Abstract
Spasmolytic polypeptide/trefoil factor 2 (TFF2)-expressing metaplasia (SPEM) is a mucous-secreting reparative lineage that emerges at the ulcer margin in response to gastric injury. Under conditions of chronic inflammation with parietal cell loss, SPEM has been found to emerge and evolve into neoplasia. Cluster-of-differentiation gene 44 (CD44) is known to coordinate normal and metaplastic epithelial cell proliferation. In particular, CD44 variant isoform 9 (CD44v9) associates with the cystine-glutamate transporter xCT, stabilizes the protein, and provides defense against reactive oxygen species (ROS). xCT stabilization by CD44v9 leads to defense against ROS by cystine uptake, glutathione (GSH) synthesis, and maintenance of the redox balance within the intracellular environment. Furthermore, p38 signaling is a known downstream ROS target, leading to diminished cell proliferation and migration, two vital processes of gastric epithelial repair. CD44v9 emerges during repair of the gastric epithelium after injury, where it is coexpressed with other markers of SPEM. The regulatory mechanisms for the emergence of CD44v9 and the role of CD44v9 during the process of gastric epithelial regeneration are largely unknown. Inflammation and M2 macrophage infiltration have recently been demonstrated to play key roles in the induction of SPEM after injury. The following review proposes new insights into the functional role of metaplasia in the process of gastric regeneration in response to ulceration. Our insights are extrapolated from documented studies reporting oxyntic atrophy and SPEM development and our current unpublished findings using the acetic acid-induced gastric injury model.
Keywords: CD44 variant isoform 9, cystine-glutamate transporter, gastric ulcers, spasmolytic polypeptide-expressing metaplasia
INTRODUCTION
In the United States, increased use of nonsteroidal anti-inflammatory drugs (NSAIDS) and Helicobacter pylori (H. pylori) infection are the leading risk factors for the development of peptic ulcer disease, especially in the elderly (4, 59). H. pylori is a Gram-negative bacterium typically acquired by oral ingestion of fecal-contaminated foods (42). The gastric epithelium possesses many protective mechanisms against ingested pathogens. However, H. pylori has evolved such that mechanisms of motility, mucosal attachment, evasion of host's immune response, and survival within the acidic environment allow for chronic infection. H. pylori produces urease to hydrolyze urea to ammonia and carbon dioxide (4, 76), and thus neutralizing the acidic environment allowing for persistent bacterial infection. H. pylori flagella and adhesins for motility and epithelial binding respectively further increase the pathogenicity of the bacteria. Moreover, most strains express the cag pathogenicity island, a genomic fragment containing genes that increases the host’s susceptibility to the development of gastric ulcers and adenocarcinoma (33, 59). Early studies demonstrate that H. pylori does not generate enough torque to swim through the mucous gel layer protecting the gastric epithelium. Instead, the bacteria achieve motility by rheological modification of the local environment that is facilitated by the coupling of the pH-dependent rheology of gastric mucin and increased pH driven by urease-mediated urea hydrolysis (9). Ultimately, the breakdown of the protective mucous layer exposes the epithelium to even further irritation from gastric acid and pepsin secretion, subsequently leading to ulceration (84).
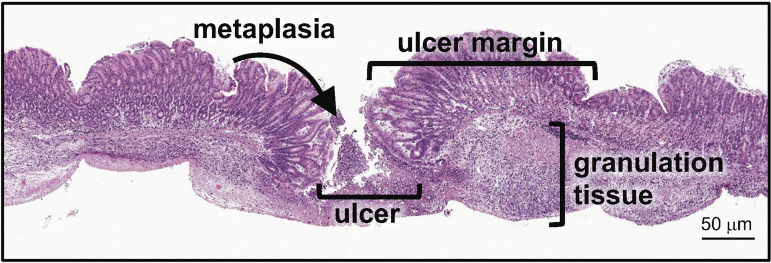
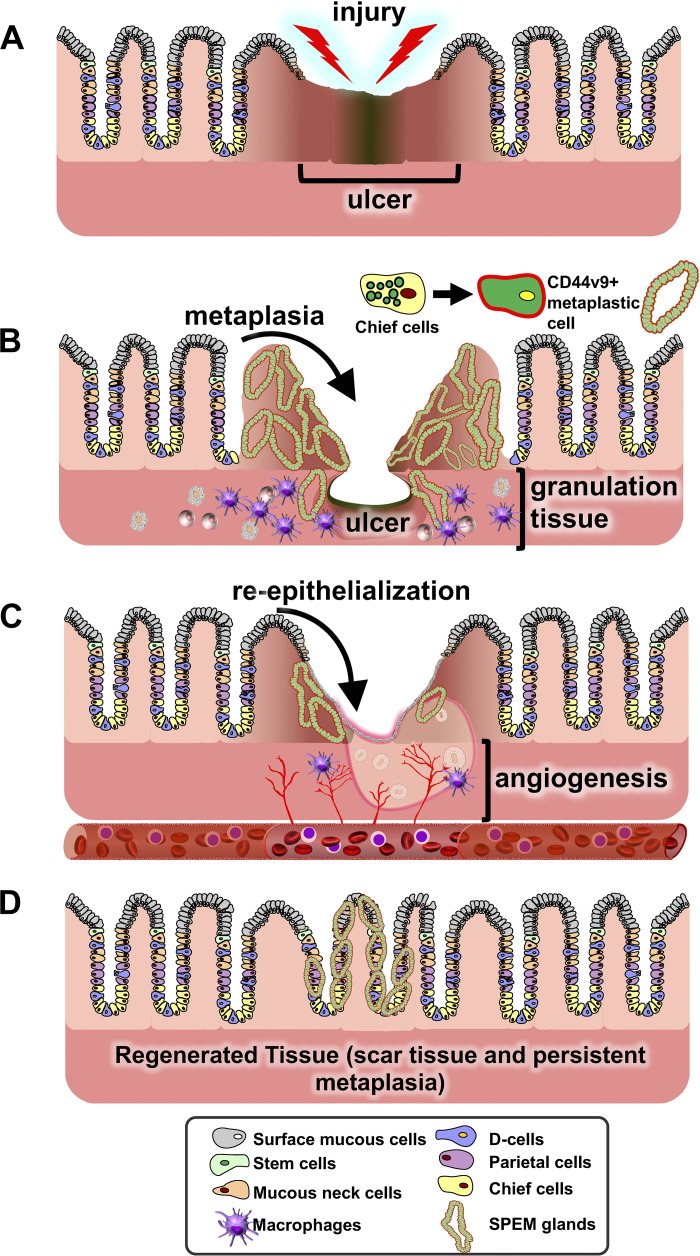
