Abstract
Circulating blood glucocorticoid levels are dynamic and responsive to stimuli that impact autonomic function. In the brain stem, vagal afferent terminals release the excitatory neurotransmitter glutamate to neurons in the nucleus of the solitary tract (NTS). Vagal afferents integrate direct visceral signals and circulating hormones with ongoing NTS activity to control autonomic function and behavior. Here, we investigated the effects of corticosterone (CORT) on glutamate signaling in the NTS using patch-clamp electrophysiology on brain stem slices containing the NTS and central afferent terminals from male C57BL/6 mice. We found that CORT rapidly decreased both action potential-evoked and spontaneous glutamate signaling. The effects of CORT were phenocopied by dexamethasone and blocked by mifepristone, consistent with glucocorticoid receptor (GR)-mediated signaling. While mRNA for GR was present in both the NTS and vagal afferent neurons, selective intracellular quenching of G protein signaling in postsynaptic NTS neurons eliminated the effects of CORT. We then investigated the contribution of retrograde endocannabinoid signaling, which has been reported to transduce nongenomic GR effects. Pharmacological or genetic elimination of the cannabinoid type 1 receptor signaling blocked CORT suppression of glutamate release. Together, our results detail a mechanism, whereby the NTS integrates endocrine CORT signals with fast neurotransmission to control autonomic reflex pathways.
Keywords: autonomic, corticosterone, glucocorticoid, GPCR, stress, synaptic
INTRODUCTION
Activation of the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis promotes necessary adaptations in physiology and behavior in response to stress (real or anticipated threats to homeostasis) (28, 61). Stress facilitates a rapid “fight or flight” response through increased sympathetic activity, resulting in increased cardiac and respiratory outputs (45), as well as limited gastrointestinal motility (40, 65). Furthermore, glucocorticoids, secreted following HPA axis activation, act in the periphery and the brain to prolong adaptation and provide negative feedback to specific brain regions that regulate HPA and autonomic activity during stress (53, 54, 58). Maintaining the balance between sympathetic and parasympathetic drive onto organ systems that control these physiological responses is mediated by key autonomic centers in the brain stem, including the nucleus of the solitary tract (NTS). The focus of this study is to investigate the mechanisms of glucocorticoid signaling in the NTS.
The NTS is the first site of integration of visceral afferent signals through primary afferent innervation from the vagus nerve via the solitary tract (ST). ST afferents release the fast excitatory neurotransmitter glutamate, which plays a crucial role in initiating coordinated autonomic reflex pathways, such as the baroreflex (1, 41). Growing evidence supports the NTS as a primary mediator of both stress-induced autonomic responses and HPA axis activation. Neurons in the NTS are rapidly activated following various physical and psychogenic stressors, including hypoxia, visceral illness, restraint, and forced swim stresses (10, 49, 59). Ablation of NTS catecholamine neurons modulates cardiovascular responses to acute and chronic stress (8, 12), indicating its role in promoting stress-induced tachycardia. The NTS also has dense projections to stress-responsive forebrain areas, including the paraventricular nucleus (PVN), amygdala, and bed nucleus of the stria terminalis (BNST) (48, 51). Stimulation of these ascending fibers induces increased firing of neuroendocrine PVN neurons (52), and ablation of NTS terminals in the PVN blunts corticosterone responses to both stress and glucoprivic challenge (21, 50). Given its role in feedforward control of HPA activity, this suggests the NTS is a key point of glucocorticoid negative-feedback following stress.
The NTS expresses both mineralocorticoid (MR) and glucocorticoid (GR) receptors (24, 26), and its position around the 4th ventricle, along with the presence of fenestrated capillaries, make it a viable site for modulation by circulating factors like glucocorticoids (39). Intra-NTS microinjection of the synthetic glucocorticoid dexamethasone produces hypertension and tachycardia (62), while chronic dorsal hindbrain corticosterone treatment augments pressor responses during acute stress and reduces baroreflex gain (4, 5, 53). While glucocorticoids clearly act in the NTS to modulate autonomic responses and HPA axis activity, their effects on underlying neurotransmission of cranial afferents remain unknown.
Glucocorticoids act within the brain to modulate synaptic transmission through putative membrane-associated GRs (15, 16). These effects occur on a faster timescale (within minutes) compared with canonical transcriptional regulation (within hours) and appear to be mediated via G protein-coupled receptor signaling (15, 16, 20). This rapid mode of signaling potentially underlies an essential mechanism by which glucocorticoids provide feedback to regulate HPA axis activity, and also to modulate the autonomic nervous system following a stressor. Thus, we hypothesized that the glucocorticoid corticosterone acts rapidly in the NTS to modulate glutamatergic neurotransmission from vagal afferents onto second-order neurons.
MATERIALS AND METHODS
Animals.
All experiments were performed in accordance with procedures approved by the Institutional Animal Use and Care Committee (IACUC) at Washington State University. Adult male C57BL/6 mice (20–30 g) were obtained from The Jackson Laboratory. Cannabinoid-type 1 (CB1) receptor knockout mice (CB1R−/−) used in this study were bred in our colony (5a). We focused on males for these initial studies due to the documented effects of sex hormones on glucocorticoid signaling and associated neural responses (30, 63). Mice were housed in a temperature-controlled (23 ± 1°C) room with ad libitum access to water and standard pellet chow. They were maintained on a 12:12-h light-dark cycle with lights on between 0700 and 1900. Mice were taken for electrophysiological experiments within 2–3 h following the onset of the light period.
Horizontal brain stem slice preparation.
Brain stem slices were isolated and prepared as previously described (2). Following induction of anesthesia by isoflurane, the brain stem was removed from just rostral to the cerebellum to the first cervical vertebra and placed in ice-cold artificial cerebral spinal fluid (aCSF) containing (mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 2 CaCl2, and 10 dextrose, bubbled with 95% O2-5% CO2. aCSF was brought to a pH of 7.40 using 1M HCl. Once chilled, the tissue was cut to remove the cerebellum, and the tissue block was mounted horizontally to a pedestal with cyanoacrylate glue and submerged in cold aCSF on a vibrating microtome (Leica VT1200S). Approximately 150 μm was removed from the dorsal surface, and then a single 250-μm thick horizontal slice was collected containing the solitary tract (ST) along with the neuronal cell bodies of the medial NTS region. Slices were cut with a sapphire knife (Delaware Diamond Knives, Wilmington, DE) and secured using a fine polyethylene mesh in a perfusion chamber with continuous perfusion of aCSF bubbled with 95% O2-5% CO2 at 32°C.
Whole cell patch-clamp recordings.
