Keywords: acute kidney injury, apoptosis, bone morphogenetic protein-7, epigenetic, histone deacetylase 4, lipopolysaccharide
Abstract
Sepsis-associated acute kidney injury (SA-AKI) is associated with high mortality rates, but clinicians lack effective treatments except supportive care or renal replacement therapies. Recently, histone deacetylase (HDAC) inhibitors have been recognized as potential treatments for acute kidney injury and sepsis in animal models; however, the adverse effect generated by the use of pan inhibitors of HDACs may limit their application in people. In the present study, we explored the possible renoprotective effect of a selective class IIa HDAC inhibitor, TMP195, in a murine model of SA-AKI induced by lipopolysaccharide (LPS). Administration of TMP195 significantly reduced increased serum creatinine and blood urea nitrogen levels and renal damage induced by LPS; this was coincident with reduced expression of HDAC4, a major isoform of class IIa HDACs, and elevated histone H3 acetylation. TMP195 treatment following LPS exposure also reduced renal tubular cell apoptosis and attenuated renal expression of neutrophil gelatinase-associated lipocalin and kidney injury molecule-1, two biomarkers of tubular injury. Moreover, LPS exposure resulted in increased expression of BAX and cleaved caspase-3 and decreased expression of Bcl-2 and bone morphogenetic protein-7 in vivo and in vitro; TMP195 treatment reversed these responses. Finally, TMP195 inhibited LPS-induced upregulation of multiple proinflammatory cytokines/chemokines, including intercellular adhesion molecule-1, monocyte chemoattractant protein-1, tumor necrosis factor-α, and interleukin-1β, and accumulation of inflammatory cells in the injured kidney. Collectively, these data indicate that TMP195 has a powerful renoprotective effect in SA-AKI by mitigating renal tubular cell apoptosis and inflammation and suggest that targeting class IIa HDACs might be a novel therapeutic strategy for the treatment of SA-AKI that avoids the unintended adverse effects of a pan-HDAC inhibitor.
INTRODUCTION
Acute kidney injury (AKI) is a syndrome characterized by the rapid deterioration of kidney function and is associated with 70–80% mortality in the intensive care unit (6, 19). AKI can be caused by many insults, and sepsis-associated AKI (SA-AKI) is a frequent complication of critically ill patients (20). The pathogenesis of SA-AKI is complex and involves systemic cytokine production, tubular epithelial cell injury, and endothelial dysfunction (2, 20). Due to limited understanding of pathophysiological mechanisms, there are no effective treatments for SA-AKI (38). Increasingly, evidence indicates that epigenetic processes are critically involved in the pathogenesis of AKI (18, 64). Thus, epigenetic regulators may be potential therapeutic targets for AKI (10, 43, 49, 64).
Epigenetics is defined as inheritable modulation in gene expression without changing DNA sequences (23, 26). Acetylation is a common epigenetic modification that occurs in both histone and nonhistone proteins and is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) (53). An imbalance of HDAC/HAT activity would result in changes in acetylation status. For example, either increased HAT activation or decreased HDAC activity increases protein acetylation (52). Eighteen different HDACs have been reported in humans and are divided into four classes based on their sequence homology: class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8), class II HDACs (class IIa, HDAC4, HDAC5, HDAC7, and HDAC9; class IIb, HDAC6 and HDAC10), class III [sirtuin (SIRT)1–SIRT7], and class IV (HDAC11) (16). Classes I, II, and IV HDACs require zinc ions as cofactors, whereas class III HDACs are NAD+-dependent enzymes (52). The different HDACs have distinct functions, unique cellular/organ distribution, and discrete physiological and pathological effects (52). HDAC inhibitors have been extensively used for the treatment of cancer and are well tolerated by the majority of patients (46).
Efforts have also been made to test the efficacy of HDAC inhibitors for treating other diseases including sepsis (55, 56). Treatment with valproic acid (VPA) or suberoylanilide hydroxamic acid, a class I/II HDAC inhibitor, showed survival benefits to mice with sepsis, as evidenced by prolonged survival time and reduced inflammatory responses and liver injury in a mouse model of cecal ligation and puncture (62); administration of the class IIb HDAC inhibitor tubastatin A (TA) was also effective in improving long-term survival, reducing “cytokine storm” and liver injury and increasing bacterial clearance in this model (31). In contrast, administration of the class I HDAC inhibitor MS-275 showed no survival benefit and organ protection (31). These data indicate that inhibition of HDAC classes IIa or IIb, but not class I, activates prosurvival pathways. However, these studies were limited to investigating the effect of HDAC inhibitors on survival, inflammation, or liver injury, and their efficacy in the treatment of SA-AKI remains unknown.
Nevertheless, there are several studies that have investigated the efficacy of HDAC inhibitors in other models of AKI. For example, treatment with VPA or trichostatin A, a class I/II HDAC inhibitor, inhibited renal tubular cell apoptosis and attenuated renal fibrosis in the animal models of ischemia-reperfusion (I/R), cold ischemia, or cisplatin-induced AKI (12, 30, 34). Administration of the class IIb HDAC inhibitor TA or 23BB was also effective in inhibiting renal damage and improved renal function in rhabdomyolysis- or cisplatin-induced AKI (13, 48, 50). We also examined the effect of the class I HDAC inhibitor MS-275 on rhabdomyolysis- and folic acid-induced AKI and found that MS-275 treatment aggravated renal damage and accelerated renal dysfunction (51). These studies indicated that HDAC inhibitors may have both renoprotective and detrimental effects in AKI (4, 39), depending on which class or isoform of HDACs is inhibited. The role of class IIa HDACs in SA-AKI has not been explored yet due to lack of effective and class-selective inhibitors.
Recently, TMP195, a first-in-class highly selective class IIa HDAC inhibitor, was developed (33). In this study, we examined the effect of TMP195 on SA-AKI in a murine model of AKI induced by lipopolysaccharide (LPS), which has been shown to induce the systemic inflammatory response syndrome with AKI and is widely used for mimicking sepsis in drug development (29, 44).
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies to acetyl-H3K14, cleaved caspase-3, and bone morphogenetic protein-7 (BMP-7) were purchased from Cell Signaling Technology (Danvers, MA). Anti-T cell immunoglobulin and mucin-containing molecule (TIM)-1 [kidney injury molecule-1 (KIM-1)] and anti-CD68 antibodies were obtained from Abcam (Cambridge, MA). Antibodies to neutrophil gelatinase-associated lipocalin (NGAL) and HDAC4 were purchased from R&D Systems (Minneapolis, MN). α-Tubulin antibodies, secondary antibodies, and all other chemicals were purchased from Sigma (St. Louis, MO). Anti-phosphorylated (p-)Bcl-2, anti-BAX, anti-β-actin, and anti-GAPDH antibodies were acquired from Santa Cruz Biotechnology (Dallas, TX).
Animals.
Male C57/black mice that weighed 20–25 g (Jackson Laboratory, Bar Harbor, ME) were housed under a 12:12-h light-dark cycle with free access to water and standard food. All animal experiments were performed in accordance with national and institutional animal care and ethical guidelines and were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital. Mice were divided into the following four groups: control, control + TMP195, LPS, and LPS + TMP195. LPS (Sigma, 20 mg·kg−1·day−1 and 1 mg/mL in 0.9% normal saline) was intraperitoneally injected, and the same volume of 0.9% normal saline alone was used as the control. TMP195 at 50 mg·kg−1·day−1 (3 mg/mL dissolved in 10% DMSO and 90% corn oil in accordance with the manufacturer’s instructions) was intraperitoneally injected immediately after the LPS injection. An equal volume of the solvent was injected in the control and LPS-alone groups. Mice were euthanized at 24 h after various treatments, and kidneys were collected for subsequent analysis. The doses of LPS and TMP195 used in this study were selected according to previous reports (17, 28).
Measurement of renal function.
Blood urea nitrogen (BUN) and serum creatinine levels were measured to estimate renal function. Colorimetric kits for BUN and creatinine (Abcam) were used to measure the serum in accordance with the manufacturer’s instructions.
Quantitative real-time PCR.
