Keywords: cross-organ sensitization, intercell interaction, neuroinflammation, sensory neurons, visceral hypersensitivity
Abstract
Inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), historically considered as regional gastrointestinal disorders with heightened colonic sensitivity, are increasingly recognized to have concurrent dysfunction of other visceral and somatic organs, such as urinary bladder hyperactivity, leg pain, and skin hypersensitivity. The interorgan sensory cross talk is, at large, termed “cross-organ sensitization.” These organs, anatomically distant from one another, physiologically interlock through projecting their sensory information into dorsal root ganglia (DRG) and then the spinal cord for integrative processing. The fundamental question of how sensitization of colonic afferent neurons conveys nociceptive information to activate primary afferents that innervate distant organs remains ambiguous. In DRG, primary afferent neurons are surrounded by satellite glial cells (SGCs) and macrophage accumulation in response to signals of injury to form a neuron-glia-macrophage triad. Astrocytes and microglia are major resident nonneuronal cells in the spinal cord to interact, physically and chemically, with sensory synapses. Cumulative evidence gathered so far indicate the indispensable roles of paracrine/autocrine interactions among neurons, glial cells, and immune cells in sensory cross-activation. Dichotomizing afferents, sensory convergency in the spinal cord, spinal nerve comingling, and extensive sprouting of central axons of primary afferents each has significant roles in the process of cross-organ sensitization; however, more results are required to explain their functional contributions. DRG that are located outside the blood-brain barrier and reside upstream in the cascade of sensory flow from one organ to the other in cross-organ sensitization could be safer therapeutic targets to produce less central adverse effects.
SENSORY INTEGRATION IN CROSS-ORGAN SENSITIZATION
The four cardinal signs, dolor (pain), calor (heat), rubor (redness), and tumor (swelling), were first recorded by Celsus in the first century A.D. to be characteristics of inflammation. In the context of colonic inflammation, excessive epithelial, immunological, and endocrinal mediators sensitize the peripheral axonal terminals of nociceptors that are located in dorsal root ganglia (DRG) (61) and lead to increases in the excitability of axons and neurons of colonic afferents, resulting in sensory hypersensitivity and pain perception. The initiation of orthodromic action potential, when reaching threshold points, evokes axon reflex and antidromic release of neuropeptides [e.g., calcitonin gene-related peptide (CGRP) and substance P (SP)] from colonic afferent neurons to the peripheral terminals in the colon where they increase local blood flow and exacerbate the inflammatory process, together resulting in petechial hemorrhages and organ hypertrophy. This inflammatory process can be acute and can also develop into chronic due to local tissue remodeling [e.g., inflammatory bowel disease (IBD)] or as a result of epigenetic modification of sensory neurons that prolongs the heightened sensitivity and persists abdominal pain even after inflammation is resolved [e.g., postinfectious irritable bowel syndrome (IBS)].
There is increasing recognition that adult and children who have bowel diseases such as acute colitis, Crohn’s disease, constipation, or functional bowel disorders often show evidence of bladder dysfunction including detrusor instability, nocturia, interstitial cystitis/painful bladder syndrome (IC/PBS), and overactive bladder (64). A subset of those patients who have bladder hyperactivity also experience concurrent IBS (64). In patients who have IBS and/or IC/PBS, symptoms like pain at the neck, back, shoulder, and lower extremity, and fibromyalgia syndrome are highly prevalent (79). Patients with IBD also have higher risk for herpes zoster virus infection to develop shingles, a painful rash on the skin (44). Likewise, varicella-zoster virus infection of skin (chickenpox) establishes latency in DRG and ganglia of the enteric nervous system, causing gastrointestinal (GI) disorders including severe abdominal pain (11). Such interorgan cross-sensitization not just restricts to pelvic organ disorders but is widely distributed throughout the body. Clinical and laboratory evidence have identified cross-sensitization between esophagus and heart, gallbladder and heart, stomach and heart, stomach and duodenum, duodenum and pancreas, esophagus and airway, colon and urinary bladder, colon and uterus, colon and urethra, prostate and urinary bladder, heart and arm muscles, colon and skin, etc. (7, 11, 22, 44, 51, 74). In these phenomena, damage (inflammation, injury, and noxious stimulation) to a specific organ causes a heightened sensation of a distant organ to chemical or mechanical or external environmental (e.g., temperature) stimuli, namely “cross-organ sensitization.”
The pain sensation is initiated in the primary afferent neurons of DRG, termed first-order sensory neurons that are morphologically pseudounipolar with their distal axonal branches (spinal nerves) innervating the peripheral organ and their proximal axonal branches (via dorsal root) innervating the dorsal horn of the spinal cord. Each branch of the axons can carry orthodromic and antidromic signals for retrograde modulation of sensory activity or anterograde release of sensory mediators, respectively, into the peripheral organ or the central nervous system. The somata of sensory neurons that are clustered within the capsulized ganglia do not form synapses but are surrounded by satellite glial cells (SGCs) and interlinked by SGC networks (58). The central nerve terminals of the DRG neuron within the spinal dorsal horn form synapses with second-order neurons that send processed signals to the thalamus for pain perception (66). After harmful stimulation of the peripheral organ, sensitization of sensory neurons leads to an initial release and subsequent production/prolonged release of chemicals and neuropeptides along both the distal and proximal axons. Mediators generated by sensory neurons can also release into the sensory ganglia by extrasynaptic secretion and thus they affect the activity of nearby neurons and nonneuronal cells (4, 62).
In the process of bowel dysfunction-associated visceral or somatic cross-organ sensitization, sensitization of colonic afferent neurons leads to cross-activation of the primary reflex pathway of other uninjured organs (e.g., the urinary bladder or lower extremity). The enhanced activity of the cross-activated primary afferent neurons facilitates the antidromic release of neurogenic factors (e.g., CGRP and SP) into the uninjured peripheral organs, causing neurogenic inflammation (e.g., mast cell activation and altered epithelial permeability) that reenforces the pain sensation of the secondarily impaired organ. Thus far, the mechanisms by which sensitization of primary afferent neurons of one organ leads to activation of primary afferent neurons that innervate a distinct organ, a fundamental process in cross-organ sensitization, are still not fully understood. More perplexingly, estrous and hormonal modulations, sympathetic inputs, and immunological disorders undoubtedly interfere in the sensory process and pain perception as well as cross-organ sensitization, and those aspects are beyond the scope of the current discussion.
Anatomically, colonic and bladder afferent neurons are housed in the same levels of DRG (70). In colon-to-bladder cross-sensitization, the intraganglionic dialog of colonic and bladder afferent neurons is transduced by a cascade of events that start with mediators releasing from the initially sensitized colonic primary afferent neurons, followed by receptor-mediated paracrine activation of nearby neuronal and nonneuronal cells, and ultimately signal relay to the primary afferent neurons of the secondarily sensitized urinary bladder. Convergent neurons that receive inputs from the colon and the bladder are also found in DRG and the spinal cord dorsal horn, though the populations of these neurons are small (24). Convergent neurons in DRG have dichotomizing afferents with their split distal axons innervating different organs; therefore, signals from one organ can be conveyed to another organ through axon reflex. Driven by sensory reflex and epigenetic regulation of sensory neurons, bowel diseases-associated urinary bladder neurogenic hypersensitivity can be initiated through increased axonal reflex along dichotomizing afferents (more evidence is required) and maintained by prolonged cross-activation of bladder afferent neurons upon insulation of the gut (70, 86). Chronic cross-organ sensitization may also involve neuroplasticity and neuroinflammation in the spinal cord in which the central nerve terminals of primary afferent neurons release sensory mediators and also receive inflammatory factors (24, 49). The spinal nerves of colonic and bladder afferent neurons exit the sensory ganglia in the same nerve bundle toward visceral organs before splitting into individual organs. The increased action potential and excitability of colonic afferent axons in bowel dysfunction may also affect the activity of the comingled bladder afferents; however, this theory needs to be proved. The anatomical and molecular pathways that direct viscerosomatic cross-sensitization such as IBS-associated somatic pain are extremely understudied. DRGs that contain primary afferent neurons to the lower extremity do not have primary afferent neurons to the distal colon. Therefore, cross talk in the spinal cord becomes the major driving force in colon-limb cross-organ sensitization. Not much information has been obtained on how signals travel transsegmentally in the spinal cord so that primary afferent nerve terminals and second-order neurons can be sensitized by mediators released into different segments of the spinal dorsal horn. Astrocytic network, microglial migration, and sensory nerve fiber sprouting are suggested in animal models of colonic inflammation and other nerve injuries. In summary, this review will discuss the established animal models in the investigation of viscerovisceral and viscerosomatic cross-organ sensitization and incorporate these models and others to link the evidence of sensory cross-activation to shed light on the known and prospective spinal mechanisms of cross-organ sensitization.
