Keywords: exosomes, IBD, miRNA, RNA-binding protein, 3′-end modification, sorting
Abstract
Exosomes represent secretory membranous vesicles used for the information exchange between cells and organ-to-organ communication. Exosome crosstalk mechanisms are involved in the regulation of several inflammatory bowel disease (IBD)-associated pathophysiological intestinal processes such as barrier function, immune responses, and intestinal flora. Functional biomolecules, mainly noncoding RNAs (ncRNAs), are believed to be transmitted between the mammalian cells via exosomes that likely play important roles in cell-to-cell communication, both locally and systemically. MicroRNAs (miRNAs) encapsulated in exosomes have generated substantial interest because of their critical roles in multiple pathophysiological processes. In addition, exosomal miRNAs are implicated in the gut health. MiRNAs are selectively and actively loaded into the exosomes and then transferred to the target recipient cell where they manipulate cell function through posttranscriptional silencing of target genes. Intriguingly, miRNA profile of exosomes differs from their cellular counterparts suggesting an active sorting and packaging mechanism of exosomal miRNAs. Even more exciting is the involvement of posttranscriptional modifications in the specific loading of miRNAs into exosomes, but the underlying mechanisms of how these modifications direct ncRNA sorting have not been established. This review gives a brief overview of the status of exosomes and exosomal miRNAs in IBD and also discusses potential mechanisms of exosomal miRNA sorting and delivering.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic and recurrent inflammatory disorder, characterized by altered microbiome and exacerbated immune responses within the gastrointestinal (GI) tract of genetically susceptible hosts (17, 48). IBD has become a global emerging disease with more than 6.8 million cases worldwide (9, 38). Currently, the incidence and prevalence of IBD is increasing in both the United States and Europe with more than 3 million people affected, characterized by higher prevalence in females (∼3.9 million) than in males (∼3 million) (9). Despite considerable research, the pathogenesis of IBD remains ill defined. Likely, the host genetic susceptibility, impaired intestinal epithelial barrier function, dysregulated immune response, and environmental factors form the basic tenets of IBD and contribute to multifactorial nature of the disease (10, 35). IBD is mainly classified into two chronic and relapsing-remitting disorders, ulcerative colitis (UC) and Crohn’s disease (CD) (15). The two forms of IBD may share similar symptoms, including protracted diarrhea, abdominal pain, and hematochezia, but have distinct pathological and clinical characteristics (17, 48). UC tends to exhibit diffused mucosal inflammation that extends proximally from the rectum to a varying degree with extensive superficial mucosal ulceration, cryptitis, and microabscesses in the lamina propria and crypts. UC is limited to the colon with intermittent bloody diarrhea as its hallmark symptom (10, 17). Inflammation associated with CD, a debilitating IBD entity, is transmural and patchy involving the entire digestive tract, predominantly terminal ileum or perianal region (11, 48).
Exosomes are phospholipid-enclosed nanovesicles produced by the lyso-endosomal system released by the cells under both normal and pathological conditions (40). Exosomes carry a repertoire of RNAs [including microRNAs (miRNAs), mRNAs, and long noncoding RNAs], DNA, protein, and lipids and possess the exceptional ability to target specific cells or tissues. The complex cargo of exosomes can modulate bioactivities in recipient cells by delivering these functional moieties, especially miRNAs, which fundamentally provide a snapshot of the content and state of the cells (22, 40). MiRNAs are tiny RNA molecules transcribed from intronic, intergenic, or exonic DNA exhibiting no coding potential but mediating gene silencing by targeting 3′-untranslated region (UTR) of reciprocal mRNAs for cleavage or translational repression (12, 70). MiRNAs are one of the key regulators of cellular function and homeostasis, and dysregulated miRNAs are involved in the pathophysiology of several immune diseases, including IBD (37, 39). MiRNAs and mRNAs can traffic among cells via exosomes (termed as exosomal shuttle RNAs) and this genetic-level information exchange is recognized as a novel means of intercellular communication (40). MiRNAs are differentially enriched in exosomes compared with their producer cells and these exosomal miRNAs are crucial regulators of autocrine, paracrine, and endocrine signaling (21, 22). Besides their key role in intercellular communication, exosome-associated miRNAs regulate many pathophysiological processes, including cancer and IBD.
Despite the importance of miRNAs in exosomal cell signaling, only a selective subset of cellular miRNAs are expressed in exosomes whereas others remain within the cells, suggesting that the loading of miRNAs into exosomes is not a random process. Almost all the RNAs inside cells are part of ribonucleoprotein (RNP) complexes making RNA-binding proteins (RBPs) crucial to RNA molecules. Moreover, previous studies have identified associations of RNAs with RBPs outside of cells, pointing to the importance of RBPs in the transport and maintenance of RNA in extracellular space. Several studies have evidenced the participation of various RBPs in shuttling of RNAs between cells via exosomes when cells are required to pack and release these specific RNA species, making RBPs important candidates for the fate and function of exosomal shuttle RNAs (esRNAs) (34, 51, 55, 61). Thus, it appears conceivable that some loading mechanisms could be involved to cargo these RNA species, especially miRNAs into extracellular vesicles, including exosomes. Moreover, the exosome-associated miRNAs reach neighboring as well as distant cells and modify their physiology, thereby regulating diverse cellular processes. Understanding this complex interplay between exosome-associated miRNAs and their target cell sorting is of high relevance to enable targeted and efficient drug delivery to block disease progression and their potential use as a disease biomarker, including IBD. In this review, we endeavor to summarize the current literature on the significance of exosomes and exosomal miRNA transfer in IBD and explore the currently proposed mechanisms controlling the sorting of exosome-enriched miRNAs.
