Abstract
Left ventricular (LV) global longitudinal strain (GLS) has emerged as a significant prognostic marker in patients after myocardial infarction (MI). Although elevated LV filling pressure after MI might alter GLS, direct evidence for this is lacking. This study aimed to clarify the association between GLS and LV filling pressure in a large animal MI model. A total of 104 Yorkshire pigs underwent both echocardiographic and hemodynamic assessments 1–4 wk after induction of large anterior MI. GLS was measured in the apical four-chamber view using a semiautomated speckle-tracking software. LV pressure-volume relationship was invasively measured using a high-fidelity pressure-volume catheter. GLS >−14% was considered impaired. Compared with pigs with LV ejection fraction (LVEF) >40% and preserved GLS (n = 29), those with LVEF >40% and impaired GLS (n = 37) and those with LVEF ≤40% (n = 38) had significantly higher LV end-diastolic pressure (15.5 ± 5.5 vs. 19.7 ± 5.8 and 19.6 ± 6.6 mmHg; P = 0.008 and P = 0.026, respectively) and higher LV mean diastolic pressure (7.1 ± 2.9 vs. 10.4 ± 4.5 and 11.1 ± 5.4 mmHg; P = 0.013 and P = 0.002, respectively). GLS was modestly correlated with τ (r = 0.21, P = 0.039) and slope of LV end-diastolic pressure–volume relationship (r = 0.43, P < 0.001). Impaired GLS was associated with higher LV end-diastolic and mean-diastolic pressures after adjusting for LVEF and baseline characteristics (P = 0.026 and P = 0.001, respectively). Impaired GLS assessed by speckle-tracking echocardiography was associated with elevated LV filling pressure after MI. GLS has an incremental diagnostic value for detecting elevated LV filling pressure and may be particularly useful for evaluating post-MI patients with preserved LVEF.
NEW & NOTEWORTHY Strain analysis was performed in 104 pigs after MI, and its relationship to invasive hemodynamic measurements was studied. Impaired longitudinal strain was associated with high ventricular filling pressure independent of LVEF in post-MI setting. Global longitudinal strain is a potential prognostic marker after MI.
Keywords: global longitudinal strain, left ventricular filling pressure, left ventricular pressure-volume relationship, myocardial infarction
INTRODUCTION
Noninvasive echocardiography plays an important role in the diagnosis, risk stratification, and measurement of treatment effects in patients with myocardial infarction (MI) (13). Myocardial strain analysis by speckle-tracking echocardiography has been developed as an angle-independent method for the evaluation of myocardial function, which allows assessments of longitudinal, circumferential, and radial myocardial strains (5). Among these, left ventricular (LV) global longitudinal strain (GLS) may be the most sensitive marker for detecting early changes in patients with cardiac pathology because subendocardial fibers are oriented longitudinally and are sensitive to ischemia (15). Recently, GLS has also emerged as a significant prognostic marker for patients after MI. Impaired GLS was associated with worse clinical outcomes even after adjusting for LV ejection fraction (LVEF) (10, 18). However, the underlying mechanisms of GLS impairment in preserved LVEF and how it provides better prognostic value remain unclear.
The mortality risk associated with Doppler-derived LV filling pressure has long been recognized (3, 12, 16, 21). Impaired GLS after MI has been demonstrated to be associated with increased neurohormonal activation assessed by N-terminal pro-brain natriuretic peptide (9), suggesting that impaired GLS after MI may reflect not only cardiac function but also alterations in cardiac load. Nevertheless, direct evidence for association of altered GLS with LV filling pressure in post-MI setting is lacking. Therefore, this study aimed to clarify the association between GLS and LV filling pressure in a large animal MI model. Specifically, we hypothesized that GLS sensitively detects elevated LV filling pressure even in subjects with preserved LVEF after an MI. Using a large number of experimental, but clinically relevant animal model of MI, we demonstrate that impaired LV GLS is associated with elevated filling pressure independent of LVEF in the post-MI setting.
METHODS
All animal protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and standards of US regulatory agencies. Protocols were approved by the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai. This is a retrospective review of the pooled data consisting of 104 Yorkshire pigs (27 males and 77 females; mean body weight, 35.8 ± 9.9 kg) that survived acute MI induction of the left anterior descending artery and underwent both echocardiographic and hemodynamic assessments 1–4 wk after MI. All pigs were intubated and ventilated with 100% oxygen, while anesthesia was maintained with propofol (10 mg/kg/h iv) throughout the procedure.
Percutaneous MI induction.
The detailed protocol of percutaneous MI induction was published previously (2, 29). Briefly, a coronary balloon was delivered to the proximal left anterior descending artery using the transfemoral approach. MI was induced by occluding the coronary artery for 60–120 min followed by reperfusion. Fifteen pigs also received autologous thrombus injection to induce transmural infarction after reperfusion (29).
Echocardiography.