ANIMAL MODELS: FOCUSING ON BOWEL DYSFUNCTION-ASSOCIATED SENSORY COMORBIDITY
Animal models are crucial for teasing out the critical components that are involved in pathogenesis, such as molecular pathways in DRG and the spinal cord in cross-organ sensitization. The common problems of animal models are that even the best one only resembles some aspects of human diseases, especially for those diseases with a complex and unknown etiology and pathophysiology. Comorbidity is such a disease in which multiple organs and systems are impaired simultaneously. In pelvic pain syndrome, it is often hard to directly diagnose the origin of the disease, let alone treatment. Strategies of questionnaire and diagnosis of exclusion are popularly used. Many clinical reports (64) and the one in which 2,682 patients who are surveyed to have IC/PBS, a chronic condition of bladder pain and frequent urination with no bladder infection, are 100 times more likely to have IBD compared with the general population (3) suggest that organic disorders of the colon are tightly associated with neurogenic pain and hyperactivity of the urinary bladder. In a well-established colitis rodent model induced by intracolonic instillation of 2,4,6-trinitrobenzene sulfonic acid (TNBS), a similar phenomenon occurs where the urinary bladder becomes hypersensitive to mechanical stimulation and also shows urinary frequency but with no signs of inflammation or macroscopic changes (67, 88), a typical characteristic of IC/PBS. This close resemblance of clinical features of IBD-associated IC/PBS by the rodent TNBS colitis model sheds light on the possibility of using animal models for the study of the underlying mechanisms of cross-organ sensitization. In fact, similar patterns for immunoreactivities of pain-related molecules in smaller-sized nociceptive neurons are identified in DRGs of humans and laboratory animals (26). Humans and experimental rodents also have consistency on certain neuron-immune cell interactions that are involved in pain sensitivity (26). To be more accurate and precise to identify the cellular and molecular events in sensory neuron cross-activation but not jeopardize data translation to humans, a good animal model of cross-organ sensitization is necessary and by logic required to meet the following criteria: 1) the induction of disease needs to be localized to a specific organ, 2) at least one organ other than the injured/irritated organ shows functional disorder, and 3) any direct injury/irritation to the cross-sensitized organ needs to be avoided so that its sensitization is “referred” from a distant diseased organ.
In animal models that satisfy the above rules, activation of primary afferent neurons of the cross-sensitized organ is secondary to the activation of primary afferent neurons of the injured organ. In line with these criteria, several other forms of cross-organ sensitization are also successfully modeled in experimental animals (Table 1). For example, localized inflammation in the prostate is induced to study prostate-to-bladder cross-organ sensitization (22). In this section, we will discuss the animal models that have the origin of disease in the large bowel and have accompanying comorbidity of referred visceral and/or somatic hypersensitivity.
Table 1.
Animal models of cross-organ sensitization
| Induction of Disease | Animals | Secondarily Impaired Organ | Time of Cross Talk | Effects | Ref. No. |
|---|---|---|---|---|---|
| Colitis by TNBS | Rat and mouse | Bladder | 1 h to 90 days | Activation | 24, 67, 86, 88 |
| Colitis by TNBS | Rat | Hindpaw | Days 14–28 | Activation | 99 |
| Colitis by TNBS | Mouse | Hindpaw | Day 7 | Activation | 48 |
| Colitis by MO | Mouse | Upper GI | Day 28 | Activation | 43 |
| Colitis by MO | Neonatal mouse | Hindpaw | Adult | Activation | 13 |
| Colitis by MO | Rat | Hindpaw | Immediate | Inhibition | 80 |
| Noxious CRD | Rat and mouse | Bladder | Immediate | Inhibition | 84, Fig. 1 |
| Noxious CRD | Mouse | Bladder | >3 h | Activation | Fig. 1 |
| Prostatitis by zymosan | Mouse | Bladder | Days 7–28 | Activation | 74 |
| Prostatitis by formalin | Rat | Bladder | Day 7 | Activation | 22 |
CRD, colorectal distension; MO, mustard oil; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
TNBS Colitis and Postcolitis
IBD and IBS are highly correlated in a way that patients with active colitis or after colitis remission demonstrate increased rectum sensitivity (5, 20). This is also true for the rodent model that is induced by TNBS. At the acute stage, TNBS-treated rats or mice have the presence of multiple serpiginous ulcers, mucosal damage, elevation of inflammatory markers that peak on days 3–4, and at this time visceral hypersensitivity is also evident (6, 15, 87). Visceral hypersensitivity persists after inflammation resolution (beyond 14 days), resembling postcolitis IBS (21, 37). Pezzone et al. (67) first document that rats having TNBS-induced colitis exhibit increased bladder contractility during the initial phase of colonic inflammation (day 1). Micturition frequency is increased throughout the course from acute (67) to the peak of colitis (88) and to postcolitis recovery in rats (86), suggesting that TNBS colitis induces acute and chronic bladder hyperactivity. Urethral pain and somatic hypersensitivity are also detected in postcolitic rats (89, 98, 99). Both thermal and mechanical hypersensitivity of hind paw appear on days 14–28 of TNBS colitis but not earlier, whereas colonic hypersensitivity appears on day 2 of colitis (99), suggesting a delayed onset of somatic cross-sensitization. This is similar to the case in IBS patients who develop leg pain and foot hypersensitivity at a late stage (79). A similar pattern is seen in mice that TNBS colitis induces bladder hyperactivity examined on day 7 of colitis, which is accompanied by colonic hypersensitivity and somatic pain (48). Interestingly, only a subset (24%) of TNBS-treated rats maintain colonic hypersensitivity and somatic pain by week 16 (98). These findings suggest the limitation of the TNBS model that a subset of animals can completely recover from disease and are not suitable for prolonged (>16 wk) studies. Another recent study in mice shows that spontaneous bladder voiding hyperactivity is detected on days 14–28 but not earlier in TNBS colitis (25). This is consistent with rats that frequent spontaneous voiding is found on day 21 of colitis (86), whereas in both rats and mice, urodynamic examination by filling cystometry shows an earlier onset of bladder hyperactivity during active colitis (50, 67, 88). The inconsistent outcome of bladder hyperactivity measured by spontaneous voiding and cystometry is not clear. It could be that surgery before cystometry exacerbates bladder hyperactivity during the acute phase of colitis. Nevertheless, the TNBS model is so far one of the best tools for mechanistic studies of bowel dysfunction-associated cross-organ sensitization due to its unique feature that this model resembles the clinical characteristics of acute colitis and IBS at different time periods, causes sensory hypersensitivity of the colon and remote uninjured organs, and does not cause direct damage but modifies the function of the distant organs (48, 88).
MO Colitis and Postcolitis
Mustard oil (MO) instillation into the distal colon of mice causes severe colitis with body weight loss, mucosal damage, and diarrhea, whereas the colonic inflammation recovers by day 14 (43). MO administration results in immediate colonic pain behavior (47) and a later development of accelerated upper GI transit after inflammation recovery (day 28) (43). Neonatal MO installation to the colon, a mimic of childhood infection, also causes visceral hypersensitivity in adults (13). These features suggest that the MO colitis model resembles some aspects of postcolitis IBS. MO-treated neonatal mice develop mechanical somatic pain during the adult stage (13), mimicking IBS-associated somatic hypersensitivity. However, MO installation in adult rats immediately inhibits thermal hypersensitivity (80). Not as extensively used as the TNBS colitis model, the MO colitis model can be another good tool for the study of cross-organ sensitization.
Noxious CRD
Colorectal distension (CRD) and constipation can alter bladder sensation in healthy individuals and patients (8, 85). Animal models following noxious CRD exhibit visceral pain and elevated expression of pain markers such as the phosphorylated extracellular signal-regulated kinase (p-ERK) in the spinal cord (28). During standard filling cystometry that measures bladder activity, noxious CRD in mice causes immediate and acute bladder inhibition that is determined by increased micturition intervals (MIs) compared with baseline (Fig. 1A, 10-min post CRD; Fig. 1B). The inhibition is followed by recovery (not shown) and subsequent development of bladder hyperactivity (Fig. 1A, 3-h post-CRD; Fig. 1B). CRD treatment also causes acute bladder inhibition in rats (84). The switch from initial reflex cross-inhibition to prolonged cross-activation occurs as well following MO-induced colonic inflammation (43, 80). The sensation of the colon and the bladder is integrated initially in DRG and driven by sensory reflexes (Fig. 1D). The signal processes in the dynamic inhibitory-activatory shift of sensory cross talk are not clear and can be an interesting paradigm in the study of the initiation and maintenance of cross-organ sensitization. The advantage of the CRD model is that it does not involve potential chemical leakage from the distal colon that may irritate the anus, nearby skin, and organs. However, stimulation of the distal gut by CRD may produce passive pressure to the urinary bladder due to decreased volume in the abdominal cavity when the colon is expanded during intracolonic balloon inflation. The smallest size of the mini intracolonic balloon can be used to minimize this adverse effect.
Fig. 1.
Acute noxious colorectal distension-induced inhibition-activation switch in bladder activity. A total of five male adult mice (25 g) were implanted with intravesical catheters according to our previous publication (88). Cystometry was performed under continuous saline infusion at 1 mL/h. After surgery, the animals were recovered for at least 2 h. The animals were then inserted with intracolonic miniballoons under light anesthesia (0.5%–1% isoflurane) and placed into a recording cage for 30 min before recording. Each animal was first recorded for baseline with mini-balloon uninflated (control). Noxious colorectal distension (CRD, 80 mmHg for 10-s with 5-s intervals and repeated for five times) was performed at the beginning of a filling phase and with saline infusion paused. Cystometry was immediately resumed after CRD. After at least four micturition cycles were obtained (average 10–20 min), cystometry was restarted on the hour for an average of 10–20 min. Among the five animals, one was only recorded for baseline and 3 h and another one was recorded for baseline and the first period without stopping saline infusion during CRD. Results were consistent in all animals that CRD-induced immediate bladder inhibition was shown as longer micturition intervals (MI) by 1.3-fold (B). Bladder hyperactivity was developed at 3 h post-CRD that reduced MI from baseline by 32% (B). One-way ANOVA and Newman–Keuls multiple comparison test was used for data analysis, with the baseline normalized as 1 (B, ***P < 0.001). The noxious stimulation of colon excites colonic afferent neurons and conveys information to the urinary bladder through primary afferents (C). The mechanisms underlying the development of bladder inhibition and the switch from inhibition to activation following mechanical or chemical irritation of the colon are unknown. CRD, colorectal distension.