Peptic ulcers are defined as an open wound in the esophageal, gastric, duodenal, or intestinal wall which penetrates through to the muscularis mucosae (12). Wound healing involves four critical and continuous processes that include 1) hemostasis by constriction of vasculature or dilation, 2) inflammation in the wound site, 3) reepithelialization by a process of cellular proliferation, migration, and remodeling, and 4) cell differentiation and maturation (84). The ulcer site can be divided into major histological areas that include 1) the margin surrounding the ulcer, 2) the granulation tissue consisting of connective tissue and infiltrating immune cells, and 3) recently identified emerging metaplastic glands at the ulcer margin that serve as a critical reparative cell lineage (Fig. 1). It was originally reported that, in response to injury, a “healing zone” was formed whereby gastric glands surrounding the ulcer dilated, expressed primarily epidermal growth factor (EGF), and began proliferating onto the granulation tissue to reepithelialize the base of the wound (84). Therefore, quick and efficient reepithelialization is critical in the gastric environment to protect from infection or chemical injury (82, 84, 97, 100). In intestinal regeneration, proliferating epithelial cells at the base of the margin begin to migrate to the granulation tissue, where they form tubes that are referred to as ulcer-associated cell lineages (UACLs) which will later differentiate and replace the lost intestinal epithelium (82, 84, 97, 101). Recently we reported that, following gastric injury, the UACLs are identified as spasmolytic polypeptide-expressing metaplasia (SPEM) that are marked by CD44 variant isoform 9 (CD44v9) (15). SPEM emerges at the ulcer margin in the regenerating gastric glands and is a transient response (15). We suggest that, in response to gastric ulceration, SPEM is a regulated mechanism that contributes to repair (6, 15) (Fig. 2).
Fig. 1.
Representative histology of the injured site within the mouse stomach. Hematoxylin and eosin stain showing metaplastic glands at the ulcer margin and areas of granulation tissue and the ulcer site. Scale bar = 50 μm.
Fig. 2.
Schematic diagram showing the progression of gastric wound healing. A: injury of the gastric epithelium either via chronic nonsteroidal anti-inflammatory drug (NSAID) use or inflammation results in ablation of the gastric epithelium and obvious ulcer margins. B: during the early stages of regeneration there is the emergence of the granulation tissue with infiltrating macrophages and the emergence of spasmolytic polypeptide-expressing metaplasia (SPEM). It is still unknown whether the SPEM glands at the ulcer margins arise from the transdifferentiation of the chief cells to a metaplastic phenotype. Reepithelialization (C) occurs before complete regeneration (D) of the gastric epithelium and transient expression of SPEM.
Coordinating these wound-healing events are growth factors secreted by immune cells and the epithelium at the ulcer margin and adjacent intact tissue (17, 82, 84). Growth factors including EGF, HGF, and FGF, are secreted and interact with their receptors at the epithelium to signal and advance the cellular proliferation and migration (82, 84, 101). Furthermore, cytoskeleton rearrangements and extracellular matrix formations will also direct cell motility (82, 84). EGF-R/MAPK(ERK) signaling plays a critical role in signaling events at the ulcer margin, and its inhibition has been shown to delay ulcer healing (82, 84, 101). In addition, the secretion of sonic hedgehog (Shh) from parietal cells acts as an endocrine factor during gastric regeneration (17). Shh signaling may orchestrate the infiltration of macrophages to the injured site that are instrumental in the repair process (17, 74, 102). Within the granulation tissue, macrophages secrete cytokines such as IL-33 and IL-13 to induce the migration of fibroblasts and the formation of new microvasculature (49, 84). Additionally, these processes in the granulation tissue are important in that they also supply extracellular matrix within the newly formed scar (65, 82). Importantly, in the context of H. pylori infection and parietal cell atrophy, alternatively activated macrophages (M2) are known to promote the progression of SPEM (61, 63) (Fig. 2). Whether macrophages induce SPEM in the setting of gastric regeneration has yet to be reported.
The following review proposes new insights into the functional role of metaplasia in the process of gastric regeneration in response to ulceration. Our insights are extrapolated from documented studies reporting oxyntic atrophy and SPEM development and our current unpublished findings using the acetic acid-induced gastric injury model.
