Introduction
A 31-year-old previously healthy woman was admitted with rapidly progressive respiratory failure from acute respiratory distress syndrome related to infection with SARS-COV-2. Over a 48-hour period, she went from requiring 2-L nasal canula oxygen to maximum ventilator support, and she developed septic shock requiring infusion of norepinephrine. On her third day of treatment, she became oliguric, her creatinine rose to 3.0 mg/dl from 0.8 mg/dl on presentation, and she had become hyperkalemic. She was significantly volume overloaded, and there was concern that volume overload contributed to her worsening respiratory status. Nephrology was consulted for management of her AKI.
No form of KRT has been shown to be superior in AKI (1). Local expertise and patient factors typically drive choice. Prolonged intermittent KRT, like sustained low-efficiency dialysis (SLED), has shown similar metabolic control, hemodynamic effects, safety, and outcomes to continuous RRT (CRRT) (2–4). During the COVID-19 pandemic, shortages of CRRT machines and supplies have made SLED an attractive option for hemodynamically unstable patients. SLED utilizes supplies and equipment that are usually available in secondary and tertiary hospitals. However, the nurses to manage the dialysis procedure are also in short supply due to the pandemic. We describe here the specifics of the techniques and implementation of “walkaway SLED.”
Walkaway Sustained Low-Efficiency Dialysis Therapy
This is a joint discipline between the critical care unit and nephrology department (Figure 1A) (5). Dialysis staff members set up the hemodialysis machine, and dialysis nurses initiate the treatment. They ensure that the procedure (machine, lines, etc.) is running well without issues, and then, they hand over the patient and treatment to the intensive care unit (ICU) nurse. During hand over, the ICU nurse is instructed how to adjust the ultrafiltration rate. The dialysis nurse then “walks away” but returns periodically to the bedside. In the meantime, the ICU nurse documents fluid removal and dialysis pressures hourly and is always assessing for alarms, ensuring connections are tight and secured, and assessing the dialysis effluent for a pink or red hue. Throughout the treatment, the dialysis nurse rounds every 2 hours on each patient on SLED and remains in the general area, so the nurse is immediately available for an emergent issue. This cooperation allows for the dialysis nurse to complete four to six “walkaway” SLED treatments simultaneously. The simultaneous SLED treatments should be in the same geographical area but could be done day or night, allowing these machines to be used around the clock.
Figure 1.
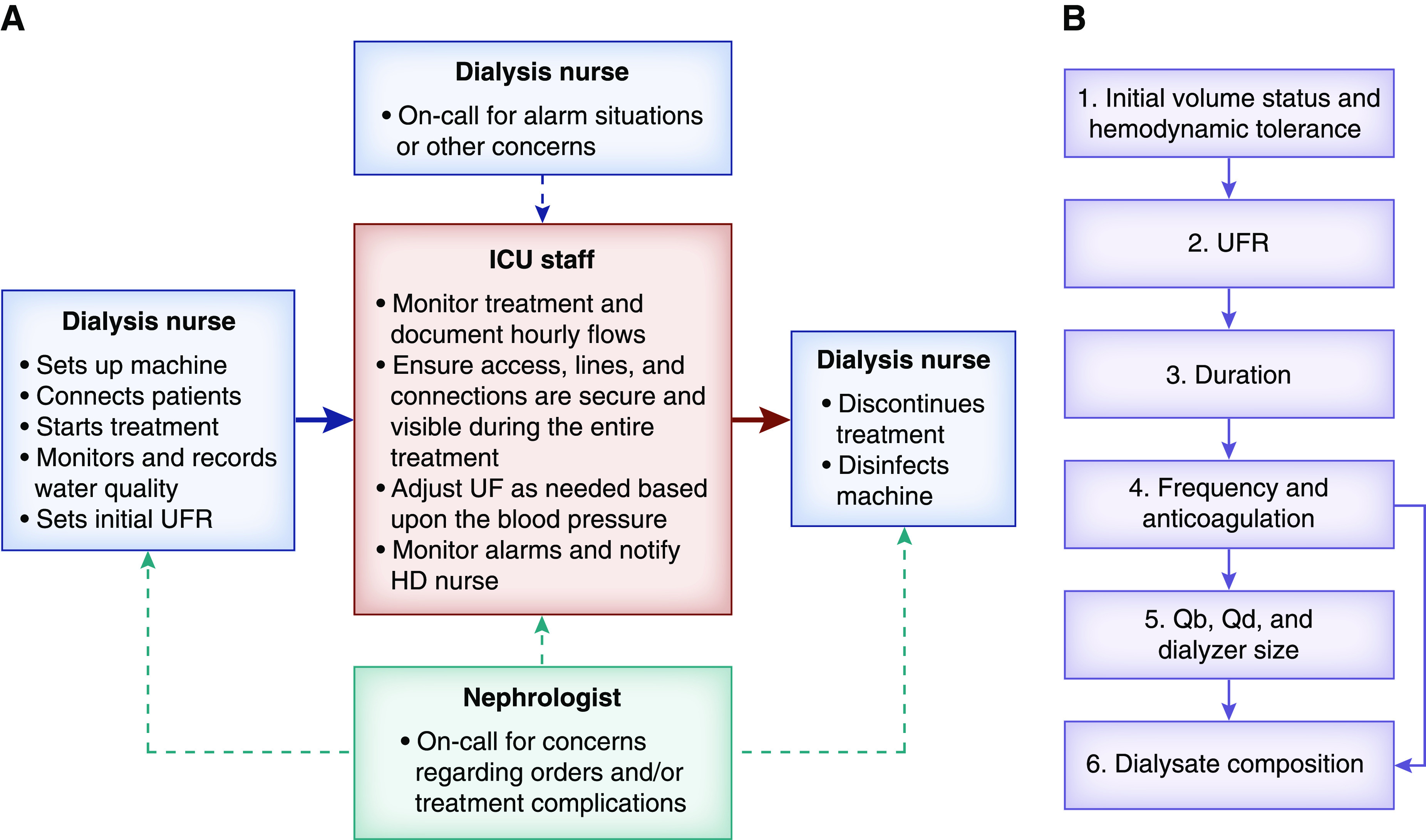
