Abstract
Exosomes are a subtype of extracellular vesicles. They range from 30 to 150 nm in diameter and originate from intraluminal vesicles. Exosomes were first identified as the mechanism for releasing unnecessary molecules from reticulocytes as they matured to red blood cells. Since then, exosomes have been shown to be secreted by a broad spectrum of cells and play an important role in the cardiovascular system. Different stimuli are associated with increased exosome release and result in different exosome content. The release of harmful DNA and other molecules via exosomes has been proposed as a mechanism to maintain cellular homeostasis. Because exosomes contain parent cell-specific proteins on the membrane and in the cargo that is delivered to recipient cells, exosomes are potential diagnostic biomarkers of various types of diseases, including cardiovascular disease. As exosomes are readily taken up by other cells, stem cell-derived exosomes have been recognized as a potential cell-free regenerative therapy to repair not only the injured heart but other tissues as well. The objective of this review is to provide an overview of the biological functions of exosomes in heart disease and tissue regeneration. Therefore, state-of-the-art methods for exosome isolation and characterization, as well as approaches to assess exosome functional properties, are reviewed. Investigation of exosomes provides a new approach to the study of disease and biological processes. Exosomes provide a potential “liquid biopsy,” as they are present in most, if not all, biological fluids that are released by a wide range of cell types.
Keywords: exosomes, methods, stem cells therapeutics
OVERVIEW OF EXOSOMES
The first observation of extracellular particles and vesicles was in platelet-free serum in 1946 (30). Since then, a substantial number of studies on extracellular vesicles (EVs) have been published (9, 43, 203, 230). Over time, numerous types of EVs have been characterized and assigned various names based on their size, biogenesis, and origin/function such as oncosomes, ectosomes, texosomes, cardiosomes, apoptotic bodies, microvesicles, and exosomes (235). There are overlaps among the different subgroups. However, it is also evident that within these EV populations there are even more subpopulations based on vesicle diameter, density, RNA, DNA, and protein cargo (198).
Currently, EVs are classified into one of three broad categories based on their biogenesis: apoptotic bodies, microvesicles, and exosomes (118). Apoptotic bodies are heterogeneous in size, with an estimated diameter ranging from 200 to 5,000 nm, and are shed from dying cells undergoing programmed cell death (4). Microvesicles, which are also referred to as ectosomes, are shed from the plasma membrane of viable cells and are 100–800 nm in diameter (165, 177). Exosomes are 30–150 nm in size and are released into the extracellular space when multivesicular bodies fuse with the plasma membrane (136). However, it has been demonstrated that vesicles smaller than 100 nm in diameter can bud directly off the plasma membrane (74, 140). EVs can be separated and purified based on their density (194), but the isolated EVs can be heterogeneous, likely due to coisolation of different EV subpopulations during the various purification steps (88). It has been suggested that the vesicles should more properly be called 2 K, 10 K, and 100 K vesicles, or potentially medium/large and small EVs, or high- and low-density EVs, as the biogenesis of each vesicle is indeterminate and might be independent of its size and density (231). Further effort is needed to distinguish among the different types of EVs in a meaningful and useful manner. Size is one parameter, but a greater understanding of the differences among EVs will help focus the field and future studies. Since the exosomal membrane retains the characteristics and orientation of the plasma membrane of the cell(s) of origin, investigation of exosomal membrane proteins can provide further insight into the parent cell. Furthermore, known membrane proteins for a given tissue can potentially be used to immunoprecipitate tissue-specific exosomes from plasma and serum samples.
Virtually all cells are capable of secreting types of EVs, and this process is conserved throughout evolution, from bacteria to humans (66, 112). The secretion of EVs was initially reported as the mechanism for eliminating unneeded proteins from maturing reticulocytes (78). However, we now know that EVs have the capacity to exchange components between cells, ranging from nucleic acids to lipids and proteins, and to act as signaling vehicles in homeostatic and pathological cellular processes (169). In the 1990s, EVs were reported to be secreted by B cells and dendritic cells and possessed immunoregulatory properties in antitumoral immune responses (26, 164). Since then, studies of EV cargo and signaling properties have been widely published across a broad range of fields, including cardiovascular disease (22, 32, 54, 65, 171, 181). There is now evidence that cells fine-tune the cargo of EVs, with the rate of secretion depending on their physiological state and exposure to various stimuli (7, 185). Once released into the extracellular space, EVs come into contact with recipient cells and elicit functional responses, promoting phenotypic changes that affect target cells’ physiological status (49, 51). EV-mediated intercellular communication can be achieved through various mechanisms, from surface-bound EV ligands binding target cell receptors to initiate signaling cascades to EV internalization either through endocytosis or by fusion with target cells (136).
The first examples of the signaling properties of EVs docking to cells were from B cells and dendritic cells, where EVs were able to present antigens to T cells and induce a specific antigenic response (3, 135, 197). Tumor-derived EVs were shown to transport fibronectin, which bound to integrins on nontransformed fibroblasts and promoted their anchorage-independent growth (one hallmark of tumorigenesis) (13). Similarly, EVs are released along the surface of trophoblasts, thereby promoting embryo implantation (193).
EVs can also transport various lipid species, including sphingomyelin, eicosanoids, fatty acids, and cholesterol, thereby mediating the regulation of cellular bioactive lipid species (166). An example of EV-mediated transfer of pathological material is amyloid proteins (162). Amyloid-β peptide and prion protein (PrP) were found to be present on the surface of EVs, whereas TAR DNA-binding protein and α-synuclein are intraluminal in EVs (56, 58, 145, 155). Consequently, EVs have been found to mediate intercellular spreading of amyloids in neurological disorders (172).
Exosomes have a diabolical role in cancer, as after being released by cancer cells they have been found to promote the spread of cancer by preparing a niche for metastases or premetastases (15, 53, 125). Furthermore, exosomes appear to avoid immune surveillance and have the potential through their cargo to spread drug resistance (53, 125). Although the lipid composition of exosomes is different from that of the parent cells, the function studies of lipids in exosomes is limited (53). Ceramide, a member of the sphingolipid family, is essential for exosome formation, and it is also enriched in EVs Lipids in the exosomal membrane may organize and form “mobile rafts” that convert exosomes into signalosomes, which promote the activation of cell signaling pathways for oncogenesis and metastasis. It is thought that ceramide may modify the function of mobile rafts and their effect on these signaling pathways (53, 125).
An early key study showing a direct link between EVs and tumor invasion of healthy tissues demonstrated that expression of epidermal growth factor receptor (EGFR)vII in glioma cells resulted in markedly enhanced vesiculation, which was detectable in the blood of tumor-bearing mice (6, 70). The resulting EVs transported this oncoprotein to adjacent tumor cells, which led to the production of angiogenic factors such as vascular endothelial growth factor (VEGF) (5). Similar studies by Skog et al. (180) demonstrated that human primary glioblastoma cell-derived EVs transferred not only EGFR but also numerous microRNAs (miRNAs) that stimulated tumor growth and angiogenesis. That group was subsequently able to isolate similar tumor-derived EVs from the blood of patients before treatment (180). Thus, exosomes released by cancer cells can be used to follow the clinical disease state. This foundational work led to the one of the first EV startup companies, Exosome Diagnostics, to develop EV-based biomarkers in the oncology field (175). It is possible that the cancer exosomes could be modified to target metastases and even the original tumor through a cytotoxic cargo. The role of exosomes in cancer is a focus of much research at this time, and new developments may lead to new therapeutic targets as well as new diagnostic and prognostic approaches.
HEART-DERIVED EXOSOMES
Our laboratory was the first to demonstrate that exosomes were produced by adult cardiomyocytes isolated from rat hearts and that these exosomes could be readily purified and characterized. We identified exosomes as the mechanism by which heat shock protein-60 (HSP60) was released from cardiomyocytes (75). The release of exosomes from the cardiomyocytes was augmented by mild hypoxic stress in the absence of necrosis, and the release was inhibited by dimethyl amiloride (DMA; an exosome inhibitor) or methyl-β-cyclodextrin (MBC; an inhibitor of lipid raft formation) with or without hypoxic stress (75). Our later work demonstrated that moderate and heavy consumption of alcoholic beverages, simulated by low and high ethanol concentrations in culture, would increase the release of exosomes from cardiomyocytes (122). Ethanol exposure induced a greater release of exosomes than hypoxia/reoxygenation. Pretreating the cardiomyocytes with a combination of three different antioxidants that inhibit reactive oxygen species (ROS) formation markedly decreased acetylcholine esterase activity, a marker of exosome abundance (122). Exosomal content reflects the cell of origin and cellular stress conditions (8, 86). Although some proteins would commonly be present in the exosomes derived from cardiomyocytes, exposure to these different stimuli resulted in differences in the exosomal protein content, when analyzed by mass spectrometry (122). Some of the proteins, such as HSP27 and HSP90, were found only in hypoxia/reoxygenation-derived exosomes, whereas several other proteins were found only in ethanol-derived exosomes. Unsurprisingly, others have shown that exosomal RNA content also differs depending on the type of stress induced, as shown in endothelial cells (47).
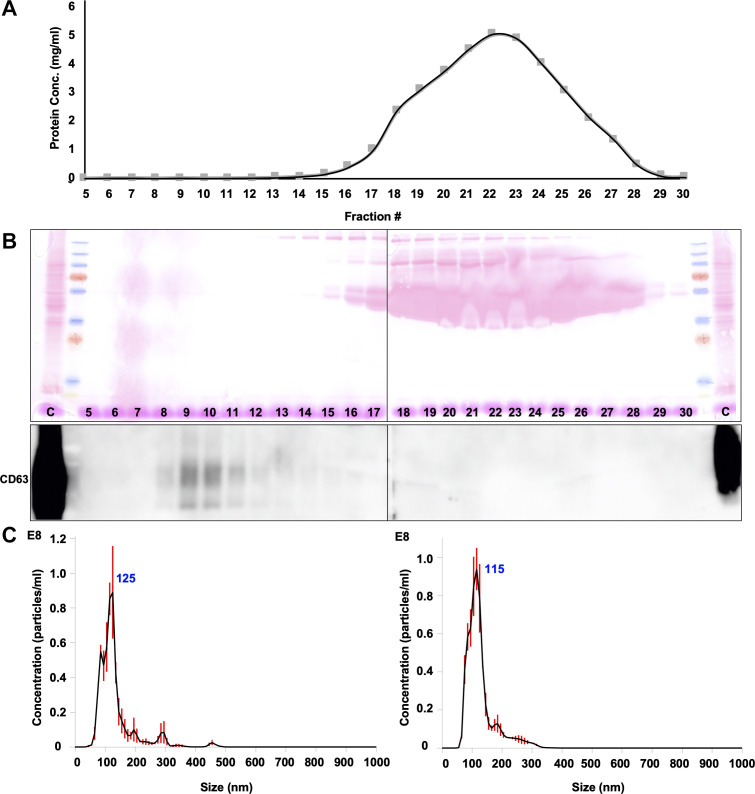
Bodily fluids and cell culture media contain not only exosomes but abundant amounts of protein, which can confound any analysis of the content of exosomes. Serum and plasma samples have a very large amount of protein, given the high concentrations of albumin and γ-globulins in the blood. It is imperative to separate the exosomes from these other molecules to have a valid analysis of the exosomal contents. We have found that the most efficient way to do this is by size exclusion chromatography (SEC) (Fig. 1), which is quite simple to perform. It separates the large exosomes, which readily pass through the column, from many proteins present in bodily fluids and in culture media. Importantly, the column matrix can be washed, autoclaved for sterility, and reused. This is a more straightforward method to isolate clean exosomes than some other approaches.
Fig. 1.
