Abstract
Extracellular vesicles (EVs) have attracted rising interests in the cardiovascular field not only because they serve as serological markers for circulatory disorders but also because they participate in important physiological responses to stress and inflammation. In the circulation, these membranous vesicles are mainly derived from blood or vascular cells, and they carry cargos with distinct molecular signatures reflecting the origin and activation state of parent cells that produce them, thus providing a powerful tool for diagnosis and prognosis of pathological conditions. Functionally, circulating EVs mediate tissue-tissue communication by transporting bioactive cargos to local and distant sites, where they directly interact with target cells to alter their function. Recent evidence points to the critical contributions of EVs to the pathogenesis of vascular endothelial barrier dysfunction during inflammatory response to injury or infection. In this review, we provide a brief summary of the current knowledge on EV biology and advanced techniques in EV isolation and characterization. This is followed by a discussion focusing on the role and mechanisms of EVs in regulating blood-endothelium interactions and vascular permeability during inflammation. We conclude with a translational perspective on the diagnostic and therapeutic potential of EVs in vascular injury or infectious diseases, such as COVID-19.
Keywords: endothelial permeability, exosomes, inflammation, microvesicles
INTRODUCTION
Extracellular vesicles (EVs) were originally considered as inert debris released by cells to dispose of metabolic wastes (61, 154). One of the seminal discoveries in the history of EV research was made in 1967, when Peter Wolf (154) identified platelet-derived vesicles as “platelet dust” having coagulative properties. The term “exosomes” was used for the first time by Trams et al. (139) in 1981 to describe EVs released from cell cultures. Other notable studies in the 1980s and 1990s by Johnstone et al. characterized EV production from maturing reticulocytes (58, 60) and described functional changes in the plasma membrane due to shedding of redundant proteins in EVs (59). Rapidly evolving research in the past few decades has revealed that EVs are in fact a nonclassical means of intercellular communication, along with the classical means of cell-cell contact and secretion of soluble molecules (96). EVs have gained significant interests in the scientific community as novel contributors to physiological and pathological processes, as they carry specialized cargos capable of altering a variety of molecular reactions (47). Unlike secreted solutes, EV cargos contain proteins and nucleic acids that are enclosed within a lipid bilayer membrane that can be easily transported in the circulation without losing biological activity. The lipophilic nature of these tiny vesicles makes them highly interactive with recipient cells as they can be easily fused with cell membranes and endocytosed (129). Hence, circulating EVs are currently recognized as not only biomarkers but also regulators in cardiovascular diseases.
Despite a recent surge in EV research, a lot of questions about EV biology and their function in circulatory homeostasis or disease remain unanswered (85). The endothelial cells (ECs) lining the luminal surface of blood vessels provide a semipermeable barrier that restricts plasma leakage and immune cell infiltration into surrounding tissues. The integrity of this barrier is an important determinant of circulatory homeostasis, and endothelial hyperpermeability is a hallmark of inflammatory response to injury or infection. EVs have recently been shown to regulate vascular inflammation and permeability response. In this review, we attempt to summarize the current knowledge of EV biology, followed by discussing the roles of circulating EVs in regulating vascular inflammation and permeability.
