Keywords: acute kidney injury, adenosine, 15-hydoxyprostaglandin dehydrogenase, prostaglandin E2, vasodilation
Abstract
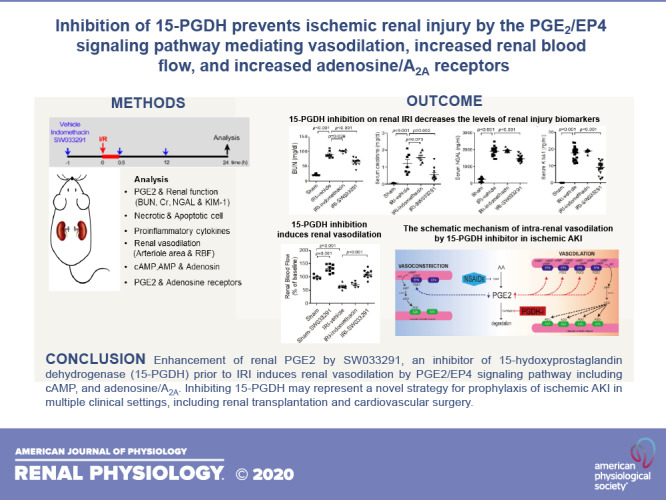
In the present study, we demonstrated the marked activity of SW033291, an inhibitor of 15-hydoxyprostaglandin dehydrogenase (15-PGDH), in preventing acute kidney injury (AKI) in a murine model of ischemia-reperfusion injury. AKI due to ischemic injury represents a significant clinical problem. PGE2 is vasodilatory in the kidney, but it is rapidly degraded in vivo due to catabolism by 15-PGDH. We investigated the potential of SW033291, a potent and specific 15-PGDH inhibitor, as prophylactic treatment for ischemic AKI. Prophylactic administration of SW033291 significantly increased renal tissue PGE2 levels and increased post-AKI renal blood flow and renal arteriole area. In parallel, prophylactic SW033291 decreased post-AKI renal morphology injury scores and tubular apoptosis and markedly reduced biomarkers of renal injury that included blood urea nitrogen, creatinine, neutrophil gelatinase-associated lipocalin, and kidney injury molecule-1. Prophylactic SW033291 also reduced post-AKI induction of proinflammatory cytokines, high-mobility group box 1, and malondialdehyde. Protective effects of SW033291 were mediated by PGE2 signaling, as they could be blocked by pharmacological inhibition of PGE2 synthesis. Consistent with activation of PGE2 signaling, SW033291 induced renal levels of both EP4 receptors and cAMP, along with other vasodilatory effectors, including AMP, adenosine, and the adenosine A2A receptor. The protective effects of SW0333291 could largely be achieved with a single prophylactic dose of the drug. Inhibition of 15-PGDH may thus represent a novel strategy for prophylaxis of ischemic AKI in multiple clinical settings, including renal transplantation and cardiovascular surgery.
INTRODUCTION
Acute kidney injury (AKI) is an important clinical problem associated with high rates of morbidity and mortality (1.7 million deaths annually) (8, 10, 14, 18, 20, 29, 36, 39, 42, 50). Considerable effort has been directed toward the development of preventive strategies for AKI using various agents and animal models (42, 50). Despite advances in prevention strategies, no specific treatment for AKI has yet been developed (50).
The main causes of AKI are hypoxia and oxidative stress due to renal ischemia-reperfusion injury (IRI) (11). During periods of transient reduction in renal blood flow (RBF), an insufficient oxygen supply can cause energy impairment (ATP depletion) in the renal outer medulla, resulting in injury and death of tubular epithelial cells due to acute tubular necrosis (ATN) and apoptosis (3–5, 10, 30). Inflammation, due to oxygen free radicals after reperfusion, leads to the extension phase of ischemic AKI (10, 31). Resistance to hypoxia and the reduction of oxidative stress are treatment targets for ischemic AKI. Various endogenous vasoactive mediators [e.g., PGE2, prostaglandin receptor, adenosine transporter, nitric oxide, and vasopressin] are potential candidate agents for treating or preventing ischemia (10, 15, 17, 19, 22, 26, 31).
The PGE2 pathway is a particularly interesting target in ischemic AKI. PGE2 exerts a vasodilatory effect in the kidney, which is mediated by EP2 or EP4 receptors and a cAMP-dependent mechanism in renal afferent arterioles (33, 41). A PGE2 cytoprotective effect against ischemic AKI has been demonstrated in several animal models (6, 28, 32, 34, 45). Beneficial effects of PGE2 are reflected by the well-known nephrotoxic activity of nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit [cyclooxygenase (COX)] enzymatic synthesis of PGE2 from an arachidonic acid precursor. However, establishing a therapeutic dose for PGE2 is complicated by PGE2 also mediating all of the cardinal signs of inflammation as well as enhancing microvascular permeability at sites of injury (14, 33, 40). Furthermore, PGE2 is rapidly degraded in vivo by the enzymatic activity of 15-hydroxyprostaglandin dehydrogenase (15-PGDH), which, by oxidizing the PGE2 15-hyodroxyl group, both catalyzes the first step in PGE2 degradation and blocks the molecule’s binding to prostaglandin receptors (9, 14, 32, 33, 38, 40, 48). Inhibition of 15-PGDH has been shown to increase endogenous PGE2 and cAMP levels and to, thereby, potentiate tissue repair in several mouse models of injury and disease (48). In this study, we examined SW033291, a potent small-molecule inhibitor of 15-PGDH, for effects in the kidney, demonstrating that SW033291 shows potent in vivo activity in increasing endogenous renal PGE2, in mediating renal vasodilation, and in conferring renal protection against ischemic AKI.
MATERIALS AND METHODS
Animals.
Male C57/BL6 mice (10 wk) were purchased from Orient Bio. The care of and experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Inje University (protocol no. 2016-010).
Induction of renal IRI.
Renal IRI was performed as previously described (2). Briefly, mice were anesthetized with isoflurane using a vaporizer, and bilateral renal arteries were clamped for 30 min (2, 49). After the ischemic time, clamps were released to induce blood reperfusion. SW033291 (5 mg/kg, Cayman, Ann Arbor, MI), indomethacin (5 mg/kg, Sigma-Aldrich), or vehicle [10% ethanol, 5% cremophor EL, and 85% D5W (5% dextrose in water)] were intraperitoneally administered three times at 1 h before, immediately after, and 12 h after AKI. Serum and kidney tissue were collected 24 h after renal IRI. These dose levels of SW033291 and indomethacin have been demonstrated as biologically effective in C57/BL6 mice in our prior published study (48). As an additional comparator, celecoxib (100 mg/kg, Pfizer), a selective COX-2 inhibitor, was also orally administered two times at 2 h before and 12 h after renal IRI. Additionally, an EP4 receptor antagonist, ONO-AE3-208 (10 mg/kg, Cayman), was administered for three doses, either concurrently with SW033291 or individually on the same schedule.
Measurement of PGE2 levels and renal function.
