Keywords: female, kidney, male, mitochondria, sex differences
Abstract
Sex differences (biological distinctions between males and females) present a complex interplay of genetic, developmental, biological, and environmental factors. More and more studies are shedding light on the importance of sex differences in normal physiology and susceptibility to cancer, cardiovascular and renal conditions, and neurodegenerative diseases. This mini-review is devoted to the role of sex dimorphisms in renal function, with a focus on the distinctions between male and female mitochondria. Here, we cover the aspects of renal mitochondrial bioenergetics where sex differences have been reported to date, for instance, biogenesis, reactive oxygen species production, and oxidative stress. Special attention is devoted to the effects of sex hormones, such as estrogen and testosterone, on mitochondrial bioenergetics in the kidney in physiology and pathophysiology.
INTRODUCTION
Biological differences between males and females resulting from genetic, epigenetic, and hormonal factors are known as sex differences (102, 114). The specific genes located in sex chromosomes are primarily responsible for the sex differences, aided by epigenetic factors and the environment, which ultimately lead to molecular signaling patterns that would be more prevalent in one of the sexes (94a). Sex differences are implicated as a crucial factor in the biology and pathophysiology of various disorders. For instance, neurodevelopmental conditions such as autism spectrum disorder or attention deficit hyperactivity disorder are more often diagnosed in males, whereas Alzheimer’s disease and anxiety disorders have a higher occurrence in females (37, 122). Sex-related differences have been observed in the onset and outcomes of various cardiovascular and kidney diseases, including diabetes, hypertension, aortic aneurism, and stroke (3, 11, 12, 23, 24, 36, 50, 78, 94, 98). Interestingly, mitochondrial dysfunction has been reported to be implicated in all these pathologies (20, 24, 34, 44, 76, 115). Moreover, in many cases, the susceptibility to certain damage has been directly linked to distinct sex differences identified in mitochondrial bioenergetics (75, 114, 120).
The kidney is notorious for functional differences specific to males or females; the onset and progression of renal diseases often depend on sex (70, 85, 99). A recent review by Layton and Sullivan (57) highlighted the advances in the understanding of renal functions that are different between sexes. Male and female kidneys exhibit dimorphic patterns of transporter expression and salt handling, differences in basal ammonia metabolism, greater nitric oxide (NO) bioavailability in females compared with males, numerous sex differences in the expression and activity of renin-angiotensin-aldosterone system (RAAS) components, as well as differential responses to injury (57). The overall incidences of cardiovascular disease, chronic kidney disease (CKD), and hypertension are more prevalent in age-matched males than in females until a female reaches menopause (71, 119). Similarly, mortality in acute kidney injury (AKI) occurs twice more frequently in males compared with females (premenopausal females have the lowest incidence of AKI) (8, 81, 85). Lima-Posada et al. (62) demonstrated that in AKI, estrogen enhances the protection of females against reactive oxygen species (ROS) generation and inflammation in renal tissue. In contrast, human studies have shown that the female sex is a predisposing risk factor in drug-induced AKI. For instance, female patients were found to be more vulnerable to nephrotoxicity induced by amikacin (but not gentamicin) (108). As mentioned by Schiffl (101) in a recent review, there are more questions than answers in sex differences in the susceptibility to AKI, the research and epidemiological data are conflicting, and there is a need for more rigorous research.
Research in type 2 diabetes (T2D) demonstrated that although males tend to have type 2 diabetes more often than females, the latter are at a higher risk to develop CKD (45, 103). Many factors have been suggested to underlie the differences that occur between males and females in T2D, obviously including sex hormones and differential expression of renal receptors and transporters. Interestingly, females with high estrogen and low testosterone levels have better protection from T2D, whereas the most protected males, inversely, exhibit low estrogen and high testosterone levels (45). In type 1 diabetes (T1D), females typically exhibit decreased estradiol levels, and this was suggested to be implicated in the loss of protection against CKD (25, 71). Moreover, the expression of components of the RAAS, which plays a pivotal role in renal disease, is also sex dependent (107). For instance, the angiotensin II (ANG II) level is sustained longer in males, which could be a reason why premenopausal females tend to have lower blood pressure (107, 119). Sex-related differences in the expression and function of various renal channels and transporters have also been widely observed (4, 10, 113).
Renal filtration and reabsorption require a lot of energy, and proper mitochondrial performance is very important to help mitigate kidney disease development (85). Mitochondrial dysfunction has been shown to be a crucial factor for the development of AKI, diabetic nephropathy, hypertensive kidney damage, and various glomerular disorders (27, 64, 85, 91, 100); mitochondria-derived ROS contribute to microvascular dysfunction in CKD (48). However, although in other organs, mitochondrial structure and functionality are known to depend on sex (114), very little is known about the connection between mitochondria and sex in the context of renal physiology and pathophysiology. The goal of this mini-review is to summarize the current mechanistic knowledge about the yet obscure link between mitochondrial function and sex in the kidney.
OVERVIEW OF MITOCHONDRIAL FUNCTION
Mitochondria are almost ubiquitous organelles that make ATP to power the cellular biochemical reactions. This is achieved by oxidizing various substrates (such as pyruvate) during aerobic respiration (111). In addition, mitochondria play a major role in signal transduction pathways, producing, among other substances, ROS and affecting cellular Ca2+ homeostasis (14, 86). Over a billion years ago, eukaryotic cells symbiotically incorporated a certain type of prokaryotic cell, which became mitochondria, by endocytosis (51, 121). This allowed the mitochondria to keep their own DNA while still enabling nuclear transcription factors to influence mitochondrial protein expression (6). If needed, mitochondria can undergo fission or fusion processes, which serve to generate more morphologically and functionally distinct mitochondria, remove dysfunctional mitochondria, or mix and unify their compartments (111). During the process of biogenesis, mitochondria collaborate with the nuclear genome to produce proteins required for mitochondrial function. Thirty-seven genes essential for mitochondria remain encoded by mitochondrial DNA (mtDNA) (42). Biogenesis is stimulated or precluded when the cell senses an imbalance in NAD+-to-NADH, AMP-to-ATP, and Ca2+-to-cAMP ratios as well as ROS and other signals, which can activate a nuclear cotranscriptional regulation factor, peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1-α), the master regulator of mitochondrial biogenesis (42). Mitochondria produce ATP in a process of oxidative phosphorylation (OXPHOS), which includes a series of redox reactions with proteins of the electron transport chain (ETC) (111). The ETC takes electrons from the tricarboxylic acid cycle, mainly fueled by glycolysis and fatty acid oxidation (FAO), and creates an electrochemical gradient by pumping protons out of the matrix and into the intermembrane space. ATP synthase then produces ATP powered by moving protons back into the matrix. The ETC is not perfect; electrons occasionally escape the ETC complexes, creating superoxide anion, which can be harmful if produced in excess (13). In addition, mitochondria uptake and store large amounts of Ca2+ from the cytosol. Ca2+ and ROS overload may result in the formation of the mitochondrial permeability transition pore (mPTP), which leads to apoptosis or necrosis of the cell (83). If mitochondria become damaged, the cell can use an autophagosome to engulf and degrade dysfunctional mitochondria in a process called mitophagy (110).
