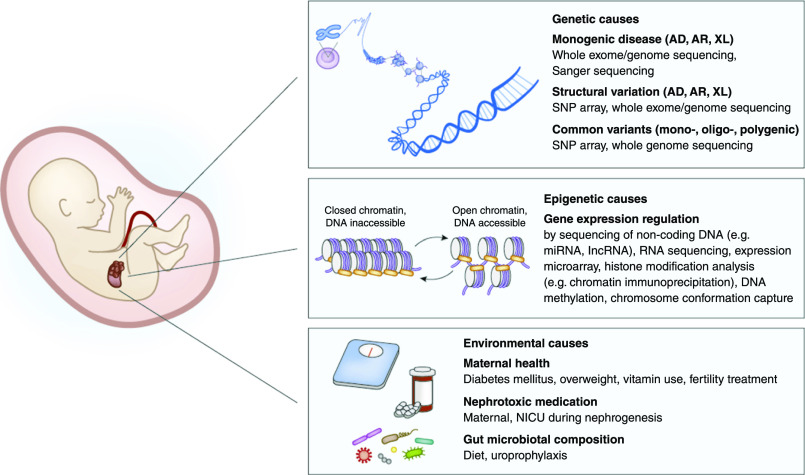
The next step will be the implementation of whole-genome sequencing (WGS) in routine diagnostics. WGS comprises the sequencing of the full DNA of an individual, without enrichment of a part of the genome. WGS has the potential to capture all classes of genetic variation—including noncoding variants and structural variants—but, in light of the huge amount of data produced, WGS will also lead to enormous challenges in interpreting the data. Moreover, WGS data should be carefully integrated with broad -omics data on gene expression, epigenetics, metabolomics, and proteomics, as well as with detailed clinical and environmental information to differentiate common benign variants from rare damaging variants and prioritize or classify them as such (Figure 2). Although still predominantly in a preclinical phase, data scientists are now developing bioinformatics pipelines that allow such integration of multiomics data with information on biology and phenotypes in machine learning systems (91–95). Given the phenotypic complexity of CAKUT, standardized classification of developmental kidney and urinary tract defects, for example according to the Human Ontology Project nomenclature (96), is essential in the development of such frameworks for clinical use. Moreover, ingenious methods that integrate other data sources (e.g., expression data from patient-derived material) will be crucial for the development of genome diagnostic modalities based on statistics and artificial intelligence (97,98). We further see great relevance for functional characterization of genetic variants in a diagnostic setting. The growing identification of variants that have an unknown effect on the disease require the appropriate model systems (99). For kidney diseases, the use of urine-derived cells is under investigation as a noninvasive tool in functional diagnostics for kidney disease (100,101). Although these experimental diagnostic tools contain great promise, successful translation and implementation of integrated diagnostics approaches to the clinic requires close interdisciplinary teamwork including clinicians, biomedical researchers, and bioinformaticians (102). It seems likely that the first integrated genomic diagnostic approaches will be introduced into clinical care in the coming decade. For clinicians caring for patients with CAKUT, we expect these diagnostic algorithms to strengthen the classic anatomic approach with genetic, epigenetic, and environmental risk factors and robust clinical parameters (e.g., imaging studies, early biomarkers for CKD progression in blood and urine), ultimately leading to a better prognosis of individuals with kidney and urinary tract malformations.
Figure 2.
Multiomics approach to define CAKUT etiology. Integration of data on genetic variation, gene expression and regulation, and environmental factors with clinical information on phenotype, imaging, and lab values will help to shape the genomic landscape of CAKUT and determine which DNA variants are involved in disease etiology and prognosis. AD, autosomal dominant; AR, autosomal recessive; CAKUT, congenital anomalies of the kidney and urinary tract; NICU, neonatal intensive care unit; lncRNA, long noncoding RNA; miRNA, micro-RNA; SNP, single nucleotide polymorphism; XL, X-linked.