EV BIOGENESIS AND TARGETING
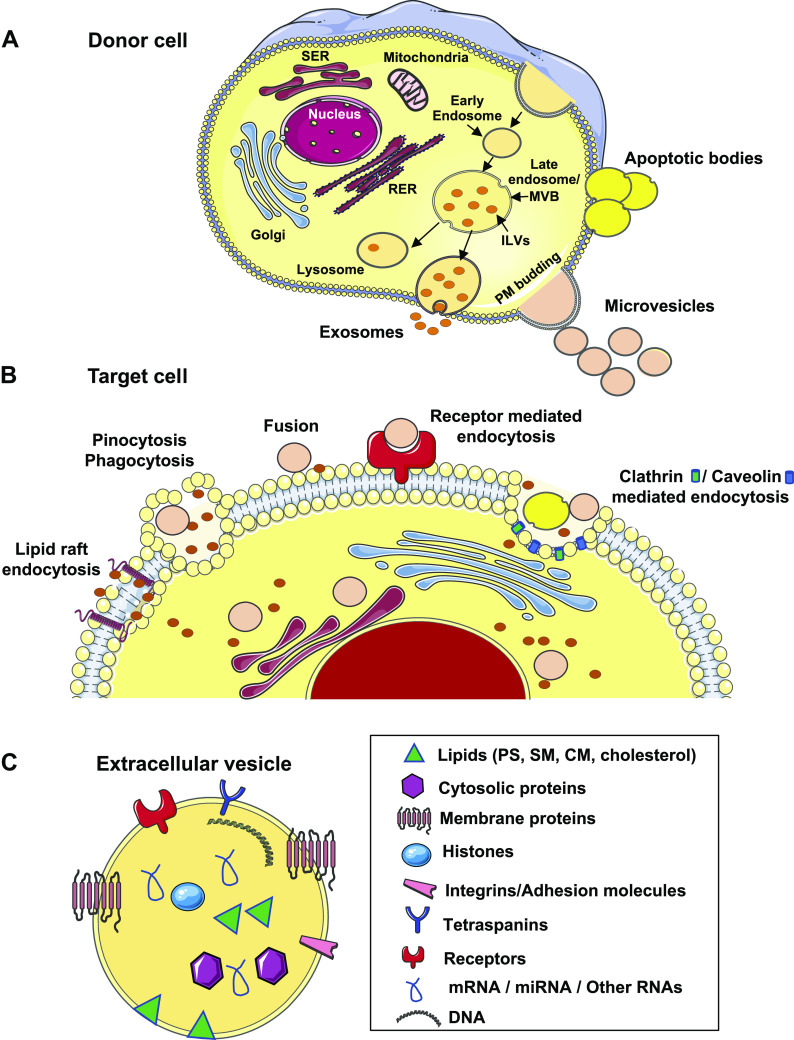
EVs are a heterogeneous collection of membranous vesicles produced by a variety of cells via complex intracellular pathways involving different subcellular compartments (Fig. 1A). The International Society of Extracellular Vesicles and Extracellular RNA Communication Consortium have classified them into three groups: 1) exosomes, small vesicles with diameters ≤100–150 nm that are formed inside multivesicular bodies (MVBs), which can be pelleted at centrifugation speed of 100,000 g (119); 2) microvesicles (MVs), medium-size vesicles of plasma membrane origin ranging in diameters up to 1,000 nm, which sediment at centrifugation speed of 20,000 g (89); and 3) apoptotic bodies, large vesicles with diameters >1,000 nm that can be collected at centrifugation speed of around 2,000 g (89).
Fig. 1.
A: generation of extracellular vesicles (exosomes, microvesicles, and apoptotic bodies) from donor cells. Exosomes are produced as a result of a fusion of a specialized subset of multivesicular bodies (MVBs) in the endosomal pathway to the plasma membrane. Microvesicles are formed by outward budding of the plasma membrane. Apoptotic bodies are formed by outward blebbing of the plasma membrane of apoptotic cells. B: different mechanisms of uptake of extracellular vesicles into the target cell. C: cargo composition of extracellular vesicles comprising lipids, proteins, and nucleic acids. CM, ceramide; MVB, multivesicular body; PS, phosphatidylserine; RER, rough endoplasmic reticulum; SER, smooth endoplasmic reticulum; SM, sphingomyelin. Some images of cells or organelles were obtained from Smart-Servier Medical Art (https://smart.servier.com).