It is important to mention that mitochondria can affect the innate immune response. There are two levels of mitochondria-mediated regulation of inflammation: first, mitochondria play a key role in the transduction of the signals downstream of pattern recognition receptors (PRRs); intracellular signaling pathways of several critical PRRs physically interact with mitochondria to act as modulators of their function. As an example, binding of Toll-like receptors leads to the recruitment of mitochondria to phagosomes of the macrophages, where mitochondria then start releasing ROS. Second, mtDNA released into the cytosol or extracellular space as a result of mitochondrial damage (e.g., mitochondrial membrane permeability increase) can be recognized by PRRs as a damage-associated molecular pattern (DAMP) and, thus, initiate a proinflammatory response (69, 74).
Mitochondria also play a pivotal role in the first steps of steroidogenesis (15, 109). Pregnenolone, the precursor/metabolic intermediate to steroids (progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids), is formed when cytochrome P-450scc, also known as CYP11A1, cleaves cholesterol to form pregnenolone in the mitochondrial intermembrane space (15, 116). Steroidogenic acute regulatory protein (STAR1) mediates the transport of cholesterol into this space, but the mechanism of this transport is still being debated (28, 61). Steroid synthesis primarily occurs in the adrenal glands, gonads, and placenta but has also been suggested to take place locally in the brain, liver, and skin because they express the required enzymes and are known to be capable of producing pregnenolone (9, 96, 104). The potential for steroid production in the kidney is yet to be fully determined; however, there is a previous report that renal tissue has steroidogenic capacity (93).
SEXUAL DIMORPHISMS IN MITOCHONDRIAL FUNCTION
The classical theory of mitochondrial inheritance postulates that mtDNA is exclusively inherited from the female; however, there have been some (yet not fully confirmed) reports about a potential possibility of its paternal inheritance (66). That said, biparental inheritance of mtDNA is likely not a common occurrence (95) and is still being debated (67). Mitochondrial sexual dimorphisms are well established; one of the reasons behind this phenomenon could be the distinct male/female sex hormones that regulate mitochondrial energy, OXPHOS, and Ca2+ homeostasis (35). Female mitochondria are typically reported to have higher functional capacity than males (however, this depends on the tissue in question and the age/hormonal status of the tested subject) (114). For example, in female heart and brain tissues, mitochondria have higher antioxidant capacity and produce less ROS than in males (46). During pressure overload, the efficiency of mitochondrial function as well as the expression of the FAO-related genes are more repressed in young male hearts compared with female hearts, which respond to pressure overload with a better adaptation of cardiac energy metabolism than males (31). In females, brain and spinal cord mitochondria have lower Ca2+ uptake than in males (47). In the liver, female mitochondria have higher ADP‐stimulated respiration rate, protein content, and cardiolipin levels as well as greater respiratory and OXPHOS capacity (18, 43). Skeletal muscles of female rats show higher mtDNA and mitochondrial protein contents, OXPHOS capacity, and glutathione peroxidase activity (19). In addition, female mitochondria in the myocardium have lower sensitivity of mPTP formation to Ca2+-induced swelling (84). In conclusion, the state of mitochondrial bioenergetics does depend on sex; however, it is also dependent on the tissue and the pathological condition in question. In most tissues, female mitochondria have been reported to have upregulated antioxidant capacity, respiratory function, and biogenesis and lower ROS production than male mitochondria.
SEX HORMONES AND MITOCHONDRIA
Sex hormones, especially female steroids, can affect mitochondria and thus contribute to different mechanisms implicated in physiology and pathophysiology (114). Estrogens are a group of female steroids that includes estradiol, estrone, and estriol; normally in premenopausal women, estrogens are synthesized in the ovaries from cholesterol, whereas postmenopausal women primarily create estrogen by aromatization, a process that converts estrogen precursors, androstenedione and testosterone, into estrogen (72). The mechanisms of estrogen’s action are primarily mediated via estrogen receptor (ER)α and ERβ. However, newly described G protein-coupled estrogen receptor 1 (GPER) can also bind estrogen (39). ERα and ERβ are found in the cytoplasm or nucleus and share a similar structure that features a ligand-binding domain, a DNA-binding domain, and two activation function (AF) domains (30). However, there are some studies that have shown that ERs as well as GPER are expressed in the cell membrane (2). Estrogen can function through genomic and nongenomic mechanisms, and both pathways have been reported to result in changes in mitochondrial bioenergetics (39). In the genomic pathway, estrogen interacts with ERα or ERβ, inducing their homo- or heterodimerization. Following dimerization, the whole complex translocates into the nucleus (Fig. 1), where it binds to estrogen response elements (EREs) in the regulatory regions of estrogen target genes. It is known that estrogen can affect transcription of nuclear-encoded mitochondrial genes (49). Interestingly, estrogen can also regulate transcription by ER-ERE-independent interaction with transcription factors such as activator protein 1 (AP1) and specificity protein 1 (SP1) (39). Moreover, it was found that ERα and ERβ can be localized in mitochondria, although it is still unclear if they can directly regulate mtDNA transcription (41). Membrane-associated ERs activate a number of intracellular protein kinases (MAPK, PKB, and PKC) and G proteins, which trigger rapid signaling cascades through the nongenomic pathway, and can also regulate mitochondrial energy homeostasis and biogenesis (39). For instance, estrogen treatment was shown to increase expression of PGC1-α, a key regulator of mitochondrial biogenesis and a coactivator of nuclear respiratory factor (NRF)-1/NRF-2 (79). Estrogen can increase the content of NRF-1 (106), which is responsible for the expression of mtDNA-specific transcription factors–transcription factor A, mitochondrial (TFAM), transcription factor B1, mitochondrial (TFB1M), and transcription factor B2, mitochondrial (TFB2M)–and thus regulates transcription of the mitochondrial genome (73). In vivo estrogen administration in ovariectomized female rats increased the levels of some crucial proteins in mitochondria of cerebrovascular cells, including mtDNA-encoded subunit I of ETC complex IV, nuclear DNA-encoded cytochrome c, and subunit IV of complex IV (106).