Real-time quantitative RT-PCR amplifications were performed in 20-μL reactions. The forward and reverse primers of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), and GAPDH were taken from our previous publication (40) and are shown in Table 1. RNA was isolated from frozen kidney cortex samples using an RNA isolation kit (Biovendor) in accordance with the manufacturer’s protocol. cDNA was reverse transcribed with a high-capacity RNA-to-cDNA kit (ThermoFisher Scientific). Quantitative PCR was performed using the Power SYBR Green PCR Master Mix and Power SYBR Green RT-PCR reagent kits (Life Technologies, Carlsbad, CA). The thermocycle program was set as previously described (40). The expression level of each gene was calculated using the ΔΔCt method (where Ct is threshold cycle) relative to GAPDH.
Table 1.
Primers for detection of TNF-α, IL-1β, MCP-1, and ICAM-1 by RT-PCR
| Gene | Sequences (Forward) | Sequences (Reverse) |
|---|---|---|
| TNF-α | 5′-TAGCCAGGAGGGAGAACAGA-3′ | 5′-TTTTCTGGAGGGAGATGTGG-3′ |
| IL-1β | 5′-CCCAAGCAATACCCAAAGAA-3′ | 5′-GCTTGTGCTCTGCTTGTGAG-3′ |
| MCP-1 | 5′-AGCACCAGCCAACTCTCACT-3′ | 5′-CGTTAACTGCATCTGGCTGA-3′ |
| ICAM-1 | 5′-CTTCCAGCTACCATCCCAAA-3′ | 5′-CTTCAGAGGCAGGAAACAGG-3′ |
| GAPDH | 5′-GGTGAAGGTCGGTGTGAACG-3′ | 5′-CTCGCTCCTGGAAGATGGTG-3′ |
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; MCP-1, monocyte chemoattractant protein-1; ICAM-1, intracellular adhesion molecule-1.
Pathological analysis.
Three micrometer-thick sections of paraffin-embedded kidney tissue were stained with periodic acid-Schiff (PAS) and examined through light microscopy. Ten consecutive interstitial fields were chosen randomly. Tubulointerstitial injury was described as interstitial inflammation and fibrosis, tubular atrophy, and dilation with cast formation. The tubulointerstitial injury index was scored on the basis of the injured areas as follows: 0, normal; 1, lesion area < 30% of the field; 2, lesion area equal to 30–60% of the field; and 3, lesion area > 60% of the field (×200). Paraffin-embedded kidney tissue was used for immunofluorescent and immunohistochemical staining based on the protocol introduced in our previous study (39) with antibodies to HDAC4 (1:200), cleaved caspase-3 (1:100), KIM-1 (1:200), and CD68 (1:200) (Table 2). For assessment of NGAL and KIM-1 expression quantitatively, the positive area was measured after staining using Image-Pro-Plus 6.0 image-analysis software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of the positive staining area and calculating and graphing the average ratio to each microscopic field (×400).
Table 2.
Antibodies used for immunofluorescence and immunohistochemical staining
| Antibody | Catalog No. | Supplier | Dilution |
|---|---|---|---|
| TIM1 (KIM-1) | 447766335 | Abcam (Cambridge, MA) | 1:200 |
| CD68 | 125212 | Abcam | 1:200 |
| NGAL | AF1857 | R&D Systems (Minneapolis, MN) | 1:200 |
| HDAC4 | AF6205 | R&D Systems | 1:200 |
| Cleaved caspase-3 | 9664 | Cell Signaling Technology (Danvers, MA) | 1:100 |
TIM-1, T cell immunoglobulin and mucin-containing molecule-1; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; HDAC4, histone deacetylase 4.
TUNEL staining of renal tubular cells.
Renal tubular epithelial cell apoptosis was performed with a TUNEL Apoptosis Assay Kit-FITC (Abcam). Kidney tissue was stained in accordance with the manufacturer’s instructions. Apoptosis of renal tubular epithelial cells was estimated as TUNEL-positive cells per high power field (×200).
Cell culture and reagents.
The murine renal proximal tubular epithelial cell line (TKPT cells) was kindly provided by Dr. E. Bello-Reuss (University of Texas Medical Branch, Galveston, TX) and inoculated in DMEM-F-12 that contained 5% FBS at 37°C in a humidified atmosphere of 5% CO2. Cells were seeded on six-well BioFlex collagen-coated culture plates, grown to 80% confluence, and then starved with DMEM-F-12 without FBS for 24 h. Starved cells were pretreated with TMP195 (1 μM) or vehicle (saline) for 1 h and then exposed to 10 μg/mL LPS (Sigma) for an additional 24 h before being harvested for protein analysis.
Western blot analysis.
Lysates of frozen kidney tissue and cultured cells were separated through 10% or 12% SDS-PAGE and then transferred to PVDF membranes. PVDF membranes were blocked with 5% milk at room temperature for 1 h and incubated with antibodies to HDAC4 (1:1,000), acetyl-H3K14 (1:500), NGAL (1:1,000), p-Bcl-2 (1:1,000), BMP-7 (1:1,000), BAX (1:1,000), and cleaved caspase-3 (1:500) (Table 3) at 4°C overnight.
Table 3.
Details of primary antibodies used for Western blot analysis
| Antibody | Catalog No. | Supplier | Dilution |
|---|---|---|---|
| Acetyl H3K14 | 7627 | Cell Signaling Technology (Danvers, MA) | 1:500 |
| Cleaved caspase-3 | 9664 | Cell Signaling Technology | 1:500 |
| BMP-7 | 4693 | Cell Signaling Technology | 1:1,000 |
| NGAL | AF1857 | R&D Systems (Minneapolis, MN) | 1:1,000 |
| α-Tubulin | T6199 | Sigma (St. Louis, MO) | 1:1,000 |
| HDAC4 | AF6205 | R&D Systems | 1:1,000 |
| p-Bcl-2 | 377576 | Santa Cruz Biotechnology (Dallas, TX) | 1:1,000 |
| BAX | 7480 | Santa Cruz Biotechnology | 1:1,000 |
| β-Actin | 47778 | Santa Cruz Biotechnology | 1:1,000 |
| GAPDH | 365062 | Santa Cruz Biotechnology | 1:1,000 |
BMP-7, bone morphogenetic protein-7; NGAL, neutrophil gelatinase-associated lipocalin; HDAC4, histone deacetylase 4; p-Bcl-2, phosphorylated Bcl-2.
Statistical analysis.
Values were presented as means ± SE. Statistical analysis was performed with one-way ANOVA followed by Tukey’s multiple-comparison test. P < 0.05 was considered significant. All experiments were repeated at least three times.
RESULTS
TMP195 treatment ameliorates renal dysfunction in a murine model of LPS-induced AKI.