All pigs underwent two- and three-dimensional echocardiography using a Philips iE-33 ultrasound system (Philips Medical Systems, Andover, MA) as previously described (14). LV volumes and LVEF were measured in three-dimensional echocardiographic data using a semiautomated border detection software (Philips QLAB 3DQ Advanced, Philips Medical Systems). The standard two-dimensional echocardiographic studies included parasternal long-axis and short-axis views to measure LV and left atrial diameters and LV wall thickness. Peak early diastolic mitral flow velocity (E), peak late diastolic mitral flow velocity (A), E/A ratio, E wave deceleration time, the ratio of peak velocity of systolic to diastolic pulmonary venous flow (S/D), mitral annulus early diastolic velocity (e´) at septal and lateral mitral annulus, and mean E/e´ were evaluated in accordance with American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) recommendations (19). GLS was measured from the apical four-chamber view using a semiautomated speckle-tracking software (Philips QLAB CMQ, Philips Medical Systems) (11) (Fig. 1). Strain analysis was performed by an investigator (T.A.) blinded to the hemodynamic data, which showed good reproducibility as previously reported (11). To adjust the effect of LV wall stress on myocardial strain, transmural wall stress was calculated from the following formula (25):
where LVESP (mmHg) is LV end-systolic pressure, LVID is end-systolic LV internal dimension (mm), and PWT is end-systolic posterior wall thickness (mm).
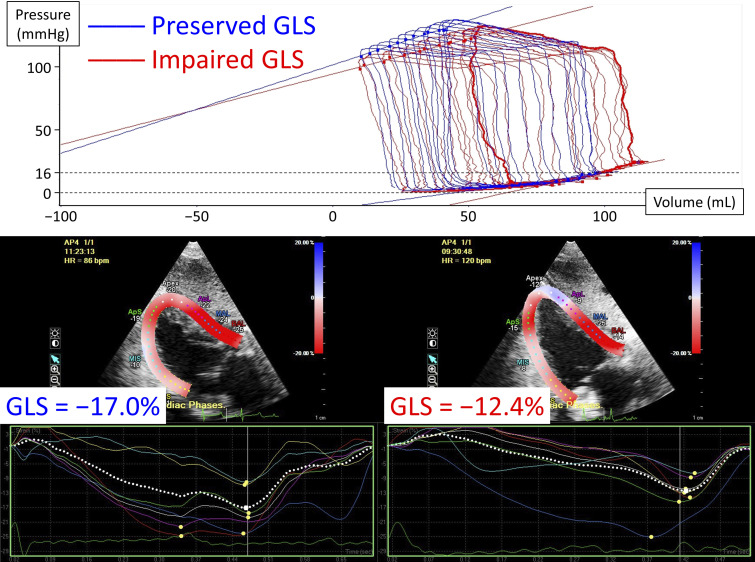
Fig. 1.
Representative images of left ventricular pressure-volume relationships during transient inferior vena cava occlusion (top) and speckle-tracking echocardiography in pigs with preserved (bottom left) or impaired global longitudinal strain (GLS, bottom right). A pig with impaired GLS (red line) has a higher left ventricular end-diastolic pressure before inferior vena cava occlusion with a steeper end-diastolic pressure-volume relationship.
Hemodynamic measurements.
A high-fidelity pressure-volume loop catheter (Millar Instruments, Houston, TX) was inserted into the LV via the right carotid artery or the femoral artery to collect continuous LV pressure-volume measurements during steady-state and transient inferior vena cava occlusion with short breath-hold (Fig. 1). All pressure-volume data were analyzed using an iox2 application (Emka Technologies, Falls Church, VA). Alpha value and parallel conductance were calibrated using cardiac output derived from Swan–Ganz catheter, end-diastolic volume derived from three-dimensional echocardiography (11), and a bolus injection of hypertonic saline (27). An elevated LV filling pressure was defined as LV end-diastolic pressure >16 mmHg (23) or LV mean diastolic pressure >12 mmHg (22). The time constant of LV isovolumic relaxation (τ) was calculated by the method of Weiss et al. (30).
Measurement of myocardial infarct size.
At the end of each study in 65 pigs, the heart was rapidly excised and sectioned from base to apex transversely into 1-cm-thick parallel slices along the long axis. These tissues were immersed in 1.0% triphenyl tetrazolium chloride for 15 min at 37°C. The infarct area was traced on each slice and expressed as a percentage of LV mass.
Statistical analysis.
The pigs were divided into three groups according to LVEF (≤40% or >40%) and GLS (≤−14% or >−14%) based on clinical criteria (10): LVEF >40% and GLS ≤−14% (preserved GLS), LVEF >40% and GLS >−14% (impaired GLS), and LVEF ≤40%. Continuous variables were presented as means ± SD. Categorical variables were presented as absolute numbers with percentages. Comparisons between groups were performed using Wilcoxon’s rank-sum test, followed by the Steel–Dwass test for continuous data and Fisher’s exact test for categorical data. Correlations between continuous variables were evaluated using Pearson’s correlation coefficients.
To test the association between GLS and LV filling pressure, multiple linear regression analyses were performed. Because myocardial strain has been shown to be affected by LVEF (6), heart rate (4), and LV end-systolic wall stress (7), model 1 was adjusted for these parameters as well as measurement time points after MI (1 wk vs. 2 wk vs. 4 wk). To investigate the effect of LV infarct size on the association between GLS and LV filling pressure, we performed exploratory analyses (model 2), which included categories of LV infarct size (≤20% of LV mass vs. >20% of LV mass vs. unavailable LV infarct size data) instead of LVEF in model 1. Since mitral E and e´ can also reflect LV filling pressure (20), additional sensitivity analyses were performed, in which E and mean e´ values were added to models 1 and 2. The linearity assumption of continuous variables was confirmed by plotting the raw residuals against each of the independent variables and the estimated variables.