METAPLASIA IN GASTRIC ULCER REPAIR
One of the pathological hallmarks associated with chronic H. pylori infection and gastritis is atrophy of the acid-secreting parietal cells (90). The development of metaplasia is one of the major etiologies associated with the progression from inflammation to cancer. While metaplasia has traditionally been studied in the development of gastric cancer, in recent years studies have emerged demonstrating its active role in gastric regeneration. Nevertheless, this article will review the current knowledge and understanding of the function of SPEM in gastric repair after injury. Because some of the many functions of the stomach are initiation of digestion and acting as a line of defense against foreign pathogens, it is critical that after gastric injury, the repair response be quick and efficient in its reestablishment of the epithelial barrier. Reparative responses to gastric injury are extensive and include cellular proliferation and migration, granular tissue infiltration by immune cells, angiogenesis, and more recently defined, SPEM (15, 82, 84).
SPEM was first reported in mice infected with Helicobacter felis as a gastric mucous cell lineage that expressed trefoil factor 2 (TFF2) also known as spasmolytic polypeptide (SP) (92), a spasmolytic polypeptide found primarily in the antrum and mucous neck cells of the corpus (48, 94). SPEM glands were then identified in 1999 by Schmidt et al. (73) within the body of the stomach and bordering gastric cancer. These glands were found to express TFF2, this time found exclusively in the antrum (48, 94). In addition to TFF2, SPEM glands have since been reported to express other markers found exclusively within the antral glands of the stomach, including CD44v9, clusterin, mucin 6 (Muc6), GSII-lectin, and HE4 (48). While these mucous-secreting SPEM glands are strikingly similar to the antral glands in terms of cellular markers and cellular composition, gastrin cells have not been observed to be expressed within the SPEM glands (25, 73). Mills and colleagues (67, 95) recently defined SPEM using the term paligenosis (from the Greek for “return to the regenerative state”). It is conceivable that paligenosis is a process that extends into the esophageal squamous cells that transdifferentiate into Barrett’s esophagus metaplasia.
One major contributor to gastric ulcer disease and metaplasia development in humans is infection by H. pylori. The organism induces a host of gastric responses, but most importantly it is a known driver of parietal cell loss and inflammation (21, 22, 92). It has previously been reported that conditions of acute injury with parietal cell loss are necessary for SPEM development (25, 93). Other groups have confirmed this by inducing parietal cell loss and injury by treatment with DMP777, L365, or tamoxifen. These groups found that parietal cell loss quickly induces SPEM development, a response that is resolved after resolution of the injury (15). Interestingly, the transition to intestinal-type metaplasia and gastric cancer has been noted to occur only with parietal cell loss in the setting of chronic inflammation (25). Furthermore, while SPEM has been discovered to progress toward intestinal-type metaplasia and gastric cancer, SPEM cell lineages have also been identified to emerge transiently at the margins of ulcers within the regenerating gastric glands (6, 15). It has therefore been postulated that SPEM may act as an initial reparative cell lineage whereas continued chronic injury with inflammation and parietal cell loss may catalyze the progression to intestinal-type metaplasia followed by neoplasia.
Studies by several groups looking at parietal cell loss have corroborated chief cell transdifferentiation to a metaplastic cell phenotype in the injured glands (10, 37, 45, 53, 68, 95). Nozaki et al. (58) first demonstrated the concept of the transition of chief cells to SPEM cells using DMP-777 treatment and lineage mapping. Other studies have also observed disruption of the normal transitioning of cells during regeneration following injury (38, 95). Furthermore, numerous other studies have demonstrated SPEM cells originating from transdifferentiated chief cells (10, 37, 45, 52, 68, 95).
One important facet of this transdifferentiation is the lack of proliferation (24, 25). The transition from mucous cell to chief cell does not require cell division but rather the chief cell undergoes transcriptional reprogramming and transitioning to the mucous-secreting SPEM lineage (24, 57). Observations of the emergence of BrdU+ve cells in the base of the glands in addition to the stem cell proliferative compartment were suggestive of two proliferating populations of cells, one being the metaplastic SPEM cells (24, 26, 55). Indeed, Ki67+ve cells were found in the base of fundic glands after acute parietal cell loss that correlates with SPEM emergence (57).
FUNCTIONAL ROLE OF SPEM IN GASTRIC REGENERATION
The cellular composition and organization of the normal gastric mucosa is complex and highly specialized. In particular, the oxyntic/fundic glands include the parietal, chief, enterochromaffin-like (ECL), somatostatin (D), surface mucous, and mucous neck cells. In particular, gastric parietal cells not only secrete acid and intrinsic factor, but also sonic hedgehog (Shh) (17, 102, 104, 106). Shh secretion and signaling in the adult stomach play a fundamental role in the maintenance of gastric function and glandular cell lineage differentiation (17, 102, 104, 106). Early studies from our group using mice with a parietal cell-specific deletion of Shh (HKCre/ShhKO) demonstrated disrupted differentiation of the chief cell lineage from the mucous neck cells as indicated by zymogen and neck cell markers that were coexpressed in the same cell population and reminiscent of SPEM (104). Notably, a similar phenotype was found in the case of SPEM development (15, 55, 57). In the circumstances of parietal cell atrophy, damage to the epithelial barrier and ulceration, there is reprogramming of zymogen/chief cells into the metaplastic SPEM lineage (15, 55, 57). SPEM is identified by the strong expression of mucin 6 (Muc6) and trefoil factor 2 (TFF2, spasmolytic polypeptide) (73, 93) and cell surface glycoprotein CD44v9 (91) that is not normally expressed in the oxyntic region of the stomach.