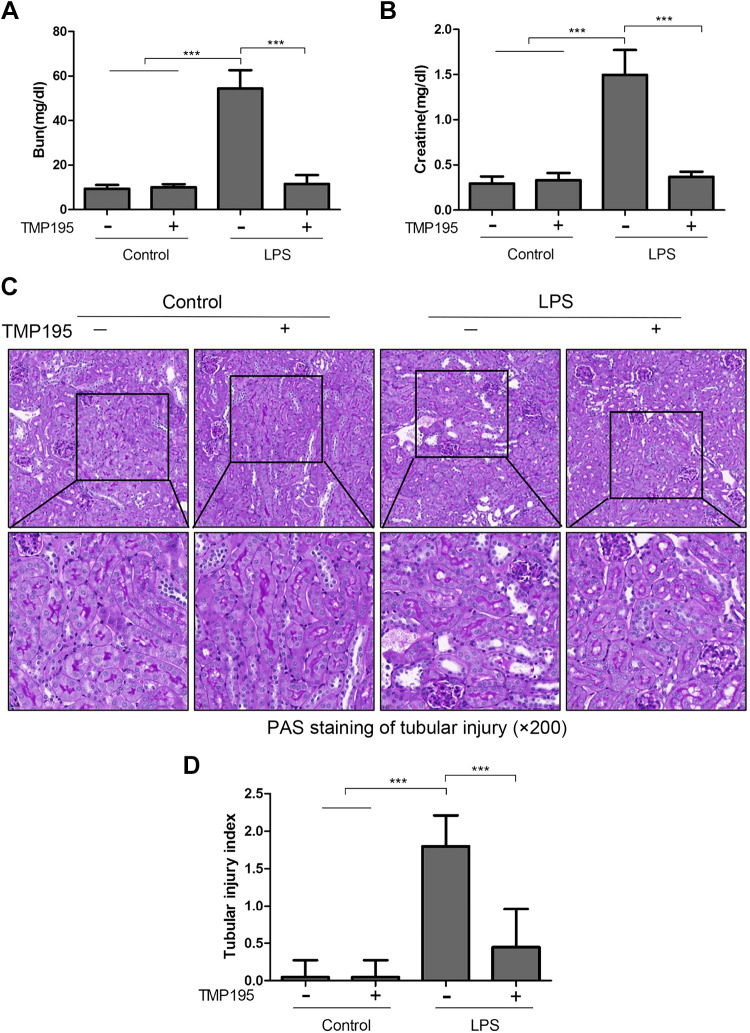
LPS is the major component of the outer membrane of gram-negative bacteria and a key factor in sepsis (septicemia) in humans (45). To investigate the therapeutic effect of TMP195 on SA-AKI, LPS (20 mg/kg) was injected into mice intraperitoneally to induce AKI. Mice were then treated with TMP195 or vehicle. Blood samples and kidney tissue were collected 24 h after LPS injection for assessment of renal function and pathological changes. As shown in Fig. 1, BUN levels in the LPS group were much higher than that in the control group (54.42 ± 8.226 vs. 9.423 ± 1.652 mg/dL). TMP195 treatment reduced the BUN level to 11.55 ± 3.957 mg/dL (P < 0.001). Similarly, the serum creatinine level was 1.497 ± 0.2759 mg/dL in the LPS-alone group, which was higher than that in the control group (0.2960 ± 0.07638 mg/dL). TMP195 treatment significantly reduced the serum creatinine level to 0.3698 ± 0.05650 mg/dL (P < 0.001; Fig. 1, A and B). PAS staining showed LPS-induced kidney damage, as indicated by dilated tubules with cast formation, cell shedding, and tubular atrophy; TMP195 administration ameliorated these pathological changes. There were no significant pathological changes in the sham-operated kidney with and without TMP195 treatment. The pathological score was calculated by averaging the grades assigned to tubules in all fields (P < 0.001; Fig. 1, C and D). TMP195 treatment also significantly reduced the pathological score in the kidney of mice exposed to LPS. Collectively, our data indicate that selective inhibition of class IIa HDACs is able to improve renal function and attenuate the renal damage induced by LPS.
Fig. 1.
TMP195 administration protects against lipopolysaccharide (LPS)-induced acute kidney injury (AKI) in mice. A and B: after various treatments for 24 h, as indicated in materials and methods, blood was collected and serum creatinine and blood urea nitrogen (BUN) were measured. C: periodic acid-Schiff (PAS) staining of the kidneys (magnfication: ×200). D: morphological changes were scored based on the scale described in material and methods. Data are presented as means ± SE; n = 5. ***P < 0.001.
TMP195 is effective in inhibiting the LPS-induced increase of HDAC4 expression and activation in the kidney.
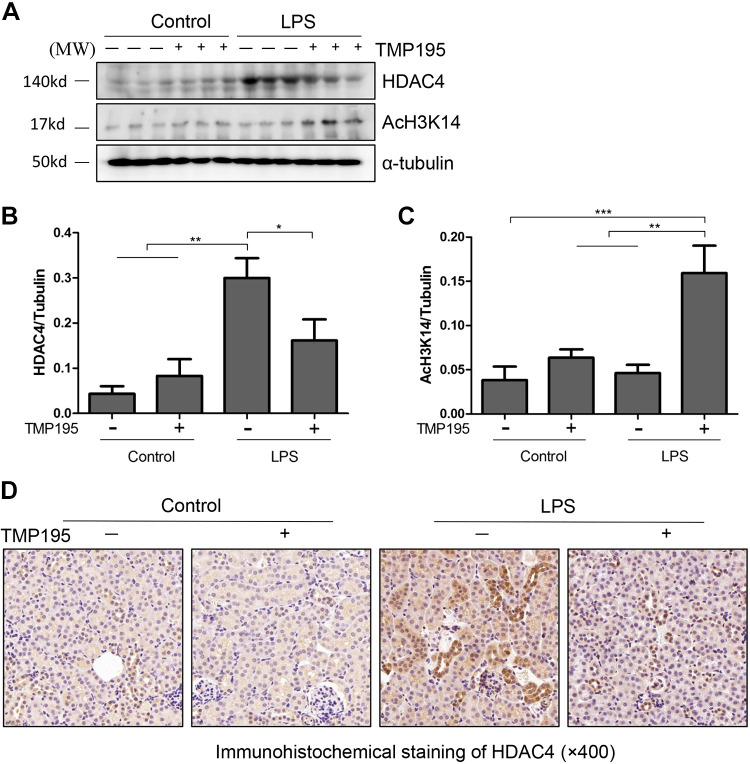
Recently, we and others have shown that among four class IIa HDAC members, only HDAC4 was abundantly expressed in the kidney after unilateral ureteral obstruction (UUO) (57) or I/R injury (21). On this basis, we examined the expression of HDAC4 in the kidney of mice after LPS injection and treatment with TMP195. As shown in Fig. 2, A and B, LPS injection dramatically increased the expression of HDAC4, which was partially reduced by TMP195 treatment. Although LPS injection did not significantly affect expression levels of acetyl histone H3K14, TMP195 administration increased its expression (Fig. 2, A and C). Immunohistochemical staining also showed increased expression of HDAC4 protein in the kidney after LPS injection, and this was inhibited by TMP195 treatment. Notably, HDAC4 was mainly distributed in the cytosol of renal tubular cells (Fig. 2D). This was consistent with its cellular location in the kidney after injury by UUO (57). These data illustrated that TMP195 can effectively suppress HDAC4 expression and induce histone H3 acetylation.
Fig. 2.
TMP195 inhibits histone deacetylase (HDAC)4 expression and promotes histone H3 acetylation in the murine model of lipopolysaccharide (LPS)-induced acute kidney injury (AKI). A: after various treatments for 24 h, as indicated in materials and methods, kidney tissue was collected and tissue lysates were subjected to immunoblot analysis with specific antibodies against HDAC4, acetyl-histone H3K14, or α-tubulin. B and C: expression levels of HDAC4 (B) and acetyl-histone H3K14 (C) were quantified by densitometry and normalized with α-tubulin. Representative immunoblots are three samples in each group. *P < 0.05; **P < 0.01; ***P < 0.001. D: immunohistochemical staining showed that HDAC4 expression was increased in the kidney with LPS injection and inhibited by TMP195 (magnification: ×400).
TMP195 treatment reduces NGAL and KIM-1 expression in the kidney with LPS-induced AKI.
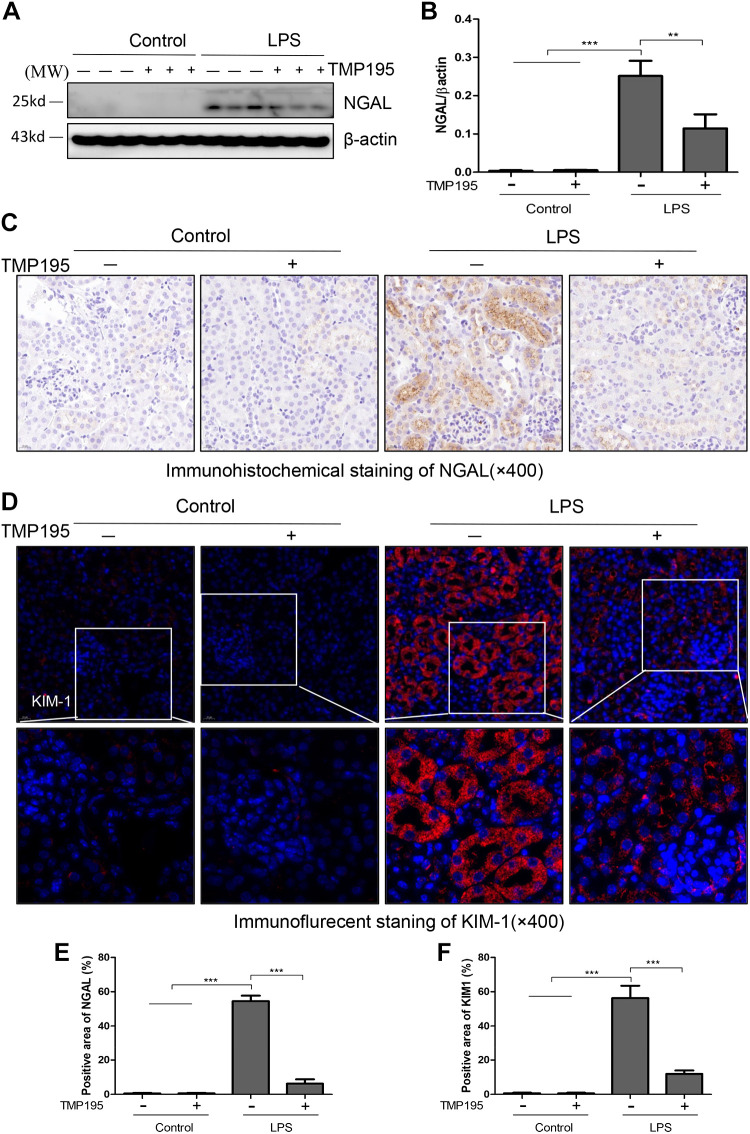
NGAL and KIM-1 are two commonly used biomarkers to reflect renal tubular damage (36). We examined the effect of TMP195 on their expression in the kidney with and without LPS injection. Immunoblot analysis did not detect a signal of NGAL in the sham-operated kidney, but abundant NGAL expression was observed in the kidney with LPS-induced AKI. TMP195 treatment reduced its expression (Fig. 3, A and B). Immunohistochemical staining also confirmed increased expression of renal NGAL in mice with LPS-induced AKI and the inhibitory effect of TMP195 on NGAL expression (Fig. 3, C and E). In line with this observation, TMP195 was also effective in inhibiting the expression of KIM-1 by immunofluorescent staining (Fig. 3, D and F).