To assess the incremental diagnostic value of GLS for detecting elevated LV filling pressure, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were derived from logistic regression models (24) including E/e´ ratio, E/A ratio, mitral E deceleration time, S/D ratio, tricuspid regurgitant velocity >2.8 m/s, and left atrial diameter with and without GLS. To determine whether mitral e´ alone negates the effect of GLS on LV filling pressure, sensitivity analysis was performed using mean e´, mitral E deceleration time, S/D ratio, tricuspid regurgitant velocity >2.8 m/s, and left atrial diameter with and without GLS. All statistical analyses were performed using JMP Pro 14.3.0 (SAS Institute Inc., Cary, NC) and JMP scripting language for NRI and IDI (https://www.jmp.com/japan/support/jmpfile/nri_idi.zip). A two-sided P value <0.05 was considered statistically significant.
RESULTS
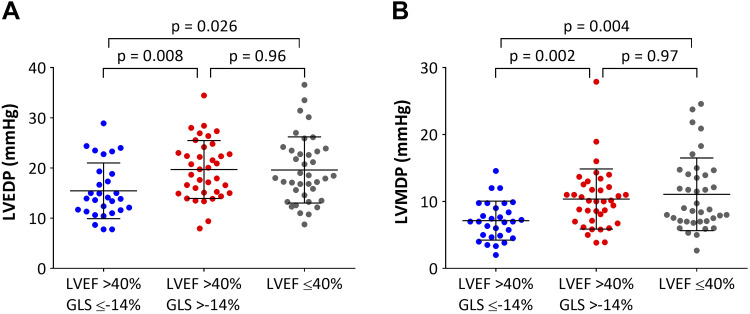
Table 1 summarizes the LV parameters of the 104 pigs after MI, of which 38 had LVEF ≤40%, and the remaining 66 had LVEF >40%. The mean value for GLS in pigs with LVEF ≤40% was significantly lower than those with LVEF >40% (−10.1 ± 3.3% vs. −13.8 ± 3.1%, P < 0.001). Pigs with LVEF >40% and preserved GLS had the highest LVEF, dP/dt maximum, and preload recruitable stroke work among the three groups. Compared with pigs with LVEF >40% and preserved GLS (n = 29), those with LVEF >40% and impaired GLS (n = 37) as well as those with LVEF ≤40% (n = 38) had significantly higher LV end-diastolic pressure (15.5 ± 5.5 vs. 19.7 ± 5.8 and 19.6 ± 6.6 mmHg; P = 0.008 and P = 0.026, respectively) and higher LV mean diastolic pressure (7.1 ± 2.9 vs. 10.4 ± 4.5 and 11.1 ± 5.4 mmHg; P = 0.013 and P = 0.002, respectively) (Fig. 2). There were no significant differences in LV end-diastolic and mean diastolic pressures between pigs with LVEF >40% and impaired GLS and those with LVEF ≤40%.
Table 1.
Baseline LV parameters
| LVEF (>40%) |
|||||
|---|---|---|---|---|---|
| Parameters | n | Preserved GLS (≤−14%) | Impaired GLS (>−14%) | LVEF (≤40%) | P Value |
| n | 29 | 37 | 38 | ||
| Longitudinal strain, % | −16.3 ± 2.6 | −11.8 ± 1.6* | −10.1 ± 3.3*† | <0.001 | |
| Heart rate at strain measurement, beats/min | 91 ± 14 | 94 ± 19 | 94 ± 18 | 0.88 | |
| Female, n (%) | 22 (76) | 31 (84) | 24 (63) | 0.12 | |
| Body weight, kg | 39.7 ± 8.1 | 34.4 ± 10.6 | 34.2 ± 9.9 | 0.073 | |
| LV 3-D echocardiography | |||||
| LVEF, % | 50.9 ± 6.6 | 47.3 ± 6.2 | 33.9 ± 5.1*† | <0.001 | |
| End-diastolic volume, mL | 100.2 ± 20.1 | 98.3 ± 23.7 | 115.8 ± 23.4*† | 0.001 | |
| End-systolic volume, mL | 49.2 ± 12.3 | 51.9 ± 14.8 | 76.3 ± 15.7*† | <0.001 | |
| Stroke volume, mL | 51.1 ± 11.4 | 46.4 ± 12.5 | 39.5 ± 10.5*† | <0.001 | |
| LV 2-D echocardiography | |||||