EXOSOMES: BIOGENESIS, COMPOSITION, AND FUNCTION
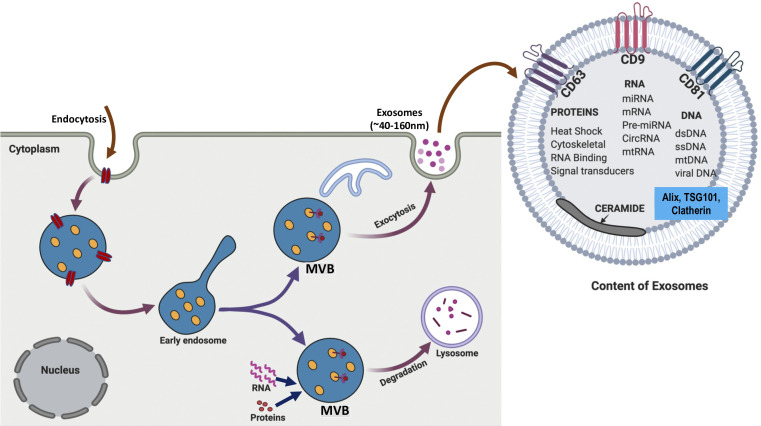
Exosomes are lipid bilayer nanovesicles with an average diameter of ∼100 nm secreted by nearly all mammalian cell types and are present in a large variety of diverse body fluids (59). Exosomes were first discovered by Pan and Johnstone (24) in 1983 while exploring reticulocyte maturation. These nanoscale messengers endowed with potent autocrine and paracrine biological activities are actively secreted by the cells through an exocytosis pathway and are involved in both normal physiology and acquired abnormalities (44, 46). Exosomes, the smallest class of the extracellular vesicles (EVs), are formed within the endosomal system involving its maturation from early to late endosomes (25, 42). First, the inward budding of cell membrane forms the early endosome, which is followed by the invagination of the endosomal membrane giving rise to the late endosome (comprising intraluminal vesicles, ILVs). The intraluminal vesicles (ILVs) identified as multivesicular bodies (MVBs) have two potential fates; they either encounter degradation by fusion with lysosomes or undergo an endocytic process involving fusion with the plasma membrane, releasing exosomes into the extracellular space (25, 42; Fig. 1).
Fig. 1.
Exosome biogenesis and release: exosomes are extracellular vesicles generated de novo by cells through endocytosis process. Exosome formation involves the inward budding of the plasma membrane encompassing bioactive molecules to form early endosomes. Early endosomes then mature to late endosomes that either fuse with lysosomes for breakdown and degradation or fuse back with plasma membranes, releasing nanovesicles called exosomes. Exosomes are composed of functional proteins, messenger RNA (mRNA) and micro RNA (miRNA). They also contain various biomarkers, including TSG101 and tetraspanins like CD63, CD9, and CD81. MVBs, multivesicular bodies.
Exosomal cargo comprises lipids, nucleic acids, and various cytosolic and cell-surface proteins and their composition varies from cell to cell (22, 25, 52). The Exocarta (version 4) database presently lists ∼194 lipids, 1,639 mRNAs, 764 miRNAs, and 4,563 proteins in the exosomes (18). Exosomes carry a specific composition of RNA, especially mRNAs and miRNAs, which can be transferred from donor cells to target recipient cells thereby altering gene expression leading to functional cellular changes (47, 55). Shuttling of exosome-derived unique miRNAs is a gene-based communication between the cells. Some of the miRNAs are more abundant in exosomes compared with cells, implying their unique and selective packaging into exosomes; these exosomal miRNAs reflect the nature and state of its producer cell (14, 25, 52).
EXOSOMES AND EXOSOMAL miRNA IN IBD
Multiple cell-to-cell interactions at the mucosal interface make the intestinal lumen a rich source of IBD-specific EVs. These EVs are absorbed by intestinal epithelial cells (IECs) and macrophages and induce proinflammatory pathways, which, in turn, facilitate local propagation of inflammation (44). Intestinal cells, including IECs, antigen presenting cells (APCs) (especially macrophages and dendritic cells), and neutrophils are potential sources of exosomes at the site of inflammation, whereas their components have significant influence on IBD progression (7, 46, 74). Dendritic cells (DCs), “the conductors of immune orchestra,” are known to produce exosomes in the course of maturation with the potential to elicit immune stimulatory or suppressive responses depending on the maturation state of these cells. DCs are the only APCs capable of activating naive T cells, whereas exosomes derived from mature DCs promote immune responses by releasing various inflammatory mediators, contributing greatly to IBD pathogenesis (7). Exosomes derived from DCs after treatment with IL-10 or Schistosoma japonicum-soluble egg antigens exert protective effects in IBD (7, 72). Also, exosomes derived from macrophages and DCs are key in potentiation of immune response to vaccines and therefore could serve as effective drug-delivery vehicles (8, 46, 74). Recent studies have demonstrated that human mesenchymal stem cells (MSCs)-derived exosomes can ameliorate colitis by alleviating proinflammatory responses and augmenting anti-inflammatory responses (46, 69). IECs are crucial participants in regulating effective communication between intestinal cells through the release of immunologically active exosomes (from apical and basolateral sides), pivotal for intestinal homeostasis (7, 60). IEC-derived exosomes prime and signal adaptive immune cells in the gut by transmitting antigenic information to these cells important for inflammatory/immunogenic response (4, 27, 46). Intestinal epithelial exosomes (IEEs) have been shown to induce antigen-specific tolerance in the gut either directly or through the activation of the functional tolerogenic DCs (indispensable for maintaining intestinal homeostasis) and antigen-specific Tregs under both physiological and pathological conditions (7, 27, 74). IEEs contribute significantly to the pathogenesis of IBD through the release and propagation of proinflammatory mediators, which also reflect severity of the disease (31, 46).
