Keywords: abdominal pain, autonomic, enteric nervous system, functional bowel disorders, glia, histamine, intestine, mast cell, neuroimmune
Abstract
Early-life adversity contributes to the development of functional bowel disorders later in life through unresolved mechanisms. Here, we tested the hypothesis that early-life adversity alters anatomical and functional interactions between mast cells and enteric glia. The effects of early-life stress were studied using the neonatal maternal separation (NMS) stress mouse model. Anatomical relationships between mast cells and enteric glia were assessed using immunohistochemistry and mast cell reporter mice (Mcpt5Cre;GCaMP5g-tdT). Immunohistochemistry was used to assess the expression of histamine, histamine 1 (H1) receptors, and glial fibrillary acidic protein. Functional responses of glia to mast cell mediators were assessed in calcium imaging experiments using Sox10CreERT2;GCaMP5g-tdT mice and cultured human enteric glial cells. NMS increases mast cell numbers at the level of the myenteric plexus and their proximity to myenteric ganglia. Myenteric glia respond to mediators released by activated mast cells that are blocked by H1 receptor antagonists in mice and humans and by blocking neuronal activity with tetrodotoxin in mouse tissue. Histamine replicates the effects of mast cell supernatants on enteric glia, and NMS increases histamine production by mast cells. NMS reduces glial responses to mast cell mediators in mouse tissue, while potentiating responses in cultured human enteric glia. NMS increases myenteric glial fibrillary acidic protein expression and reduces glial process length but does not cause neurodegeneration. Histamine receptor expression is not altered by NMS and is localized to neurons in mice, but glia in humans. Early-life stress increases the potential for interactions between enteric glia and mast cells, and histamine is a potential mediator of mast cell-glial interactions through H1 receptors. We propose that glial-mast cell signaling is a mechanism that contributes to enteric neuroplasticity driven by early-life adversity.
NEW & NOTEWORTHY Early-life adversity places an individual at risk for developing functional gastrointestinal disorders later in life through unknown mechanisms. Here, we show that interactions between mast cells and glia are disrupted by early-life stress in mice and that histamine is a potential mediator of mast cell-glial interactions.
INTRODUCTION
Functional bowel disorders are common disturbances of neurogastroenterology that cause debilitating pain and altered intestinal functions (24). The etiology of functional bowel disorders is complex and may involve genetic, environmental, psychosocial, and inflammatory triggers (25, 41). Stress during the early-life critical developmental period is particularly dangerous and is strongly associated with the development of irritable bowel syndrome (IBS) later in life in humans (11, 17, 18) and animals (45, 53, 54, 56). How early-life stress drives IBS pathophysiology remains poorly understood.
Mast cells, hematopoietic-derived innate immune cells, have been implicated as a major immune player in the pathogenesis of functional bowel disorders. Furthermore, intestinal mast cells are persistently increased in number and activation in adults from rodent and porcine models of early-life adversity, thus highlighting a potential interaction between functional bowel disorders, early-life adversity, and mast cells (6, 7, 53). Mast cells contribute to the maintenance of intestinal homeostatic functions, such as host defense, vascular permeability, peristalsis, tissue repair, and nociception (8, 73). However, increased numbers and excessive degranulation and release of mast cell mediators can have a profound impact on gut functions, such as increased sensory nociception, intestinal permeability, and altered motility and secretion, which are thought to underly clinical symptoms of functional bowel diseases such as IBS (3, 4, 20, 33, 52, 57). Mast cells release mediators that include histamine, serotonin, proteases, and neurotrophins that contribute to dysmotility and visceral pain by sensitizing enteric neurons and the terminals of sensory neurons in dorsal root ganglia (2, 4, 16, 48, 72). Mast cell activation and close proximity to enteric nerve fibers in IBS patients correlated with worse symptom severity (22, 51). On the basis of this evidence, it is plausible that increased mast cell activity in IBS could contribute to altered intestinal functions through effects on cells in the enteric nervous system.
The sensitization of enteric neurons by mast cell mediators is well characterized, but whether mast cells exert similar effects on enteric glia remains unknown. Similar to mast cells, enteric glia regulate homeostasis within the enteric nervous system, and alterations in glial functions contribute to the pathophysiology of IBS (21, 36, 46). Enteric glia regulate enteric neural reflexes that coordinate motility and secretions, neuroinflammation, and nociception (13, 30, 42, 43, 69), and changes in glial morphology are associated with neuroplasticity following early-life stress (28). Therefore, it is possible that mast cells drive neuroplasticity, in part, through actions on glial cells.
Here, we tested the hypothesis that the long-lasting effects of early-life adversity include changes to mast cell-glial interactions. We tested our hypothesis using the neonatal maternal separation and early weaning (NMS) stress model, mast cell reporter mice, and functional calcium imaging with genetically encoded indicators in enteric glia. Our results show that mast cell abundance at the level of the myenteric plexus is increased in adult mice that previously experienced NMS, and that these mast cells are closely associated with myenteric glia. Mast cells from NMS mice release a greater amount of mediators, including histamine, that activate Ca2+ responses in mouse and human enteric glia through H1 receptors. However, the mechanisms and location of H1 receptors differ between mice and humans. These data suggest that communication between mast cells and enteric glia is affected by early life stress and could contribute to the increased risk and severity of IBS in individuals with a history of early-life adversity.
MATERIALS AND METHODS
Animals.
All experimental protocols were approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC) in specific pathogen-free conditions in facilities accredited by the Association for Assessment and Accreditation for Laboratory Care (AAALAC) International. Mice were maintained in a temperature-controlled environment (Optimice cage system; Animal Care Systems, Centennial, CO) on a 12:12-h light-dark cycle with access to water and a minimal phytoestrogen diet (Diet Number 2919; Envigo, Indianapolis, IN) ad libitum.
Sox10CreERT2;GCaMP5g-tdT and Mcpt5Cre;GCaMP5g-tdT mice were generated as previously described (44) to express the genetically encoded Ca2+ indicator GCaMP5g in enteric glia and mast cells, respectively. Briefly, Sox10CreERT2 mice (a gift from Dr. Vassilis Pachnis, The Francis Crick Institute, London, UK) and Mcpt5Cre mice [a gift from Dr. Soman Abraham, Duke University and developed by Dr. Axel Roers (59)] were crossed with PC::G5-tdT mice (B6;129S6-Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd/J; Jackson Laboratory, stock number 024477; RRID:IMSR_JAX:024477) to generate Sox10CreERT2+/−;GCaMP5g-tdT+/− and Mcpt5Cre+/−;GCaMP5g-tdT+/− mice (hereafter referred to as Sox10CreERT2;GCaMP5g-tdT and Mcpt5Cre;GCaMP5g-tdT mice, respectively). Mice of both sexes were used for experiments when they reached 8–12 wk of age. Mice were genotyped by Transnetyx (Cordova, TN). Sox10CreERT2;GCaMP5g-tdT mice were fed tamoxifen citrate (400 mg/kg) for 1 wk to induce GCaMP5g-tdTomato (GCaMP5g-tdT) expression and allowed to recover for at least 1 wk before experiments.
Human enteric glial cells.
In vitro studies of human enteric glial cells (hEGCs) were approved by the Institutional Ethics Review Board at The Ohio State University under the Institutional Review Board no. 2012H0231 and no. 2017H0441. hEGCs were prepared from isolated ganglia, as previously described (47, 65) and were derived from five surgical specimens obtained from patients after informed patient consent was obtained. Surgical specimens were obtained from colectomy cases (open or laparoscopic) from distal colon (descending or sigmoid). Individuals ranged in age from 50 to 66 yr and included both male and female subjects.
Neonatal maternal separation and early weaning model of early life stress.
C57BL/6 pregnant nulliparous dams (The Jackson Laboratory, Bar Harbor, ME) were isolated before giving birth. The first day the pups were observed was designated as postnatal day 0 (PD0). At postnatal day (PD1) female and male C57BL/6 wild-type mice pups were randomly assigned to two groups: 1) normally handled (NH), in which mice pups were raised under standard protocols undisturbed and weaned at PD28 or 2) neonatal maternal separation (NMS), in which mice pups were subjected to 3-h periods of neonatal maternal separation on PD1–16 plus early weaning at PD17. Pups in the NH group remained undisturbed with their mother in their home cages during the entire NMS period and received no special handling other than necessary changes to their bedding, water, and food. Pups in the NMS group were separated from their mother for 3 h each day (Zeitgeber + 3–6) and were placed in a separate cage in individual cups containing small amounts of their own bedding. NMS mothers were placed in a separate clean cage with food and water during the separation. After the 3-h separation period, the mother and pups were returned to their original cage and remained untouched until next day of separation. Adult mice were euthanized for experiments at 10 wk of age.
Bone marrow-derived mast cells isolation and generation.
Bone marrow progenitor cells (BMMCs) were harvested from femurs of 10-wk-old female and male C57BL/6 mice and cultured in 182 cm2 tissue vented culture flasks with complete media (cRPMI) containing RPMI 1640 with l-glutamine (Corning, Corning, NY), supplemented with 10% heat-inactivated FBS (Gibco Waltham, MA), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM HEPES, 1 mM nonessential amino acids, and 1 mM sodium pyruvate (Corning, Corning, NY), 5 ng/mL recombinant mouse IL-3, and 5 ng/mL recombinant mouse stem cell factor (SCF) (R&D Systems Minneapolis, MN). Cells were incubated in a humidified 5% CO2 and 95% air at 37°C. The media were changed twice a week to eliminate adherent cells by transferring the cell suspension to a 50-mL conical polypropylene centrifuge tube, and centrifuging for 5 min at 1,500 rpm at 20°C. The culture flasks were changed every time the medium was changed. After 4 wk of culture, <95% of the cells were identified as mast cells, as determined by toluidine blue staining (1%, pH 1), and cell-surface expression of FcεRI and c-Kit was confirmed by flow cytometry. Experiments were carried out within 6–7 wk of culture initiation.