After reperfusion for 24 h, kidney tissues (∼20 mg) were homogenized in cold PBS containing indomethacin (10 μg/mL) and then centrifuged for 10 min at 12,000 rpm. Protein concentrations of supernatant were determined by BCA assay (ThermoFisher Scientific, Rockford, IL). The PGE2 level was measured using a PGE2 ELISA kit (R&D Systems, Minneapolis, MN) in triplicate. PGE2 levels were expressed as nanograms of PGE2 per milligram of protein. Renal function was assessed by determining the serum levels of blood urea nitrogen (BUN; Abcam), serum creatinine (Agilent 6410 LC-MS/MS, Agilent, Santa Clara, CA), lipocalin-2 (R&D Systems), and kidney injury molecule-1 (KIM-1; R&D Systems) after reperfusion for 24 h. Measurement of serum creatinine was by HPLC. Serum creatinine was assessed using an Agilent 6410 LC-MS/MS system coupled with an Agilent 1200 series HPLC system (Agilent). The sample preparation for serum creatinine was that 50 μL of serum samples were mixed with 100 μL of acetonitrile that contained creatinine-d3 as an internal standard (5 μg/mL). The mixture was centrifuged at 13,000 rpm for 10 min after vigorous vortex, and 1 μL of supernatant was then injected into the LC-MS/MS system. The chromatography separation was performed on a Hypersil GOLD C18 column (150 × 2.1 mm, 3 μm, Thermo Scientific) using a mobile phase of 0.1% formic acid solution and acetonitrile [70:30 (vol/vol)]. The flow rate was 0.2 mL/min. Electrospray ionization was performed in the positive ion mode. The capillary voltage was set at 4,000 V. Nitrogen as the nebulizing gas was set at 15 psi, and the drying gas temperature was set at 300°C, with a flow rate of 6 L/min. The multiple-reaction monitoring mode using specific precursor/product ion transition was used for quantification. Detection of ions was performed by monitoring m/z 114.1 → 44.1 for creatinine and 117.1 → 77.1 for creatinine-d3. The collision energies were 10 and 15 eV for creatinine and creatinine-d3, respectively. The peak areas for all analytes were integrated automatically using Agilent Mass Hunter Analysis software (version B.01.04).
Necrotic and apoptotic cell death assays.
To evaluate necrosis, 5-μm-thick paraffin sections were stained with hematoxylin and eosin. Tubular injury of individual slides was scored semiquantitatively according to a scoring system by a pathologist in a blinded manner. The scoring system was as follows: 0, no damage; 1, patchy isolated unicellular necrosis; 2, tubular necrosis < 25%; 3, tubular necrosis 25–50%; and 4, tubular necrosis > 50%. To analyze the frequency of apoptosis, 5-μm-thick paraffin sections were subjected to a TUNEL assay (Millipore, Temecula, CA) according to the manufacturer’s protocol. TUNEL-positive cells were counted in at least five separate fields (×64 magnification) in the outer medulla, and the apoptosis index (in %, number of apoptosis cells/total number of cells) was calculated using GENASIS software.
Measurement of proinflammatory cytokine levels.
Inflammatory cytokine mRNA and protein levels were measured by real-time PCR and ELISA. Kidney tissue and serum were harvested after reperfusion for 24 h. Total RNA was extracted from frozen kidney tissue using TRIzol reagent (ThermoFisher Scientific) according to the manufacturer’s protocol. RNA was converted to cDNA using oligo-dT primers. IL-17, TNF-α, and IL-1β mRNA levels were determined by real-time PCR with SYBR Green PCR master mix with the following primers: TNF-α, forward 5′-CCACATCTCCCTCCAGAAAA-3′ and reverse 5′-AGGGTCTGGGCCATAGAACT-3′; IL-1β, forward 5′-TCACAGCAGCACATCAACAA-3′ and reverse 5′-TGTCCTCATC-CTGGAAGGTC-3′; IL-17, forward 5′-TCCAGAAGGCCCTCAGACTA-3′ and reverse 5′-AGCATCTTCTCGACCCTGAA-3′; and GAPDH, forward 5′-TTCACCACCATGGAGAAGGC-3′ and reverse 5′-GGCATGGACTGTGGTCATGA-3′. Serum IL-17 (R&D Systems), TNF-α (R&D Systems), and IL-1β (R&D Systems) were measured using commercial ELISA kits according to the manufacturer’s instructions.
Assessment of renal vasodilation.
The inner arteriolar area of the outer medulla was determined using α-smooth muscle actin (α-SMA)-stained sections. Kidneys were removed without perfusion under inhalational isoflurane anesthesia, divided into three pieces horizontally, immediately fixed in 10% formalin, and then embedded in paraffin. The inner area of α-SMA-positive vessels in the outer medulla (×25) was measured using ImageJ (25, 44). The results are expressed as average areas of renal arteries in the outer medulla. As a surrogate for RBF, we used noninvasive laser-Doppler flowmetry (periflux system 5000, Perimed) to measure flux, placing laser-Doppler probes at a fixed position on the kidney surface and recording renal cortical measurements (21). Renal-Doppler flux (RDF) was measured before (1 h after administration of vehicle, indomethacin, and SW033291), during, and 24 h after renal IRI. The relative increase represents the percent increase in RDF from baseline to peak for each test.
Measurement of cAMP, AMP, and adenosine levels.
After reperfusion for 24 h, kidney tissues were harvested, homogenized in 10 volumes of 0.1 M HCl, and centrifuged for 10 min at 12,000 rpm. cAMP levels in kidney tissues and serum were measured using a cAMP Complete ELISA kit (Enzo Life Science, Farmingdale, NY). AMP and adenosine in kidney tissue were assessed by Agilent 6410 LC-MS/MS. Serum adenosine was measured using an assay kit (Abnova, Walnut, CA).
Assessment of PGE2 and adenosine receptors.
PGE2 receptors (EP1, EP2, EP3, and EP4) and adenosineA2A receptor mRNA levels were determined by real-time PCR with SYBR Green PCR master mix and the following primers: EP1, forward 5′-CCTGGCACCTGGTGTTTTAT-3′ and reverse 5′-TGGCTGAAGTGATGGATGAG-3′; EP2, forward 5′-ATGCTCCTGCTGCTTATCGT-3′ and reverse 5′-TAATGGCCAGGAGAATGAGG-3′; EP3, forward 5′-GAGAGCAAGCGCAAGAAGTC-3′ and reverse 5′-ATGGTTAGCCGAAGAAGGT-3′; EP4, forward 5′-TGGCTGTCACTGACCTTCTG-3′ and reverse 5′-TGCATAGATGGCGAAGAGTG-3′; and A2A, forward 5′-GAAGCAGATGGAGAGCCAAC-3′ and reverse 5′-AATTGCTGTGGGAGAGGATG-3′. Their protein levels were determined by Western blot assay and immunofluorescence analysis.
Measurement of reactive oxygen species levels.
Reactive oxygen species (ROS) levels were determined by measurement of malondialdehyde (MDA), the end product of lipid peroxidation in kidney lysates. Free MDA reacts with thiobarbituric acid (TBA) at 95°C to generate an MDA-TBA adduct, which can be quantified colorimetrically at a wavelength of 532 nm. MDA levels were measured in kidney lysates using a lipid peroxidation (MDA) assay kit (Abcam, Cambridge, UK). Results were corrected for total protein level and are expressed as μM MDA/g protein.
Statistical analysis.
Statistical analyses were performed with one-way ANOVA followed by a Bonferroni post hoc test when three or more experimental groups were compared. Data are presented as means ± SE, using Student‘s t-test for two-group analysis. Values of P < 0.05 were considered indicative of statistical significance.
RESULTS
15-PGDH inhibition ameliorates renal dysfunction in mice with ischemic AKI.