Although exosomal cargo could exert both pro- or anti-inflammatory effects in intestinal tissues, in active IBD, this cargo is enriched with proinflammatory contents (30). Intestinal exosomal miRNAs are intriguing because of their crucial role in IBD pathogenesis as well as in the modulation of intestinal immune system, intestinal barrier functions, and gut-microbiome (46, 74). In IBD, exosomal miRNAs transfer among immune cells such as, DCs, T cells, and macrophages and modulate their functionality, thereby coordinating the immune system in IBD (46, 74). Exosomal miR-155 and miR-146a exchange between DCs and modulate inflammation as well as mediate targeted gene expression in recipient DCs (74). Studies have shown that miR-223 is enriched in exosomes and acts as a double-edged sword by promoting IBD progression through activation of IL-32 inflammatory cascade (62) and by conferring protection against colitis through regulation of differentiation and function of the intestinal CX3C chemokine receptor 1 (CX3CCR1) in macrophages and DCs (76). Also, exosomal miR-223 promotes IBD progression by acting as a modulator of intestinal epithelial barrier through claudin 8 downregulation, an event that breaches the integrity of intestinal tight junctions (62). miR-21 is highly enriched miRNA in exosomes released from different intestinal cells and is regarded as one of the most dysregulated genes in active IBD (2). Studies have shown that the levels of miR-21 are significantly increased in the inflamed colonic mucosa compared with non-inflamed one in the active IBD (in both UC and CD) (2). In 2014, Wu et al. (68) demonstrated that miR-21 deletion reduces dextran sodium sulfate (DSS)-induced colitis but aggravates inflammation in 2,4,6-trinitrobenzenesulfonic acid (TNBS) and the T-cell transfer models of colitis, suggesting the protective and inflammatory roles of miR-21 (46). Another study in 2016 revealed that miR-21 levels significantly decrease upon UC remission compared with the active UC cases in mucosal T cells, indicating its role in transition from between active and recovery phases of the disease (2, 46). Furthermore, we have recently shown that substance P (SP), a neuropeptide/hormone that plays important roles in the pathophysiology of colitis, stimulates the release of exosomes from IECs (56). Importantly, the proliferative and migratory effects of SP-released exosomes on naive colonic epithelial cells is exerted at least in part on miR-21 present in these exosomes (3).
EXOSOMAL-miRNA SORTING AND POSTTRANSCRIPTIONAL MODIFICATION
Exosomes contain substantial amount of RNAs and the transfer of these functional moieties, especially miRNAs from their cell of origin to recipient cell via exosomes is quite selective. Exosomal miRNA content does not match their intracellular profile indicating that a tightly regulated sorting and packaging mechanism governs this process. Some researchers have revealed that the 3′-UTR fragment enrichment in RNAs might have important roles in their specific sorting in exosomes (73). Several studies have shown the involvement of various RBPs as well as the membranous proteins in the selective packaging of miRNAs into the exosomes, but mechanistic details are scarce (58). Almost all the RNAs in the cells are bound to RBPs and these interactions between RBPs and their cognate RNAs are dominant among exosomal-RNAs as well. Heterogeneous nuclear ribonucleoproteins (hnRNPs), the key proteins in cellular nucleic acid metabolism, are a large family of RBPs that assemble to the newly created transcripts in the nucleus of the eukaryotic cell (58). Several pieces of evidence point to the involvement of various hnRNPs in the posttranscriptional processing, biogenesis, transport, and localization of cytoplasmic miRNAs into the exosomes (13). In 2018, Statello et al. (55) established the presence of various hnRNP family proteins in exosomes, including hnRNPA2B1, hnRNPM, hnRNPA1, hnRNPD, hnRNPH1, and hnRNPU.
Heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) gene, through alternate splicing, encodes two isoforms, hnRNPB1 and hnRNPA2, both comprising an RNA-binding domain (66). HnRNPA2B1 is a ubiquitously expressed RBP implicated in numerous biological processes in the normal or the cancerous cells, including multiple roles in RNA processing and trafficking (26, 61). hnRNPA2B1 binds to m(6)A (N6-methyladenosine) marks on m(6)A-bearing miRNAs and modulates their RNA splicing and processing events similar to Methyltransferase Like 3 (METTL3), the m6A “writer” (66). Villaroya-Beltri et al. (61) demonstrated the importance of hnRNPA2B1 in coordinating the sorting of specific repertoire of miRNAs into the exosomes through binding to their EXO-motifs which are over-represented in EXO-miRNAs. In accordance with this study, hnRNPA2B1 gets preferentially sumoylated in exosomes enabling it to recognize and bind specific miRNA-binding motifs, EXO-motifs, thereby exerting its regulatory control over miRNA sorting (16, 61). The GGAG/UGCA motifs of miRNAs-198 and -601 enable their binding with sumoylated hnRNPA2B1 and this attachment localizes the hnRNPA2B1-miR-198 and -miR601 complex into exosomes (16, 61). In addition, hnRNPA2B1 also specifically recognizes AGG/UAG motifs of miRNAs-17 and -93 and exhibits its strong binding through these motifs (16, 32). Other two hnRNP family proteins, hnRNPA1 (a nucleocytoplasmic shuttling protein with many roles in RNA metabolism) and hnRNPC are also implicated in the sorting of specific miRNAs into the exosomes, for example, sumoylated form of hnRNPA1 recognizes the specific RNA sequence allowing its binding to the EXO-miRNA miR-198 (16, 61). Studies have shown another hnRNP family member, hnRNPQ or synaptotagmin-binding cytoplasmic RNA-interaction protein (SYNCRIP), to be strongly and specifically associated with some exosome-enriched miRNAs, such as miR-347a and miR-194-2-3p (51). SYNCRIP is believed to be associated with miR-347a and other EXO-miRNAs through strong binding between its N-terminal unit for RNA recognition (NURR) domain and GGCU sequence of exosome-associated miRNAs suggesting the involvement of SYNCRIP in loading specific miRNAs into the exosomes (51). The other important protein implicated in the binding and sorting of miRNAs into the exosomes is Ago2 protein, a highly specialized RBP and the key effector protein of RNA-induced silencing complex (RISC). Besides its role in transcriptional and posttranscriptional gene silencing, Ago2 protein greatly contributes in maintaining chromosome integrity as well as in the maturation of siRNAs and miRNAs (41). Recent studies have shown that circulating Ago2 complexes serve as a significant carrier of miRNAs suggesting their role in the miRNA stabilization (16). Intriguingly, Ago2 protein has also been implicated in the sorting of miRNAs into exosomes and this process of Ago2 and Ago2-associated miRNAs sorting is regulated by Kristen Rat Sarcoma Virus (KRAS) signaling (41). Another important RBP, Y-Box Binding Protein 1 (YBX-1), is known to be associated with the exosomal miRNA sorting and packaging, including the specific export of miR-133 into exosomes of endothelial progenitor cells under the influence of hyperoxia/reperfusion (34) as well as the packaging of miR-223 into HEK293T-derived exosomes (16). In 2017, Teng et al. (57) demonstrated the role of major vault protein (MVP), a ribonucleoprotein, in the sorting of miR-193a into the exosomes of colon cancer cells promoting cancer invasion and progression (Fig. 2).
