Abstract
Inhalation of organic dust is an occupational hazard leading to the development of respiratory symptoms and respiratory diseases. Bioaerosols from concentrated animal feeding operations are rich in bacteria and could carry bacterial extracellular vesicles (EVs) that could induce lung inflammation. It is not known if organic dust contains bacterial EVs and whether they modulate lung inflammation. Herein, we show that poultry organic dust contains bacterial EVs (dust EVs) that induce lung inflammation. Treatment of airway epithelial cells, THP-1-monocytes and -macrophages with dust EVs rapidly induced IL-8, IL-6, ICAM-1, proIL-1β, and TNF-α levels. In airway epithelial cells, induction of inflammatory mediators was due to increased mRNA levels and NF-κB activation. Induction of inflammatory mediators by dust EVs was not inhibited by polymyxin B. Single and repeated treatments of mice with dust EVs increased lung KC, IL-6, and TNF-α levels without significantly altering IL-17A levels. Increases in cytokines were associated with enhanced neutrophil infiltration into the lung. Repeated treatments of mice with dust EVs increased lung mean linear intercept and increased collagen deposition around airways indicating lung remodeling. Peribronchial cell infiltrates and airway epithelial thickening were also observed in treated mice. Because bacterial EVs are nanometer-sized particles, they can reach and accumulate in the bronchiolar and alveolar regions causing lung injury leading to the development of respiratory diseases. Our studies have provided new evidence for the presence of bacterial EVs in organic dust and for their role as one of the causative agents of organic dust-induced lung inflammation and lung injury.
Keywords: cytokines, gene expression, lung remodeling, occupational lung diseases
INTRODUCTION
Agricultural workers particularly livestock workers are at risk of exposure to high levels of airborne dust in their work environment (1, 54). Modern livestock farms practice high-density animal farming operations known as concentrated animal feeding operations (CAFOs) to achieve efficiency and enhance productivity to provide low-cost source of meat, eggs, and milk (18). The air inside CAFOs is contaminated with high levels of airborne dust and its constituents (19). Lack of use of personal protective equipment further exposes workers to high levels of airborne dust. In fact, exposure to airborne dust in CAFOs is associated with the development of acute and chronic respiratory symptoms and respiratory diseases (1, 54). Agricultural workers, particularly poultry workers, experience a high incidence of lower and upper respiratory tract symptoms and respiratory diseases such as bronchitis, organic dust toxic syndrome, hypersensitivity pneumonitis, and occupational asthma (47, 51). The prevalence of chronic obstructive pulmonary disease (COPD) is also high among agricultural workers (17).
Dust in livestock farms known as organic dust is a complex mixture of particulate matter containing animal dander, feathers, and food particles (1, 22). It also contains microorganisms and their byproducts such as endotoxin, peptidoglycan, and mycotoxin as well as gases such as ammonia, methane, and hydrogen sulfide (1, 22). Organic dusts from different sources increase inflammatory cytokines in lungs of human subjects (30, 55) and experimental animals (8, 34, 46) and in lung cells in vitro (6, 41, 48, 56). Several dust constituents have been identified and quantified from various organic dusts (44); however, their relative roles in the inflammatory outcomes are not well understood. Endotoxin, a major constituent of animal farm and grain dusts, does not appear to play an independent role in the induction of cytokines by organic dust extracts in lung epithelial cells in vitro (16, 48). Lung inflammation in human subjects could not be correlated with endotoxin levels in the environment (10). Aqueous poultry farm dust extracts contain low concentrations of peptidoglycan yet stimulate inflammatory mediators markedly (6), and peptidoglycan was found to be a weak stimulator of IL-8 and IL-6 in human lung epithelial cells (48) indicating that peptidoglycan may not be a major player in the induction of inflammatory mediator levels in lung epithelial cells in vitro. Recent studies have demonstrated that proteases in aqueous extracts of swine (49) and poultry (37) farm dusts are involved in the induction of inflammatory cytokine production indicating that they may be one of the major components of animal farm dusts promoting inflammatory responses.
Poultry farming, which involves raising domesticated chickens, turkey, ducks, and geese for meat and egg production, is a major component of agriculture world-wide and employs several thousand workers in the United States alone. Poultry farm workers experience higher prevalence and greater severity of respiratory symptoms than swine workers (47, 51) that could be due to the presence of higher levels of dust in the poultry farm environment (47). The concentration of airborne dust and the concentrations of dust constituents such as endotoxin, total fungi, total bacteria, ammonia, and CO2 were found to be significantly higher in poultry farms than in swine farms (47). Poultry bioaerosols contain bacteria with preponderance of Gram-positive bacteria (40, 50). With the overall goal of understanding mechanisms of lung inflammatory responses to organic dust exposure, our research using poultry (broiler) farm dust as a model agricultural dust has demonstrated that aqueous extracts of dust increased the expression of several immune and inflammatory genes in lung epithelial and THP-1 monocytic cells via activation of protein kinase signaling (6, 16). Protease activities present in dust extracts and increased oxidative stress were found to activate NF-κB and STAT-3 transcription factors to induce inflammatory gene expression in lung epithelial cells (37, 38). Interestingly, poultry organic dust extracts induced cytokines and chemokines but reduced surfactant protein levels in lung epithelial cells (36).
Poultry farm dust contains high concentrations of bacteria (50) that could release extracellular vesicles into the environment. Bacterial extracellular vesicles (EVs) are bilayered membrane vesicles that are released in a constitutive and a regulated manner by Gram-negative and Gram-positive bacteria (25). Bacterial EVs range in size between 20 and 300 nm and can contain proteins, lipids, DNA, RNA, virulence factors, and other molecules and are known to be involved in diverse functions including intercellular communication, biofilm formation, and modulation of host inflammatory responses (25). Because of the presence of high concentrations of airborne bacteria in poultry farms (50), we hypothesized that poultry farm dust contains bacterial EVs and they contribute to the induction of inflammatory mediators and inflammatory responses in the lung. To date, there is no information on the identification of bacterial EVs in agricultural dust and their involvement in the elicitation of inflammatory responses in the lung. In this study, we sought to identify bacterial EVs in poultry organic dust and determine their role in the modulation of inflammatory responses in lung epithelial cells in vitro and in mouse lungs. We found that bacterial EVs are indeed present in poultry organic dust and are derived from Gram-negative and Gram-positive bacteria. Bacterial EVs isolated from poultry organic dust (hereafter referred to as dust EVs) were found to increase inflammatory mediators in lung epithelial cells and THP-1-monocytes and -macrophages. Induction of inflammatory gene expression in lung epithelial cells was due to activation of NF-κB. The increase in inflammatory gene expression was not inhibited by endotoxin neutralizing agent polymyxin B. Intranasal instillation of dust EVs into mice increased inflammatory cytokine levels that were associated with elevated neutrophil counts in the bronchoalveolar lavage (BAL) fluid (BALF). Long-term treatment of mice with bacterial EVs caused enlargement of alveoli and increased deposition of collagen around airways.
MATERIALS AND METHODS
Chemicals and reagents.
Polymyxin B (Sigma-Aldrich) was dissolved in cell culture medium. The NF-κB inhibitor Bay 11-7082 (Calbiochem) was dissolved in dimethylsulfoxide.
Antibodies.
Prointerleukin-1β (proIL-1β) (Cell Signaling Technology), intercellular adhesion molecule-1 (ICAM-1) (Santa Cruz Biotechnology), phospho-NF-κB p65 (S536) (Cell Signaling Technology), NF-κB p65 (Santa Cruz Biotechnology), and actin (Santa Cruz Biotechnology) antibodies were used as primary antibodies in Western blotting analysis. AP-linked mouse and rabbit secondary antibodies (Cell signaling Technology) were used as secondary antibodies in Western blotting analysis.
Dust samples.
Dust settled on vertical surfaces inside poultry houses were collected with permission from two different commercial broiler poultry farms located in East Texas when the birds were ∼49–56 days of age and stored at −70°C. The poultry houses are constructed of solid side walls and equipped with tunnel ventilation.
Isolation of bacterial extracellular vesicles.
Bacterial EVs were isolated from dust samples essentially as described previously (26). Dust was suspended in Dulbecco’s phosphate-buffered saline (D-PBS) without calcium and magnesium (1:10, wt/vol) and mixed on a rotating mixer overnight at 4°C. The dust suspension was centrifuged twice at 800 g for 10 min at 4°C, and the resulting supernatant was centrifuged again at 10,000 g for 10 min at 4°C. The supernatant was filtered with a 0.22-µm syringe filter, and the filtrate was centrifuged at 150,000 g for 3 h at 4°C. The supernatant was completely decanted, and the walls of the centrifuge tube were rinsed with cold D-PBS. The pellet was washed by resuspending in 10 mL cold D-PBS, and the suspension was centrifuged at 150,000 g for 3 h at 4°C. The supernatant was completely decanted, the pellet was carefully suspended in cold D-PBS, and the suspension was stored in aliquots at −70°C. Protein concentration of the vesicles was determined by Bradford assay and their size distribution and particle number were determined by nanoparticle tracking analysis using Nanosight NS300 instrument (Malvern Pananalytical).