Two complex mechanisms have been identified in the generation of exosomes. The first pathway is dependent on the endosomal sorting complex required for transport (ESCRT) machinery, which consists of four subunits (ESCRT-0, ESCRT-I, -II, and -III) that recruit ubiquitinated proteins to the early endosome and act in concert with other associated proteins (e.g., VPS4, VTA1, ALIX) in the assembly of exosome cargo to intraluminal vesicles (ILVs). Invagination of late endosomal membranes results in the formation of ILVs within larger endosomal multivesicular bodies (30). Once fusion of MVBs to the plasma membrane occurs, ILVs are released into the extracellular space as exosomes (96). Exosome biogenesis also involves the syndecan-syntenin-ALIX pathway and proteins such as heparanase, syndecans, heparan sulfate, ADP-ribosylation factor 6, phospholipase D2, and syntenin (136). The second pathway in exosome biogenesis is ESCRT independent and mediated by sphingomyelinase-dependent ceramide production (136). Exosome release is facilitated by multiple proteins, such as Rab GTPases, diacylglycerol kinase-α, and SNARE proteins (30, 136).
Microvesicles (MVs) are formed via blebbing or budding of the plasma membrane. This process is less well characterized compared with exosomes; rather, it is considered a sequela of loss of membrane asymmetry as a result of cell activation or injury. The outer leaflet of plasma membrane is enriched in lipids, including phosphatidylcholine and sphingomyelin, whereas the inner leaflet contains phosphatidylserine (PS) and phosphatidylethanolamine (29). This asymmetry is maintained by coordinated activity of membrane phospholipid transporter, flippase (inward translocation of PS), floppase (outward translocation of PS), and scramblase (bidirectional transporter that shuttles phospholipids along concentration gradient and Ca2+ influx) (29). Cell activation or injury causes increased Ca2+ influx, enhanced activity of floppases, and diminished activity of flippases, leading to PS outward exposure. This reaction is coupled with activation of caspases and/or calpains. The membrane budding process is facilitated by cytoskeletal remodeling, which involves RhoA, ADP-ribosylation factors 1 and 6, extracellular signal-regulated kinase, and myosin light chain kinase (MLCK) (79, 142). There is also a report that sphingomyelinase converts sphingomyelin to ceramide, which in turn regulates membrane curvature and MV release (12). MVs expressing PS on their surface are identified by positive annexin V labeling. PS+ MVs are known to be procoagulant, as they provide a negatively charged surface for prothrombinase and tenase complexes to bind, thereby promoting coagulation (104). In addition to the PS+ MVs, vesicles that do not expose PS on their surface exist. The mechanism behind the generation of PS− MVs is unclear. A study suggests their formation is via smaller endosomal vesicle binding to plasma membrane or via cleavage of cytoskeleton components during basal conditions, as opposed to activated states that produce PS+ MVs (104). In addition to annexin V, a recent study in cancer cell-culture models described annexin 1 and annexin 2 as novel MV markers that were exclusively present in MVs and not in exosomes (57). However, it must be noted that characterization of EVs is heavily dependent on methods of EV isolation and purification to reliably separate vesicular from nonvesicular fractions and soluble proteins in biofluids.
Apoptotic bodies are larger vesicles released by apoptotic cells via membrane blebbing, with or without cytoskeletal spikes (23). They are generally distinguished from other MVs based on size. Further details are discussed in other reviews (41, 135).
EVs have been detected in various biofluids, including plasma, urine, bile, saliva, bronchoalveolar lavage, ascitic fluid, lymph, and postoperative drainage fluid (67, 125). A variety of cells have been identified as parent cells capable of generating EVs, including platelets, leukocytes, endothelial cells, epithelial cells, hepatocytes, brain cells, and cancer cells. Most of them also serve as recipients or effectors of EV targeting. Once docked onto the recipient cell surface, EVs are internalized via membrane fusion or endocytosis; the latter occurs via clathrin-mediated endocytosis, caveolin-mediated endocytosis, lipid raft-mediated endocytosis, macropinocytosis, or phagocytosis (1, 90) (Fig. 1B). The level and mechanism of uptake vary depending on the recipient cell type, state of activation, and presence of receptors. For example, PS+ EVs target endothelial cells via developmental endothelial locus-1, or the tyrosine kinase receptor AXL that recognizes Gas6 on EV surface (35, 49). Heparan sulfate proteoglycans on the recipient cell surface also participate in EV uptake and internalization (11). The circulation kinetics and biodistribution profile of EVs in vivo depend on the route of administration, cell type of origin, and surface protein composition (152). A study shows that EVs labeled with the infrared lipophilic dye DiR, when intravenously injected in mice at a concentration of 1 × 1010 particles/g body wt, are mainly distributed in the liver, spleen, gastrointestinal (GI) tract, and lungs 24 h after injection (152).