Fig. 2.
Potential modes for miRNA sorting into the exosomes. Route I represents the sorting of specific miRNAs (containing EXOmotifs) into exosomes through their binding to hnRNPA2B1. The RNA-binding protein (RBP) hnRNPA2B1 undergoes preferential sumoylation imperative for loading of the miRNAs; e.g., miR-198 and miR-601 with the EXOmotif (GGAG), into the exosomes. The miRNAs with the CLmotif (UGCA) are retained in the cytosol. Route II represents the interaction of another RNA-binding protein SYNCRIP/hnRNPQ with the selected miRNAs and their exosomal sorting. The RNA-binding protein SYNCRIP is a component of hepatocyte-exosomal machinery responsible for sorting of miRNAs. The binding of SYNCRIP to these specific miRNAs is determined by the extra-seed motif/HEXOmotif present in the HEXOmiRNAs. Route III represents Ago-2-dependent sorting of exosomal miRNAs. Ago2 protein together with other assembly proteins (RBPs) bind to the mature miRNA and form a complex called miRISC. The complex includes target miRNA, miRNA-repressible mRNA, GW-182 proteins, and Ago-2 (miRNA effector protein). The GW-182 protein, main component of RISC, is co-localized with multivesicular bodies (MVBs) and therefore may facilitate exosomal sorting of miRNAs (Let-7b, miR-100, miR-720a). A, B, and C represent the exosomes released from the donor cell with specific repertoire of miRNAs and the associated proteins (RBPs). Exosome A contains miRNAs (with EXOmotifs) that are bound to RNA-binding protein hnRNPA2B1. Exosome B contains the RNA-binding protein SYNCRIP/hnRNPQ and associated HEXOmiRNAs (with HEXOmotifs). Exosome C contains miRNAs that are bound to Ago2 protein. Ago2, Argonaute2; SYNCRIP, synaptotagmin-binding cytoplasmic RNA-interaction protein.
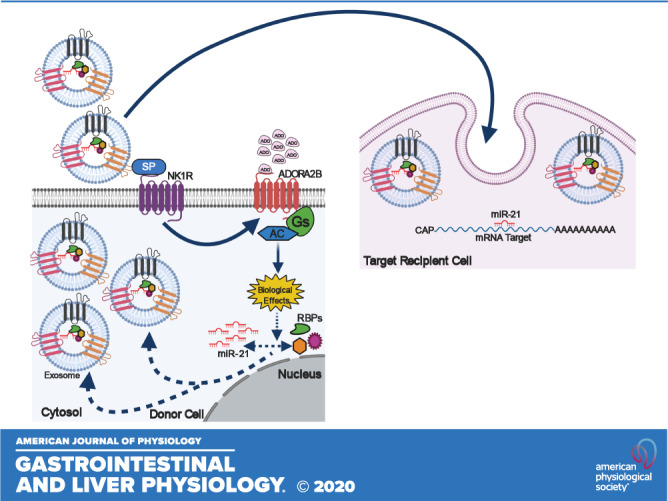
Apart from RBPs, membrane proteins have also been implicated in the sorting of mature miRNAs into the exosomes. One important candidate membrane protein involved in exosomal-miRNA trafficking is caveolin-1, a 21- to 24-kDa protein, which is a principle component of caveolae membranes and a key player in membrane trafficking (16, 32). A recent finding by Lee et al. (32) has established the ability of caveolin-1 to escort hnRNPA2B1- and hnRNPA2B1-associated miRNAs into microvesicles in response to hyperoxia. Another important candidate protein is nSMase2, a hydrolase that produces ceramide at the inner leaflet of plasma membrane and thus regulates myriad cellular activities via ceramide signaling, including exosome biogenesis and regulation of miRNA content in the exosomes (16). Studies have indicated that nSMase2 upregulation or downregulation significantly affects the miRNA number in exosomes, for example, the levels of miR-16 and -146a within the exosome were significantly altered upon changes in nSMNase2 levels (16). Another feature that promotes sorting of miRNAs into exosomes is the 3′-end sequence of candidate miRNA isoforms with possible sorting signal associated with it. Recently, scientists demonstrated that the posttranscriptional modification of miRNAs may exert opposite effects and regulate the distribution of miRNAs between cells and their released exosomes. According to this study, 3′-end adenylation of miRNAs results in their cellular retention whereas 3′-end uridylation facilitates the exosomal incorporation of miRNAs (29). Another study has recently showed that the dominant 3′-end uridylation of miR-2909 in prostate cancer cells enables its exosomal recruitment as compared with the bladder cancer cells (63, 64). Wani et al. demonstrated that the miR-2909 inclusion or exclusion from exosomes could be linked to the differential distribution of adenosine kinase between the cells and the exosomes, which modulates cellular adenosine pool affecting 3′-end adenylation to uridylation ratio of miRNAs. In IBD, the repression of adenosine transporter, equilibrative nucleoside transporter 2 (ENT2), in colonic intestinal epithelium decreases intracellular adenosine levels and enhances extracellular adenosine signaling through the adenosine A2b receptor (Adora2b) (1). Importantly, Ado/Adora2b signaling in colonic epithelial cells (CECs) is pivotal for epithelial barrier function and mucosal healing in IBD (1). Previous findings from our laboratory revealed that the SP\NK1R activation selectively recruits miR-21 into human colonic epithelial exosomes, compared with other miRs expressed only in the cytosol (3). Unpublished evidence from our group also suggested a SP-dependent upregulation of Adora2b in colonic epithelial cells in vivo and in vitro. Taken together, these data reveal that SP/NK1R signaling could regulate intracellular adenosine levels in CECs in IBD through increased extracellular adenosine signaling (Ado/Adora2b) (Fig. 3). This SP-induced decrease in intracellular adenosine could in turn modulate the adenylation/uridylation ratio of SP-induced miRNAs and hence their localization and transport, providing a possible explanation for the selective SP-associated exosomal miRNA cargo in colonic epithelial cells.
Fig. 3.