Included in the many functions of the stomach are initiation of digestion and action as a first line of defense against foreign pathogens. It is therefore critical that after gastric injury, the repair response be efficient in its reestablishment of the epithelial barrier (6, 15, 84). In addition to simply being present at the gastric ulcer margin after injury, SPEM has been observed to play a functional role in the regenerative process (6, 15). A study by our group showed that CD44v9 emerges within the gastric epithelium in response to injury and contributes to tissue regeneration, and is a reliable marker of SPEM (6, 15). We observed that ulcer repair was impaired in CD44-deficient mice as documented by the lack of epithelial gland regeneration, persistence of ulceration, and loss of epithelial proliferation (6). We further used an injury/orthotopic organoid transplantation model to transplant CD44v9-expressing gastric organoids within the gastric epithelium of CD44-deficient mice and subsequently induced epithelial cell proliferation and regeneration (6). Wound healing requires a complex process of biological and molecular events, one of which includes proliferation (19, 43, 84, 98). The emergence of CD44v9 at the ulcer margin correlated with proliferation, and this is of importance given that CD44 is known to coordinate progenitor cell proliferation within the normal and metaplastic gastric epithelium (51, 56, 57, 105).
Shown to have a critical role in wound healing are reactive oxygen species (ROS). ROS can come in many different flavors, including peroxide, hydrogen peroxide, and hydroxyl ions and are produced mainly within the mitochondria as a derivative of oxygen metabolism (87). ROS levels are highly regulated as they are deleterious to cells outside of basal levels (7, 14, 30). However, it has been documented that at moderately increased levels of ROS, wound healing benefits from ROS-mediated actions such as increased cellular proliferation and migration, monocyte and neutrophil chemoattraction, bacteriostatic properties, and vascular dilation/constriction abilities (78, 86). Integral to the protection against ROS is antioxidant production and homeostatic maintenance of cellular ROS levels (78, 86). The deleterious effects of ROS are primarily caused by their oxidizing capacity. ROS-mediated oxidation of molecules such as DNA, proteins, and lipids in their vicinity can alter conformation and therefore their functional abilities (28, 70, 71, 81). Protection against ROS activities are antioxidant proteins, which have the ability to donate their own electrons to negate any electron capturing by ROS. There are a host of antioxidants available including vitamin C and E, superoxide dismutase, and glutathione (GSH) to name a few (77, 87). Fundamental in the synthesis of GSH is the cystine-glutamate exchange transporter (xCT) (30, 91). Interestingly, CD44v9 a known marker of SPEM during gastric regeneration (6, 15), stabilizes xCT and regulates the intracellular levels of GSH to protect the cell from ROS-induced oxidative stress in gastric cancer cells (30, 91). Additionally, xCT has recently been found to contribute to gastric metaplasia induction after injury and is also highly expressed in gastric tumor stem cells and may mediate drug resistance in tumor cells (11, 46, 91).
CD44v9 is known to interact with the cystine-glutamate transporter xCT that regulates the intracellular level of reduced glutathione (30). CD44-expressing human gastrointestinal cancer cells exhibit defense against ROS via an enhanced capacity for glutathione synthesis (30, 34, 50). Oxidative stress in response to injury contributes to the development of epithelial apoptosis and necrosis, leading to ulceration (7). Thus we propose that the emergence of CD44v9 during wound repair potentiates defense against ROS by stabilizing xCT and thus promotes the effective regeneration of the gastric epithelium. However, the precise role of CD44v9 during gastric regeneration has yet to be reported. The orthotopic transplantation of CD44v9-positive SPEM lineage-expressing organoids into aged mice that were deficient in SPEM glands showed successful rescue of repair loss typically observed in the elderly stomach (15). Recent investigations have demonstrated a contribution of the CD44v9-xCT system in chief cell transdifferentiation, or plasticity, to the metaplastic cell phenotype, and when inhibited there is a loss of SPEM development (46). In these recent studies, the induction of acute gastric injury in response to L635 or DMP-777 resulted in the upregulation of xCT as an early event during chief cell transition to SPEM that was crucial for ROS detoxification and proliferation (46). The results clearly demonstrated that maintenance of the redox balance is fundamental for the reprogramming, and plasticity, of chief cells to SPEM and ROS detoxification in response to injury (46). Given the functional role of CD44v9 during regeneration of the gastric epithelium (6, 15), future studies identifying chief cell plasticity during ulcer repair are necessary.
The emergence of SPEM is also important in the pathogenesis of H. pylori infection and the progression of disease to gastric cancer. Recent studies have shown that SPEM also contributes to increased susceptibility to H. pylori colonization and infection. A report by Sáenz et al. (71a) demonstrated that H. pylori binds to the gastric epithelium via glycosylated receptor sialyl-Lewis X (S-LewX) that is expressed on SPEM glands. H. pylori adheres to the gastric epithelium via sialic acid-binding adhesin (SAbA) binding to mucin α-2,3-sialyl-glycan S-LewX during inflammation (40). Of relevance to this review, protein glycosylation plays a critical role in selectin-selectin ligand-mediated leukocyte capture and rolling, and integrin-mediated leukocyte adhesion (80, 96). A necessary event during regeneration of the gastric epithelium is infiltration of immune cells, including macrophages and T cells, to the site of injury (6, 15, 17, 102). Given the likely expression of S-LewX/SPEM glands during regeneration, we postulate a functional role of this emerging cell lineage in immune cell trafficking during repair. Indeed, other studies have reported that O-glycosylation that controls S-LewX expression may have a function in dendritic cell and monocyte migration (35). Reports that have revealed the importance of sialyation on chemokine receptor-triggered neutrophil arrest, and α4β1 integrin function (23, 79, 96) suggests a role of glycosylation beyond leukocyte trafficking. These findings open new research areas that may be of interest and relevance to immune cell trafficking under various pathological conditions and ulcer repair.