Fig. 3.
TMP195 inhibits renal neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) expression in the murine model of lipopolysaccharide (LPS)-induced acute kidney injury (AKI). A: after various treatments for 24 h, as indicated in materials and methods, kidney tissues were collected and tissue lysates were subjected to immunoblot analysis with specific antibodies against NGAL or β-actin. B: expression levels of NGAL were quantified by densitometry and normalized with β-actin. Representative immunoblots are three samples in each group. C and D: immunostaining showed that NGAL or KIM-1 expression was increased in the kidney of mice with LPS injection and inhibited by TMP195. E and F: tubules with positive NGAL (E) or KIM-1 (F) staining were counted in 10 high-power fields (×400) and expressed as means ± SD. **P < 0.01; ***P < 0.001.
TMP195 ameliorates renal tubular apoptosis in the kidney with LPS-induced AKI.
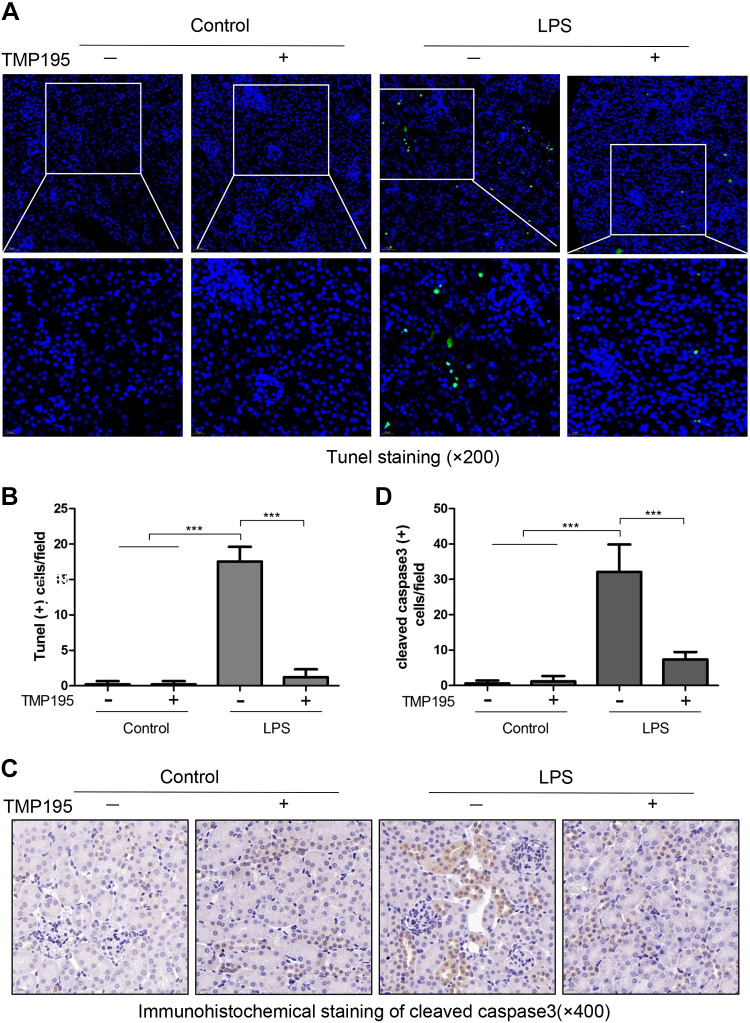
To investigate the effect of TMP195 on apoptosis of renal tubular epithelial cells in LPS-induced AKI, we performed TUNEL staining of kidney tissue. The number of TUNEL-positive cells increased in renal tubular cells of mice exposed to LPS; TMP195 treatment completely blocked this response. Calculation of TUNEL-positive cells indicated that TMP195 largely reduced the number of TUNEL-positive cells in the kidney with LPS administration (Fig. 4, A and B). Immunohistochemical staining also demonstrated an increase in the number of renal tubular cells with cleaved caspase-3 in mice following LPS injection; this reduced significantly by TMP195 treatment (Fig. 4, C and D). These data, together with the efficacy of TMP195 in suppressing NGAL and KIM-1 expression, suggest that TMP195 acts as a potent agent to inhibit renal tubular cell injury and death.
Fig. 4.
TMP195 ameliorates renal tubular apoptosis in the murine model of lipopolysaccharide (LPS)-induced acute kidney injury (AKI). A: after various treatments for 24 h, as indicated in materials and methods, kidney tissue was collected and subjected to TUNEL staining. B: TUNEL-positive cells were calculated and expressed as the number per high-power field. C and D: immunohistochemical staining of cleaved caspase-3 expression in renal tubular epithelial cells (C) and cleaved caspase-3-positive cells was calculated and expressed as the number per high-power field (D) (magnification: ×200). ***P < 0.001.
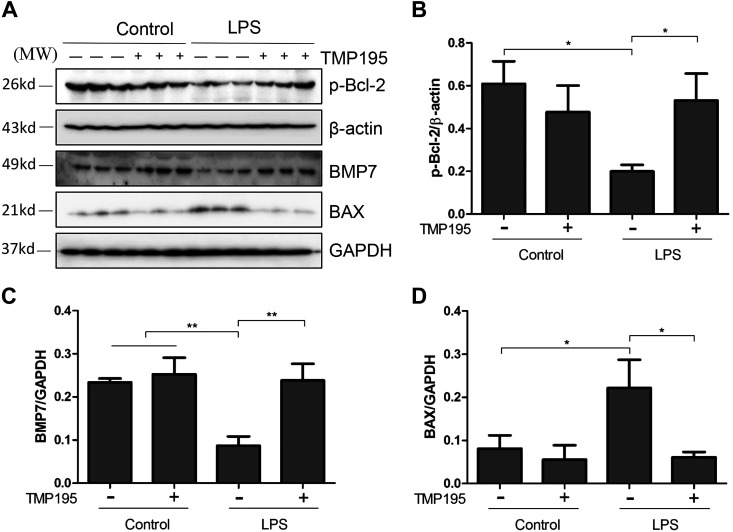
TMP195 inhibited expression of BAX and preserves expression of p-Bcl-2 and BMP-7 in the kidney with LPS-induced AKI.
BAX is believed to interact with mitochondrial membranes and induce damage, whereas Bcl-2 and BMP-7 are two renoprotective proteins (8). It has been documented that Bcl-2 phosphorylation increases cell survival and that BMP-7 is able to protect the kidney against renal injury induced by multiple stimuli (8, 35). To understand whether TMP195-elicited renoprotection is related to regulation of these proteins, we examined the effect of TMP195 on the expression of BAX and BMP-7 as well as phosphorylation of Bcl-2. As shown in Fig. 5, A–D, p-Bcl-2, BAX, and BMP-7 expression levels were detected in the normal kidney; LPS administration led to decreased Bcl-2 phosphorylation and BMP-7 expression but increased Bax2 expression. TMP195 treatment largely reversed these responses. It should be noted that TMP195 could significantly enhance the expression of BMP-7 in the control kidney. These data suggest that TMP195 may inhibit apoptosis through a mechanism associated with suppression of BAX, preservation of p-Bcl-2, and upregulation of BMP7.