Exosome isolation using size exclusion chromatography (SEC). Endothelial cells were cultured in medium containing 5% exosome-depleted FBS. The supernatant was collected and ultrafiltered after 24 h and then processed in SEC with 10 mL of bed volume. Each 500 μL of fraction was collected after the sample was added on top of the column. A: protein concentration of each fraction was determined using Nanodrop. B: Western blot was performed using 2 separate gels to detect the distribution of vast number of proteins and exosomes in fraction 5–30. Ponceau red stain confirmed that the vast number of proteins were eluted in later fractions, whereas anti-CD63 detected exosomes in early fractions. C, parent cell lysates as positive control. C: nanoparticle tracking analysis confirmed the exosome sizes in F9 (left) and F10 (right).
The biological content of exosomes is protected by their lipid bilayer. Despite exposure to various physiological and pathological environments, such as ethanol consumption, change in pH, as seen with diabetic ketoacidosis, or fever, exosomes remained very stable and did not release their contents (122). Given the stability of exosomes, they are reliable carriers of the functional content from the releasing cells to recipient cells. For example, both in vitro hypotonic mechanical stretch and in vivo pressure overload induced the cardiac secretion of exosomes containing angiotensin II (ANG II) type 1 receptors (AT1Rs) (159). The AT1R-enriched exosomes were biochemically functional, showing internalization of AT1Rs in the recipient cells after ANG II stimulation. Injection of AT1R-enriched exosomes modulated blood pressure in response to ANG II infusion in AT1R knockout mice (159). Valadi et al. (203) demonstrated the presence of mRNA and miRNA in the exosomes derived from different cell types and species and that mouse RNA was transferable via exosomes to human cells, in which the new mouse proteins were then confirmed. Another study identified 1,520 mRNA and 343 chromosomal DNA sequences in the exosomes derived from adult mouse cardiomyocytes in culture (216). The stained DNA and RNA of cardiomyocyte exosomes were detected in the target fibroblasts within 3 h of incubation, and induced changes in the expression of 333 genes, demonstrating that cardiomyocytes can communicate intercellularly via exosomes (216).
Cardiac remodeling is a complex process involving cardiomyocyte hypertrophy and cardiac fibrosis that lead to alterations in ventricular function and structure in response to hypertension, valve disease, ischemic cardiac disease, or cardiac damage. Cardiac fibroblasts are essential in pathological cardiomyocyte hypertrophy in the mouse heart when subjected to transverse aortic constriction in vivo (190). Newborn rat cardiomyocytes cultured in fibroblast-conditioned medium manifested hypertrophy, reduced contractile activity, and increased expression of vimentin (105). This effect of cardiac fibroblasts on cardiomyocyte hypertrophy was likely mediated through paracrine or autocrine pathways, involving cardiac fibroblast-associated cytokines, chemokines, and miRNAs (20, 105, 190). Cardiac fibroblasts have been found to secrete exosomes enriched in star (*) miRNAs, which normally are degraded intracellularly. MiR-21*, one of the fibroblast exosomal derived RNA molecules, was analyzed as a potent paracrine mediator repressing sorbin and SH3 domain-containing protein-2 (SORBS2) and PDZ and LIM domain 5 (PDLIM5) in cardiomyocytes. Silencing either SORBS2 or PDLIM5 in cardiomyocytes resulted in significant cellular hypertrophy (20). In one in vivo study, miR-21* was significantly increased in the pericardial fluid of mice with left ventricular pressure overload–induced hypertrophy, and antagonism of miR-21* in mice was able to attenuate ANG II-induced cardiac hypertrophy (20). ANG II has been shown to enhance exosome release from cardiac fibroblasts that in turn augment ANG II production in cardiomyocytes (120). After administration of exosome inhibitors GW4869 and DMA in ANG-II-treated adult mice, both basal and ANG II-induced exosome release from the isolated cardiac fibroblasts was inhibited, and ANG II-induced cardiac hypertrophy and fibrosis were also prevented (120).
Since cardiomyocytes secrete exosomes, researchers also investigated the role of cardiomyocyte-derived exosomes in the progression of heart failure. Among heart-specific miRNAs, miR-208a levels were shown to be consistently higher in both serum and heart tissue in heart failure induced by either doxorubicin injection or left anterior descending (LAD) coronary artery ligation (236). The increase in miR-208a was confirmed as coming from cardiomyocytes stressed by hypoxia or ANG II in vitro and in the released exosomes, whereas the expression of miR-208a did not change in fibroblasts. The exosomes derived from the stressed cardiomyocytes promoted cardiac fibroblast proliferation, differentiation into myofibroblasts in vitro, and increased cardiac fibrosis in normal rats. Inhibiting miR-208a mitigated these effects in vitro and preserved cardiac function in the infarcted heart (236). Diabetes is a well-known cause of microvascular insufficiency and a risk factor of heart failure. Endothelial dysfunction and angiogenesis impairment are associated with the development of heart failure. Cardiomyocytes isolated from type 2 diabetic rats as well as cardiomyocytes treated with high glucose have been shown to release exosomes enriched in antiangiogenic miR-320 (219). Endothelial cells that incorporated these exosomes exhibited inhibited proliferation, migration, and tube-like formation with the downregulation of IGF-I, HSP20, and Ets2 which are targets of exosomal miR-320 (219).
In contrast to the potentially deleterious effects of the exosomes released in pathological conditions, modified exosomes have a more protective role when released from the heart in the case of diabetes. Cardiac-specific overexpression of HSP20 promoted the production of exosomes, which encapsulated higher levels of antioxidant proteins, protecting cardiomyocytes and endothelial cells against hyperglycemia-induced stress (218). Injection of exosomes derived from cardiomyocytes overexpressing HSP20 attenuated cardiac dysfunction and remodeling in mice whose diabetes was induced by streptozocin (STZ) (218). As discussed, external stimuli can alter the secretion of exosomes, as well as their cargo. Glucose starvation enhanced exosome production by H9C2 cardiomyocytes (64). These exosomes promoted endothelial cell proliferation and tube formation. The enhancement correlated with the upregulated exosomal expressions of miR-17, -19a, -19b, -20a, -30c, and -126, which promote angiogenesis (64). Matrix metalloproteinase-9 (MMP-9), a member of the zinc-metalloproteinase family, is secreted by various cell types, including cardiomyocytes, fibroblasts, and endothelial cells. MMP-9 knockout in diabetic mice has been shown to improve cardiac repair (130). Exercise was later found to stimulate exosome production and downregulate MMP-9 expression in the diabetic heart (31). Altered expression of MMP-9 was associated with markedly upregulated miR-29b and miR-455 in the exosomes secreted after exercise (31).
Circulating exosomes secreted from other cell types can impact the heart or vasculature. Platelets are a major source of plasma exosomes (237). In sepsis, exosome release from platelets was increased secondarily to the increase in nitric oxide (NO) and lipopolysaccharide (LPS). These exosomes subsequently induced caspase-3 activation and apoptosis in endothelial cells, leading to vascular dysfunction, which is a key mediator in sepsis induced shock, which leads to death (62). They also negatively affected myocardial function, as shown in studies of isolated rabbit hearts and rat papillary muscle (14). This may be secondary to the intrinsic production of NO by these septic platelet-derived exosomes (14). Endothelial cells and immune cells also contribute to plasma exosomes. Pretreatment with preconditioned endothelial cell-derived exosomes protected rat cardiomyocytes from cell death secondarily to simulated ischemia/reoxygenation (46). Exosomes can be derived from both activated endothelial cells and apoptotic endothelial cells. These exosomes unsurprisingly, given their different origins, play different roles. The ratio of circulating EVs derived from activated versus apoptotic endothelial cells might be associated with the severity of heart failure (24). As immune cells play sophisticated roles in the progression of various diseases, including cardiovascular disease, interest in the immune response and its modulation by immune cell-derived exosomes on the injured heart is increasing and was recently addressed in a comprehensive review (232).
CARDIAC REGENERATION VIA EXOSOMES
Nearly 20 years of stem cell research on the treatment of myocardial infarction has generated promising data in animal models as well as in clinical trials (35, 102, 184, 186). This body of work has shown the safety of these therapies, whereas demonstrable efficacy is yet to be fully determined (25, 60, 87). The transplanted stem cells can attenuate expansion of infarct size and preserve the indexes of global cardiac function, thus preventing left ventricular remodeling (40, 199, 246). Some reports suggest that the underlying mechanism of action of transplanted bone marrow-derived stem cells is their ability to adopt a competent cardiomyocyte phenotype, providing de novo myocardial regeneration (45, 241). However, this hypothesis has been challenged by several research groups (127). More conventional studies have established that bone marrow-derived mesenchymal stem cells (MSCs) secrete bioactive molecules such as growth factors and cytokines that potentiate tissue repair and possess immunoregulatory properties (44, 113). Such canonical secretory signaling proteins have been the main research focus of MSCs’ therapeutic properties. However, an increasing number of studies support that much of the tissue healing and immunoregulatory properties of MSCs are derived from the exosomes they secrete (108, 109, 173).
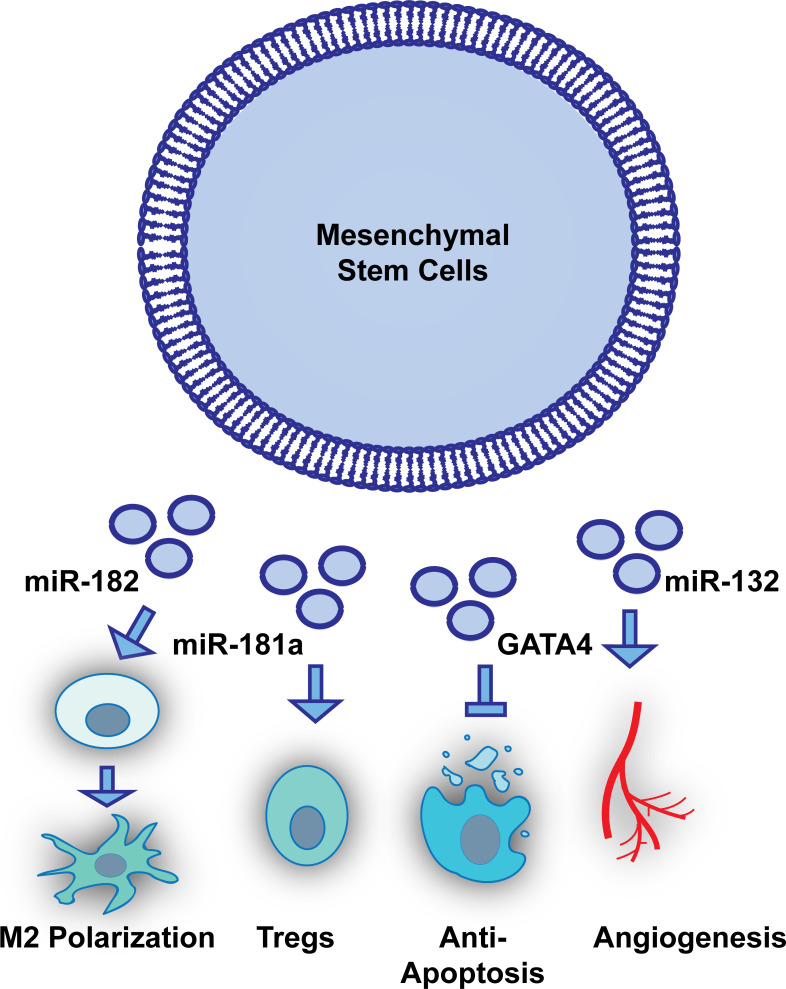
Several studies have established that exosomes isolated and purified from medium that has been conditioned by MSCs possess angiogenic and regenerative properties both in vitro and in animal models of myocardial infarction (107, 121, 195, 245). Several studies have demonstrated that the regenerative properties of MSC-derived exosomes can be enhanced through cell culture-based priming conditions, such as hypoxia, serum deprivation, and cytokine stimulation (33, 67, 119). Additional studies have shown that engineering of the miRNA cargo of exosomes is feasible and may enhance MSC exosomes’ regenerative properties, including enhanced angiogenic ability of endothelial cells and reduced proliferation rates of activated T cells (Fig. 2) (104, 121, 221, 243). Other groups have used genetic engineering approaches to enrich the protein cargo of MSC exosomes (110, 157, 226). For example, MSCs transduced with GATA4 produced exosomes presenting higher levels of GATA4 (ExoGATA4), which significantly induced expression of antiapoptotic miR-19a and miR-451 (238). GATA4 is a transcription factor highly expressed in cardiomyocytes and important for cardiomyocyte survival and function in the adult heart (156). ExoGATA4 enhanced cardioprotection by augmentation of viable cardiomyocytes after exposure to hypoxic stress, improving cardiac function and reducing infarction size in the heart following permanent LAD ligation (238). ExoGATA4 were distributed in the border zone of ischemic rat myocardium, where miR-19a was also upregulated (238). Some groups have also used MSC exosomes to precondition cardiac progenitor cells (CPCs.) After being preconditioned with MSC exosomes, CPCs were found to stimulate proliferation, migration, and angiotube formation in a dose-dependent manner (242). CPCs preconditioned with MSC exosomes enhanced differentiation into neovasculature, reduced fibrotic area, and preserved the cardiac function (242).