INFLAMMATION AND REGULATION IN SPEM
Significant research has been devoted to study the immune response during SPEM emergence in response to gastric injury (6, 15, 61, 63). It is known that immune cells secrete cytokines at the wound site that initiate preprogrammed processes of ulcer repair such as angiogenesis, cellular proliferation and migration, reepithelialization, and tissue remodeling (84). A significant driver of SPEM emergence is interleukin 33 (IL-33) (61). IL-33 expression is found to be increased in gastric epithelial cells after parietal cell loss and IL-33 macrophages have been associated with SPEM emergence (61). In particular, in mouse models where the functional subunit of the IL-33 receptor is removed and parietal cell loss is induced, SPEM emergence is inhibited (61). IL-33 upregulates type II cytokines such as IL-13 in the polarization of macrophages. IL-13 has also been correlated with metaplasia development in the stomach, and indeed treatment with IL-13 in mice where the IL-33 receptor is dysfunctional leads to the development of SPEM after acute parietal cell loss (61). These foundational studies were the first to clearly demonstrate that induction of gastric metaplasia, in response to parietal cell atrophy, is coordinated through the IL-33 and IL-13 signaling network and infiltrating M2 macrophages (6, 61, 63).
The immune response is crucial for repair in tissues that include the kidney (39, 69) and skin (41). In particular, macrophages are key immune cells that secrete necessary cytokines, chemokines, and proangiogenic factors that are necessary regulators for repair (82). Published studies from our laboratory (17, 102) document that hedgehog (Hh) ligand Shh may act as a macrophage chemoattractant during repair. In support of a macrophage chemoattractant role for Shh, mice expressing a tamoxifen-inducible parietal cell-specific deletion of Shh exhibit decreased macrophage infiltration and neovascularization that coincides with failure to repair and regenerate the gastric epithelium in response to injury (102). In addition, another study by our group (74) using bone marrow chimera experiments with donor cells collected from mice that have a myeloid cell-specific deletion of the Hh signal transduction protein smoothened (LysMCre/SmoKO) demonstrates that Shh signals to the macrophages to initiate recruitment during the initiation of gastritis in response to H. pylori.
Typically, inflammatory cues within the regional microenvironment can prime the macrophage into a reparative phenotype. Inflammatory monocytes are recruited in response to cytokine cues and undergo differentiation into two distinct subsets of macrophages that are categorized as either classically activated (M1) or alternatively activated (M2) (69). M2 macrophages represent various phenotypes that are further subdivided into M2a, M2b, and M2c (44). Typically, TH2 cytokines induce the polarization of macrophages to an M2 phenotype (27). IL-33 is an alarmin for the rapid induction of IL-13-driven immunity (8, 66). It is known that IL-13 and IL-4 mediate alternative M2 macrophage activation through a common receptor, IL-4Rα (27). Mice that lack the expression of parietal cell-derived Shh exhibit diminished growth factor and cytokine responses following injury and thus suggest that Shh may be upstream of factors that promote tissue repair, including IL-33 (17). Moreover, unpublished data from our group demonstrate that macrophage-derived Indian hedgehog (Ihh) promotes the emergence of SPEM cells during repair. In support of this hypothesis, first we have shown that Ihh induces epithelial cell proliferation within the stomach (20), and second it is published that in response to human schistosomiasis macrophage-derived Ihh induces an M2 macrophage phenotype (60). Thus M2 macrophages may secrete trophic factors, including Hh ligands, and exhibit a specific cytokine and inflammatory profile that suppresses the proinflammatory response and promotes wound healing (69). For example, during skeletal muscle regeneration, macrophages are first recruited as phagocytic cells with elevated release of proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) and then rapidly convert into an anti-inflammatory phenotype to promote myogenesis (5). During regeneration in the injured kidney, macrophages produce Wnt7b that stimulate tissue repair (39). Importantly, in the stomach, studies of mouse models and human metaplastic tissues indicate that M2 macrophages promote the advancement of SPEM in the presence of inflammation and parietal cell atrophy (63). Whether M2 macrophages promote the emergence of SPEM during gastric repair in response to injury remains to be clearly identified.
DIAGNOSIS AND TREATMENT OF PEPTIC ULCER DISEASE
Sixty-five–95% of patients with gastric ulcers and 80–95% of patients with duodenal ulcers are infected with H. pylori (88). H. pylori has also been detected in 20–45% of asymptomatic patients (88). Symptoms such as abdominal pain and stomach pain after eating as well as a previous history of NSAID use can be used to determine whether a patient has peptic ulcer disease. Although the presence of a gastric or duodenal ulcer can only be determined by endoscopy, stool sample culture analysis and the rapid urease test are typically used to detect H. pylori infection (89). Not all H. pylori infections result in the clinical presentation of ulcer disease. Although it is not clear why asymptomatic infected patients do not develop H. pylori-induced inflammation and injury, the pathogenesis of H. pylori may indicate why asymptomatic patients exist. H. pylori has evolved to avoid the host’s acute immune response. Following infection, most of the bacteria are in the mucosa of the gastric epithelium (4). Rather, inflammation and apoptosis caused by chronic and persistent infection results in ulceration of the muscularis mucosae. In addition to using several virulence factors including molecular mimicry, some strains of H. pylori are more virulent than others, especially those that possess the cag pathogenicity island (4, 33, 59). Current treatments for peptic ulcers include antibiotic treatment for H. pylori-positive patients followed by either proton pump inhibitor medications, antacids, acid blockers, or cytoprotective medications that protect the lining of the stomach.