| Left atrial dimension, mm | 44.6 ± 6.0 | 42.0 ± 7.8 | 43.5 ± 6.5 | 0.16 | |
| Septal e´, cm/s | 8.9 ± 2.3 | 8.2 ± 1.6 | 7.1 ± 2.5* | 0.017 | |
| Lateral e´, cm/s | 10.5 ± 3.1 | 9.1 ± 2.2 | 8.0 ± 2.3* | 0.003 | |
| Mean e´, cm/s | 9.6 ± 2.2 | 8.7 ± 1.7 | 7.5 ± 2.0* | 0.001 | |
| E/e´ ratio | 8.3 ± 1.9 | 8.6 ± 2.7 | 8.8 ± 2.5 | 0.73 | |
| E/A ratio | 1.18 ± 0.37 | 1.17 ± 0.35 | 1.08 ± 0.45 | 0.18 | |
| Mitral E deceleration time, ms | 103 | 175 ± 72 | 143 ± 67 | 151 ± 68 | 0.085 |
| S/D ratio | 102 | 1.15 ± 0.29 | 1.22 ± 0.39 | 1.22 ± 0.43 | 0.77 |
| Tricuspid regurgitant velocity >2.8 m/s, n (%) | 0 (0) | 8 (22)* | 4 (11) | 0.017 | |
| LV pressure catheter | |||||
| End-systolic pressure, mmHg | 104.8 ± 16.7 | 101.0 ± 19.2 | 88.7 ± 19.0*† | 0.003 | |
| End-diastolic pressure, mmHg | 15.5 ± 5.5 | 19.7 ± 5.8* | 19.6 ± 6.6* | 0.006 | |
| Mean diastolic pressure, mmHg | 7.1 ± 2.9 | 10.4 ± 4.5* | 11.1 ± 5.4* | 0.001 | |
| Elevated LV filling pressure‡, n (%) | 10 (34) | 25 (68)* | 26 (68)* | 0.001 | |
| dP/dt maximum, mmHg/s | 1892 ± 503 | 1605 ± 484* | 1344 ± 455*† | <0.001 | |
| dP/dt minimum, mmHg/s | −1915 ± 488 | −1748 ± 527 | −1350 ± 494*† | <0.001 | |
| Stroke work, mmHg × mL | 4596 ± 1535 | 3289 ± 1445* | 2575 ± 1335* | <0.001 | |
| Preload recruitable stroke work, mmHg | 47.2 ± 19.1 | 38.9 ± 12.3 | 32.7 ± 13.0* | 0.002 | |
| τ, ms | 54.8 ± 10.2 | 60.2 ± 14.5 | 64.5 ± 18.0* | 0.028 | |
| End-systolic pressure-volume relationship, mmHg/mL | 1.09 ± 0.68 | 1.23 ± 0.58 | 1.00 ± 0.48 | 0.096 | |
| End-diastolic pressure-volume relationship, mmHg/mL | 0.34 ± 0.23 | 0.52 ± 0.39* | 0.60 ± 0.35* | <0.001 | |
| Cardiac output derived from Swan-Ganz catheter, L/min | 4.1 ± 1.3 | 3.1 ± 1.3* | 3.0 ± 1.2* | <0.001 | |
| End-systolic wall stress, dyn/mm2 | 97.9 ± 41.1 | 106.6 ± 30.1 | 113.1 ± 49.2 | 0.14 | |
| Interval between MI and measurements, n (%) | 0.037 | ||||
| 1 wk | 11 (38) | 9 (24) | 11 (29) | ||
| 2 wk | 1 (3) | 4 (11) | 11 (29) | ||
| 4 wk | 17 (59) | 24 (65) | 16 (42) | ||
| Infarct size, %LV mass | 65 | 17.5 ± 6.7 | 21.3 ± 6.9 | 24.1 ± 4.8* | 0.002 |
Values are means ± SD or n (%). A, peak velocity at atrial contraction; D, diastolic forward flow; E, peak early filling velocity; e´, velocity of mitral annulus early diastolic motion; GLS, global longitudinal strain; LV, left ventricular: LVEF, left ventricular ejection fraction; MI, myocardial infarction; S, systolic forward flow.
P < 0.05 vs. LVEF >40% and preserved GLS.
†P < 0.05 vs. LVEF >40% and impaired GLS.
Elevated LV filling pressure is defined as LV end-diastolic pressure >16 mmHg or LV mean diastolic pressure >12 mmHg.
Fig. 2.
Scatterplots of left ventricular end-diastolic pressure (LVEDP, A) and left ventricular mean diastolic pressure (LVMDP, B) for the three groups. Horizontal bars indicate the means and standard deviation.