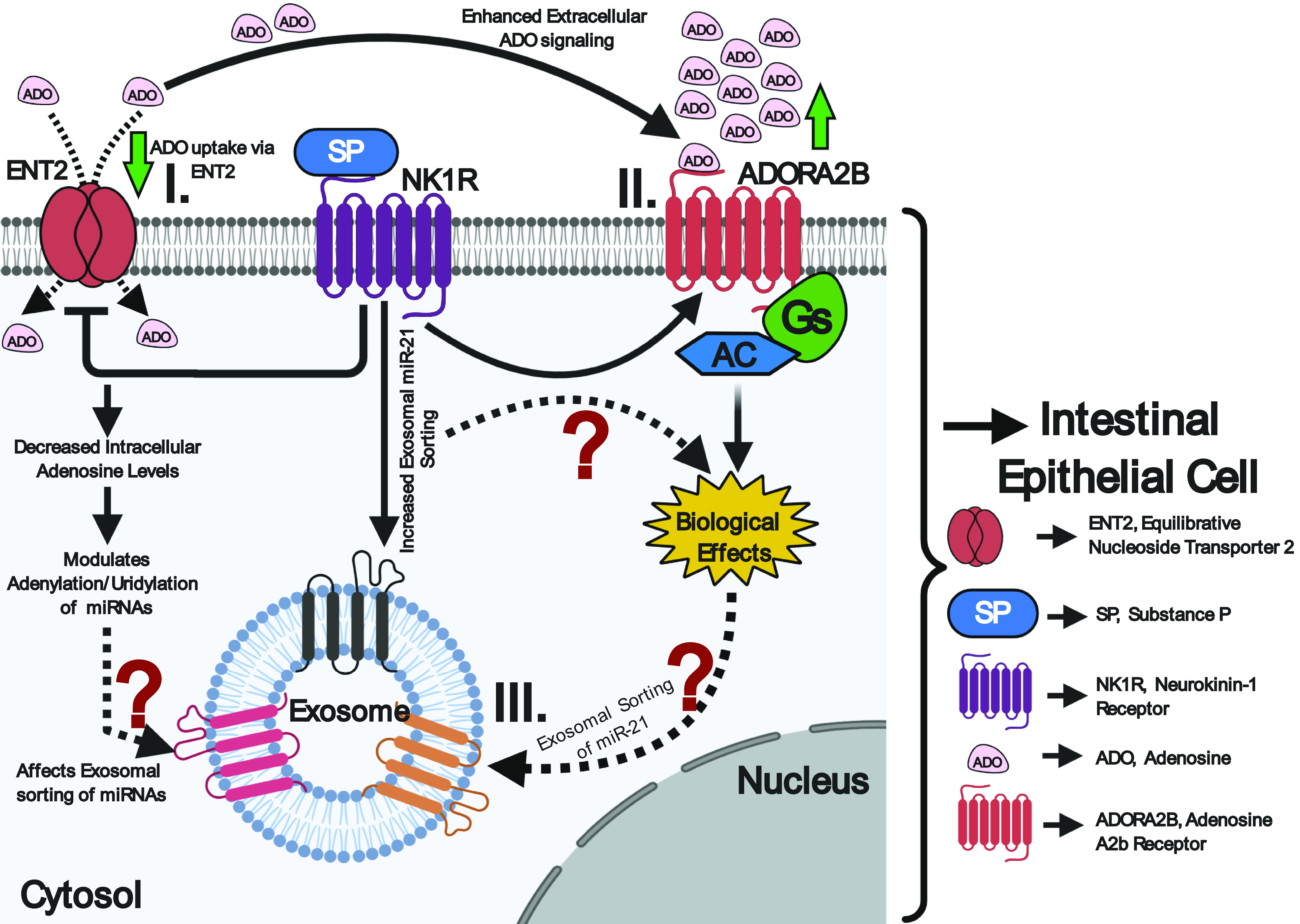
Representative diagram showing the possible effects of SP/NK1R signaling on adenosine uptake in colonic epithelial cells. I: SP/NK1R signaling represses ENT2 in colonic epithelial cells resulting in decreased intracellular adenosine in these cells. Decreased adenosine leads to alterations of adenylation to uridylation ratio, affecting exosomal sorting of micro RNAs (miRNAs). II: the decreased adenosine uptake by ENT2 enhances the extracellular adenosine signaling through adenosine A2b receptor (Adora2b). III: SP\NK1R activation selectively recruits miR-21 into human colonic epithelial exosomes possibly through the involvement of the Ado/Adora2b signaling cascade.
APPLICATIONS
Therapeutic Applications
The intrinsic ability of the exosomes to be loaded with multifarious cargos, particularly miRNAs, which could be used as therapeutic agents for diseases, like cancer and IBD, enables them to serve as excellent delivery vehicle for various therapeutic molecules (5, 50). The presence of multiple adhesion proteins on exosomal membranes as well as their ability to cross the blood-brain barrier opens the possibility that exosomes may be effective for drug- and gene delivery (5, 74). Apart from being optimal candidates as vectors for gene therapy, these nanovesicles can even be engineered to deliver specific miRNA and siRNA or pharmacological molecules for different therapeutic applications in cancer and IBD (28, 50, 58). Exosomes obtained from different sources (including tissues and body fluids) exhibit excellent host biocompatibility and can very well circumvent immunogenicity making them promising therapeutic agents in various diseases, like IEC, or mesenchymal cell-derived exosomes exhibit no immunogenicity and are very well tolerated upon repeated administration in mice during IBD (25, 74). Exosomes released from various cell types in IBD could be exploited as a new therapeutic strategy to dampen inflammation through various mechanisms, including induction and/or amplification of immune responses (T-cell activation, etc.) and increasing the epithelial barrier function. Also, exosomes derived from different cell types in IBD are known to possess cytoprotective and reparative properties identical to their parent cell making them excellent therapeutic agents to treat IBD. Studies have evidenced the protective roles of macrophage and MSC-derived exosomes in limiting DSS-induced colitis in mice by increasing the number of anti-inflammatory mediators (69, 71). MSC-derived exosomes (obtained from bone marrow) show protective effects against IBD by reducing mRNA and protein levels of various proinflammatory mediators, like NF-κB, IL-6, COX2, TNF-α, and IL-1β, whereas increase the levels of protective cytokines, such as IL-10. Studies have shown the miR-146a from MSC-derived exosomes is capable of inhibiting NF-κB pathway activation and limit inflammation. Similarly, DC-derived exosomes under the influence of IL-10 could ameliorate TNBS-induced colitis in mice by promoting Treg expansion (72). Intravenous injection of IEC-derived exosomes from untreated to DSS-treated mice induces immunosuppressive effects through Treg and immunosuppressive cell activation (31). Transforming growth factor β (TGFβ)-containing exosomes from IECs alleviate DSS-induced colitis, which is mediated by the direct binding of TGFβ1 to the epithelial cell adhesion molecules (EpCAM) expressed by epithelial cells of the mice (23). DC-derived exosomes with modified expression of TGF-β1 have therapeutic effects in IBD by inhibiting IBD development through attenuation of Th17 cell capacity (74). Since exosomal miRNAs modulate functions of DCs, T cells, and macrophages, as well as NF-κB-related signaling pathways, which could be targeted to modulate immune dysfunction, exosomal miRNAs serve as a promising new direction for IBD treatment (31).