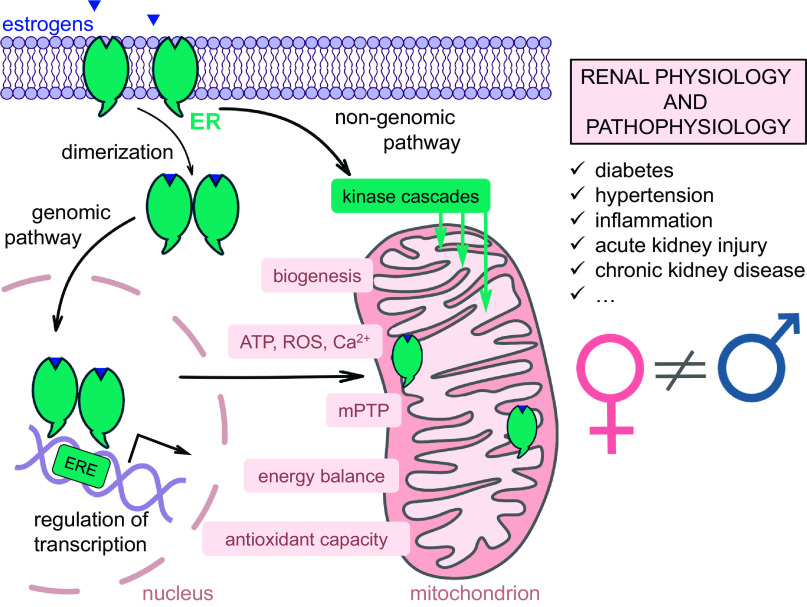
Fig. 1.
Schematic illustration of the role that estrogens play in mitochondrial bioenergetics. Estrogen affects mitochondria bioenergetics through two different pathways. In a genomic pathway, estrogen binds to estrogen receptor (ER)α or ERβ, which induces dimerization of the receptors and translocation of the whole complex to the nucleus. In the nucleus, the dimer of the receptors binds to estrogen response elements (ERE) and affects transcription of nuclear-encoded mitochondrial genes. ERα and ERβ have been shown to localize in mitochondria, but it is yet unclear if the complex can directly regulate transcription of mtDNA-encoded genes. The “nongenomic pathway” involves rapid activation of various kinases (for instance, MAPK or phosphatidylinositol 3-kinase) by membrane-associated ERs, which in turn can affect mitochondrial function. Both ER-associated pathways regulate different parameters of mitochondrial bioenergetics, such as biogenesis, energy balance, antioxidant capacity, formation and stability of the mitochondria transition pore (mPTP), ATP and reactive oxygen species (ROS) production, and Ca2+ handling, all of which are important for renal physiology, and especially so in disease states.
Besides estrogens, male hormones can also influence mitochondrial function. For instance, androgen receptors (ARs) were recently shown to localize to mitochondria, where they can negatively regulate the expression and function of mitochondrial complexes (5). Very little is known about the role of male hormones in mitochondrial function, especially in the kidney, and there is a need for more research. However, it is clear that sex and sex hormones play a role in mitochondrial bioenergetics (see Fig. 1 for a schematic explanation of the role of estrogen in this process). Kidney-specific sex dimorphisms in mitochondrial function are discussed below.
SEX DIFFERENCES IN RENAL MITOCHONDRIAL BIOENERGETICS
Estrogen and Related Mechanisms
It is known that female sex hormones play a major role in cardiorenal protection in premenopausal women during AKI, hypertension, diabetes, and other diseases that affect the kidney (16, 85). Estrogen has a vasoprotective effect (22), which is linked to greater NO bioavailability in females (57). The mechanism of estrogen-mediated protection is being actively studied, and a large body of evidence has already been accumulated regarding the modulation of renal mitochondrial bioenergetics by estrogen. All three ERs have been reported to be expressed in the kidney (21, 63); ERα, ERβ, and GPER have been suggested to play a role in renal protection by modulating redox signaling (75), although the mechanisms are not completely clear.
Several studies have reported changes in functional parameters of mitochondria after estrogen or ER agonist treatment. For instance, in renal tissue of ovariectomized ICGN mice, selective estrogen modulators, such as raloxifene, upregulated thioredoxin reductase and superoxide dismutase (SOD) activities as well as expression of genes associated with mitochondrial β-oxidation (82). In cultured human proximal tubular cells, 17β-estradiol and ER agonists decreased methotrexate-induced nephrotoxicity, potentially via upregulation of SOD activity (53). In podocytes, icariin, a GPER agonist, protected the integrity of the mitochondrial membrane and ameliorated apoptosis induced by high glucose treatment (92). The renal mitochondrial injury induced by sepsis was lower in female compared with male 9-wk-old CD1 mice (68). Estradiol suppressed destabilization of mPTP induced by transforming growth factor-β1 (TGF-β1) in cultured mesangial cells isolated from male C57BL/6 mice (80). Administration of potential nephrotoxins trichloroethylene and perchloroethylene inhibited state 3 and increased state 4 respiration of renal cortical mitochondria isolated from male Fisher 344 rats, whereas mitochondria from female Fisher 344 rats showed similar values when experimental and control groups were compared (55, 56). This sex difference in mitochondrial leak respiration might be one of the key contributing factors in sex-dependent susceptibility to nephrotoxins: increased mitochondria leak respiration (state 4) was suggested to cause intrarenal hypoxia and initiate CKD (32).