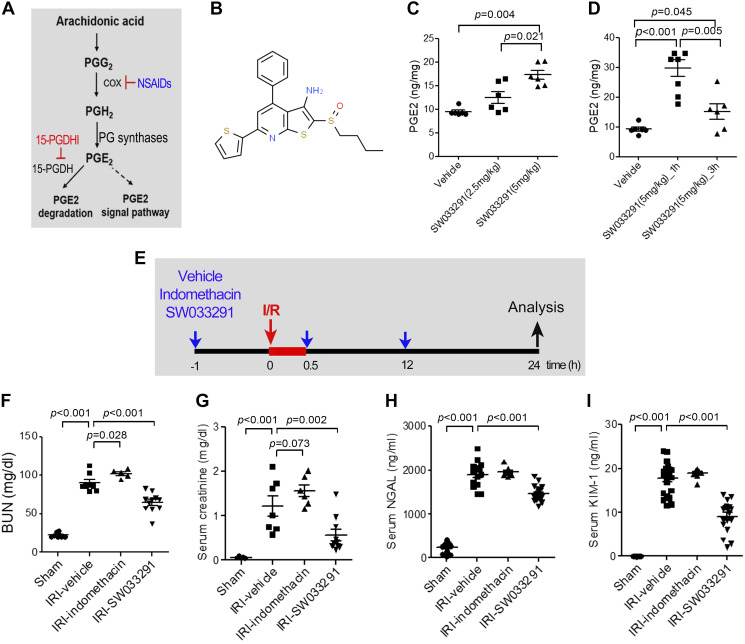
Endogenous PGE2 levels are decreased by blocking PGE2 synthesis with NSAIDs that inhibit COX-1 and/or COX-2 but are increased by blocking PGE2 degradation by inhibiting 15-PGDH (Fig. 1A). SW033291 is a potent and specific chemical inhibitor of 15-PGDH with a subnanomolar IC50 value (Fig. 1B) (48). Treatment of mice with SW033291 induced a dose-dependent increase in renal PGE2 at 3 h after intraperitoneal injection of 2.5 or 5 mg/kg SW033291 (Fig. 1C) and showed a peak at 1 h with 5 mg/kg SW033291 tripling endogenous renal PGE2 (Fig. 1D). To interrogate effects of inhibition of 15-PGDH on protection from IRI, mice were subjected to 30 min of IRI and administered three doses of SW033291 (IRI-SW033291) versus vehicle (IRI-vehicle), versus indomethacin (IRI-indomethacin), or versus celecoxib (IRI-celecoxib). Treatments were administered beginning 1 h before, immediately after, and 12 h after renal IRI (Fig. 1E) (with the exception that the celecoxib dose was administered 2 h before and 12 h after renal IRI). IRI-vehicle mice exhibited significant ischemic AKI, as indicated by increases in BUN, creatinine, neutrophil gelatinase-associated lipocalin (NGAL), and KIM-1 (Fig. 1, F–I). However, IRI-SW033291 markedly protected the kidney from IRI, significantly reducing BUN, creatinine, NGAL, and KIM-1 compared with IRI-vehicle mice (Fig. 1, F–I). In contrast, inhibition of endogenous PGE2 production with administration of either indomethacin or celecoxib significantly aggravated IRI, as reflected by the increase in BUN of the indomethacin-treated group (Fig. 1F) and the increase of BUN and creatinine in the celecoxib-treated group (see Supplemental Fig. S1, A and B, available online at https://doi.org/10.6084/m9.figshare.13088993.v1). In summary, inhibition of 15-PGDH increased endogenous renal PGE2 levels and ameliorated renal dysfunction from ischemic AKI compared with renal dysfunction being worsened by inhibiting COX-1 and COX-2 with indomethacin or COX-2 alone with celecoxib.
Fig. 1.
15-Hydoxyprostaglandin dehydrogenase (15-PGDH) inhibition with renal ischemia-reperfusion (I/R) injury (IRI) decreases levels of renal injury biomarkers. A: the arachidonic acid prostaglandin (PG) biosynthesis pathway and biological activity of 15-PGDH inhibitor. Cox, cyclooxygenase; NSAIDs, nonsteroidal anti-inflammatory drugs. B: chemical structure of SW033291. C: pharmacological inhibition of 15-PGDH with SW033291 was confirmed by endogenous PGE2 levels in kidney tissue at 3 h after intraperitoneal injection of 2.5 or 5 mg/kg SW033291 or vehicle. D: PGE2 levels in kidney tissue at 1 and 3 h after intraperitoneal injection of 5 mg/kg SW033291 or vehicle. E: experimental setup. Mice were subjected to bilateral renal IRI for 30 min and were injected with vehicle, SW033291, or indomethacin at 1 h before, immediately after, and 12 h after renal IRI. F−I: serum levels of blood urea nitrogen (BUN; F), creatinine (G), neutrophil gelatinase-associated lipocalin (NGAL; H), and kidney injury molecule 1 (KIM-1; I). Renal function was evaluated at 24 h after renal IRI. n = 8–15 animals/group. Data are presented as means ± SE. Analysis was performed using Student’s t test.
15-PGDH inhibition attenuates renal necrosis and apoptosis in mice with ischemic AKI.
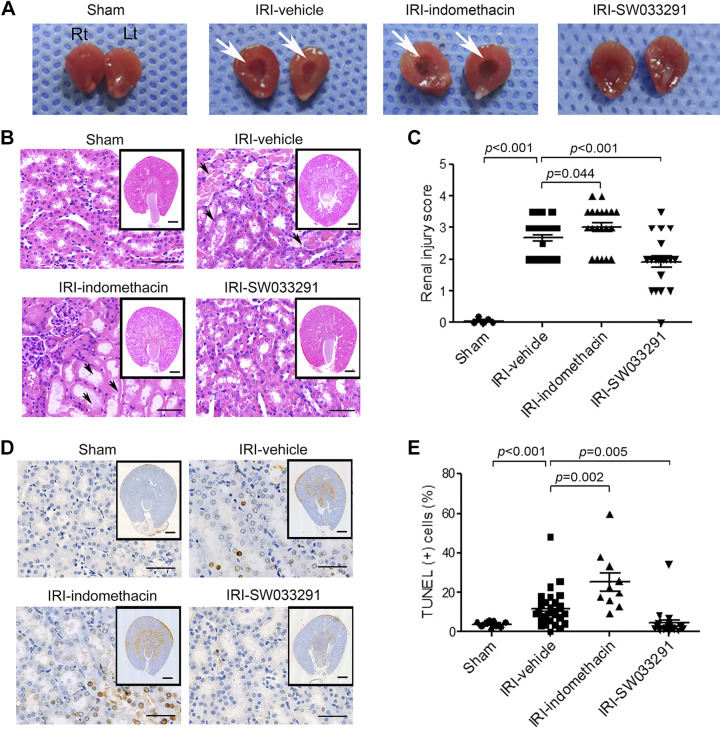
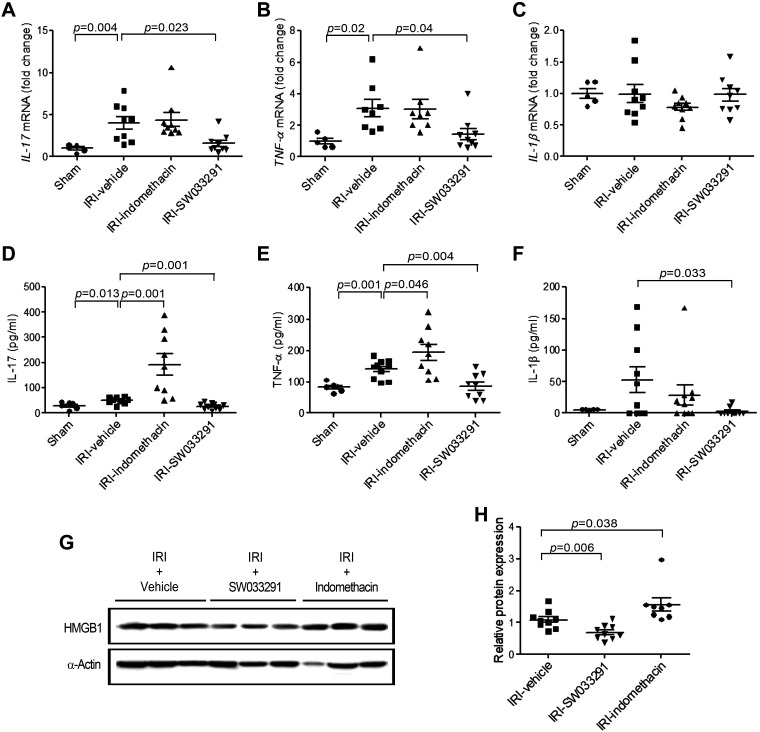
During renal IRI, tubular epithelial cells undergo injury, apoptosis, and acute tubular necrosis. Postischemic congestion that persists in the outer medulla further exacerbates renal injury by worsening hypoxia (5, 10, 12, 30). By gross pathology, IRI-vehicle mice showed increased tissue congestion in the outer medulla versus sham mice. This congestion was ameliorated by treatment with SW033291 but was worsened by treatment with indomethacin (Fig. 2A). Histopathological assessment of IRI-vehicle revealed features of acute tubular damage with tubular dilatation, extensive tubular necrosis, and apoptosis (Fig. 2, B–E). In contrast, SW033291 treatment markedly alleviated renal injury, reducing the histological renal injury score and the count of TUNEL-positive apoptotic cells (Fig. 2, C and E). Additionally, high-mobility group box 1 (HMGB1) promotes kidney damage after IRI and induces proinflammatory cytokines (1a, 37, 46). Similarly, compared with IRI-vehicle mice, SW033291 treatment reduced HMGB1 levels (Fig. 3, G and H) and blocked induction of IL-17, TNF-α, and IL-1β (protein only) (Fig. 3, A–F) (24, 35). In contrast to the effects of SW033291, treatment of IRI mice with indomethacin increased HMGB1 (Fig. 3, G and H). In summary, prophylactic SW0333291 protects from AKI, reducing tubular damage in the outer medulla, ATN, apoptosis, HMGB1, and downstream inflammatory cytokines that promote kidney damage after IRI.