Isolation of DNA from bacterial EVs and DNA sequencing.
Isolation of DNA and 16S rRNA gene sequencing and taxonomic profiling of bacteria were performed by Omega Bioservices (Norcross, GA).
Electron microscopy.
Negative staining electron micrographs of bacterial vesicles were obtained at the Electron Microscopy Core Facility, University of Texas Southwestern Medical Center (Dallas, TX). For negative staining, bacterial vesicles were extracted from dust samples using HEPES-buffered saline instead of phosphate-buffered saline as phosphate would react with uranyl acetate to produce dark precipitate in the sample. Bacterial vesicles were suspended in 0.125 M cacodylate buffer, pH 7.4 containing 2.5% glutaraldehyde and processed for negative staining. Images were obtained with a JEOL 1400 Plus electron microscope.
Cell culture.
Beas2B (ATCC CRL 9609) bronchial epithelial cells were grown on plastic culture dishes coated with fibronectin, bovine type I collagen, and bovine serum albumin and maintained in LHC 9 medium (Invitrogen) containing penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin B (0.25 µg/mL). Normal human bronchial epithelial cells (NHBE) from female or male donors were purchased from Lonza (Walkersville, MD) or Lifeline Cell Technology (Walkersville, MD) and grown on plastic culture dishes under submerged conditions in bronchial epithelial growth medium. A549 alveolar epithelial (ATCC CCL185) and THP-1 monocytic (ATCC TIB-202) cells were maintained in F12K medium and RPMI 1640 medium containing 0.05 mM β-mercaptoethanol, respectively, in the presence of 10% fetal bovine serum and 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. Human umbilical vein endothelial cells (HUVEC) (Lonza) were grown in EGM-2 complete medium (Lonza), and when 80–90% confluent, cells were maintained overnight in EBM-2 basal medium (Lonza) and treated in the same medium. THP-1 monocytes were differentiated into macrophages by treatment with 200 nM phorbol 12-myristate 13-acetate (PMA) for 48 h, washed, and rested in growth medium without PMA for 5 days (11). THP-1 macrophages were rinsed two times with serum-free RPMI 1640 medium containing antibiotics and antimycotics and incubated in the same medium for 3 h before treatment. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% room air and medium was changed once every 2 to 3 days. Beas2B, NHBE, and THP-1 cells were maintained in serum-free RPMI 1640 medium, and A549 cells were maintained in serum-free F12K medium overnight before treatments. Both media contained penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin B (0.25 µg/mL). Adherent cells were 80–90% confluent at the time of treatment.
Animals.
Animal studies had been previously approved by our Institutional Animal Care and Use Committee. Female C57BL6 mice (8–10 wk old, ∼18–20 g) were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained under standard housing conditions with a 12:12-h light-dark cycle and fed standard mouse diet and water. Mice were acclimatized for 1 wk before treatments, weighed, and anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (8.5 mg/kg) at a dose of 0.1 mL/20 g weight. Deep anesthesia was confirmed by the absence of response to toe pinch and 50 μL PBS or 50 μL PBS containing bacterial EVs equivalent to 0.1–1 μg protein content were administered into the mice via intranasal instillation. Bacterial EVs were administered once or once daily Monday–Friday for 9 days (∼2 wk). Mice that were repeatedly exposed were euthanized 3 h after administration of bacterial EVs on the final day.
Bronchoalveolar lavage fluid.
Mice were euthanized by injecting Beuthanasia/Euthasol (5 µL/g weight ip) and exsanguinated by severing renal artery. BAL fluid was collected by slowly instilling and aspirating 0.5 mL PBS containing 2.6 mM EDTA into the lung via the trachea. This was repeated three additional times, and the first two and the last two fractions were pooled separately and centrifuged at 800 g for 10 min at 4°C, and the supernatant was stored at – 80°C. The pooled samples of the first two fractions were used for ELISA measurements. The cells from all the BAL fluid fractions were pooled, suspended in 0.5 mL PBS containing 2.6 mM EDTA, and erythrocytes were lysed. BAL cells cleared of erythrocytes were counted using a hematocytometer. One-hundred microliters of cell suspension were applied onto glass slides using a cytospin centrifuge. Slides were stained with Diff-Quick (Hema 3 manual staining kit, Fisher Scientific) to visualize and count various cell types.
Flow cytometry.
BALF cells were stained with antibodies against surface markers for leukocytes and analyzed by flow cytometry to determine differential cell counts. BALF cell suspensions were prepared and stained as described previously (29). Briefly, cells were suspended in FACS buffer containing fc-blocking antibodies for 20 min at 4°C. Antibodies were obtained from BioLegend or BD Biosciences. Cells were washed and resuspended in 50 μL FACS buffer containing antibodies against CD45: PerCP-Cy5.5; CD3: PE-CF594; Ly6G: PE; CD11b: APC-Cy7; F4/80: APC; Siglec-F: FITC; and CD11c: PE-Cy7, and incubated for 15 min at 4°C. After incubation, cells were washed with FACS buffer and analyzed using BD LSRFortessa X-20 flow cytometer. The data were analyzed using FlowJo software (Version 10.6.1, TreeStar)
Lung homogenization.
After BAL fluid collection, the lungs were excised for the isolation of total RNA and preparation of tissue homogenate. Lung was cut into small pieces on ice with a scissor and homogenized (OMNI Tissue Homogenizer) in 10 vol (wt/vol) of ice-cold tissue homogenization buffer (10 mM Tris·HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 1× protease and phosphatase inhibitor cocktail). Tissue homogenate was subjected to freeze thaw and then centrifuged at 16 000 g for 10 min, and supernatant was stored at –80°C.
Histology.
Mouse lungs were cleared of blood by injecting 10 mL of D-PBS into the right heart ventricle and then instilled with Excell Plus fixative (American MasterTech) under a constant hydrostatic pressure of 20 cm. Lungs were stored in the fixative for at least 2 days before processing for sectioning. Lung sections of 5 μm were obtained, and sections were subjected to hematoxylin and eosin (H and E) staining. Collagen was visualized by performing trichrome staining. Images were captured with a Cytation 5 Imaging Reader (BioTek). Mean liner intercept (Lm) was determined using ImageJ software (National Institutes of Health). To determine the mean linear intercept, a total of 40 horizontal lines were randomly drawn across 2 different images of a lung section at ×200 magnification, and Lm was calculated as the length of the line divided by the total number of alveolar wall-line intersections.
Enzyme-linked immunosorbent assay.
The levels of cytokines IL-6, IL-8, and TNF-α in cell culture medium, bronchoalveolar lavage (BAL) fluid and lung homogenates were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems) according to the manufacturer’s instructions.
Western immunoblotting.
Equal amounts of cellular proteins (20−30 µg) were separated by SDS-PAGE on 10% NuPAGE Bis-Tris gels (Life Technologies) alongside protein markers using MOPS as the running buffer. Separated proteins were transferred to Hybond-PVDF membranes (0.2 µm) (GE Healthcare) by electroblotting. Protein bands were detected by the enhanced chemifluorescence detection method (GE HealthCare Life Sciences) and visualized by scanning with Bio-Rad Molecular Imager FX or Bio-Rad Chemidoc MP Imager and quantified using Quantity One Software or ImageLab software (Bio-Rad). Membranes were reprobed with actin antibodies to assess for equal loading and transfer of proteins.
RNA isolation and qRT-PCR.
Lung cells and lung tissues were homogenized in 1 mL of TRI-Reagent for the isolation of total RNA, and RNA purity was checked by analyzing A260/A280 and A260/A230 ratios. For the determination of mRNA levels, total RNA was first digested with DNase (Turbo DNA-free kit, Ambion) for removal of genomic DNA and purified total RNA was reverse transcribed to synthesize cDNA using iScript Reverse Transcription kit (Bio-Rad). Levels of IL-8, IL-6, IL-1β, ICAM-1, and actin mRNAs and 18S rRNA were quantified by TaqMan gene expression assays using reaction conditions of 40 cycles of 95°C for 30 s, 95°C for 5 s, and 60°C for 30 s. Levels of mRNAs were normalized to actin or 18S rRNA levels.
Cell transfection and luciferase reporter assay.
Cells were transfected with IL-8 promoter plasmids (5) using Lipofectamine 3000 and P3000 reagent (Invitrogen) according to the manufacturer’s protocol. Luciferase activity in cell lysates was measured by chemiluminescent assay (Promega) and normalized to the total protein content.
MTS assay.
Cell viability was determined using MTS assay (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay) (Promega) according to the manufacturer’s protocol.
Statistical analysis.
Data were analyzed using GraphPad Prism software. Each experiment was performed at least three times independently, and the data are shown as means ± SD/SE. Two-tailed paired t test or one-way way analysis of variance (ANOVA) using Tukey’s multiple comparison test, as appropriate, was used to analyze statistical significance between the treatment groups and P value of < 0.05 was considered statistically significant.
RESULTS
Isolation and characterization of dust EVs.