Whole cell recordings were performed on NTS neurons contained in horizontal brain stem slices using an upright Nikon FN1 microscope with a Nikon DS-Qi1Mc digital camera and NIS-elements AR imaging software. Recording electrodes (3.0–4.5 MΩ) were filled with an intracellular solution containing (mM): 10 CsCl, 130 Cs-methanesulfonate, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 2 Na2ATP, and 0.2 Na2GTP. For measurements of excitatory postsynaptic currents (EPSCs), all neurons were studied under voltage-clamp conditions with an Axopatch 200A or MultiClamp 700A amplifier (Molecular Devices, Union City, CA) and held at VH = −60 mV in whole cell patch configuration. Only recordings with a series resistance of <20 MΩ were used for experiments to ensure good access and maintenance of voltage clamp. Signals were filtered with a 1-kHz bezel filter and sampled at 20 kHz using Axon pClamp10 software (Molecular Devices). Liquid junction potentials were not corrected. Extracellular solution (aCSF) was continuously perfused at 32 ± 1°C throughout the experiment, and specific drugs were bath applied. Experimental protocols used within-subject designs for pharmacological treatments and between-subject treatments for control versus knockout mice.
Functional Identification of Second-Order NTS Neurons.
Second-order neurons in the NTS receive direct innervation from solitary tract (ST)-afferents, which include the primary vagal afferent terminals, and can be identified on the basis of the precision of shock-evoked synchronous glutamate. To selectively activate ST-afferent fibers, a concentric bipolar stimulating electrode (200 μm outer tip diameter; Frederick Haer, Bowdoinham, ME) was placed on distal portions of the visible ST rostral to the recording region. The ST received constant current shocks every 6 s (shock duration 60 μs) using a Master-8 isolated stimulator (A.M.P.I., Jerusalem, Israel). During each trial the ST was stimulated 5 times at a shock frequency of 25 Hz to visualize evoked synchronous glutamate release and perform associated analyses. Suprathreshold shocks triggered a long-latency (∼3–7 ms) EPSC, resulting from action potential-driven synchronous release of glutamate vesicles activating postsynaptic α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptors in these recording conditions. Latency is the time between the shock artifact and onset of the synchronous EPSC. Synaptic “jitter” is the standard deviation of ST-EPSC latencies for 30 trials. Jitters of <200 μs identify monosynaptic afferent inputs onto the NTS neuron (17a). Direct ST synaptic release also demonstrated pronounced frequency-dependent depression (FDD) due to the intrinsically high probability of release (Pr) from the afferents (43).
Cell-attached recordings.
To assay changes in action potential firing, without disrupting the internal environment of the NTS neurons, we used cell-attached recording techniques. Negative pressure was applied to a neuron to gain a loose seal, but not giga-ohm, and extracellular changes in voltage due to action potential firing were recorded in current-clamp configuration during control ACSF and corticosterone (CORT) exposure. During these recordings, the membrane was not ruptured, and no extrinsic current was injected.
Gene expression analysis.
Nodose ganglia and brains collected from mice were used for quantitative real-time-PCR (qRT-PCR) for gene products encoding glucocorticoid (NR3C1), mineralocorticoid (NR3C2), and CB1 (CNR1) receptors. Samples were run in triplicate from n = 5 mice for nodose and n = 3 mice for NTS tissue. Control amplification of water was run in parallel, and melt curves were examined to ensure single product amplification. Using a microsurgical knife, we cut out the NTS from a horizontal brain stem slice in ice-cold aCSF. Following removal, NTS and nodose ganglia were placed in RNAlater (Thermo Fisher Scientific) and stored at 4°C before RNA extraction. For quantitative RT-PCR (qRT-PCR), mRNA isolation was performed using QIAzol extraction and RNeasy micro kit (Qiagen). mRNA concentrations were measured using spectrophotometry, and samples were diluted to the same final concentration. Immediately following extraction, mRNA was treated with Ambion DNase treatment and removal (Life Technologies), and cDNA synthesis was performed with QuantiTect cDNA reverse transcription kit (Qiagen). Real-time PCR assays were performed using the TaqMan chemistry and commercially available validated assays from Life Technologies (NR3C1, Mm00433832_m1; NR3C2, Mm01241596_m1; CNR1, Mm01212171_s1). Biological replicates were each run in triplicate on a Life Technologies/Applied Biosystems Viia7 real-time PCR machine with a 20-μL reaction volume. Samples were then compared using the ΔCT method of relative quantification, with GAPDH (GAPDH, Mm99999915_g1) used to normalize between biological replicates.
Experimental design and statistical analyses.
For brain stem slice recordings, the digitized waveforms of synaptic events were analyzed using an event detection and analysis program (MiniAnalysis, Synaptosoft, Decatur, GA) for all quantal synaptic currents and Clampfit 10 (Molecular Devices) for all ST-stimulated currents. All events >10 pA were counted for frequency values. Fitting of quantal EPSC amplitudes and decay kinetics (90–10%) was performed using a fitting protocol (MiniAnalysis) on >500 discrete events. For statistical comparisons, we used Sigma Stat software (Systat Software, San Jose, CA). The data were tested for normality and equal variance, and the appropriate parametric or nonparametric statistics were used, including, ANOVA with post hoc analysis, t tests, Mann-Whitney rank sum test, and linear regression analysis. Specific tests used are indicated in the results. Comparisons were considered statistically different with an α level of P < 0.05.
Chemicals and drugs.
The chemicals used for this series of studies were purchased from retail distributors, including Sigma-Aldrich (GDPβS) and Tocris [corticosterone, dexamethasone, mifepristone (RU486), spironolactone (SC9420), AM251, and AM4113] and Aobious (DO34). The general salts used for making bath solutions were purchased from Sigma-Aldrich. All drugs not soluble in H2O were dissolved in ≤ 0.1% DMSO. Concentrations of drugs used were based on previously published work (14, 15, 25).
RESULTS
CORT inhibits action-potential-driven synchronous and asynchronous glutamate release.