CONCLUSIONS AND CLINICAL RELEVANCE
Normal gastric processes such as acid secretion, motility, and epithelial cell proliferation and regeneration are known to be disrupted in the elderly population (13). The elderly population also exhibits increased susceptibility to ulcer incidence due to increased H. pylori infection and NSAID use (13). In the case of chronic peptic ulcer disease where ulcers do not heal with treatment, complications such as vitamin deficiencies, bleeding ulcers, and hemorrhaging may result and disproportionally affect the elderly. Despite evidence showing that ulcers and subsequent complications are a burden that is focused in the elderly, there is a lack of knowledge as to the molecular mechanisms that disrupt normal repair even after H. pylori eradication within the aged stomach (32, 72). There are a number of mechanisms of action that prevent the gastric mucosa in the elderly from healing normally after being subjected to injury. For example, aging is associated with impaired gastric angiogenesis (1–3, 85) and decreased regenerative capacity, suggesting changes in epithelial stem cells (29, 36). However, the aged-related changes affecting gastric epithelial stem cells are unclear. We have shown that during repair of the stomach in response to ulceration there is the emergence of SPEM cells at the base of the ulcer margin (6, 15), precisely where the ulcer-associated cell lineages (UACLs) have been reported in the intestine (82, 84, 98, 100). Importantly, we observe that in the elderly mouse gastric epithelium, SPEM emergence does not occur at the critical stage of repair (within 3 days post-injury) (15). Rather, SPEM emerges in an unregulated mechanism at the abnormal, and metaplastic, regenerative site of the aged gastric epithelium. Thus SPEM represents the major reparative lineage responsible for wound healing after severe gastric ulcer injury, a response that is unregulated in the aged gastric epithelium, leading to increased risk for the development of disease, including H. pylori infection and cancer.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK083402-08.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.T. and Y.Z. prepared figures; E.T., M.D.-A., S.T.H., and Y.Z. drafted manuscript; E.T., M.D.-A., S.T.H., and Y.Z. edited and revised manuscript; E.T., M.D.-A., S.T.H., and Y.Z. approved final version of manuscript.
REFERENCES
- 1.Ahluwalia A, Jones MK, Deng X, Sandor Z, Szabo S, Tarnawski AS. An imbalance between VEGF and endostatin underlies impaired angiogenesis in gastric mucosa of aging rats. Am J Physiol Gastrointest Liver Physiol 305: G325–G332, 2013. doi: 10.1152/ajpgi.00127.2013. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aberrant, ectopic expression of VEGF and VEGF receptors 1 and 2 in malignant colonic epithelial cells. Implications for these cells growth via an autocrine mechanism. Biochem Biophys Res Commun 437: 515–520, 2013. doi: 10.1016/j.bbrc.2013.06.096. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia A, Jones MK, Szabo S, Tarnawski AS. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol 65: 209–215, 2014. [PubMed] [Google Scholar]
- 4.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 150: 64–78, 2016. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertaux-Skeirik N, Wunderlich M, Teal E, Chakrabarti J, Biesiada J, Mahe M, Sundaram N, Gabre J, Hawkins J, Jian G, Engevik AC, Yang L, Wang J, Goldenring JR, Qualls JE, Medvedovic M, Helmrath MA, Diwan T, Mulloy JC, Zavros Y. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol 242: 463–475, 2017. doi: 10.1002/path.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94: 329–354, 2014. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzzelli JN, Chalinor HV, Pavlic DI, Sutton P, Menheniott TR, Giraud AS, Judd LM. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell Mol Gastroenterol Hepatol 1: 203–221.e3, 2015. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci USA 106: 14321–14326, 2009. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology 150: 918–30.e13, 2016. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458: 780–783, 2009. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol 6: 125–130, 1991. doi: 10.1111/j.1440-1746.1991.tb01451.x. [DOI] [PubMed] [Google Scholar]
- 13.Dumic I, Nordin T, Jecmenica M, Stojkovic Lalosevic M, Milosavljevic T, Milovanovic T. Gastrointestinal tract disorders in older age. Can J Gastroenterol Hepatol 2019: 6757524, 2019. doi: 10.1155/2019/6757524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J 14: 89–96, 2017. doi: 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engevik AC, Feng R, Choi E, White S, Bertaux-Skeirik N, Li J, Mahe MM, Aihara E, Yang L, DiPasquale B, Oh S, Engevik KA, Giraud AS, Montrose MH, Medvedovic M, Helmrath MA, Goldenring JR, Zavros Y. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol 2: 605–624, 2016. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engevik AC, Feng R, Yang L, Zavros Y. The acid-secreting parietal cell as an endocrine source of Sonic Hedgehog during gastric repair. Endocrinology 154: 4627–4639, 2013. doi: 10.1210/en.2013-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743, 2005. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 20.Feng R, Aihara E, Kenny S, Yang L, Li J, Varro A, Montrose MH, Shroyer NF, Wang TC, Shivdasani RA, Zavros Y. Indian Hedgehog mediates gastrin-induced proliferation in stomach of adult mice. Gastroenterology 147: 655–666.e9, 2014. doi: 10.1053/j.gastro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110: 155–166, 1996. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 22.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res 63: 942–950, 2003. [PubMed] [Google Scholar]