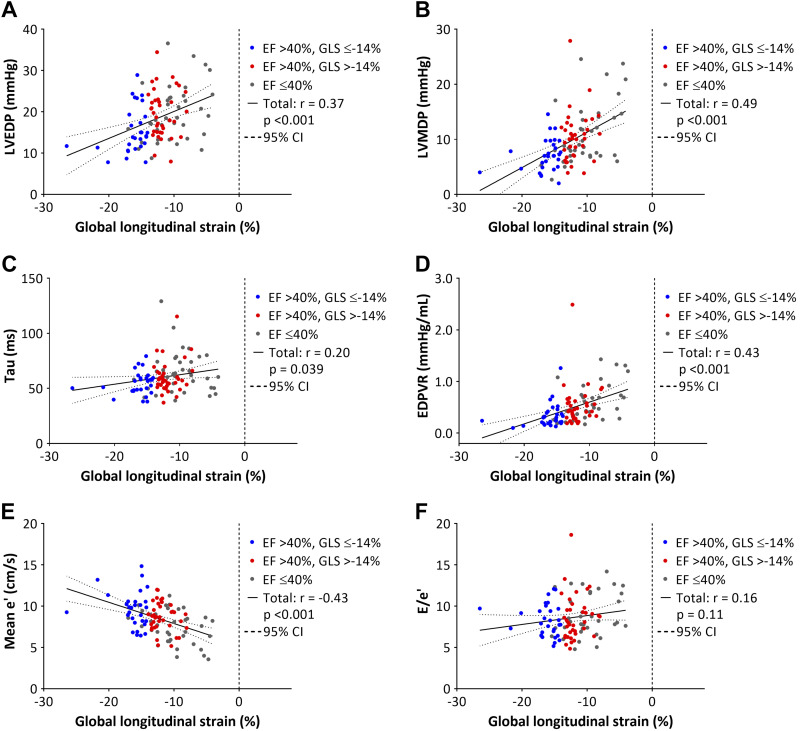
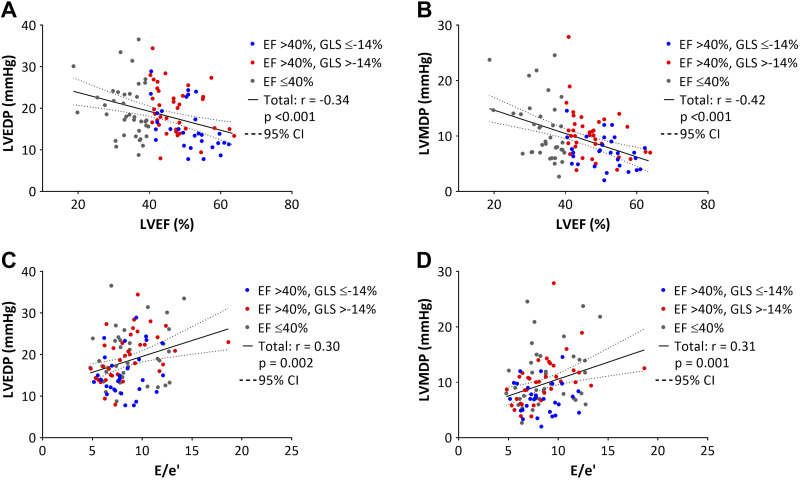
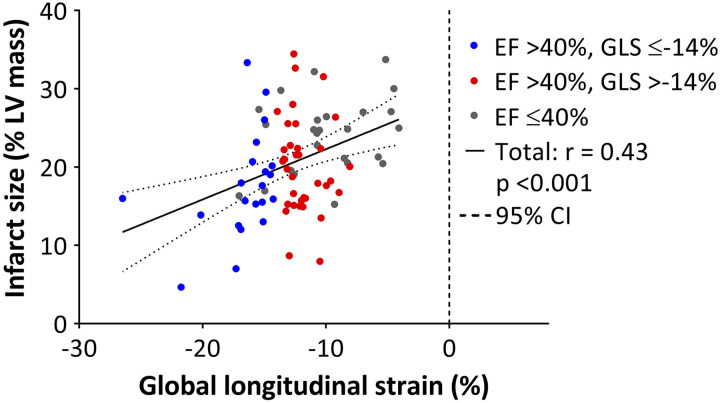
Figure 3 shows the relationships between GLS, LV filling pressure, invasively obtained parameters of LV diastolic function, mean e´, and E/e´. GLS was significantly correlated with LV end-diastolic and mean diastolic pressures, τ, the slope of end-diastolic pressure-volume relationship, and mean e´ (P < 0.05 for all). Regarding the conventional echocardiographic parameters, LVEF and E/e´ ratio were modestly correlated with LV filling pressures (Fig. 4). There were no significant correlations between E/A and S/D ratios, mitral E velocity deceleration time, left atrial dimension, and LV filling pressures. In 65 pigs that were immediately euthanized after the measurements, GLS correlated significantly with LV infarct size (r = 0.43, P < 0.001) (Fig. 5).
Fig. 3.
Relationships between global longitudinal strain (GLS) and left ventricular (LV) end-diastolic pressure (LVEDP, A), LV mean diastolic pressure (LVMDP, B), the time constant of LV isovolumic relaxation (τ, C), slope of end-diastolic pressure volume relationship (EDPVR, D), mean e´ (E), E/e´ (F). CI, confidence interval; EF, ejection fraction.
Fig. 4.
Relationships between left ventricular (LV) end-diastolic pressure (LVEDP; A and C) or LV mean diastolic pressure (LVMDP; B and D) and LV ejection fraction (EF) or E/e´ ratio. CI, confidence interval.
Fig. 5.
Relationship between left ventricular (LV) infarct size and global longitudinal strain (GLS). CI, confidence interval.
GLS as a determinant of LV filling pressure.
Table 2 shows the results of multiple linear regression analysis performed to estimate LV filling pressure. In both models 1 and 2, the impairment of GLS was independently associated with elevated LV end-diastolic pressure as well as LV mean diastolic pressure. In contrast, LVEF and heart rate did not show significant association with LV filling pressures. LV infarct size was significantly associated only with LV mean diastolic pressure. When mitral E and mean e´ were included in the multivariable analyses, GLS was still significantly associated with LV filling pressure except for the model estimating LV end-diastolic pressure with LV infarct size (Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.12895046).
Table 2.