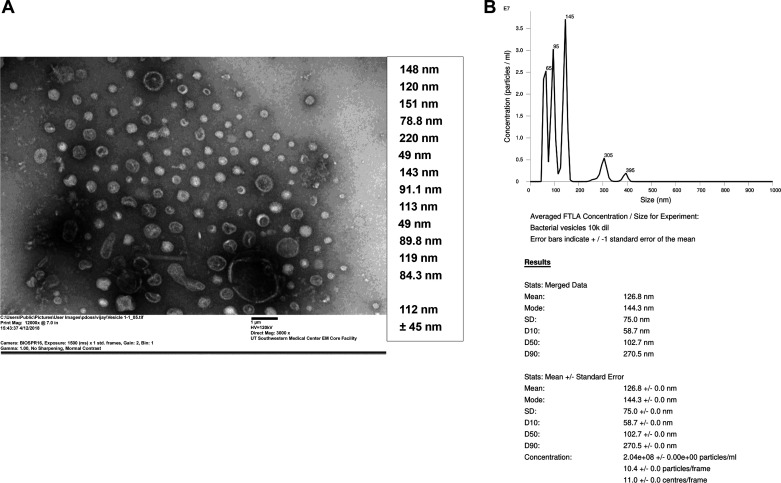
Bacterial EVs were isolated from poultry organic dust and characterized with regards to their size distribution and shape by negative staining electron microscopy and by nanoparticle tracking analysis. Negative staining electron microscopy and measurements by NanoSight NS300 instrument indicated that the dust EVs are heterogenous in size with the majority of the vesicles in the size range of 65–145 nm and the remaining vesicles in sizes of 305 and 395 nm (Fig. 1, A and B). The vesicles appeared spherical in shape with bilayer membrane structure.
Fig. 1.
Negative staining electron microscopy and size analyses of dust extracellular vesicles (EVs). A: dust EVs were visualized by negative staining electron microscopy using uranyl acetate. A representative electron micrograph with sizes of dust EVs that were randomly selected for measurement is shown. B: a representative size distribution of dust EVs obtained by nanoparticle tracking analysis (NTA) using NanoSight NS300 instrument. EVs (n = 15) isolated from 3 different dust samples were analyzed by NTA.
Genotyping of dust EVs.
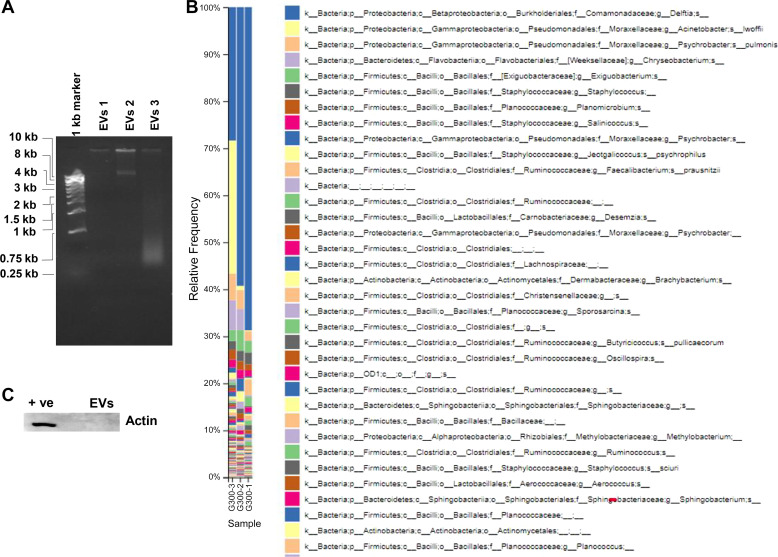
Analysis of dust EVs by agarose gel electrophoresis and ethidium bromide staining showed that they contained high molecular weight DNA as well as a fragment of ∼10 kb indicating that the high molecular weight DNA could be chromosomal DNA (Fig. 2A). Genotyping of dust EV DNAs isolated from the two different dust samples collected from two different poultry farms were performed by 16S rRNA gene sequencing. Genotyping included sequencing of unwashed and washed vesicles isolated from one of the dust samples. The taxonomic profile derived from 16S rRNA sequence data indicated that the vesicles are derived from Gram-positive and Gram-negative bacteria (Fig. 2B). Taxonomic data also showed that the different dust EV samples shared similar bacterial species but with varying frequency. Actin could not be detected by Western blotting (Fig. 2C), and 18S rRNA could not be detected by qRT-PCR in dust EVs (data not shown) indicating that they may not contain mammalian and chicken EVs. Protein determinations and counting of EVs by nanoparticle tracking analysis indicated that 1 μg protein of isolated bacterial EVs was equivalent to 32.76 ± 6.5 × 108 vesicles (n = 5).
Fig. 2.
Agarose gel electrophoresis of dust extracellular vesicles (EVs) and genotyping of DNA isolated from dust EVs. A: dust EVs alongside DNA markers were subjected to agarose gel electrophoresis and DNA was visualized by ethidium bromide staining. B: DNAs isolated from dust EVs isolated from 2 different dust samples were genotyped by 16S ribosomal RNA gene sequencing and relative abundance of bacterial species is shown. EVs 1, EVs (unwashed) from sample dust 1; EVs 2, EVs (unwashed) from sample dust 2; EVs 3, EVs (washed) from sample dust 2. C: EVs (1 μg protein) alongside Beas2B cell lysate as a positive control (+ve) were analyzed for the presence of actin by Western blotting.
Dust EVs induce lung inflammatory mediator levels in a concentration- and time-dependent manner.
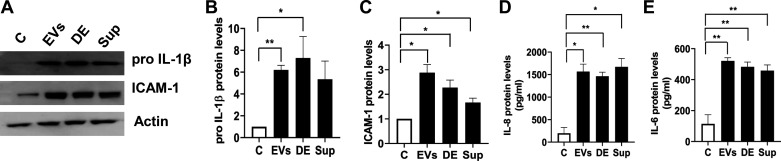
Lung epithelium not only acts as a barrier to external agents but also controls lung inflammation and innate immune responses in cooperation with immune cells through production of cytokines and other immunomodulatory factors (33). In preliminary experiments, we evaluated EVs isolated from three different dust samples, collected from two poultry farms located in different regions of East Texas, for their abilities to induce inflammatory mediator levels in Beas2B cells. We found that the three different dust EVs increased proIL-1β and ICAM-1 protein levels by a similar degree in comparison with cells treated with PBS alone, indicating the inductive capacities of EVs are largely independent of the sources of the dust samples (data not shown). To determine the relative contributions, we assessed the effects of isolated EVs, the supernatant obtained after pelleting the EVs, and the whole dust extract to induce inflammatory mediators in Beas2B cells (Fig. 3). The levels of cellular proIL-1β and ICAM-1 and secreted IL-6 protein levels in cells treated with the supernatant were reduced compared with cells treated with whole dust extract but were still significantly elevated than in control cells. This suggested that soluble factors and perhaps EVs that have not been completely cleared by ultracentrifugation could be contributing to the induction of inflammatory mediators.
Fig. 3.
Effects of dust extracellular vesicles (EVs), total dust extract, and dust extract cleared of EVs on the induction of inflammatory mediator protein levels in Beas2B cells. Cells were untreated (C) or treated with dust EVs (EVs) (0.5 μg protein/mL), dust extract (DE) (0.25%), or dust extract cleared of EVs by ultracentrifugation (sup) (0.25%) for 5 h. The levels of proIL-1β and ICAM-1 in cell lysates and the levels of IL-8 and IL-6 in cell medium were determined. A representative Western blot is shown. All data shown are means ± SE (n = 4). *P < 0.05 and **P < 0.01 according to one-way ANOVA using Tukey’s multiple-comparison test.
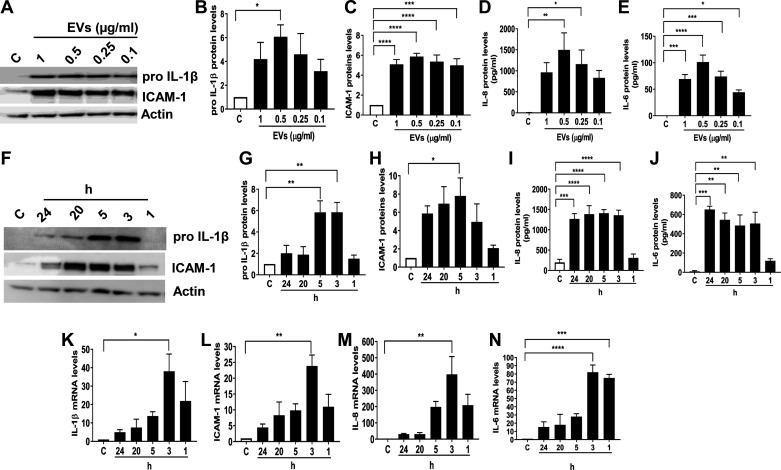
To determine if the dust EVs elicit inflammatory responses, we first investigated their effects on the expression of inflammatory mediators such as IL-1β, ICAM-1, IL-6, and IL-8 in Beas2B bronchial epithelial cells. Cells were treated with 0.1–1 μg/mL dust EVs (equivalent to 0.1–1 μg/mL protein content) for 5 h, and the cellular and secreted levels of inflammatory mediators were determined by Western immunoblotting and ELISA, respectively. Results demonstrated that dust EVs increased proIL-1β, ICAM-1, IL-8, and IL-6 levels in a concentration-dependent manner in Beas2B (Fig. 4, A–E). To determine the time-dependent effects of dust EVs on the expression of inflammatory markers, Beas2B cells were treated with 0.5 μg/mL of dust EVs for 1–24 h and the protein levels of proIL-1β, ICAM-1, IL-6, and IL-8 were determined (Fig. 4, F–J). Results demonstrated that the cellular levels of proIL-1β and ICAM-1 peaked at 3 or 5 h and declined thereafter. At 24 h posttreatment, proIL-1β levels were significantly reduced, however ICAM-1 levels were still elevated. Secreted IL-6 and IL-8 levels were significantly elevated at 3 h and remained at this level till 24 h posttreatment. To determine if the increases in inflammatory mediator protein levels are due to alterations in gene expression, we investigated the effects of dust EVs on the levels of IL-1β, ICAM-1, IL-6, and IL-8 mRNAs in Beas2B cells (Fig. 4, K–N). Results showed that treatment with dust EVs rapidly increased mRNA levels in as little as 1 h after treatment and the mRNA levels peaked at 3 h and steadily decreased by 24 h. These data indicate that the induction of inflammatory mediators by dust EVs are due to increased gene expression.