The level and activity of mitochondrial ornithine aminotransferase, which takes part in amino acid metabolism, were higher in female kidneys of OF-1 Swiss mice (60) and in proximal tubules of female compared with male 6-wk-old Sprague-Dawley rats (59), although this sex difference was compensated by higher renal mass in male rats. Another enzyme of amino acid metabolism, arginase II, was found to localize in mitochondria of proximal tubules, and its activity was higher in female 9-wk-old OF-1 Swiss mice and 6-wk-old Sprague-Dawley rats compared with age-matched males (58, 59). The metabolic pathways mentioned above are important to the signaling cascades that are central to mitochondria-mediated regulation of inflammation and need further investigation.
Interestingly, gene expression of two key regulators of mitochondrial biogenesis (AMP-activated protein kinase and sirtuin-1) in the 22-mo-old female rat kidney was significantly lower compared with the male kidney, but there were no significant differences in Pgc1-α and Nrf2 expression (123). Therefore, there are distinct age-related changes in renal mitochondrial bioenergetics in females that have not been reported in age-matched males.
Androgens and Renal Mitochondrial Function
Along with estrogen, testosterone also plays an important role in renal physiology; however, the data are not perfectly consistent. Recently, Harris et al. (40) reported that testosterone has dramatic effects on the kidney and decreases ammonia excretion through its effects on key proteins of ammonia metabolism. Low-dose testosterone administration protected male rats against renal ischemia-reperfusion injury (87); furthermore, an age-related decrease in testosterone levels in males leads to reduced kidney function (52). However, while testosterone administration induced podocyte apoptosis in ovariectomized mice, pretreatment with estrogen protected these cells (26). The role of androgens in mitochondrial bioenergetics is unclear and has many controversies in not only the renal system but also the whole organism. There are no data that would indicate the presence of ARs in renal mitochondria, but there are ARs in mitochondria from skeletal muscle cell line (90), sperm cells (105), and human prostate cancer cell line (5).
Although some studies have shown some negative effects of testosterone on mitochondria, there are many discrepancies in the literature. For instance, tubular epithelial cells treated with testosterone exhibited hypoxia-inducible factor (HIF)-1α and HIF-2α activation and increased apoptosis and necrosis in vitro (89). HIF-1α induces a shift to aerobic glycolysis in the hypoxia condition (17) and can indirectly initiate the opening of the mPTP and mitochondrial dysfunction (89, 112). Testosterone also induced apoptosis of vascular smooth muscle cells via increased mitochondrial ROS generation (65). Interestingly, the level of bioavailable testosterone in men was associated with higher risk of CKD and albuminuria and lower estimated glomerular filtration rate, but this is not observed in women (124).
Other studies have shown that testosterone can, on the contrary, play a protective role. Low levels of testosterone in the blood correlated with decreased mitochondrial membrane potential and SOD expression and high levels of ROS production in leukocytes of males with T2D (97). In the skeletal muscle of C57 male mice, testosterone administration beneficially increased expression levels of PGC1-α and some genes of the ETC (7). Moreover, testosterone was able to alleviate blood pressure by inducing production of NO, which elicits vasodilation (77). Interestingly, androgen deprivation therapy (ADT) administered to patients with prostate cancer antagonizes testosterone and can be a reason for the glomerular damage and AKI observed during this therapy. However, ADT induces hypogonadism, which also decreases estrogen levels, which could be a contributing factor to exacerbated kidney injury during ADT (54). We are looking forward to new data that would resolve the existing controversies regarding the effects of testosterone on the kidney and mitochondrial bioenergetics in renal cells.
Mitochondria and the Renal Renin-Angiotensin System
Overactivation of the renin-angiotensin system (RAS) is a key feature in many renal diseases. Interestingly, expression of ANG II type 1 (AT1) and type 2 (AT2) receptors has been shown in the mitochondria (1, 29). Under normal conditions, ANG II decreases mitochondrial oxygen consumption via an AT2 receptor-mediated cascade. During T1D, however, ANG II, acting through the AT1 receptor cascade, leads to increased mitochondrial oxygen consumption due to leak respiration, which can contribute to hypoxia and, therefore, kidney injury (33). As described above, in males, ANG II levels are sustained longer, whereas the AT1-to-AT2 receptor ratio is lower in female kidneys (107), allowing us to speculate that there are sex differences in mediating of this ANG II-mitochondria signaling pathway, which will require further studies.
Iron Deficiency and Sex-Related Discrepancies in Renal Mitochondrial Function
Mitochondria play a critical role in iron metabolism, serving as a nexus for heme and iron-sulfur (FeS) cluster biogenesis [for more detail, see the excellent review by Paul et al. (88)]. In mitochondria, iron can be stored or used for heme synthesis and FeS cluster formation. Disruption of mitochondrial iron metabolism results in cellular stress or death, as heme and FeS clusters participate in redox metabolism and are crucial cofactors for a variety of cellular metabolic processes. Therefore, systemic iron balance disturbances can lead to changes in mitochondrial function; recently, some interesting sex-dependent alterations have been reported in relation to renal mitochondrial iron handling. Woodman et al. (117) showed that fetal kidneys from male iron-deficient mice are more susceptible to mitochondrial dysfunction and oxidative stress than fetal kidneys from female iron-deficient mice. Specifically, male iron-deficient kidneys exhibited globally reduced mitochondrial content and respiration as well as increased cytosolic superoxide and lower levels of NO. In female iron-deficient kidneys, ETC complex II was downregulated, whereas mitochondrial oxidative stress was increased. Moreover, the same group later showed that perinatal iron deficiency in males leads to a more pronounced sensitivity to a high-salt diet; males on the high-salt diet had higher mitochondrial superoxide and lower NO in the renal medulla compared with those on the normal-salt diet, whereas females did not show any changes (118). Therefore, there are sex-specific alterations in mitochondrial content and bioenergetics as well as oxidant status in response to perinatal iron deficiency. These sex discrepancies may contribute to the programming of predisposition to cardiovascular disease.