Fig. 2.
15-Hydoxyprostaglandin dehydrogenase inhibition ameliorates renal tubular cell death in mice with ischemic acute kidney injury. Before and after renal ischemia-reperfusion injury (IRI), mice were injected intraperitoneally three times with vehicle, SW033291 (5 mg/kg), or indomethacin (5 mg/kg). Assessments were performed at 24 h after renal IRI. A: representative gross appearance of the right (Rt) and left (Lt) kidneys of mice injected with vehicle (IRI-vehicle), indomethacin (IRI-indomethacin), or SW033291 (IRI-SW033291) before and after renal IRI. Vascular congestion in the renal medulla is indicated by white arrows. B: representative image of tubular injury in the outer zone of the renal medulla (hematoxylin and eosin staining). Scale bars in the enlarged images = 50 μm; scale bars in insets = 500 μm. C: statistical analysis of tubular injury scores (n = 20 per group). D: representative images of apoptosis in the outer zone of the renal medulla (TUNEL staining). Scale bar in the enlarged images = 25 μm; scale bars in insets = 500 μm.;. E: statistical analysis of apoptosis (n = 20 per group). Data are presented as means ± SE. Analysis was performed using Student’s t test.
Fig. 3.
15-Hydoxyprostaglandin dehydrogenase inhibitor pretreatment ameliorates the inflammatory response in mice with ischemic acute kidney injury. Before and after renal ischemia-reperfusion injury (IRI), mice were injected intraperitoneally three times with vehicle, SW033291 (5 mg/kg), or indomethacin (5 mg/kg). Assessments were performed at 24 h after renal IRI. A−F: proinflammatory cytokine mRNA by real-time PCR (A–C) and protein levels by ELISA (D–F). A and D: IL-17; B and E: TNF-α; C and F: IL-1β. G: Western blots of high-mobility group box 1 (HMGB1; 29 kDa) in kidney tissue (representative of three experiments). H: statistical analysis of HMGB1 levels in kidney tissue (n = 9 per group). n = 9 animals/group. Data are presented as means ± SE. Analysis was performed using Student’s t test.
15-PGDH inhibition induces renal vasodilatation in the outer medulla concomitant with induction of a PGE2/EP4 receptor and adenosine/A2A receptor signaling pathway.
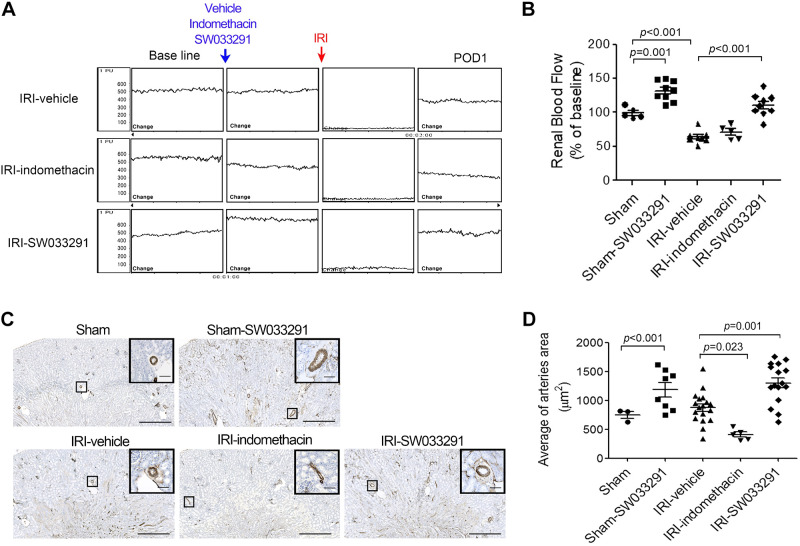
To investigate the mechanism of the renal protective effect of the 15-PGDH inhibitor shown in Figs. 1–3, we interrogated the effects of inhibition of 15-PGDH on renal hemodynamics using measurement of renal cortical Doppler flux as a surrogate to infer RBF. SW033291 increased inferred RBF above baseline within 1 h after the first injection, reflecting elevated RBF just before when AKI was initiated (Fig. 4, A and B). These benefits persisted throughout the 24-h course of study. Thus, at 24 h following IRI, vehicle-treated mice demonstrated a nearly 40% decrease in RDF-inferred RBF (vs. sham mice) (Fig. 4, A and B), whereas, in marked contrast, treatment of mice with SW033291 completely preserved RDF-inferred RBF at this time point (Fig. 4, A and B). SW033291 increases in cortical RDF-inferred RBF at 24 h were paralleled by findings of significantly increased renal arteriolar area in the outer medulla of SW033291-treated mice at 24 h post-IRI (compared with both post-IRI-vehicle-treated mice and sham mice; Fig. 4, C and D). Again, in contrast, both indomethacin or celecoxib administration reduced RDF (Fig. 4, A and B, and Supplemental Fig. S1E). In summary, these findings suggest that SW033291 increased RBF via a PGE2-mediated vasodilatory mechanism.
Fig. 4.
15-Hydoxyprostaglandin dehydrogenase inhibition induces renal vasodilation in the outer medulla of mice with ischemic acute kidney injury. To quantify vasodilation, the inner arteriolar area in the outer medulla was identified by α-smooth muscle actin staining. Assessments were performed at postoperative day 1 (POD1), 24 h after renal ischemia-reperfusion injury (IRI). A: representative images of the change in renal blood flow, as assessed by renal Doppler flux, with administration of vehicle, indomethacin, and SW033291. B: statistical analysis of renal blood flow in sham animals at time 0, in sham animals administered SW033291 (sham-SW033291) at 1 h postadministration of drug, and in cohorts subject to IRI and administered vehicle, indomethacin, and SW033291, which were then assayed at 24 h post-IRI. C: representative images of an arteriole in the outer zone of the renal medulla. Zoomed images are enlargements of the outlined areas. Scale bars in the enlarged images = 50 μm; scale bars in insets = 500 μm. D: statistical analysis of the inner arteriolar area of the outer medulla. Data are presented as means ± SE. Analysis was performed using Student’s t test.