Walkaway sustained low-efficiency dialysis (SLED) implementation and prescription. (A) Flow diagram of the interactive parts of walkaway SLED. (B) Decision-making sequence in walkaway SLED. HD, hemodialysis; ICU, intensive care unit; Qb, blood flow; Qd, dialysate flow; UF, ultrafiltration; UFR, ultrafiltration rate.
Sustained Low-Efficiency Dialysis Prescription
The strategy in SLED is that it is intermittent and prolonged, hence the term prolonged intermittent KRT. A priority decision-making tree for the SLED prescription is shown in Figure 1B. SLED is performed three to seven times weekly for 6–18 hours, but it is usually in the 8- to 10-hour range. The length and frequency of the sessions depend on hemodynamic stability (ultrafiltration rate tolerance), the amount of fluid to be removed, and the tolerance to fluid accumulation in the interdialytic interval.
We set blood flow at 300 ml/min or the maximum the catheter will allow for the theoretical antithrombotic benefit of a higher blood flow causing less stasis within the blood pathway. We set the dialysate flow at 300 ml/min as it is the lowest dialysate flow the Fresenius 2008T and 2008K machines will allow without a retrofit. With this dialysate flow for 6–8 hours, one achieves the necessary small solute clearance typically administered to catabolic patients with AKI. However, if this duration obligates an intolerable ultrafiltration rate, we recommend continuing the ultrafiltration as isolated ultrafiltration as an extension to the SLED procedure. The dialysis nurse performs this conversion. Thus, dialysate is conserved, and the patient’s fluid removal rate is lowered as tolerated to achieve the net fluid removal goal. This is why the duration and frequency of treatments may vary from patient to patient and day to day.
Anticoagulation of the circuit has been critical for successful treatments during COVID-19, due to the prothrombotic nature of the illness (6). Regional citrate anticoagulation or heparin can be used (7,8). In a patient not already on systemic anticoagulation, our protocol uses a heparin bolus followed by a heparin infusion. If the patient is already on a heparin drip, we convert the infusion site for the drip to be in the most proximal site of the extracorporeal circuit. This strategy ensures that the highest concentration of heparin is in the dialyzer and the venous drip chamber, the most frequent sites for circuit thrombosis. Regional citrate can also be used, but we would not recommend starting a new protocol for SLED in your unit in the middle of a worldwide emergency unless you already have considerable experience with regional citrate anticoagulation in CRRT.
Dialysate composition is patient specific. The potassium dialysate concentration may need to be in the 3- to 4-mEq/L range to avoid hypokalemia. It can be difficult to predict potassium removal as well as potassium and acid generation. Checking laboratory values during the procedure and further adjusting dialysate composition may be needed.