Diagnostic Applications
Exosomes exist in a variety of body fluids, including blood, plasma, amniotic fluid, breast milk, saliva, and urine (6). Exosomes and exosomal content, especially proteins and miRNAs, have emerged as a new class of biomarkers for early and minimally invasive diagnosis of an array of diseases, such as cancer and IBD (45, 64, 74). In IBD, exosomal miRNAs have proven useful as potential biomarkers because of their role in immunity and the intestinal barrier function. At present, salivary and urinary diagnosis is drawing increasing attention because of its noninvasive nature and ease of collection. For example, miR-2909 in urinary exosomes of patients with prostate cancer has been recognized as a noninvasive and specific biomarker for prostate cancer (64). Reports also highlight the involvement of the oral cavity in IBD, such as the co-occurrence of oral lesions with IBD-associated intestinal damage (49). Salivary exosomes exhibit aberrant expression of various components in many systemic pathologies (19, 43), particularly IBD (31, 75), and therefore serve as important diagnostic tools. For example, salivary exosomal Proteasome 20S Subunit Alpha 7 (PSMA7) has been reported as the most promising IBD biomarker, which exhibits an increased expression in the diseased state (7, 75). In addition, salivary exosomal PMSA7 has higher expression in UC than in CD patients and therefore could be used in differential diagnosis between the two forms of IBD. Studies have reported that the exosomes from colonic lumen of patients with IBD exhibit higher expression of various proinflammatory cytokine mRNAs, and proteins positively correlated with disease severity and hence could greatly contribute to accurate disease diagnosis and monitoring of disease progression (31, 44). In patients with IBD, both the sera- and intestinal epithelial cell-derived exosomes show a marked expression of Annexin A1 (ANXA1) making ANXA1-containing exosomes promising candidates as biomarker for active IBD (33, 46). In 2019, Wu et al. (67) have investigated that exosomes and exosomal miRNAs in bovine milk are implicated in the gut health and could be crucial contributory candidates to IBD pathogenesis. Besides miRNAs and proteins, exosomal long noncoding RNAs (lncRNAs) could also contribute to IBD diagnosis since these lncRNAs are highly deregulated in IBD. One such lncRNA is nuclear paraspeckle assembly transcript 1 (NEAT1), which is highly deregulated in IBD and contributes to intestinal epithelial barrier injury (36, 46). LncRNA NEAT1 is highly expressed in IBD as is evidenced by its elevated levels in the DSS-induced mouse intestinal tissues, serum, as well as exosomes (36). TNF-α-induced HT-29 and NCM460 inflammatory cell models also exhibit a marked increase in the levels of lncRNA NEAT1, suggesting its potential role as a biomarker for the diagnosis and subsequent treatment of IBD (36). Further investigations are required to expand the role of exosomal cargo in IBD for a better understanding of IBD pathogenesis and provide further insights into the potential and efficacy of exosomal-miRNAs-based prevention and treatment options.
FUTURE PROSPECTUS
Cells are selective in sorting their miRNA content into secreted exosomes and therefore a cellular miRNA-sorting and packaging mechanism may exist to deliver miRNAs to the exosomes (54). The discordant enrichment of miRNAs into exosomes indicates their active sorting into these nanovesicles, which involves particular sorting machinery, especially RBPs that recognize specific miRNA motifs and assist miRNA sorting (20). Thus far, several mechanisms have been identified in the selective sorting of exosomal miRNAs, including the involvement of various RBPs, Argonaute2 (Ago2) protein, and membranous proteins involved in EV biogenesis, but the specific details of how these RBPs sort specific miRNAs into exosomes remains unclear (47, 55). Nonetheless, RBPs are pivotal for the regulation of miRNA biogenesis, profiling, and sorting. Studies have shown that the knockout of RBPs, such as Ago2 or other hnRNPs (like Major Vault Protein and Y-Box1, etc.) in multiple cell lines, including DKs-8, HEK 293FT, and CT26 cells affect preferential secretion of miRNAs into exosomes (20, 53, 57). Hence, the miRNA-RBP interactions and RBP-mediated miRNA sorting into exosomes may provide exciting new ways to understand the association of miRNAs with development and progression of the diseases, such as IBD. Most interestingly, recent studies have suggested the posttranscriptional modifications of miRNAs as possible mechanisms governing distribution of subsets of miRNA species between the cells and their secreted exosomes (29). Nevertheless, the differential sorting of miRNAs into exosomes and the functional role of exosomal miRNAs still await adequate elucidation; thus further investigations are necessary to gain a better understanding of the associated molecular mechanisms.
CONCLUSIONS
Exosomes are important mediators of intercellular communication and therefore could be harnessed for both diagnostic and therapeutic purposes. The increased content of exosomes in biological fluids (serum, saliva) of patients with IBD relative to the healthy controls defines the underlying importance of exosomes in IBD diagnosis (65, 74, 75). The role of exosomal miRNAs as ligands has increased the inquisitiveness to explore the molecular mechanisms associated with the molecular cargo sorting in exosomes. Keeping in view the comprehensive role of exosomes and exosomal miRNAs in the progression of IBD, it is imperative to explore the link between the sorting of specific miRNAs into intestinal exosomes and profile of exosomal biomarkers of IBD for diagnosing and monitoring disease activity as well as the potential of miRNA-based therapeutics. Exosomes are able to attenuate IBD through efficient regulation of a number of biological processes, including modulation of immune cells and IEC barrier functions, and therefore could serve as emerging therapeutic options for IBD.