In summary, CAKUT is characterized by a variable clinical outcome that reflects the complex genetic, epigenetic, and environmental basis of this frequently occurring human developmental defect. Although the advent of next-generation sequencing and bioinformatic tools has improved our understanding of the molecular landscape of CAKUT, the etiology currently remains unknown for the majority of cases. Therefore, integration of large-scale human genetic, epigenetic, and environment studies is essential to further define the molecular architecture of CAKUT. When the findings of such studies are combined with diagnostic imaging studies and biochemical parameters of disease progression, clinical decisions will comprise more accurate assessment of risk factors and complications of CAKUT, as well as realistic predictions of kidney function survival and overall prognosis. Moreover, insight into the molecular background opens doors to develop new therapeutic strategies that prevent or halt disease progression and improve the clinical outcome of these patients. Yet, to be embraced fully by clinicians, such complex precision-medicine approaches still have to overcome practical challenges such as awareness, fast interpretation of genome-wide genetic data, and short test-to-diagnosis turnover times, as well as financial hurdles and societal-ethical issues regarding return of results and incidental findings. Despite these challenges of modern clinical medicine, we strongly feel that understanding the molecular basis of CAKUT is the way forward for precision medicine for individuals with developmental kidney and urinary tract defects.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by the European Union’s Seventh Framework Programme, grant 305608 (EURenOmics) (to N.V.A.M. Knoers), and Dutch Kidney Foundation, grant 15OKG18 (to K.Y. Renkema).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV: Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11: 720–731, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Sanna-Cherchi S, Westland R, Ghiggeri GM, Gharavi AG: Genetic basis of human congenital anomalies of the kidney and urinary tract. J Clin Invest 128: 4–15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F: Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 29: 695–704, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis TK, Hoshi M, Jain S: To bud or not to bud: The RET perspective in CAKUT. Pediatr Nephrol 29: 597–608, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichikawa I, Kuwayama F, Pope JC 4th, Stephens FD, Miyazaki Y: Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int 61: 889–898, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Mackie GG, Stephens FD: Duplex kidneys: A correlation of renal dysplasia with position of the ureteral orifice. J Urol 114: 274–280, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn C: Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 5: 306–314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lissauer D, Morris RK, Kilby MD: Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med 12: 464–470, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Pope JC 4th, Brock JW 3rd, Adams MC, Stephens FD, Ichikawa I: How they begin and how they end: Classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol 10: 2018–2028, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R: Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: Results of the ESCAPE study. J Am Soc Nephrol 17: 2864–2870, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Sanna-Cherchi S, Caridi G, Weng PL, Scolari F, Perfumo F, Gharavi AG, Ghiggeri GM: Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol 22: 1675–1684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Ven AT, Vivante A, Hildebrandt F: Novel insights into the pathogenesis of monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 29: 36–50, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harambat J, Bonthuis M, Groothoff JW, Schaefer F, Tizard EJ, Verrina E, van Stralen KJ, Jager KJ: Lessons learned from the ESPN/ERA-EDTA Registry. Pediatr Nephrol 31: 2055–2064, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Wühl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F: Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol 8: 67–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westland R, Schreuder MF, van Goudoever JB, Sanna-Cherchi S, van Wijk JA: Clinical implications of the solitary functioning kidney. Clin J Am Soc Nephrol 9: 978–986, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM: Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Chevalier RL: When is one kidney not enough? Kidney Int 76: 475–477, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birth defects monitoring program (BDMP)/commission on professional and hospital activities (CPHA) surveillance data, 1988-1991. Teratology 48: 658–675, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Garne E, Dolk H, Loane M, Boyd PA; EUROCAT: EUROCAT website data on prenatal detection rates of congenital anomalies. J Med Screen 17: 97–98, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Yamaçake KG, Nguyen HT: Current management of antenatal hydronephrosis. Pediatr Nephrol 28: 237–243, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Roig M, Ridley DE, McCracken C, Arlen AM, Cooper CS, Kirsch AJ: Vesicoureteral reflux index: Predicting primary vesicoureteral reflux resolution in children diagnosed after age 24 months. J Urol 197: 1150–1157, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Westland R, Schreuder MF, Ket JC, van Wijk JA: Unilateral renal agenesis: A systematic review on associated anomalies and renal injury. Nephrol Dial Transplant 28: 1844–1855, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Schreuder MF, Westland R, van Wijk JA: Unilateral multicystic dysplastic kidney: A meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant 24: 1810–1818, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Bulum B, Ozçakar ZB, Ustüner E, Düşünceli E, Kavaz A, Duman D, Walz K, Fitoz S, Tekin M, Yalçınkaya F: High frequency of kidney and urinary tract anomalies in asymptomatic first-degree relatives of patients with CAKUT. Pediatr Nephrol 28: 2143–2147, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Madariaga L, Morinière V, Jeanpierre C, Bouvier R, Loget P, Martinovic J, Dechelotte P, Leporrier N, Thauvin-Robinet C, Jensen UB, Gaillard D, Mathieu M, Turlin B, Attie-Bitach T, Salomon R, Gübler MC, Antignac C, Heidet L: Severe prenatal renal anomalies associated with mutations in HNF1B or PAX2 genes. Clin J Am Soc Nephrol 8: 1179–1187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Materna-Kiryluk A, Kiryluk K, Burgess KE, Bieleninik A, Sanna-Cherchi S, Gharavi AG, Latos-Bielenska A: The emerging role of genomics in the diagnosis and workup of congenital urinary tract defects: A novel deletion syndrome on chromosome 3q13.31-22.1. Pediatr Nephrol 29: 257–267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Ven AT, Connaughton DM, Ityel H, Mann N, Nakayama M, Chen J, Vivante A, Hwang DY, Schulz J, Braun DA, Schmidt JM, Schapiro D, Schneider R, Warejko JK, Daga A, Majmundar AJ, Tan W, Jobst-Schwan T, Hermle T, Widmeier E, Ashraf S, Amar A, Hoogstraaten CA, Hugo H, Kitzler TM, Kause F, Kolvenbach CM, Dai R, Spaneas L, Amann K, Stein DR, Baum MA, Somers MJG, Rodig NM, Ferguson MA, Traum AZ, Daouk GH, Bogdanović R, Stajić N, Soliman NA, Kari JA, El Desoky S, Fathy HM, Milosevic D, Al-Saffar M, Awad HS, Eid LA, Selvin A, Senguttuvan P, Sanna-Cherchi S, Rehm HL, MacArthur DG, Lek M, Laricchia KM, Wilson MW, Mane SM, Lifton RP, Lee RS, Bauer SB, Lu W, Reutter HM, Tasic V, Shril S, Hildebrandt F: Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 29: 2348–2361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbitsky M, Westland R, Perez A, Kiryluk K, Liu Q, Krithivasan P, Mitrotti A, Fasel DA, Batourina E, Sampson MG, Bodria M, Werth M, Kao C, Martino J, Capone VP, Vivante A, Shril S, Kil BH, Marasà M, Zhang JY, Na YJ, Lim TY, Ahram D, Weng PL, Heinzen EL, Carrea A, Piaggio G, Gesualdo L, Manca V, Masnata G, Gigante M, Cusi D, Izzi C, Scolari F, van Wijk JAE, Saraga M, Santoro D, Conti G, Zamboli P, White H, Drozdz D, Zachwieja K, Miklaszewska M, Tkaczyk M, Tomczyk D, Krakowska A, Sikora P, Jarmoliński T, Borszewska-Kornacka MK, Pawluch R, Szczepanska M, Adamczyk P, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Dobson MG, Darlow