- 23.Frommhold D, Ludwig A, Bixel MG, Zarbock A, Babushkina I, Weissinger M, Cauwenberghs S, Ellies LG, Marth JD, Beck-Sickinger AG, Sixt M, Lange-Sperandio B, Zernecke A, Brandt E, Weber C, Vestweber D, Ley K, Sperandio M. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J Exp Med 205: 1435–1446, 2008. doi: 10.1084/jem.20070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 317: 2759–2764, 2011. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138: 2207–2210, 2010. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldenring JR, Ray GS, Coffey RJ Jr, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000. doi: 10.1016/S0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 27.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 28.Grishko VI, Druzhyna N, LeDoux SP, Wilson GL. Nitric oxide-induced damage to mtDNA and its subsequent repair. Nucleic Acids Res 27: 4510–4516, 1999. doi: 10.1093/nar/27.22.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinsull SM. Effect of colloidal bismuth subcitrate on age related gastric lesions in the rat. Gut 32: 355–360, 1991. doi: 10.1136/gut.32.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19: 387–400, 2011. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Jones JI, Hawkey CJ. Physiology and organ-related pathology of the elderly: stomach ulcers. Best Pract Res Clin Gastroenterol 15: 943–961, 2001. doi: 10.1053/bega.2001.0251. [DOI] [PubMed] [Google Scholar]
- 33.Jones KR, Whitmire JM, Merrell DS. A tale of two toxins: Helicobacter pylori CagA and VacA modulate host pathways that impact disease. Front Microbiol 1: 115, 2010. doi: 10.3389/fmicb.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju HQ, Lu YX, Chen DL, Tian T, Mo HY, Wei XL, Liao JW, Wang F, Zeng ZL, Pelicano H, Aguilar M, Jia WH, Xu RH. Redox regulation of stem-like cells though the CD44v-xCT axis in colorectal cancer: mechanisms and therapeutic implications. Theranostics 6: 1160–1175, 2016. doi: 10.7150/thno.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julien S, Grimshaw MJ, Sutton-Smith M, Coleman J, Morris HR, Dell A, Taylor-Papadimitriou J, Burchell JM. Sialyl-Lewis(x) on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: a mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. J Immunol 179: 5701–5710, 2007. doi: 10.4049/jimmunol.179.9.5701. [DOI] [PubMed] [Google Scholar]
- 36.Kirkwood TB. Intrinsic ageing of gut epithelial stem cells. Mech Ageing Dev 125: 911–915, 2004. doi: 10.1016/j.mad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, Goh J, Wong E, Denil SLIJ, Murakami K, Barker N. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 19: 774–786, 2017. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem 271: 3671–3676, 1996. doi: 10.1074/jbc.271.7.3671. [DOI] [PubMed] [Google Scholar]
- 39.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297: 573–578, 2002. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 216: 753–762, 2011. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 323: 1311–1315, 1984. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 43.Martin P. Wound healing–aiming for perfect skin regeneration. Science 276: 75–81, 1997. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 44.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 13: 453–461, 2008. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 45.Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Voon DC, Hiai H, Unno M, So JB, Zhu F, Srivastava S, Teh M, Yeoh KG, Osato M, Ito Y. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology 152: 218–231.e14, 2017. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Meyer AR, Engevik AC, Willet SG, Williams JA, Zou Y, Massion PP, Mills JC, Choi E, Goldenring JR. Cystine/glutamate antiporter (xCT) is required for chief cell plasticity after gastric injury. Cell Mol Gastroenterol Hepatol 8: 379–405, 2019. doi: 10.1016/j.jcmgh.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol 596: 3861–3867, 2018. doi: 10.1113/JP275512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michailidou M, Brown HK, Lefley DV, Evans A, Cross SS, Coleman RE, Brown NJ, Holen I. Microvascular endothelial cell responses in vitro and in vivo: modulation by zoledronic acid and paclitaxel? J Vasc Res 47: 481–493, 2010. doi: 10.1159/000313876. [DOI] [PubMed] [Google Scholar]
- 50.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 32: 5191–5198, 2013. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 51.Nam KT, Lee HJ, Mok H, Romero-Gallo J, Crowe JE Jr, Peek RM Jr, Goldenring JR. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 136: 1288–1296, 2009. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RMJ Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037.e9, 2010. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nam KTVA, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology 132: 1804–1819, 2007. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 55.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology 127: 582–594, 2004. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 56.Nomura S, Settle SH, Leys CM, Means AL, Peek RM Jr, Leach SD, Wright CV, Coffey RJ, Goldenring JR. Evidence for repatterning of the gastric fundic epithelium associated with Ménétrier’s disease and TGFalpha overexpression. Gastroenterology 128: 1292–1305, 2005. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 58.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134: 511–522, 2008. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peek RM., Jr Helicobacter pylori strain-specific modulation of gastric mucosal cellular turnover: implications for carcinogenesis. J Gastroenterol 37, Suppl 13: 10–16, 2002. doi: 10.1007/BF02990093. [DOI] [PubMed] [Google Scholar]
- 60.Pereira TA, Xie G, Choi SS, Syn WK, Voieta I, Lu J, Chan IS, Swiderska M, Amaral KB, Antunes CM, Secor WE, Witek RP, Lambertucci JR, Pereira FL, Diehl AM. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver Int 33: 149–161, 2013. doi: 10.1111/liv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen CP, Meyer AR, De Salvo C, Choi E, Schlegel C, Petersen A, Engevik AC, Prasad N, Levy SE, Peebles RS, Pizarro TT, Goldenring JR. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 67: 805–817, 2018. doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 146: 1727–38.e8, 2014. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev 70: 427–451, 1990. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 66.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 125: 752–754, 2010. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 67.Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 157: 349–364.e1, 2019. doi: 10.1053/j.gastro.2019.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology 154: 839–843.e2, 2018. doi: 10.1053/j.gastro.2017.11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richter C, Tanaka T, Yada RY. Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem J 335: 481–490, 1998. doi: 10.1042/bj3350481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269: 26066–26075, 1994. [PubMed] [Google Scholar]