BMMC IgE-DNP stimulation.
BMMCs (2 × 106 cells/mL) were sensitized with 0.8 µg/mL of mouse monoclonal anti-2,4-dinitrophenol (DNP) IgE antibody and later stimulated with 0 and 62 ng/mL of DNP-HSA for 1 h (inactivated and activated BMMCs, respectively). Cell culture supernatants and cell lysates (RIPA buffer lysis) were stored at −80°C for further analysis. Viability of the cells throughout experiments >98% was assessed by trypan blue dye exclusion test in an automated Cell Counter (LUNA, San Mateo, CA). Histamine release levels from activated BMMCs were measured using the Oxford Biomedical Research histamine EIA (cat. no. EA31, Oxford, MI).
Circular muscle myenteric plexus whole mount preparation.
Colons were carefully removed from euthanized mice and immediately placed in ice-cold DMEM/Ham’s F-12 nutrient mixture (DMEM; Gibco, Thermo Fisher Scientific, Waltham, MA) containing 3 µM nicardipine and 1 µM scopolamine. Colons were then transferred to a Sylgard-coated petri dish (Dow-Corning, Midland, MI) filled with chilled DMEM, secured with insect pins, and opened along the mesenteric border. The full thickness of the colon was pinned flat, mucosa facing down, and the serosa and longitudinal muscle were removed by microdissection (see Ref. 26). The resulting live whole mount consists of intact myenteric plexus lying atop circular muscle, submucosal plexus, and mucosa.
Ca2+ imaging.
Live whole mounts of the colonic myenteric plexus were prepared for Ca2+ imaging, as previously described (44). For fluorescence imaging, we used an upright Olympus BX51WI fixed-stage microscope (Olympus, Center Valley, PA) fitted with a ×20 water-immersion objective [XLUMPlanFI N, 1.0 numerical aperture (n.a.)] and a Lambda DG-4 Plus xenon light source (Sutter, Novato, CA). Fluorescence of GCaMP5g was excited by light passed through a 485-nm, 20-nm band-pass filter and detected by reflected light passing through a 515-nm, long-pass filter. tdTomato fluorescence was excited by light passed through a 535-nm, 20-nm band-pass filter and detected by reflected light passing through a 610-nm, 75-nm bandpass emission filter. Glial cells were identified by tdTomato fluorescence in Sox10CreERT2;GCaMP5g-tdT samples. Images of GCaMP5g fluorescence were acquired at a rate of 1 Hz with an Andor Neo sCMOS camera controlled by Metamorph 7.1 software (Molecular Devices, San Jose, CA). Whole mounts were continually superfused with 37°C Krebs buffer consisting of (in mM): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, 8 glucose (pH adjusted to 7.4 with NaOH) with 3 µM nicardipine and 1 µM scopolamine at a flow rate of 2–3 mL/min. BMMC supernatants (1:5), histamine (10 µM), and ADP (100 µM) were diluted in Krebs buffer and bath applied. The voltage-gated sodium channel inhibitor tetrodotoxin (TTX; 1 µM) and the histamine type 1 receptor (H1R) antagonist desloratadine [10 µM, based off previous studies (9, 70)] were diluted in Krebs buffer and applied for 5 min before imaging.
hEGCs were incubated with 2 µM Fluo-4/AM (Molecular Probes, Eugene, OR) in DMEM with no FBS for 30 min at 37°C and replaced with fresh media for an additional 30 min. For fluorescence imaging, we used an upright Nikon Eclipse FN1 microscope (Nikon, Tokyo, Japan) with a ×20 water-immersion objective (Nikon Fluor, 0.50 n.a.). The Fluo-4 signal was detected through a Fura-4 filter cube/dichroic set (excitation filter model ET480/×40 center wavelength at 480-nm with 40-nm bandwidth, T510lpxrxt beamsplitter, and ET535/50-nm emission filter center at 535-nm with 50-nm bandwidth), and images of Fluo-4 fluorescence were acquired at 7 Hz with an ANDOR iXon Ultra 897 EMCCD camera controlled by NIS Elements Advanced Research software (Nikon). Cells were continuously superfused with 36.5°C ± 0.5°C oxygenated Krebs buffer consisting of (in mM): 120 NaCl, 6.0 KCl, 1.2 MgCl2, 1.35 NaH2PO4, 14.4 NaHCO3, 2.5 CaCl2, and 12.7 glucose) at a flow rate of 4.0 mL/min. BMMC supernatants and histamine (1:5 and 10 µM, respectively) were diluted in Krebs buffer and bath applied. Pyrilamine (1 µM) was based off previous studies (4, 14, 72) and diluted in Krebs buffer and applied for 30 min before application of histamine or supernatant. The washout period was at least 30–45 min.
Whole mount immunohistochemistry.
Colons from euthanized NH and NMS mice were collected, fixed overnight in Zamboni’s fixative at 4°C, and processed for immunohistochemistry (IHC), as described previously (31). Antibody details are supplied in Table 1. Briefly, longitudinal muscle myenteric plexus (LMMP) preparations were rinsed three times (10 min each) in PBS containing 0.1% Triton X-100 (PBST) followed by a 45-min incubation in blocking solution [containing 4% normal donkey serum (NDS), 0.4% Triton X-100, and 1% BSA]. Primary antibodies were diluted in blocking solution and were applied overnight at room temperature. LMMPs were rinsed three times with PBS after removal of primary antibodies the following day and secondary antibodies (diluted in blocking solution) were applied for 2 h at room temperature. Finally, LMMPs were rinsed in 0.1 M phosphate buffer and mounted on slides with bicarbonate-buffered glycerol consisting of a 1:3 mixture of 142.8 mM sodium bicarbonate and 56.6 mM carbonate to glycerol. Images were acquired through the ×60 (Plan-Apochromat, 1.42 n.a.) oil-immersion objective of an inverted FluoView FV1000 confocal microscope (Olympus, Center Valley, PA).
Table 1.
Primary and secondary antibodies used in study
| Antibody | Source | Catalog No. | Resource ID | Dilution |
|---|---|---|---|---|
| Primary antibodies | ||||
| Chicken anti-GFAP | Abcam, Cambridge, MA | ab4674 | AB_304558 | 1:1,000 |
| Rabbit anti-Histamine | Millipore Sigma, Burlington, MA | H7403 | AB_260077 | 1:200 |
| Goat anti-H1R | LifeSpan Biosciences, Seattle, WA | LS-B1745 | AB_2092486 | 1:200 |
| Rabbit anti-H1R | Alomone, Israel | AHR-001 | AB_2039915 | 1:200 |
| Biotin mouse anti-HuC/D | Abcam, Cambridge, MA | A21272 | AB_2535822 | 1:200 |
| Rabbit anti-dsRed | Takara, Mountain View, CA | 632496 | AB_10015246 | 1:1,000 |
| Mouse anti-S100β | Millipore Sigma, St. Burlington, MA | S2532 | AB_477499 | 1:200 |
| Goat anti-tdTomato | Sicgen, Portugal | AB8181–200 | AB_2722750 | 1:1,000 |
| Secondary antibodies | ||||
| Streptavidin Dylight 405 | Jackson Laboratories, West Grove, PA | 016–470–084 | AB_2337248 | 1:400 |
| Donkey anti-chicken Alexa Fluor 488 | Jackson Laboratories, West Grove, PA | 703–545–155 | AB_2340375 | 1:400 |
| Donkey anti-goat Alexa Fluor 488 | ThermoFisher, Waltham, MA | A11055 | AB_2534102 | 1:400 |
| Donkey anti-mouse Alexa Fluor 568 | ThermoFisher, Waltham, MA | A10037 | AB_2534013 | 1:400 |
| Donkey anti-rabbit Alexa Fluor 594 | Jackson Laboratories, West Grove, PA | 711–585–152 | AB_2340621 | 1:400 |
GFAP, glial fibrillary acidic protein; H1R, histamine type 1 receptor.
hEGC immunocytochemistry.
hEGCs were seeded onto laminin-coated glass-bottom dishes (MatTek, Ashland, MA) and cultured for 7–10 days. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature and processed for immunocytochemistry (ICC), as previously described (37). Antibody details are supplied in Table 1. Briefly, cells were permeabilized for 1 h in blocking solution (containing 10% NDS and 0.5% Tween 20 in PBS). Primary antibodies were diluted in 1% NDS blocking solution and applied overnight at 4°C. hEGCs were rinsed three times with PBS after removal of primary antibodies the following day and secondary antibodies (diluted in 1% NDS blocking solution) were applied for 2 h at room temperature. Finally, hEGCs were rinsed in PBS and incubated for 5 min with DAPI (NucBlue Fixed Cell ReadyProbes Reagent, Thermo Fisher Scientific) followed by a quick wash with PBS. Fresh PBS was added before images were acquired through the ×20 (Nikon Fluor, 0.5 n.a.) objective of a Nikon Eclipse Ti-S inverted microscope (Nikon).
Chemicals and reagents.
Chemicals and reagents were purchased from Millipore Sigma (Burlington, MA) unless otherwise stated.
Data analysis.
Raw Ca2+ imaging files were analyzed with Metamorph 7.1 software, where regions of interest (ROIs) were drawn around enteric glial cells within a ganglion and the relative fluorescence intensity was measured. Analysis and generation of traces were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA) and NIS Elements Advanced Research software (Nikon). Traces represent the change in fluorescence (ΔF/F) over time (as described by Ref. 61) for glial cells within a single ganglion and hEGCs from a single experiment, where appropriate. N values represent individual glial cells and numbers of experiments in different cultures for Ca2+ imaging experiments (Sox10CreERT2;GCaMP5g-tdT, and hEGCs, respectively).