Multiple linear regression analysis to estimate left ventricular end-diastolic and mean diastolic pressures
|
Model 1: Including LVEF |
Model 2: Including LV Infarct Size |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |
| Dependent variable: LV end-diastolic pressure | ||||||
| Intercept | 26.44 | 16.38 to 36.51 | <0.001 | 17.89 | 7.25 to 28.53 | 0.001 |
| LVEF, per 5% | −0.18 | −1.04 to 0.68 | 0.68 | – | – | – |
| Global longitudinal strain, % | 0.50 | 0.06 to 0.93 | 0.026 | 0.37 | 0.02 to 0.72 | 0.037 |
| Heart rate, per 10 beats/min | −0.22 | −0.96 to 0.52 | 0.55 | −0.10 | −0.82 to 0.63 | 0.79 |
| LV end-systolic wall stress, per 10 dyn/mm2 | 0.23 | −0.08 to 0.54 | 0.14 | 0.31 | 0.03 to 0.59 | 0.033 |
| Interval between MI and measurements* | ||||||
| 2 wk | 2.77 | −0.84 to 6.37 | 0.13 | 2.98 | −0.50 to 6.46 | 0.093 |
| 4 wk | −1.96 | −4.74 to 0.82 | 0.16 | −0.47 | −3.45 to 2.51 | 0.75 |
| LV infarct size, % of LV mass† | ||||||
| >20% | – | – | – | 4.72 | 1.27 to 8.17 | 0.008 |
| Unavailable | – | – | – | 2.38 | −0.55 to 5.31 | 0.11 |
| Dependent variable: LV mean diastolic pressure | ||||||
| Intercept | 13.28 | 6.34 to 20.23 | <0.001 | 9.98 | 2.41 to 17.56 | 0.010 |
| LVEF, per 5% | −0.07 | −0.67 to 0.52 | 0.81 | – | – | – |
| Global longitudinal strain, % | 0.50 | 0.20 to 0.80 | 0.001 | 0.46 | 0.21 to 0.70 | <0.001 |
| Heart rate, per 10 beats/min | 0.14 | −0.37 to 0.65 | 0.59 | 0.21 | −0.30 to 0.73 | 0.42 |
| LV end-systolic wall stress, per 10 dyn/mm2 | 0.28 | 0.06 to 0.49 | 0.012 | 0.31 | 0.10 to 0.51 | 0.003 |
| Interval between MI and measurements* | ||||||
| 2 wk | 1.46 | −1.03 to 3.95 | 0.25 | 1.59 | −0.89 to 4.06 | 0.21 |
| 4 wk | −2.15 | −4.07 to −0.23 | 0.028 | −1.79 | −3.91 to 0.33 | 0.097 |
| LV infarct size, %LV mass† | ||||||
| >20% | – | – | – | 1.47 | −0.98 to 3.93 | 0.24 |
| Unavailable | – | – | – | 0.99 | −1.09 to 3.08 | 0.35 |
LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Reference interval is 1 wk after MI.
Reference LV infarct size is ≤20% of LV mass.
Reclassification analysis of adding GLS to the conventional echocardiographic parameters for detecting elevated LV filling pressure resulted in a significant improvement with NRI of 0.42 [95% confidence interval (CI), 0.03–0.81; P = 0.038] and IDI of 0.11 (95% CI, 0.04–0.18; P = 0.002) (Table 3). Similarly, when mean e´ was included in the reclassification analysis, adding GLS significantly improved the prediction with NRI of 0.50 (95% CI, 0.12–0.89; P = 0.013) and IDI of 0.07 (95% CI, 0.02–0.12; P = 0.012) (Supplemental Table S2).
Table 3.
Reclassification in pigs with or without elevated left ventricular filling pressure based on models with or without global longitudinal strain in 101 pigs
| Model Without GLS |
Model With GLS |
|||
|---|---|---|---|---|
| Probability | <30% | 30–70% | >70% | Total |
| Pigs with elevated LV filling pressure† | ||||
| <30% | 0 | 0 | 0 | 0 |
| 30–70% | 1 | 28 | 15 | 44 |
| >70% | 0 | 4 | 12 | 16 |
| Total | 1 | 32 | 27 | 60 |
| Pigs without elevated LV filling pressure | ||||
| <30% | 1 | 1 | 0 | 2 |
| 30–70% | 6 | 24 | 4 | 34 |
| >70% | 0 | 3 | 2 | 5 |
| Total | 7 | 28 | 6 | 41 |
| Net reclassification improvement | 0.42 (95% CI, 0.03–0.81; P = 0.038) | |||
| Integrated discrimination improvement | 0.11 (95% CI, 0.04–0.18; P = 0.002) | |||
Three of 104 pigs had missing data for mitral E deceleration time (n = 1) or S/D ratio (n = 2). CI, confidence interval; GLS, global longitudinal strain; LV, left ventricular.
Elevated LV filling pressure was defined as LV end-diastolic pressure >16 mmHg or LV mean diastolic pressure >12 mmHg.
DISCUSSION
This study showed that pigs with reduced LVEF or impaired GLS after MI had a significantly elevated LV filling pressure compared with those with preserved LVEF and GLS. Association between impaired GLS and elevated LV filling pressure was independent of LVEF or LV infarct size, which explains the superior performance of GLS over LVEF in predicting clinical outcome after MI by detecting elevated LV filling pressure. GLS also had an incremental diagnostic value for detecting elevated LV filling pressure over conventional echocardiographic parameters including E/e´ and E/A ratios, mitral E deceleration time, and tricuspid regurgitant velocity.