Other Models
A variety of other means are used to induce localized colonic inflammation and visceral pain such as intracolonic administration of zymosan, hydrogen sulfide (H2S), deoxycholic acid (DCA), acetic acid, or capsaicin. These models are less characterized for cross-organ sensitization. Stress including early life stress can affect the function of visceral organs and are risk factors for the development of IBS and visceral comorbidities. This is mainly due to the mechanisms of systemic sympathomedullary and hypothalamic-pituitary-adrenal (HPA) regulation. Dextran sulfate sodium (DSS) in drinking water induces colitis in rodents and is widely used to model IBD. DSS also induces kidney and liver damages (19). Visceral comorbidity following DSS treatment could be a result of systemic metabolic changes (19). After DSS treatment, the bladder afferent neurons are sensitized (24). However, there is less evidence to show whether DSS metabolites affect the urinary bladder directly instead of colon-to-bladder sensory cross talk in the primary afferent pathway.
MECHANISMS: INTRAGANGLIONIC DIALOG
Devor and Wall (17) described that previously silent DRG neurons can be cross-excited (cross-depolarization or cross-excitation) when axons of neighboring neurons of the same ganglion are stimulated repetitively. In vivo imaging of DRG neurons in live mice shows that 2–5 sensory neurons are activated as a cluster in inflammatory and neuropathic pain (42). The activity-coupled neuronal clusters include nociceptive neurons (directly receiving noxious stimuli) and Aβ-low-threshold mechanoreceptors (LTMRs) (not directly sensitized by inflammation) (42). In the cluster, some neurons have delayed activation after the first event of neuronal activation (42). In cross-organ sensitization, localized colonic inflammation or localized prostatic inflammation that sensitizes specific primary afferent neurons of the inflamed organ also leads to the activation of bladder afferent neurons without apparent signs of bladder inflammation (22, 70, 86). Although colonic (prostatic) afferent neurons and bladder afferent neurons are housed in the same DRG, there is a lack of evidence whether colonic (prostatic) afferent neurons and bladder afferent neurons are anatomically clustered [within 1 µm distance (42)]. Nonetheless, the question remains, however not clear, on how activation of primary afferent neurons of an organ (e.g., the distal colon or prostate) causes cross-activation of primary afferent neurons of another organ (e.g., the urinary bladder). More evidence favors a mechanism by which diffusible chemical mediators from the initially sensitized neurons are secreted within the sensory ganglia and received by neighboring cells (4, 62). These nearby cells can be neurons and nonneuronal cells such as SGCs and resident macrophages. In this section, we will discuss the paracrine/autocrine mechanisms within the sensory ganglia that underlie mediator-mediated intercell cross talk and suggest their roles in cross-organ sensitization.
Paracrine/Autocrine Mediators
A recent review summarizes a list of chemicals that are released from the somata of neurons within DRG or trigeminal ganglia under inflammatory or neuropathic pain condition (58). These chemicals include ATP, SP, CGRP, glutamate, γ-aminobutyric acid (GABA), galanin, nitric oxide (NO), and brain-derived neurotrophic factor (BDNF) (58). Many of these factors have not been studied for their roles in cross-organ sensitization. Evidence on ATP, CGRP, and BDNF as paracrine mediators in sensory cross talk and cross-organ sensitization is best documented.
After nerve injury, pannexin-1 is upregulated in DRG neurons (93) which allows ATP release (14). In prostate-to-bladder cross-sensitization, prostate inflammation increases P2X2 expression in bladder afferent neurons (22), thereby enhancing the responsiveness of these neurons to paracrine ATP signals and leading to sensory neuron cross-activation. DRG ATP that acts on bladder afferent neurons in this context could be also from other cells such as SGCs (27).
In DRG, BDNF is generated by nociceptive neurons. TNBS colitis increases BDNF expression in colonic afferent neurons through target-derived retrograde NGF signaling (90) and promotes the coexpression of BDNF with the transient receptor potential cation channel subfamily V member 1 (TrpV1) (88). BDNF secretion by DRG neurons and trigeminal sensory neurons is measured in the cultured medium (2, 30, 52). Direct delivery of the BDNF antibody into the injured DRG or interference of the proBDNF-sortilin complex in DRG reduces mechanical allodynia and inflammatory pain (30, 100). The fact that BDNF neutralization by antibodies (100) and interference of BDNF-receptor interactions (30) only occur extracellularly suggests a paracrine/autocrine role of BDNF within DRG in the regulation of sensory activity. In colon-to-bladder cross-organ sensitization, peripheral delivery of the BDNF antibody, which can reach DRG outside the blood-brain barrier but is virtually excluded from the central nervous system due to its large size, attenuates TNBS colitis-induced CGRP elevation through phospholipase Cγ (PLCγ) but not Akt in bladder-afferent neurons (29, 70, 86). Partial deletion of the BDNF gene also blocks TNBS colitis-induced bladder hyperactivity (86).
The CGRP release from sensory neurons is potentiated by the activation of TrpV1 (59). CGRP receptors are also expressed by DRG neurons and trigeminal sensory neurons (55). The CGRP antibody and its receptor antagonist that enter sensory ganglia with no central action are approved to be effective for migraine therapy (12, 40), suggesting a paracrine/autocrine action of CGRP in sensory neuron activation. CGRP8–37, a receptor antagonist, reduces TNBS colitis-induced colonic hypersensitivity (16). Understanding of the paracrine role of CGRP in DRG can take advantage to repurpose the peripherally acting CGRP antagonists for treatment of the chronic sensory comorbidities and cross-organ sensitization.
SGC Networks
Sensory neurons do not form synaptic contacts with one another but are enwrapped individually or in a cluster containing two or three neurons (<10% of the total population) by an envelope of SGCs, the major nonneuronal cells in naïve DRG (27). The space between neuronal somata and the sheath of SGCs is ∼20 nm (49), in a similar way to those at the synapses (a 20 to 40-nm gap). The proximity of DRG neurons and SGCs endows SGCs to efficiently receive neuronal mediators and in turn send gliotransmitters back to neurons. After peripheral inflammation, BDNF high affinity receptor TrkB.T1, the truncated form of TrkB, is increased in the SGCs of DRG (66), ensuring the responsiveness of SGCs to endogenous BDNF paracrine signals. The N-methyl-d-aspartate receptors (NMDARs) (67) and purinergic receptors (49) are also expressed in SGCs to mediate glutamate and ATP signals, respectively. Receptor-mediated SGC activation is a fundamental process in facilitating gliotransmission, whereas the formation of gap junctions by hemichannels between the cytoplasma of two adjacent SGCs allows for bidirectional flow of Ca2+, other ions, and small signaling molecules within the SGC networks.
An individual DRG neuron is typically surrounded by two or three SGCs (27). The neuron(s) and the adjacent SGCs are termed as the “neuron-glia unit.” Dye permeability assay shows that under physiological conditions, SGCs in the same neuron–glia unit are highly coupled (26.2%), with fewer (3.2%) SGC coupling between neuron-glia units (34). Both intra-and interunit SGC coupling are increased in aging (36) and following partial colonic obstruction (35). Dinitrosulfonate benzoate (DNBS)-induced colitis increases interunit SGC coupling by 6.6-fold after 10–12 days of induction of colitis (33). The increased SGC coupling in colitis is preferentially around colonic afferent neurons with a twofold increase (33). The newly formed interneuron SGC coupling following colitis may provide a passage to convey nociceptive information from colonic afferent neurons to SGC-coupled remote neurons such as bladder afferent neurons to promote chronic colon-to-bladder cross-organ sensitization, whereas this notion needs to be confirmed by reducing gap junction expression and activities to block chronic SGC coupling. In another scenario where DRG neurons are acutely coactivated in a cluster following complete Freund adjuvant (CFA)-induced inflammatory pain, the coupled activation of DRG neurons is reduced by the Cre-based deletion of connexin 43 (Cx43) from peripheral glia including SGCs (42). Interestingly, Cx43 in SGCs declines in old age (68) whereas the dye coupling among SGCs increases with age (36). This suggests that additional gap junction proteins other than Cx43 may participate in SGC dye coupling in aging.
Gliotransmitters released by SGCs following inflammation or nerve injury include nerve growth factor (NGF) (100), neurotrophin-3 (NT-3) (100), tumor necrosis factor-α (TNF-α) (78), ATP, glutamate, NO, interleukins, and fractalkine (27). TNF-α release from SGCs is promoted by the activation of P2X7 receptor cation channels (92). SGCs also possess immune-like functions and express chemokines, major histocompatibility complex (23), and Toll-like receptors (60). After TNBS-induced colonic inflammation, TNF-α is upregulated on day 4 in DRG, presumably from SGCs (78). The chronic responses of TNF-α in DRG to colonic inflammation are not known, which limits the understanding of neuroinflammation in chronic visceral pain and cross-organ sensitization.