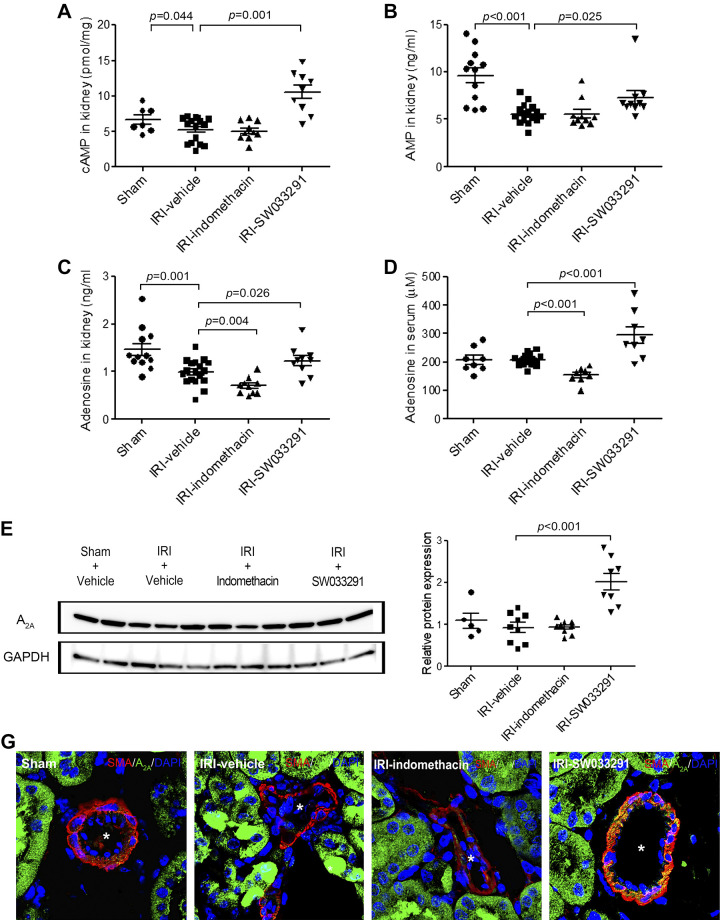
PGE2 induces cAMP levels by signaling via EP2 and EP4 receptors, a pathway that is well characterized as inducing vasodilation in the renal afferent arteriole (33, 41). Thus, we interpreted the coincident induction of PGE2 and of increased RDF at 1 h after SW033291 as likely reflecting this established biology (Figs. 1D and 4B). This biology further motivated us to examine cAMP as a potential downstream mechanism for the persistent SW033291-induced increase in RDF-inferred RBF at 24 h post-IRI. At 24 h post-IRI, both vehicle- and indomethacin-treated mice showed reduced renal cAMP versus sham mice (Fig. 5A). However, in parallel with RDF-inferred increased RBF, cAMP levels at 24 h were also elevated over sham mice in IRI-SW033291-treated mice (Fig. 5A). Moreover, at 24 h post-IRI, SW033291-treated mice also showed increased levels of the cAMP breakdown product AMP (Fig. 5B). Furthermore, an additional vasodilatory molecule, adenosine, was also decreased in the kidney at 24 h after IRI, and, like cAMP, this decrease was also reversed in mice treated with SW033291 (Fig. 5C). Increased adenosine was, furthermore, detectable at 24 h post-IRI in serum SW033391-treated mice (Fig. 5D). Moreover, at 24 h, SW0332391 also induced the adenosine A2A receptor (compared with both sham and IRI mice) (Fig. 5, E and F), with immunohistochemistry localizing the upregulated adenosine A2A receptors to the membrane of α-SMA-positive vascular smooth muscle cells that directly regulate constriction or dilation of renal arterioles (Fig. 5G).
Fig. 5.
15-Hydoxyprostaglandin dehydrogenase inhibitor promoted adenosine production and upregulated the expression of adenosine A2A receptors in the renal arterioles in the outer medulla via the cAMP/AMP signaling pathway. Assessments were performed at 24 h after renal ischemia-reperfusion injury (IRI). A and B: statistical analysis of cAMP (A) and AMP levels (B) in kidney tissue. n = 12–18 animals/group. C and D: statistical analysis of renal (C) and serum (D) adenosine levels. n = 6–10 animals/group. E: Western blots for A2A receptor protein (45 kDa) in kidney tissue (representative of three experiments). F: statistical analysis of A2A receptor protein levels in kidney tissue (n = 9 per group). G: representative confocal microscopy images of kidneys immunostained for A2A receptor and α-smooth muscle actin (α-SMA). A2A receptor-positive cells were observed in α-SMA-positive cells in the renal arteriolar outer medulla (yellow color). *α-SMA-positive renal arterioles in the outer medullar. Scale bars = 25 μm. Data are presented as means ± SE. Analysis was performed using Student’s t test.
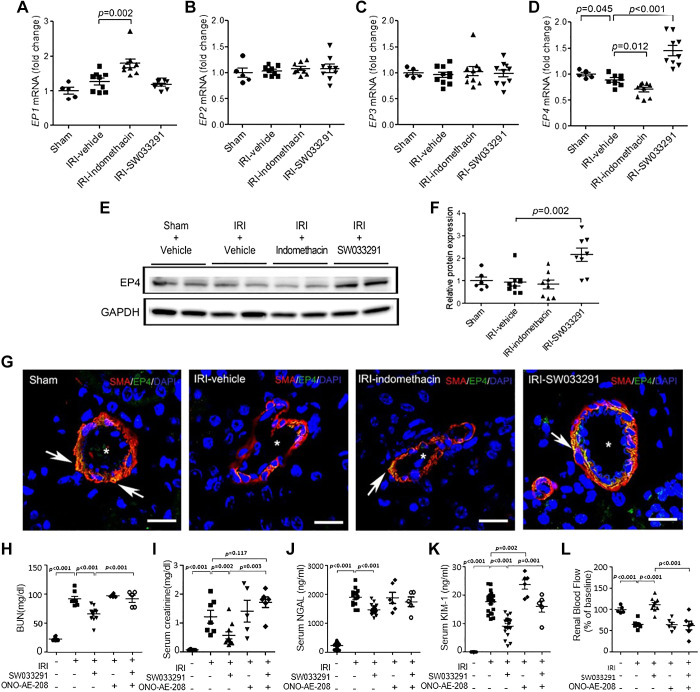
To inspect for upstream elements of the PGE2 signaling pathway that might correlate with SW033291-induced signaling events at the 24-h post IRI time point, we looked for simultaneous effects of SW0332391 on the expression of PGE2 receptors. Intriguingly, we found that at 24 h post-IRI, mice treated with SW033291 had significantly increased EP4 receptor expression, at both mRNA and protein levels, with EP4 receptor protein more than twice as high in SW033291- versus vehicle-treated mice (Fig. 6, D–F) (compared with both sham and vehicle-treated IRI mice). Indomethacin, in contrast, reduced EP4 receptor mRNA levels (Fig. 6, D–F) and moreover increased EP1 receptor mRNA levels, a receptor known to be involved in vasoconstriction (Fig. 6A). SW033291 and indomethacin had no effect on levels of EP2 and EP3 receptors (Fig. 6, B and C). EP4 receptors induced by SW033291 were localized to the membrane of α-SMA-positive vascular smooth muscle cells that directly regulate constriction or dilation of renal arterioles (Fig. 6G).
Fig. 6.
15-Hydoxyprostaglandin dehydrogenase inhibitor treatment promoted the expression of EP4 receptors in the renal arteriolar outer medulla. Assessments were performed at 24 h after renal ischemia-reperfusion injury (IRI). A−D: statistical analysis of EP1 (A), EP2 (B), EP3 (C), and EP4 (D) receptor mRNA levels in kidney tissue by real-time PCR. n = 6–10 animals/group. E: Western blots for EP4 receptor protein (73 kDa) in kidney tissue (representative of three experiments). F: statistical analysis of EP4 receptor protein levels in kidney tissue (n = 8 per group except sham, where n = 6). G: representative confocal microscopy images for EP4 receptors (green), α-smooth muscle actin (α-SMA; red), and DAPI (blue)-stained kidney sections. EP4 receptor-positive cells were observed in α-SMA-positive cells in the renal arteriolar outer medulla (arrow). *α-SMA-positive renal arterioles in the outer medullar. Scale bars = 25 μm. H–L: effects of the EP4 inhibitor ONO-AE3-208 on SW033291 amelioration of IRI-induced renal injury. Data are presented as means ± SE. Analysis was performed using Student’s t test.