Fig. 2.
Engineering of the exosomal cargo is feasible to study mesenchymal stem cell (MSC) exosomes’ regenerative properties and may enhance regenerative efficacy in myocardial injury. Figure shows some examples that modulating or overexpressing miRNAs or proteins in MSC exosomes can protect the heart from ischemia injury directly through reducing cardiomyocyte apoptosis (238) and promoting endothelial angiogenesis (121) or indirectly through immunomodulation, such as M2 macrophage polarization (243) or regulatory T (Treg) cell activation (221).
CPCs alone release exosomes enriched with GATA4-responsive-miR-451 (34). These exosomes inhibited oxidative stress induced caspase-3/7 activation in H9C2 cardiomyocytes and protected the mouse heart by significantly reducing myocardial apoptosis after ischemia/reperfusion (I/R) (34). Preconditioning CPCs with oxidative stress induced higher levels of exosomal secretion and resulted in significantly upregulated miR-21 in these exosomes compared with those derived from untreated CPCs (233). Whereas oxidative stress caused the reduction of miR-21 and increase of cleaved caspase-3 in H9C2 cells, pretreatment with CPC-derived exosomes was protective of the oxidatively stressed H9C2 cells, showing increased miR-21 and resistance to apoptosis. This protective function was amplified in exosomes derived from oxidatively stressed CPCs. The underlying mechanism may involve programmed cell death 4 (PDCD4), a target gene of miR-21 (233). Another group found that 11 miRNAs were upregulated in the exosomes derived from CPCs in response to hypoxia (71). The exosomes from hypoxic CPCs induced endothelial tube formation and reduced profibrotic gene expression in TGFβ-stimulated fibroblasts in vitro and improved cardiac function and reduced fibrosis in the I/R-injured heart (71).
As induced pluripotent stem cells (iPSCs) have a high capacity of cell differentiation that is beneficial to cardiac repair and regeneration, the therapeutic efficacy of exosomes derived from iPSCs or iPSC-derivatives is gaining interest. The iPSC-derived exosomes have been shown to be enriched with cardioprotective miRNAs, miR-21 and miR-210, which are regulated by Nanog and hypoxia-inducible factor-1α. These exosome miRNAs were transferrable to H9C2 cells and protected the cells from oxidative stress-induced apoptosis. Intramyocardial injection of iPSC-derived exosomes immediately after myocardial infarction and before reperfusion was cytoprotective via suppressing apoptosis in the injured heart (220). Another study demonstrated that iPSC-derived EVs, including the fraction of exosomes, were enriched with miRNAs and proteins that are proangiogenic and cytoprotective. The iPSC-EVs enhanced angiogenic capacity, migration, and survival in cardiac endothelial cells. While iPSCs exerted cytoprotective effects on the heart undergoing I/R, iPSC-EVs induced superior cardiac repair, showing attenuated left ventricle dysfunction and hypertrophy and improved neovascularization (2).
CONSIDERATIONS FOR REGENERATIVE EXOSOME SOURCE MATERIAL
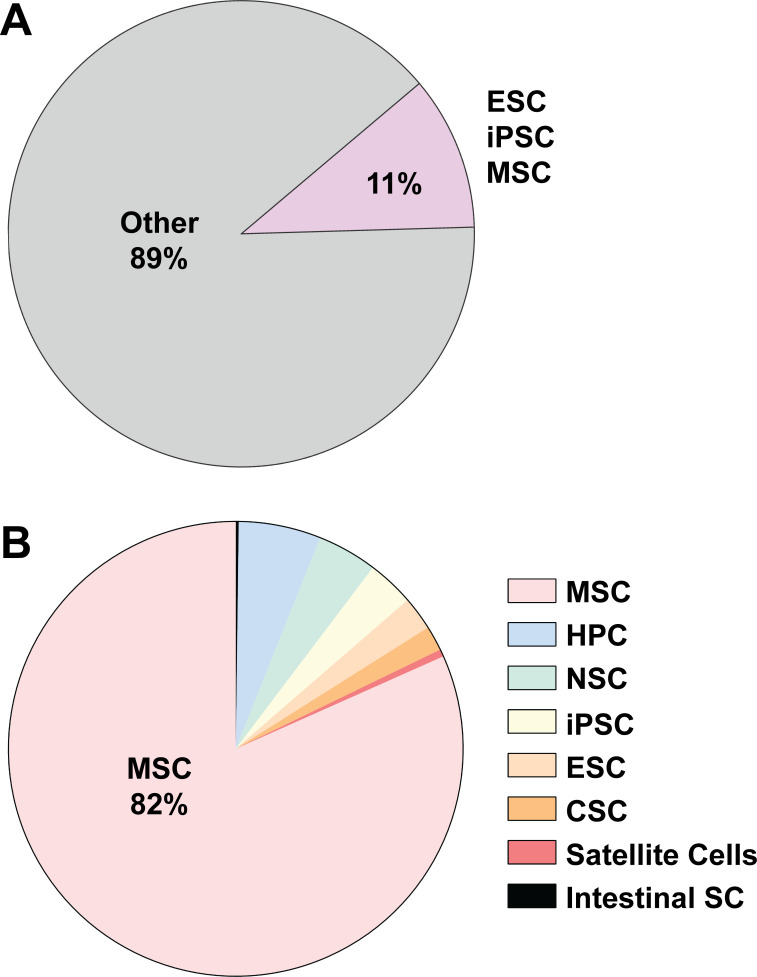
There is growing interest in the evaluation of stem cell-derived EVs (Fig. 3) in terms of elucidating their biological role in development and disease and as novel therapeutic approaches (10, 52, 131, 153, 176, 198, 239). The verification of stem cell lines should be given careful consideration due to their inherent sensitivity to the dynamic microenvironmental stimuli experienced during culture (80, 160, 214, 228). For example, MSCs are the most widely studied stem cell source of EV studies to date (52). MSCs possess limited doubling capacity and specific culturing density requirements and have been observed to modulate their morphology and therapeutic properties with continuous culturing (37, 84, 215). Therefore, studies of EVs derived from stem cells necessitate special attention to these factors, which should be reported in detail to allow readers to interpret the state of the parental cell line.
Fig. 3.
Distribution of extracellular vesicle (EV) publications across stem cell classes. A and B: PubMed searches using key terms exosomes, microvesicles, extracellular vesicles, stem cells, MSCs, HPCs, NSCs, iPSCs, ESCs, CSCs, satellite cells, and intestinal stem cells were used to determine the fraction of EV publications associated with different types of stem cells. MSC, mesenchymal stem cell; HPC, hematopoietic progenitor cell; NSC, neural stem cell; iPSC, induced pluripotent stem cell; ESC, embryonic stem cell; CSC, cancer stem cell.
Stem cell lines should be validated for the presence of definitive markers associated with their respective cell type (97, 234). Generally reported markers for verifying pluripotency in embryonic stem cells (ESCs) and iPSCs are OCT4, SOX2, Nanog, SSEA4, TRA-1–60 (PODXL), and TRA-1–81 (PODXL) (29, 76, 244). Some studies also report the presence of additional markers, including SSEA3, LIN28, and SSEA5 (42, 143, 192). For iPSC validation, a verified ESC line like H9 should be used as a positive control for marker expression using either qPCR or immunocytochemistry analysis (114, 117). The karyotypic stability of ESC/iPSC lines should also be established to verify the absence of gross chromosomal abnormalities (188). The surface markers that define MSCs are CD73, CD90, and CD105, which are typically reported as more than 90% positive expression (128). In addition, the trilineage differential potential of MSCs should be demonstrated (adipocyte, chondrocyte, osteoblast) (170).
EXOSOME ISOLATION
Ultracentrifugation.
Ultracentrifugation has been used as a “gold standard”. Samples are cleaned of debris with low-speed centrifugation first (500–10,000 g), and then ultracentrifugation is applied to pellet the exosomes, with or without a sucrose cushion, or to separate exosomes in sucrose gradient (1.15–1.19 g/mL) (196). The advantage of ultracentrifugation for exosome isolation is its capacity for large sample volumes (Table 1). The speed and duration are determined depending on rotor dimensions. One of the most commonly used ultracentrifuge rotors, SW28 Ti (Beckman Coulter), takes two 100,000 g centrifugations with a minimum of 70 min per run (one for pelleting exosomes and contaminant proteins, followed by one for washing exosomes in PBS) (196). However, the sequential centrifugation requires a significant amount of work and expensive ultracentrifuge equipment. In addition, viscous samples have lower sedimentation efficiency. Increasing ultracentrifugation duration might improve pellet output, but the particle sizes are variable due to protein aggregation or damaged particles. When isolating exosomes from serum samples or cell conditioned media containing serum supplements, these serum proteins might also be pulled down and therefore affect exosome analysis. Serum-free media may be used when isolating exosomes from cell culture. However, serum deprivation will likely affect cell physiology. The duration of serum deprivation needs evaluation depending on the cell type and experimental requirements. It is generally considered that EV isolation via density gradients such as sucrose or OptiPrep yields the most purified exosome populations compared with precipitation and immunoaffinity methods (1, 92, 205). Therefore, the use of density gradient isolation methods can be considered in studies aiming to characterize and investigate the factors specifically packaged into exosomes (230). However, such isolation methods are thought to greatly diminish the functional properties of the resulting vesicle population, thereby limiting the utility of this approach for many studies (27). Alternatively, SEC, which is less expensive because the column matrix can be repeatedly reused after a simple washing, has been shown to be comparable to the density gradient method in terms of exosome purity and yield (116). SEC has also been used to remove OptiPrep remnants in isolated EV samples (211).
Table 1.
Comparison of exosome isolation methods
| Isolation Method | Principle | Pro | Con |
|---|---|---|---|
| Size exclusion chromatography (SEC) | Separate exosomes from other proteins based on size difference | Good exosome purity and reproducibility. Good yield. Good method to remove nonexosomal proteins before proteomics work |
May require ultrafiltration prior and post-SEC. |
| Anion exchange chromatography (AEC) | Separate exosomes from other proteins based on the surface charge | Scalable. Good exosome purity and reproducibility. No pre-AEC concentration of sample required. | Requires ultrafiltration concentration post-AEC. |
| Affinity purification | Pull down exosomes using antibodies that bind to exosome surface antigens | Specific and high exosome purity. Possible to selectively isolate subset of exosomes. | Low yields and sample capacity. Require prior analysis to select exosome surface markers. |
| Ultracentrifugation | Sequential centrifugation based on exosome density and size | Large sample capacity and exosome yield. Sucrose gradient can increase exosome purity. | Lengthy process and labor intensive. High equipment cost. Reduced efficiency when sample viscosity increased. Exosomes might be damaged and can clump together due to high speed centrifugation. |
| Polyethylene glycol-based reagents | Precipitate exosomes based on less solubility in the reagent | Easy procedure. Only basic benchtop centrifuge needed. High yield. | Coprecipitated proteins and reagents can contaminate exosomes and affect downstream applications. |
| Tangential flow filtration | Generate fluidics flow that is tangential to a filter membrane | Scalable. Moderate exosome purity. No expensive, specialized equipment. High yield. | Yield may be reduced due to substantial contact with plasticware. Some smaller vesicles lost with 100-kDa molecular weight cut-off membranes. |
Commercial polyethylene glycol-based reagents.