GRANTS
This work was supported by The National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants DK60729, DK47373, and DK110003, CURE: DDRC P30 DK 41301 (to C. Pothoulakis); Career Development Award from the Crohn’s and Colitis Foundation of America, Inc. (to I. K. Man Law), the Blinder Research Foundation for Crohn’s Disease (to C. Pothoulakis), the Eli and Edythe Broad Chair (to C. Pothoulakis), and a Research Grant from the Broad Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.W. conceived and designed research; S.W. prepared figures; S.W. drafted manuscript; S.W., I.K.M.L., and C.P. edited and revised manuscript; S.W. and C.P. approved final version of manuscript.
REFERENCES
- 1.Aherne CM, Collins CB, Rapp CR, Olli KE, Perrenoud L, Jedlicka P, Bowser JL, Mills TW, Karmouty-Quintana H, Blackburn MR, Eltzschig HK. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight 3: e121521, 2018. doi: 10.1172/jci.insight.121521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando Y, Mazzurana L, Forkel M, Okazaki K, Aoi M, Schmidt PT, Mjösberg J, Bresso F. Downregulation of microRNA-21 in colonic CD3+ T cells in UC remission. Inflamm Bowel Dis 22: 2788–2793, 2016. doi: 10.1097/MIB.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 3.Bakirtzi K, Man Law IK, Fang K, Iliopoulos D, Pothoulakis C. MiR-21 in substance P-induced exosomes promotes cell proliferation and migration in human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 317: G802–G810, 2019. doi: 10.1152/ajpgi.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui TM, Mascarenhas LA, Sumagin R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers 6: e1431038, 2018. doi: 10.1080/21688370.2018.1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology 16: 81, 2018. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappello F, Logozzi M, Campanella C, Bavisotto CC, Marcilla A, Properzi F, Fais S. Exosome levels in human body fluids: a tumor marker by themselves? Eur J Pharm Sci 96: 93–98, 2017. doi: 10.1016/j.ejps.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Chang X, Wang SL, Zhao SB, Shi YH, Pan P, Gu L, Yao J, Li ZS, Bai Y. Extracellular vesicles with possible roles in gut intestinal tract homeostasis and IBD. Mediators Inflamm 2020: 1945832, 2020. doi: 10.1155/2020/1945832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther 25: 1665–1675, 2017. doi: 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, , et al. ; GBD 2017; Inflammatory Bowel Disease Collaborators . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5: 17–30, 2020. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 14: 739–749, 2017. doi: 10.1038/nrgastro.2017.110. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc 92: 1088–1103, 2017. doi: 10.1016/j.mayocp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 20: 21–37, 2019. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geuens T, Bouhy D, Timmerman V. The hnRNP family: insights into their role in health and disease. Hum Genet 135: 851–867, 2016. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42: 9195–9208, 2014. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 578: 527–539, 2020. doi: 10.1038/s41586-020-2025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groot M, Lee H. Sorting mechanisms for micrornas into extracellular vesicles and their associated diseases. Cells 9: 1044, 2020. doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Q A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019: 7247238, 2019. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashed MH, Bayraktar E, K Helal G, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci 18: 538, 2017. doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Y, Jia L, Zheng Y, Li W. Salivary exosomes: emerging roles in systemic disease. Int J Biol Sci 14: 633–643, 2018. doi: 10.7150/ijbs.25018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Liu C, Bi ZY, Zhou Q, Zhang H, Li LL, Zhang J, Zhu W, Song YY, Zhang F, Yang HM, Bi YY, He QQ, Tan GJ, Sun CC, Li DJ. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol Cancer 19: 102, 2020. doi: 10.1186/s12943-020-01199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingenito F, Roscigno G, Affinito A, Nuzzo S, Scognamiglio I, Quintavalle C, Condorelli G. The role of exo-miRNAs in cancer: a focus on therapeutic and diagnostic applications. Int J Mol Sci 20: 4687, 2019. doi: 10.3390/ijms20194687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell 177: 428–445.e18, 2019. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol 31: 233–239, 2013. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 25.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 367: eaau6977, 2020. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinge CM, Piell KM, Tooley CS, Rouchka EC. HNRNPA2/B1 is upregulated in endocrine-resistant LCC9 breast cancer cells and alters the miRNA transcriptome when overexpressed in MCF-7 cells. Sci Rep 9: 9430, 2019. doi: 10.1038/s41598-019-45636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima M, Costantini TW, Eliceiri BP, Chan TW, Baird A, Coimbra R. Gut epithelial cell-derived exosomes trigger posttrauma immune dysfunction. J Trauma Acute Care Surg 84: 257–264, 2018. doi: 10.1097/TA.0000000000001748. [DOI] [PubMed] [Google Scholar]
- 28.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed 7: 1525–1541, 2012. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX, Würdinger T, Meijer GA, Pegtel DM. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep 8: 1649–1658, 2014. 10.2147/IJN.S29661 doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Kubiritova Z, Radvanszky J, Gardlik R. Cell-free nucleic acids and their emerging role in the pathogenesis and clinical management of inflammatory bowel disease. Int J Mol Sci 20: 3662, 2019. doi: 10.3390/ijms20153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larabi A, Barnich N, Nguyen HTT. Emerging role of exosomes in diagnosis and treatment of infectious and inflammatory bowel diseases. Cells 9: 1111, 2020. doi: 10.3390/cells9051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med 216: 2202–2220, 2019. doi: 10.1084/jem.20182313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, Argyris I, Rijcken E, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Farokhzad OC, Neish AS, Nusrat A. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 125: 1215–1227, 2015. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin F, Zeng Z, Song Y, Li L, Wu Z, Zhang X, Li Z, Ke X, Hu X. YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT. Stem Cell Res Ther 10: 263, 2019. doi: 10.1186/s13287-019-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, , et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47: 979–986, 2015. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Tang A, Wang X, Chen X, Zhao L, Xiao Z, Shen S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int J Mol Med 42: 2903–2913, 2018. doi: 10.3892/ijmm.2018.3829. [DOI] [PubMed] [Google Scholar]