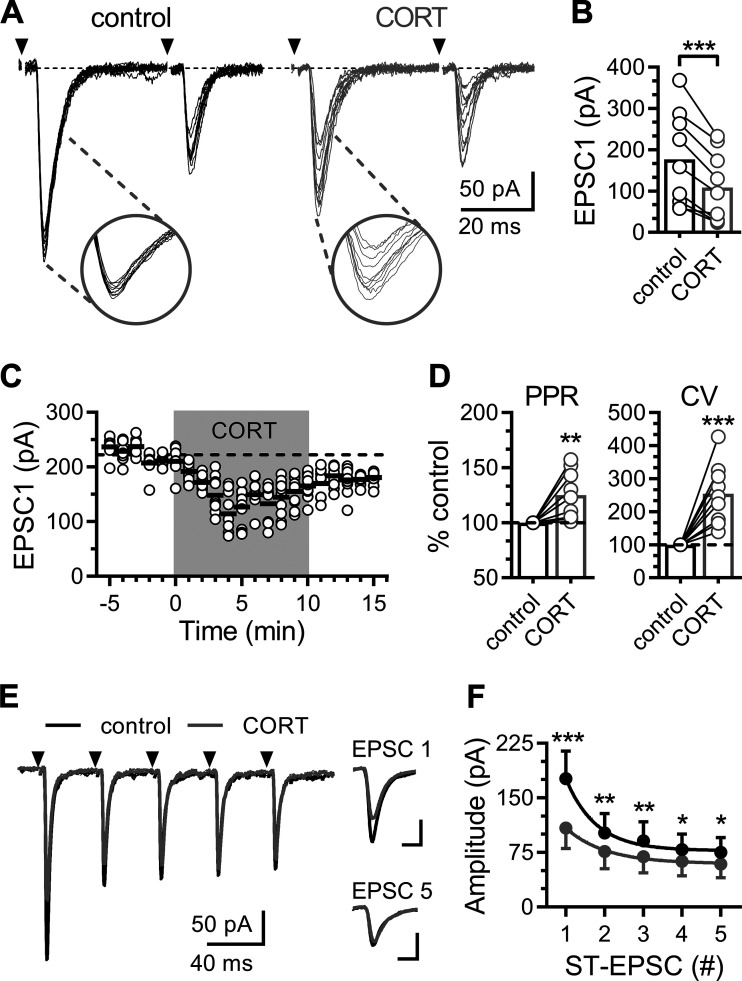
Suprathreshold stimulation of the ST-evoked large amplitude EPSCs in NTS neurons (Fig. 1). ST-EPSCs on second-order NTS neurons were identified by input latency and jitter. All neurons reported in this experiment received direct monosynaptic afferent inputs with a mean latency of 3.9 ± 0.3 ms and a jitter of 93 ± 6 μs. Within minutes of bath application, CORT (1 μM) decreased the amplitude of ST-evoked EPSCs in 9 of 13 (∼70%) neurons tested (Fig. 1, A–C). Neurons were considered responsive when CORT decreased the EPSC amplitude by at least 20%. Across all CORT-inhibited neurons, the average EPSC amplitude was significantly decreased from 177 ± 38 pA to 109 ± 28 pA (56 ± 6% of control). CORT-induced inhibition of EPSC1 amplitude was accompanied with an increase in variability by 254 ± 31% and paired-pulse ratio (PPR; EPSC2 / EPSC1) by 125 ± 7% of baseline (Fig. 1D). We never observed an enhancement of ST-evoked EPSCs in the presence of CORT. The direction of change in the coefficient of variance (CV; σ/EPSC1 mean amplitude) and PPR are consistent with a decrease in the probability of glutamate release from the presynaptic terminal. To explore and expand our observations about these changes to the initial release probability, we delivered trains of stimulations (5 shocks at 25 Hz), which deplete the readily releasable pool and promote vesicle mobilization. Repeated ST-evoked release produced pronounced FDD (Fig. 1, E and F), which is consistent with the reported high initial Pr state at this synapse (3, 43). CORT exposure significantly decreased EPSC amplitude across the train of stimuli (Fig. 1, E and F), suggesting it may also impact vesicle recruitment to the membrane.
Fig. 1.
Corticosterone rapidly suppresses evoked glutamate release. A: representative solitary tract-excitatory post-synaptic currents (ST-EPSC) traces recorded in artificial cerebrospinal fluid (ACSF; control; black traces) and following bath application of corticosterone (CORT; 1 µM; gray traces). Each trace displays 10 trials overlaid (shock artifact has been removed) with arrows indicating the time of ST stimulation. Insets highlight the amplitude variability of EPSC1. B: plot of the average amplitude of the initial ST evoked EPSC (EPSC1). CORT decreases EPSC1 amplitude (n = 9 neurons/6 mice; P < 0.001, paired t test). C: plot of individual EPSC1 amplitudes (open circles) over time. Black lines represent average EPSC1 amplitude of 10 consecutive trials over 1 min, and gray shading indicates time of CORT exposure. D: plots of the average paired-pulse ratio (PPR; EPSC 2/1) and the coefficient of variance (CV) of EPSC1 normalized to baseline. CORT increases both the PPR (n = 9 neurons / 6 mice; P = 0.008, paired t test) and CV (P < 0.001, paired t test). E: representative trace showing ST-evoked EPSCs during a train of ST stimulations (five shocks at 20 Hz). Traces are an average of 10 consecutive trials. Scales for inset traces are 5 ms and 100 pA. F: curves for frequency dependent depression (FDD) of mean ST-EPSC amplitude across five ST shocks. CORT significantly decreased the FDD response (P < 0.001, two-way ANOVA), as well as the size of each ST-EPSC at each shock (P < 0.001; P = 0.001; P = 0.004; P = 0.026; P = 0.026; using Holm-Sidak multiple-comparison test). Values are expressed as means ± SE *P < 0.05, **P < 0.01, ***P < 0.001.
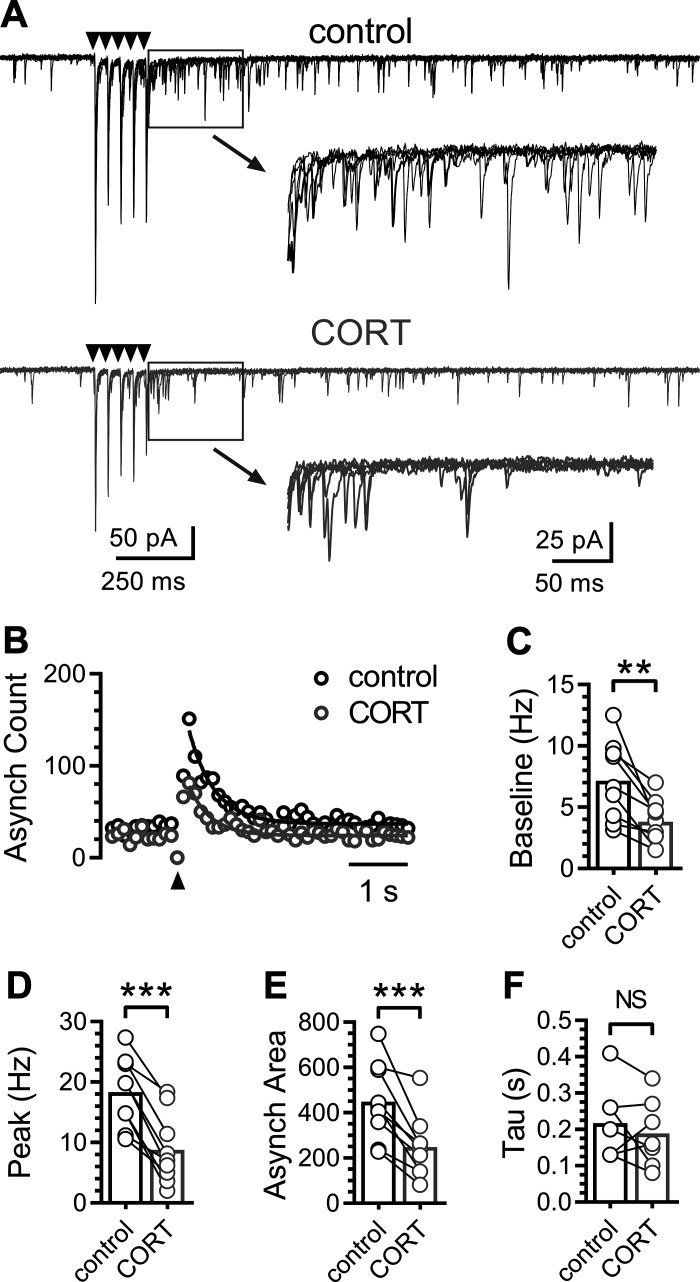
The majority of ST afferents are known to exhibit a large asynchronous glutamate release profile following trains of stimuli (Fig. 2) (42). Peak asynchronous frequency was calculated as the peak frequency in a 100-ms bin after the last stimulation and the area under the cure was calculated by using the total baseline subtracted EPSC count over 5 s following stimulation. Stimulation of ST afferents produced reliable asynchronous release (9 of 13 neurons) that was consistently inhibited by CORT in neurons, where synchronous release was also inhibited (Fig. 2A). The asynchronous frequency peaked rapidly just following ST stimulation and decayed back to baseline after a few seconds (Fig. 2B). CORT reduced the basal frequency to 56 ± 6% (Fig. 2C), the peak frequency to 46 ± 7% (Fig. 2D), and the integrated area of asynchronous EPSCs to 54 ± 6% of control (Fig. 2E). However, CORT did not alter the decay kinetics (93 ± 16% of control) of the asynchronous profile (Fig. 2F). Unlike ST-EPSC amplitude, asynchronous EPSC count was reduced in all neurons, in which it was reliably expressed. This profile suggests a slight preference for inhibition of quantal release pathways over evoked release.