Macrophage-SGC-Neuron Triad
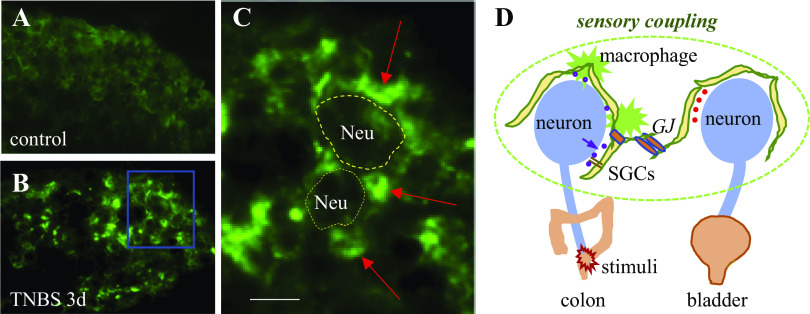
The level of macrophages in DRG in the physiological state is low. TNBS-induced colonic inflammation facilities macrophages clustering around DRG neurons (Fig. 2). Local nerve injury by chronic tube compression that raises sensory activity and widespread pain also increases macrophages in DRG (72). The proliferation of resident macrophages is driven by colony stimulating factor (CSF1) that is released by the injured sensory neurons following peripheral nerve injury and leads to macrophage clustering around the injured neurons (91). Targeted deletion of CSF1 from sensory neurons reduces macrophage expansion, suggesting a paracrine action of CSF1 in mediating sensory neuron–macrophage cross talk (91).
Fig. 2.
Macrophage accumulation around DRG neurons following colitis. We generated mice by the Csf1r-Cre-based expression of GFP to label CSF1R expressing macrophages and microglia. We induced colonic inflammation by TNBS in adult female mice according to publications (25, 47). Control animals received EtOH vehicle treatment. Three days later, animals were perfused by transcardiac Krebs buffer followed by 4% paraformaldehyde. T13 to L2 DRGs were extracted and sectioned for 10-µm thickness for visualization of GFP-labeled macrophages. Macrophages in control animals were low (A). Macrophages clustered around DRG neurons following TNBS treatment (B and C, indicated by the red arrows; bar = 20 µm). Macrophages and SGCs surround DRG neurons to form a network to regulate DRG neuron intraganglionic interaction (D). SGCs are coupled by gap junctions (GJs). Mediators released by colonic afferent neurons following colonic inflammation can act on SGCs and macrophages; likewise, gliotransmitters and inflammatory factors released by SGCs and/or macrophages can also act on DRG neurons (D). DRG, dorsal root ganglia; GFP, green fluorescent protein; SGCs, satellite glial cells; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
In addition to the effects of sensory neurons on macrophage expansion and activation, macrophages in turn can also affect sensory neuron activity by releasing various inflammatory factors. After sciatic nerve injury, the number of macrophages in DRG gradually increases by day 7 and persists up to 28 days, which contributes to the upregulation of TNF-α, IL-6, IL-1β, oncomodulin (OCM), and leukemia inhibitory factor (LIF) (46). The persistent upregulation of macrophage-derived proinflammatory factors is a key mediator in the development of chronic pain. Depletion of DRG macrophages also reduces nerve injury-induced long-lasting mechanical hypersensitivity (91). In colon-to-bladder cross-organ sensitization, the intrathecal administration of minocycline attenuates colitis-induced bladder hyperactivity (56). Minocycline is a nonspecific antibiotic that decreases injury-induced macrophages/microglia activation. The effects of minocycline on inhibiting macrophage activity in this context have not been examined.
When macrophages proliferate/migrate near DRG neurons, they develop close contact with SGCs. Following sciatic nerve transection, macrophage accumulation around DRG neurons precedes SGC activation (45); therefore, macrophages may be more prominent than SGCs in the initiation of nerve injury pain (45, 91). The elongated macrophage processes physically adhere to SGCs at the chronic stage (14 days) after axotomy (45). TNF-α produced by macrophages in DRG downregulates the inwardly rectifying K+ channel Kir4.1 in SGCs and contributes to SGC activation (76); together, macrophages and SGCs orchestrate to maintain the chronic pain state (76, 91). Investigation of the ensemble of neuron-macrophage-SGC-neuron in sensory ganglia is fast growing. The progress in this area may shed light on the understanding of sensory neuron cross-activation, an intricate process in cross-organ sensitization.
STORY ON DICHOTOMIZING AFFERENTS
In double dye neuronal tracing of organ-specific primary afferent neurons in DRG, a unique group of neurons are identified to contain both dyes that are injected individually into two distinct organs, suggesting that these neurons (convergent neurons) have dichotomizing distal axons splitting into the two different organs. The number of convergent neurons for each pair of cross-interacted organs are diverse, but in general are small, and are organ-dependent (Table 2). The most extensively characterized organs are the urinary bladder and the distal gut for their convergent inputs to both DRG and the spinal cord (24). Results from a number of laboratories including ours demonstrate that colon and bladder convergent primary afferent neurons compose ∼10–15% of the single dye-labeled either bladder afferent neurons or colonic afferent neurons (24, 70). Similar proportions of sensory neurons have dichotomizing axons projecting to both the prostate and the urinary bladder (22, 74), and the colon and the uterus (9). Double labeling of the suprabasal epidermis and the viscera in the peritoneal cavity also identifies a small number of (not being quantified) convergent neurons in DRG (11). Interestingly, the degree of primary afferent convergency to DRG is very much organ-specific. In double labeling of the pancreas and the duodenum, the percentage of convergent neurons in ratio to single labeled neurons is ∼50% (51), whereas the percentage of double labeled neurons is less than 3% of either of the single dye labeled neurons that have axons innervating the lower back skin and the urinary bladder (75), or the knee and the lower back muscle (63), or the deep back muscle and intervertebral disk, facet joint, or sacroiliac joint (82). Especially for the urinary bladder and back skin double labeling, ∼0.05–0.1% of dichotomizing neurons are identified in DRG even though these primary afferent neurons are housed in the same DRG (75). A more comprehensive study on double injection of the different areas of the stomach and the small intestine reveals that the percentage of convergent neurons depends on the distance between the injection sites (94), which ranges from 6% (fundus and duodenum with a distance larger than 3 cm) to 48% (fundus and corpus with a distance of 1 cm) in DRG (94). The percentage of double labeled neurons of fundus and pylorus (distance of 2 cm) is in the middle range (36% for DRG) (94). This notion is also reflected in double labeling of other organs: the facet joint and the lower back muscle have 7–17% dichotomizing afferents (83), whereas the peroneal and tibial nerves have ∼50% of labeled neurons being double labeled (73). The number of convergent neurons in nodose ganglia projecting to the stomach and the small intestine also depends on the distance of the two injection sites in the organs (94). Postmortem examination reveals dye spreads between the two injected sites when they are anatomically close (94); this limits the technique of using double dye tracing to identify spinal dichotomizing afferents of two anatomically adjacent organs.
Table 2.
Organs/tissues with dichotomizing afferents from the highest to lowest percentage
| Organs/Tissues | Species | Approximate Range, % | Ref. No. |
|---|---|---|---|
| Peroneal and tibial nerves | Pigeon | 50 | 73 |
| Pancreas and duodenum | Rat | 40–50 | 51 |
| Fundus and corpus | Mouse | 48 | 94 |
| Fundus and pylorus | Mouse | 36 | 94 |
| Prostate and bladder | Rat and mouse | 17–20 | 22, 74 |
| Colon and bladder | Rat and mouse | 10–15 | 24, 70 |
| Colon and uterus | Rat | 10–15 | 9 |
| Facet joint and lower back muscle | Rat | 7–17 | 83 |
| Colon and urethra | Rat | 7–9 | 89 |
| Fundus and duodenum | Mouse | 6 | 94 |
| Knee and lumbar muscle | Rat | 1 | 63 |
| Bladder and lower back skin | Rat | 0.05–0.1 | 75 |
The experiments to depict the role of dichotomizing afferents in cross-organ sensitization are currently limited to few descriptive characterizations. After colonic inflammation, the levels of CGRP are increased (70) and the voltage and current thresholds for action potential firing are decreased (57) in colon-bladder convergent neurons. Prostatic inflammation also enhances the responsiveness of prostate-bladder convergent neurons to capsaicin, downregulates the slow KA current density and Kv1.4 expression, and upregulates TrpV1, TrpA1, and P2X2 expression in the double labeled prostate-bladder primary afferent neurons (22). The changes in these neurochemical coding and ion channel properties in the dichotomizing afferents are much like those in single dye labeled uninjured urinary bladder afferent neurons following colonic or prostatic inflammation (22, 57, 70). Because of the lack of specific molecular characteristics that distinguish the convergent neurons from the single dye labeled neurons of a specific organ, pinpointing the cause-effect relationship of the dichotomizing afferents in mediating cross-organ sensitization is significantly hindered. Single-cell RNA-seq has been used to identify subtypes of colonic afferent neurons (31). This technique may also be applicable to explore unique neurochemical coding in dichotomizing afferents to identify specific molecular targets to study the functional role of dichotomizing afferents in cross-organ sensitization.