EV CARGO COMPOSITION
EV cargos are enriched in nucleic acids, proteins, and lipids (Fig. 1C). Studies related to EV cargo packaging under different conditions are reported in public databases, including ExoCarta (91), Vesiclepedia (63), and EVpedia (63).
The nucleic acid contents of EVs include DNA (chromosomal and mitochondrial), mRNAs, miRNAs, short noncoding RNAs, tRNA fragments, piwi-interacting RNA, vault RNA, and Y RNA (1). The RNAs are enclosed in the lipid membrane rendering them resistant to RNAse degradation. The RNAs also exist in combination with ribonucleoproteins such as Argonaute 2 (AGO2), or high-/low-density lipoproteins (142). The existence of miRNAs in EVs indicates that EVs may influence gene expression in distal tissues via their cargo miRNA.
The protein composition of EVs has been studied using proteomic approaches. EV proteins are mainly derived from endosomes, plasma membranes, and cytosolic components. In contrast, those from the nucleus, endoplasmic reticulum, and Golgi apparatus are mostly absent (30). Some proteins are cell specific as shown in Table 1; others are commonly detectable irrespective of cell origin. For example, exosomes contain tetraspanins (CD9, CD63, CD81, CD37), heat shock proteins (Hsp 70, 90), tumor susceptibility gene 101 protein, major histocompatibility complex II, flotillin, and ALG-2-interacting protein X (ALIX) (1). Among them, CD9 is one of the most abundant exosomal proteins (65). Larger EVs are enriched with cytosolic components (enzymes, histones, ribosomal proteins, and proteasomes), transmembrane molecules (integrins, receptor kinases, and G proteins), and cytoskeletal elements (actin, cofilin, and tubulin) (30).
Table 1.
Cell-specific EV markers
| Cell of origin | Markers |
|---|---|
| Platelet | CD41+, CD61+, CD42b+, P-selectin+ |
| Endothelial cell | CD144+, CD106+, E-selectin+, CD31+/CD41-, CD144+/CD105+ |
| Neutrophil | CD66b+, CD11b+, L-selectin+, CD15+ (human), Ly6g+ (mouse) |
| Monocyte | CD14+, CD16+, Ly6c+ (mouse) |
| Lymphocyte | CD3+, CD4/8+ for T cells, CD19+ for B cells |
| Red blood cell | CD235a+ (human), TER-119+ (mouse) |
EV, extracellular vesicle.
Although the lipid profile of EVs has not been fully established, current known lipids include sphingomyelin, phosphatidylserine, cholesterol, diglycerides, saturated fatty acids, glycerophospholipids, ganglioside GM3, ceramides, and their derivatives (30, 76).