Fig. 2.
Corticosterone decreases solitary tract (ST)-evoked asynchronous glutamate release. A: representative recordings showing synchronous and asynchronous glutamate release during control (black traces) and following exposure to corticosterone (CORT; 1 μM; gray traces). Each trace displays five trials overlaid (shock artifact has been removed), with arrows indicating the time of ST stimulation. Inset: asynchronous excitatory post-synaptic currents (EPSCs) immediately following ST-evoked EPSCs. B: event histogram of a representative experiment showing the summed count of quantal EPSCs over time (50 trials summed, 100-ms bins). C: plot of baseline frequency of sEPSCs one second before ST-stimulation (n = 9 neurons / 5 mice). CORT decreased the number of basal spontaneous EPSCs before ST stimulation (P = 0.003, paired t test). Plots of the peak frequency (baseline subtracted) of asynchronous quantal glutamate events during a 100-ms bin (D) and area of summed asynchronous events five seconds following ST stimulation (E). CORT decreased the both peak asynchronous frequency (P < 0.001, paired t test) and the total area of the asynchronous period (P < 0.001, paired t test). F: there was no significant difference in the decay of asynchronous release (P = 0.083, using paired t test). Values are expressed as means ± SE. **P < 0.01, ***P < 0.001.
Corticosterone rapidly suppresses spontaneous glutamate release without changing the event amplitudes or decay kinetics.
We next investigated the effects of CORT on constitutive spontaneous glutamate release by recording spontaneous EPSCs (sEPSCs) in NTS neurons (Fig. 3). Bath application of CORT (1 µM) rapidly and robustly decreased the frequency of sEPSCs in all NTS neurons recorded (n = 13 of 13 neurons / 6 mice) (Fig. 3A). On average, CORT decreased the frequency from 7.5 ± 1.1 to 3.9 ± 0.7 Hz (50 ± 5%) (Fig. 3, B and C). The sEPSC frequency remained decreased at 46 ± 5% of control frequency after a washout period of 10–20 min. Neither the sEPSC amplitude (94 ± 4% of control) nor tau (100 ± 5% of control) were significantly changed by CORT exposure (Fig. 3, D–F).
Fig. 3.
Corticosterone rapidly decreases quantal glutamate release onto nucleus of the solitary tract (NTS) neurons. A: representative recordings showing spontaneous excitatory post-synaptic currents (sEPSCs) during artificial cerebrospinal fluid (ACSF) control and following bath application of 1 µM corticosterone (CORT). B: normalized time course plot (50-s bin width) of EPSC frequency (○) and amplitude (●); normalized to the baseline period 3 min before CORT exposure (n = 6 neurons / 3 mice). Gray shading indicates time of CORT application. C: plot of average sEPSC frequency during control and after CORT (n = 13 neurons / 6 mice). CORT significantly decreased the sEPSC frequency within 5 min of application (P < 0.001, paired t test). D: representative average sEPSC waveforms during control (black) and after CORT (gray). Waveforms are the average of 250 discrete events. Plots of sEPSC amplitude (E) and tau (F) during control and after CORT (n = 13 neurons / 6 mice). CORT application did not significantly change the amplitude (P = 0.11, paired t test) nor tau (P = 0.49, using paired t test). Values are expressed as means ± SE. ***P < 0.001. NS, not significant.
Postsynaptic glucocorticoid and presynaptic CB1 receptors are required for the effects of CORT on sEPSC frequency.