PERSPECTIVES OF RESEARCH INTO RENAL MITOCHONDRIAL SEX DIFFERENCES
To date, sex dimorphisms have been observed in all major pathologies including cardiovascular and renal disorders, hypertension, obesity, diabetes, neurodegenerative and autoimmune diseases, cancer, and others. It is becoming increasingly clear that sex differences play a critical role both in normal physiology and pathophysiology. In many cases, there is abundant evidence to implicate and target mitochondrial sexual dimorphisms in disease progression; however, little is known about their role in renal mitochondrial function. A more indepth understanding of the mechanisms that lead to observed sex differences in progression and susceptibility to kidney diseases in males and females is very important for the development of more successful therapies. Mitochondrial dysfunction and related damaging factors, such as oxidative stress and inflammation, can result from and cause renal tissue damage. It is essential to provide a mechanistic explanation of sexual dimorphisms in mitochondrial function and the timeline for their development. There is a growing need to study the role of male and female hormones in mitochondrial bioenergetics in the kidney, which will contribute to creating a solid premise for tailored treatment of renal disease in humans. New discoveries and research efforts devoted to this topic will stimulate individualized approaches to the use of pharmacology (based on age, sex, and hormonal status) and, in a more distant future, improve renal outcomes.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01HL148114 and R00DK105160, the Dialysis Clinic Incorporated Reserve Fund (C-4153), and the American Physiological Society Research Career Enhancement and Lazaro J Mandel awards (to D.V.I.). In addition, this work was partially supported through NIH Grants R01DK115749, P30DK072476, and a COBRE Pilot and Feasibility award (all to K.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.F.S., R.S., I.A.Y., and D.V.I. prepared figures; R.F.S., R.S., I.A.Y., K.S., and D.V.I. drafted manuscript; R.F.S., R.S., I.A.Y., K.S., and D.V.I. edited and revised manuscript; R.F.S., R.S., I.A.Y., K.S., and D.V.I. approved final version of manuscript.
REFERENCES
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amenyogbe E, Chen G, Wang Z, Lu X, Lin M, Lin AY. A review on sex steroid hormone estrogen receptors in mammals and fish. Int J Endocrinol 2020: 5386193, 2020. doi: 10.1155/2020/5386193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 37: 746–756, 2017. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL, Sullivan JC, Maric-Bilkan C, Rankin TL, Kimmel PL, Star RA; participants of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop on “Sex and the Kidneys” . Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 15: 776–783, 2019. doi: 10.1038/s41581-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajpai P, Koc E, Sonpavde G, Singh R, Singh KK. Mitochondrial localization, import, and mitochondrial function of the androgen receptor. J Biol Chem 294: 6621–6634, 2019. doi: 10.1074/jbc.RA118.006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barshad G, Marom S, Cohen T, Mishmar D. Mitochondrial DNA transcription and its regulation: an evolutionary perspective. Trends Genet 34: 682–692, 2018. doi: 10.1016/j.tig.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi VE. Testosterone, myocardial function, and mortality. Heart Fail Rev 23: 773–788, 2018. doi: 10.1007/s10741-018-9721-0. [DOI] [PubMed] [Google Scholar]
- 8.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313: F740–F755, 2017. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouguen G, Dubuquoy L, Desreumaux P, Brunner T, Bertin B. Intestinal steroidogenesis. Steroids 103: 64–71, 2015. doi: 10.1016/j.steroids.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Breljak D, Brzica H, Sweet DH, Anzai N, Sabolic I. Sex-dependent expression of Oat3 (Slc22a8) and Oat1 (Slc22a6) proteins in murine kidneys. Am J Physiol Renal Physiol 304: F1114–F1126, 2013. doi: 10.1152/ajprenal.00201.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buford TW. Hypertension and aging. Ageing Res Rev 26: 96–111, 2016. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jiménez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ, Rundek T. Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab 38: 2179–2191, 2018. doi: 10.1177/0271678X18793324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg 1859: 940–950, 2018. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Chandel NS. Mitochondria as signaling organelles. BMC Biol 12: 34, 2014. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien Y, Rosal K, Chung BC. Function of CYP11A1 in the mitochondria. Mol Cell Endocrinol 441: 55–61, 2017. doi: 10.1016/j.mce.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A, Koya D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol 166: 1629–1636, 2005. doi: 10.1016/S0002-9440(10)62473-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun SY, Johnson C, Washburn JG, Cruz-Correa MR, Dang DT, Dang LH. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1α and HIF-2α target genes. Mol Cancer 9: 293, 2010. doi: 10.1186/1476-4598-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chweih H, Castilho RF, Figueira TR. Tissue and sex specificities in Ca2+ handling by isolated mitochondria in conditions avoiding the permeability transition. Exp Physiol 100: 1073–1092, 2015. doi: 10.1113/EP085248. [DOI] [PubMed] [Google Scholar]
- 19.Colom B, Alcolea MP, Valle A, Oliver J, Roca P, García-Palmer FJ. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell Physiol Biochem 19: 205–212, 2007. doi: 10.1159/000099208. [DOI] [PubMed] [Google Scholar]
- 20.Cooper HA, Cicalese S, Preston KJ, Kawai T, Okuno K, Choi ET, Kasahara S, Uchida HA, Otaka N, Scalia R, Rizzo V, Eguchi S. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc Res 2020: cvaa133, 2020. doi: 10.1093/cvr/cvaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidoff M, Caffier H, Schiebler TH. Steroid hormone binding receptors in the rat kidney. Histochemistry 69: 39–48, 1980. doi: 10.1007/BF00508365. [DOI] [PubMed] [Google Scholar]