Fig. 4.
Effects of dust extracellular vesicles (EVs) on inflammatory mediator protein and mRNA levels in Beas2B cells. A–E: cells were untreated (C) or treated with medium containing different concentrations of EVs (μg/mL protein) for 5 h and the levels of proIL-1β and ICAM-1 in cell lysates, and the levels of IL-6 and IL-8 in cell medium were determined. F–J: cells were treated with EVs (0.5 μg protein/mL) for 1–24 h, and the levels of proIL-1β and ICAM-1 in cell lysates and the levels of IL-6 and IL-8 in cell medium were determined. K–N: cells were treated with EVs (0.5 μg protein/mL) for 1–24 h, and IL-1β, ICAM-1, IL-6, and IL-8 mRNA levels were determined. Representative Western blots are shown. All data shown are means ± SE (n = 4–5). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 according to one-way ANOVA using Tukey’s multiple-comparison test.
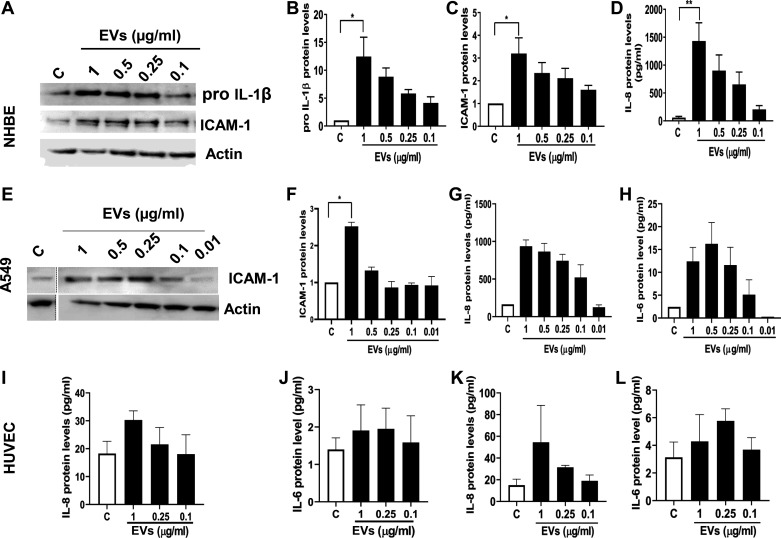
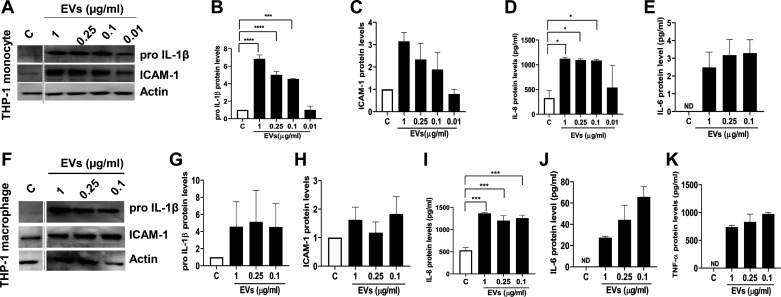
We examined the effects of dust EVs on inflammatory mediators in A549 alveolar and Beas2B bronchial epithelial cell lines, primary normal human bronchial epithelial cells, primary human umbilical vein endothelial cells (HUVEC), THP-1 monocytes, and THP-1 macrophages to understand how different cell types in the lung might respond. As in the case of Beas2B cells, dust EVs increased the expression of inflammatory mediators in normal human bronchial epithelial cells (NHBE) (Fig. 5, A–D) and A549 alveolar epithelial cells (Fig. 5, E–H). In NHBE and A549 cells, IL-6 and proIL-1β could not be detected, respectively, under control or treated conditions. In primary human umbilical vein endothelial cells (HUVEC), dust EVs modestly increased IL-8 and IL-6 protein levels (Fig. 5, I–L) without any effect on ICAM-1 protein levels (data not shown). In THP-1 monocytes and THP-1 macrophages, dust EVs markedly induced proIL-1β, IL-6, IL-8, and TNF-α protein levels, but the inductive effect on ICAM-1 levels in THP-1 monocytes was stronger than in THP-1 macrophages (Fig. 6, A–K). Treatment of Beas2B and NHBE cells with 0.1–1.0 μg/mL of dust EVs for 24 h did not reduce the viability of cells as determined by MTS assay, indicating that the induction of inflammatory mediators is not associated with cell toxicity (data not shown). We did not determine the effects of dust EVs on the viabilities of A549, HUVEC, THP-1 monocytes, and THP-1 macrophages. Morphological appearance of A549, HUVEC, and THP-1 cells and their total cell protein concentrations did not change after treatment with dust EVs indicating that cell viabilities were not affected (data not shown).
Fig. 5.
Effects of dust extracellular vesicles (EVs) on inflammatory mediator protein levels in normal human bronchial epithelial cells (NHBE), A549 alveolar epithelial cells, and human umbilical vein endothelial cells (HUVEC). NHBE (A–D) and A549 (E–H) cells were untreated (C) or treated with medium containing different concentrations of EVs (μg/mL protein) for 5 h and the levels of proIL-1β, ICAM-1, and actin in cell lysates, and the levels of IL-6 and IL-8 in cell medium were determined. Representative Western blots with noncontiguous lanes demarcated by broken lines are shown. HUVEC were untreated (C) or treated with medium containing different concentrations of EVs for 5 h (I and J) and 24 h (K and L), and the levels of IL-8 and IL-6 in cell medium were determined. All data shown are means ± SD/SE (n = 4 for NHBE cells, n = 2 for A549, n = 3 for HUVEC). *P < 0.05 and **P < 0.01 according to one-way ANOVA using Tukey’s multiple-comparison test.
Fig. 6.
Effects of dust extracellular vesicles (EVs) on inflammatory mediator protein levels in THP-1 monocytes and THP-1 macrophages. THP-1 monocytes (A–E) and THP-1 macrophages (F–K) were untreated (C) or treated with medium containing different concentrations of EVs (μg/mL protein) for 5 h and the levels of proIL-1β, ICAM-1, and actin in cell lysates and the levels of IL-8, IL-6, and TNF-α in cell medium were determined. Noncontiguous lanes in Western blots are demarcated by broken lines. All data shown are means ± SE (n = 3). *P < 0.05, ***P < 0.001, and ****P < 0.0001 according to one-way ANOVA using Tukey’s multiple-comparison test. IL-6 and TNF-α levels in control medium were below detection [not detected (ND)].
Dust EVs increase inflammatory mediators in NF-κB-dependent manner.
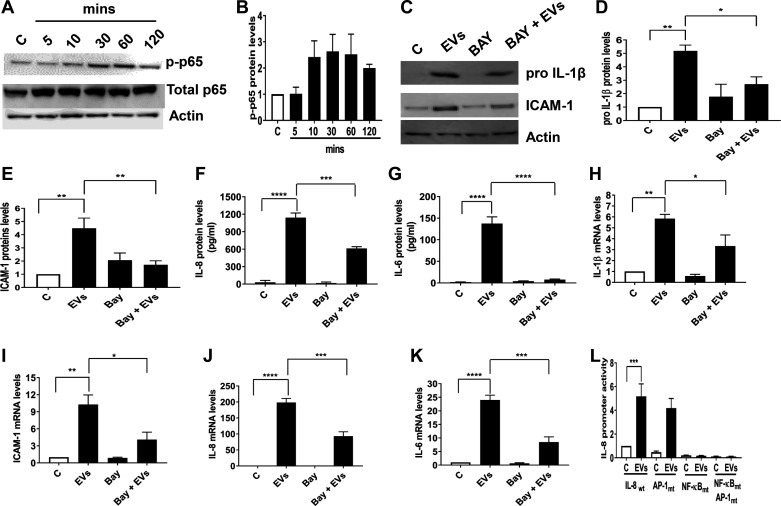
To gain insights into mechanisms by which dust EVs induce inflammatory mediator expression, we focused on the involvement of NF-κB transcription factor as NF-κB plays critical roles in the regulation of immune and inflammatory genes (31). Treatment of Beas2B cells with EVs increased ser536-phosphorylation of NF-κB p65 subunit indicating NF-κB activation (Fig. 7, A and B). Inhibition of NF-κB activation with BAY 11-7082 reduced proIL-1β, ICAM-1, IL-6, and IL-8 protein (Fig. 7, C–G) and mRNA levels (Fig. 7, H–K) indicating that NF-κB activation controls the induction of inflammatory mediator levels by EVs. Transient transfection analysis of wild-type, AP-1, and NF-κB mutant human IL-8 promoters (−546/+44 bp) in Beas2B cells showed that whereas the wild-type promoter activity was robustly induced, the NF-κB mutant promoter was not at all responsive to treatment with EVs consistent with the involvement of NF-κB in the regulation of inflammatory mediator expression (Fig. 7L).