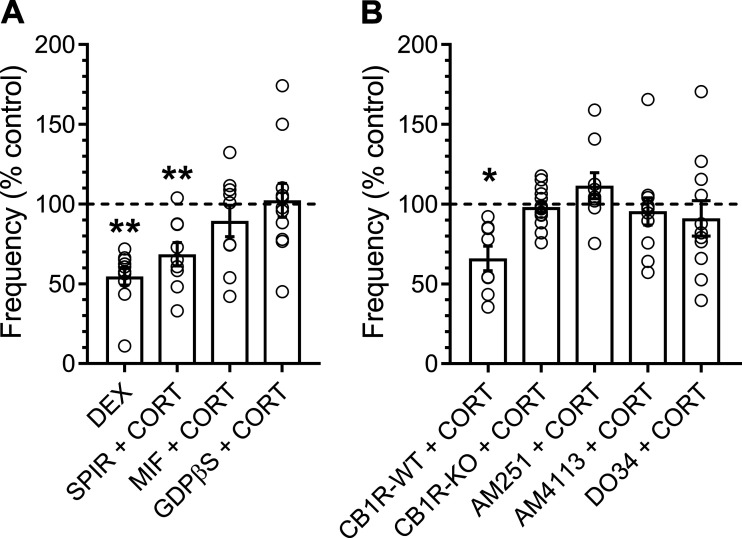
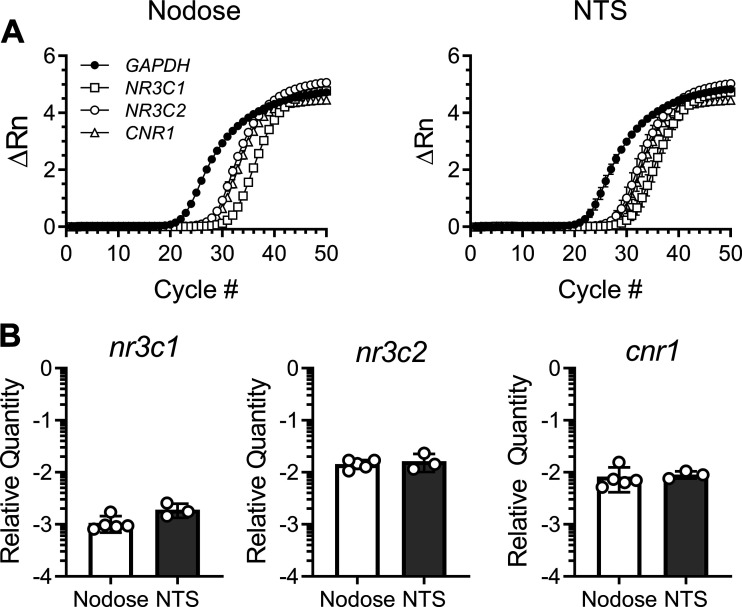
We next investigated the cellular mechanisms mediating the rapid effects of CORT on the frequency of spontaneous glutamate release (Fig. 4). CORT binds to, and has agonist properties at, both MRs and GRs (32). RT-qPCR confirmed that MR (gene name: NR3C2) and GR (gene name: NR3C1) and are abundantly expressed in cell bodies of the vagal afferent neurons in the nodose ganglia and the NTS (Fig. 5). Similar to CORT, the synthetic GR agonist dexamethasone (DEX; 1 µM) significantly decreased sEPSC frequency by 50 ± 5% and also failed to alter sEPSC amplitude (control: 26 ± 4 pA vs. DEX: 27 ± 3 pA, n = 10 neurons/3 mice, P = 0.21, paired t test) (Fig. 4A). Pretreatment with the competitive MR antagonist spironolactone (300 nM) attenuated, but did not reverse, the effects of CORT, whereas the GR antagonist mifepristone (10 μM) eliminated the ability of CORT to significantly decrease the frequency of spontaneous glutamate release (Fig. 4A). In earlier studies, it has been suggested that this effect may be mediated via postsynaptically localized GRs and requires a G protein-dependent signaling cascade (16). Disruption of intracellular postsynaptic GPCR signaling, with the addition of 500 µM guanosine 5′-[β-thio]diphosphate trilithium salt (GDPβS) to the patch pipette, prevented the rapid decrease in frequency induced by CORT (Fig. 4A).
Fig. 4.
Postsynaptic glucocorticoid and presynaptic cannabinoid-type 1 (CB1) receptors are required for the effects of corticosterone (CORT) on spontaneous excitatory postsynaptic currents (sEPSC) frequency. Plots show means ± SE change in sEPSC frequency in the presence of various agonists and antagonists. A: bath application of dexamethasone (DEX; 1 µM) inhibited sEPSC frequency similar to CORT (n = 10 neurons / 3 mice; P = 0.001, paired t test). The mineralocorticoid receptor antagonist spironolactone (SPIR; 300 nM) failed to block the inhibitory effects of CORT (n = 9 neurons / 4 mice; P = 0.006, paired t test), while the glucocorticoid receptor antagonist mifepristone (MIF; 10 µM) blocked the decrease in sEPSC frequency (n = 9 neurons / 5 mice; P = 0.26, paired t test). Intracellular application of the G protein inhibitor GDPβS (500 µM) also blocked the effects on spontaneous release (n = 10 neurons / 3 mice; P = 0.63, Wilcoxon test). B: CB1R-wild-type (WT) mice exhibited a decrease in sEPSC frequency to CORT exposure (n = 9 neurons / 3 mice; P = 0.017, paired t test), while genetic deletion of the CB1 receptor (CB1R-KO) prevented CORT-induced decrease in sEPSC frequency (n = 13 neurons / 4 mice; P = 0.09, paired t test). Both CB1 receptor antagonists AM251 (n = 9 neurons / 4 mice; P = 0.13, Wilcoxon test) and AM4113 (n = 11 neurons / 3 mice; P = 0.37, Wilcoxon test) blocked the effects of CORT on frequency. Intracellular application of the DAG lipase inhibitor DO34 (10 μM) also prevented the effects of CORT on sEPSC frequency (n = 11 neurons / 6 mice; P = 0.12, using paired t test). *P < 0.05, **P < 0.01.
Fig. 5.
Gene expression. A: plots show the ΔRn (baseline subtracted fluorescent reading normalized to the passive reference dye, ROX) of each gene across 50 cycles in cDNA from nodose ganglia (left) and nucleus of the solitary tract (NTS) tissue (right). B: RT-quantitative PCR analysis of genes encoding glucocorticoid receptor (NR3C1), mineralocorticoid receptor (NR3C2), and cannabinoid type-1 receptor (CNR1) from isolated nodose ganglia (n = 5 mice) and NTS slices (n = 3 mice). Gene expression is normalized to GAPDH and shown as 2−ΔCt. Data are expressed as means + SD.
The rapid effects of CORT on neurotransmission are reported to couple through presynaptic CB1 receptor signaling at some synapses (15, 16, 31, 58). Using qRT-PCR, we confirmed abundant CB1 receptor (gene name CNR1) expression in both nodose ganglion neurons and in the NTS (Fig. 5). In brain stem slices taken from control mice [CB1R-wild-type (WT)], we confirmed the ability of CORT to inhibit the frequency of spontaneous glutamate release (release was inhibited in five of eight neurons), whereas, CORT produced no statistically significant change in sEPSC frequency or amplitude in slices taken from CB1R-knockout (KO) mice (Fig. 4B). Bath application of the CB1 receptor antagonists AM251 (10 µM) or AM4113 (10 µM) also blocked the CORT-induced suppression of sEPSC frequency (Fig. 4B). AM251 or AM4113 alone produced statistically significant decreases in sEPSC frequency (67 ± 11% and 86 ± 5% of control, respectively, AM251: n = 9 neurons/ 4 mice, P = 0.05, paired t test; AM4113: n = 11 neurons/3 mice, P = 0.008, paired t test). However, the addition of CORT to either of these conditions failed to further suppress the frequency of spontaneous glutamate release. The coupling of postsynaptic GR activation with presynaptic CB1 receptor signaling is reported to be mediated by retrograde release of the endocannabinoid (eCB) 2-arachidonoylglycerol (2-AG). 2-AG is canonically produced postsynaptically by diacylglycerol lipase (DAGL). Therefore, we applied the DAGL inhibitor DO34 (10 µM) via the patch pipette to selectively block its production. Disrupting 2-AG synthesis with DO34 prevented the CORT-induced decrease in sEPSC frequency. Together, these data demonstrate that loss of CB1 signaling prevents the effects of CORT on sEPSC frequency.
Net effect of CORT on NTS neurons is to inhibit action potential firing.