GLS has gained increasing attention for risk stratification or to monitor treatment effects in various settings. Furthermore, impaired GLS has been identified as a significant predictor of adverse cardiovascular events after MI, independent of LVEF (8, 10, 18). However, mechanisms underlying the association between impaired GLS and worse outcomes after MI remained unclear. We used a clinically relevant swine model of MI with direct measurements of LV pressure and volume to explore the cause. Importantly, invasive pressure measurement is essential for accurate assessment of LV filling pressure, but its systematic assessment in humans shortly after MI is ethically challenging. Thus, the pig model offers unique opportunities to examine the relationships between echocardiographic and hemodynamic parameters in a post-MI setting. To our knowledge, this is the first study showing direct association between GLS and invasively measured LV filling pressure after MI. The early detection of elevated LV filling pressure using GLS may help identify patients at high risk of adverse cardiovascular events and also might be useful for appropriately titrating their medications, such as angiotensin converting enzyme inhibitors and diuretics.
Previous studies using angle-dependent Doppler imaging showed that E/e´ ratio correlated with LV filling pressure in a wide spectrum of patients with cardiac diseases (1, 22). However, this correlation is not sufficiently high enough, especially for those with preserved LVEF; therefore, the recently published ASE/EACVI guidelines recommended the combined approach of Doppler velocities and left atrium size for estimating LV filling pressure (20). Given that speckle-tracking echocardiography allows angle-independent evaluation of myocardial abnormalities, GLS may provide additional information on the guideline-recommended parameters to estimate LV filling pressure. Our findings demonstrated that GLS has an incremental diagnostic value for detecting elevated LV filling pressure over conventional echocardiographic parameters including mitral E and mean e´. Since elevated LV filling pressure after MI is associated with worse outcome (3, 12, 16, 21), GLS may offer additive diagnostic benefit or could even be a more sensitive marker for risk stratification after MI.
The LV infarct size was moderately correlated with GLS, which is in agreement with previous studies that used late gadolinium enhancement in cardiovascular magnetic resonance (26) or 99Tc-sestamibi single-photon emission computed tomography (17) to estimate infarct size. Infarct size quantification is clinically important because of its close association with all-cause mortality and hospitalization rates for heart failure after MI (28). GLS by speckle-tracking echocardiography is semiautomatically calculated using a commercially available software without time-consuming process and contrast agents, which is an important advantage over cardiovascular magnetic resonance imaging and myocardial perfusion imaging in the clinical setting. However, our results indicate that caution should be exercised when interpreting impaired GLS after MI because estimation of LV infarct size using GLS is influenced by elevated LV filling pressure.
Study limitations.
This study has several limitations. First, the pigs in this study were evaluated within 4 wk after MI. Because this is a subacute healing phase post MI, our findings may not apply to the chronic MI setting. Second, GLS was calculated using the single plane image due to the technical difficulties in obtaining multiple longitudinal images in pigs. Therefore, GLS may be more or less sensitive in detecting elevated LV filling pressure when the average of three apical views is used. Third, we did not include circumferential and radial strain values in this study because GLS is the most validated and widely used parameter in the strain analysis (5).
Conclusion.
Impaired GLS assessed by speckle-tracking echocardiography was significantly associated with elevated LV filling pressure after MI. GLS had an incremental diagnostic value for detecting elevated LV filling pressure over conventional echocardiographic parameters in a pig model of MI and may be particularly useful for evaluating post-MI patients with preserved LVEF. Further studies are required to expand our findings in an effort to improve the management after MI.
GRANTS
This work was supported by National Institutes of Health R01 HL139963 (to K. Ishikawa), American Heart Association-Scientist Development Grant 17SDG33410873 (to K. Ishikawa), fellowships from the Uehara memorial Foundation (to T. Aikawa), the Kanzawa Medical Research Foundation (to T. Aikawa), the Suginome Memorial Foundation (to T. Aikawa), Nakayama Foundation for Human Science (to T. Aikawa), and Deutsche Herzstiftung (to O. Bikou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.A. and K.I. conceived and designed research; T.A., T.K., K.P.Y., S.M., O.B., S.T., and K.I. performed experiments; T.A. analyzed data; T.A. and K.I. interpreted results of experiments; T.A. prepared figures; T.A. drafted manuscript; K.P.Y., S.T., K.F., and K.I. edited and revised manuscript; T.A., T.K., K.P.Y., S.M., O.B., S.T., K.F., and K.I. approved final version of manuscript.