Fig. 5.
TMP195 inhibits expression of BAX, restores expression of phosphorylated (p-)Bcl-2, and promotes expression of bone morphogenetic protein-7 (BMP-7) in the murine kidney of lipopolysaccharide (LPS)-induced acute kidney injury (AKI). A: after various treatments for 24 h, as indicated in materials and methods, kidney tissue was collected and tissue lysates were subjected to immunoblot analysis with specific antibodies against p-Bcl-2, β-actin, BMP-7, BAX, or GAPDH. B: expression levels of p-Bcl-2 were quantified by densitometry and normalized with β-actin. C and D: expression levels of BMP-7 and BAX were quantified by densitometry and normalized with GAPDH. Representative immunoblots are three samples in each group. *P < 0.05; **P < 0.01.
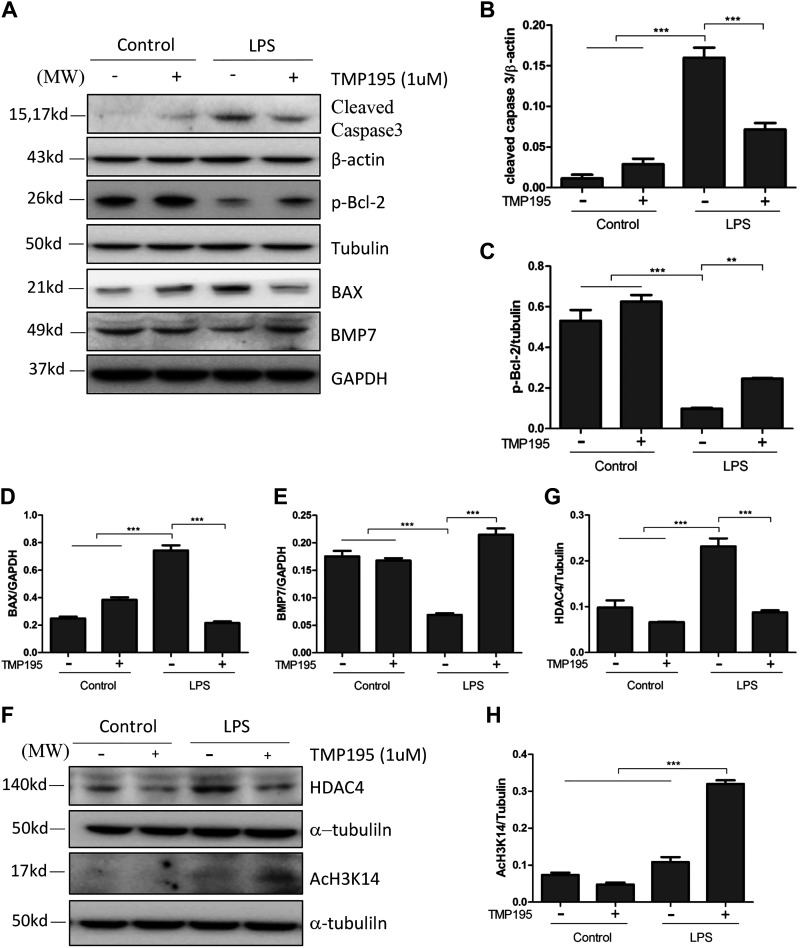
TMP195 inhibited the LPS-induced increase of cleaved caspase-3 and BAX and preserved expression of p-Bcl-2 and BMP-7 in cultured proximal renal tubular cells exposed to LPS.
Having shown that TMP195 had an antiapoptotic effect in LPS-induced AKI, we further examined the effect of this agent on the expression of apoptosis-related proteins in cultured renal epithelial cells. Consistent with its role in vivo, LPS induced increased expression of BAX and cleaved caspase-3 and reduced the expression of p-Bcl-2 and BMP-7. TMP195 treatment inhibited caspase-3 cleavage and reduced BAX to the basal level, whereas it prevented BMP-7 downregulation and partially restored p-Bcl-2 levels (Fig. 6, A–E). In addition, TMP195 was sufficient to inhibit LPS-induced HDAC4 upregulation, resulting in greater histone acetylation of histone H3 (Fig. 6, F–H). These in vitro data enforce the renoprotective effect of TMP195 in the kidney.
Fig. 6.
Effect of TMP195 on the expression of BAX, phosphorylated (p-)Bcl-2, bone morphogenetic protein-7 (BMP-7), histone deacetylase (HDAC)4, and acetyl histone H3K14 in cultured murine renal epithelial cells exposed to lipopolysaccharide (LPS). Serum-starved murine proximal tubular cells were exposed to LPS for 24 h in the absence or presence of TMP195 and then harvested. Cell lysates were subjected to immunoblot analysis with antibodies to cleaved caspase-3, p-Bcl-1, BAX, BMP-7, β-actin, α-tubulin, or GAPDH (A); HDAC4 and acetyl-H3K14 (F); as well as HDAC4, acetyl-histone H3K14, and α-tubulin. B: expression levels of cleaved caspase-3 were quantified by densitometry and normalized with β-actin. C: expression levels of p-Bcl2 were quantified by densitometry and normalized with α-tubulin. D and E: expression levels of BAX (D) and BMP-7 (E) were quantified by densitometry and normalized with GAPDH. G and H: expression levels of HDAC4 (G) and acetyl histone H3K14 (H) were quantified by densitometry and normalized with α-tubulin. **P < 0.01; ***P < 0.001.
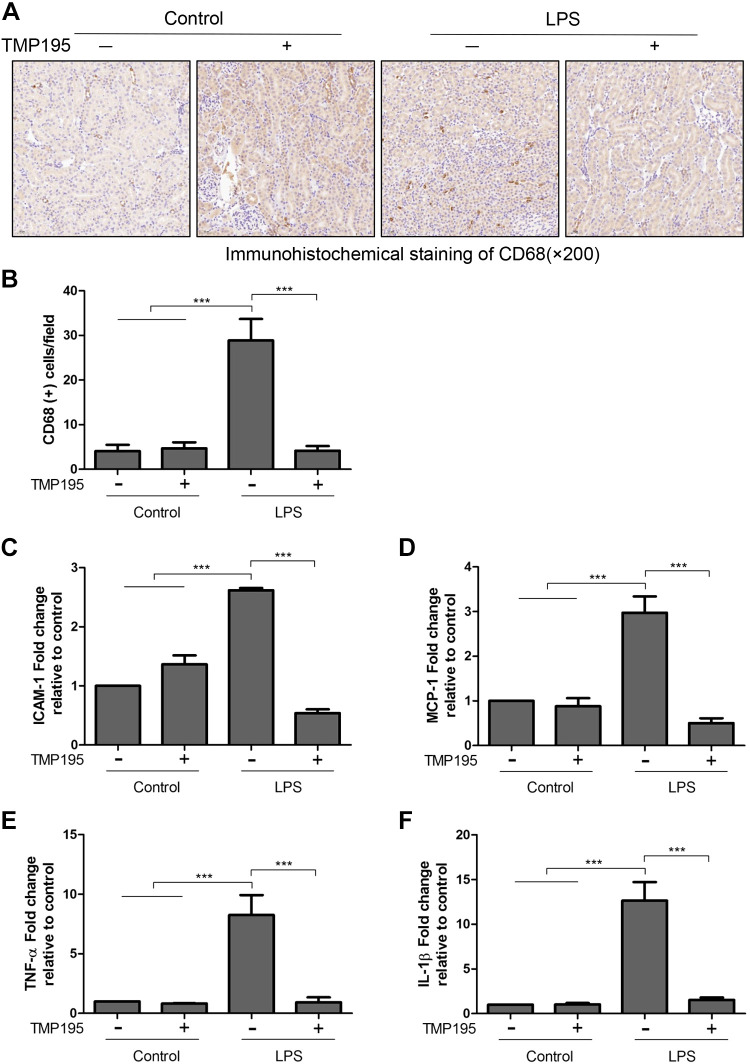
TMP195 inhibits infiltration of macrophages and expression of cytokines and chemokines in the kidney with LPS-induced AKI.