TECHNIQUES IN EV ISOLATION AND CHARACTERIZATION
Studying EVs has been challenging, owing to their small size and underdeveloped standardization protocols. Currently, methods of EV isolation include ultracentrifugation, density gradient centrifugation, sequential filtration, immunoaffinity isolation, size exclusion chromatography, polymer-based precipitation, and microfluidics (Table 2) (69). The most commonly used method is differential centrifugation by which different-sized EVs are collected under different centrifugation speeds: apoptotic bodies (2,000–2,500 g), MVs (10,000–20,000 g), and exosomes (100,000–120,000 g), respectively. This method allows processing of larger sample volumes but has limitations in separation of different types of EVs, as sedimented EVs could be contaminated with protein aggregates. EV purity can be enhanced via ultracentrifugation on a sucrose or iodixanol gradient as well as by size exclusion chromatography (SEC), each of which reduces soluble protein contamination (87). Both these techniques have their challenges; ultracentrifugation at speeds ∼100,000 g for prolonged times could damage EVs, whereas SEC that involves passing EVs through a column with nanosized pores could cause sample clogging or damage (87). Moreover, the latter is time-consuming and does not allow large sample volumes. Commercially available isolation kits use precipitation methods for fast isolation; however, their purity and specificity are often debated. In contrast, isolation by immunoaffinity method, i.e., EV capture by antibody-coated beads, produces high-quality EVs, but the yield is generally low because EVs that do not express the particular marker are missed during immunocapture (75).
Table 2.
Common methods of EV isolation and characterization
| Advantages | Disadvantages | Ref. | |
|---|---|---|---|
| Methods of isolation | |||
| Ultracentrifugation, differential centrifugation |
Large sample volumes, cost effective | Non-EV impurities, specialized equipment, physical damage to EVs | (32) |
| Density gradient ultracentrifugation | Pure EVs with minimal contamination from soluble protein and nonvesicular fractions | Labor-intensive and complex procedure, particles with same sedimentation rate cannot be separated | (69, 102) |
| Ultrafiltration | Large sample volumes, concurrent processing of multiple samples | Filter plugging, impurities, deformation of vesicles | (80) |
| Size exclusion chromatography | Pure EVs, separation of EV fraction from soluble proteins | Low sample volumes, labor-intensive, specialized equipment and complexity, column plugging | (75, 101) |
| Polymer-based precipitation | Quick and simple procedure | Impurities, additional chemicals | (69) |
| Immunocapture | Purity and high selectivity | Selective subsets of EVs only, high cost of antibodies, EV damage after elution | (32) |
| Microfluidics | Rapidness, purity, efficiency | Specialized equipment and complexity, low sample capacity | (158) |
| Methods of isolation | |||
| Nanoparticle tracking analysis (NTA); tunable resistive pulse sensing (TRPS) | Assessment of size distribution and particle concentration, fluorescent labeling for NTA | Cell debris, impurities can be mistaken for EVs, specialized equipment and training, high cost | (156) |
| Microscopy (EM, confocal, atomic force, super-resolution) | Direct visualization and study of uptake in cells, size distribution and morphology assessment, immunogold/fluorescent labeling | Dye aggregates can be mistaken for EVs, specialized equipment and training, high cost | (82) |
| Flow cytometry (conventional, nano-FACS, imaging flow cytometry) | Multicolor fluorescent labeling, estimation of particle concentration with counting beads, high resolution with nano-FACS, and imaging FCM | Smaller EVs (<200 nm) are missed with conventional flow cytometers, specialized equipment and training | (87) |
| Molecular studies (Western blot, qPCR, ELISAs, mass spectrometry, arrays) | Bulk EV cargo assessment | Cannot differentiate between different subsets of EVs | (32) |
EM, electron microscopy; EV, extracellular vesicle; FCM, flow cytometry; qPCR, quantitative polymerase chain reaction.