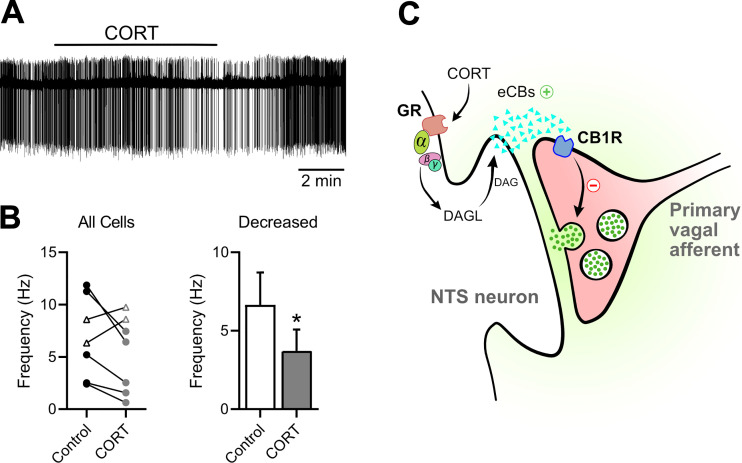
Using cell attached extracellular recording configuration, we measured spontaneous action potential firing in NTS neurons. We found bath application of CORT (1 μM) rapidly decreased the ongoing action potential firing in five of seven neurons recorded (Fig. 6A). On average, across all recordings, CORT decreased firing by 51 ± 7% (Fig. 6B). In two neurons recorded, CORT increased action potential firing, suggesting potential additional points of synaptic regulation by CORT. Figure 6C provides a diagrammatic model of CORT-induced inhibition at NTS synapses with the postsynaptic signal transduction and involvement of presynaptic CB1 receptors.
Fig. 6.
Corticosterone (CORT) rapidly suppresses action-potential firing in nucleus of the solitary tract (NTS) neurons. A: representative extracellular recordings showing constitutive action-potential firing during artificial cerebrospinal fluid (ACSF) control and following treatment with CORT (1 μM). B: on average, CORT significantly decreased action-potential firing in the majority of neurons recorded (n = 5 of 7 neurons/3 mice, P = 0.02, using paired t test). *P < 0.05. C: schematic diagram illustrating the putative mechanisms through which CORT inhibits glutamate release onto NTS neurons. CB1R, cannabinoid-type 1 receptor; DAG, diacylglycerol; DAGL, diacylglycerol lipase; eCBs, endocannabinoids; GR, glucocorticoid receptor.
DISCUSSION
We demonstrate that acute application of CORT inhibits fast glutamatergic synaptic transmission from ST afferents via postsynaptic activation of G protein-coupled GR receptors and the subsequent recruitment of presynaptic CB1 receptors. As a result, a relatively brief exposure to CORT resulted in prolonged suppression of postsynaptic excitability of NTS neurons. Thus, we posit that this mechanism provides one way by which CORT can rapidly modulate visceral, afferent, driven autonomic output and behaviors coordinated at the level of the NTS.
NTS neurons integrate primary visceral afferent information to drive autonomic reflexes during stress and also regulate HPA axis activity (27, 61). However, the acute physiological stress response, which is ultimately protective, differs from chronic stress responses that pose long-term problems to allostasis of the animal. It is possible that repeated instances of stress could lead to increased glucocorticoid effects in the NTS that could produce long-term changes that would affect visceral processing and autonomic function. The NTS contains a large proportion of ascending catecholaminergic fibers (7, 47). Sustained inhibition of firing rate of these neurons would indicate that acute glucocorticoid feedback in the NTS potentially leads to a reduced excitatory drive onto PVN neuroendocrine cells and stress-associated limbic areas, such as the amygdala and the BNST (8, 12, 51). In support of this, blockade of NTS GRs results in exacerbated HPA responses and increases helplessness behavior following acute and chronic stress (22), showing corticosterone does act in the NTS as a point of HPA axis negative feedback and regulation of behavioral responses to stress. In addition to being the primary stress hormone in rodents, corticosterone also expresses a robust circadian rhythm peaking just before the dark phase. Our recordings were made during the light, corresponding to the lowest endogenous CORT levels, so it is possible that the observed response on glutamate release is larger than what would normally occur during periods when endogenous CORT levels are higher. However, circadian rhythms of glucocorticoids (which oscillate at lower than stress concentrations) extensively bind to the high-affinity MR, as opposed to the lower-affinity GR (11, 13). Circadian expression of GR, however, could also lead to diurnal differences in this response. GR mRNA peaks during the inactive phase of the animal, indicating the largest response to stress-induced increases in CORT would occur during the light (33). This certainly would have interesting implications for how the stress circuitry responds to neuroendocrine signals across the circadian day.
Cranial visceral afferents within the ST release the fast neurotransmitter glutamate to NTS neurons via multiple release pathways. Spontaneous, action potential-independent neurotransmission conveys a large portion of the total charge transfer at these synapses and contributes to overall neuronal excitability (56). The relatively sustained suppression of this form of glutamate release by CORT provides a long-lasting point of control over synaptic transmission. Given the contrast between fast signaling of membrane-delimited CORT effects and the slow / long-term changes of transcriptionally mediated effects, this rapid but durable change in spontaneous glutamate release begins to span the time difference between these signaling modalities. The observation that CORT impacted this form of release onto every NTS neuron recorded suggests a prominent and conserved role for this point of synaptic control.
The solitary tract synapse also exhibits action potential-driven synchronous and asynchronous glutamatergic transmission. The intrinsically high initial Pr coupled with pronounced asynchronous release contribute to high-fidelity transduction of incoming visceral signals (17a). Like the spontaneous vesicle release pathway, CORT rapidly inhibited synchronous and asynchronous glutamate release. We observed a reduction in ST-evoked EPSC amplitude and an increase in both EPSC1 variance and PPR. Consistent with previous work showing that reducing extracellular calcium concentration or blocking N-type voltage-activated calcium channel (VACCs) also produces a similar response, the data suggest that the effect of CORT is mediated by a reduction in presynaptic Pr (3, 42, 43). We posit that CORT signaling via presynaptic CB1 receptors is mediated by signaling cascades described in other synapses in cerebellar Purkinje neurons (36), hippocampal neurons (55, 57), and neurons in the trigeminal nucleus (37). Evoked and quantal postsynaptic currents are inhibited by CB1 receptors via Gi/Go-coupled inhibition of N- and P/Q-type VACC (37, 57, 60). Release from ST afferents are also driven by these VACC subtypes and are likely impacted similarly.
We also found that CORT decreases the frequency-dependent-depression of consecutive ST-EPSCs (EPSC2-EPSC5), indicating a reduction in vesicle mobilization to the membrane. This is an interesting result, as we would expect that the lower initial Pr induced by CORT should lead to decreased vesicle depletion after the first shock. On the basis of current studies, it remains unclear whether this effect is secondary to CB1 receptor-induced decreases in terminal calcium-dependent processes, or if it results from direct activation of presynaptic GRs (46). We predict that the overall net effect of decreased Pr and vesicle mobilization would result in a sustained inhibition of synaptic transmission that is maintained even during strong trains of action potential stimulation.