Cell counts, distance, ganglionic expression, and glial morphology data were analyzed offline using FIJI software (National Institutes of Health, Bethesda, MD). Cell counts were performed using the cell counter plug-in of FIJI software. Mast-cell and histamine+ cell numbers are presented as number per ×20 high-powered field (HPF) calculated by counting the number of tdTomato-immunoreactive mast cells in Mcpt5Cre;GCaMP5g-tdT mice and histamine+ cells in C57BL/6 mice, respectively. Distance to ganglia was measured from center of the mast cell soma to the edge of the nearest ganglion, and zoning distance was based on the width of mast cells. Enteric neuron numbers are presented as ganglionic packing density, which was calculated by tracing the ganglionic area and counting the number of HuC/D-immunoreactive neurons within the defined ganglionic area. The relative ganglionic expression of glial fibrillary acidic protein (GFAP), histamine, and H1R were measured by recording the mean gray values of GFAP, histamine, and H1R within a defined ganglionic area. Glial morphology was characterized by measuring total process length and process thickness, as previously described (21). Cell counts and ganglionic expression data expressed as arbitrary fluorescence units (AFU) were performed on a minimum of 10 ganglia per animal and averaged to obtain a value for that animal. N values represent the number of cells in experiments assessing glial morphology, the number of cell cultures in hEGCs, and the number of animals in other experiments.
Statistical analysis.
Data were analyzed using Prism 6 and are shown as means SE. Data were analyzed by one-way or two-way ANOVA with a Bonferroni post hoc test or Student’s t test, where appropriate. A P value <0.05 was considered significant, and a P value <0.07 but >0.05 was considered a trend.
RESULTS
Mast cell numbers increase at the level of the myenteric plexus following NMS and are in close proximity to enteric glia.
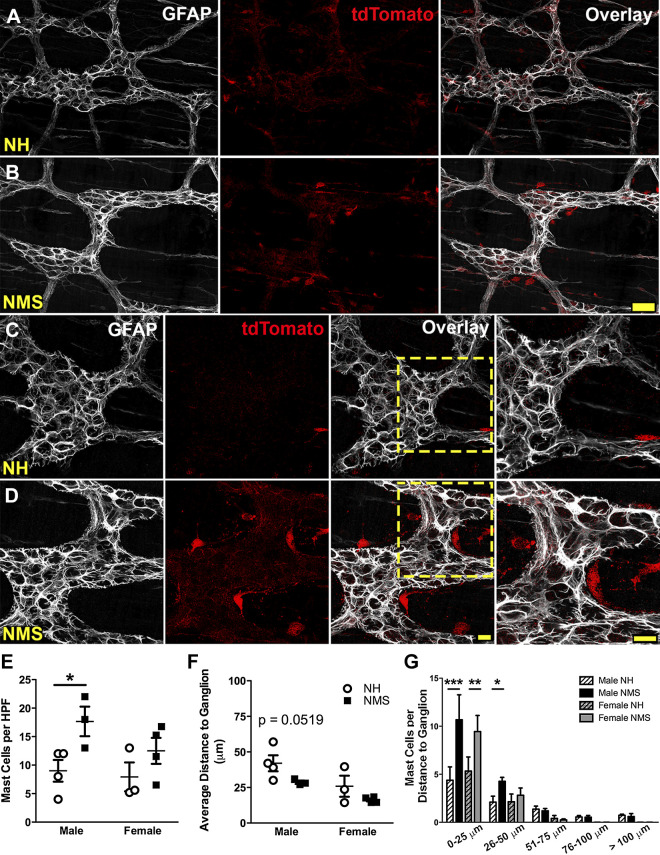
We used Mcpt5Cre;GCaMP5g-tdT mice to genetically tag mast cells as a means to accurately study their association with enteric glia, which were identified by post hoc labeling for glial fibrillary acidic protein (GFAP) (Fig. 1). tdTomato+ mast cells at the level of the myenteric plexus in the colon were predominantly localized near myenteric ganglia with an average of 9.0 ± 1.9 and 7.9 ± 2.5 cells per ×20 optical field in NH male and female controls, respectively (Fig. 1, A, C, and E). Male mice exposed to NMS had a significantly greater (by 97%) number of mast cells (P < 0.05) compared with NH male mice (Fig. 1, B, D, and E). We next assessed the proximity of mast cells to myenteric ganglia by measuring the distance of each mast cell to the closest ganglion. Overall, the average distance of all mast cells to ganglia trended toward a decrease in NMS male mice, but did not reach statistical significance (NH: 42.0 ± 5.6 vs. NMS: 28.7 ± 1.2 µm, P = 0.0519, Fig. 1F). We reasoned that this data set might be skewed by cells that are distant from myenteric ganglia and might not accurately reflect the true proximity of mast cells to ganglia. To test this possibility, we analyzed the number of mast cells in bins of various distances from myenteric ganglia (Fig. 1G). Here, the data clearly show an increase in the number of mast cells less than 25 µm in male and female NMS animals (P < 0.001 and P < 0.01, respectively; Fig. 1G) and within 50 µm to myenteric ganglia in male NMS animals (P < 0.05; Fig. 1G). Together, these data support prior observations suggesting increased mast cells following NMS (5, 6, 53) and extend these observations to show that mast cells are increased in the myenteric plexus. The close apposition between mast cells and enteric neurons and glia in NMS animals (Fig. 1D) suggests an increased potential for interactions.
Fig. 1.
Mast cells are more abundant and are localized close to myenteric ganglia in animals exposed to neonatal maternal separation (NMS). A–D: confocal images of the colonic myenteric plexus in samples from Mcpt5Cre;GCaMP5g-tdT mice showing glial fibrillary acidic protein (GFAP) immunoreactivity (grayscale; A–D) to mark enteric glia and tdTomato immunoreactivity (red, A–D) to mark mast cells. Images show representative fields from male normally handled (NH; A and C) and NMS (B and D) mice. Mast cell numbers and proximity to enteric glia (B and D, overlay and zoom) increase in NMS mice. Scale bars (B and D) = 40 µm and 20 µm and apply to A and B and C and D, respectively. E–G: quantification of mast cells (E), average distance to ganglia (F), and the number of mast cells present at various distances from myenteric ganglia (G) in male and female NH and NMS mice. n = 3 or 4 animals per group, *P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA.
Effects of mast cell mediators on glial Ca2+ responses.
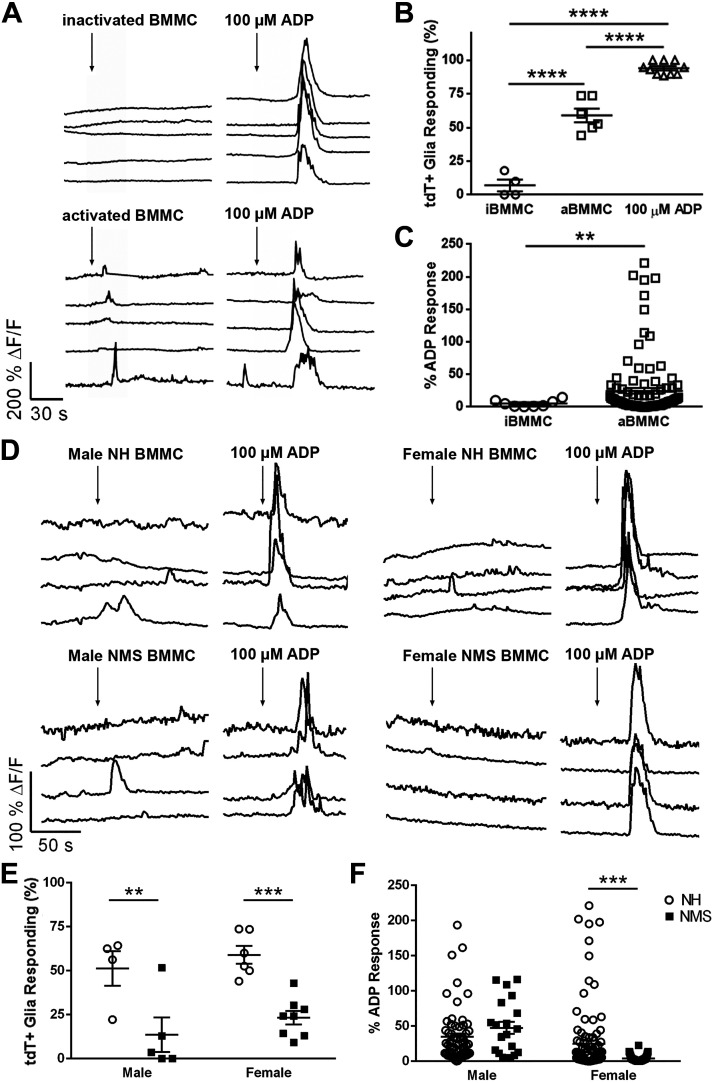
Enteric glia express receptors for many mast cell-derived mediators such as histamine, proteases, serotonin, and growth factors (30, 72). The abundance and close proximity of mast cells to glia following NMS could increase the potential for mast cell mediators to influence glia (Fig. 1). We began testing how mast cell mediators influence glial activity by challenging whole mount preparations of myenteric plexus from Sox10CreERT2;GCaMP5g-tdT mice with supernatants from cultured mast cells and recording glial activity with the genetically encoded Ca2+ indicator GCaMP5g (Fig. 2). We tested inactivated and activated mast cell supernatants from BMMCs and used the P2Y1 receptor agonist ADP as a positive control to drive broad glial activity (13, 43, 44). Glial responses to inactivated mast cell supernatants were rare and only occurred in 7.0 ± 4.6% of cells (Fig. 2, A, top, and B). These responses were minor and were only 5.4 ± 1.8% as large as ADP responses (Fig. 2C). Exposing enteric glia to supernatants derived from activated mast cells evoked Ca2+ responses in more than half (59.0 ± 5.1%) of enteric glia (Fig. 2, A, bottom, and B), and these responses were 24.8 ± 4.5% as large as glial responses to ADP (Fig. 2C). Therefore, activated mast cells derived from normal animals release substances that stimulate enteric glia.