EV characterization approaches include nanoparticle tracking analysis (NTA), tunable resistive pulse sensing (TRPS), microscopy, flow cytometry, and molecular studies (Table 2) (156). NTA is a commonly used technique to quantitate EV numbers with a size resolution threshold of ∼30 nm. Particles are visualized by scattering of light and based on Brownian motion; the average particle size is then calculated by the Stokes–Einstein equation, according to which a particle’s size is inversely proportional to its diffusion capacity (126). TRPS is another particle analysis technique combining the Coulter principle with size-tunable pores and uses information obtained from the resistive pulse signal to determine particle numbers, size, and surface charge (70). Further confirmation of EV numbers, size, and morphology can be obtained by transmission electron microscopy (TEM), cryo-EM, and atomic force microscopy. Interaction of EVs with target cells can be determined by confocal microscopy (156). In addition, super-resolution microscopy, such as stimulated emission depletion (STED) microscopy, uses technology that bypasses the diffraction limit of light microscopy and thus is able to image small structures 20–40 nm in size; it also enables dynamic monitoring of EV interaction with recipient cells (109). As EVs are membranous and rich in nucleic acids, lipophilic dyes (DiR, PKH26, Di-8-Anepps) and nucleic acid dyes (Sytox RNA) have been used to stain EVs. A caveat is that dye aggregates can form in the EV size range, confounding the reading. Other dyes used for EV labeling include amine reactive fluorescent label and 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE); these dyes show less aggregates than lipid or nucleic acid dyes but have more background noise, and they require repeated washes, causing reduced EV yield (103).
EVs can also be quantitatively characterized by multicolor flow cytometry using antibodies specific to EV markers, sizing reference polystyrene beads, and EV counting beads (87). Conventional flow cytometry has a limitation in detecting particles smaller than 200 nm; the problem is minimized with the new generation of high-resolution flow cytometers (nano-FACS) or imaging flow cytometers equipped to quantitate EVs as small as 20–40 nm (87, 115). In addition, quantitative PCR, Western blotting, ELISAs, and mass spectrometry are commonly used to quantitatively measure specific EV components.
ENDOTHELIAL BARRIER STRUCTURE AND FUNCTION