TMP195 has been reported to regulate macrophage differentiation (30). We hypothesized that this agent would also be able to influence infiltration of macrophages and production of some proinflammatory factors. Immunohistochemical staining showed that accumulation of CD68-positive monocytes and macrophages in the renal interstitium of mice exposed to LPS was reduced by TMP195 (Fig. 7, A and B). The RT-PCR analysis also indicated that LPS exposure increased the expression of ICAM-1, MCP-1, TNF-α, and IL-1β in the kidney, which was largely suppressed by TMP195 treatment (Fig. 7, C–F). Thus, inhibition of inflammatory responses may also contribute to TMP195-elicited renal protection in LPS-induced AKI.
Fig. 7.
TMP195 inhibits infiltration of macrophages and reduces expression of multiple proinflammatory factors in a murine kidney model of lipopolysaccharide (LPS)-induced acute kidney injury (AKI). After various treatments for 24 h, as indicated in materials and methods, kidney tissue was collected and subjected to immunohistochemical staining for CD68-positive monocyte macrophages (A), which were expressed as the number per high-power field (B) (magnification: ×200). C−F: RT-PCR was conducted for intracellular adhesion molecule-1 (ICAM-1; C), monocyte chemoattractant protein-1 (MCP-1; D), TNF-α, tumor necrosis factor-α (TNF-α; E), and interleukin-1β (IL-1β; F), and the value for each factor was expressed as a percentage of the control sample. ***P < 0.001.
DISCUSSION
Sepsis is a leading cause of mortality in intensive care units. Patients who survive the acute episode of blood loss also exhibit a systemic inflammatory response syndrome, leading to AKI (6, 42). Although prior work has shown that treatment with pan HDAC inhibitors and some isoform-selective HDAC inhibitor offers a survival advantage and attenuated expression of proinflammatory mediators (62), there is still a lack of studies on the role of class IIa HDACs in SA-AKI. In this study, we demonstrated that HDAC4, a major isoform of class IIa HDAC, was highly expressed in the kidney with SA-AKI induced by LPS. We showed that TMP195 treatment improved renal dysfunction, attenuated tubular cell damage and apoptosis, alleviated the expression of multiple cytokines/chemokines, and reduced macrophage infiltration in the kidney of LPS-induced AKI in mice. These results suggest that class IIa HDAC activity was involved in the injury and death of renal epithelial tubular cells and the inflammatory responses of the kidney after acute septic injury. Thus, targeting class IIa HDACs might be a potential therapeutic approach for SA-AKI.
This study is the first to demonstrate that SA-AKI can be attenuated by a class IIa HDAC inhibitor. Previously, it has been reported that treatment with VPA, a nonselective HDACI that predominantly inhibits HDAC classes I and IIa (9) and improves outcomes in various animal models of lethal hemorrhage, sepsis, and combined insults (14, 32, 37). VPA treatment also improves renal function and attenuates renal damage and inflammatory responses in animal models of AKI induced by sepsis (63) and I/R (3). These data suggest that inhibition of classes I and IIa may contribute to the VPA-elicited renoprotective effect. However, later studies showed that selective inhibition of class I HDACs by MS275 failed to improve survival in a hemorrhagic shock model (11) and accelerated renal dysfunction and kidney damage in murine models of nephrotoxin and rhabdomyolysis-induced AKI (51). These findings, together with the results from the current study, strongly suggest that the VPA-associated survival advantage and renoprotective effect may result from inhibition of class IIa rather than class I HDACs. Supporting this statement, recent studies also showed that treatment with MC1568, another class IIa HDAC inhibitor, attenuated renal tubular damage and renal fibrosis induced by ureteral obstruction and improved survival in a rat model of hemorrhagic shock (11).
In humans, four class IIa HDAC isoforms (10, 23, 38, 64) have been identified. Unlike class I HDACs, which are ubiquitously distributed, class IIa HDACs are more tissue specific and mainly expressed in the heart, muscle, and brain (41). Recently, we and others compared their expression in normal and injured adult kidneys and found that HDAC5, HDAC7, and HDAC9 were expressed at low levels in the normal kidney and slightly increased following acute or chronic kidney injury, but only HDAC4 was dramatically upregulated (21, 57). In this study, we particularly examined the effect of LPS on the expression of HDAC4 in the mouse kidney and found that LPS exposure also increased its expression, whereas TMP195 treatment reduced its expression, which was accompanied by increased histone H3 acetylation. These data imply that HDAC4 might be the major isoform of class IIa HDACs that mediate the pharmacological effect of TMP195 in injured kidneys. In line with this speculation, overexpressing activated HDAC4 in mice promoted myocardial I/R injury and exacerbates cardiac dysfunction (59, 61), whereas specific inhibition of HDAC4 by transplantation of HDAC4 siRNA-treated cardiac stem cells facilitated myocardial repair and preserved cardiac performance (60). In addition, HDAC4 knockdown with siRNA improved the survival of oxygen-glucose deprivation-treated neurons (58). To date, there is still no specific HDAC4 inhibitor that can be used to identify the role of HDAC4 in AKI, and global HDAC4 knockout mice are not viable. Further investigation using HDAC4 conditional knockout mice may help address the role of HDAC4 in AKI and other pathological conditions.
Currently, mechanisms of class IIa HDAC inhibition-elicited protection against SA-AKI remain incompletely understood. Our data suggested that inhibition of renal tubular apoptosis plays an essential role. This is evident by our observations that TMP195 treatment inhibits expression of NGAL and KIM-1, two major biomarkers of tubular injury, and reduced the number of TUNEL-positive tubular cells in the kidney. TMP195 also effectively inhibited the expression of BAX and cleaved caspase-3, two mediators of apoptotic signaling pathways, in the kidney and cultured renal tubular cells. Moreover, TMP195 reversed LPS-induced dephosphorylation of Bcl-2 and downregulation of BMP-7 expression. Thus, it appears that TMP195 is capable of suppressing the function of proapoptotic proteins and facilitates the action of antiapoptotic proteins. Although the mechanism of how TMP195 regulates the expression of these proteins positively or negatively is not clear, the reciprocal regulation of the expression of proapoptotic and antiapoptotic proteins may create a prosurvival phenotype of renal tubular cells and help them resist injury and apoptosis.
Another mechanism responsible for TMP195-induced renoprotection may be through suppression of inflammation. In response to LPS, endothelial and renal tubular cells release a large amount of proinflammatory cytokines and chemokines, such as TNF-α, IL-6, MCP-1, and ICAM-1. In this study, we found that TMP195 treatment suppressed renal expression of all those proinflammatory cytokines/chemokines in mice with LPS-induced AKI. In addition, TMP195 was also effective in inhibiting accumulation of macrophages in the injured kidney. In agreement with our observations, administration of some pan inhibitors with an inhibitory effect on class IIa HDACs also reduced inflammatory responses in other animal models of AKI, such as I/R (3, 12), animal models of other organ injury (i.e., acute lung injury) (7, 15, 24, 27), or LPS-induced multiple organ dysfunction (47). Thus, the decrease in inflammatory cytokines may be an important mechanism by which class IIa HDAC inhibition mediates organ protection. The mechanism by which TMP195 suppresses the inflammatory responses remains unclear but may be associated with its inhibition of the expression and activation of NF-κB, a transcription factor related to production of multiple inflammatory cytokines/chemokines. A very recent study showed that TMP195 inhibits activation of IKK and inflammatory responses (5). Our recent studies also demonstrated that treatment with MC1568, another selective inhibitor of class IIa HDACs, reduces the phosphorylation level of NF-κB in the kidney after UUO (57).
It has been previously reported that endogenously expressed BMP-7 plays a pivotal role in protection of the kidney from injuries and is required for renal regeneration and prevention of renal fibrosis (54). We speculated that the renoprotective effect of TMP195 may also be associated with regulation of BMP-7 expression. In this study, we found that TMP195 treatment not only prevented LPS-induced BMP-7 downregulation but also enhanced its expression. Thus, preservation of BMP-7 by class IIa HDAC inhibition may provide dual effects to the injured kidney: early protection and late fibrosis prevention. The antifibrotic effect of BMP-7 has been extensively studied in various models of chronic kidney disease. Our recent study also showed that inhibition of class IIa HDACs by MC1568 prevented downregulation of BMP-7 in the kidney after UUO injury (57). Thus, prevention of BMP-7 downregulation by pharmacological modulation like inhibition of class IIa HDACs may have important implications for the treatment of acute and chronic kidney injury.