JM, Puri P, Barton DE, Furth SL, Warady BA, Gucev Z, Lozanovski VJ, Tasic V, Pisani I, Allegri L, Rodas LM, Campistol JM, Jeanpierre C, Alam S, Casale P, Wong CS, Lin F, Miranda DM, Oliveira EA, Simões-E-Silva AC, Barasch JM, Levy B, Wu N, Hildebrandt F, Ghiggeri GM, Latos-Bielenska A, Materna-Kiryluk A, Zhang F, Hakonarson H, Papaioannou VE, Mendelsohn CL, Gharavi AG, Sanna-Cherchi S: The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet 51: 117–127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivante A, Hwang DY, Kohl S, Chen J, Shril S, Schulz J, van der Ven A, Daouk G, Soliman NA, Kumar AS, Senguttuvan P, Kehinde EO, Tasic V, Hildebrandt F: Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol 28: 69–75, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, Zhang J, Nestor J, Krithivasan P, Lam WY, Mitrotti A, Piva S, Kil BH, Chatterjee D, Reingold R, Bradbury D, DiVecchia M, Snyder H, Mu X, Mehl K, Balderes O, Fasel DA, Weng C, Radhakrishnan J, Canetta P, Appel GB, Bomback AS, Ahn W, Uy NS, Alam S, Cohen DJ, Crew RJ, Dube GK, Rao MK, Kamalakaran S, Copeland B, Ren Z, Bridgers J, Malone CD, Mebane CM, Dagaonkar N, Fellström BC, Haefliger C, Mohan S, Sanna-Cherchi S, Kiryluk K, Fleckner J, March R, Platt A, Goldstein DB, Gharavi AG: Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 380: 142–151, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F: Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int 85: 1429–1433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolaou N, Pulit SL, Nijman IJ, Monroe GR, Feitz WF, Schreuder MF, van Eerde AM, de Jong TP, Giltay JC, van der Zwaag B, Havenith MR, Zwakenberg S, van der Zanden LF, Poelmans G, Cornelissen EA, Lilien MR, Franke B, Roeleveld N, van Rooij IA, Cuppen E, Bongers EM, Giles RH, Knoers NV, Renkema KY: Prioritization and burden analysis of rare variants in 208 candidate genes suggest they do not play a major role in CAKUT. Kidney Int 89: 476–486, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Ishiwa S, Sato M, Morisada N, Nishi K, Kanamori T, Okutsu M, Ogura M, Sako M, Kosuga M, Kamei K, Ito S, Nozu K, Iijima K, Ishikura K: Association between the clinical presentation of congenital anomalies of the kidney and urinary tract (CAKUT) and gene mutations: An analysis of 66 patients at a single institution. Pediatr Nephrol 34: 1457–1464, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Stokman MF, Renkema KY, Giles RH, Schaefer F, Knoers NV, van Eerde AM: The expanding phenotypic spectra of kidney diseases: Insights from genetic studies. Nat Rev Nephrol 12: 472–483, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR: Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9: 358–364, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Barua M, Stellacci E, Stella L, Weins A, Genovese G, Muto V, Caputo V, Toka HR, Charoonratana VT, Tartaglia M, Pollak MR: Mutations in PAX2 associate with adult-onset FSGS. J Am Soc Nephrol 25: 1942–1953, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivante A, Chacham OS, Shril S, Schreiber R, Mane SM, Pode-Shakked B, Soliman NA, Koneth I, Schiffer M, Anikster Y, Hildebrandt F: Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol 34: 1607–1613, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI: Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet 17: 384–385, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, van’t Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D: HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20: 1123–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raaijmakers A, Corveleyn A, Devriendt K, van Tienoven TP, Allegaert K, Van Dyck M, van den Heuvel L, Kuypers D, Claes K, Mekahli D, Levtchenko E: Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant 30: 835–842, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Verhave JC, Bech AP, Wetzels JF, Nijenhuis T: Hepatocyte nuclear factor 1β-associated kidney disease: More than renal cysts and diabetes. J Am Soc Nephrol 27: 345–353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarrei M, MacDonald JR, Merico D, Scherer SW: A copy number variation map of the human genome. Nat Rev Genet 16: 172–183, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, González