- 71a.Sáenz JB, Vargas N, Mills JC. Tropism for spasmolytic polypeptide-expressing metaplasia allows Helicobacter pylori to expand its intragastric niche. Gastroenterology 156: 160–174, 156, 2019. doi: 10.1053/j.gastro.2018.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salles N. Is stomach spontaneously ageing? Pathophysiology of the ageing stomach. Best Pract Res Clin Gastroenterol 23: 805–819, 2009. doi: 10.1016/j.bpg.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79: 639–646, 1999. [PMC free article] [PubMed] [Google Scholar]
- 74.Schumacher MA, Donnelly JM, Engevik AC, Xiao C, Yang L, Kenny S, Varro A, Hollande F, Samuelson LC, Zavros Y. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 142: 1150–1159.e6, 2012. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott DR, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology 114: 58–70, 1998. doi: 10.1016/S0016-5085(98)70633-X. [DOI] [PubMed] [Google Scholar]
- 77.Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells 17: 132–139, 2004. [PubMed] [Google Scholar]
- 78.Shen H-M, Pervaiz S. Reactive oxygen species in cell fate decisions. In: Essentials of Apoptosis, edited by Dong Z, Yin XM. Totowa, NJ: Humana, 2009, p. 199–221. [Google Scholar]
- 79.Sperandio M, Frommhold D, Babushkina I, Ellies LG, Olson TS, Smith ML, Fritzsching B, Pauly E, Smith DF, Nobiling R, Linderkamp O, Marth JD, Ley K. Alpha 2,3-sialyltransferase-IV is essential for L-selectin ligand function in inflammation. Eur J Immunol 36: 3207–3215, 2006. doi: 10.1002/eji.200636157. [DOI] [PubMed] [Google Scholar]
- 80.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev 230: 97–113, 2009. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stadtman ER, Levine RL. Protein oxidation. Ann NY Acad Sci 899: 191–208, 2000. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 82.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology 102: 695–698, 1992. doi: 10.1016/0016-5085(92)90123-G. [DOI] [PubMed] [Google Scholar]
- 84.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 50, Suppl 1: S24–S33, 2005. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 85.Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol 29, Suppl 4: 112–123, 2014. doi: 10.1111/jgh.12734. [DOI] [PubMed] [Google Scholar]
- 86.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 10: 1343–1374, 2008. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turrens JF. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med 25: 211–220, 2004. doi: 10.1016/j.mam.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 88.Tytgat GN, Rauws EA. The role of Campylobacter pylori in gastroduodenal diseases. A “believer”’s point of view. Gastroenterol Clin Biol 13: 118B–121B, 1989. [PubMed] [Google Scholar]
- 89.Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 3: 9, 2015. doi: 10.3978/j.issn.2305-5839.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venerito M, Varbanova M, Röhl F-W, Reinhold D, Frauenschläger K, Jechorek D, Weigt J, Link A, Malfertheiner P. Oxyntic gastric atrophy in Helicobacter pylori gastritis is distinct from autoimmune gastritis. J Clin Pathol 69: 677–685, 2016. doi: 10.1136/jclinpath-2015-203405. [DOI] [PubMed] [Google Scholar]
- 91.Wada T, Ishimoto T, Seishima R, Tsuchihashi K, Yoshikawa M, Oshima H, Oshima M, Masuko T, Wright NA, Furuhashi S, Hirashima K, Baba H, Kitagawa Y, Saya H, Nagano O. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 104: 1323–1329, 2013. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114: 675–689, 1998. doi: 10.1016/S0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 93.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 12: 189–197, 2009. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 62: 1270–1279, 2013. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Willet SG, Lewis MA, Miao ZF, Liu D, Radyk MD, Cunningham RL, Burclaff J, Sibbel G, Lo HG, Blanc V, Davidson NO, Wang ZN, Mills JC. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 37: e98311, 2018. doi: 10.15252/embj.201798311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woodard-Grice AV, McBrayer AC, Wakefield JK, Zhuo Y, Bellis SL. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J Biol Chem 283: 26364–26373, 2008. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wright CL, Riddell RH. Histology of the stomach and duodenum in Crohn’s disease. Am J Surg Pathol 22: 383–390, 1998. doi: 10.1097/00000478-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Wright NA. Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos Trans R Soc Lond B Biol Sci 353: 925–933, 1998. doi: 10.1098/rstb.1998.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature 343: 82–85, 1990. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 101.Wright NA, Pike CM, Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion 46, Suppl 2: 125–133, 1990. doi: 10.1159/000200375. [DOI] [PubMed] [Google Scholar]
- 102.Xiao C, Feng R, Engevik AC, Martin JR, Tritschler JA, Schumacher M, Koncar R, Roland J, Nam KT, Goldenring JR, Zavros Y. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest 93: 96–111, 2013. [Erratum in Lab Invest 93: 264, 2013]. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561.e8, 2010. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshizawa N, Takenaka Y, Yamaguchi H, Tetsuya T, Tanaka H, Tatematsu M, Nomura S, Goldenring JR, Kaminishi M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest 87: 1265–1276, 2007. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 106.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol 295: G99–G111, 2008. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]