The population of single labeled bladder afferents very much outweighs the dichotomizing afferents that project to both the colon and the urinary bladder or the prostate and the urinary bladder, suggesting that the activation of single labeled bladder afferent neurons following colonic inflammation (57, 70, 86) or prostatic inflammation (22) may be a major driving force in colon-to-bladder or prostate-to-bladder cross-organ sensitization. The activation of single labeled bladder afferent neurons in these disease contexts is prolonged during remission of organ inflammation, therefore they are more likely to participate in the maintenance of chronic cross-organ sensitization. The anatomical and morphological characteristics of dichotomizing afferents may endow them an axon-axon reflex through their bifurcated axons to convey information from the injured organ to the uninjured organ to initiate neurogenic inflammation. Interestingly, the double-labeled and single-labeled bladder afferent neurons have the same neurochemicals and ion channels regulated in the chronic stages following inflammation (22, 57, 70). It is likely that they share similar activation mechanisms by autocrine/paracrine factors and have similar physiological functions in chronic cross-organ sensitization.
MECHANISMS: SPINAL TRANSEGMENTAL CROSS TALK
It is perplexing how bowel dysfunction leads to pain in the legs and hypersensitivity of the feet. Since the primary afferent neurons from the colon and the lower extremities locate in different spinal segments, this viscerosomatic cross-organ sensitization should involve a central mechanism by which inputs from the diseased colon are centrally organized and relayed along the spinal column to reach the sensory reflex circuits of the somatic organs. Spinal central sensitization is a fundamental process to convey perception-related information including pain. Spinal central sensitization is triggered by excessive mediators released into the spinal cord dorsal horn due to peripheral sensory nerve stimulation (49). These mediators in the spinal cord modulate the activity of nearby neurons, nonneuronal cells, and the central nerve terminals of primary afferent neurons of a distant, uninjured organ to cause cross-organ sensitization.
Activation of the NMDAR is a hallmark of spinal central sensitization (49). In a clinical study of IBS-associated cutaneous hypersensitivity in the foot, administration of dextromethorphan, an NMDAR antagonist, nearly completely blocks the referred somatic hypersensitivity (96). The activity of the NMDAR is modulated by phosphorylation of its subunit NR1. In TNBS-induced colitis, NR1 expression and phosphorylation are upregulated in the spinal cord (53, 97). Bladder inflammation also increased NR1 phosphorylation in the spinal dorsal horn (41). In an animal model of nerve injury pain, a Cre-based deletion of NMDAR subunit GluN1, a required subunit for NMDAR functionality, blocks the development of chronic pain but has little effects on the onset of acute pain hypersensitivity (38). The NMDAR activity is increased by BDNF (53), CGRP (41), and glutamate (49). The NMDAR-mediated central sensitization involves NMDAR-facilitated loss of γ-aminobutyric acid (GABA)ergic spinal dorsal horn neurons due to excessive Ca2+ influx-induced neurotoxicity, causing disinhibition (38). However, activation of the NMDAR is not the only pathway to induce spinal central sensitization. The activation (phosphorylation) of cAMP response element-binding protein (CREB), a molecular switch of cellular function, is promoted independently in the spinal cord by the NMDAR and the phosphoinositide 3-kinase (PI3K)/Akt pathway that are both triggered by CGRP (41). In this section, we will discuss the possible mechanisms by which spinal central sensitization is carried on in the spinal cord beyond segmental discrimination to cause cross-sensitization of two organs (e.g., the colon and lower extremity) whose primary afferent neurons project to different segments of the spinal cord.
Sensory Fiber Sprouting
The central axons of peptidergic nociceptive neurons can be visualized by CGRP immunoreactivity. The peptidergic nerve terminals communicate with nearby cells by the release of bioactive chemicals. Besides classical synaptic transmission, neurotransmitters are released by volume transmission in which bioactive substances “leak” from varicosities and diffuse into extracellular space (81). The extrasynaptic neurotransmitters can travel long distances, up to millimeters, to interact with their receptors that are more sensitive than receptors on the postsynaptic terminals (81). Extracellular vesicles (i.e., exosomes) released by DRG neurons and nerve terminals are one of the forms of volume transmission to carry bioactive compounds to nearby cells (77). A recent study using high-frequency stimulation (HFS) to activate mouse sciatic nerves shows that CGRP-immunoreactive nerve fibers in the spinal cord contain more varicosities after HFS (95). In addition, the HFS of sciatic nerves that sensitizes sensory neurons in L4-L5 DRG promotes CGRP nerve growth not only into the L4-L6 spinal segments but also extending to the L3 and S1 spinal dorsal horn which do not contain axonal terminals of the L4-L5 DRGs (95). The extension of CGRP sensory fibers to these distant spinal segments can sensitize these spinal regions and lead to transsegmental spinal cross-activation. Increased intensity of colonic afferent central terminals and CGRP nerve fiber sprouting in the spinal cord are also detected following TNBS-induced colitis (28, 69). Examined on day 7 following TNBS treatment, there is a greater density of CGRP fibers in the L1 and S1 spinal dorsal horn (69): CGRP fibers extend toward deep laminae and central commissure in the L1 spinal cord; CGRP fibers also sprout into the Lissauer’s tract of the S1 spinal cord that has a critical role in the regulation of bladder activity (69). Although CGRP mRNA is not increased in L6 DRG in TNBS colitis, CGRP nerve fibers and Akt phosphorylation are increased in the L6 spinal cord (69) where CGRP activates Akt and the NMDAR (41), leading to spinal central sensitization and spinal transsegmental sensory cross talk.
Microglia-Astrocyte-Sensory Nerve Interplay
The two most abundant nonneuronal cells in the spinal cord are microglia and astrocytes. Microglia are primary resident immune cells and the first line of defense with macrophage-like characteristics. In the physiological state, microglia are actively involved in immune surveillance to maintain neuronal homeostasis. In response to injury signals, microglia undergo microgliosis that involves morphological changes, proliferation, and hypertrophy. Astrocytes in the spinal cord are connected by gap junctions to form a network. They are star-shaped with leaflet terminals to enwrap synapses. It is estimated that a single mature rodent astrocyte is in contact with more than 100k individual synapses which mediates signal cross talk between these synapses by exchanging chemicals between nerves and astrocytes. Upon receiving presynaptic signals from the primary afferent neurons following injury, astrocytes undergo astrogliosis that involves morphological changes, proliferation, and differentiation into reactive astrocytes and scar-forming astrocytes. The reactive astrocytes actively participate in neuroplasticity through releasing gliotransmitters that linger nearby or travel long distances to interact with microglia and neurons.
A recent study shows that microglia in the spinal cord participate in colon-to-bladder cross-organ sensitization (56). Rats that received TNBS treatment of the distal colon show urinary bladder hyperactivity examined on day 7 of colitis. Concomitantly, the immunoreactivity of CD11b, a microglial marker, is increased in the L6 spinal cord by colitis. The mRNA expression of IL-1β, CCL3, and BDNF in the spinal cord is also upregulated by colitis. Intrathecal administration of minocycline attenuates colitis-induced bladder hyperactivity and reduces CD11b and the microglia mediators in the spinal cord. Minocycline treatment also reduces the numbers of mast cells in the urinary bladder that are evoked by colitis (56). The number of astrocytes is also upregulated in the spinal cord 14 days following colitis (54). In bladder inflammation-induced chronic referred pain in rats, both astrocytes and microglia are elevated in the dorsal horn of the spinal cord (18). In these nonneuronal cells, BDNF receptors are highly expressed to mediate p38/JNK signaling to lead to the production of TNF-α and IL-1β (18). Intrathecal inhibition of astrocytes with L-α-aminoadipate (LAA) reduces cystitis-induced referred mechanical allodynia (18).
Microgliosis in the spinal cord can be triggered by multiple sensory mediators released by primary afferent neurons such as CSF1, cytokines and chemokines (CCL2, CXCL1, and CCL21), ATP, and fractalkine (10). In turn, microglia, upon activation, produce diverse inflammatory mediators such as BDNF, TNF-α, and interleukins (10) that can act on nearby cells and nerves to facilitate neuroplasticity. For example, BDNF released by microglia promotes CGRP sensory fiber sprouting from the injured spinal segments to the uninjured segments to facilitate transsegmental cross-sensitization (95). In addition to microglia that produce BDNF and TNF-α, BDNF and TNF-α are also anterogradely transported from sensory ganglia to the spinal cord; together, they drive astrogliosis following noxious peripheral stimulation. Astrocytic TrkB.T1 mediates BDNF-induced astrocyte morphogenesis (32). IL-1α and TNF-α released by microglia are each essential to transform naïve astrocytes into reactive astrocytes (39). Mediators produced by astrocytes, such as glial cell line-derived neurotrophic factor (GDNF), lipocalin (LCN2), and chemokines (CCL2 and CXCL10), also act on microglia to promote microglia activation and motility (39). The bidirectional intercell cross talk of microglia and astrocytes orchestrates the processes of synaptic information and maintenance of synaptic strength.