- 22.Davis CM, Fairbanks SL, Alkayed NJ. Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res 4: 381–389, 2013. doi: 10.1007/s12975-012-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis FM, Daugherty A, Lu HS. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol 39: e83–e90, 2019. doi: 10.1161/ATVBAHA.119.312000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz A, López-Grueso R, Gambini J, Monleón D, Mas-Bargues C, Abdelaziz KM, Viña J, Borrás C. Sex differences in age-associated type 2 diabetes in rats−role of estrogens and oxidative stress. Oxid Med Cell Longev 2019: 6734836, 2019. doi: 10.1155/2019/6734836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorman JS, Steenkiste AR, Foley TP, Strotmeyer ES, Burke JP, Kuller LH, Kwoh CK; Familial Autoimmune and Diabetes (FAD) Study . Menopause in type 1 diabetic women: is it premature? Diabetes 50: 1857–1862, 2001. doi: 10.2337/diabetes.50.8.1857. [DOI] [PubMed] [Google Scholar]
- 26.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ, Karl M. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011. doi: 10.1038/ki.2010.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducasa GM, Mitrofanova A, Fornoni A. Crosstalk between lipids and mitochondria in diabetic kidney disease. Curr Diab Rep 19: 144, 2019. doi: 10.1007/s11892-019-1263-x. [DOI] [PubMed] [Google Scholar]
- 28.Elustondo P, Martin LA, Karten B. Mitochondrial cholesterol import. Biochim Biophys Acta Mol Cell Biol Lipids 1862: 90–101, 2017. doi: 10.1016/j.bbalip.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Escobales N, Nuñez RE, Javadov S. Mitochondrial angiotensin receptors and cardioprotective pathways. Am J Physiol Heart Circ Physiol 316: H1426–H1438, 2019. doi: 10.1152/ajpheart.00772.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyster KM. The estrogen receptors: an overview from different perspectives. Methods Mol Biol 1366: 1–10, 2016. doi: 10.1007/978-1-4939-3127-9_1. [DOI] [PubMed] [Google Scholar]
- 31.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298: R1597–R1606, 2010. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 32.Franzén S, Pihl L, Fasching A, Palm F. Intrarenal activation of endothelin type B receptors improves kidney oxygenation in type 1 diabetic rats. Am J Physiol Renal Physiol 314: F439–F444, 2018. doi: 10.1152/ajprenal.00498.2017. [DOI] [PubMed] [Google Scholar]
- 33.Friederich-Persson M, Persson P. Mitochondrial angiotensin II receptors regulate oxygen consumption in kidney mitochondria from healthy and type 1 diabetic rats. Am J Physiol Renal Physiol 318: F683–F688, 2020. doi: 10.1152/ajprenal.00417.2019. [DOI] [PubMed] [Google Scholar]
- 34.Gaignard P, Fréchou M, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R. Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol 30: e12497, 2018. doi: 10.1111/jne.12497. [DOI] [PubMed] [Google Scholar]
- 35.Gaignard P, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R. Role of sex hormones on brain mitochondrial function, with special reference to aging and neurodegenerative diseases. Front Aging Neurosci 9: 406, 2017. doi: 10.3389/fnagi.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension 68: 1322–1327, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology 44: 9–21, 2019. doi: 10.1038/s41386-018-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res 2015: 916585, 2015. doi: 10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris AN, Lee HW, Verlander JW, Weiner ID. Testosterone modulates renal ammonia metabolism. Am J Physiol Renal Physiol 318: F922–F935, 2020. doi: 10.1152/ajprenal.00560.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia G, Aroor AR, Sowers JR. Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog Mol Biol Transl Sci 127: 229–249, 2014. doi: 10.1016/B978-0-12-394625-6.00009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem 47: 69–84, 2010. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justo R, Boada J, Frontera M, Oliver J, Bermúdez J, Gianotti M. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am J Physiol Cell Physiol 289: C372–C378, 2005. doi: 10.1152/ajpcell.00035.2005. [DOI] [PubMed] [Google Scholar]
- 44.Kauppila TES, Kauppila JHK, Larsson NG. Mammalian mitochondria and aging: an update. Cell Metab 25: 57–71, 2017. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37: 278–316, 2016. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalifa AR, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 5: e13125, 2017. doi: 10.14814/phy2.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, Magranè J, Starkov AA, Manfredi G. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain 135: 2865–2874, 2012. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkman DL, Muth BJ, Ramick MG, Townsend RR, Edwards DG. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am J Physiol Renal Physiol 314: F423–F429, 2018. doi: 10.1152/ajprenal.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105: 1342–1351, 2008. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong WKF, Bax JJ, Michelena HI, Delgado V. Sex differences in bicuspid aortic valve disease. Prog Cardiovasc Dis 63: 452–456, 2020. doi: 10.1016/j.pcad.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol 13: 89, 2015. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurita N, Horie S, Yamazaki S, Otani K, Sekiguchi M, Onishi Y, Takegami M, Ono R, Konno S, Kikuchi S, Fukuhara S. Low testosterone levels and reduced kidney function in japanese adult men: the Locomotive Syndrome and Health Outcome in Aizu Cohort study. J Am Med Dir Assoc 17: 371.e1−371.e6, 2016. 10.1016/j.jamda.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Kurt AH, Bozkus F, Uremis N, Uremis MM. The protective role of G protein-coupled estrogen receptor 1 (GPER-1) on methotrexate-induced nephrotoxicity in human renal epithelium cells. Ren Fail 38: 686–692, 2016. doi: 10.3109/0886022X.2016.1155398. [DOI] [PubMed] [Google Scholar]
- 54.Lapi F, Azoulay L, Niazi MT, Yin H, Benayoun S, Suissa S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA 310: 289–296, 2013. doi: 10.1001/jama.2013.8638. [DOI] [PubMed] [Google Scholar]
- 55.Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Krause RJ, Parker JC. Renal and hepatic toxicity of trichloroethylene and its glutathione-derived metabolites in rats and mice: sex-, species-, and tissue-dependent differences. J Pharmacol Exp Ther 297: 155–164, 2001. [PubMed] [Google Scholar]
- 56.Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Sicuri AR, Parker JC. Renal toxicity of perchloroethylene and S-(1,2,2-trichlorovinyl)glutathione in rats and mice: sex- and species-dependent differences. Toxicol Appl Pharmacol 179: 163–171, 2002. doi: 10.1006/taap.2001.9358. [DOI] [PubMed] [Google Scholar]
- 57.Layton AT, Sullivan JC. Recent advances in sex differences in kidney function. Am J Physiol Renal Physiol 316: F328–F331, 2019. doi: 10.1152/ajprenal.00584.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levillain O, Balvay S, Peyrol S. Localization and differential expression of arginase II in the kidney of male and female mice. Pflugers Arch 449: 491–503, 2005. doi: 10.1007/s00424-004-1336-8. [DOI] [PubMed] [Google Scholar]