Currently, there is still no HDAC-based approach or agent available for treatment of kidney disease. However, growing evidence suggests that inhibition of class II HDACs may have therapeutic potential. Our previous and current studies have demonstrated that class IIa HDACs mediate both acute and chronic kidney injury (57). Studies by us and others have also shown that pharmacological inhibition of HDAC6, a member of class IIb HDACs, attenuated renal damage and improved renal function in murine models of AKI (13, 48, 50). Given that pharmacological inhibition of both class IIa and IIb HDACs is able to offer a protective effect in acute and chronic kidney injury, a class II-selective HDAC inhibitor would be preferred over pan HDAC inhibitors to lessen toxic effects, such as thrombocytopenia, neutropenia, diarrhea, nausea, vomiting, and fatigue (1). As such, TMP195 or other class II-selective HDAC inhibitors are worthy of further development as therapeutic treatment for acute and chronic kidney injury. In this study, we did not observe obvious toxicities such as changes of feeding behavior, activity, and body weight in mice treated with TMP195 during our experimental period (24 h) of this study. Nevertheless, we cannot rule out the possibility that a longer use of TMP195 would cause some side effects. Further investigation is needed to examine the toxicity of TMP195 in detail in the future.
In summary, this study provides evidence for renoprotective effect of class IIa HDAC inhibition with TMP195 in a murine model of LPS-induced AKI. The action of TMP195 may be through a mechanism associated with antiapoptosis and antiinflammation and preservation of endogenous BMP-7. Further investigation of the efficacy of TMP195 in different animal models of AKI would gain a better understanding of other possible mechanisms leading to enhanced renoprotection with class IIa HDAC inhibition and speed up the translational application of HDAC inhibitors in treating this and other kidney diseases.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 81670623 and 81830021 (to S.Z.), National key R&D Program of China Grant 2018YFA0108802 (to S.Z.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01DK113256-01A1 (to S.Z.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Z. and S.Z. conceived and designed research; W.Z. and Y. G. performed experiments; W.Z. analyzed data; W.Z. and S.Z. interpreted results of experiments; W.Z. prepared figures; W.Z. drafted manuscript; G.B. and S.Z. edited and revised manuscript; W.Z., Y.G., G.B., and S.Z. approved final version of manuscript.
REFERENCES
- 1.Afifi S, Michael A, Azimi M, Rodriguez M, Lendvai N, Landgren O. Role of histone deacetylase inhibitors in relapsed refractory multiple myeloma: a focus on vorinostat and panobinostat. Pharmacotherapy 35: 1173–1188, 2015. doi: 10.1002/phar.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol 35: 2–11, 2015. doi: 10.1016/j.semnephrol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amirzargar MA, Yaghubi F, Hosseinipanah M, Jafari M, Pourjafar M, Rezaeepoor M, Rezaei H, Roshanaei G, Hajilooi M, Solgi G. Anti-inflammatory effects of valproic acid in a rat model of renal ischemia/reperfusion injury: alteration in cytokine profile. Inflammation 40: 1310–1318, 2017. doi: 10.1007/s10753-017-0574-9. [DOI] [PubMed] [Google Scholar]
- 4.Arany I, Herbert J, Herbert Z, Safirstein RL. Restoration of CREB function ameliorates cisplatin cytotoxicity in renal tubular cells. Am J Physiol Renal Physiol 294: F577–F581, 2008. doi: 10.1152/ajprenal.00487.2007. [DOI] [PubMed] [Google Scholar]
- 5.Asare Y, Campbell-James TA, Bokov Y, Yu LL, Prestel M, El Bounkari O, Roth S, Megens RTA, Straub T, Thomas K, Yan G, Schneider M, Ziesch N, Tiedt S, Silvestre-Roig C, Braster Q, Huang Y, Schneider M, Malik R, Haffner C, Liesz A, Soehnlein O, Bernhagen J, Dichgans M. Histone deacetylase 9 activates IKK to regulate atherosclerotic plaque vulnerability. Circ Res 127: 811–823, 2020. doi: 10.1161/CIRCRESAHA.120.316743. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. Acute kidney injury in sepsis. Intensive Care Med 43: 816–828, 2017. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 7.Bhatti UF, Williams AM, Kathawate RG, Chang P, Zhou J, Biesterveld BE, Wu Z, Dahl J, Liu B, Li Y, Alam HB. Comparative analysis of isoform-specific and non-selective histone deacetylase inhibitors in attenuating the intestinal damage after hemorrhagic shock. Trauma Surg Acute Care Open 4: e000321, 2019. doi: 10.1136/tsaco-2019-000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogner C, Kale J, Pogmore J, Chi X, Shamas-Din A, Fradin C, Leber B, Andrews DW. Allosteric regulation of BH3 proteins in Bcl-xL complexes enables switch-like activation of Bax. Mol Cell 77: 901−912.e9, 2020. doi: 10.1016/j.molcel.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784, 2006. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 10.Bomsztyk K, Denisenko O. Epigenetic alterations in acute kidney injury. Semin Nephrol 33: 327–340, 2013. doi: 10.1016/j.semnephrol.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang P, Weykamp M, Dennahy IS, Williams AM, Bhatti UF, Liu B, Nikolian VC, Li Y, Alam HB. Histone deacetylase inhibitors: isoform selectivity improves survival in a hemorrhagic shock model. J Trauma Acute Care Surg 84: 795–801, 2018. doi: 10.1097/TA.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costalonga EC, Silva FM, Noronha IL. Valproic acid prevents renal dysfunction and inflammation in the ischemia-reperfusion injury model. BioMed Res Int 2016: 5985903, 2016. doi: 10.1155/2016/5985903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Huang R, Guo F, Liang Y, Xiang J, Lei S, Shi M, Li L, Liu J, Feng Y, Ma L, Fu P. Selective histone deacetylase 6 inhibitor 23BB alleviated rhabdomyolysis-induced acute kidney injury by regulating endoplasmic reticulum stress and apoptosis. Front Pharmacol 9: 274, 2018. doi: 10.3389/fphar.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukudome EY, Kochanek AR, Li Y, Smith EJ, Liu B, Kheirbek T, Lu J, Kim K, Hamwi K, Velmahos GC, Alam HB. Pharmacologic resuscitation promotes survival and attenuates hemorrhage-induced activation of extracellular signal-regulated kinase 1/2. J Surg Res 163: 118–126, 2010. doi: 10.1016/j.jss.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukudome EY, Li Y, Kochanek AR, Lu J, Smith EJ, Liu B, Kim K, Velmahos GC, deMoya MA, Alam HB. Pharmacologic resuscitation decreases circulating cytokine-induced neutrophil chemoattractant-1 levels and attenuates hemorrhage-induced acute lung injury. Surgery 152: 254–261, 2012. doi: 10.1016/j.surg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 338: 17–31, 2004. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, Davis SP, Lobera M, Nolan MA, Letai A. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 543: 428–432, 2017. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo C, Dong G, Liang X, Dong Z. Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat Rev Nephrol 15: 220–239, 2019. doi: 10.1038/s41581-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höcherl K, Schmidt C, Kurt B, Bucher M. Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol 298: F196–F204, 2010. doi: 10.1152/ajprenal.90607.2008. [DOI] [PubMed] [Google Scholar]
- 20.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14: 607–625, 2018. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 21.Hyndman KA, Kasztan M, Mendoza LD, Monteiro-Pai S. Dynamic changes in histone deacetylases following kidney ischemia-reperfusion injury are critical for promoting proximal tubule proliferation. Am J Physiol Renal Physiol 316: F875–F888, 2019. doi: 10.1152/ajprenal.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inche AG, La Thangue NB. Chromatin control and cancer-drug discovery: realizing the promise. Drug Discov Today 11: 97–109, 2006. doi: 10.1016/S1359-6446(05)03691-3. [DOI] [PubMed] [Google Scholar]
- 24.Ji MH, Li GM, Jia M, Zhu SH, Gao DP, Fan YX, Wu J, Yang JJ. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation 36: 1453–1459, 2013. doi: 10.1007/s10753-013-9686-z. [DOI] [PubMed] [Google Scholar]