Fig. 2.
Mast cell supernatants evoke Ca2+ responses in myenteric glia. Data show Ca2+ imaging of myenteric glia in whole-mount preparations of colon isolated from Sox10CreERT2;GCaMP5g-tdT mice. A: representative traces showing glial Ca2+ responses as reported by GCaMP fluorescence (black traces) within a myenteric ganglion. Cells were challenged with supernatants obtained from cultures of bone marrow-derived mast cells (BMMC) at rest (inactivated, i) or following activation with DNP (activated, a). ADP (100 µM) served as a positive control. ΔF/F, change in fluorescence. B and C: quantification of the effects of BMMC supernatants on the percentage of glia [tdT-positive cells (tdT+)] responding to iBMMC, aBMMC, and ADP (B) and the percent of ADP response (C). D: representative traces showing glial Ca2+ responses as reported by GCaMP fluorescence (black traces) within a myenteric ganglion. Cells were challenged with activated supernatants obtained from cultures of BMMC from normally handled (NH) and neonatal maternal separation (NMS) male and female mice. ADP (100 µM) served as a positive control. ΔF/F, change in fluorescence. E and F: quantification of the effects of activated BMMC supernatants on the percentage of glia (tdT+ cells) responding to BMMC and ADP (E) and the percent of ADP response (F). Data are representative of recordings in n = 21–115 glial cells from five to eight ganglia from four mice. **P < 0.01, ***P < 0.001, ****P < 0.0001, Student’s t test and one-way and two-way ANOVA.
We next tested whether supernatants from activated BMMCs derived from mice that underwent NMS differ in their ability to stimulate enteric glia (Fig. 2, D–F). In males and females, the percentage of enteric glia responding to supernatants derived from activated mast cells from NMS mice was lower than observed for mast cell supernatants from NH mice (Males: % responding: 51.3 ± 9.8% NH vs. 13.6 ± 9.8% NMS, P < 0.01; Females: % responding: 58.9 ± 5.1% NH vs. 23.2 ± 3.9% NMS, P < 0.001; Fig. 2, D and E). The magnitude of glial responses to activated mast cell supernatants from male NMS mice was unchanged (P > 0.05; Fig. 2F) but was lower for supernatants from activated mast cells derived from female NMS mice (% ADP response: 47.4 ± 8.7 NH vs. 3.8 ± 0.5 NMS, P < 0.001; Fig. 2F). These data show that NMS causes changes in mast cells that affect their production of mediators that ultimately activate enteric glia.
Histamine is a mediator of mast cell-glia communication.
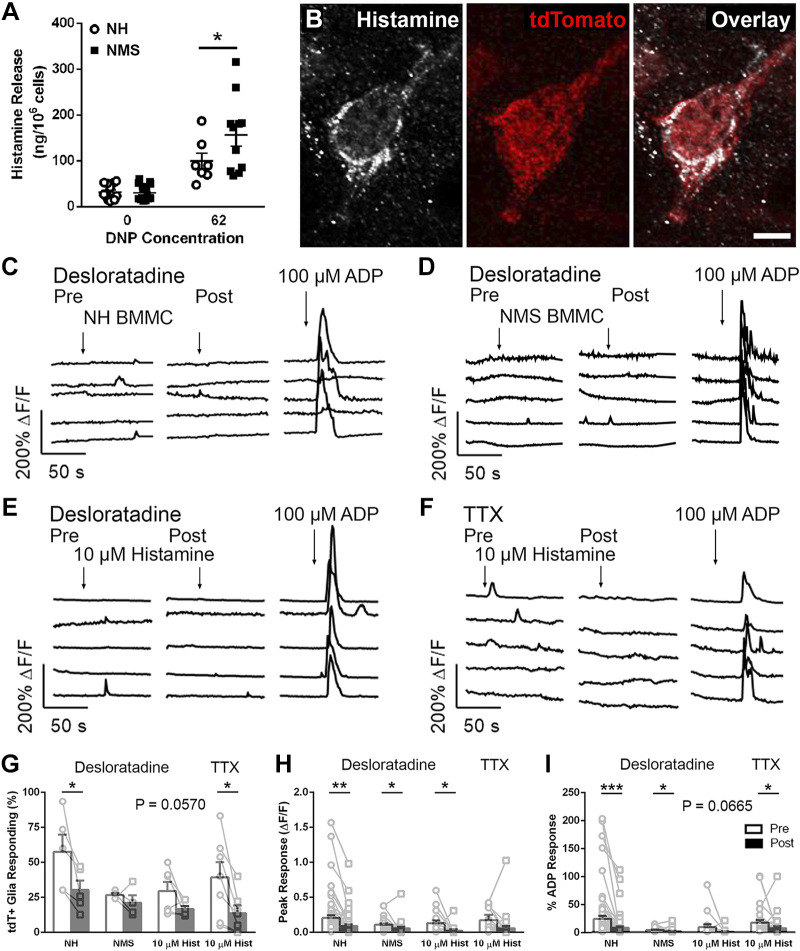
Histamine is a well-known mast cell mediator that contributes to visceral pain by sensitizing sensory nerves (3, 72), and mast cells are increased in IBS (20, 57). Histamine also reduces activity within the enteric nervous system through presynaptic inhibition at cholinergic synapses (40, 62). Given that histamine released during antigenic degranulation of mast cells produces presynaptic inhibition in enteric microcircuits (27), is a mediator that reduces enteric neuron activity in IBS (49) while enhancing activity in dorsal root ganglion (DRG) sensory neurons (4), and both enteric and DRG neurons communicate with enteric glia (21), we hypothesized that an increase in histamine may explain the dichotomous effects of mast cell mediators on glia (Fig. 3). tdTomato+ mast cells at the level of the myenteric plexus displayed histamine immunoreactivity in the colons of Mcpt5Cre;GCaMP5g-tdT mice (Fig. 3B), supporting its potential role in mediating interactions. Cultured BMMCs derived from mice exposed to NMS exhibited a greater ability to release histamine upon sensitization and stimulation with IgE/DNP (ng/106 cells: 99.01 ± 17.82 NH vs. 156.46 ± 24.39 NMS, P < 0.05, Fig. 3A). Conversion of these values to molarity indicates that NMS mast cells produce local concentrations of histamine in the 10–20 µM range, and we chose this concentration for subsequent experiments.
Fig. 3.
Histamine is a mediator of mast cell-glia communication. A: activated bone marrow-derived mast cells (BMMCs) [2×106 cells/mL, stimulated with 0 and 62 ng/mL 2,4-dinitrophenol (DNP)] from neonatal maternal separation (NMS) mice release more histamine compared with activated BMMCs from normally handled (NH) mice. B: confocal images of the colon from Mcpt5Cre;GCaMP5g-tdT mice showing histamine immunoreactivity (grayscale) and tdTomato immunoreactivity (red) to mark mast cells. Scale bar (B, overlay) = 20 µm. C and D: representative traces of Ca2+ responses in myenteric glial cells evoked by exposure to activated BMMC supernatant from NH and NMS mice pre- and post-histamine type 1 receptor (H1R) antagonism with desloratadine (10 µM, left and right, respectively). E: representative traces of Ca2+ responses in myenteric glial cells evoked by exposure to histamine (10 µM) pre- and post-H1R antagonism with desloratadine (10 µM, left and right, respectively). F: representative traces of Ca2+ responses in myenteric glial cells evoked by exposure to histamine (10 µM) pre- and post-TTX (1 µM, left and right, respectively). G–I: quantification of the effects of H1R antagonism on the number of responding cells (G), peak glial Ca2+ responses evoked (H), and glial responses normalized to ADP responses (I). Data are representative of recordings in n = 17–63 glial cells from at least three ganglia from at least three mice and 7–16 BMMC cultures. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test.
On the basis of the increased ability of mast cells to release histamine following NMS, we hypothesized that histamine is a mediator of mast cell-glial interactions. To this end, we tested whether Ca2+ responses in myenteric glia driven by mast cells supernatants are sensitive to histamine type 1 receptor (H1R) antagonism. In support of this, glial Ca2+ responses evoked by activated mast cell supernatants from NH and NMS mice were reduced by the H1R antagonist desloratadine (10 µM; Fig. 3, C and D). Overall, the percentage of cells responding to mast cell supernatants from NH mice decreased following H1R antagonism (% responding: 57.5 ± 12.3 Pre vs. 30.3 ± 6.7 Post, P < 0.05), but the percentage of cells responding to mast cell supernatants from NMS mice was unaffected (Fig. 3G). H1R antagonism decreased maximal glial responses to mast cell supernatants from NH and NMS mice (average peak of responding glial cells: 0.24 ± 0.05 ∆F/F Pre vs. 0.09 ± 0.02 ∆F/F Post and 0.11 ± 0.02 ∆F/F Pre vs. 0.06 ± 0.02 ∆F/F Post, P < 0.01 and P < 0.05, NH and NMS, respectively; Fig. 3H) and glial responses when normalized to maximal responses evoked by ADP (% ADP response: 24.5 ± 5.7 Pre vs. 8.6 ± 2.6 Post and 4.7 ± 0.8 Pre vs. 2.4 ± 0.9 Post, P < 0.001 and P < 0.05, NH and NMS, respectively, Fig. 3I). These data show that glial Ca2+ responses evoked by supernatants from activated mast cells derived from either NH or NMS mice are driven, in part, by histamine.