Since ultracentrifuges are expensive, and using them for isolation is a lengthy process, commercial polyethylene glycol (PEG)-based reagents have been developed to precipitate exosomes with a standard countertop centrifuge. However, the exosome-enriched pellet is contaminated with residual PEG polymer and other soluble proteins (154). An alternate method to enrich exosomes using the countertop centrifuge is centrifugal ultrafiltration. Smaller impurities are allowed to pass through the cellulose membrane of the ultrafilter system, with exosomes retained above the filter. The purity of the exosome samples will depend on the choice of filter pore sizes, whereas smaller pores can provide higher exosome yield but with more large protein complexes and aggregates.
Tangential flow filtration.
In a similar fashion to many of the other EV isolation techniques, tangential flow filtration (TFF) has been widely used in the virology field for some time (142). TFF systems generally use a peristaltic pump to generate fluidic flow that is tangential to a filter membrane. Polyethersulfone membranes with a molecular mass cutoff of 100 kDa are most commonly used for EV isolations (77, 81). Although there is no direct conversion from kilodaltons to nanometers, a 100-kDa pore roughly equates to a 10-nm diameter. TFF is fairly efficient at concentrating EVs and allows for a buffer exchange to wash away residual medium contaminants. It should be noted that the more plasticware the source material is exposed to, the more opportunity there is for EV loss due to their charged and sticky surfaces.
Size exclusion chromatography.
SEC, as discussed above, facilitates the removal of contaminating extracellular proteins and provides purer exosomes (21). SEC uses porous resin gravity or low-pressure systems. With this method, proteins equilibrate with the resin pores, delaying their passage through the column. On the other hand, exosomes are far too large to equilibrate with the resin pores and therefore pass through the column without delay (137). Sample particles separated in SEC are collected in fractions. The resolution (particle separation) is dependent on the selection of resins and packing dimensions. For example, some of the earliest reports of SEC-based isolation utilized Sepharose CL-2B resin, which has a mean pore size of ∼75 nm (111). More recent studies have reported that the use of Sepharose 4B or CL-4B resin, which has a smaller mean pore size of ∼42 nm, may be better suited for the separation of smaller EV fractions (23, 111). Generally, exosomes are collected in the earlier fractions, whereas the bulk of serum proteins elute in later fractions. Sample volumes ranging from 0.5 to 4% of packed resin volume can provide good resolution separation, and high resolution can be achieved if the sample volume does not exceed 2% (17). If sample starting volume is large, centrifugal ultrafiltration can be used to concentrate samples before the SEC (23). The sample should be equilibrated in the SEC elution buffer in the final step of ultrafiltration.
Anion exchange chromatography.
Anion exchange chromatography (AEC) has been previously used to isolate virus-like particles (VLPs) from cell culture supernatants and separate them away from contaminates (91, 147). AEC takes advantage of the net negative charge observed on the surface of EVs (from glycans/phospho/sulfo groups) to bind EVs to a positively charged chromatographic matrix (79, 101). This allows for minimally processed, precleared conditioned cell culture media to be run on AEC to maximize EV collection under relatively gentle isolation conditions. EVs can then be eluted by using a buffer that possess greater ionic strength than that of the surrounding mobile phase (e.g., higher sodium concentration). Differing glycosylation patterns from parental cell lines and other source material can affect the net charge of the cell and, presumably, the EV surface. Therefore, the optimal ion concentration in the elution buffer needs to be empirically determined for each source EV material. Filtration devices can be used to concentrate the EV fractions and perform a buffer exchange as needed.
Affinity purification.
The affinity purification method employs antibodies that capture exosomes by binding to specific surface markers, therefore limiting protein contamination in exosome isolation (18, 141). This can be done with antibodies targeting CD63 or other established surface markers. A further advantage of affinity purification is to pull exosomes derived from specific cell sources from serum samples by identifying a surface protein marker of interest. This approach is enhanced by the sequential use of two different antibodies to known proteins on the surface of the cell of interest. Although it has high specificity, it requires prior analysis of exosome surface markers and presumable exosome quantities and might result in selection bias from the total population. Furthermore, the yield of exosomes will be much lower with this method but potentially much more specific to the cells of interest.
BIOPHYSICAL AND BIOCHEMICAL CHARACTERIZATION OF EXOSOMES
Electron microscopy.
In electron microscopy (EM), a beam of electrons is focused by electromagnetic or electrostatic lenses to illuminate the sample of interest in a vacuum chamber (39). Because the wavelength of electrons is significantly shorter than that of visible light, EM has greater resolution and magnification than light microscopes (38). Ergo, EM has been widely used for particle sizing due to its excellent nanometer resolution (Table 2) (38, 167). Transmission electron microscopy (TEM) is the most common EM method (187). In TEM, the incident electrons pass through the extremely thin sections of sample or are scattered out depending on the sample structure. The transmitted electrons are magnified and projected to create an image on a fluorescent screen or a camera on the bottom of a TEM. Heavy metal stains may be required to enhance the image contrast, where immunolabeling with nanogold particles enables the recognition of samples presenting biomarkers. This approach will not work for regular monitoring of isolated exosomes, as it is very time consuming to prepare samples for EM and then analyze. However, it can be worthwhile to verify by a second method that exosomes are present in a sample using EM and antibodies to exosome markers (122).
Table 2.
Comparison of exosome characterization methods
| Technique | Principle | Resolution | Pro | Con |
|---|---|---|---|---|
| Electron microscope | Scattered electrons | 0.5 nm | Characterize exosome size and morphology. Possible for immunolabeling. |
Sample handling may result in morphological artifacts. |
| Atomic force microcopy | Reflected laser beam based on deflection of the cantilever | 1 nm | Characterize exosome size, morphology, and topology. Possible for immunocharacterization. | Probe and sample may be damaged in contact mode. |
| Dynamic light scattering | Brownian motion of the suspended particles | 1 nm–6 μm | Analyze exosome size distribution. Fast and easy procedure. |
No absolute quantification. Polydisperse populations results in less accuracy. Sensitive to large particles. |
| Nanoparticle tracking analysis | Brownian motion of the suspended particles. Analysis of particle distribution based on the Stokes–Einstein equation | 30 nm–1 μm | Characterize exosome size, size distribution, and concentration. May use for fluorescence detection, allowing immunocharacterization. | Requires sample exosomes within a limited concentration range. |
| Resistive pulse sensing | Change in electrical resistance | Depends on the selection of nanopore sizes on the elastic membrane | Characterize exosome size, size distribution, and concentration. | Heterogenous particles might need different nanopore sizes for measurements. |
| Flow cytometry | Scattered light | *100–200 nm | High throughput. Particle size and count. Possible for fluorescence detection, allowing immunocharacterization. | *Requires high-resolution flow cytometry. High particle concentrations might reduce the accuracy of event count. |
| Protein detection | Antibody affinity | N/A | Phenotyping. Western blot: semi-quantitation. ELISA: quantitation. |
Low sensitivity. No size information. |
| Mass spectrometry | N/A | Global characterization of protein content. Quantitative. | Purity of sample influences robustness of data. Requires specialized equipment and data analysis techniques. | |
| RNA detection | qPCR | N/A | Highly sensitive for both mRNA and miRNA. High throughput. | Normalization transcripts need to be empirically derived. |
| Transcriptomics | N/A | Highly sensitive and quantitative. | Need large sample size. Low throughput. |
N/A, not applicable.
Although TEM is able to provide morphological and biochemical information with the use of immunostaining, sample preparation has been reported to cause morphological artifacts, such as cup-shaped appearance of exosomes (90). To avoid the drawback of exosome deformation due to dehydration and chemical fixatives, cryoelectron microscopy (Cryo-EM) can be applied to image cryoembedded exosomes. The very low temperature in this technique can additionally protect the exosomes from radiological damage caused by the incident electron beam (187). Cryoelectron tomography has been reported to verify the spherical structure of exosomes (41) and ultrastructural components within exosomes (89).
Atomic force microcopy.
Atomic force microcopy (AFM) drives a cantilever with probe tips at its free end to assess nanoparticle morphology (179). When the probe tips interact with the particles while moving through, the deflection of the cantilever reflects a laser beam (152). The laser reflection is recorded and then analyzed for particle surface structure with resolutions down to ∼1 nm. This method has been applied to characterize exosomes derived from human saliva (174) or EVs derived from iPSCs (2). When the AFM tips are functionalized by being coated with antibody, this method can be used to characterize specific individual exosomes. The same group further used anti-CD63 IgG-coated AFM tips to detect exosomal CD63, showing stronger interaction force between the functionalized tips and the exosomes compared with the controls (174).
Dynamic light scattering.
The dynamic light scattering (DLS) system detects scattered laser light caused by Brownian motion of the suspended particles (151). It quantifies the average particle sizes ranging from nanometer to micrometer based on monodisperse populations (55). However, the size profile is less accurate if obtained from heterogeneous particle populations, where larger particles scatter more light.
Nanoparticle tracking analysis.
Similar to DLS, nanoparticle tracking analysis (NTA) also tracks suspended particles based on Brownian motion (16). This system is equipped with a camera to record the laser light scattered by moving particles at a fixed flow rate driven by a hydraulic pump (187). Each particle is tracked in the recorded video to analyze particle distribution by calculating the particle velocity based on the Stokes–Einstein equation. With this feature, the number and size of heterogeneous vesicle populations can be quantified more accurately with optimal camera and analysis settings (212). The sizing ranges from 30 to 1,000 nm (59). Furthermore, NTA can track fluorescently labeled exosomes, allowing for characterizing exosome phenotypes when labeled with specific antibody-conjugated quantum dots (50, 148). However, the accuracy may be affected by sample handling, and the sample concentration range is limited (107–109 particles/mL) (59). If the sizes of contaminants are close to the exosome size, the contaminants may be not distinguishable from isolated exosomes and might result in overestimation of the number of exosomes.
Resistive pulse sensing.
Resistive pulse sensing (RPS) is capable of accessing particle concentration and size distribution via measuring individual particles (12). When particles pass through the pore filled with conductive fluid in a membrane, the increased electrical resistance produced by each individual is detected by the resistive pulse sensor for analyzing particle size (11). An advanced technique, tunable resistive pulse sensing (TRPS), utilizes a size-tunable pore created in an elastic membrane (183) to improve measurement sensitivity and resolution (168). If the sample consists of heterogeneous particle sizes, multiple membranes of specific pore size ranges may be needed.
Flow cytometry.
Flow cytometry is one of the most commonly used methods to qualitatively and quantitatively characterize single cells in a high-throughput and high-sensitivity manner (146). However, limitations in cytometer sensitivity, assay specificity, and the lack of proper reporting of experimental details have produced a body of scientific literature whose rigor is not always clear (132, 204). Recently, a working group of EV flow cytometry researchers from the International Society for Extracellular Vesicles (ISEV), the International Society for the Advancement of Cytometry (ISAC), and the International Society on Thrombosis and Hemostasis (ISTH) developed a framework for the experimental design and minimum amount of information that should be reported with EV flow cytometry studies (MIFlowCyto-EV) (225). Some cytometers are more sensitive than others in detecting EVs (222). Beckman’s CytoFLEX and Apogee’s MICRO-PLUS are considered two of the more sensitive cytometers currently on the market, with several newer cytometer models that may be even more sensitive and specialized toward EV work making their way to the market (224).
Calibration beads for standardizing EV size and molecules of equivalent soluble fluorophore (MESF) beads are utilized to standardize reporting of EV fluorochrome brightness (68, 223). Cellarcus now has EV flow cytometry kits available that contain many of the controls outlined in the new MIFlowCyto-EV standards. In general, groups should perform titration dilutions not only of the antibodies used but also of their EV samples to ensure that concentrated EV preparations are not producing artifacts through the “swarming” effect (48, 68). Researchers should also perform detergent lysis (e.g., 0.3% Triton X) control of EV preparations to verify that the particle population being detected is lipid membrane based (150).