Fig. 7.
Effects of dust extracellular vesicles (EVs) on NF-κΒ p65 phosphorylation and the effects of NF-κΒ inhibitor BAY 11-7082 on the induction of inflammatory mediator protein and mRNA levels in Beas2B cells. A and B: cells were untreated (C) or treated with medium containing EVs (1 μg protein/mL) for 5–120 min, and the levels of phospho-NF-κΒ p65, total p65 and actin were determined by Western blotting. Cells were first incubated with BAY 11-7082 (5 μM) for 1 h and then treated with EVs (0.5 μg protein/mL) for 5 h. The levels of proIL-1β and ICAM-1 in cell lysates and the levels of IL-8 and IL-6 in cell medium were determined (C–G), and the levels of IL-1β, ICAM-1, IL-8, and IL-6 mRNAs were determined (H–K). L: cells were transiently transfected with human IL-8 promoter plasmids (−546/+44 bp) linked to luciferase reporter gene and incubated in control medium (C) or medium containing dust EVs (0.25 μg protein/mL) (EVs) for 24 h. Luciferase activities in cell lysates were measured and normalized to total cell protein content. Wt, wild type; AP-1mt, AP-1 site mutant; NF-κBmt, NF-κB site mutant. All data shown are means ± SE (n = 3–4). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, according to one-way ANOVA using Tukey’s multiple-comparison test.
Effects of polymyxin B.
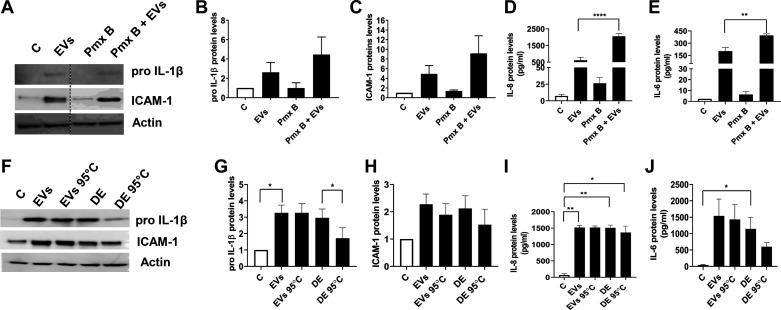
Our genotyping data indicated that the EVs are derived from Gram-positive and Gram-negative bacteria and could contain several microbe associated molecular patterns (MAMPs) and other molecules. To determine the contribution of lipopolysaccharide (LPS) from EVs, we tested the effects of polymyxin B, a peptide antibiotic that is known to bind and neutralize LPS on the induction of inflammatory mediators. We found that polymyxin B treatment did not block induction of inflammatory mediators by dust EVs indicating that the induction may not be due to LPS (Fig. 8, A–E). On the contrary, polymyxin B appeared to potentiate the inductive effects of EVs. To verify the neutralizing effects of polymyxin B on LPS, we tested the effects of polymyxin B on the ability of Escherichia coli LPS (1 μg/mL) to induce IL-6, IL-8, and TNF-α secretion in THP-1 cells. We found that polymyxin B (10 μg/mL) inhibited IL-6 and TNF-α levels by >90% and 75%, respectively, but had no effect on IL-8 levels (data not shown). We also examined the effects of heat inactivation of EVs to determine the involvement of heat-labile factors such as proteases on the induction of IL-1β, ICAM-1, IL-6, and IL-8. We previously found that protease activities in poultry organic dust are involved in the induction of inflammatory mediators (37). Heating EVs at 95°C for 10 min did not reduce induction of inflammatory mediator levels compared with untreated EVs whereas heating dust extract reduced the induction of inflammatory mediators (Fig. 8, F–J).
Fig. 8.
Effects of polymyxin B (Pmx B) and heating on dust extracellular vesicle (EV) induction of inflammatory mediator protein levels in Beas2B cells. A–E: to determine the effects of Pmx B, cells were first incubated with Pmx B (10 μg/mL) for 30 min and then treated with EVs (0.25 μg protein/mL) for 5 h. Control cells (C) were left untreated. The levels of proIL-1β and ICAM-1 in cell lysates and the levels of IL-8 and IL-6 in cell medium were determined. A representative Western blot with noncontiguous lanes demarcated by a broken line is shown. Data shown are means ± SE (n = 6). **P < 0.01 and ****P < 0.0001 according to one-way ANOVA using Tukey’s multiple-comparison test. F–J: cells were untreated (C) or treated with medium containing EVs (0.5 μg protein/mL), EVs (0.5 μg protein/mL) heated at 95°C for 10 min (EVs 95°C), dust extract (0.25%) (DE), or dust extract (0.25%) heated at 95°C for 10 min (DE 95°C) for 5 h, and the levels proIL-1β and ICAM-1 in cell lysates and the levels of IL-6 and IL-8 proteins in cell medium were determined. Data shown are means ± SE (n = 4). A representative Western blot is shown. *P < 0.05 and **P < 0.01 according to one-way ANOVA using Tukey’s multiple-comparison test.
Effects on lung inflammatory mediators and inflammatory cell counts in mice.
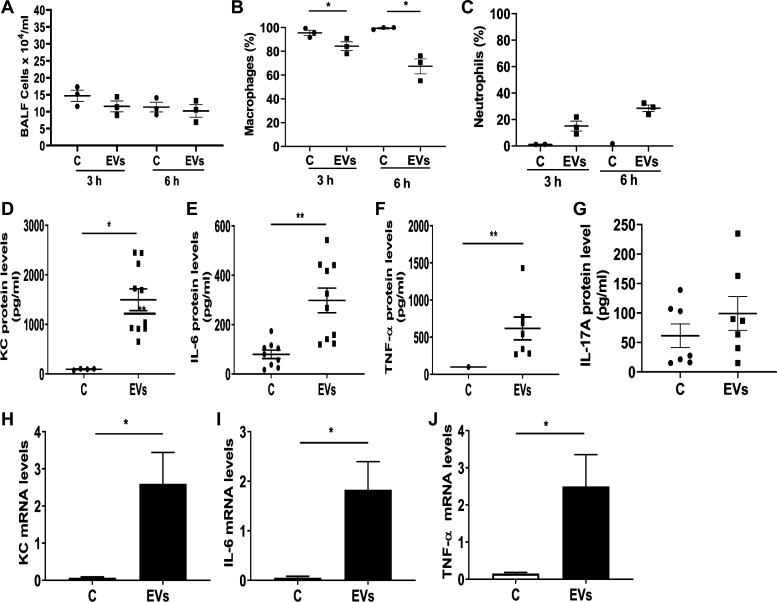
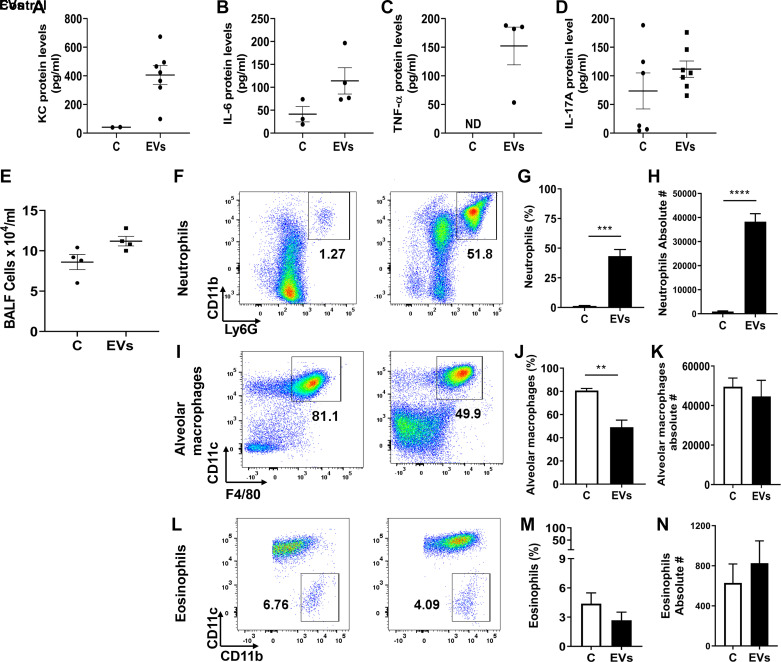
In efforts to understand the effects of dust EVs on lung inflammatory responses in vivo, we initially determined the effects of single doses of EVs (0.01, 0.1, or 1 μg protein) on cytokine levels at 3 h after treatment and the time-course effects of a single dose of EVs (1 μg protein) on BALF cytokine levels at 3, 6, and 24 h after treatment. We found that dust EVs increased IL-6, KC, and TNF-α levels in a concentration-dependent manner and the cytokine levels peaked at 3 h and decreased thereafter to control levels at 24 h after treatment (data not shown). In mice treated with dust EVs (1 μg protein), total BALF cell counts did not change, however macrophage counts decreased, and neutrophil counts increased over time (Fig. 9, A–C). Administration of a single dose (1 μg protein) of dust EVs significantly elevated BALF IL-6, KC, and TNF-α levels at 3 h after treatment (Fig. 9, D–F). Although BALF IL-17A levels were somewhat elevated in treated mice, the increase was statistically not significant (Fig. 9G). Determination of mRNA levels showed that treatment with dust EVs increased IL-6, KC, and TNF-α mRNA levels (Fig. 9, H and J) indicating that gene expression controls the induction of inflammatory mediators. To model repeated exposure, dust EVs were administered once daily (Monday–Friday) for ∼2 wk and lung inflammatory responses were assessed by measuring BALF cytokine levels and BALF inflammatory cells. The levels of KC, IL-6, and TNF-α, although significantly elevated (Fig. 10, A–C), were reduced by ∼50% than their levels in BALF from mice that were treated just once (Fig. 9, D–F ) suggesting that the reduced responses could be due to adaptation. As in the case of mice treated once for 3 h, BALF IL-17A levels were somewhat elevated, but the increase was not statistically significant (Fig. 10D). BALF total cell counts were increased by ∼30% (Fig. 10E); however, neutrophils counts were markedly increased accounting for nearly 40% of BALF cells (Fig. 10, F–H). Macrophage and eosinophil numbers were not significantly altered (Fig. 10, I–N).