JR, Gratacòs M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME: Global variation in copy number in the human genome. Nature 444: 444–454, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golzio C, Katsanis N: Genetic architecture of reciprocal CNVs. Curr Opin Genet Dev 23: 240–248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG: Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91: 987–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verbitsky M, Sanna-Cherchi S, Fasel DA, Levy B, Kiryluk K, Wuttke M, Abraham AG, Kaskel F, Köttgen A, Warady BA, Furth SL, Wong CS, Gharavi AG: Genomic imbalances in pediatric patients with chronic kidney disease. J Clin Invest 125: 2171–2178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westland R, Verbitsky M, Vukojevic K, Perry BJ, Fasel DA, Zwijnenburg PJ, Bökenkamp A, Gille JJ, Saraga-Babic M, Ghiggeri GM, D’Agati VD, Schreuder MF, Gharavi AG, van Wijk JA, Sanna-Cherchi S: Copy number variation analysis identifies novel CAKUT candidate genes in children with a solitary functioning kidney. Kidney Int 88: 1402–1410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, Yan Z, Mitrotti A, Martino J, Steers NJ, Fasel DA, Vukojevic K, Deng R, Racedo SE, Liu Q, Werth M, Westland R, Vivante A, Makar GS, Bodria M, Sampson MG, Gillies CE, Vega-Warner V, Maiorana M, Petrey DS, Honig B, Lozanovski VJ, Salomon R, Heidet L, Carpentier W, Gaillard D, Carrea A, Gesualdo L, Cusi D, Izzi C, Scolari F, van Wijk JA, Arapovic A, Saraga-Babic M, Saraga M, Kunac N, Samii A, McDonald-McGinn DM, Crowley TB, Zackai EH, Drozdz D, Miklaszewska M, Tkaczyk M, Sikora P, Szczepanska M, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Darlow JM, Puri P, Barton D, Casolari E, Furth SL, Warady BA, Gucev Z, Hakonarson H, Flogelova H, Tasic V, Latos-Bielenska A, Materna-Kiryluk A, Allegri L, Wong CS, Drummond IA, D’Agati V, Imamoto A, Barasch JM, Hildebrandt F, Kiryluk K, Lifton RP, Morrow BE, Jeanpierre C, Papaioannou VE, Ghiggeri GM, Gharavi AG, Katsanis N, Sanna-Cherchi S: Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med 376: 742–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brockschmidt A, Chung B, Weber S, Fischer DC, Kolatsi-Joannou M, Christ L, Heimbach A, Shtiza D, Klaus G, Simonetti GD, Konrad M, Winyard P, Haffner D, Schaefer F, Weber RG: CHD1L: A new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT). Nephrol Dial Transplant 27: 2355–2364, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Haller M, Au J, O’Neill M, Lamb DJ: 16p11.2 transcription factor MAZ is a dosage-sensitive regulator of genitourinary development. Proc Natl Acad Sci U S A 115: E1849–E1858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG: Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46: 1187–1196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Darlow JM, Darlay R, Dobson MG, Stewart A, Charoen P, Southgate J, Baker SC, Xu Y, Hunziker M, Lambert HJ, Green AJ, Santibanez-Koref M, Sayer JA, Goodship THJ, Puri P, Woolf AS, Kenda RB, Barton DE, Cordell HJ: Genome-wide linkage and association study implicates the 10q26 region as a major genetic contributor to primary nonsyndromic vesicoureteric reflux [published correction appears in Sci Rep 8: 459, 2018]. Sci Rep 7: 14595, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Eerde AM, Duran K, van Riel E, de Kovel CG, Koeleman BP, Knoers NV, Renkema KY, van der Horst HJ, Bökenkamp A, van Hagen JM, van den Berg LH, Wolffenbuttel KP, van den Hoek J, Feitz WF, de Jong TP, Giltay JC, Wijmenga C: Genes in the ureteric budding pathway: Association study on vesico-ureteral reflux patients. PLoS One 7: e31327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Kiryluk K: Genome-wide polygenic risk predictors for kidney disease. Nat Rev Nephrol 14: 723–724, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S: Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 50: 1219–1224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Arensbergen J, Pagie L, FitzPatrick VD, de Haas M, Baltissen MP, Comoglio F, van der Weide RH, Teunissen H, Võsa U, Franke L, de Wit E, Vermeulen M, Bussemaker HJ, van Steensel B: High-throughput identification of human SNPs affecting regulatory