Currently, several EV flow cytometry groups use carboxyfluorescein succinimidyl ester (CFSE)-based dyes as a general stain to discern EVs from other particulate matter in EV preparations as well as cytometer fluidics (48). CFSE is able to passively diffuse into EVs, where its acetate groups are cleaved by esterases, which are then converted to fluorescent esters. CFSE is retained within EVs due to its covalent binding with adjacent molecules via succinimidyl groups (69, 134). This covalent bonding allows CFSE to be retained within EVs for long periods of time and limits its transfer outside of EVs (133). The CFSE+ fraction is then gated on as the EV population for further downstream analysis by fluorochrome-conjugated monoclonal antibodies (124). Given the small surface area of EVs and the lack of robust sensitivity of current cytometers in the submicrometer range, the usage of the brightest fluorochromes available for detecting EV surface protein expression [e.g., R-phycoerythrin (PE) or Brilliant Violet 421 (BV421)] is advantageous (134). Careful application of compensation and fluorescence minus one control are critical for rigorous data acquisition as well as unstained controls in addition to stained buffer controls. Optimization of voltages on cytometers, filtration of buffers, and cytometer fluidics with 20–30-nm filters can greatly reduce background noise due to the presence of non-EV particles. Furthermore, some cytometer companies have specialized filter sets to enhance sensitivity of EV detection (e.g., Fisher’s Attune NxT Small Particle Side Scatter Filter).
Protein detection.
Immune-based assays, such as Western blot analysis and enzyme-linked immunosorbent assay (ELISA), are widely used to characterize exosomes or exosome subpopulations. Western blot is an antibody-based, semiquantitative or qualitative method to analyze the presence of specific proteins in EV preparations. When doing Western blot analysis, exosomes and the lysates of their producing cells should be loaded side by side in a defined amount for comparing the abundance of detected proteins. Exosome-specific ELISAs provide plates that are precoated with antibodies that can capture exosomes and include lyophilized EV standards for the quantification analysis. Numerous EV-associated proteins (e.g., CD9, CD63, CD81, syntenin-1, flotillin-1) have been assessed systematically (88) and summarized in Minimal Information for Studies of Extracellular Vesicles 2018, including EV-negative control marker proteins (198). Mass spectrometry-based proteomics approaches have also been widely employed in the characterization of EV preparations (19, 36). Characterization of membrane proteins by use of such approaches requires consideration of the lysis buffer used and the medium in which the peptides are analyzed on the mass spectrometer. Ionic detergents are often required to sufficiently separate out membrane-restricted proteins from lipid components, but such ionic detergents are not compatible with most mass spectrometer methodologies (229). However, EVs may be lysed in strong ionic detergents if it is followed up by a column-based buffer exchange step (229).
RNA detection.
EVs are packaged with various subtypes of RNA, including mRNA, miRNA, tRNA, snoRNA, and other classes of noncoding RNA (85). Frequently, quantitative polymerase chain reaction (qPCR) is a common method to detect and quantify RNAs of interest within EV preparations (96). For EVs derived from plasma and other bodily fluids, careful attention should be given to non-EV particles that can coisolate with EVersus Particles such as high- and low-density lipoproteins are abundant in blood and can also be associated with miRNA (213). Therefore, EVs need to be rigorously separated from such contaminants with density gradients to limit the contribution of non-EV-associated RNA (201). To date, there is no consensus on which EV-associated transcripts may serve as effective normalization controls (202). Therefore, effective normalization controls for each class of EV preparation need to be empirically determined and verified for reproducibility. Other methods such as RNA-Seq do not require such normalization genes but are rather normalized to the total number of reads sequenced. To date, several groups have reported on both the detection and EV-based transfer of mRNA transcripts, whereas other groups have had trouble detecting full-length mRNA transcripts in their EV preparations. It is not clear what accounts for this discrepancy, but controls should be used to limit the detection of artifacts, which may be facilitated by longer read lengths and paired end sequencing analyses.
EVALUATION OF EV’S FUNCTIONAL PROPERTIES
Isolation media.
The media used for exosome isolations should be given careful consideration. Historically, most published preclinical reports of stem cell-based therapies have utilized serum-containing media (52). However, the use of serum to isolate exosomes is problematic (100). Fetal bovine serum (FBS) is highly enriched for bovine exosomes. Most studies utilizing serum-containing media attempt to preclear the media of bovine EVs via overnight ultracentrifugation (163). However, such depletion strategies are not 100% efficient, resulting in residual FBS-derived exosomes that coisolate with stem cell-derived EVs secreted into the conditioned media (115). Such xenocontaminants interfere with studies investigating the functional properties of exosomes derived from stem cells and limit the future clinical applicability of such EV isolations. There is a growing appreciation that exosomes isolated from serum-free media greatly diminish these issues, yet exposing stem cells to serum deprivation induces cellular stress responses and potentially influences the factors packaged into the resulting exosomes (144, 149). Future studies are needed to investigate the effects of such serum deprivation techniques and whether they greatly influence the composition of the resulting exosomes.
Dosing regimens.
Methods of dosing for functional studies should be taken into consideration for studies assessing the functional properties of stem cell-derived exosomes. Some groups determine the administered dose based on the number of EVs present, while other laboratories use total exosome protein content to determine dosing regimens (52). Studies using vesicle concentration methods should verify the accuracy and reproducibility of the methods used to determine vesicle concentration, as previously this has been a limiting factor. The rationale for the use of protein as an appropriate determinant of exosome dosing regimens is based on proteinaceous mass compared with their total RNA content (200). These two classes of macromolecules, protein and RNA, are generally attributed with being the chief mediators of exosomes’ functional properties. The preponderance of total protein compared with total RNA among stem cell-derived exosomal populations provides the rationale for using protein as a metric for dosing.
Formulation.
Formulation issues associated with exosome cryostorage have garnered increasing attention of late (103). Many published reports utilize PBS as a cryostorage medium for isolated stem cell-derived exosomes for downstream functional assessments. However, PBS is not an ideal cryostorage medium for biologics, as it experiences a significant drop in pH as it approaches freezing temperatures (99, 158). Increasingly, stem cell-derived exosome research groups have started utilizing excipients and carriers to increase their stability. Proteins such as HSA/BSA and sugars such as trehalose are commonly used to stabilize various biologics during product processing and may aid in exosome stability during storage (28). Comprehensive comparison studies of the numerous excipients and carriers available for exosome storage have yet to be published, but such future studies are warranted. Many groups working with stem cell-derived exosomes have also anecdotally reported in personal communications to us that exosomal yield seems to decrease as a function of the amount of plasticware they were exposed to. Limiting exposure to plasticware, using low protein-binding plasticware and preblocking of nonspecific protein binding may potentially aid in minimizing exosomal loss during processing.
In vitro bioassays.
Numerous groups have reported multiple functional properties of EV preparations from various source materials, including culture-conditioned media (181, 210). Several have reported the use in vitro immune assays, where generally primary peripheral blood mononuclear cells (PBMCs) or splenocytes, or a subpopulation thereof, are stimulated with an antigen (e.g., LPS) or other stimulant (e.g., phytohemagglutinin) and then treated with a concentrated dose of EVs (72, 98). Outcome measures include cytokine expression analysis, flow cytometry analysis of activation or differentiation markers, and colorimetric activity assays. EVs have also been assessed for their cytoprotective properties in vitro, using a variety of reagents to generate a stress response (e.g., ROS, endoplasmic reticulum stress) in the target cell population, followed by assessments of gene expression or cellular viability rates (73). It is also important to provide evidence that EVs are being taken up by the target cell population, which can be accomplished by fluorescently labeling EVs with various esterase and lipid-incorporating dyes (63). For EV uptake studies using lipid-incorporating dyes, it is important to have appropriate controls demonstrating that the observed EV uptake is not an artifact attributable to dye aggregates forming in the buffers used, or due to dye leakage out of EVs (which can occur in serum-containing media) (191).
Animal studies.
To date, there are over 200 animal studies that have investigated the ability of EVs to modulate tissue physiology in vivo (52). Although these studies have examined a diverse set of diseases and EVs derived from numerous sources, it is clear that EVs have the wide-ranging ability to influence both molecular phenotypes and clinical measures of disease burden in animals. Although most EV animal studies have reported intravenous administration, EV groups should also consider other routes of delivery, including subcutaneous and intraperitoneal, as well as the timing of the EV dosages relative to disease state, both of which may strongly influence outcome measures. When heart disease is treated, the delivery route is an important factor for effective EV homing to the heart. The intravenously delivered exosomes have been shown to be rapidly cleared from the blood circulation, followed by predominant distribution to the liver and then to the lungs (189). To compensate for the unspecific delivery, a larger dose of intravenously delivered exosomes is often used (206, 207). However, a more effective approach to increase the efficacy of intravenously injected exosomes is to modify the exosome surface by either tagging or expressing peptides that target the cells of interest (94, 95, 206, 217). Intramyocardial administration might be an alternative approach, as the intramyocardial injected exosomes that were released by cardiosphere-derived cells were demonstrated to improve heart function in both mouse (83) and porcine (61) models of myocardial infarction. However, the risks of translating the intramyocardial delivery in animal studies to a clinical setting, which will require injection of the exosomes into the heart muscle, are not insignificant (206).
Notably, it is known that bovine exosomes are present in milk, and these exosomes and their miRNAs can be found in milk consumers across species (e.g., humans and mice) (240). Although epidemiological evidence correlates bovine milk intake with the development of chronic diseases of civilization, including obesity, type 2 diabetes mellitus, osteoporosis, common cancers (e.g., prostate, breast, liver, B cells) as well as Parkinson’s disease (126), an immune response to the presence of bovine milk exosomes in humans has not been identified despite often daily consumption of milk. In the mouse and porcine studies of milk exosome tracking, the labeled exosomes accumulated in liver, spleen, and brain following suckling, oral gavage, and intravenous administration (123). Repeated challenge of bovine exosomes in mice neither showed any systemic toxicity nor elevated inflammatory cytokines (182). Since bovine milk is a biocompatible and scalable source of exosomes, and the bovine exosomes are protective to the bioactive cargo in a strong acidic environment (e.g., gastric acid), these exosomes are considered effective vehicles to deliver small drug molecules against lung tumor xenografts in vivo via oral or intraperitoneal routes (138).
Although the literature is limited, the bovine milk exosomes open a window of therapeutic exosomes derived from different species, and the lack of evidence of an immune response to exogenous exosomes increases the likelihood of safe repeated dosing with exosomes for therapeutics. Nevertheless, studies employing exosomes to repeatedly deliver therapy should include monitoring for an immune/inflammatory response, given the nascent nature of this approach to therapy. Going forward, it will also be critical for groups to optimize methods, determine dose-response curves, and investigate potential high-dose toxicities as well as potential immunogenicity of EVs derived from different species.
Biodistribution assessment.
To date, there have been few published reports on the biodistribution and pharmacokinetics of administered stem cell-derived EVs (57, 93, 106, 129, 227). Such studies often rely on fluorescently labeled lipid-incorporating dyes in conjunction with in vivo optical imaging (e.g., IVIS), assuming that the lipid-incorporating dyes stay embedded in the EV membrane throughout the duration of the experiment (161). Yet several studies investigating commonly used lipid-incorporating dyes have demonstrated that up to 75% of the dye dissociated from the vesicles when incubated in serum (139, 178, 191). Amphiphilic lipid-anchored fluorophores, such as PKH dyes used for poststaining of EVs, also possess the capability to form nanoparticles that are hardly distinguishable from real fluorescently stained EVs (161). Few published reports have investigated the biodistribution of radiolabeled exosomes (57, 82, 209). Another possibility is to label EVs with chemiluminescence-emitting proteins that permit in vivo quantitative evaluation (189). Thus, further studies elucidating the pharmacokinetics of exosome-based therapies are necessary. Nonetheless, current exosome biodistribution methodologies require substantial manipulation of EV populations, which may result in the attenuation of exosome function as a result of degradation or abnormal conformation of proteinaceous factors responsible for cellular uptake. Studies examining the postinsertion function of the resulting exosome fractions are important for validation.