Fig. 9.
Effects of administration of dust extracellular vesicles (EVs) on inflammatory cell counts and inflammatory mediator protein levels in lungs of mice. Mice were administered PBS or dust EVs (1 μg protein) via intranasal instillation and bronchoalveolar lavage (BAL) fluid (BALF) was collected at 3 and 6 h after instillation and total RNAs from lung tissues were isolated. A–C: BAL fluid total cell counts were determined using a hemocytometer and macrophage and neutrophil counts were determined by Diff Quick staining. Percentages of macrophages and neutrophils in total BALF cells are shown. Data are means ± SE (n = 3). D–G: KC, IL-6, TNF-α and IL-17A levels in BALF samples collected at 3 h were determined by ELISA. Data shown are means ± SE (n = 7–10). H–J: KC, IL-6, and TNF-α mRNA levels in lungs collected at 3 h were determined by qRT-PCR. All data are shown as means ± SE (n = 6). *P < 0.05 and **P < 0.01 according to one-way ANOVA using Tukey’s multiple-comparison test.
Fig. 10.
Effects of repeated administration of dust extracellular vesicles (EVs) on inflammatory mediator protein levels and on inflammatory cell counts in lungs of mice. Mice were administered 50 μL PBS or 50 μL dust EVs (1 μg protein) once daily (Monday–Friday) for ∼2 wk and bronchoalveolar lavage (BAL) fluid (BALF) and BALF cells were obtained. A–D: KC, IL-6, TNF-α, and IL-17A levels were determined by ELISA. KC, IL-6, and TNF-α levels in some BAL samples from untreated mice were below detection limits. ND, not detected. Data shown are means ± SE (n = 4–7). E–N: total cell counts were determined using a hemocytometer and neutrophil, and macrophage and eosinophil counts were determined by flow cytometry. Flow cytometry plots, percentages, and absolute numbers of neutrophils, macrophages, and eosinophils are shown. All data are shown as means ± SE (n = 4). **P < 0.01, ***P < 0.001 and ****P < 0.0001 according to one-way ANOVA using Tukey’s multiple-comparison test.
Effects on lung histology.
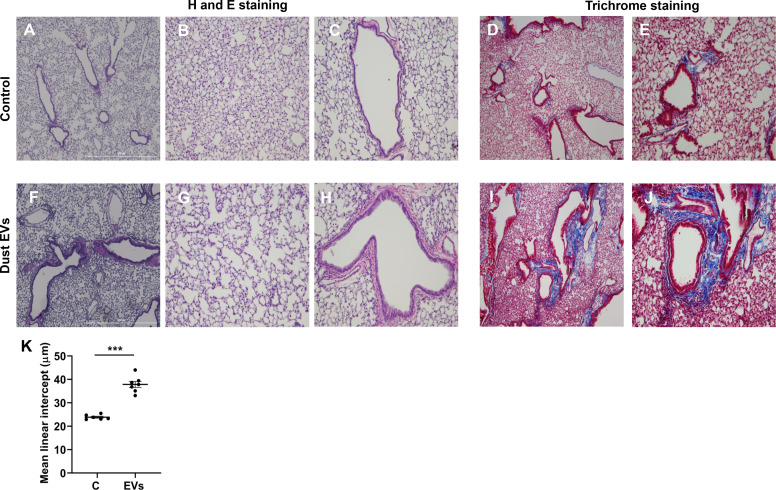
Histological examination of lung sections from control mice and mice exposed to dust EVs daily (Monday–Friday) for ∼2 wk showed that in the treated mice alveoli were enlarged (Fig. 11G) compared with control mice (Fig. 11B) and airways showed signs of increased epithelial thickness (Fig. 11, F and H) compared with control mice (Fig. 11, A and C). Peribronchial cell infiltrates were also present in the treated mice (Fig. 11, F and H). The mean linear intercept was increased in lungs of treated mice (Fig. 11K) consistent with air space enlargement. Lung sections from treated mice stained more prominently for collagen the around airways (Fig. 11, I and J) compared with control mice (Fig. 11, D and E) indicating increased collagen deposition.
Fig. 11.
Effects of repeated administration of dust extracellular vesicles (EVs) on lung histology and collagen staining. Mice were administered 50 μL PBS or 50 μL dust EVs (1 μg protein) once daily (Monday–Friday) for ∼2 wk and lung sections were subjected to hematoxylin and eosin (H and E) staining. Collagen was visualized by trichrome staining. Representative images of H&E- and trichrome-stained lung sections from control and treated mice are shown at ×40 and ×100 magnification. A–C: H&E-stained sections from control mice. D and E: trichrome-stained sections from control mice. F–H: H&E-stained sections from dust EV-treated mice. I and J: trichrome-stained sections from dust EV-treated mice (n = 6 for control and n = 7 for EV-treated mice). K: images of lung sections from control and dust EV-treated mice were analyzed using ImageJ software to determine the mean linear intercept (Lm). Data shown are means ± SE (n = 6–7). ***P < 0.001 using t test.
DISCUSSION
Our studies demonstrate for the first time that bacteria-derived EVs are present in agricultural dust and they induce inflammatory responses in lung epithelial cells, macrophages and in mouse lungs. The dust EVs did not appear to contain mammalian-derived EVs indicating that bacteria-derived EVs per se are responsible for the induction of inflammatory mediators. Fungi also produce EVs; however, their concentration in bioaerosols from broiler poultry buildings is very low (14) suggesting that dust EVs may only contain trace levels of fungi derived EVs if any. It is not known if mites found in poultry houses produce EVs, but they are known to carry bacteria (20) that could release EVs contributing to the bacterial EVs found in dust.
Our data indicated that bacterial EVs are important constituents of poultry organic dust involved in eliciting inflammatory responses and development of lung injury. They further suggest that the dust EVs interact with lung epithelial cells and lung macrophages to modulate inflammatory responses. As macrophages are phagocytic cells, it is likely that they may be involved in the phagocytic clearance of dust EVs, and alveolar epithelial cells could contribute to their clearance via transcytosis into lymph and blood stream. It is not known whether the dust EVs interact with and modulate the functions of dendritic cells and lymphocytes that are also found on the lung epithelial surface. Studies in mice found that amorphous silica nanoparticles (13) are cleared via transcytosis across alveolar epithelial cells. After translocation across the lung epithelium, dust EVs can potentially interact with fibroblasts, endothelial and other cells to modulate inflammatory responses. Much of our knowledge on the characterization of bacterial EVs and their effects on host immune responses comes from studies on EVs derived from individual Gram-negative and Gram-positive bacterial species. Information on bacterial EVs in environmental samples and their effects on lung inflammation is limited. Particularly, there appears to be no information on the occurrence of bacterial EVs in agricultural dusts and their effects on lung inflammation. Recent studies demonstrated that E. coli-derived EVs from indoor house dust elicited neutrophilic inflammation causing emphysema in mice (26). Fecal EVs consisting of mammalian and bacterial origin EVs caused sepsis-like local and systemic inflammation in mice that was attributed to EVs derived from Gram-negative and Gram-positive bacteria (43).