Dialysis Access
For walkaway SLED, we use a central venous dialysis catheter. An arteriovenous (AV) fistula or AV graft in a patient with ESKD could be used with appropriate monitoring and a cutaneous blood detector at the venous needle site. There is risk of dislodgement and bleeding or injury to the AV access. Our program has a specific policy for the use of such accesses with CRRT and SLED treatments. However, with the current pandemic and the stresses on the nursing staff, we are not utilizing AV accesses. To do so, we would want a one nurse-one patient ratio. Thus, for our walkaway SLED program, we allow only central venous catheters.
Complications
Hypotension may still occur, even with the slower ultrafiltration rate. Thus, it is important for the ICU nurses to be closely monitoring hemodynamics and to feel confident in adjusting the ultrafiltration rate just like they would for CRRT.
Hypophosphatemia may also occur, similar to what is seen in CRRT. This is more likely with prolonged or more frequent treatments. Phosphate supplementation may be needed. We do not add phosphate to the dialysate in SLED (9).
Alarms
Dialysis alarms are a major source of anxiety for ICU nurses. We have created a nine-page laminated document that stays at the bedside with the dialysis machine and ICU nurses to help remind them of what to do in different situations. The main focus is alerting the dialysis nurse of problems. The sections in the document are titled: (1) low arterial pressure or high venous pressure, (2) any other audible and visual alarm, (3) power failure, (4) patient code, and (5) emergency termination. The rest of the document includes where to find the different flows and pressures on the machine that they need to record every hour to ensure that this information is not lost when the treatment is terminated. We are happy to share this document if requested.
Conclusions
Walkaway SLED represents another tool in the nephrologist’s tool box. When CRRT equipment or supplies are scarce and a hemodynamically unstable patient needs KRT, SLED is a great option. If dialysis nurses are an additional scarcity, walkaway SLED is an attractive option. Collaboration between the ICU and dialysis unit is critical for the success of such a program.
Disclosures
A. Burgner reports consulting for Bayer regarding creating education material on diabetic nephropathy. T. Golper reports receiving personal fees for serving on the advisory boards for Akebia and NxStage and receiving royalties from UpToDate during the conduct of the study. T. Golper also reports being course director for Home Dialysis University and being on the scientific advisory boards for IQVIA.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M; Alberta Kidney Disease Network: Renal replacement therapy in patients with acute renal failure: A systematic review. JAMA 299: 793–805, 2008. 10.1001/jama.299.7.793 [DOI] [PubMed] [Google Scholar]
- 2.Marshall MR, Creamer JM, Foster M, Ma TM, Mann SL, Fiaccadori E, Maggiore U, Richards B, Wilson VL, Williams AB, Rankin AP: Mortality rate comparison after switching from continuous to prolonged intermittent renal replacement for acute kidney injury in three intensive care units from different countries. Nephrol Dial Transplant 26: 2169–2175, 2011. 10.1093/ndt/gfq694 [DOI] [PubMed] [Google Scholar]
- 3.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK: Urea kinetics during sustained low-efficiency dialysis in critically ill patients requiring renal replacement therapy. Am J Kidney Dis 39: 556–570, 2002. 10.1053/ajkd.2002.31406 [DOI] [PubMed] [Google Scholar]
- 4.Fliser D, Kielstein JT: Technology insight: Treatment of renal failure in the intensive care unit with extended dialysis. Nat Clin Pract Nephrol 2: 32–39, 2006. 10.1038/ncpneph0060 [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Baldwin I, Fealy N: Prolonged intermittent renal replacement therapy in the intensive care unit. Crit Care Resusc 4: 281–290, 2002. [PubMed] [Google Scholar]
- 6.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H: Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 191: 148–150, 2020. 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark JA, Schulman G, Golper TA: Safety and efficacy of regional citrate anticoagulation during 8-hour sustained low-efficiency dialysis. Clin J Am Soc Nephrol 3: 736–742, 2008. 10.2215/CJN.03460807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berbece AN, Richardson RM: Sustained low-efficiency dialysis in the ICU: Cost, anticoagulation, and solute removal. Kidney Int 70: 963–968, 2006. 10.1038/sj.ki.5001700 [DOI] [PubMed] [Google Scholar]
- 9.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK: Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int 60: 777–785, 2001. 10.1046/j.1523-1755.2001.060002777.x [DOI] [PubMed] [Google Scholar]