CONCLUSION
Exosomes play a critical role in cell-to-cell communication, as they encapsulate bioactive molecules from the parent cells. Interest in exosomes and their potential physiological and pathological impacts is augmenting in many areas of biological research. Both heart cells and other cell types in different conditions can detrimentally or favorably mediate the status of heart health or diseases through exosomes. Stem cell-derived exosomes have been shown as a regenerative mediator in cardiac repair. Understanding the composition of exosomal cargo enables the investigation of signaling mechanisms and possible genetic modifications in the exosome-releasing cells to improve treatment outcomes. To achieve this, numerous methods of exosome isolation (Table 1) and characterization (Table 2) have been developed. While each method has advantages and disadvantages, adjunct techniques are often employed to enhance the quality and quantity of isolated exosomes. When therapeutic exosomes in preclinical or clinical studies are being applied, several factors should be carefully considered to ensure safety, reproducibility, and efficiency. Overall, it is likely that the investigation and therapeutic use of exosomes has just begun, and we can anticipate more advances in the future.
GRANTS
This work was supported by National Institute on Aging Grant AG-056710-01,= (to A. A. Knowlton), American Heart Association Grant 18POST34020069 (to Y. Lin), National Center for Advancing Translational Sciences Grant UL1 TR-001860 and linked award KL2 TR-001859 (to J. D. Anderson), as well as CIRM Grant EDUC2-08390, UC Office of the President’s Multi-Campus Research Program Grant MRP-17-454909, STAIR Grant, STAIR-Plus Grant, CTSC Rapid Translational Grant UL1 TR-001860, U2C ES-030158, T32 Cardio National Heart, Lung, and Blood Institute Grant T32-HL-086350, and the Denny and Jeanene Dickenson Fellowship.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and J.D.A. conceived and drafted the manuscript. Y.L. and J.D.A. prepared the figures. Y.L., L.M.A.R. and S.V.G. performed the experiments. L.M.A.R. and A.A.K. proofread the manuscript. A.A.K. supervised and revised the article. All authors approved the final manuscript.
REFERENCES
- 1.Abramowicz A, Widlak P, Pietrowska M. Proteomic analysis of exosomal cargo: the challenge of high purity vesicle isolation. Mol Biosyst 12: 1407–1419, 2016. doi: 10.1039/C6MB00082G. [DOI] [PubMed] [Google Scholar]
- 2.Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res 122: 296–309, 2018. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol 120: 1418–1424, 2007. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113: 1–11, 2013. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA 106: 3794–3799, 2009. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10: 619–624, 2008. [Erratum in Nat Cell Biol 10: 752, 2008]. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 7.Alexander M, Ramstead AG, Bauer KM, Lee S-H, Runtsch MC, Wallace J, Huffaker TB, Larsen DK, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Rab27-dependent exosome production inhibits chronic inflammation and enables acute responses to inflammatory stimuli. J Immunol 199: 3559–3570, 2017. doi: 10.4049/jimmunol.1700904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alibhai FJ, Tobin SW, Yeganeh A, Weisel RD, Li RK. Emerging roles of extracellular vesicles in cardiac repair and rejuvenation. Am J Physiol Heart Circ Physiol 315: H733–H744, 2018. doi: 10.1152/ajpheart.00100.2018. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 41: 59–72, 1969. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtiö J, Nolta JA. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells 34: 601–613, 2016. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JL, Quinn JA. The relationship between particle size and signal in coulter-type counters. Rev Sci Instrum 42: 1257–1258, 1971. doi: 10.1063/1.1685356. [DOI] [Google Scholar]
- 12.Anderson W, Kozak D, Coleman VA, Jämting ÅK, Trau M. A comparative study of submicron particle sizing platforms: accuracy, precision and resolution analysis of polydisperse particle size distributions. J Colloid Interface Sci 405: 322–330, 2013. doi: 10.1016/j.jcis.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA 108: 4852–4857, 2011. [Erratum in Proc Natl Acad Sci USA 108: 17569, 2011]. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azevedo LC, Janiszewski M, Pontieri V, Pedro MA, Bassi E, Tucci PJ, Laurindo FR. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care 11: R120, 2007. doi: 10.1186/cc6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer 141: 220–230, 2017. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 16.Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M, Pogge von Strandmann E. Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles 8: 1596016, 2019. doi: 10.1080/20013078.2019.1596016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Y. Detecting protein-protein interactions by gel filtration chromatography In: Protein-Protein Interactions Methods and Applications (Meyerkord CL, Fu H, editors). New York: Humana Press, p. 223–232. [DOI] [PubMed] [Google Scholar]
- 18.Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO, Skog J, Maguire CA. Heparin affinity purification of extracellular vesicles. Sci Rep 5: 10266, 2015. doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandu R, Oh JW, Kim KP. Mass spectrometry-based proteome profiling of extracellular vesicles and their roles in cancer biology. Exp Mol Med 51: 1–10, 2019. doi: 10.1038/s12276-019-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranyai T, Herczeg K, Onódi Z, Voszka I, Módos K, Marton N, Nagy G, Mäger I, Wood MJ, El Andaloussi S, Pálinkás Z, Kumar V, Nagy P, Kittel Á, Buzás EI, Ferdinandy P, Giricz Z. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 10: e0145686, 2015. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belting M, Christianson HC. Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease. J Intern Med 278: 251–263, 2015. doi: 10.1111/joim.12393. [DOI] [PubMed] [Google Scholar]
- 23.Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, Wouters EF, Savelkoul PH, Lopez-Iglesias C, Koenen RR, Rohde GGU, Stassen FR. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep 7: 15297, 2017. doi: 10.1038/s41598-017-15717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berezin AE, Berezin AA. Extracellular endothelial cell-derived vesicles: emerging role in cardiac and vascular remodeling in heart failure. Front Cardiovasc Med 7: 47, 2020. doi: 10.3389/fcvm.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290: H2196–H2203, 2006. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12: 1659–1668, 2011. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 27.Böing AN, van der Pol E, Grootemaat AE, Coumans FAW, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles 3: 23430, 2014. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chrétien D, Jegou D, Bach J-M. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep 6: 36162, 2016. doi: 10.1038/srep36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauffman G, De Rycke M, Sermon K, Liebaers I, Van de Velde H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum Reprod 24: 63–70, 2009. doi: 10.1093/humrep/den351. [DOI] [PubMed] [Google Scholar]
- 30.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem 166: 189–197, 1946. [PubMed] [Google Scholar]
- 31.Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med 19: 2153–2161, 2015. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen GH, Xu J, Yang Y-J. Exosomes: promising sacks for treating ischemic heart disease? Am J Physiol Heart Circ Physiol 313: H508–H523, 2017. doi: 10.1152/ajpheart.00213.2017. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, Guo ZK, Zhong J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One 9: e115316, 2014. doi: 10.1371/journal.pone.0115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431: 566–571, 2013. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94: 92–95, 2004. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev 34: 474–490, 2015. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 37.Choi JS, Lee BJ, Park HY, Song JS, Shin SC, Lee JC, Wang SG, Jung JS. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell Physiol Biochem 36: 85–99, 2015. doi: 10.1159/000374055. [DOI] [PubMed] [Google Scholar]
- 38.Chuo ST, Chien JC, Lai CP. Imaging extracellular vesicles: current and emerging methods. J Biomed Sci 25: 91, 2018. doi: 10.1186/s12929-018-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cizmar P, Yuana Y. Detection and characterization of extracellular vesicles by transmission and cryo-transmission electron microscopy In: Extracellular Vesicles: Methods and Protocols, ed. by Kuo WP and Jia S. New York: Humana, p. 221–232. [DOI] [PubMed] [Google Scholar]
- 40.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2: CD006536, 2012. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Coleman BM, Hanssen E, Lawson VA, Hill AF. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J 26: 4160–4173, 2012. doi: 10.1096/fj.11-202077. [DOI] [PubMed] [Google Scholar]
- 42.Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, Zanetta C, Faravelli I, Bresolin N, Comi GP. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res 318: 1528–1541, 2012. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles 2: 20677, 2013. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287–2299, 2008. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 45.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation 112: 214–223, 2005. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 46.Davidson SM, Riquelme JA, Zheng Y, Vicencio JM, Lavandero S, Yellon DM. Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Sci Rep 8: 15885, 2018. doi: 10.1038/s41598-018-34357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 1: 18396, 2012. doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Rond L, van der Pol E, Hau CM, Varga Z, Sturk A, van Leeuwen TG, Nieuwland R, Coumans FA. Comparison of generic fluorescent markers for detection of extracellular vesicles by flow cytometry. Clin Chem 64: 680–689, 2018. doi: 10.1373/clinchem.2017.278978. [DOI] [PubMed] [Google Scholar]
- 49.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 4: 1131–1143, 2015. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine (Lond) 7: 780–788, 2011. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eitan E, Green J, Bodogai M, Mode NA, Bæk R, Jørgensen MM, Freeman DW, Witwer KW, Zonderman AB, Biragyn A, Mattson MP, Noren Hooten N, Evans MK. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep 7: 1342, 2017. doi: 10.1038/s41598-017-01386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 38: 15–21, 2020. doi: 10.1002/stem.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elsherbini A, Bieberich E. Ceramide and exosomes: a novel target in cancer biology and therapy. Adv Cancer Res 140: 121–154, 2018. doi: 10.1016/bs.acr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol 71: 24–30, 2015. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdbrügger U, Lannigan J. Analytical challenges of extracellular vesicle detection: a comparison of different techniques. Cytometry A 89: 123–134, 2016. doi: 10.1002/cyto.a.22795. [DOI] [PubMed] [Google Scholar]
- 56.Falker C, Hartmann A, Guett I, Dohler F, Altmeppen H, Betzel C, Schubert R, Thurm D, Wegwitz F, Joshi P, Verderio C, Krasemann S, Glatzel M. Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J Neurochem 137: 88–100, 2016. doi: 10.1111/jnc.13514. [DOI] [PubMed] [Google Scholar]
- 57.Faruqu FN, Wang JT, Xu L, McNickle L, Chong EM, Walters A, Gurney M, Clayton A, Smyth LA, Hider R, Sosabowski J, Al-Jamal KT. Membrane radiolabelling of exosomes for comparative biodistribution analysis in immunocompetent and immunodeficient mice - A novel and universal approach. Theranostics 9: 1666–1682, 2019. doi: 10.7150/thno.27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li D, Thal DR, Walther P, Ludolph AC, Danzer KM, Weishaupt JH. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 211: 897–911, 2015. doi: 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filipe V, Hawe A, Jiskoot W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm Res 27: 796–810, 2010. doi: 10.1007/s11095-010-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 27: 1114–1122, 2006. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 61.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38: 201–211, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gambim MH, do Carmo AO, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 11: R107, 2007. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gangadaran P, Hong CM, Ahn BC. Current perspectives on in vivo noninvasive tracking of extracellular vesicles with molecular imaging. BioMed Res Int 2017: 9158319, 2017. doi: 10.1155/2017/9158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One 10: e0138849, 2015. doi: 10.1371/journal.pone.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garikipati VN, Shoja-Taheri F, Davis ME, Kishore R. Extracellular vesicles and the application of system biology and computational modeling in cardiac repair. Circ Res 123: 188–204, 2018. doi: 10.1161/CIRCRESAHA.117.311215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gill S, Catchpole R, Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev 43: 273–303, 2019. doi: 10.1093/femsre/fuy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35: 1747–1759, 2017. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 68.Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA, Thalmann S, Welsh JA, Probst C, Guerin C, Boulanger CM, Jones JC, Hanenberg H, Erdbrügger U, Lannigan J, Ricklefs FL, El-Andaloussi S, Giebel B. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles 8: 1587567, 2019. doi: 10.1080/20013078.2019.1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorgun C, Reverberi D, Rotta G, Villa F, Quarto R, Tasso R. Isolation and flow cytometry characterization of extracellular-vesicle subpopulations derived from human mesenchymal stromal cells. Curr Protoc Stem Cell Biol 48: e76, 2019. doi: 10.1002/cpsc.76. [DOI] [PubMed] [Google Scholar]