Bacterial EVs contain a variety of biological molecules such as proteins, lipids, polysaccharides, and nucleic acids (28). Nucleic acids include DNA and different classes of RNAs such as small noncoding RNAs and transfer RNA-derived small RNAs (tsRNA) (12). Dust EVs contained high molecular weight DNA indicating the presence of bacterial chromosomal DNA. Published studies have shown that bacterial EVs contain bacterial chromosomal DNA with the majority of the DNA present on the external surface of the EVs and smaller amounts of DNA present in the lumen (4). Chromosomal DNA within the EVs was reported to be enriched for specific segments of the bacterial chromosome coding for proteins important for virulence, stress response, and antibiotic resistance (4). It will be interesting to determine if bacterial proteins are expressed after uptake of dust EVs by lung epithelial and other cells. Upon interaction with cell surfaces, bacterial EVs are endocytosed via clathrin-, lipid raft-, and dynamin-mediated pathways and transfer their luminal contents to recipient cells (3, 24). Various microbe-associated molecular patterns (MAMPs) such as LPS, peptidoglycan, flagellin, lipids, and nucleic acids found in bacterial EVs can interact with immune and nonimmune cells by binding to different pattern recognition receptor (PRR) families including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and NOD-like receptors (NLRs) to modulate immune responses (24, 28).
Our studies demonstrated that NF-κB activation is important for the induction of inflammatory mediators by dust EVs. We previously found that poultry organic dust extracts activate NF-κB to induce inflammatory gene expression in lung epithelial cells (16). Mechanisms by which dust EVs activate NF-κB and the EV component(s) responsible for the activation are not known. MAMPs such as LPS, peptidoglycan, lipoteichoic acid, flagellin, and nucleic acids present in dust EVs can activate NF-κB to modulate inflammatory mediator expression. MAMPs bind to TLRs to activate MyD88- and/or TRIF-dependent intracellular signaling pathways to activate NF-κB (39). Interestingly induction of IL-6 and IL-8 by Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Helicobacter pylori EVs in human epithelial cells was mediated in a TLR2- and TLR9-independent manner via nucleotide-binding oligomerization domain-containing protein-1 (NOD1) and NF-κB signaling (23). EVs from pathogenic and nonpathogenic E. coli were found to induce expression of adhesion molecules and cytokines via NF-κB activation in endothelial cells (52). Previous studies have demonstrated that PRRs such as TLR and NOD2 modulate production of lung cell inflammatory mediators and lung inflammatory responses by organic dust (45). We found that induction of inflammatory mediators by dust EVs was not inhibited by polymyxin B treatment indicating that LPS may not be important for the induction. These data are similar to our previous finding that IL-8 induction by poultry organic dust extract was insensitive to polymyxin B inhibition (16). However, these results should be interpreted with caution as LPS present in dust EVs is likely to be heterogenous as they are derived from different species of bacteria and hence may display differential sensitivity to polymyxin B inhibition. Indeed, inhibition of LPS by polymyxin B is known to be dependent on LPS origin (7).
Our studies indicated that long-term exposure of mice to dust EVs resulted in an enlargement of alveoli, increased collagen staining around the airways, and increased thickness of the bronchial epithelium. Increased infiltration of neutrophils into the lung in mice exposed to dust EVs and their degranulation resulting in the release of proteases and reactive oxygen species could have caused damage to lung structural integrity. Additionally, release of neutrophil-derived cytokines could exacerbate inflammatory outcomes. Vesicles derived from E. coli (26, 32) and P. aeruginosa (42) increase neutrophil infiltration into mouse lungs to induce inflammation. Progression of chronic lung diseases such as COPD, asthma, and bronchiectasis are attributed to excessive neutrophilic inflammation (21). Interactions between oxidative stress, protease/antiprotease balance, and cellular apoptosis pathways are maintained under homeostatic conditions; however, aberrant interactions among the pathways could underlie alveolar destruction in emphysema (53). Increased proliferation of airway epithelial cells contributing to thickening of the epithelium was observed in asthmatic human subjects (9).
The uptake and clearance of dust EVs in lungs of mice are not known and remain to be investigated. It is not known in which regions of the tracheo-bronchial tree that the dust EVs would be deposited. However, on the basis of their size (50–150 nm) it can be predicted that they may be deposited mainly in the alveolar and bronchiolar regions of the lung. Inhaled nanoparticles are known to be deposited in the entire respiratory tract from airway to alveolar regions (15). Due to their deposition in the distal regions of the lung where mucus velocity is reduced, inhaled nanoparticles may not be cleared rapidly and may accumulate with prolonged exposure. The inhaled nanoparticles deposited on distal lung surfaces will be subsequently transported into blood circulation and can reach other organs such as heart, liver, and brain where they could exert adverse effects. Kinetic studies on the clearance of inhaled iridium nanoparticles in rats (27) and mice (2) revealed that only 30% of the deposited particles were cleared within 24 h following aerosol inhalation. Administration of 100-nm carbon particles to human lungs by shallow aerosol bolus inhalation resulted in the clearance of only 25% of the nanoparticles within 24 h, while 75% of the particles were retained for more than 24 h (35). On the basis of these data, it can be speculated that repeated inhalation of dust EVs could lead to their accumulation in the bronchiolar and alveolar regions thereby promoting sustained low-level inflammation causing injury to the lung parenchyma and associated lung dysfunction.
Our studies have provided new evidence for the presence of bacterial EVs in poultry organic dust and their contribution to induction of lung inflammatory responses. However, our studies are not without limitations. Use of only female mice and submerged culture conditions for airway epithelial cells, which prevents formation of pseudostratified airway epithelia, could have influenced inflammatory responses. Our studies were limited to bacterial vesicles isolated from poultry organic dust. Isolation of bacterial vesicles from other agricultural dusts and studies of their effects on lung inflammation would lend further support to the involvement of bacterial EVs in the modulation of lung inflammatory responses by organic dust. The levels of bacterial EVs in the poultry environment are not known as is the exposure levels of workers. Information on these parameters would help in the design of future animal studies to model human exposure to bacterial vesicles in poultry farms.
In summary, our studies have found that poultry organic dust contains bacteria-derived EVs that promoted inflammatory responses in lung epithelial and THP-1 cells in vitro and in mouse lungs. Activation of NF-κB was found to underlie the increased production of inflammatory mediators by dust EVs. LPS, an important component of bacterial EVs, appeared not to be important for the increased production of inflammatory mediators. Exposure of mice to dust EVs induced cytokine production causing increased neutrophil infiltration into lungs. Repeated exposure of mice to dust EVs caused structural changes in the lung including enlargement of the alveoli, increased collagen deposition around airways, and thickening of airway epithelium. Owing to the nanometer particle size of the EVs and their potential retention in the lung for prolonged periods of time, dust EVs could be considered as a significant contributing factor for the development of lung inflammatory outcomes.
GRANTS
This research was supported by Centers for Disease Control and the National Institute of Occupational Safety and Health Grant U54 OH007541.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.B. conceived and designed research; V.M., R.M., K.N., W.K., S.M. and V.B. performed experiments; V.M., R.M., K.N., W.K., S.K., S.M. and V.B. analyzed data; V.M., R.M., K.N., W.K., S.K., S.M. and V.B. interpreted results of experiments; V.M., R.M. and W.K. prepared figures; V.B. drafted manuscript; V.M., K.N., S.K. and V.B. edited and revised manuscript; V.M., R.M., K.N., W.K., S.K., S.M. and V.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Usha Pendurthi, Nagarjun Konduru, and Shabbir Ansari for help in size distribution analyses of extracellular vesicles.