- 59.Levillain O, Hus-Citharel A, Garvi S, Peyrol S, Reymond I, Mutin M, Morel F. Ornithine metabolism in male and female rat kidney: mitochondrial expression of ornithine aminotransferase and arginase II. Am J Physiol Renal Physiol 286: F727–F738, 2004. doi: 10.1152/ajprenal.00315.2003. [DOI] [PubMed] [Google Scholar]
- 60.Levillain O, Ventura G, Déchaud H, Hobeika M, Meseguer A, Moinard C, Cynober L. Sex-differential expression of ornithine aminotransferase in the mouse kidney. Am J Physiol Renal Physiol 292: F1016–F1027, 2007. doi: 10.1152/ajprenal.00408.2006. [DOI] [PubMed] [Google Scholar]
- 61.Liang JJ, Rasmusson AM. Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic Stress (Thousand Oaks) 2: 2470547018818555, 2018. doi: 10.1177/2470547018818555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lima-Posada I, Portas-Cortés C, Pérez-Villalva R, Fontana F, Rodríguez-Romo R, Prieto R, Sánchez-Navarro A, Rodríguez-González GL, Gamba G, Zambrano E, Bobadilla NA. Gender differences in the acute kidney injury to chronic kidney disease transition. Sci Rep 7: 12270, 2017. doi: 10.1038/s41598-017-09630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58: 665–671, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am 101: 169–193, 2017. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol 306: H1485–H1494, 2014. doi: 10.1152/ajpheart.00809.2013. [DOI] [PubMed] [Google Scholar]
- 66.Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J, Chen SM, Chien YH, Hwu WL, Fan PC, Wong LJ, Atwal PS, Huang T. Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci USA 115: 13039–13044, 2018. doi: 10.1073/pnas.1810946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutz-Bonengel S, Parson W. No further evidence for paternal leakage of mitochondrial DNA in humans yet. Proc Natl Acad Sci USA 116: 1821–1822, 2019. doi: 10.1073/pnas.1820533116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacMillan-Crow LA, Mayeux PR. Female mice exhibit less renal mitochondrial injury but greater mortality using a comorbid model of experimental sepsis. Intern Med Rev (Wash D C) 4: 10.18103/imr.v4i10.768, 2018. doi: 10.18103/imr.v4i10.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, Fujii R, Ishidate F, Tanaka T, Tanaka Y, Hirokawa N, Nangaku M, Inagi R. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep 29: 1261−1273.e6, 2019. doi: 10.1016/j.celrep.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 70.Maric-Bilkan C. Sex differences in diabetic kidney disease. Mayo Clin Proc 95: 587–599, 2020. doi: 10.1016/j.mayocp.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 71.Maric C, Sullivan S. Estrogens and the diabetic kidney. Gend Med 5, Suppl A: S103−S113, 2008. doi: 10.1016/j.genm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masood DE, Roach EC, Beauregard KG, Khalil RA. Impact of sex hormone metabolism on the vascular effects of menopausal hormone therapy in cardiovascular disease. Curr Drug Metab 11: 693–714, 2010. doi: 10.2174/138920010794233477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22: 609–622, 2008. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer A, Laverny G, Bernardi L, Charles AL, Alsaleh G, Pottecher J, Sibilia J, Geny B. Mitochondria: an organelle of bacterial origin controlling inflammation. Front Immunol 9: 536, 2018. doi: 10.3389/fimmu.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell T, De Miguel C, Gohar EY. Sex differences in redox homeostasis in renal disease. Redox Biol 31: 101489, 2020. doi: 10.1016/j.redox.2020.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyao M, Cicalese S, Cooper HA, Eguchi S. Endoplasmic reticulum stress and mitochondrial biogenesis are potential therapeutic targets for abdominal aortic aneurysm. Clin Sci (Lond) 133: 2023–2028, 2019. doi: 10.1042/CS20190648. [DOI] [PubMed] [Google Scholar]
- 77.Molinari C, Battaglia A, Grossini E, Mary DA, Vassanelli C, Vacca G. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs. J Physiol 543: 365–372, 2002. doi: 10.1113/jphysiol.2002.022756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 124: 2145–2154, 2011. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res 75: 478–486, 2007. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 80.Negulescu O, Bognar I, Lei J, Devarajan P, Silbiger S, Neugarten J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int 62: 1989–1998, 2002. doi: 10.1046/j.1523-1755.2002.00679.x. [DOI] [PubMed] [Google Scholar]
- 81.Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol 19: 131, 2018. doi: 10.1186/s12882-018-0937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishi Y, Satoh M, Nagasu H, Kadoya H, Ihoriya C, Kidokoro K, Sasaki T, Kashihara N. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int 83: 662–673, 2013. doi: 10.1038/ki.2012.475. [DOI] [PubMed] [Google Scholar]
- 83.Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun 460: 72–81, 2015. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 84.Ostadal B, Drahota Z, Houstek J, Milerova M, Ostadalova I, Hlavackova M, Kolar F. Developmental and sex differences in cardiac tolerance to ischemia-reperfusion injury: the role of mitochondria 1. Can J Physiol Pharmacol 97: 808–814, 2019. doi: 10.1139/cjpp-2019-0060. [DOI] [PubMed] [Google Scholar]
- 85.Pan JS, Sheikh-Hamad D. Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch 7: 10.18103/mra.v7i2.1898, 2019. doi: 10.18103/mra.v7i2.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pathak T, Trebak M. Mitochondrial Ca2+ signaling. Pharmacol Ther 192: 112–123, 2018. doi: 10.1016/j.pharmthera.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patil CN, Wallace K, LaMarca BD, Moulana M, Lopez-Ruiz A, Soljancic A, Juncos LA, Grande JP, Reckelhoff JF. Low-dose testosterone protects against renal ischemia-reperfusion injury by increasing renal IL-10-to-TNF-α ratio and attenuating T-cell infiltration. Am J Physiol Renal Physiol 311: F395–F403, 2016. doi: 10.1152/ajprenal.00454.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and iron: current questions. Expert Rev Hematol 10: 65–79, 2017. doi: 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng Y, Fang Z, Liu M, Wang Z, Li L, Ming S, Lu C, Dong H, Zhang W, Wang Q, Shen R, Xie F, Zhang W, Yang C, Gao X, Sun Y. Testosterone induces renal tubular epithelial cell death through the HIF-1α/BNIP3 pathway. J Transl Med 17: 62, 2019. doi: 10.1186/s12967-019-1821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pronsato L, Boland R, Milanesi L. Non-classical localization of androgen receptor in the C2C12 skeletal muscle cell line. Arch Biochem Biophys 530: 13–22, 2013. doi: 10.1016/j.abb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, Zhang W, D’Agati V, Schlondorff D, Haraldsson B, Böttinger E, Daehn I. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 66: 763–778, 2017. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qiao C, Ye W, Li S, Wang H, Ding X. Icariin modulates mitochondrial function and apoptosis in high glucose-induced glomerular podocytes through G protein-coupled estrogen receptors. Mol Cell Endocrinol 473: 146–155, 2018. doi: 10.1016/j.mce.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 93.Quinkler M, Bumke-Vogt C, Meyer B, Bähr V, Oelkers W, Diederich S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrinol Metab 88: 2803–2809, 2003. doi: 10.1210/jc.2002-021970. [DOI] [PubMed] [Google Scholar]