element activity. Nat Genet 51: 1160–1169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Susztak K: Understanding the epigenetic syntax for the genetic alphabet in the kidney. J Am Soc Nephrol 25: 10–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J, Liu CL, Kim J, Susztak K: Understanding the kidney one cell at a time. Kidney Int 96: 862–870, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li SY, Park J, Guan Y, Chung K, Shrestha R, Palmer MB, Susztak K: DNMT1 in Six2 progenitor cells is essential for transposable element silencing and kidney development. J Am Soc Nephrol 30: 594–609, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohl S, Chen J, Vivante A, Hwang DY, Shril S, Dworschak GC, Van Der Ven A, Sanna-Cherchi S, Bauer SB, Lee RS, Soliman NA, Kehinde EO, Reutter HM, Tasic V, Hildebrandt F: Targeted sequencing of 96 renal developmental microRNAs in 1213 individuals from 980 families with congenital anomalies of the kidney and urinary tract. Nephrol Dial Transplant 31: 1280–1283, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jovanovic I, Zivkovic M, Kostic M, Krstic Z, Djuric T, Kolic I, Alavantic D, Stankovic A: Transcriptome-wide based identification of miRs in congenital anomalies of the kidney and urinary tract (CAKUT) in children: The significant upregulation of tissue miR-144 expression. J Transl Med 14: 193, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dötsch J, Alejandre-Alcazar M, Janoschek R, Nüsken E, Weber LT, Nüsken KD: Perinatal programming of renal function. Curr Opin Pediatr 28: 188–194, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Safi-Stibler S, Gabory A: Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin Cell Dev Biol 97: 172–180, 2020 [DOI] [PubMed] [Google Scholar]
- 68.Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT: Prenatal risk factors for childhood CKD. J Am Soc Nephrol 25: 2105–2111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dart AB, Ruth CA, Sellers EA, Au W, Dean HJ: Maternal diabetes mellitus and congenital anomalies of the kidney and urinary tract (CAKUT) in the child. Am J Kidney Dis 65: 684–691, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Groen In ’t Woud S, Renkema KY, Schreuder MF, Wijers CH, van der Zanden LF, Knoers NV, Feitz WF, Bongers EM, Roeleveld N, van Rooij IA: Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: A case-control study. Birth Defects Res A Clin Mol Teratol 106: 596–603, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Macumber I, Schwartz S, Leca N: Maternal obesity is associated with congenital anomalies of the kidney and urinary tract in offspring. Pediatr Nephrol 32: 635–642, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Schreuder MF, Bueters RR, Huigen MC, Russel FG, Masereeuw R, van den Heuvel LP: Effect of drugs on renal development. Clin J Am Soc Nephrol 6: 212–217, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Rhone ET, Carmody JB, Swanson JR, Charlton JR: Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 27: 1485–1490, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Al Khodor S, Shatat IF: Gut microbiome and kidney disease: A bidirectional relationship. Pediatr Nephrol 32: 921–931, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamdy RF, Pohl HG, Forster CS: Antibiotic prophylaxis prescribing patterns of pediatric urologists for children with vesicoureteral reflux and other congenital anomalies of the kidney and urinary tract. Urology 136: 225–230, 2020 [DOI] [PubMed] [Google Scholar]
- 76.Hsu CN, Lu PC, Hou CY, Tain YL: Blood pressure abnormalities associated with gut microbiota-derived short chain fatty acids in children with congenital anomalies of the kidney and urinary tract. J Clin Med 8: E1090, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss MM, Van der Zwaag B, Jongbloed JD, Vogel MJ, Brüggenwirth HT, Lekanne Deprez RH, Mook O, Ruivenkamp CA, van Slegtenhorst MA, van den Wijngaard A, Waisfisz Q, Nelen MR, van der Stoep N: Best practice guidelines for the use of next-generation sequencing applications in genome diagnostics: A national collaborative study of Dutch genome diagnostic laboratories. Hum Mutat 34: 1313–1321, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Hildebrandt F: Decade in review--Genetics of kidney diseases: Genetic dissection of kidney disorders. Nat Rev Nephrol 11: 635–636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neveling K, Feenstra I, Gilissen C, Hoefsloot LH, Kamsteeg EJ, Mensenkamp AR, Rodenburg RJ, Yntema HG, Spruijt L, Vermeer S, Rinne T, van Gassen KL, Bodmer D, Lugtenberg D, de Reuver R, Buijsman W, Derks RC, Wieskamp N, van den Heuvel B, Ligtenberg MJ, Kremer H, Koolen DA, van de Warrenburg BP, Cremers FP, Marcelis CL, Smeitink JA, Wortmann SB, van Zelst-Stams WA, Veltman JA, Brunner HG, Scheffer H, Nelen MR: A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum Mutat 34: 1721–1726, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Mallett AJ, McCarthy HJ, Ho G, Holman K, Farnsworth E, Patel C, Fletcher JT, Mallawaarachchi A, Quinlan C, Bennetts B, Alexander SI: Massively parallel sequencing and targeted exomes in familial kidney disease can diagnose underlying genetic disorders. Kidney Int 92: 1493–1506, 2017 [DOI] [PubMed] [Google Scholar]
- 81.Snoek R, van Eerde AM, Knoers NVAM: Importance of reliable variant calling and clear phenotyping when reporting on gene panel testing in renal disease. Kidney Int 92: 1325–1327, 2017 [DOI] [PubMed] [Google Scholar]
- 82.Mansilla MA, Sompallae RR, Nishimura CJ, Kwitek AE, Kimble MJ, Freese ME, Campbell CA, Smith RJ, Thomas CP: Targeted broad-based genetic testing by next-generation sequencing informs diagnosis and facilitates management in patients with kidney diseases [published online ahead of print Nov 18, 2019]. Nephrol Dial Transplant doi: 10.1093/ndt/gfz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dillon OJ, Lunke S, Stark Z, Yeung A, Thorne N, Gaff C, White SM, Tan TY, Tan TY; Melbourne Genomics Health Alliance: Exome sequencing has higher diagnostic yield compared to simulated disease-specific panels in children with suspected monogenic disorders. Eur J Hum Genet 26: 644–651, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lawrence L, Sincan M, Markello T, Adams DR, Gill F, Godfrey R, Golas G, Groden C, Landis D, Nehrebecky M, Park G, Soldatos A, Tifft C, Toro C, Wahl C, Wolfe L, Gahl WA, Boerkoel CF: The implications of familial incidental findings from exome sequencing: The NIH Undiagnosed Diseases Program experience. Genet Med 16: 741–750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vrijenhoek T, Middelburg EM, Monroe GR, van Gassen KLI, Geenen JW, Hövels AM, Knoers NV, van Amstel HKP, Frederix GWJ: Whole-exome sequencing in intellectual disability; cost before and after a diagnosis. Eur J Hum Genet 26: 1566–1571, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lata S, Marasa M, Li Y, Fasel DA, Groopman E, Jobanputra V, Rasouly H, Mitrotti A, Westland R, Verbitsky M, Nestor J, Slater LM, D’Agati V, Zaniew M, Materna-Kiryluk A, Lugani F, Caridi G, Rampoldi L, Mattoo A, Newton CA, Rao MK, Radhakrishnan J, Ahn W, Canetta PA, Bomback AS, Appel GB, Antignac C, Markowitz GS, Garcia CK, Kiryluk K, Sanna-Cherchi S, Gharavi AG: Whole-exome sequencing in adults with chronic kidney disease: A pilot study. Ann Intern Med 168: 100–109, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Eerde AM, Krediet CT, Rookmaaker MB, van Reekum FE, Knoers NV, Lely AT: Pre-pregnancy advice in chronic kidney disease: Do not forget genetic counseling. Kidney Int 90: 905–906, 2016 [DOI] [PubMed] [Google Scholar]
- 88.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, Yeung A, Peters H, Mordaunt D, Cowie S, Amor DJ, Savarirayan R, McGillivray G, Downie L, Ekert PG, Theda C, James PA, Yaplito-Lee J, Ryan MM, Leventer RJ, Creed E, Macciocca I, Bell KM, Oshlack A, Sadedin S, Georgeson P, Anderson C, Thorne N, Gaff C, White SM, White SM; Melbourne Genomics Health Alliance: A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med 18: 1090–1096, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Sumathipala D, Strømme P, Gilissen C, Corominas J, Frengen E, Misceo D: TBCK encephaloneuropathy with abnormal lysosomal storage: Use of a structural variant bioinformatics pipeline on whole-genome sequencing data unravels a 20-year-old clinical mystery. Pediatr Neurol 96: 74–75, 2019 [DOI] [PubMed] [Google Scholar]