If histamine is a mediator of mast cell-glia interactions, then histamine should be sufficient to activate enteric glia. We tested whether histamine activates enteric glia by challenging whole mount preparations of myenteric plexus from Sox10CreERT2;GCaMP5g-tdT mice with histamine (10 µM) pre- and post-H1R antagonism (Fig. 3E). Overall, glial responses to histamine were infrequent (29.4 ± 6.6% cells responding), and this observation is consistent with what has been observed in prior work with cultured guinea pig enteric glia [31% (35)]. The percentage of cells responding to histamine trended toward a decrease following H1R antagonism but did not reach statistical significance (% responding: 29.4 ± 6.6 Pre vs. 16.7 ± 2.2 Post, P = 0.0570; Fig. 3G). However, H1R antagonism did decrease maximal glial responses (average peak of responding glial cells: 0.13 ± 0.04 ∆F/F Pre vs. 0.02 ± 0.02 ∆F/F Post, P < 0.05; Fig. 3H). Normalizing these histamine responses to ADP trended toward a decrease following H1R antagonism but did not reach statistical significance (% ADP response: 9.9 ± 5.1 Pre vs. 1.5 ± 1.2 Post, P = 0.0665; Fig. 3I). These results show that histamine produces glial activity that is similar to that evoked by supernatants from NMS mast cells.
H1Rs are expressed by sensory neurons innervating myenteric ganglia (72) that communicate with enteric glia to drive neuroinflammation (21). We tested whether glial responses to histamine required the activation of neuronal H1Rs by repeating the histamine experiments in the presence of TTX (1 µM, Fig. 3F). Overall, TTX reduced the percentage of enteric glial cells responding to histamine (% responding: 39.3 ± 10.9 Pre vs. 13.7 ± 5.4 Post, P < 0.05; Fig. 3G). TTX did not decrease the maximal glial responses to histamine (average peak of responding glial cells: 0.17 ± 0.08 ∆F/F Pre vs. 0.06 ± 0.03 ∆F/F Post, P > 0.05; Fig. 3H). However, TTX did decrease glial responses to histamine when normalized to ADP responses (% ADP response: 17.8 ± 4.7 Pre vs. 8.0 ± 3.6 Post, P < 0.05; Fig. 3I). On the basis of these data, histamine appears to evoke glial responses through direct mechanisms and through indirect mechanisms that involve communication between sensory nerves and enteric glia.
Effects of NMS on enteric neuron survival, glial reactivity, and histamine signaling proteins.
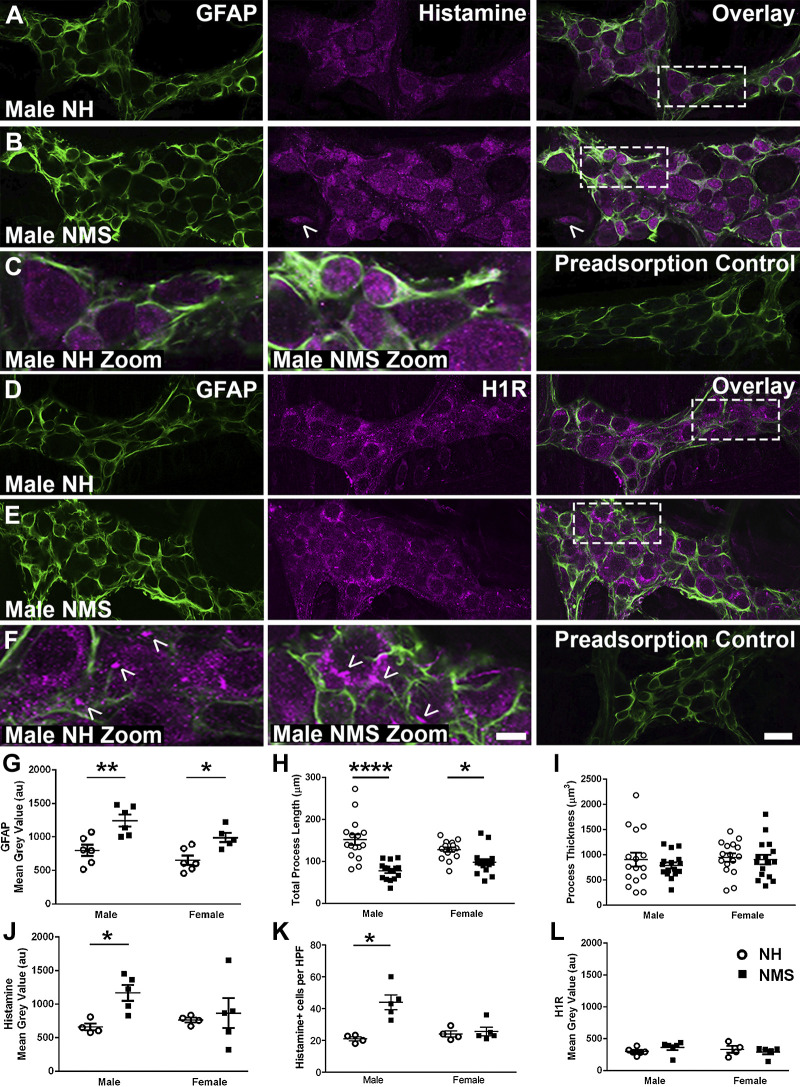
Neuroplasticity driven by neuroinflammation contributes to the pathophysiology of IBS and involves reactive gliosis and enteric neuron death (21, 67). These effects are driven by communication between sensory nerves and enteric glia (21), and our data above suggest that similar mechanisms may contribute to the effects of mast cells on the ENS following NMS. We assessed whether mice that underwent NMS showed lasting signs of neuroinflammation, as quantified by assessing GFAP expression and glial morphology as measures of glial reactivity, and by assessing enteric neuron survival by quantifying neuron numbers with HuC/D labeling (Fig. 4). Male and female mice that underwent NMS exhibited comparable neuron density to control NH mice (P > 0.05, data not shown). However, ganglionic GFAP protein expression, a marker of glial reactivity, was increased in both male and female mice exposed to NMS (Fig. 4, A–F, and G). Likewise, glial process length was decreased in both male and female mice exposed to NMS while glial process thickness was unchanged (Fig. 4, H and I). These observations are consistent with prior work, suggesting reactive gliosis in the rat NMS model (28, 64) and show that early-life adversity drives a glial phenotype consistent with reactive gliosis in mice without causing overt neurodegeneration.
Fig. 4.
Neonatal maternal separation (NMS) alters glial fibrillary acidic protein (GFAP) expression, glial process length, and histamine content in the mouse colon myenteric plexus. A–F: confocal images showing GFAP immunoreactivity (green, A–F), histamine immunoreactivity (magenta, A–C), and histamine type1 receptor (H1R) immunoreactivity (magenta, D–F) in the myenteric plexus of male normally handled (NH) and NMS mice (A–F). GFAP immunoreactivity (green, A–F) and histamine immunoreactivity, primarily glial (magenta, A–C), increase in male NMS mice. Note histamine+ cell outside myenteric ganglion of male NMS mice (B). H1R immunoreactivity is primarily localized to neuron cell bodies and neuronal varicosities (D–F, arrowheads in zoom). Scale bars (F, ×60 and zoom) = 20 µm and 5 µm and applies to A–F (×60 and zoom, respectively). G–L: quantification of GFAP (G), process length (H), process thickness (I), histamine (J), histamine+ cells per high-powered field (HPF) (K), and H1R (L) mean gray values in male and female NH and NMS mice. n = 16 glia and n = 4–6 animals per group, *P < 0.05, **P < 0.01, ****P < 0.0001, two-way ANOVA.
Given that our data suggested that histamine could contribute to the effects of mast cell mediators on glia, we quantified ganglionic histamine and H1R expression to determine whether alterations in histamine stores or response elements could contribute to neuroplasticity following NMS. Ganglionic histamine immunoreactivity was primarily localized to enteric glia and a subset of enteric neurons in NH mice (Fig. 4, A–C). Labeling was lost when the primary antibody was preadsorbed with an excess of 5.4 mM histamine (50), confirming the specificity of this antibody for histamine (Fig. 4C). Male mice exposed to NMS exhibited a marked increase in ganglionic histamine immunoreactivity in myenteric glia and a subset of enteric neurons [arbitrary fluorescence units (AFU): 661.78 ± 51.88 male NH vs. 1168.97 ± 117.94 male NMS, P < 0.05, Fig. 4, A–C, and J], but histamine immunoreactivity was not changed in female mice exposed to NMS (P > 0.05; Fig. 4J). Histamine+ cell number outside the myenteric plexus increased in male mice exposed to NMS (histamine+ cells per HPF: 21.00 ± 1.23 male NH vs. 43.93 ± 4.61 male NMS, P < 0.05; Fig. 4K), while histamine+ cell number was not changed in female mice exposed to NMS (P > 0.05; Fig. 4K). Ganglionic H1R immunoreactivity was primarily localized to neuronal cell bodies and varicosities (Fig. 4, D–F). However, punctate labeling was also observed in GFAP+ glial processes, so it is possible that a subset of glia express H1Rs. H1R labeling was lost when the primary antibody was preadsorbed with 5× molar excess of control peptide (Fig. 4F). H1R expression was not changed in male or female NMS mice (P > 0.05, Fig. 4L). These data suggest that reactive gliosis and sex-specific alterations in histamine handling by glia could contribute to the pathophysiology of NMS.