To interrogate the functional role of EP4 receptors in mediating SW033291 effects, we conducted experiments of IRI mice coadministered the three-dose schedule of SW033291 together with an EP4 receptor antagonist, ONO-AE3-208. Coadministration of ONO-AE3-208 substantially blocked the beneficial effects of SW033291 on reversing IRI-induced increases of BUN, serum creatinine, and serum Kim-1 and on reversing IRI-induced decreases in RDF-inferred RBF (Fig. 6, H–L).
In overview, induction of renal vasodilation by 15-PGDH inhibition was well correlated with increased PGE2 levels at 1 h postadministration of drug, and, at 24 h, it was well correlated with induction of downstream mediators that included cAMP, AMP, adenosine, and adenosine A2A and EP4 receptors, with induction of both EP4 and A2A receptors targeted to vascular smooth muscle cells and with EP4 function required for drug activity.
Pretreatment with a single 15-PGDH inhibitor dose mitigates renal dysfunction.
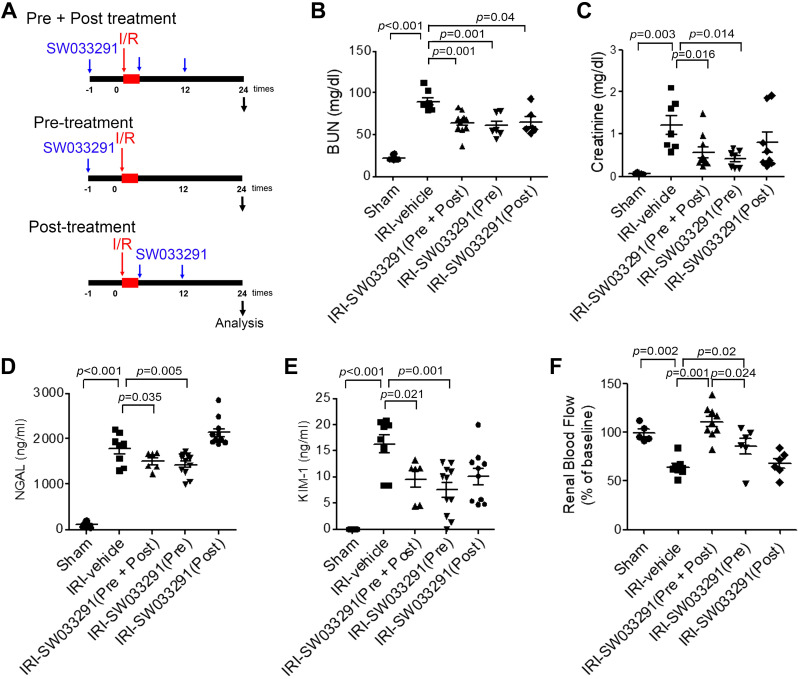
To interrogate the contributions of SW033291 administered just before versus just after AKI, we compared the effects of administeration of a single dose of SW033291 given 1 h before IRI (Pre) versus two doses given immediately after IRI (Post) versus our standard three-dose regimen given 1 h before, immediately after, and 12 h after IRI (Pre + Post) (Fig. 7A). Surprisingly, IRI-SW033291 (Pre) was as effective in ameliorating AKI as IRI-SW033291 (Pre + Post) (Fig. 7, B–E), as assessed by BUN, creatinine, NGAL, and KIM-1, although IRI-SW033291 (Pre) provided somewhat less protection from reduction of RDF-inferred RBF versus IRI-SW033291 (Pre + Post) (Fig. 7F). IRI-SW033291 (Post) was insufficient to ameliorate AKI in the absence of concomitant pretreatment with drug. These findings suggest that a single dose of SW033291 administered before IRI can provide prophylaxis from inducing AKI.
Fig. 7.
15-Hydoxyprostaglandin dehydrogenase inhibitor pretreatment mitigates renal dysfunction after renal ischemia-reperfusion injury (IRI). A: experimental setup for the three different injection protocols. Mice were injected with vehicle or SW033291 (5 mg/kg) according to three different injection protocols. Renal function was assessed at 24 h after renal IRI. B−D: serum levels of blood urea nitrogen (BUN; B), creatinine (C), neutrophil gelatinase-associated lipocalin (NGAL; D), and kidney injury molecule-1 (KIM-1; E). n = 9–11 animals/group. F: renal blood flow. Data are presented as means ± SE. Analysis was performed with Student’s t test.
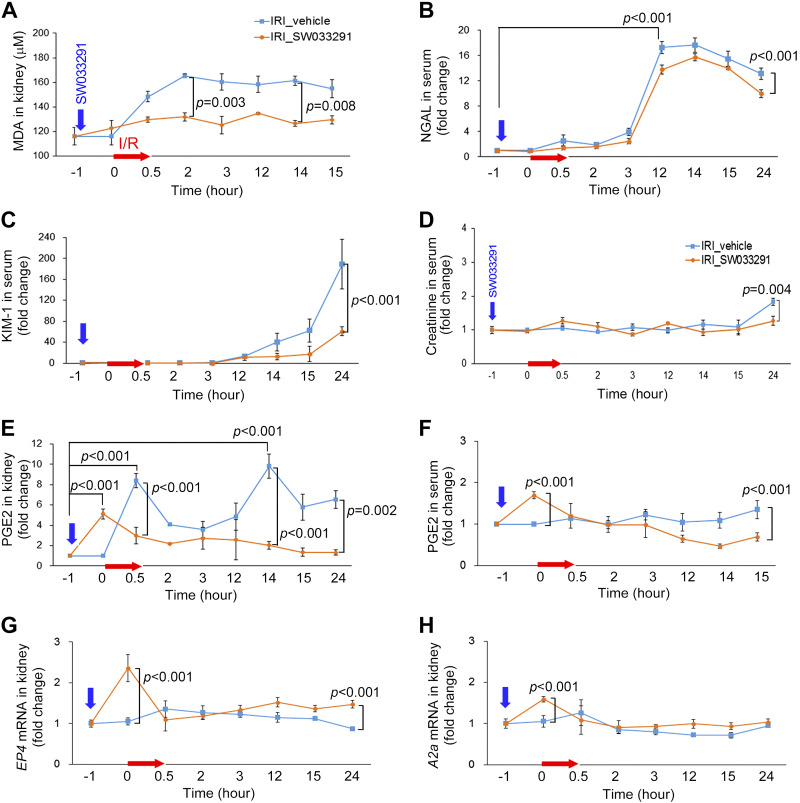
Pretreatment with a single 15-PGDH inhibitor dose attenuates AKI-induced oxidative stress and blocks injury-induced increases in renal PGE2.
MDA, a marker of oxidative stress, began to increase immediately after renal IRI of vehicle-treated mice and peaked at 2 h at 48% above baseline. A single pre-IRI dose of SW033291 blunted and reduced this increase to only 14% (Fig. 8A). IRI-SW033291 (Pre) mice also showed notable protection from the induction of NGAL, KIM-1, and creatinine (Fig. 8, B–D). In vehicle-treated mice, IRI induced two peaks of renal PGE2, an immediate post-IRI peak plus a 14-h post-IRI peak (Fig. 8E). Intriguingly, a single pre-IRI dose of SW033291 induced an early PGE2 peak just before IRI and was then sufficient to substantially block both of the later post-IRI peaks of PGE2 (Fig. 8E). Moreover, the single pretreatment dose of SW033291 also significantly decreased both renal and serum PGE2 levels at 24 h (Fig. 8, E and F). Furthermore, pretreatment with SW033291 increased pre-IRI levels of both EP4 and A2A receptors (Fig. 8, G and H). In overview, induction of endogenous PGE2 by administration of a prophylactic 15-PGDH inhibitor before IRI increases EP4 and A2A receptors, induces vasodilatation, attenuates post-IRI oxidative stress, and, thereby, reduces multiple markers of renal injury.
Fig. 8.