- 70.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23: 1541–1557, 2009. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 116: 255–263, 2015. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol 40: 72–81, 2015. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Grindheim AK, Vedeler A. Extracellular vesicles released from cells exposed to reactive oxygen species increase annexin A2 expression and survival of target cells exposed to the same conditions. Commun Integr Biol 9: e1191715, 2016. doi: 10.1080/19420889.2016.1191715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gulinelli S, Salaro E, Vuerich M, Bozzato D, Pizzirani C, Bolognesi G, Idzko M, Di Virgilio F, Ferrari D. IL-18 associates to microvesicles shed from human macrophages by a LPS/TLR-4 independent mechanism in response to P2X receptor stimulation. Eur J Immunol 42: 3334–3345, 2012. doi: 10.1002/eji.201142268. [DOI] [PubMed] [Google Scholar]
- 75.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292: H3052–H3056, 2007. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 76.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Schöler HR, Martin U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 5: 434–441, 2009. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 77.Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot M-C, Wollacott R, Sapp E, Dubuke ML, Li X, Shaffer SA, DiFiglia M, Wang Y, Aronin N, Khvorova A. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther 26: 2838–2847, 2018. doi: 10.1016/j.ymthe.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol 200: 367–371, 2013. [Erratum in J Cell Biol 201: 485, 2013]. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heath N, Grant L, De Oliveira TM, Rowlinson R, Osteikoetxea X, Dekker N, Overman R. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci Rep 8: 5730, 2018. doi: 10.1038/s41598-018-24163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8: 3274–3284, 2009. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, Alt E, Vykoukal J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J Chromatogr A 1371: 125–135, 2014. doi: 10.1016/j.chroma.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 82.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, Oh HJ, Ha S, Lee YS, Jeong JM, Gho YS, Lee DS. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci Rep 5: 15636, 2015. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res 68: 4229–4238, 2008. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janas T, Janas MM, Sapoń K, Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett 589: 1391–1398, 2015. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 86.Jansen F, Li Q. Exosomes as diagnostic biomarkers in cardiovascular diseases In: Exosomes in Cardiovascular Diseases: Biomarkers, Pathological and Therapeutic Effects, ed. by (Xiao J and Cretoiu S. Singapore: Springer Nature, p. 61–70. [Google Scholar]
- 87.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367: 113–121, 2006. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 88.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell 177: 428–445.e18, 2019. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, Xue Y, Xu T, Zhu HJ, Simpson RJ. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 13: 1672–1686, 2013. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 90.Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp 131: 56482, 2018. doi: 10.3791/56482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalbfuss B, Wolff M, Morenweiser R, Reichl U. Purification of cell culture-derived human influenza A virus by size-exclusion and anion-exchange chromatography. Biotechnol Bioeng 96: 932–944, 2007. doi: 10.1002/bit.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 13: 3354–3364, 2013. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 93.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546: 498–503, 2017. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanki S, Jaalouk DE, Lee S, Yu AY, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol 50: 841–848, 2011. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim H, Yun N, Mun D, Kang JY, Lee SH, Park H, Park H, Joung B. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun 499: 803–808, 2018. doi: 10.1016/j.bbrc.2018.03.227. [DOI] [PubMed] [Google Scholar]
- 96.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA 8: e1413, 2017. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol 20: 102–107, 2007. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 98.Ko JH, Kim HJ, Jeong HJ, Lee HJ, Oh JY. Mesenchymal stem and stromal cells harness macrophage-derived amphiregulin to maintain tissue homeostasis. Cell Reports 30: 3806–3820.e6, 2020. doi: 10.1016/j.celrep.2020.02.062. [DOI] [PubMed] [Google Scholar]
- 99.Kolhe P, Amend E, Singh SK. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol Prog 26: 727–733, 2010. doi: 10.1002/btpr.377. [DOI] [PubMed] [Google Scholar]
- 100.Kornilov R, Puhka M, Mannerström B, Hiidenmaa H, Peltoniemi H, Siljander P, Seppänen-Kaijansinkko R, Kaur S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J Extracell Vesicles 7: 1422674, 2018. doi: 10.1080/20013078.2017.1422674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kosanović M, Milutinović B, Goč S, Mitić N, Janković M. Ion-exchange chromatography purification of extracellular vesicles. Biotechniques 63: 65–71, 2017. doi: 10.2144/000114575. [DOI] [PubMed] [Google Scholar]
- 102.Krishna KA, Krishna KS, Berrocal R, Rao KS, Sambasiva Rao KR. Myocardial infarction and stem cells. J Pharm Bioallied Sci 3: 182–188, 2011. doi: 10.4103/0975-7406.80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kusuma GD, Barabadi M, Tan JL, Morton DA, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol 9: 1199, 2018. doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev 26: 617–631, 2017. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 105.LaFramboise WA, Scalise D, Stoodley P, Graner SR, Guthrie RD, Magovern JA, Becich MJ. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol 292: C1799–C1808, 2007. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 106.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8: 483–494, 2014. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 4: 214–222, 2010. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6: 481–492, 2011. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 109.Lai RC, Yeo R, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40: 82–88, 2015. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv 31: 543–551, 2013. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Lane RE, Korbie D, Trau M, Hill MM. Optimizing size exclusion chromatography for extracellular vesicle enrichment and proteomic analysis from clinically relevant samples. Proteomics 19: e1800156, 2019. doi: 10.1002/pmic.201800156. [DOI] [PubMed] [Google Scholar]
- 112.Lawson C, Kovacs D, Finding E, Ulfelder E, Luis-Fuentes V. Extracellular vesicles: evolutionarily conserved mediators of intercellular communication. Yale J Biol Med 90: 481–491, 2017. [PMC free article] [PubMed] [Google Scholar]
- 113.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31: 890–896, 2003. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 114.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461: 402–406, 2009. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehrich BM, Liang Y, Khosravi P, Federoff HJ, Fiandaca MS. Fetal bovine serum-derived extracellular vesicles persist within vesicle-depleted culture media. Int J Mol Sci 19: 3538, 2018. doi: 10.3390/ijms19113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lobb RJ, Becker M, Wen SW, Wong CSF, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 4: 27031, 2015. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, Manos PD, Datta S, Lander ES, Schlaeger TM, Daley GQ, Rinn JL. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet 42: 1113–1117, 2010. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913, 2014. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med 49: e346, 2017. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 89, Pt B: 268–279, 2015. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, Yu Y, Huang H, Hu Y, Yang Z, Yang J, Shen Z. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int 2018: 3290372, 2018. doi: 10.1155/2018/3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol 304: H954–H965, 2013. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 8: 11321, 2018. doi: 10.1038/s41598-018-29780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marcoux G, Duchez AC, Cloutier N, Provost P, Nigrovic PA, Boilard E. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci Rep 6: 35928, 2016. doi: 10.1038/srep35928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer 18: 75, 2019. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Melnik BC, Schmitz G. Exosomes of pasteurized milk: potential pathogens of Western diseases. J Transl Med 17: 3, 2019. doi: 10.1186/s12967-018-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Menasché P. Challenging the cardiac differentiation of bone marrow cells: a clinical perspective. Mol Ther 16: 1000–1001, 2008. doi: 10.1038/mt.2008.75. [DOI] [PubMed] [Google Scholar]
- 128.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14: 141–145, 2014. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 129.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EV, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3: e99263, 2018. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mishra PK, Chavali V, Metreveli N, Tyagi SC. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol 90: 353–360, 2012. doi: 10.1139/y11-131. [DOI] [PubMed] [Google Scholar]
- 131.Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, Park SS. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res 42: 1358–1367, 2017. doi: 10.1080/02713683.2017.1319491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morales-Kastresana A, Jones JC. Flow cytometric analysis of extracellular vesicles In: Exosomes and Microvesicles: Methods and Protocols (Hill AF, editor). New York: Humana Press, p. 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morales-Kastresana A, Musich TA, Welsh JA, Telford W, Demberg T, Wood JCS, Bigos M, Ross CD, Kachynski A, Dean A, Felton EJ, Van Dyke J, Tigges J, Toxavidis V, Parks DR, Overton WR, Kesarwala AH, Freeman GJ, Rosner A, Perfetto SP, Pasquet L, Terabe M, McKinnon K, Kapoor V, Trepel JB, Puri A, Kobayashi H, Yung B, Chen X, Guion P, Choyke P, Knox SJ, Ghiran I, Robert-Guroff M, Berzofsky JA, Jones JC. High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer. J Extracell Vesicles 8: 1597603, 2019. doi: 10.1080/20013078.2019.1597603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, Rosner A, Demberg T, Watson DC, Karpova TS, Freeman GJ, DeKruyff RH, Pavlakis GN, Terabe M, Robert-Guroff M, Berzofsky JA, Jones JC. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep 7: 1878, 2017. doi: 10.1038/s41598-017-01731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104: 3257–3266, 2004. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 136.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3: 24641, 2014. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods 411: 55–65, 2014. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 371: 48–61, 2016. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Münter R, Kristensen K, Pedersbæk D, Larsen JB, Simonsen JB, Andresen TL. Dissociation of fluorescently labeled lipids from liposomes in biological environments challenges the interpretation of uptake studies. Nanoscale 10: 22720–22724, 2018. doi: 10.1039/C8NR07755J. [DOI] [PubMed] [Google Scholar]
- 140.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA 109: 4146–4151, 2012. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep 6: 33935, 2016. doi: 10.1038/srep33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Negrete A, Pai A, Shiloach J. Use of hollow fiber tangential flow filtration for the recovery and concentration of HIV virus-like particles produced in insect cells. J Virol Methods 195: 240–246, 2014. doi: 10.1016/j.jviromet.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8: 267–280, 2011. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nie Y, Han BM, Liu XB, Yang JJ, Wang F, Cong XF, Chen X. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci 7: 762–768, 2011. doi: 10.7150/ijbs.7.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Niu M, Li Y, Li G, Zhou L, Luo N, Yao M, Kang W, Liu J. A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur J Neurol 27: 967–974, 2020. doi: 10.1111/ene.14208. [DOI] [PubMed] [Google Scholar]
- 146.Nolan JP, Duggan E. Analysis of individual extracellular vesicles by flow cytometry In: Flow Cytometry Protocols (Hawley TS, Hawley RG, editors). New York: Humana Press, p. 79–92. [DOI] [PubMed] [Google Scholar]
- 147.Norling L, Lute S, Emery R, Khuu W, Voisard M, Xu Y, Chen Q, Blank G, Brorson K. Impact of multiple re-use of anion-exchange chromatography media on virus removal. J Chromatogr A 1069: 79–89, 2005. doi: 10.1016/j.chroma.2004.09.072. [DOI] [PubMed] [Google Scholar]
- 148.Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, Bath LE, Webb DJ, Gregory CD, Bailey MA, Dear JW. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol 591: 5833–5842, 2013. doi: 10.1113/jphysiol.2013.264069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oskowitz A, McFerrin H, Gutschow M, Carter ML, Pochampally R. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res (Amst) 6: 215–225, 2011. doi: 10.1016/j.scr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Osteikoetxea X, Sódar B, Németh A, Szabó-Taylor K, Pálóczi K, Vukman KV, Tamási V, Balogh A, Kittel Á, Pállinger É, Buzás EI. Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem 13: 9775–9782, 2015. doi: 10.1039/C5OB01451D. [DOI] [PubMed] [Google Scholar]
- 151.Palmieri V, Lucchetti D, Gatto I, Maiorana A, Marcantoni M, Maulucci G, Papi M, Pola R, De Spirito M, Sgambato A. Dynamic light scattering for the characterization and counting of extracellular vesicles: a powerful noninvasive tool. J Nanopart Res 16: 2583, 2014. doi: 10.1007/s11051-014-2583-z. [DOI] [Google Scholar]
- 152.Parisse P, Rago I, Ulloa Severino L, Perissinotto F, Ambrosetti E, Paoletti P, Ricci M, Beltrami AP, Cesselli D, Casalis L. Atomic force microscopy analysis of extracellular vesicles. Eur Biophys J 46: 813–820, 2017. doi: 10.1007/s00249-017-1252-4. [DOI] [PubMed] [Google Scholar]