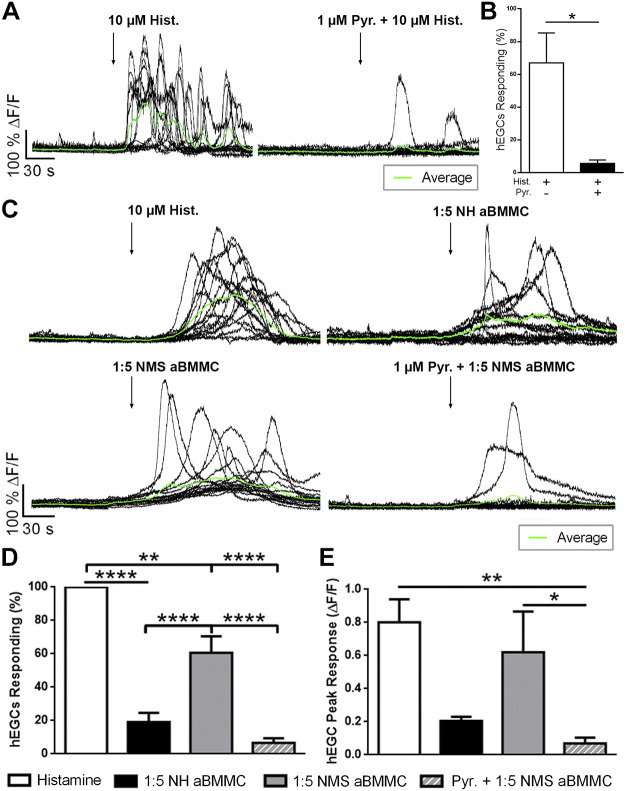
Histamine and mast cell supernatants evoke Ca2+ responses in hEGCs through histamine type 1 receptors.
Our data show that mast cells release mediators that influence the activity of enteric glia in mice. To determine whether these results are translatable to humans, we tested the effects of histamine and mast cell supernatants on hEGC activity using Ca2+ imaging (Fig. 5). Histamine induced robust Ca2+ responses in cultured hEGCs, and these responses were blocked by H1R antagonism with pyrilamine (1 µM, P < 0.05; Fig. 5, A and B). Next, we determined whether mast cell supernatants induce Ca2+ responses in hEGCs (Fig. 5, C–E). Supernatants from activated mast cells isolated from NMS mice elicited activity in a significantly greater population of glia than supernatants from NH mice (% cells responding: 20% NH vs. 60% NMS, P < 0.05, Fig. 5, C and D). H1R antagonism blocked the majority of responses evoked by NMS-activated supernatants (% responding cells: 60.7% Pre vs. 6.6% Post, P < 0.0001, Fig. 5D) and by NH-activated supernatants in hEGCs from a separate culture (% responding cells: 44.9 ± 5.79% Pre vs. 16.2 ± 2.41% Post; P < 0.001). Peak response of the responding hEGCs were used as a measurement of the magnitude of glial Ca2+ responses. Glial Ca2+ responses evoked by activated mast cell supernatants from NMS mice were similar in magnitude to those evoked by histamine (average peak response: 0.80 ± 0.13 ∆F/F histamine vs. 0.62 ± 0.24 ∆F/F NMS aBMMC, P > 0.05, Fig. 5E). H1R antagonism reduced the peak response in hEGCs evoked by NMS-activated supernatants (average peak response: 0.62 ± 0.24 ∆F/F Pre vs. 0.07 ± 0.03 ∆F/F Post, P < 0.05, Fig. 5E) and by NH-activated supernatants in hEGCs from a separate culture (average peak response: 0.78 ± 0.08 ∆F/F Pre vs. 0.35 ± 0.04 ∆F/F Post, P < 0.05). Together, these data show that human enteric glia have the capacity to detect and respond to histamine through H1R pathways and that NMS mast cells stimulate greater glial activity through this mechanism.
Fig. 5.
Histamine and mast cell supernatants induce Ca2+ responses in human enteric glial cells (hEGCs) through histamine type 1 receptors. A: representative traces showing Ca2+ responses in hEGCs (individual cells and experimental average traces in black and green, respectively) evoked by histamine (10 µM) and the effects of histamine type1 receptor (H1R) antagonism on those responses (pyrilamine, 1 µM). ΔF/F, change in fluorescence. B: quantification of the effect of H1R antagonism on the percentage of hEGCs responding to histamine. C: representative traces showing Ca2+ responses in hEGCs evoked by histamine, activated mast cell (BMMC) supernatants from normally handled (NH) and neonatal maternal separation (NMS) mice, and the effects of H1R antagonism in glia responsive to exogenous histamine. D and E: quantification of the percentage of hEGCs responding (D) and their peak responses (ΔF/F; E). Data are representative of recordings in 260–301 hEGCs from n = 5–14 experiments. *P < 0.05, **P < 0.01, ****P < 0.0001 Student’s t test and one-way ANOVA.
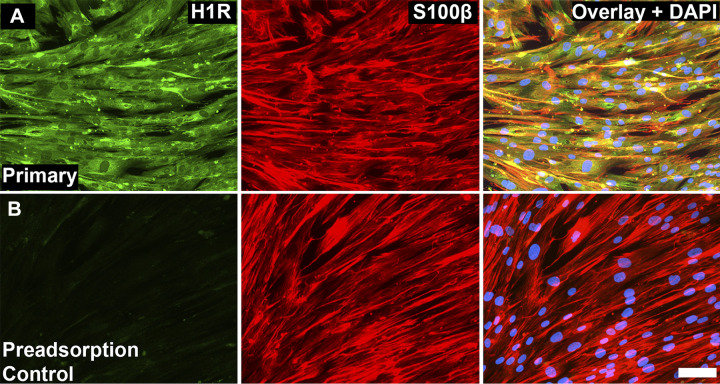
Human enteric glia display histamine type 1 receptor immunoreactivity.
Our data show that H1R expression in the mouse colon is mainly localized to neurons and possibly a subset of enteric glia. However, human enteric glia display more robust activity in response to histamine and mast cell mediators that is sensitive to H1R antagonism. These observations would suggest that human enteric glia display more widespread H1R expression than their mouse or guinea pig counterparts. We tested this concept by assessing H1R expression in human enteric glia (Fig. 6). S100β-immunoreactive hEGCs displayed robust H1R immunoreactivity, and labeling was lost when the primary antibody was preadsorbed with 5× molar excess of control peptide (Fig. 6, A and B, respectively). These data support the conclusion that human enteric glia display widespread H1R expression, which allows them to directly respond to histamine in humans.
Fig. 6.
Histamine type 1 receptors (H1R) localize to human enteric glial cells (hEGCs). A and B: representative epifluorescence images of H1R immunoreactivity (green), S100β immunoreactivity (red), and DAPI staining (blue) in cultured hEGCs. Scale bar (B; overlay) = 100 µm and applies to A and B. n = 3 cell cultures.
DISCUSSION
Early-life adversity is a major risk factor for the subsequent development of functional gastrointestinal disorders such as IBS in humans (11, 17, 18). Likewise, early-life stress in animals produces features of functional gastrointestinal disorders, including dysmotility and visceral hypersensitivity (45, 53, 54, 56). Although the underlying mechanisms are unresolved, mast cell activation and changes in glial activity have been separately linked to early-life adversity and IBS. Here, we show that these processes are linked and that increased communication between mast cells and glia in the mouse colon may contribute to the heightened pathophysiology of functional GI diseases in individuals with a history of early-life adversity. This conclusion is supported by prior work that showed perturbing glial metabolism with the drug fluorocitrate corrects heightened intestinal motor responses in the rat NMS model (28). Our data show that mast cells are more abundant at the level of the myenteric plexus following NMS and are in close apposition to enteric glia. These mast cells have an enhanced ability to release mediators, including histamine, which we identify as a mediator of mast cell-glial interactions. The ability of histamine and mediators released by activated mast cells to drive Ca2+ responses in both mouse and human enteric glia suggests that this mechanism is translationally relevant, although the specific mechanisms vary depending on species.
Enteric mast cells regulate innate immunity during general inflammatory responses (71), and an increase in mast cells is considered an important factor in the pathophysiology of diarrhea-predominant IBS (IBS-D) (3, 51, 57, 66). Increased mast cell numbers and degranulation alter intestinal homeostatic functions in IBS (3, 4, 20, 33, 52, 57) and have a major influence on neuronal activity. However, conflicting data in human cohorts suggest varying roles of mast cells in IBS (57, 60). Mast cells are localized close to enteric and primary afferent nerve fibers in the intestine (19), and mast cell mediators contribute to the sensitization of enteric and sensory afferent nerve fibers in IBS (4, 7, 14, 16, 55, 72). Similarly, chronic stress in adult rats produces persistent visceral hypersensitivity through mechanisms that involve an increase in mast cells (10). Our observations show that similar mechanisms contribute to neuroimmune disruption in animals that experience early-life stress. In this study, we used Mcpt5Cre;GCaMP5g-tdT mice to genetically tag mast cells and observed an increase in mast cell numbers at the level of the myenteric plexus following NMS. Similarly, we observed an increase in histamine+ cells surrounding myenteric ganglia in male mice exposed to NMS. Importantly, mast cells in mice that experienced early life stress are closely associated with enteric glia and produce more histamine than mast cells from control animals. Given that enteric glia surround and interact with nociceptive nerve fibers (21), these interactions could impact visceral sensitivity. Together, these observations support an increased potential for interaction between mast cells and enteric glia, which may be a mechanistic link between early-life adversity and increased risk and severity of functional bowel diseases.
One mechanism by which enteric glia could impact visceral sensitivity is by regulating the availability of histamine surrounding enteric neurons. Interestingly, ganglionic histamine immunoreactivity appeared to be concentrated in myenteric glia and increases in male animals following NMS. Histamine also appeared to increase in a subset of enteric neurons following NMS and may be due to histamine accumulation following excitatory or inhibitory signaling effects (7, 14, 55). Similar populations of neuroglia such as astrocytes regulate histamine availability in the nervous system and degrade histamine through mechanisms that involve the enzyme histamine N-methyltransferase (HNMT) (74). HNMT expression is altered in the mucosa of the small intestine in individuals with IBS-D, suggesting that changes in histamine degradation could contribute to IBS. Interestingly, the difference in glial histamine content was only observed in male mice. Sex is an important biological factor that influences susceptibility to early-life stress and IBS (38, 39, 52, 53). Sex differences in mast cells, themselves, also occur, and female mast cells possessed higher concentrations of granule mediators, including histamine (39). Thus, sex-dependent differences in glial histamine handling and mast cell histamine secretion could influence IBS pathophysiology.