REFERENCES
- 1.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69: 1937–1948, 2017. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Bikou O, Watanabe S, Hajjar RJ, Ishikawa K. A pig model of myocardial infarction: catheter-based approaches. Methods Mol Biol 1816: 281–294, 2018. doi: 10.1007/978-1-4939-8597-5_22. [DOI] [PubMed] [Google Scholar]
- 3.Cerisano G, Bolognese L, Buonamici P, Valenti R, Carrabba N, Dovellini EV, Pucci PD, Santoro GM, Antoniucci D. Prognostic implications of restrictive left ventricular filling in reperfused anterior acute myocardial infarction. J Am Coll Cardiol 37: 793–799, 2001. doi: 10.1016/S0735-1097(00)01203-1. [DOI] [PubMed] [Google Scholar]
- 4.Cifra B, Mertens L, Mirkhani M, Slorach C, Hui W, Manlhiot C, Friedberg MK, Dragulescu A. Systolic and diastolic myocardial response to exercise in a healthy pediatric cohort. J Am Soc Echocardiogr 29: 648–654, 2016. doi: 10.1016/j.echo.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 69: 1043–1056, 2017. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Delgado V, Mollema SA, Ypenburg C, Tops LF, van der Wall EE, Schalij MJ, Bax JJ. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J Am Soc Echocardiogr 21: 1244–1250, 2008. doi: 10.1016/j.echo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, Ovize M, Derumeaux G. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr 10: 914–921, 2009. doi: 10.1093/ejechocard/jep095. [DOI] [PubMed] [Google Scholar]
- 8.Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Kutty S, Gutberlet M, Hasenfuß G, Thiele H, Schuster A. Cardiac magnetic resonance myocardial feature tracking for optimized prediction of cardiovascular events following myocardial infarction. JACC Cardiovasc Imaging 11: 1433–1444, 2018. doi: 10.1016/j.jcmg.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Ersbøll M, Valeur N, Mogensen UM, Andersen M, Greibe R, Møller JE, Hassager C, Søgaard P, Køber L. Global left ventricular longitudinal strain is closely associated with increased neurohormonal activation after acute myocardial infarction in patients with both reduced and preserved ejection fraction: a two-dimensional speckle tracking study. Eur J Heart Fail 14: 1121–1129, 2012. doi: 10.1093/eurjhf/hfs107. [DOI] [PubMed] [Google Scholar]
- 10.Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 61: 2365–2373, 2013. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Hammoudi N, Watanabe S, Bikou O, Ceccaldi A, Fish K, Yamada KP, Miyashita S, Lebreton G, Hajjar RJ, Ishikawa K. Speckle-tracking echocardiographic strain analysis reliably estimates degree of acute LV unloading during mechanical LV support by Impella. J Cardiovasc Transl Res 12: 135–141, 2019. doi: 10.1007/s12265-018-9812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillis GS, Møller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43: 360–367, 2004. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, , et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39: 119–177, 2018. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa K, Aguero J, Oh JG, Hammoudi N, Fish LA, Leonardson L, Picatoste B, Santos-Gallego CG, Fish KM, Hajjar RJ. Increased stiffness is the major early abnormality in a pig model of severe aortic stenosis and predisposes to congestive heart failure in the absence of systolic dysfunction. J Am Heart Assoc 4: e001925, 2015. doi: 10.1161/JAHA.115.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liou K, Negishi K, Ho S, Russell EA, Cranney G, Ooi SY. Detection of obstructive coronary artery disease using peak systolic global longitudinal strain derived by two-dimensional speckle-tracking: a systematic review and meta-analysis. J Am Soc Echocardiogr 29: 724–735.e4, 2016. doi: 10.1016/j.echo.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Møller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation 114: 438–444, 2006. doi: 10.1161/CIRCULATIONAHA.105.601005. [DOI] [PubMed] [Google Scholar]
- 17.Munk K, Andersen NH, Nielsen SS, Bibby BM, Bøtker HE, Nielsen TT, Poulsen SH. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr 12: 156–165, 2011. doi: 10.1093/ejechocard/jeq168. [DOI] [PubMed] [Google Scholar]
- 18.Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Bøtker HE, Nielsen TT, Poulsen SH. Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr 25: 644–651, 2012. doi: 10.1016/j.echo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD.. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17: 1321–1360, 2016. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Nijland F, Kamp O, Karreman AJ, van Eenige MJ, Visser CA. Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: a serial Doppler echocardiographic study. J Am Coll Cardiol 30: 1618–1624, 1997. doi: 10.1016/S0735-1097(97)00369-0. [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102: 1788–1794, 2000. doi: 10.1161/01.CIR.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 23.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539–2550, 2007. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 25.Reichek N, Wilson J, St John Sutton M, Plappert TA, Goldberg S, Hirshfeld JW. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation 65: 99–108, 1982. doi: 10.1161/01.CIR.65.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Sjøli B, Ørn S, Grenne B, Vartdal T, Smiseth OA, Edvardsen T, Brunvand H. Comparison of left ventricular ejection fraction and left ventricular global strain as determinants of infarct size in patients with acute myocardial infarction. J Am Soc Echocardiogr 22: 1232–1238, 2009. doi: 10.1016/j.echo.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Steendijk P, Staal E, Jukema JW, Baan J. Hypertonic saline method accurately determines parallel conductance for dual-field conductance catheter. Am J Physiol Heart Circ Physiol 281: H755–H763, 2001. doi: 10.1152/ajpheart.2001.281.2.H755. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol 67: 1674–1683, 2016. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe S, Fish K, Kovacic JC, Bikou O, Leonardson L, Nomoto K, Aguero J, Kapur NK, Hajjar RJ, Ishikawa K. Left ventricular unloading using an Impella CP improves coronary flow and infarct zone perfusion in ischemic heart failure. J Am Heart Assoc 7: e006462, 2018. [Erratum in J Am Heart Assoc 7: e004250, 2018]. doi: 10.1161/JAHA.117.006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 58: 751–760, 1976. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]