- 26.Kaur P, Shorey LE, Ho E, Dashwood RH, Williams DE. The epigenome as a potential mediator of cancer and disease prevention in prenatal development. Nutr Rev 71: 441–457, 2013. doi: 10.1111/nure.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Li Y, Jin G, Chong W, Liu B, Lu J, Lee K, Demoya M, Velmahos GC, Alam HB. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation 83: 243–248, 2012. doi: 10.1016/j.resuscitation.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingsley SM, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep 18: 26, 2016. doi: 10.1007/s11908-016-0535-8. [DOI] [PubMed] [Google Scholar]
- 29.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int 59: 2243–2249, 2001. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 30.Levine MH, Wang Z, Bhatti TR, Wang Y, Aufhauser DD, McNeal S, Liu Y, Cheraghlou S, Han R, Wang L, Hancock WW. Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Am J Transplant 15: 965–973, 2015. doi: 10.1111/ajt.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Zhao T, Liu B, Halaweish I, Mazitschek R, Duan X, Alam HB. Inhibition of histone deacetylase 6 improves long-term survival in a lethal septic model. J Trauma Acute Care Surg 78: 378–385, 2015. doi: 10.1097/TA.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Li Y, Chong W, Deperalta DK, Duan X, Liu B, Halaweish I, Zhou P, Alam HB. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock 41: 104–108, 2014. doi: 10.1097/SHK.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobera M, Madauss KP, Pohlhaus DT, Wright QG, Trocha M, Schmidt DR, Baloglu E, Trump RP, Head MS, Hofmann GA, Murray-Thompson M, Schwartz B, Chakravorty S, Wu Z, Mander PK, Kruidenier L, Reid RA, Burkhart W, Turunen BJ, Rong JX, Wagner C, Moyer MB, Wells C, Hong X, Moore JT, Williams JD, Soler D, Ghosh S, Nolan MA. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol 9: 319–325, 2013. doi: 10.1038/nchembio.1223. [DOI] [PubMed] [Google Scholar]
- 34.Ma T, Huang C, Xu Q, Yang Y, Liu Y, Meng X, Li J, Ye M, Liang H. Suppression of BMP-7 by histone deacetylase 2 promoted apoptosis of renal tubular epithelial cells in acute kidney injury. Cell Death Dis 8: e3139, 2017. doi: 10.1038/cddis.2017.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manson SR, Austin PF, Guo Q, Moore KH. BMP-7 signaling and its critical roles in kidney development, the responses to renal injury, and chronic kidney disease. Vitam Horm 99: 91–144, 2015. doi: 10.1016/bs.vh.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Moledina DG, Parikh CR. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol 38: 3–11, 2018. doi: 10.1016/j.semnephrol.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolian VC, Georgoff PE, Pai MP, Dennahy IS, Chtraklin K, Eidy H, Ghandour MH, Han Y, Srinivasan A, Li Y, Alam HB. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Trauma Acute Care Surg 83: 1066–1073, 2017. doi: 10.1097/TA.0000000000001612. [DOI] [PubMed] [Google Scholar]
- 38.O’Kane D, Baldwin GS, Bolton DM, Ischia JJ, Patel O. Preconditioning against renal ischaemia reperfusion injury: the failure to translate to the clinic. J Nephrol 32: 539–547, 2019. doi: 10.1007/s40620-019-00582-6. [DOI] [PubMed] [Google Scholar]
- 39.Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 41.Parra M. Class IIa HDACs—new insights into their functions in physiology and pathology. FEBS J 282: 1736–1744, 2015. doi: 10.1111/febs.13061. [DOI] [PubMed] [Google Scholar]
- 42.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96: 1083–1099, 2019. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy MA, Natarajan R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int 88: 250–261, 2015. doi: 10.1038/ki.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13: 110–116, 2000. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 45.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 8: 217–225, 1994. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 46.Sanaei M, Kavoosi F. Histone deacetylases and histone deacetylase inhibitors: molecular mechanisms of action in various cancers. Adv Biomed Res 8: 63, 2019. doi: 10.4103/abr.abr_142_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang Y, Jiang YX, Ding ZJ, Shen AL, Xu SP, Yuan SY, Yao SL. Valproic acid attenuates the multiple-organ dysfunction in a rat model of septic shock. Chin Med J (Engl) 123: 2682–2687, 2010. [PubMed] [Google Scholar]
- 48.Shi Y, Xu L, Tang J, Fang L, Ma S, Ma X, Nie J, Pi X, Qiu A, Zhuang S, Liu N. Inhibition of HDAC6 protects against rhabdomyolysis-induced acute kidney injury. Am J Physiol Renal Physiol 312: F502–F515, 2017. doi: 10.1152/ajprenal.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang C, Dong Z. Epigenetic regulation in acute kidney injury: new light in a dark area. Kidney Int 88: 665–668, 2015. doi: 10.1038/ki.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang J, Shi Y, Liu N, Xu L, Zang X, Li P, Zhang J, Zheng X, Qiu A, Zhuang S. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 132: 339–359, 2018. doi: 10.1042/CS20171417. [DOI] [PubMed] [Google Scholar]
- 51.Tang J, Yan Y, Zhao TC, Gong R, Bayliss G, Yan H, Zhuang S. Class I HDAC activity is required for renal protection and regeneration after acute kidney injury. Am J Physiol Renal Physiol 307: F303–F316, 2014. doi: 10.1152/ajprenal.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang J, Zhuang S. Epigenetics in acute kidney injury. Curr Opin Nephrol Hypertens 24: 351–358, 2015. doi: 10.1097/MNH.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang J, Zhuang S. Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin Sci (Lond) 133: 597–609, 2019. doi: 10.1042/CS20180465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujimura T, Idei M, Yoshikawa M, Takase O, Hishikawa K. Roles and regulation of bone morphogenetic protein-7 in kidney development and diseases. World J Stem Cells 8: 288–296, 2016. doi: 10.4252/wjsc.v8.i9.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Knethen A, Brüne B. Histone deacetylation inhibitors as therapy concept in sepsis. Int J Mol Sci 20: E346, 2019. doi: 10.3390/ijms20020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams AM, Dennahy IS, Bhatti UF, Biesterveld BE, Graham NJ, Li Y, Alam HB. Histone deacetylase inhibitors: a novel strategy in trauma and sepsis. Shock 52: 300–306, 2019. doi: 10.1097/SHK.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong C, Guan Y, Zhou X, Liu L, Zhuang MA, Zhang W, Zhang Y, Masucci MV, Bayliss G, Zhao TC, Zhuang S. Selective inhibition of class IIa histone deacetylases alleviates renal fibrosis. FASEB J 33: 8249–8262, 2019. doi: 10.1096/fj.201801067RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan H, Denton K, Liu L, Li XJ, Benashski S, McCullough L, Li J. Nuclear translocation of histone deacetylase 4 induces neuronal death in stroke. Neurobiol Dis 91: 182–193, 2016. doi: 10.1016/j.nbd.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Wang H, Zhao Y, Wang J, Dubielecka PM, Zhuang S, Qin G, Chin YE, Kao RL, Zhao TC. Myocyte-specific overexpressing HDAC4 promotes myocardial ischemia/reperfusion injury. Mol Med 24: 37, 2018. doi: 10.1186/s10020-018-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang LX, DeNicola M, Qin X, Du J, Ma J, Tina Zhao Y, Zhuang S, Liu PY, Wei L, Qin G, Tang Y, Zhao TC. Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am J Physiol Cell Physiol 307: C358–C372, 2014. doi: 10.1152/ajpcell.00187.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang LX, Du J, Zhao YT, Wang J, Zhang S, Dubielecka PM, Wei L, Zhuang S, Qin G, Chin YE, Zhao TC. Transgenic overexpression of active HDAC4 in the heart attenuates cardiac function and exacerbates remodeling in infarcted myocardium. J Appl Physiol 125: 1968–1978, 2018. doi: 10.1152/japplphysiol.00006.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, Deperalta DK, Velmahos GC, Alam HB. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery 154: 206–213, 2013. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng Q, Liu W, Liu Z, Zhao H, Han X, Zhao M. Valproic acid protects septic mice from renal injury by reducing the inflammatory response. J Surg Res 192: 163–169, 2014. doi: 10.1016/j.jss.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang S. Epigenetic targeting for acute kidney injury. Nephrology (Carlton) 23, Suppl 4: 21–25, 2018. doi: 10.1111/nep.13466. [DOI] [PubMed] [Google Scholar]