Excitatory effects of mast cell mediators have been observed in enteric neurons (7, 14, 55) and DRG sensory neurons (4, 16, 72). However, the main effects of histamine on enteric and DRG neurons differ. Histamine can promote long-lasting excitation of certain enteric neurons through cell body effects, but primarily acts to suppress synaptic transmission through actions at presynaptic terminals of nicotinic synapses (40, 62). Histamine released during antigenic degranulation of mast cells exerts its effects on enteric microcircuits in this manner (27). In contrast, histamine primary acts to sensitize dorsal root ganglion (DRG) sensory neurons through H1R-dependent mechanisms (2, 4, 72). These differences in the effects of histamine likely underlie the apparent contradictory effects of supernatants derived from individuals with IBS. Ostertag et al. (49) showed that an IBS cocktail containing histamine reduces the responsiveness of submucosal neurons, while other studies focusing on DRG sensory neurons have shown excitatory effects (2, 4, 72). Similarly, IBS supernatants have an inhibitory effect on glial activity and decrease ATP-induced Ca2+ activity in cultured rat enteric glia (36).
Our data show that mediators released by activated mast cells excite enteric glia in mice and humans but that their ability to do so differs following exposure to NMS and between mice and humans. Glial responses to histamine and mast cell mediators in mouse tissue involve multicellular signaling between intrinsic enteric neurons, extrinsic primary afferent neurons, and enteric glia, since suppressing neuronal activity with TTX reduced the ability of enteric glia to respond to histamine. Likewise, histamine receptors are broadly expressed by intrinsic and extrinsic neurons (7, 72) that communicate with glia (21, 32), and our data show that H1R immunoreactivity is mainly localized to neuron cell bodies and varicosities in the mouse colon myenteric plexus. Therefore, increased histamine secretion by mast cells following NMS would function to decrease neuron-glia communication through inhibitory effects on synaptic transmission (40, 62). In contrast, cultured human enteric glia display widespread H1R expression and respond to histamine directly. Since there is no cellular intermediary in this interaction, human enteric glia display robust responses to histamine that are potentiated when mast cells release additional histamine following NMS. Whether the differences in H1R expression observed here reflect species differences or differences in experimental settings is not entirely clear; however, RNA sequencing data suggest that the former is most likely. Available transcriptional profiles of human and mouse enteric glia show significant HRH1 expression in human enteric glia, while it is undetectable in enteric glia from mice (21, 23, 75).
A subset of mouse enteric glia does exhibit responses to histamine that are resistant to TTX, suggesting direct actions. Similar Ca2+ responses to histamine have been observed in cultures of guinea pig (35) and rat enteric glia (36). Such direct actions may explain the ability of IBS supernatants to reduce the expression of connexin-43 in cultures of rat enteric glia (43) and promote glial proliferation in response to histamine in vivo (34). Mast cells release a number of mediators that include serotonin, proteases, and neurotrophins (2, 4, 48, 73) that may also influence glial responses in situ. While the current study demonstrated that NMS mast cells release more histamine and NMS mast cell supernatants evoked greater Ca2+ activity in enteric glia, a limitation of the current study is that the effect of mediators released by mast cells were assessed only in MP preparations from NH mice but not NMS mice. It is plausible that NMS tissue might have a different response to mast cell mediators, compared with NH tissue. NMS mast cells exert an inhibitory effect similar to the IBS supernatants, and this might be an important question for future work. It is also possible that histamine may alter the potential of glial Ca2+ signaling by other yet unstudied mast cell mediators. A potential limitation is the difference between primary derived BMMCs used in this study and native intestinal mast cells. BMMCs are derived directly from bone marrow progenitors and develop in culture lacking exposure to tissue maturation factors and are phenotypically less mature, exhibiting reduced abundance of gene expression (1) and reduced concentrations of granule mediators such as histamine (39). Despite the reduced levels of mediators, BMMCs express a similar repertoire of mediators and receptors compared with tissue mast cells.
The increase in GFAP expression and decrease in total glial process length observed in the myenteric plexus of animals exposed to early-life stress is consistent with a “reactive” gliosis phenotype in enteric glia. Similar changes in glial morphology and GFAP content have been reported in the rat model of NMS (28, 64). The increase in GFAP immunoreactivity and accompanying structural changes occurred in both male and female mice after NMS and suggest a transition to a reactive glial-like phenotype (12, 29, 63, 68). Reactive gliosis is considered beneficial by protecting neural networks and integrity from damage during disease and injury (15, 63). Interestingly, reactive gliosis is driven by intense activity in nociceptive sensory neurons in the myenteric plexus (21), and mast cell mediators sensitize and activate visceral sensory nerves (4, 16). The activation of reactive gliosis in the context of acute inflammation also produces enteric neurodegeneration, but we did not observe a loss of enteric neurons in NMS mice. The NMS model does not induce significant inflammation, other than mast cell activation, and this supports the relevance of the model in terms of IBS and is a potential reason that we did not observe neurodegeneration. Mast cells do reduce the survival of enteric neurons in culture, but this effect is mediated by PAR2, IL6, and prostaglandin D2 and does not involve histamine (58). It is also possible that NMS causes intrinsic changes to glia that are not driven by interactions with mast cells or histamine. This conclusion is consistent with our observations here since features of gliosis were observed in both males and females, but the density of histamine-positive cells was only increased in males. Therefore, glial intrinsic changes could work in tandem with changes in mast cells to contribute to the effects of early-life stress on gastrointestinal functions.
In conclusion, our results identify intercellular signaling mechanisms between mast cells, enteric glia, and neurons that are mediated by histamine and altered by early-life stress. Higher numbers of mast cells surrounding enteric ganglia increase the potential for mast cell mediators, including histamine, to influence glia through direct and indirect mechanisms. Altering glial activity is anticipated to disrupt neuronal signaling and contribute to neuroplasticity given the key roles of glia in the regulation of neuronal signaling and homeostasis. Understanding the mechanisms whereby early-life stress alters mast cell-glial interactions, and the development of visceral pain and dysmotility will provide new insight into the pathogenesis and increased risk for functional gastrointestinal disorders such as IBS, especially at-risk individuals with a history of early-life adversity.
GRANTS
This work was supported by National Institutes of Health Grants R01DK103723 and R01DK120862 to B. D. Gulbransen; USDA-NIFA 2019-07035 to A. J. Moeser; R01HD072968 and R21AI140413 to A. J. Moeser and A. J. Robison; and R01DK113943 to F. L. Christofi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.R., F.L.C., A.J.M., and B.D.G. conceived and designed research; J.L.M., E.A.M., N.M., N.D. and I.G. performed experiments; J.L.M., E.A.M., N.M., N.D., I.G., F.L.C., A.J.M., and B.D.G. analyzed data; J.L.M., E.A.M., N.M., N.D., I.G., A.J.R., F.L.C., A.J.M., and B.D.G. interpreted results of experiments; J.L.M. and E.A.M. prepared figures; J.L.M. and E.A.M. drafted manuscript; J.L.M., E.A.M., N.M., N.D., I.G., A.J.R., F.L.C., A.J.M., and B.D.G. edited and revised manuscript; J.L.M., E.A.M., N.M., N.D., I.G., A.J.R., F.L.C., A.J.M., and B.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Kyan Thelen for technical assistance.