- 153.Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res 56: 148–165, 2017. doi: 10.1016/j.preteyeres.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M, Singh S, Singh AP. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep 9: 5335, 2019. doi: 10.1038/s41598-019-41800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287: 43108–43115, 2012. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perrino C, Rockman HA. GATA4 and the two sides of gene expression reprogramming. Circ Res 98: 715–716, 2006. doi: 10.1161/01.RES.0000217593.07196.af. [DOI] [PubMed] [Google Scholar]
- 157.Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles 7: 1522236, 2018. doi: 10.1080/20013078.2018.1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pikal-Cleland KA, Rodríguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric β-galactosidase. Arch Biochem Biophys 384: 398–406, 2000. doi: 10.1006/abbi.2000.2088. [DOI] [PubMed] [Google Scholar]
- 159.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131: 2120–2130, 2015. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA 105: 4329–4334, 2008. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pužar Dominkuš P, Stenovec M, Sitar S, Lasič E, Zorec R, Plemenitaš A, Žagar E, Kreft M, Lenassi M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim Biophys Acta Biomembr 1860: 1350–1361, 2018. doi: 10.1016/j.bbamem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 162.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103: 11172–11177, 2006. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rani S. MicroRNA profiling of exosomes isolated from biofluids and conditioned media In: RNA Mapping: Methods and Protocols (Alvarez ML, Nourbakhsh M, editors). New York: Humana Press, p. 131–144. [DOI] [PubMed] [Google Scholar]
- 164.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172, 1996. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200: 373–383, 2013. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841: 108–120, 2014. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 167.Rikkert LG, Nieuwland R, Terstappen LW, Coumans FA. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J Extracell Vesicles 8: 1555419, 2019. doi: 10.1080/20013078.2018.1555419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roberts GS, Kozak D, Anderson W, Broom MF, Vogel R, Trau M. Tunable nano/micropores for particle detection and discrimination: scanning ion occlusion spectroscopy. Small 6: 2653–2658, 2010. doi: 10.1002/smll.201001129. [DOI] [PubMed] [Google Scholar]
- 169.Russell AE, Sneider A, Witwer KW, Bergese P, Bhattacharyya SN, Cocks A, Cocucci E, Erdbrügger U, Falcon-Perez JM, Freeman DW, Gallagher TM, Hu S, Huang Y, Jay SM, Kano SI, Lavieu G, Leszczynska A, Llorente AM, Lu Q, Mahairaki V, Muth DC, Noren Hooten N, Ostrowski M, Prada I, Sahoo S, Schøyen TH, Sheng L, Tesch D, Van Niel G, Vandenbroucke RE, Verweij FJ, Villar AV, Wauben M, Wehman AM, Yin H, Carter DR, Vader P. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles 8: 1684862, 2019. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 28: 788–798, 2010. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 171.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109: 724–728, 2011. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136: 41–56, 2018. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24: 1635–1647, 2015. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sharma S, Rasool HI, Palanisamy V, Mathisen C, Schmidt M, Wong DT, Gimzewski JK. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 4: 1921–1926, 2010. doi: 10.1021/nn901824n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sheridan C. Exosome cancer diagnostic reaches market. Nat Biotechnol 34: 359–360, 2016. doi: 10.1038/nbt0416-359. [DOI] [PubMed] [Google Scholar]
- 176.Showalter MR, Wancewicz B, Fiehn O, Archard JA, Clayton S, Wagner J, Deng P, Halmai J, Fink KD, Bauer G, Fury B, Perotti NH, Apperson M, Butters J, Belafsky P, Farwell G, Kuhn M, Nolta JA, Anderson JD. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem Biophys Res Commun 512: 729–735, 2019. doi: 10.1016/j.bbrc.2019.03.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575–581, 2009. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 178.Simonsen JB. Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J Extracell Vesicles 8: 1582237, 2019. doi: 10.1080/20013078.2019.1582237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Skliar M, Chernyshev VS. Imaging of extracellular vesicles by atomic force microscopy. J Vis Exp 151: 59254, 2019. doi: 10.3791/59254. [DOI] [PubMed] [Google Scholar]
- 180.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L Jr, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476, 2008. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sluijter JP, Davidson SM, Boulanger CM, Buzás EI, de Kleijn DP, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 114: 19–34, 2018. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Somiya M, Yoshioka Y, Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles 7: 1440132, 2018. doi: 10.1080/20013078.2018.1440132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sowerby SJ, Broom MF, Petersen GB. Dynamically resizable nanometre-scale apertures for molecular sensing. Sens Actuators B Chem 123: 325–330, 2007. doi: 10.1016/j.snb.2006.08.031. [DOI] [Google Scholar]
- 184.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 361: 45–46, 2003. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 185.Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, Gutwein P, Ludwig A, Rubinstein E, Altevogt P. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J 393: 609–618, 2006. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, Kögler G, Wernet P. Intrakoronare, humane autologe Stammzelltransplantation zur Myokardregeneration nach Herzinfarkt. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med Wochenschr 126: 932–938, 2001. doi: 10.1055/s-2001-16579-1. [DOI] [PubMed] [Google Scholar]
- 187.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci 18: 1153, 2017. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Taapken SM, Nisler BS, Newton MA, Sampsell-Barron TL, Leonhard KA, McIntire EM, Montgomery KD. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat Biotechnol 29: 313–314, 2011. doi: 10.1038/nbt.1835. [DOI] [PubMed] [Google Scholar]
- 189.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 165: 77–84, 2013. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 190.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 120: 254–265, 2010. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takov K, Yellon DM, Davidson SM. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J Extracell Vesicles 6: 1388731, 2017. doi: 10.1080/20013078.2017.1388731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tang C, Lee AS, Volkmer J-P, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol 29: 829–834, 2011. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol 11: 548–563, 2014. doi: 10.1038/cmi.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56: 293–304, 2012. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 195.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37: 2415–2424, 2015. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 196.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 30: 3.22.1–3.22.29, 2006. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 197.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166: 7309–7318, 2001. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 198.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YX, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DPV. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res (Amst) 6: 206–214, 2011. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 200.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 46: 843–853, 2018. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tsatsaronis JA, Franch-Arroyo S, Resch U, Charpentier E. Extracellular vesicle RNA: a universal mediator of microbial communication? Trends Microbiol 26: 401–410, 2018. doi: 10.1016/j.tim.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 202.Turchinovich A, Drapkina O, Tonevitsky A. Transcriptome of extracellular vesicles: state-of-the-art. Front Immunol 10: 202, 2019. doi: 10.3389/fimmu.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 204.van der Vlist EJ, Nolte-’t Hoen EN, Stoorvogel W, Arkesteijn GJA, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc 7: 1311–1326, 2012. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 205.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 3: 24858, 2014. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8: 1869–1878, 2018. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vandergriff AC, de Andrade JB, Tang J, Hensley MT, Piedrahita JA, Caranasos TG, Cheng K. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int 2015: 960926, 2015. doi: 10.1155/2015/960926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Varga Z, Gyurkó I, Pálóczi K, Buzás EI, Horváth I, Hegedűs N, Máthé D, Szigeti K. Radiolabeling of extracellular vesicles with 99mTc for quantitative in vivo imaging studies. Cancer Biother Radiopharm 31: 168–173, 2016. doi: 10.1089/cbr.2016.2009. [DOI] [PubMed] [Google Scholar]
- 210.Venkat P, Cui C, Chen Z, Chopp M, Zacharek A, Landschoot-Ward J, Culmone L, Yang XP, Xu J, Chen J. CD133+Exosome treatment improves cardiac function after stroke in type 2 diabetic mice. Transl Stroke Res, 2020. doi: 10.1007/s12975-020-00807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, Van den Broecke R, Denys H, De Wever O, Hendrix A. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep 7: 2704, 2017. doi: 10.1038/s41598-017-02599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vestad B, Llorente A, Neurauter A, Phuyal S, Kierulf B, Kierulf P, Skotland T, Sandvig K, Haug KB, Øvstebø R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles 6: 1344087, 2017. doi: 10.1080/20013078.2017.1344087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13: 423–433, 2011. [Erratum in Nat Cell Biol 17: 104, 2015]. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18: 728–742, 2017. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3: e2213, 2008. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7: e34653, 2012. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc 7: e008737, 2018. doi: 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 65: 3111–3128, 2016. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74: 139–150, 2014. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192: 61–69, 2015. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, Dai Q, Kang L, Xu B. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci 232: 116632, 2019. [Erratum in Life Sci 256: 118045, 2020]. doi: 10.1016/j.lfs.2019.116632. [DOI] [PubMed] [Google Scholar]
- 222.Welsh JA, Holloway JA, Wilkinson JS, Englyst NA. Extracellular vesicle flow cytometry analysis and standardization. Front Cell Dev Biol 5: 78, 2017. doi: 10.3389/fcell.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Welsh JA, Horak P, Wilkinson JS, Ford VJ, Jones JC, Smith D, Holloway JA, Englyst NA. FCMPASS software aids extracellular vesicle light scatter standardization. Cytometry A 97: 569–581, 2020. doi: 10.1002/cyto.a.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Welsh JA, Jones JC, Tang VA. Fluorescence and light scatter calibration allow comparisons of small particle data in standard units across different flow cytometry platforms and detector settings. Cytometry A 97: 592–601, 2020. doi: 10.1002/cyto.a.24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Welsh JA, Van Der Pol E, Arkesteijn GJA, Bremer M, Brisson A, Coumans F, Dignat-George F, Duggan E, Ghiran I, Giebel B, Görgens A, Hendrix A, Lacroix R, Lannigan J, Libregts SF, Lozano-Andrés E, Morales-Kastresana A, Robert S, De Rond L, Tertel T, Tigges J, De Wever O, Yan X, Nieuwland R, Wauben MH, Nolan JP, Jones JC. MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments. J Extracell Vesicles 9: 1713526, 2020. doi: 10.1080/20013078.2020.1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release 238: 166–175, 2016. doi: 10.1016/j.jconrel.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 227.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CIE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 352: 438–441, 1991. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 229.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods 6: 359–362, 2009. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 230.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2: 20360, 2013. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8: 1648167, 2019. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol 10: 648, 2019. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 7: e2277, 2016. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109: 466–471, 2012. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res 10: 4350–4366, 2018. [PMC free article] [PubMed] [Google Scholar]
- 237.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res 114: 325–332, 2014. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 238.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182: 349–360, 2015. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yuan O, Lin C, Wagner J, Archard JA, Deng P, Halmai J, Bauer G, Fink KD, Fury B, Perotti NH, Walker JE, Pollock K, Apperson M, Butters J, Belafsky P, Farwell DG, Kuhn M, Nolta J, Anderson JD. Exosomes derived from human primed mesenchymal stem cells induce mitosis and potentiate growth factor secretion. Stem Cells Dev 28: 398–409, 2019. doi: 10.1089/scd.2018.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci 7: 245–262, 2019. doi: 10.1146/annurev-animal-020518-115300. [DOI] [PubMed] [Google Scholar]
- 241.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 21: 3197–3207, 2007. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 242.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc 5: e002856, 2016. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res 115: 1205–1216, 2019. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhao W, Ji X, Zhang F, Li L, Ma L. Embryonic stem cell markers. Molecules 17: 6196–6236, 2012. doi: 10.3390/molecules17066196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int 2015: 761643, 2015. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zohlnhöfer D, Ott I, Mehilli J, Schömig K, Michalk F, Ibrahim T, Meisetschläger G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schömig A; REVIVAL-2 Investigators . Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA 295: 1003–1010, 2006. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]