Astrocytes, microglia, and presynaptic and postsynaptic terminals constitute a “quadripartite synapse” unit. Factors produced by microglia and astrocytes including BDNF, cytokines, and others (10, 39) act as paracrine mediators in cell-cell and cell-nerve cross talk. TNF-α produced by microglia in the spinal cord increases cyclooxygenase-2 (Cox-2) to facilitate the production of prostaglandins. Upregulation of gap junction Cx43 in astrocytes and opening of Cx43 hemichannels leads to release of ATP, glutamate, and chemokines. The presynaptic terminals of DRG neurons contain receptors for BDNF, TNF-α, glutamate, CGRP, and prostaglandins thereby the sensory neurons can be sensitized or resensitized by mediators that are released by nonneuronal cells and sensory nerve sprouts in the spinal cord in a retrograde fashion, forming a feed-forward loop to enhance the excitability of sensory neurons that project to different organs (Fig. 3). One example of the spinal retrograde sensitization of sensory neurons is that intrathecal administration of NMDA or prostaglandin E2 produces bilateral paw hypernociception, which is abolished following Nav1.8 sodium channel antisense-induced downregulation of the Nav1.8 channels in DRG (65). The postsynaptic second-order neurons in the spinal cord are also responsive to astrocytic and microglial mediators. The mediators released by the activated nonneuronal cells, in a similar manner as volume transmission, diffuse long distances to act on presynaptic nerve terminals of primary afferent neurons that innervate a variety of organs, causing sensory cross-activation and widespread pain.
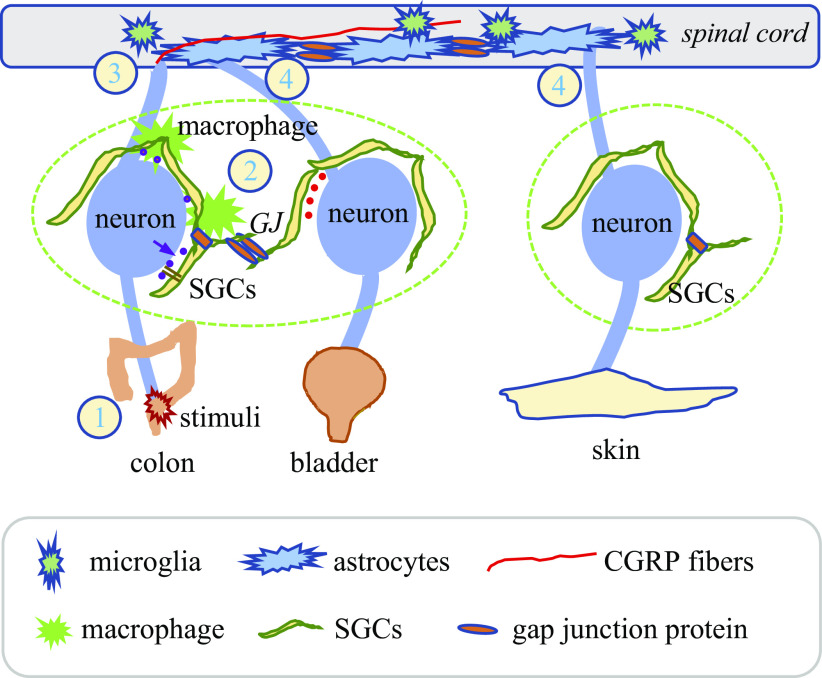
Fig. 3.
Possible mechanisms of cross-organ sensitization involve intraganglionic signal cross talk and spinal central sensitization. Upon noxious stimulation of the distal colon such as TNBS-induced colonic inflammation (1), sensory mediators released by colonic afferent neurons act on adjacent macrophages and SGCs to convey sensory information to nearby neurons to mediate sensory neuron cross-activation within DRG (2). Sensory mediators released into the spinal dorsal horn induce microgliosis and astrogliosis (3). Activation of nonneuronal cells (macrophages, SGCs, microglia, and astrocytes) produces inflammatory factors, causing neuroinflammation. The formation of gap junctions between astrocytes also conveys information to a distant area in the spinal cord. The close contact of spinal factors to the central terminals of primary afferent neurons leads to retrograde sensitization of these neurons (4). CGRP fiber sprouting transsegmentally and volume transmission are also critical contributors in spinal central sensitization and cross-sensitization of organs when their primary afferent neurons do not locate in the same DRG (e.g., colon and lower extremity). CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglia; SGCs, satellite glial cells; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
SPINAL CONVERGENCY AND WALLERIAN DEGENERATION
Spinal neurons that are responsive to both CRD and urinary bladder distension are identified in the superficial dorsal horn (14% recorded neurons) and deep lamina (29% recorded neurons) (71). Among these convergent spinal neurons, 45% are excited by distension of both organs, whereas the rest exhibit mixed patterns of excitation and inhibition (71). A recent study on characterizing the synaptic architecture of the spinal dorsal horn using a series of sophisticated genetic tracing shows that each interneuron can receive inputs from at least one to three primary afferent classes (1). This study specifically examines the LTMRs in mediating mechanosensory activity (1). It may also present a nature for other types of sensory neurons such as nociceptors. Indeed, convergent nociceptive inputs to the spinal cord are identified in both humans and animals. The functional role of sensory convergency in the spinal cord in cross-organ sensitization needs further investigation. In a similar situation to dichotomizing afferents, the intervention of the convergent neurons in the spinal cord is challenging due to lack of specific approaches to separate them from neurons projecting to a single organ.
Anatomically, nerve fibers of bladder afferent neurons and colonic afferent neurons exit DRG in the same nerve bundles and comingle with each other before separating to innervate each specific organ. In a model of neuropathic pain in which partial L5 nerve transection can induce L4 DRG hypersensitivity, Wallerian degeneration is suggested to mediate L5-to-L4 cross-sensitization. In this context, the injury of L5 afferents releases inflammatory mediators to affect nearby L4 fibers in the same bundle, and subsequently excites the uninjured L4 afferent neurons. Whether Wallerian degeneration is also involved in colon-bladder cross-organ sensitization is not yet examined.
CONCLUDING REMARKS
Cross-organ sensitization is suggested to underlie the comorbidity of pelvic organs and somatic secondary hyperalgesia in patients and experimental animals. This involves a process of communicating sensory information from the primarily injured organ to a distant uninjured organ, in which a cascade of neuronal activation occurs in DRG and the spinal cord, and beyond. To tease out the complex neural interaction in cross-organ sensitization, the use of animal models is necessary. Thus far, TNBS-colitis is mostly utilized for the study of bowel dysfunction-associated bladder hyperactivity and somatic pain. Other models are also developed and briefly tested. The knowledge obtained from these animal models and other pain models suggest that sensory neuron cross-activation involving SGCs and macrophages is the initial step in cross-organ sensitization (Fig. 2). Mediators released distally by sensitized DRG neurons cause neurogenic inflammation of the peripheral organ. Mediators released within the sensory ganglia act on nearby neurons and nonneuronal cells in a paracrine manner to facilitate sensory neuron cross-activation. Sensory mediators released centrally into the spinal cord lead to spinal central sensitization that is strengthened by the activation of astrocytes and microglia (Fig. 3). Bidirectional interactions between macrophages and SGCs in DRG and between astrocytes and microglia in the spinal cord are prominent contributors to neuroinflammation. Astrocytic networks and volume transmission from astrocytes and sensory nerve terminals that sprout transsegmentally along the spinal cord convey sensory information from one level of DRG to another. Retrograde sensitization of primary afferent neurons by spinal mediators finally finishes a possible feed-forward loop in viscerovisceral and viscerosomatic cross-organ sensitization. Sensory information from two distinct organs can also converge on neurons in DRG and the spinal cord. Dichotomizing afferents of two adjacent organs are identified by anatomical labeling, however there is a lack of evidence on their functional roles in mediating cross-organ sensitization. DRG locates outside the blood-brain barrier and is the first hub for pain generation and cross-organ sensitization. Recently, electrical stimulation of DRG is proved to be effective to alleviate pain. Targeting DRG to block the initiation of cross-organ sensitization could be a safer approach to have less adverse effects on the central nervous system. Although some information has been obtained to suggest the involvement of sensory cross talk in DRG in cross-organ sensitization, much is descriptive which is likely due to the limitation of delivery of drugs specifically to DRG. Cre-based genetic intervention may help to pinpoint the mechanisms of sensory neuron cross talk within DRG. An emerging and clinically important area for further investigation includes the interaction of neurons, glial cells, and immune cells within DRG that may contribute to cross-organ sensitization and chronic pain at large.
GRANTS
This review was supported by National Institutes of Health (NIH) Grants R01 DK118137 and CHRB 236-06-18, Virginia Commonwealth University’s Clinical Translational Science Award KL2TR002648, the Center for Clinical and Translational Research Endowment Fund, and NIH P30 DA033934 (subcontract).
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Y.Q. conceived and designed review; L.Y.Q. prepared figures; L.Y.Q. and N.T. drafted manuscript; L.Y.Q. edited and revised manuscript; L.Y.Q. and N.T. approved final version of manuscript.