REFERENCES
- 1.Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158, Suppl 1: S1–S76, 1998. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini F, Semmler-Behnke M, Jakob T, Schulz H, Behrendt H, Kreyling W. Total and regional deposition of ultrafine particles in a mouse model of allergic inflammation of the lung. Inhal Toxicol 20: 585–593, 2008. doi: 10.1080/08958370801949167. [DOI] [PubMed] [Google Scholar]
- 3.Anand D, Chaudhuri A. Bacterial outer membrane vesicles: new insights and applications. Mol Membr Biol 33: 125–137, 2016. doi: 10.1080/09687688.2017.1400602. [DOI] [PubMed] [Google Scholar]
- 4.Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, Whitchurch CB, Stinear TP, Stacey KJ, Ferrero RL. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep 7: 7072, 2017. doi: 10.1038/s41598-017-07288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggaram V, Gottipati KR, Wang X, Samten B. Early secreted antigenic target of 6 kDa (ESAT-6) protein of Mycobacterium tuberculosis induces interleukin-8 (IL-8) expression in lung epithelial cells via protein kinase signaling and reactive oxygen species. J Biol Chem 288: 25500–25511, 2013. doi: 10.1074/jbc.M112.448217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boggaram V, Loose DS, Gottipati KR, Natarajan K, Mitchell CT. Gene expression profiling of the effects of organic dust in lung epithelial and THP-1 cells reveals inductive effects on inflammatory and immune response genes. Physiol Genomics 48: 281–289, 2016. doi: 10.1152/physiolgenomics.00096.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaillon JM, Haeffner-Cavaillon N. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol Immunol 23: 965–969, 1986. doi: 10.1016/0161-5890(86)90127-6. [DOI] [PubMed] [Google Scholar]
- 8.Cleave J, Willson PJ, Town J, Gordon JR. Fractionation of swine barn dust and assessment of its impact on the respiratory tract following repeated airway exposure. J Toxicol Environ Health A 73: 1090–1101, 2010. doi: 10.1080/15287394.2010.482916. [DOI] [PubMed] [Google Scholar]
- 9.Cohen L, e X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M; NHLBI Severe Asthma Research Program (SARP) . Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med 176: 138–145, 2007. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormier Y, Duchaine C, Israël-Assayag E, Bédard G, Laviolette M, Dosman J. Effects of repeated swine building exposures on normal naive subjects. Eur Respir J 10: 1516–1522, 1997. doi: 10.1183/09031936.97.10071516. [DOI] [PubMed] [Google Scholar]
- 11.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5: e8668, 2010. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauros-Singorenko P, Blenkiron C, Phillips A, Swift S. The functional RNA cargo of bacterial membrane vesicles. FEMS Microbiol Lett 365: 365, 2018. doi: 10.1093/femsle/fny023. [DOI] [PubMed] [Google Scholar]
- 13.Detampel P, Ganguly A, Tehranian S, Green F, Singha S, Santamaria P, Jeje AA, Cho CS, Petri B, Amrein MW. In vivo clearance of nanoparticles by transcytosis across alveolar epithelial cells. PLoS One 14: e0223339, 2019. doi: 10.1371/journal.pone.0223339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutkiewicz J. Bacteria and fungi in organic dust as potential health hazard. Ann Agric Environ Med 4: 11–16, 1997. [Google Scholar]
- 15.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol 7: 2, 2010. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottipati KR, Bandari SK, Nonnenmann MW, Levin JL, Dooley GP, Reynolds SJ, Boggaram V. Transcriptional mechanisms and protein kinase signaling mediate organic dust induction of IL-8 expression in lung epithelial and THP-1 cells. Am J Physiol Lung Cell Mol Physiol 308: L11–L21, 2015. doi: 10.1152/ajplung.00215.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillien A, Puyraveau M, Soumagne T, Guillot S, Rannou F, Marquette D, Berger P, Jouneau S, Monnet E, Mauny F, Laplante JJ, Dalphin JC, Degano B. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J 47: 95–103, 2016. doi: 10.1183/13993003.00153-2015. [DOI] [PubMed] [Google Scholar]
- 18.Hart J, Mayda C. The industrialization of livestock production in the United States. Southeast Geogr 38: 58–78, 1998. doi: 10.1353/sgo.1998.0014. [DOI] [Google Scholar]
- 19.Heederik D, Sigsgaard T, Thorne PS, Kline JN, Avery R, Bønløkke JH, Chrischilles EA, Dosman JA, Duchaine C, Kirkhorn SR, Kulhankova K, Merchant JA. Health effects of airborne exposures from concentrated animal feeding operations. Environ Health Perspect 115: 298–302, 2007. doi: 10.1289/ehp.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubert J, Erban T, Kopecky J, Sopko B, Nesvorna M, Lichovnikova M, Schicht S, Strube C, Sparagano O. Comparison of microbiomes between red poultry mite populations (Dermanyssus gallinae): predominance of bartonella-like bacteria. Microb Ecol 74: 947–960, 2017. doi: 10.1007/s00248-017-0993-z. [DOI] [PubMed] [Google Scholar]
- 21.Jasper AE, McIver WJ, Sapey E, Walton GM. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000 Res 8: 557, 2019. doi: 10.12688/f1000research.18411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Just N, Duchaine C, Singh B. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J Occup Med Toxicol 4: 13, 2009. doi: 10.1186/1745-6673-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, Hutton ML, Davies JK, Crack PJ, Hertzog PJ, Philpott DJ, Girardin SE, Whitchurch CB, Ferrero RL. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol 12: 372–385, 2010. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol 15: 375–387, 2015. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40: 97–104, 2015. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, Jee YK, Oh YM, Kim YK. Extracellular vesicles derived from Gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol 194: 3361–3368, 2015. doi: 10.4049/jimmunol.1402268. [DOI] [PubMed] [Google Scholar]
- 27.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A 65: 1513–1530, 2002. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 28.Kuipers ME, Hokke CH, Smits HH, Nolte-’t Hoen EN. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front Microbiol 9: 2182, 2018. doi: 10.3389/fmicb.2018.02182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kujur W, Gurram RK, Haleem N, Maurya SK, Agrewala JN. Caerulomycin A inhibits Th2 cell activity: a possible role in the management of asthma. Sci Rep 5: 15396, 2015. doi: 10.1038/srep15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson BM, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax 52: 638–642, 1997. doi: 10.1136/thx.52.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651, 2009. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Yoon YJ, Kim JH, Dinh NT, Go G, Tae S, Park KS, Park HT, Lee C, Roh TY, Di Vizio D, Gho YS. Outer membrane vesicles derived from Escherichia coli regulate neutrophil migration by induction of endothelial IL-8. Front Microbiol 9: 2268, 2018. doi: 10.3389/fmicb.2018.02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J 10: 2139–2146, 1997. doi: 10.1183/09031936.97.10092139. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell CT. Organic Dust Induced Lung Inflammatory Responses in Mice (Master’s thesis). Tyler, TX: University of Texas Health Science Center at Tyler, 2015. [Google Scholar]
- 35.Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Häussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med 177: 426–432, 2008. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan K, Gangam K, Meganathan V, Gottipati KR, Mitchell C, Boggaram V. Organic dust inhibits surfactant protein expression by reducing thyroid transcription factor-1 levels in human lung epithelial cells. Innate Immun 25: 118–131, 2019. doi: 10.1177/1753425919827360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natarajan K, Gottipati KR, Berhane K, Samten B, Pendurthi U, Boggaram V. Proteases and oxidant stress control organic dust induction of inflammatory gene expression in lung epithelial cells. Respir Res 17: 137, 2016. doi: 10.1186/s12931-016-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan K, Meganathan V, Mitchell C, Boggaram V. Organic dust induces inflammatory gene expression in lung epithelial cells via ROS-dependent STAT-3 activation. Am J Physiol Lung Cell Mol Physiol 317: L127–L140, 2019. doi: 10.1152/ajplung.00448.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4: a006049, 2012. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien KM, Chimenti MS, Farnell M, Tabler T, Bair T, Bray JL, Nonnenmann MW. High throughput genomic sequencing of bioaerosols in broiler chicken production facilities. Microb Biotechnol 9: 782–791, 2016. doi: 10.1111/1751-7915.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmberg L, Larsson BM, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53: 260–264, 1998. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park KS, Lee J, Jang SC, Kim SR, Jang MH, Lötvall J, Kim YK, Gho YS. Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 49: 637–645, 2013. doi: 10.1165/rcmb.2012-0370OC. [DOI] [PubMed] [Google Scholar]
- 43.Park KS, Lee J, Lee C, Park HT, Kim JW, Kim OY, Kim SR, Rådinger M, Jung HY, Park J, Lötvall J, Gho YS. Sepsis-like systemic inflammation induced by nano-sized extracellular vesicles from feces. Front Microbiol 9: 1735, 2018. doi: 10.3389/fmicb.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 73: 684–700, 2010. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol 12: 126–132, 2012. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296: L1085–L1095, 2009. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure assessment and lung function in pig and poultry farmers. Occup Environ Med 58: 405–410, 2001. doi: 10.1136/oem.58.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 93: 289–296, 2002. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 49.Romberger DJ, Heires AJ, Nordgren TM, Souder CP, West W, Liu XD, Poole JA, Toews ML, Wyatt TA. Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am J Physiol Lung Cell Mol Physiol 309: L388–L399, 2015. doi: 10.1152/ajplung.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seedorf J, Hartung J, Schroder M, Linkert KH, Phillips VR, Holder MR, Sneath RW, Short JL, White RP, Pedersen S, Takai H, Johnsen JO, Metz JH, GroorKoerkamp PW, Uenk GH, Wathes CM. Concentrations and emissions of airborne endotoxins and microorganisms in livestock buildings in Northern Europe. J Agric Eng Res 70: 97–109, 1998. doi: 10.1006/jaer.1997.0281. [DOI] [Google Scholar]
- 51.Simpson JC, Niven RM, Pickering CA, Fletcher AM, Oldham LA, Francis HM. Prevalence and predictors of work related respiratory symptoms in workers exposed to organic dusts. Occup Environ Med 55: 668–672, 1998. doi: 10.1136/oem.55.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res 184: 458–466, 2013. doi: 10.1016/j.jss.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 53.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 3: 503–510, 2006. doi: 10.1513/pats.200603-054MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Von Essen S, Donham K. Illness and injury in animal confinement workers. Occup Med 14: 337–350, 1999. [PubMed] [Google Scholar]
- 55.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 10: 381–387, 1997. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- 56.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cepsilon in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293: L1163–L1170, 2007. doi: 10.1152/ajplung.00103.2007. [DOI] [PubMed] [Google Scholar]