- 94.Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens 27: 176–181, 2018. doi: 10.1097/MNH.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 94a.Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Maas AH, Kautzky-Willer A, Knappe-Wegner D, Kintscher U, Ladwig KH, Schenck-Gustafsson K, Stangl V, Stangl V; EUGenMed Cardiovascular Clinical Study Group . Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 37: 24–34, 2016. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 95.Rius R, Cowley MJ, Riley L, Puttick C, Thorburn DR, Christodoulou J. Biparental inheritance of mitochondrial DNA in humans is not a common phenomenon. Genet Med 21: 2823–2826, 2019. doi: 10.1038/s41436-019-0568-0. [DOI] [PubMed] [Google Scholar]
- 96.Rossetti MF, Cambiasso MJ, Holschbach MA, Cabrera R. Oestrogens and progestagens: synthesis and action in the brain. J Neuroendocrinol 28: 28, 2016. doi: 10.1111/jne.12402. [DOI] [PubMed] [Google Scholar]
- 97.Rovira-Llopis S, Bañuls C, de Marañon AM, Diaz-Morales N, Jover A, Garzon S, Rocha M, Victor VM, Hernandez-Mijares A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic Biol Med 108: 155–162, 2017. doi: 10.1016/j.freeradbiomed.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 98.Roy-O’Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology 159: 3120–3131, 2018. doi: 10.1210/en.2018-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 455: 397–429, 2007. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 100.Saxena S, Mathur A, Kakkar P. Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy. J Cell Physiol 234: 19223–19236, 2019. doi: 10.1002/jcp.28712. [DOI] [PubMed] [Google Scholar]
- 101.Schiffl H. Gender differences in the susceptibility of hospital-acquired acute kidney injury: more questions than answers. Int Urol Nephrol 52: 1911–1914, 2020. doi: 10.1007/s11255-020-02526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seifarth JE, McGowan CL, Milne KJ. Sex and life expectancy. Gend Med 9: 390–401, 2012. doi: 10.1016/j.genm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Shepard BD. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am J Physiol Renal Physiol 317: F456–F462, 2019. doi: 10.1152/ajprenal.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 137: 107–123, 2013. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod 20: 3481–3487, 2005. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- 106.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68: 959–965, 2005. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 107.Sullivan JC. Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008. doi: 10.1152/ajpregu.00864.2007. [DOI] [PubMed] [Google Scholar]
- 108.Sweileh WM. Gender differences in aminoglycoside induced nephrotoxicity: a prospective, hospital-based study. Curr Clin Pharmacol 4: 229–232, 2009. doi: 10.2174/157488409789375339. [DOI] [PubMed] [Google Scholar]
- 109.Tugaeva KV, Sluchanko NN. Steroidogenic Acute Regulatory Protein: Structure, Functioning, and Regulation. Biochemistry (Mosc) 84, Suppl 1: S233–S253, 2019. doi: 10.1134/S0006297919140141. [DOI] [PubMed] [Google Scholar]
- 110.Um JH, Yun J. Emerging role of mitophagy in human diseases and physiology. BMB Rep 50: 299–307, 2017. doi: 10.5483/BMBRep.2017.50.6.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Bliek AM, Sedensky MM, Morgan PG. Cell biology of the mitochondrion. Genetics 207: 843–871, 2017. doi: 10.1534/genetics.117.300262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 20: 5454–5468, 2000. doi: 10.1128/MCB.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond) 131: 803–822, 2017. doi: 10.1042/CS20160485. [DOI] [PubMed] [Google Scholar]
- 115.Wei PZ, Szeto CC. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta 496: 108–116, 2019. doi: 10.1016/j.cca.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Weng JH, Chung BC. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids 111: 54–59, 2016. doi: 10.1016/j.steroids.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 117.Woodman AG, Mah R, Keddie D, Noble RMN, Panahi S, Gragasin FS, Lemieux H, Bourque SL. Prenatal iron deficiency causes sex-dependent mitochondrial dysfunction and oxidative stress in fetal rat kidneys and liver. FASEB J 32: 3254–3263, 2018. doi: 10.1096/fj.201701080R. [DOI] [PubMed] [Google Scholar]
- 118.Woodman AG, Mah R, Keddie DL, Noble RMN, Holody CD, Panahi S, Gragasin FS, Lemieux H, Bourque SL. Perinatal iron deficiency and a high salt diet cause long-term kidney mitochondrial dysfunction and oxidative stress. Cardiovasc Res 116: 183–192, 2020. doi: 10.1093/cvr/cvz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol Regul Integr Comp Physiol 305: R459–R463, 2013. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol 16: 263–275, 2018. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Youle RJ. Mitochondria−striking a balance between host and endosymbiont. Science 365: eaaw9855, 2019. doi: 10.1126/science.aaw9855. [DOI] [PubMed] [Google Scholar]
- 122.Young LJ, Pfaff DW. Sex differences in neurological and psychiatric disorders. Front Neuroendocrinol 35: 253–254, 2014. doi: 10.1016/j.yfrne.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Zawada I, Masternak MM, List EO, Stout MB, Berryman DE, Lewinski A, Kopchick JJ, Bartke A, Karbownik-Lewinska M, Gesing A. Gene expression of key regulators of mitochondrial biogenesis is sex dependent in mice with growth hormone receptor deletion in liver. Aging (Albany NY) 7: 195–204, 2015. doi: 10.18632/aging.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao JV, Schooling CM. The role of testosterone in chronic kidney disease and kidney function in men and women: a bi-directional Mendelian randomization study in the UK Biobank. BMC Med 18: 122, 2020. doi: 10.1186/s12916-020-01594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]