REFERENCES
- 1.Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168: 295–310.e19, 2017. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374: 450–453, 1995. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 3.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49, Suppl: 52–57, 1997. doi: 10.1016/S0090-4295(99)80332-X. [DOI] [PubMed] [Google Scholar]
- 4.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci 16: 4733–4741, 1996. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari R, Attari F, Razjouyan H, Etemadi A, Amjadi H, Merat S, Malekzadeh R. Ulcerative colitis and irritable bowel syndrome: relationships with quality of life. Eur J Gastroenterol Hepatol 20: 46–50, 2008. doi: 10.1097/MEG.0b013e3282f16a62. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, Papalois A, Pikoulis E. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond) 11: 9–15, 2016. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci 153: 106–115, 2010. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter D, Beer-Gabel M. Lower urinary tract symptoms in chronically constipated women. Int Urogynecol J Pelvic Floor Dysfunct 23: 1785–1789, 2012. doi: 10.1007/s00192-012-1812-1. [DOI] [PubMed] [Google Scholar]
- 9.Chaban VV Visceral sensory neurons that innervate both uterus and colon express nociceptive TRPv1 and P2X3 receptors in rats. Ethn Dis 18, Suppl 2: S2–S20, 2008. [PubMed] [Google Scholar]
- 10.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 100: 1292–1311, 2018. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol 17: 578–589, 2011. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen SL, Ernstsen C, Olesen J, Kristensen DM. No central action of CGRP antagonising drugs in the GTN mouse model of migraine. Cephalalgia 40: 924–934, 2020. doi: 10.1177/0333102420914913. [DOI] [PubMed] [Google Scholar]
- 13.Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain 151: 540–549, 2010. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 75: 583–593, 2013. doi: 10.1016/j.neuropharm.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One 10: e0123810, 2015. doi: 10.1371/journal.pone.0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 55: 940–945, 2006. doi: 10.1136/gut.2005.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol 64: 1733–1746, 1990. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- 18.Ding H, Chen J, Su M, Lin Z, Zhan H, Yang F, Li W, Xie J, Huang Y, Liu X, Liu B, Zhou X. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J Neuroinflammation 17: 19, 2020. doi: 10.1186/s12974-020-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong F, Zhang L, Hao F, Tang H, Wang Y. Systemic responses of mice to dextran sulfate sodium-induced acute ulcerative colitis using 1H NMR spectroscopy. J Proteome Res 12: 2958–2966, 2013. doi: 10.1021/pr4002383. [DOI] [PubMed] [Google Scholar]
- 20.Drewes AM, Frøkjaer JB, Larsen E, Reddy H, Arendt-Nielsen L, Gregersen H. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm Bowel Dis 12: 294–303, 2006. doi: 10.1097/01.MIB.0000209365.09189.04. [DOI] [PubMed] [Google Scholar]
- 21.Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol 303: G817–G824, 2012. doi: 10.1152/ajpgi.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funahashi Y, Takahashi R, Mizoguchi S, Suzuki T, Takaoka E, Ni J, Wang Z, DeFranco DB, de Groat WC, Tyagi P, Yoshimura N. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol 597: 2063–2078, 2019. doi: 10.1113/JP277452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graus F, Campo E, Cruz-Sanchez F, Ribalta T, Palacin A. Expression of lymphocyte, macrophage and class I and II major histocompatibility complex antigens in normal human dorsal root ganglia. J Neurol Sci 98: 203–211, 1990. doi: 10.1016/0022-510X(90)90261-K. [DOI] [PubMed] [Google Scholar]
- 24.Grundy L, Brierley SM. Cross-organ sensitization between the colon and bladder: to pee or not to pee? Am J Physiol Gastrointest Liver Physiol 314: G301–G308, 2018. doi: 10.1152/ajpgi.00272.2017. [DOI] [PubMed] [Google Scholar]
- 25.Grundy L, Harrington AM, Castro J, Garcia-Caraballo S, Deiteren A, Maddern J, Rychkov GY, Ge P, Peters S, Feil R, Miller P, Ghetti A, Hannig G, Kurtz CB, Silos-Santiago I, Brierley SM. Chronic linaclotide treatment reduces colitis-induced neuroplasticity and reverses persistent bladder dysfunction. JCI Insight 3: e121841, 2018. doi: 10.1172/jci.insight.121841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberberger RV, Barry C, Dominguez N, Matusica D. Human dorsal root ganglia. Front Cell Neurosci 13: 271, 2019. doi: 10.3389/fncel.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 21: 485–498, 2020. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington AM, Brierley SM, Isaacs N, Hughes PA, Castro J, Blackshaw LA. Sprouting of colonic afferent central terminals and increased spinal mitogen-activated protein kinase expression in a mouse model of chronic visceral hypersensitivity. J Comp Neurol 520: 2241–2255, 2012. doi: 10.1002/cne.23042. [DOI] [PubMed] [Google Scholar]
- 29.Hashmi F, Liu M, Shen S, Qiao LY. EXPRESS: Phospholipase C gamma mediates endogenous brain-derived neurotrophic factor - regulated calcitonin gene-related peptide expression in colitis-induced visceral pain. Mol Pain 12: 1744806916657088, 2016. doi: 10.1177/1744806916657088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho IHT, Liu X, Zou Y, Liu T, Hu W, Chan H, Tian Y, Zhang Y, Li Q, Kou S, Chan CS, Gin T, Cheng CHK, Wong SH, Yu J, Zhang L, Wu WKK, Chan MTV. A novel peptide interfering with proBDNF-sortilin interaction alleviates chronic inflammatory pain. Theranostics 9: 1651–1665, 2019. doi: 10.7150/thno.29703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hockley JRF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, Smith ESJ. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68: 633–644, 2019. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt LM, Hernandez RD, Pacheco NL, Torres Ceja B, Hossain M, Olsen ML. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. eLife 8: e44667, 2019. doi: 10.7554/eLife.44667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain 14: 49.e1–49.e11, 2010. doi: 10.1016/j.ejpain.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Huang TY, Cherkas PS, Rosenthal DW, Hanani M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res 1036: 42–49, 2005. doi: 10.1016/j.brainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol 289: G670–G678, 2005. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- 36.Huang TY, Hanani M, Ledda M, De Palo S, Pannese E. Aging is associated with an increase in dye coupling and in gap junction number in satellite glial cells of murine dorsal root ganglia. Neuroscience 137: 1185–1192, 2006. doi: 10.1016/j.neuroscience.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58: 1333–1341, 2009. doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- 38.Inquimbert P, Moll M, Latremoliere A, Tong CK, Whang J, Sheehan GF, Smith BM, Korb E, Athié MCP, Babaniyi O, Ghasemlou N, Yanagawa Y, Allis CD, Hof PR, Scholz J. NMDA Receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Reports 23: 2678–2689, 2018. doi: 10.1016/j.celrep.2018.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist 25: 227–240, 2019. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [125I] galcanezumab in male rats. Cephalalgia 39: 1241–1248, 2019. doi: 10.1177/0333102419844711. [DOI] [PubMed] [Google Scholar]
- 41.Kay JC, Xia CM, Liu M, Shen S, Yu SJ, Chung C, Qiao LY. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Exp Neurol 250: 366–375, 2013. doi: 10.1016/j.expneurol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 91: 1085–1096, 2016. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimball ES, Palmer JM, D’Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol 288: G1266–G1273, 2005. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- 44.Kotton CN Nailing down the shingles in IBD. Inflamm Bowel Dis 13: 1178–1179, 2007. doi: 10.1002/ibd.20161. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan A, Bhavanam S, Zochodne D. An intimate role for adult dorsal root ganglia resident cycling cells in the generation of local macrophages and satellite glial cells. J Neuropathol Exp Neurol 77: 929–941, 2018. doi: 10.1093/jnen/nly072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci 33: 15095–15108, 2013. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 92: 335–342, 2001. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 48.Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- 49.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10: 895–926, 2009. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei Q, Pan XQ, Villamor AN, Asfaw TS, Chang S, Zderic SA, Malykhina AP. Lack of transient receptor potential vanilloid 1 channel modulates the development of neurogenic bladder dysfunction induced by cross-sensitization in afferent pathways. J Neuroinflammation 10: 772, 2013. doi: 10.1186/1742-2094-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C, Zhu Y, Shenoy M, Pai R, Liu L, Pasricha PJ. Anatomical and functional characterization of a duodeno-pancreatic neural reflex that can induce acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 304: G490–G500, 2013. doi: 10.1152/ajpgi.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin YT, Ro LS, Wang HL, Chen JC. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation 8: 126, 2011. doi: 10.1186/1742-2094-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Kay JC, Shen S, Qiao LY. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 12: 151, 2015. doi: 10.1186/s12974-015-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucarini E, Parisio C, Branca JJV, Segnani C, Ippolito C, Pellegrini C, Antonioli L, Fornai M, Micheli L, Pacini A, Bernardini N, Blandizzi C, Ghelardini C, Di Cesare Mannelli L. Deepening the mechanisms of visceral pain persistence: an evaluation of the gut-spinal cord relationship. Cells 9: 1772, 2020. doi: 10.3390/cells9081772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 120: 677–694, 2003. doi: 10.1016/S0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 56.Majima T, Funahashi Y, Kawamorita N, Takai S, Matsukawa Y, Yamamoto T, Yoshimura N, Gotoh M. Role of microglia in the spinal cord in colon-to-bladder neural crosstalk in a rat model of colitis. Neurourol Urodyn 37: 1320–1328, 2018. doi: 10.1002/nau.23484. [DOI] [PubMed] [Google Scholar]