Pretreatment with a single dose of 15-hydoxyprostaglandin dehydrogenase inhibitor attenuates the increase of PGE2 level and renal damage after renal ischemia-reperfusion injury (IRI). Shown are expression changes of related factors in renal tissue or serum after ischemic acute kidney injury. A: malondialdehyde (MDA) levels in kidney tissue. B–D: neutrophil gelatinase-associated lipocalin (NGAL; B), kidney injury molecule-1 (KIM-1; C), and creatinine (D) levels in serum. E and F: renal PGE2 levels (E) and serum PGE2 levels (F). G and H: EP4 receptor (G) and A2A receptor (H) mRNA levels in kidney tissue. n = 4–8 animals/group. Data are presented as means ± SE. Analysis was performed using Student’s t test.
DISCUSSION
In this study, we showed that pretreatment with a 15-PGDH inhibitor (SW033291) markedly protected the kidney from induction of the classic injury hallmarks of ischemic AKI. In particular, we demonstrated that administration of SW033291 improved renal hemodynamics (Fig. 4), decreased induction of oxidative stress (Fig. 8A), reduced induction of inflammation (Fig. 3), attenuated multiple markers of renal damage, and preserved renal function (Figs. 1 and 2). These benefits were also associated with the prophylactic use of a single pre-IRI dose of SW033291, which showed notable protection from induction of NGAL, KIM-1, and creatinine (Fig. 7, C–E). The activity of SW033291 in preventing renal injury is likely to be an on-target effect due to modulation of PGE2, as efficacy of SW033291 was blocked by inhibiting EP4 signaling with ONO-AE3-208 and as treatment with indomethacin, a COX antagonist, and celecoxib, a COX-2 antagonist, acted opposite to SW033291 and further exacerbated renal injury (Figs. 1, 2, and 6, H–L, and Supplemental Fig. S1). SW033291 has been shown to enhance tissue repair in tissue injury models of other organs (48). However, in this study, we do not draw conclusions regarding potential therapeutic activity of SW033291 for use following AKI injury, as this investigation focused on a model of prevention of renal injury and did not test the effects of long-term administration of SW033291given only postinjury.
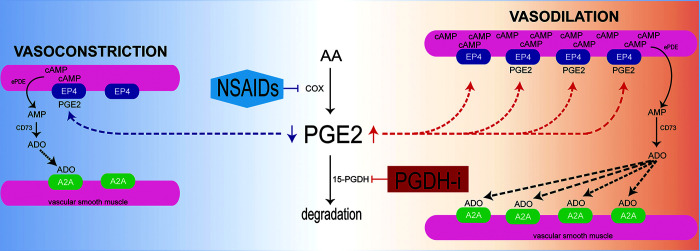
PGE2 is known to regulate renal hemodynamics and inflammation, but its rapid degradation in vivo has been an obstacle to therapeutic applications. Our data demonstrate that pretreatment with a single dose of SW033291 can induce PGE2 and reduce renal damage from ischemic AKI (Fig. 7). Specifically, inhibition of 15-PGDH and increasing endogenous PGE2 before ischemic IRI provides a protective effect in the kidney via increasing renal vasodilation and RBF that would be expected to enhance oxygen delivery capacity and increase resistance to hypoxia. These results suggest that the renal protective effect of PGE2, in part, relates to the timing of its increase. RBF is determined by the vasodilator-vasoconstriction balance, with PGE2 and adenosine both acting as significant endogenous vasoactive mediators (15, 17, 19, 22, 26). PGE2 exerts a vasodilatory effect in the kidney, and the use of NSAIDs in the early postoperative period is associated with renal dysfunction due to reduced RBF from suppression of synthesis of endogenous renal PGE2 (48). Our findings are consistent with observations that in most blood vessels, the vasodilation effect of PGE2 is mediated by the EP4 receptor, which, via coupling to Gαs, can directly activate adenylate cyclase and cAMP production (13, 23, 41, 43, 47). Our results are also consistent with the role of adenosine and its A2A receptor as important regulators of renal vasodilatation and mediators of protection from ischemic AKI (1, 16, 24). Induction of cAMP by SW033291 is, thus, likely the direct result of induction of renal PGE2 and activation of EP4 receptors. Although further study will be required to elucidate the mechanism by which SW033291 also induces EP4 receptors, adenosine, and adenosine A2A receptors, the induction of EP4 receptors and adenosine by SW033291 versus their inhibition by indomethacin strongly suggests that both of these effects are likely downstream of PGE2 signaling.
We recognize certain limitations of this study. We identify that SW033291 is able to persistently induce vasodilatation and vasodilatory factors, such as cAMP and adenosine, for up to 23 h following the peak in drug-induced renal PGE2. However, we do not have a complete understanding of the mechanism of this persistent effect of biological hysteresis. We speculate that the PGE2-induced increase that we found in EP4 and A2A receptors on vascular smooth muscle cells may help maintain the persistent increase in cAMP and vasodilation that we observed, but SW033291 and PGE2 activation of additional pathways may also contribute. We also note that the elevation of PGE2 at 24 h observed in vehicle-treated mice had much less vasodilatory effect than the early induction of PGE2 in SW033291-treated mice. We hypothesize that this may well be due to opposing effects of the multiple inflammatory cytokines that we found induced following IRI in vehicle-treated mice. Additionally, we acknowledge certain technical limitations of these experiments. First, we used an assay of renal Doppler flux, a measure of blood velocity, as an indirect measurement of RBF. We attempted to minimize any variability in RDF by standardizing the position on the renal surface at which these measurements were taken. Second, we measured RDF at the renal cortex and measured arteriolar area in the renal medulla. These assays need not be concordant. However, the finding that after SW033291 treatment, the two independent assays gave concordant results is consistent with SW033291 having a general effect on vasodilation throughout the kidney. Third, we cannot exclude that some of the effects seen with indomethacin and celecoxib may be due to inhibiting production of prostaglandins other than PGE2 (e.g., PGI2, PGD2, PGF2α, and thromboxane A2). However, the reversal of SW033291 effects with the EP4 inhibitor ONO-AE3-208 supports the hypothesis that PGE2 induction is required for and essential to SW033291 protection from renal ischemia. Fourth, SW033291 was administered systemically, and although SW033291 induces both renal PGE2 and renal EP4 receptors, we cannot exclude that some of the effects observed in the kidney may be secondary to systemic effects of increased PGE2. Finally, although we documented effects of SW033291 on renal hemodynamics and demonstrated EP4 receptors on vascular smooth muscle of renal arterioles (Fig. 6G), PGE2 receptors are also expressed on renal tubules (7). Thus, we can not rule out the possibility that direct effects of PGE2 on renal tubules may also have contributed to the protection we observed.
In review, our results support the role of 15-PGDH inhibition in renal vasodilation due to PGE2-dependent increases in PGE2/EP4 and adenosine/A2A receptors plus the induction of cAMP/AMP (Fig. 9). Most significantly, our results demonstrate that enhancement of renal PGE2 by 15-PGDH inhibition before IRI induces renal vasodilation, which we suggest enhances resistance to hypoxia and results in prophylaxis against ischemic AKI. Inhibition of 15-PGDH may provide a novel pharmacological approach for prophylaxis against ischemic AKI in various clinical settings, including renal transplantation, shock, and cardiovascular surgery.
Fig. 9.
Shematic mechanism of intrarenal vasodilation by the 15-hydoxyprostaglandin dehydrogenase (15-PGDH) inhibitor (PGDH-i) in ischemic acute kidney injury. 15-PGDH inhibitor pretreatment increases endogenous PGE2 by inhibiting degradation of PGE2 prior to an ischemic event in the kidney. Endogenous PGE2 induces vasodilation through the activated EP4 receptor. Activation of EP4 receptors increases the intracellular cAMP level in vascular smooth muscle cells and the effect on vasodilation. Increased cAMP is converted to the adenosine (ADO) substrate AMP, which, in turn, increases the endovascular adenosine level. ADO activates A2A receptors to induce vasodilation. On the other hand, nonsteroidal anti-inflammatory drugs (NSAIDs),cyclooxygenase (cox) inhibitors, lead to vasoconstriction, in contrast to the hemodynamic effects of 15-PGDH inhibitors. AA, arachidonic acid; ePDE, extracellular phosphodiesterase; RBC, red blood cell.