Previous studies have shown that glucocorticoids rapidly modulate fast neurotransmission in several brain regions (6, 14–16, 31, 32, 38). Combined with the present findings, this model for noncanonical glucocorticoid signaling is strengthened. The rapid effects of glucocorticoids, as with many other steroids, are thought to be mediated by a G protein-dependent mechanism (35, 64). We found that postsynaptic quenching of G protein signaling prevented the rapid effects of CORT, indicating the involvement of a Gα subunit, and providing the strongest and most direct evidence that rapid CORT effects are generated initially by the postsynaptic neuron. Previous work suggests that this is likely mediated by Gαs activation, as PKA-specific inhibitors prevent similar actions of CORT at other synapses (38). The nongenomic GR signaling cascade comprises a host of signaling molecules (including Src kinase, PLC, PKC, and IP3 receptors and ERK-MAP kinase) (25, 44).
This is consistent with convergent mechanisms by which both glucocorticoid-mediated and activity/calcium-dependent eCB synthesis could act to control neurotransmission, as well as a host of other cellular processes (34). As such, inclusion of a DAGL inhibitor in the patch pipette to inhibit 2-AG production prevented the effects of CORT on spontaneous EPSC frequency. Although the overall effect was absent in the presence of DO34, some neurons (5/11 neurons) still showed blunted to normal responses to CORT. It is possible that this effect is partly mediated by another messenger, such as anandamide, or that this could be due to CORT acting on nearby neurons. Harris et al. (25) found that DO34 completely blocked the effects of CORT when it was bath applied. In the present study, DO34 was only applied to the patched neuron, so it is possible that 2-AG release from a neighboring neuron could act locally on those around it. These results implicate 2-AG as a retrograde eCB messenger coupling the GR signaling cascade to presynaptic CB1 receptor control over spontaneous glutamate release.
Taken together with previous work, our results illuminate potential additional synapse-specific differences in CORT signaling. Studies in the dorsal motor nucleus of the vagus (DMNX), which contains the cell bodies of vagal efferent neurons downstream of the NTS, demonstrate that glucocorticoids enhance glutamate and GABA neurotransmission through the retrograde release of anandamide (AEA) and subsequent activation of presynaptic transient receptor potential vanilloid type 1 (TRPV1) receptor (14). In contrast with these findings, we did not observe an increase in sEPSC frequency in any of the neurons recorded. TRPV1 is abundantly expressed in the central terminals of vagal afferent neurons (23). As such, the TRPV1 agonist capsaicin produces distinct effects on sEPSC frequency and ST-EPSC synaptic responses, which were not observed following CORT application (17, 29). Furthermore, while AEA activates TRPV1 on vagal afferent cell bodies, we have previously shown that bath perfusion onto slices failed to increase glutamate release from central terminals onto NTS neurons (19). Direct activation of CB1R produces a consistent inhibition of evoked ST-EPSCs (18). Similarly, depolarization-induced eCB production inhibits sIPSC frequency in NTS neurons through retrograde activation of presynaptic CB1R (9). It has yet to be determined whether differences in signaling exist between DMNX and NTS, or even hypothalamic, neurons that explain these functional differences in CORT signaling on fast synaptic transmission.
The broad effects of CORT via CB1 on multiple forms of terminal glutamate release position glucocorticoids as a potent regulator of this synapse. The NTS is composed of a heterogeneous collection of neuron subtypes mediating many physiological reflexes. Given that CORT impacts synaptic transmission on all neurons recorded, we predict that it would have diverse effects on nearly all physiological processes coordinated by the NTS and distinct neuron types. Further work would be needed to elucidate the individual effects of CORT on specific reflex pathways. Nevertheless, the mechanism uncovered here highlights how stress hormones may interact with neurons of the brain stem to impact parasympathetic outflow along with anxiety and depression-like behaviors.
GRANTS
This work was supported by grants from the National Institutes of Health (DK092651 to JHP and DK119811 to INK) and the National Science Foundation (CAREER 1553067 to INK).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.J.R., I.N.K., and J.H.P. conceived and designed research; F.J.R., R.A.A., C.W.K., M.I.S., and J.H.P. performed experiments; F.J.R., R.A.A., C.W.K., M.I.S., and J.H.P. analyzed data; F.J.R., R.A.A., M.I.S., I.N.K., and J.H.P. interpreted results of experiments; F.J.R. prepared figures; F.J.R., R.A.A., I.N.K., and J.H.P. drafted manuscript; F.J.R., R.A.A., C.W.K., I.N.K., and J.H.P. edited and revised manuscript; F.J.R., R.A.A., C.W.K., M.I.S., I.N.K., and J.H.P. approved final version of manuscript.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.Appleyard SM, Bailey TW, Doyle MW, Jin Y-H, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey TW, Jin Y-H, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol 296: R1445–R1454, 2009. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechtold AG, Scheuer DA. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am J Physiol Regul Integr Comp Physiol 290: R1003–R1011, 2006. doi: 10.1152/ajpregu.00345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Bowles NP, Karatsoreos IN, Li X, Vemuri VK, Wood J-A, Li Z, Tamashiro KLK, Schwartz GJ, Makriyannis AM, Kunos G, Hillard CJ, McEwen BS, Hill MN. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc Natl Acad Sci 112: 285–290, 2015. doi: 10.1073/pnas.1421420112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid glucocorticoid-induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric-related hypothalamic preautonomic neurons. Front Neurosci 7: 3, 2013. doi: 10.3389/fnins.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buller KM, Day TA. Involvement of medullary catecholamine cells in neuroendocrine responses to systemic cholecystokinin. J Neuroendocrinol 8: 819–824, 1996. doi: 10.1046/j.1365-2826.1996.05252.x. [DOI] [PubMed] [Google Scholar]
- 8.Bundzikova-Osacka J, Ghosal S, Packard BA, Ulrich-Lai YM, Herman JP. Role of nucleus of the solitary tract noradrenergic neurons in post-stress cardiovascular and hormonal control in male rats. Stress 18: 221–232, 2015. doi: 10.3109/10253890.2015.1013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-Y, Bonham AC, Dean C, Hopp FA, Hillard CJ, Seagard JL. Retrograde release of endocannabinoids inhibits presynaptic GABA release to second-order baroreceptive neurons in NTS. Auton Neurosci 158: 44–50, 2010. doi: 10.1016/j.autneu.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505, 1995. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 11.Dallman MF, Levin N, Cascio CS, Akana SF, Jacobson L, Kuhn RW. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology 124: 2844–2850, 1989. doi: 10.1210/endo-124-6-2844. [DOI] [PubMed] [Google Scholar]
- 12.Daubert DL, McCowan M, Erdos B, Scheuer DA. Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J Physiol 590: 4881–4895, 2012. doi: 10.1113/jphysiol.2012.232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 19: 269–301, 1998. doi: 10.1210/er.19.3.269. [DOI] [PubMed] [Google Scholar]