- 57.Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18: 936–948, 2006. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 58.Matsuka Y, Afroz S, Dalanon JC, Iwasa T, Waskitho A, Oshima M. The role of chemical transmitters in neuron-glia interaction and pain in sensory ganglion. Neurosci Biobehav Rev 108: 393–399, 2020. doi: 10.1016/j.neubiorev.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci 29: 4981–4992, 2009. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitterreiter JG, Ouwendijk WJD, van Velzen M, van Nierop GP, Osterhaus ADME, Verjans GMGM. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur J Immunol 47: 1181–1187, 2017. doi: 10.1002/eji.201746989. [DOI] [PubMed] [Google Scholar]
- 61.Najjar SA, Davis BM, Albers KM. Epithelial-neuronal communication in the colon: Implications for visceral pain. Trends Neurosci 43: 170–181, 2020. doi: 10.1016/j.tins.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh EJ, Weinreich D. Chemical communication between vagal afferent somata in nodose Ganglia of the rat and the Guinea pig in vitro. J Neurophysiol 87: 2801–2807, 2002. doi: 10.1152/jn.2002.87.6.2801. [DOI] [PubMed] [Google Scholar]
- 63.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Calcitonin gene-related peptide immunoreactive neurons with dichotomizing axons projecting to the lumbar muscle and knee in rats. Eur Spine J 12: 576–580, 2003. doi: 10.1007/s00586-003-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panicker JN, Marcelissen T, von Gontard A, Vrijens D, Abrams P, Wyndaele M. Bladder-bowel interactions: Do we understand pelvic organ cross-sensitization? International Consultation on Incontinence Research Society (ICI-RS) 2018. Neurourol Urodyn 38, Suppl 5: S25–S34, 2019. doi: 10.1002/nau.24111. [DOI] [PubMed] [Google Scholar]
- 65.Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci USA 100: 2923–2928, 2003. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peirs C, Seal RP. Neural circuits for pain: Recent advances and current views. Science 354: 578–584, 2016. doi: 10.1126/science.aaf8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 128: 1953–1964, 2005. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Procacci P, Magnaghi V, Pannese E. Perineuronal satellite cells in mouse spinal ganglia express the gap junction protein connexin43 throughout life with decline in old age. Brain Res Bull 75: 562–569, 2008. doi: 10.1016/j.brainresbull.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Qiao LY, Grider JR. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Exp Neurol 219: 93–103, 2009. doi: 10.1016/j.expneurol.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol 204: 667–679, 2007. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport 15: 467–471, 2004. doi: 10.1097/00001756-200403010-00017. [DOI] [PubMed] [Google Scholar]
- 72.Schmid AB, Coppieters MW, Ruitenberg MJ, McLachlan EM. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J Neuropathol Exp Neurol 72: 662–680, 2013. doi: 10.1097/NEN.0b013e318298de5b. [DOI] [PubMed] [Google Scholar]
- 73.Schmid H, Taylor DC, Pierau FK. Tracing of sensory neurones and spinal motoneurones of the pigeon by injection of fluorescent dyes into peripheral nerves. Cell Tissue Res 232: 9–19, 1983. doi: 10.1007/BF00222370. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz ES, La JH, Young EE, Feng B, Joyce S, Gebhart GF. Chronic prostatitis induces bladder hypersensitivity and sensitizes bladder afferents in the mouse. J Urol 196: 892–901, 2016. doi: 10.1016/j.juro.2016.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibata Y, Ugawa S, Imura M, Kubota Y, Ueda T, Kojima Y, Ishida Y, Sasaki S, Hayashi Y, Kohri K, Shimada S. TRPM8-expressing dorsal root ganglion neurons project dichotomizing axons to both skin and bladder in rats. Neuroreport 22: 61–67, 2011. doi: 10.1097/WNR.0b013e3283424c9c. [DOI] [PubMed] [Google Scholar]
- 76.Silva JR, Lopes AH, Talbot J, Cecilio NT, Rossato MF, Silva RL, Souza GR, Silva CR, Lucas G, Fonseca BA, Arruda E, Alves-Filho JC, Cunha FQ, Cunha TM. Neuroimmune-glia interactions in the sensory ganglia account for the development of acute herpetic neuralgia. J Neurosci 37: 6408–6422, 2017. doi: 10.1523/JNEUROSCI.2233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, Vacca V, Pitcher T, Grist J, Al-Ahdal H, Wong LF, Perretti M, Lai J, Mouritzen P, Heppenstall P, Malcangio M. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun 8: 1778, 2017. doi: 10.1038/s41467-017-01841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song DD, Li Y, Tang D, Huang LY, Yuan YZ. Neuron-glial communication mediated by TNF-α and glial activation in dorsal root ganglia in visceral inflammatory hypersensitivity. Am J Physiol Gastrointest Liver Physiol 306: G788–G795, 2014. doi: 10.1152/ajpgi.00318.2013. [DOI] [PubMed] [Google Scholar]
- 79.Stabell N, Stubhaug A, Flægstad T, Nielsen CS. Increased pain sensitivity among adults reporting irritable bowel syndrome symptoms in a large population-based study. Pain 154: 385–392, 2013. doi: 10.1016/j.pain.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Traub RJ, Wang G. Colonic inflammation decreases thermal sensitivity of the forepaw and hindpaw in the rat. Neurosci Lett 359: 81–84, 2004. doi: 10.1016/j.neulet.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 81.Trueta C, De-Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol 3: 319, 2012. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Umimura T, Miyagi M, Ishikawa T, Kamoda H, Wakai K, Sakuma T, Sakai R, Kuniyoshi K, Ochiai N, Kishida S, Nakamura J, Eguchi Y, Iwakura N, Kenmoku T, Arai G, Orita S, Suzuki M, Sakuma Y, Kubota G, Oikawa Y, Inoue G, Aoki Y, Toyone T, Takahashi K, Ohtori S. Investigation of dichotomizing sensory nerve fibers projecting to the lumbar multifidus muscles and intervertebral disc or facet joint or sacroiliac joint in rats. Spine 37: 557–562, 2012. doi: 10.1097/BRS.0b013e3182293178. [DOI] [PubMed] [Google Scholar]
- 83.Wakai K, Ohtori S, Yamashita M, Yamauchi K, Inoue G, Suzuki M, Orita S, Eguchi Y, Ochiai N, Kishida S, Takaso M, Fukui Y, Hayashi Y, Aoki Y, Kuniyoshi K, Nakamura J, Ishikawa T, Arai G, Miyagi M, Kamoda H, Takahashi K. Primary sensory neurons with dichotomizing axons projecting to the facet joint and the low back muscle in rats. J Orthop Sci 15: 402–406, 2010. doi: 10.1007/s00776-010-1465-1. [DOI] [PubMed] [Google Scholar]
- 84.Wyndaele M, De Wachter S, De Man J, Minagawa T, Wyndaele JJ, Pelckmans PA, De Winter BY. Mechanisms of pelvic organ crosstalk: 1. Peripheral modulation of bladder inhibition by colorectal distention in rats. J Urol 190: 765–771, 2013. doi: 10.1016/j.juro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 85.Wyndaele M, De Winter BY, Pelckmans PA, De Wachter S, Van Outryve M, Wyndaele JJ. Exploring associations between lower urinary tract symptoms (LUTS) and gastrointestinal (GI) problems in women: a study in women with urological and GI problems vs a control population. BJU Int 115: 958–967, 2015. doi: 10.1111/bju.12904. [DOI] [PubMed] [Google Scholar]
- 86.Xia C, Shen S, Hashmi F, Qiao LY. Colitis-induced bladder afferent neuronal activation is regulated by BDNF through PLCγ pathway. Exp Neurol 285: 126–135, 2016. doi: 10.1016/j.expneurol.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia CM, Colomb DG Jr, Akbarali HI, Qiao LY. Prolonged sympathetic innervation of sensory neurons in rat thoracolumbar dorsal root ganglia during chronic colitis. Neurogastroenterol Motil 23: 801–e339, 2011. doi: 10.1111/j.1365-2982.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xia CM, Gulick MA, Yu SJ, Grider JR, Murthy KS, Kuemmerle JF, Akbarali HI, Qiao LY. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation 9: 520, 2012. doi: 10.1186/1742-2094-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshikawa S, Kawamorita N, Oguchi T, Funahashi Y, Tyagi P, Chancellor MB, Yoshimura N. Pelvic organ cross-sensitization to enhance bladder and urethral pain behaviors in rats with experimental colitis. Neuroscience 284: 422–429, 2015. doi: 10.1016/j.neuroscience.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 90.Yu SJ, Grider JR, Gulick MA, Xia CM, Shen S, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Exp Neurol 238: 209–217, 2012. doi: 10.1016/j.expneurol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11: 264, 2020. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA 104: 9864–9869, 2007. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Laumet G, Chen SR, Hittelman WN, Pan HL. Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. J Biol Chem 290: 14647–14655, 2015. doi: 10.1074/jbc.M115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong F, Christianson JA, Davis BM, Bielefeldt K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig Dis Sci 53: 194–203, 2008. doi: 10.1007/s10620-007-9843-z. [DOI] [PubMed] [Google Scholar]
- 95.Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, Mai CL, Lin ZJ, Liu Y, Murugan M, Eyo UB, Umpierre AD, Xin WJ, Chen T, Li M, Wang H, Richardson JR, Tan Z, Liu XG, Wu LJ. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Reports 27: 3844–3859.e6, 2019. doi: 10.1016/j.celrep.2019.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Q, Price DD, Callam CS, Woodruff MA, Verne GN. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J Pain 12: 297–303, 2011. doi: 10.1016/j.jpain.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Q, Price DD, Caudle RM, Verne GN. Spinal NMDA NR1 subunit expression following transient TNBS colitis. Brain Res 1279: 109–120, 2009. doi: 10.1016/j.brainres.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 134: 9–15, 2008. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci 53: 429–435, 2008. doi: 10.1007/s10620-007-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci 12: 100–105, 2000. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]