- 37.Lou C, Li Y. Functional role of microRNA-135a in colitis. J Inflamm (Lond) 15: 7, 2018. doi: 10.1186/s12950-018-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol 35: 380–389, 2020. doi: 10.1111/jgh.14872. [DOI] [PubMed] [Google Scholar]
- 39.Martínez C, Rodiño-Janeiro BK, Lobo B, Stanifer ML, Klaus B, Granzow M, González-Castro AM, Salvo-Romero E, Alonso-Cotoner C, Pigrau M, Roeth R, Rappold G, Huber W, González-Silos R, Lorenzo J, de Torres I, Azpiroz F, Boulant S, Vicario M, Niesler B, Santos J. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 66: 1537–1538, 2017. doi: 10.1136/gutjnl-2016-311477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK signaling controls Ago2 sorting into exosomes. Cell Rep 15: 978–987, 2016. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meldolesi J Exosomes and ectosomes in intercellular communication. Curr Biol 28: R435–R444, 2018. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 43.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis 16: 34–38, 2010. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitsuhashi S, Feldbrügge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis 22: 1587–1595, 2016. doi: 10.1097/MIB.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedaeinia R, Manian M, Jazayeri MH, Ranjbar M, Salehi R, Sharifi M, Mohaghegh F, Goli M, Jahednia SH, Avan A, Ghayour-Mobarhan M. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther 24: 48–56, 2017. doi: 10.1038/cgt.2016.77. [DOI] [PubMed] [Google Scholar]
- 46.Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, Xu W, Mao F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc 95: 1287–1307, 2020. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramanathan S, Shenoda BB, Lin Z, Alexander GM, Huppert A, Sacan A, Ajit SK. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J Extracell Vesicles 8: 1650595, 2019. doi: 10.1080/20013078.2019.1650595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc 94: 155–165, 2019. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowland M, Fleming P, Bourke B. Looking in the mouth for Crohn’s disease. Inflamm Bowel Dis 16: 332–337, 2010. doi: 10.1002/ibd.20983. [DOI] [PubMed] [Google Scholar]
- 50.Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin 39: 501–513, 2018. doi: 10.1038/aps.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A, Tripodi M. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep 17: 799–808, 2016. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 52.Sanz-Rubio D, Martin-Burriel I, Gil A, Cubero P, Forner M, Khalyfa A, Marin JM. Stability of circulating exosomal miRNAs in healthy subjects. Sci Rep 8: 10306, 2018. doi: 10.1038/s41598-018-28748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 5: e19276, 2016. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Reports 8: 1432–1446, 2014. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 55.Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A, Lundqvist C, Lindfors L, Collén A, Sunnerhagen P, Ragusa M, Purrello M, Di Pietro C, Tigue N, Valadi H. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One 13: e0195969, 2018. doi: 10.1371/journal.pone.0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, Deng Z, Kumar A, Zhang L, Merchant ML, Yan J, Miller DM, Zhang HG. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun 8: 14448, 2017. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 59.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 60.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121: 337–349, 2001. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 61.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4: 2980, 2013. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Chao K, Ng SC, Bai AH, Yu Q, Yu J, Li M, Cui Y, Chen M, Hu JF, Zhang S. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol 17: 58, 2016. doi: 10.1186/s13059-016-0901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wani S, Kaul D. Cancer cells govern miR-2909 exosomal recruitment through its 3′-end post-transcriptional modification. Cell Biochem Funct 36: 106–111, 2018. doi: 10.1002/cbf.3323. [DOI] [PubMed] [Google Scholar]
- 64.Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol 259: 135–139, 2017. doi: 10.1016/j.jbiotec.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 65.Wong WY, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP, Tai WC. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics 16: 1131–1145, 2016. doi: 10.1002/pmic.201500174. [DOI] [PubMed] [Google Scholar]
- 66.Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, Ma J. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9: 420, 2018. doi: 10.1038/s41467-017-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu D, Kittana H, Shu J, Kachman SD, Cui J, Ramer-Tait AE, Zempleni J. Dietary depletion of milk exosomes and their microRNA cargos elicits a depletion of miR-200a-3p and elevated intestinal inflammation and chemokine (C-X-C motif) ligand 9 expression in Mdr1a−/− mice. Curr Dev Nutr 3: nzz122, 2019. doi: 10.1093/cdn/nzz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu F, Dong F, Arendovich N, Zhang J, Huang Y, Kwon JH. Divergent influence of microRNA-21 deletion on murine colitis phenotypes. Inflamm Bowel Dis 20: 1972–1985, 2014. doi: 10.1097/MIB.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Qiu W, Xu X, Kang J, Wang J, Wen Y, Tang X, Yan Y, Qian H, Zhang X, Xu W, Mao F. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res 10: 2026–2036, 2018. [PMC free article] [PubMed] [Google Scholar]
- 70.Yang A, Bofill-De Ros X, Shao TJ, Jiang M, Li K, Villanueva P, Dai L, Gu S. 3′ Uridylation confers miRNAs with non-canonical target repertoires. Mol Cell 75: 511–522.e4, 2019. doi: 10.1016/j.molcel.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang R, Liao Y, Wang L, He P, Hu Y, Yuan D, Wu Z, Sun X. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol 10: 2346, 2019. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang X, Meng S, Jiang H, Chen T, Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol 45: 1168–1177, 2010. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 73.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 312: L110–L121, 2017. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D. Exosome-induced regulation in inflammatory bowel disease. Front Immunol 10: 1464, 2019. doi: 10.3389/fimmu.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng X, Chen F, Zhang Q, Liu Y, You P, Sun S, Lin J, Chen N. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell 8: 686–695, 2017. doi: 10.1007/s13238-017-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou H, Xiao J, Wu N, Liu C, Xu J, Liu F, Wu L. MicroRNA-223 regulates the differentiation and function of intestinal dendritic cells and macrophages by targeting C/EBPβ. Cell Reports 13: 1149–1160, 2015. doi: 10.1016/j.celrep.2015.09.073. [DOI] [PubMed] [Google Scholar]