- 14.Derbenev AV, Smith BN. Dexamethasone rapidly increases GABA release in the dorsal motor nucleus of the vagus via retrograde messenger-mediated enhancement of TRPV1 activity. PLoS One 8: e70505, 2013. doi: 10.1371/journal.pone.0070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23: 4850–4857, 2003. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146: 4292–4301, 2005. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- 17.Doyle MW, Bailey TW, Jin Y-H, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci 22: 8222–8229, 2002. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- 18.Fawley JA, Hofmann ME, Andresen MC. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J Neurosci 34: 8324–8332, 2014. doi: 10.1523/JNEUROSCI.0315-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick AJ, Fowler DK, Wu S-W, Shaffer FJ, Lindberg JEM, Kinch DC, Peters JH. Direct anandamide activation of TRPV1 produces divergent calcium and current responses. Front Mol Neurosci 10: 200, 2017. doi: 10.3389/fnmol.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ffrench-Mullen JM. Cortisol inhibition of calcium currents in guinea pig hippocampal CA1 neurons via G-protein-coupled activation of protein kinase C. J Neurosci 15: 903–911, 1995. doi: 10.1523/JNEUROSCI.15-01-00903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillet S, Alonso G, Le Borgne R, Barbanel G, Malaval F, Assenmacher I, Szafarczyk A. Effects of discrete lesions in the ventral noradrenergic ascending bundle on the corticotropic stress response depend on the site of the lesion and on the plasma levels of adrenal steroids. Neuroendocrinology 58: 408–419, 1993. doi: 10.1159/000126570. [DOI] [PubMed] [Google Scholar]
- 22.Ghosal S, Bundzikova-Osacka J, Dolgas CM, Myers B, Herman JP. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology 45: 142–153, 2014. doi: 10.1016/j.psyneuen.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11: 946–958, 1999. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 24.Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, Okret S, Yu ZY, Goldstein M, Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA 83: 9779–9783, 1986. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris C, Weiss GL, Di S, Tasker JG. Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiol Stress 10: 100158, 2019. doi: 10.1016/j.ynstr.2019.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol 13: 349–372, 1993. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman JP. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell Mol Neurobiol 38: 25–35, 2018. doi: 10.1007/s10571-017-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24: 151–180, 2003. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y-H, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmakar S, Jin Y, Nagaich AK. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) α and activator protein 1 (AP1) in dexamethasone-mediated interference of ERα activity. J Biol Chem 288: 24020–24034, 2013. doi: 10.1074/jbc.M113.473819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karst H, Berger S, Erdmann G, Schütz G, Joëls M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 107: 14449–14454, 2010. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207, 2005. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch CE, Leinweber B, Drengberg BC, Blaum C, Oster H. Interaction between circadian rhythms and stress. Neurobiol Stress 6: 57–67, 2016. doi: 10.1016/j.ynstr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M, Lutz B. Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learn Mem 17: 43–49, 2009. doi: 10.1101/lm.1663410. [DOI] [PubMed] [Google Scholar]
- 35.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with G α i and G βγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol 21: 1370–1380, 2007. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 36.Lévénès C, Daniel H, Soubrié P, Crépel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol 510: 867–879, 1998. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y-C, Huang C-C, Hsu K-S, Takahashi T. Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices. J Physiol 555: 85–96, 2004. doi: 10.1113/jphysiol.2003.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malcher-Lopes R, Di S, Marcheselli VS, Weng F-J, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26: 6643–6650, 2006. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci 9: 390, 2015. doi: 10.3389/fnins.2015.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahata M, Saegusa Y, Sadakane C, Yamada C, Nakagawa K, Okubo N, Ohnishi S, Hattori T, Sakamoto N, Takeda H. Administration of exogenous acylated ghrelin or rikkunshito, an endogenous ghrelin enhancer, improves the decrease in postprandial gastric motility in an acute restraint stress mouse model. Neurogastroenterol Motil 26: 821–831, 2014. doi: 10.1111/nmo.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta H, Talman WT. Both NMDA and non-NMDA receptors in the NTS participate in the baroreceptor reflex in rats. Am J Physiol Regul Integr Comp Physiol 267: R1065–R1070, 1994. doi: 10.1152/ajpregu.1994.267.4.R1065. [DOI] [PubMed] [Google Scholar]
- 42.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu J, Wang P, Jing Q, Zhang W, Li X, Zhong Y, Sun G, Pei G, Chen Y. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem Biophys Res Commun 287: 1017–1024, 2001. doi: 10.1006/bbrc.2001.5691. [DOI] [PubMed] [Google Scholar]
- 45.Dos Reis DG, Fortaleza EAT, Tavares RF, Corrêa FMA. Role of the autonomic nervous system and baroreflex in stress-evoked cardiovascular responses in rats. Stress 17: 362–372, 2014. doi: 10.3109/10253890.2014.930429. [DOI] [PubMed] [Google Scholar]
- 46.Revest J-M, Kaouane N, Mondin M, Le Roux A, Rougé-Pont F, Vallée M, Barik J, Tronche F, Desmedt A, Piazza PV. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol Psychiatry 15: 1140–1151, 2010. doi: 10.1038/mp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reyes BAS, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res 1117: 69–79, 2006. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 153: 1–26, 1978. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 49.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 277: R582–R590, 1999. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 50.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357–1367, 2003. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- 51.Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci 11: 1317–1323, 1999. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 52.Saphier D, Feldman S. Catecholaminergic projections to tuberoinfundibular neurones of the paraventricular nucleus: III. Effects of adrenoceptor agonists and antagonists. Brain Res Bull 26: 863–870, 1991. doi: 10.1016/0361-9230(91)90250-N. [DOI] [PubMed] [Google Scholar]
- 53.Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension 49: 127–133, 2007. doi: 10.1161/01.HYP.0000250088.15021.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheuer DA, Mifflin SW. Glucocorticoids modulate baroreflex control of renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 280: R1440–R1449, 2001. doi: 10.1152/ajpregu.2001.280.5.R1440. [DOI] [PubMed] [Google Scholar]
- 55.Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16: 4322–4334, 1996. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 30: 14470–14475, 2010. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol 82: 1286–1294, 1999. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- 58.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14: 398–406, 2011. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388: 169–190, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 60.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 78: 43–50, 1997. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 61.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L-L, Ou C-C, Chan JYH. Receptor-independent activation of GABAergic neurotransmission and receptor-dependent nontranscriptional activation of phosphatidylinositol 3-kinase/protein kinase Akt pathway in short-term cardiovascular actions of dexamethasone at the nucleus tractus solitarii of the rat. Mol Pharmacol 67: 489–498, 2005. doi: 10.1124/mol.104.005595. [DOI] [PubMed] [Google Scholar]
- 63.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159: 883–895, 2009. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zagar Y, Chaumaz G, Lieberherr M. Signaling cross-talk from Gbeta4 subunit to Elk-1 in the rapid action of androgens. J Biol Chem 279: 2403–2413, 2004. doi: 10.1074/jbc.M309132200. [DOI] [PubMed] [Google Scholar]
- 65.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol 296: R1358–R1365, 2009. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]