REFERENCES
- 1.Akula S, Paivandy A, Fu Z, Thorpe M, Pejler G, Hellman L. Quantitative in-depth analysis of the mouse mast cell transcriptome reveals organ-specific mast cell heterogeneity. Cells 9: 211, 2020. doi: 10.3390/cells9010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balemans D, Aguilera-Lizarraga J, Florens MV, Jain P, Denadai-Souza A, Viola MF, Alpizar YA, Van Der Merwe S, Vanden Berghe P, Talavera K, Vanner S, Wouters MM, Boeckxstaens GE. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 316: G338–G349, 2019. doi: 10.1152/ajpgi.00116.2018. [DOI] [PubMed] [Google Scholar]
- 3.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 53: 501–506, 2004. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut 57: 582–590, 2008. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- 7.Bell A, Althaus M, Diener M. Communication between mast cells and rat submucosal neurons. Pflugers Arch 467: 1809–1823, 2015. doi: 10.1007/s00424-014-1609-9. [DOI] [PubMed] [Google Scholar]
- 8.Boeckxstaens GE The emerging role of mast cells in irritable bowel syndrome. Gastroenterol Hepatol (NY) 14: 250–252, 2018. [PMC free article] [PubMed] [Google Scholar]
- 9.Bosma R, Moritani R, Leurs R, Vischer HF. BRET-based β-arrestin2 recruitment to the histamine H1 receptor for investigating antihistamine binding kinetics. Pharmacol Res 111: 679–687, 2016. doi: 10.1016/j.phrs.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289: G42–G53, 2005. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol 10: 385–390.e1, 2012. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley JS Jr, Parr EJ, Sharkey KA. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res 289: 455–461, 1997. doi: 10.1007/s004410050891. [DOI] [PubMed] [Google Scholar]
- 13.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cenac N, Altier C, Motta JP, d’Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 59: 481–488, 2010. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Di Lorenzo C, Farrugia G, Hamilton FA, Mawe GM, Pasricha PJ, Wiley JW. Functional bowel disorders: a roadmap to guide the next generation of research. Gastroenterology 154: 723–735, 2018. doi: 10.1053/j.gastro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol 103: 765–774, 2008. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook RD, Burnstock G. The ultrastructure of Auerbach’s plexus in the guinea-pig. II. Non-neuronal elements. J Neurocytol 5: 195–206, 1976. doi: 10.1007/BF01181656. [DOI] [PubMed] [Google Scholar]
- 20.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol 104: 392–400, 2009. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 21.Delvalle NM, Dharshika C, Morales-Soto W, Fried DE, Gaudette L, Gulbransen BD. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol Gastroenterol Hepatol 6: 321–344, 2018. doi: 10.1016/j.jcmgh.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, Neunlist M, De Ponti F, Stanghellini V, Barbara G. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 148: 1002–1011.e4, 2015. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell 182: 1606–1622.e23, 2020. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology 150: 1257–1261, 2016. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers 2: 16014, 2016. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried DE, Gulbransen BD. In situ Ca2+ imaging of the enteric nervous system. J Vis Exp 2015: 52506, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology 107: 1602–1609, 1994. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- 28.Fujikawa Y, Tominaga K, Tanaka F, Tanigawa T, Watanabe T, Fujiwara Y, Arakawa T. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol Motil 27: 1010–1023, 2015. doi: 10.1111/nmo.12577. [DOI] [PubMed] [Google Scholar]
- 29.Gabella G Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J Neurocytol 13: 73–84, 1984. doi: 10.1007/BF01148319. [DOI] [PubMed] [Google Scholar]
- 30.Grubišić V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol 595: 3409–3424, 2017. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Kamphuis JBJ, Guiard B, Leveque M, Olier M, Jouanin I, Yvon S, Tondereau V, Rivière P, Guéraud F, Chevolleau S, Noguer-Meireles MH, Martin JF, Debrauwer L, Eutamène H, Theodorou V. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 158: 652–663.e6, 2020. doi: 10.1053/j.gastro.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Keles N, Yavuz Arican R, Coskun M, Elpek GO. Histamine induces the neuronal hypertrophy and increases the mast cell density in gastrointestinal tract. Exp Toxicol Pathol 64: 713–716, 2012. doi: 10.1016/j.etp.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem 66: 604–612, 1996. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- 36.Lilli NL, Quénéhervé L, Haddara S, Brochard C, Aubert P, Rolli-Derkinderen M, Durand T, Naveilhan P, Hardouin JB, De Giorgio R, Barbara G, Bruley des Varannes S, Coron E, Neunlist M. Glioplasticity in irritable bowel syndrome. Neurogastroenterol Motil 30: e13232, 2018. doi: 10.1111/nmo.13232. [DOI] [PubMed] [Google Scholar]
- 37.Liñán-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel-Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R, Christofi FL. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI Infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis 22: 1812–1834, 2016. doi: 10.1097/MIB.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 107: 991–1000, 2012. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 39.Mackey E, Ayyadurai S, Pohl CS, D’ Costa S, Li Y, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ 7: 60, 2016. doi: 10.1186/s13293-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer CJ, Wood JD. Properties of mechanosensitive neurons within Auerbach’s plexus of the small intestine of the cat. Pflugers Arch 357: 35–49, 1975. doi: 10.1007/BF00584543. [DOI] [PubMed] [Google Scholar]
- 41.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 12: 592–605, 2015. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClain J, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClain JL, Gulbransen BD. The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function, and the expression of key proteins. J Neurophysiol 117: 365–375, 2017. doi: 10.1152/jn.00507.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medland JE, Pohl CS, Edwards LL, Frandsen S, Bagley K, Li Y, Moeser AJ. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil 28: 1317–1329, 2016. doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales-Soto W, Gulbransen BD. Enteric glia: a new player in abdominal pain. Cell Mol Gastroenterol Hepatol 7: 433–445, 2019. doi: 10.1016/j.jcmgh.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric glial cells: a new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm Bowel Dis 22: 433–449, 2016. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostertag D, Annahazi A, Krueger D, Michel K, Demir IE, Ceyhan GO, Zeller F, Schemann M. Tryptase potentiates enteric nerve activation by histamine and serotonin: Relevance for the effects of mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil 29: e13070, 2017. doi: 10.1111/nmo.13070. [DOI] [PubMed] [Google Scholar]
- 49.Ostertag D, Buhner S, Michel K, Pehl C, Kurjak M, Götzberger M, Schulte-Frohlinde E, Frieling T, Enck P, Phillip J, Schemann M. Reduced responses of submucous neurons from irritable bowel syndrome patients to a cocktail containing histamine, serotonin, TNFα, and tryptase (IBS-cocktail). Front Neurosci 9: 465, 2015. doi: 10.3389/fnins.2015.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panula P, Kaartinen M, Mäcklin M, Costa E. Histamine-containing peripheral neuronal and endocrine systems. J Histochem Cytochem 33: 933–941, 1985. doi: 10.1177/33.9.3894504. [DOI] [PubMed] [Google Scholar]
- 51.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci 18: 204–210, 2003. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SH, Videlock EJ, Shih W, Presson AP, Mayer EA, Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil 28: 1252–1260, 2016. doi: 10.1111/nmo.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pohl CS, Medland JE, Mackey E, Edwards LL, Bagley KD, DeWilde MP, Williams KJ, Moeser AJ. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol Motil 29: e13118, 2017. doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prusator DK, Andrews A, Greenwood-Van Meerveld B. Neurobiology of early life stress and visceral pain: translational relevance from animal models to patient care. Neurogastroenterol Motil 28: 1290–1305, 2016. doi: 10.1111/nmo.12862. [DOI] [PubMed] [Google Scholar]
- 55.Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol 547: 531–542, 2003. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, Van Langendonck N, Gillet M, Cartier C, Baron M, Sommer C, Mallet V, Zill M, Robert H, Laurent F, Ellero-Simatos S, Théodorou V, Ménard S. Early life stress in mice is a suitable model for irritable bowel syndrome but does not predispose to colitis nor increase susceptibility to enteric infections. Brain Behav Immun 73: 403–415, 2018. doi: 10.1016/j.bbi.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Robles A, Perez Ingles D, Myneedu K, Deoker A, Sarosiek I, Zuckerman MJ, Schmulson MJ, Bashashati M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil 31: e13718, 2019. doi: 10.1111/nmo.13718. [DOI] [PubMed] [Google Scholar]
- 58.Sand E, Themner-Persson A, Ekblad E. Mast cells reduce survival of myenteric neurons in culture. Neuropharmacology 56: 522–530, 2009. doi: 10.1016/j.neuropharm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Müller W, Testa G, Roers A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res 17: 307–315, 2008. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sundin J, Nordlander S, Eutamene H, Alquier-Bacquie V, Cartier C, Theodorou V, Le Nevé B, Törnblom H, Simrén M, Öhman L. Colonic mast cell numbers, symptom profile, and mucosal expression of elements of the epithelial barrier in irritable bowel syndrome. Neurogastroenterol Motil 31: e13701, 2019. doi: 10.1111/nmo.13701. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- 62.Tamura K, Palmer JM, Wood JD. Presynaptic inhibition produced by histamine at nicotinic synapses in enteric ganglia. Neuroscience 25: 171–179, 1988. doi: 10.1016/0306-4522(88)90016-4. [DOI] [PubMed] [Google Scholar]
- 63.Thacker M, Rivera LR, Cho HJ, Furness JB. The relationship between glial distortion and neuronal changes following intestinal ischemia and reperfusion. Neurogastroenterol Motil 23: e500–e509, 2011. doi: 10.1111/j.1365-2982.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 64.Tominaga K, Fujikawa Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Watanabe T, Fujiwara Y, Arakawa T. Structural changes in gastric glial cells and delayed gastric emptying as responses to early life stress and acute adulthood stress in rats. Life Sci 148: 254–259, 2016. doi: 10.1016/j.lfs.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 65.Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D’Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 63: 105–115, 2014. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 66.Vicario M, González-Castro AM, Martínez C, Lobo B, Pigrau M, Guilarte M, de Torres I, Mosquera JL, Fortea M, Sevillano-Aguilera C, Salvo-Romero E, Alonso C, Rodiño-Janeiro BK, Söderholm JD, Azpiroz F, Santos J. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 64: 1379–1388, 2015. doi: 10.1136/gutjnl-2013-306236. [DOI] [PubMed] [Google Scholar]
- 67.Videlock EJ, Mahurkar-Joshi S, Hoffman JM, Iliopoulos D, Pothoulakis C, Mayer EA, Chang L. Sigmoid colon mucosal gene expression supports alterations of neuronal signaling in irritable bowel syndrome with constipation. Am J Physiol Gastrointest Liver Physiol 315: G140–G157, 2018. doi: 10.1152/ajpgi.00288.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Boyen GB, Steinkamp M, Reinshagen M, Schäfer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53: 222–228, 2004. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, Akhtar S, Zuo XL, Tan XD, Li YQ. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep 6: 20320, 2016. doi: 10.1038/srep20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang YH, Taché Y, Harris AG, Kreutner W, Daly AF, Wei JY. Desloratadine prevents compound 48/80-induced mast cell degranulation: visualization using a vital fluorescent dye technique. Allergy 60: 117–124, 2005. doi: 10.1111/j.1398-9995.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 71.Wechsler JB, Szabo A, Hsu CL, Krier-Burris RA, Schroeder HA, Wang MY, Carter RG, Velez TE, Aguiniga LM, Brown JB, Miller ML, Wershil BK, Barrett TA, Bryce PJ. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal Immunol 11: 861–870, 2018. doi: 10.1038/mi.2017.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, Valdez-Morales EE, Nasser Y, Van Veldhoven PP, Vanbrabant W, Van der Merwe S, Mols R, Ghesquière B, Cirillo C, Kortekaas I, Carmeliet P, Peetermans WE, Vermeire S, Rutgeerts P, Augustijns P, Hellings PW, Belmans A, Vanner S, Bulmer DC, Talavera K, Vanden Berghe P, Liston A, Boeckxstaens GE. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 150: P875–887.e9, 2016. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 73.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 65: 155–168, 2016. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 74.Yoshikawa T, Naganuma F, Iida T, Nakamura T, Harada R, Mohsen AS, Kasajima A, Sasano H, Yanai K. Molecular mechanism of histamine clearance by primary human astrocytes. Glia 61: 905–916, 2013. doi: 10.1002/glia.22484. [DOI] [PubMed] [Google Scholar]
- 75.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22, 2018. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]