- 92.Porcu E, Rüeger S, Lepik K, Santoni FA, Reymond A, Kutalik Z; eQTLGen Consortium; BIOS Consortium: Mendelian randomization integrating GWAS and eQTL data reveals genetic determinants of complex and clinical traits. Nat Commun 10: 3300, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deelen P, van Dam S, Herkert JC, Karjalainen JM, Brugge H, Abbott KM, van Diemen CC, van der Zwaag PA, Gerkes EH, Zonneveld-Huijssoon E, Boer-Bergsma JJ, Folkertsma P, Gillett T, van der Velde KJ, Kanninga R, van den Akker PC, Jan SZ, Hoorntje ET, Te Rijdt WP, Vos YJ, Jongbloed JDH, van Ravenswaaij-Arts CMA, Sinke R, Sikkema-Raddatz B, Kerstjens-Frederikse WS, Swertz MA, Franke L: Improving the diagnostic yield of exome-sequencing by predicting gene-phenotype associations using large-scale gene expression analysis. Nat Commun 10: 2837, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nair V, Kretzler M: Decoding the genetic determinants of gene regulation in the kidney. Kidney Int 95: 16–18, 2019 [DOI] [PubMed] [Google Scholar]
- 95.Stephens ZD, Lee SY, Faghri F, Campbell RH, Zhai C, Efron MJ, Iyer R, Schatz MC, Sinha S, Robinson GE: Big data: Astronomical or genomical? PLoS Biol 13: e1002195, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Köhler S, Doelken SC, Mungall CJ, Bauer S, Firth HV, Bailleul-Forestier I, Black GC, Brown DL, Brudno M, Campbell J, FitzPatrick DR, Eppig JT, Jackson AP, Freson K, Girdea M, Helbig I, Hurst JA, Jähn J, Jackson LG, Kelly AM, Ledbetter DH, Mansour S, Martin CL, Moss C, Mumford A, Ouwehand WH, Park SM, Riggs ER, Scott RH, Sisodiya S, Van Vooren S, Wapner RJ, Wilkie AO, Wright CF, Vulto-van Silfhout AT, de Leeuw N, de Vries BB, Washingthon NL, Smith CL, Westerfield M, Schofield P, Ruef BJ, Gkoutos GV, Haendel M, Smedley D, Lewis SE, Robinson PN: The Human Phenotype Ontology project: Linking molecular biology and disease through phenotype data. Nucleic Acids Res 42: D966–D974, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Renkema KY, Knoers NV: Genetics of kidney disease in 2016: Ingenious tactics to unravel complex kidney disease genetics. Nat Rev Nephrol 13: 67–68, 2017 [DOI] [PubMed] [Google Scholar]
- 98.Qiu C, Huang S, Park J, Park Y, Ko YA, Seasock MJ, Bryer JS, Xu XX, Song WC, Palmer M, Hill J, Guarnieri P, Hawkins J, Boustany-Kari CM, Pullen SS, Brown CD, Susztak K: Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat Med 24: 1721–1731, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rasouly HM, Groopman EE, Heyman-Kantor R, Fasel DA, Mitrotti A, Westland R, Bier L, Weng C, Ren Z, Copeland B, Krithivasan P, Chung WK, Sanna-Cherchi S, Goldstein DB, Gharavi AG: The burden of candidate pathogenic variants for kidney and genitourinary disorders emerging from exome sequencing. Ann Intern Med 170: 11–21, 2019 [DOI] [PubMed] [Google Scholar]
- 100.Mulder J, Sharmin S, Chow T, Rodrigues DC, Hildebrandt MR, D’Cruz R, Rogers I, Ellis J, Rosenblum ND: Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids [published online ahead of print Oct 19, 2019]. Pediatr Res doi: 10.1038/s41390-019-0618-y [DOI] [PubMed] [Google Scholar]
- 101.Oud MM, Latour BL, Bakey Z, Letteboer SJ, Lugtenberg D, Wu KM, Cornelissen EAM, Yntema HG, Schmidts M, Roepman R, Bongers EMHF: Cellular ciliary phenotyping indicates pathogenicity of novel variants in IFT140 and confirms a Mainzer-Saldino syndrome diagnosis. Cilia 7: 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haghighi A, Krier JB, Toth-Petroczy A, Cassa CA, Frank NY, Carmichael N, Fieg E, Bjonnes A, Mohanty A, Briere LC, Lincoln S, Lucia S, Gupta VA, Söylemez O, Sutti S, Kooshesh K, Qiu H, Fay CJ, Perroni V, Valerius J, Hanna M, Frank A, Ouahed J, Snapper SB, Pantazi A, Chopra SS, Leshchiner I, Stitziel NO, Feldweg A, Mannstadt M, Loscalzo J, Sweetser DA, Liao E, Stoler JM, Nowak CB, Sanchez-Lara PA, Klein OD, Perry H, Patsopoulos NA, Raychaudhuri S, Goessling W, Green RC, Seidman CE, MacRae CA, Sunyaev SR, Maas RL, Vuzman D; Undiagnosed Diseases Network, Brigham and Women’s Hospital FaceBase Project, Brigham Genomic Medicine (BGM): An integrated clinical program and crowdsourcing strategy for genomic sequencing and Mendelian disease gene discovery. NPJ Genom Med 3: 21, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]