GRANTS
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (HI15C2212) (to K.B.B.), the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science (NRF-2015R1D1A4A01019877) (to H.J.K.), the 2015 Research Grant of Inje University Busan Paik Hospital (to K.B.B.), the 2017 creative research program of Inje University (to K.B.B.), and a grant from the National Cancer Institute R35CA197442 (to S.D.M.).
DISCLOSURES
S.M. is a founder, shareholder, consultant, recipient of a sponsored research grant award from, and a member of the Board of Directors of Rodeo Therapeutics and has a royalty interest in technology on the use of 15-PGDH inhibitors for renal protection from technology optioned to Rodeo Therapeutics. These conflicts are overseen and managed by Case Western Reserve University in accord with institutional polices. K.B.B. has a royalty interest in technology on the use of 15-PGDH inhibitors for renal protection from technology optioned to Rodeo Therapeutics. This conflict is overseen and managed by Inje University in accord with institutional polices.
AUTHOR CONTRIBUTIONS
H.K., K.B., S.P., and S.D.M. conceived and designed research; H.K., S.K., M.K., M.K. and D.K. performed experiments; H.K., M.K. and D.K. analyzed data; H.K. and B.K. interpreted results of experiments; H.K. and B.K. prepared figures; H.K. drafted manuscript; K.B., H.B., and S.D.M. edited and revised manuscript; K.B. and S.D.M. approved final version of manuscript.
REFERENCES
- 1.Al-Mashhadi RH, Skøtt O, Vanhoutte PM, Hansen PB. Activation of A2 adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009. doi: 10.1038/ki.2008.684. [DOI] [PubMed] [Google Scholar]
- 1a.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci 33: 304–311, 2012. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y, Doi K, Maehara N, Kusunoki S, Takahata A, Noiri E, Suzuki Y, Yahagi N, Nishiyama A, Gunaratnam L, Takano T, Miyazaki T. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med 22: 183–193, 2016. doi: 10.1038/nm.4012. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 6.Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol 7: 8–17, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Breyer MD, Zhang Y, Guan YF, Hao CM, Hebert RL, Breyer RM. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl 67: S88–S94, 1998. doi: 10.1046/j.1523-1755.1998.06718.x. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggins KG, Latour A, Nguyen MS, Audoly L, Coffman TM, Koller BH. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med 8: 91–92, 2002. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 10.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Eckle T. Ischemia and reperfusion−from mechanism to translation. Nat Med 17: 1391–1401, 2011. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem 278: 12151–12156, 2003. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 14.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875, 2001. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 15.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther 315: 1150–1157, 2005. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- 17.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol 285: F590–F599, 2003. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 18.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 20.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakoki M, Kim HS, Arendshorst WJ, Mattson DL. l-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol Regul Integr Comp Physiol 287: R1478–R1485, 2004. doi: 10.1152/ajpregu.00386.2004. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy-Lydon TM, Crawford C, Wildman SS, Peppiatt-Wildman CM. Renal pericytes: regulators of medullary blood flow. Acta Physiol (Oxf) 207: 212–225, 2013. doi: 10.1111/apha.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan KM, Kothari P, Du B, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase-dependent microsomal prostaglandin E synthase-1 expression in macrophages: role of TNF-α and the EP4 prostanoid receptor. J Immunol 188: 1970–1980, 2012. doi: 10.4049/jimmunol.1102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 25.Landgraf SS, Wengert M, Silva JS, Zapata-Sudo G, Sudo RT, Takiya CM, Pinheiro AA, Caruso-Neves C. Changes in angiotensin receptors expression play a pivotal role in the renal damage observed in spontaneously hypertensive rats. Am J Physiol Renal Physiol 300: F499–F510, 2011. doi: 10.1152/ajprenal.00384.2010. [DOI] [PubMed] [Google Scholar]
- 26.Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv 7: 581–591, 2014. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Mauk RH, Patak RV, Fadem SZ, Lifschitz MD, Stein JH. Effect of prostaglandin E administration in a nephrotoxic and a vasoconstrictor model of acute renal failure. Kidney Int 12: 122–130, 1977. doi: 10.1038/ki.1977.89. [DOI] [PubMed] [Google Scholar]
- 29.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385: 2616–2643, 2015. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 30.Molitoris BA. Actin cytoskeleton in ischemic acute renal failure. Kidney Int 66: 871–883, 2004. doi: 10.1111/j.1523-1755.2004.00818.x. [DOI] [PubMed] [Google Scholar]
- 31.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int 66: 496–499, 2004. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 32.Nasrallah R, Hassouneh R, Hébert RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol 27: 666–676, 2016. doi: 10.1681/ASN.2015050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori K, Kida T, Hori M, Ozaki H, Murata T. Multiple roles of the PGE2-EP receptor signal in vascular permeability. Br J Pharmacol 171: 4879–4889, 2014. doi: 10.1111/bph.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papanicolaou N, Callard P, Bariety J, Milliez P. The effect of indomethacin and prostaglandin (PGE2) on renal failure due to glycerol in saline-loaded rats. Clin Sci Mol Med 49: 507–510, 1975. doi: 10.1042/cs0490507. [DOI] [PubMed] [Google Scholar]
- 35.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative Consensus XIII Work Group . Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27: 371–379, 2016. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 37.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider R, Meusel M, Renker S, Bauer C, Holzinger H, Roeder M, Wanner C, Gekle M, Sauvant C. Low-dose indomethacin after ischemic acute kidney injury prevents downregulation of Oat1/3 and improves renal outcome. Am J Physiol Renal Physiol 297: F1614–F1621, 2009. doi: 10.1152/ajprenal.00268.2009. [DOI] [PubMed] [Google Scholar]
- 39.Sharples EJ, Thiemermann C, Yaqoob MM. Mechanisms of disease: cell death in acute renal failure and emerging evidence for a protective role of erythropoietin. Nat Clin Pract Nephrol 1: 87–97, 2005. doi: 10.1038/ncpneph0042. [DOI] [PubMed] [Google Scholar]
- 40.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol 90: 579–586, 2012. doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang L, Loutzenhiser K, Loutzenhiser R. Biphasic actions of prostaglandin E2 on the renal afferent arteriole : role of EP3 and EP4 receptors. Circ Res 86: 663–670, 2000. doi: 10.1161/01.RES.86.6.663. [DOI] [PubMed] [Google Scholar]
- 42.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 43.Thieme K, Majumder S, Brijmohan AS, Batchu SN, Bowskill BB, Alghamdi TA, Advani SL, Kabir MG, Liu Y, Advani A. EP4 inhibition attenuates the development of diabetic and non-diabetic experimental kidney disease. Sci Rep 7: 3442, 2017. doi: 10.1038/s41598-017-03237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomlinson RE, Shoghi KI, Silva MJ. Nitric oxide-mediated vasodilation increases blood flow during the early stages of stress fracture healing. J Appl Physiol 116: 416–424, 2014. doi: 10.1152/japplphysiol.00957.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21: 1878–1890, 2010. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoyama U, Iwatsubo K, Umemura M, Fujita T, Ishikawa Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev 65: 1010–1052, 2013. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Desai A, Yang SY, Bae KB, Antczak MI, Fink SP, Tiwari S, Willis JE, Williams NS, Dawson DM, Wald D, Chen WD, Wang Z, Kasturi L, Larusch GA, He L, Cominelli F, Di Martino L, Djuric Z, Milne GL, Chance M, Sanabria J, Dealwis C, Mikkola D, Naidoo J, Wei S, Tai HH, Gerson SL, Ready JM, Posner B, Willson JK, Markowitz SD. Tissue regeneration. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348: aaa2340, 2015. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Li Q, Liu D, Huang Q, Cai G, Cui S, Sun X, Chen X. GDF11 improves tubular regeneration after acute kidney injury in elderly mice. Sci Rep 6: 34624, 2016. doi: 10.1038/srep34624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]