Plasma fluid and proteins move across the endothelial barrier via the paracellular (major) and/or transcellular pathway (Fig. 2). The paracellular pathway is controlled by cell-cell junctions, including the adherens junction (AJ) formed by homophilic dimers of vascular-endothelial (VE)-cadherin linked to actin through catenins (α, β, γ, and p120), as well as tight junction (TJ) composed of occludin, claudins, and zonula occludens (ZO-1,2,3) that connect to the actin cytoskeleton (50, 167). Proteins associated with junction barrier also include junctional adhesion molecules (JAMs), platelet endothelial cell adhesion molecule-1, CD99, and nectins (143). The transcellular pathway is controlled by caveolae, vesiculo-vacuolar organelles (VVOs), and transmembrane molecules like gp60 that mediate transcytosis of albumin across the endothelium (Fig. 2) (111). Caveolin-1 is a major caveolar protein that plays a dual role in barrier regulation. On the one hand, it stabilizes caveolae and facilitates transcytosis; on the other hand, it inhibits endothelial nitric oxide (NO) synthase (eNOS), thereby reducing NO-induced paracellular permeability (121).
Fig. 2.
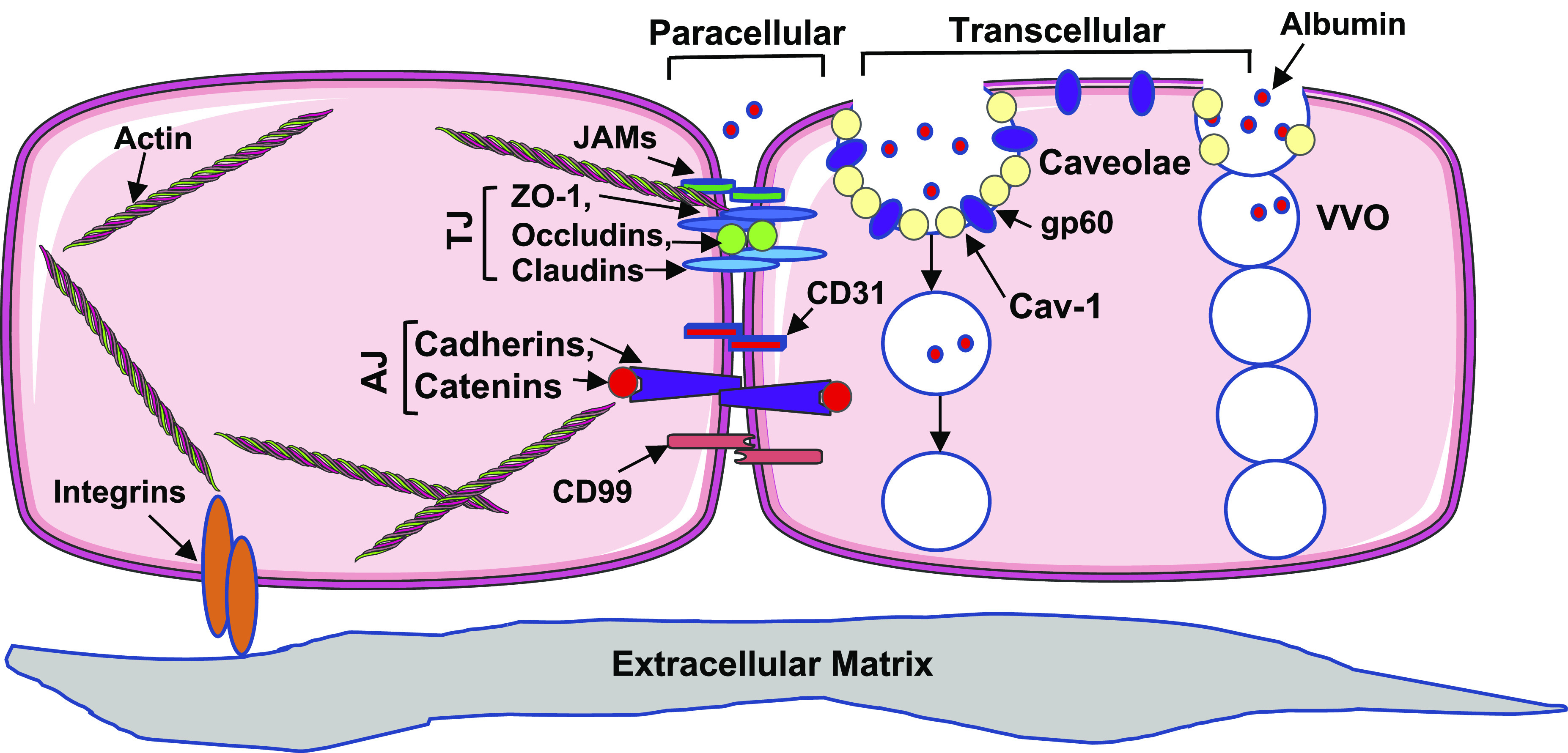
The paracellular and transcellular transport pathways for endothelial permeability. The paracellular pathway is maintained by interendothelial junctions comprising adherens junctions (AJs) and tight junctions (TJs), which are anchored to the actin cytoskeleton. The transcellular pathway comprises caveolae containing caveolin-1 (Cav-1) and vesiculo-vacoular organelles (VVOs). JAMs, junctional adhesion molecules. Some images of cells or organelles were obtained from Smart-Servier Medical Art (https://smart.servier.com).
During infection or acute inflammation, the junction proteins undergo posttranslational modifications or conformational changes, disrupting barrier integrity and enhancing permeability. Among multiple forms of posttranslational modifications, phosphorylation is best characterized; for example, VE-cadherin/catenin phosphorylation by protein kinases (c-Src, PKC) leads to AJ disassociation (36). Likewise, phosphorylation of caveolar proteins has been shown to increase EC transcellular permeability (36, 71). In addition, cytoskeleton modifications, for example, myosin light chain phosphorylation catalyzed by MLCK, trigger actomyosin contraction and intercellular gap formation (122). Recently, other forms of posttranslational modifications, including eNOS-mediated nitrosylation (48) and palmitoyltransferase-mediated palmitoylation (15), have been shown to regulate endothelial permeability. In particular, our laboratory has reported a novel function of DHHC-containing palmitoyl acyltransferases (PATs) in mediating endothelial inflammation and barrier disruption during inflammation (15).
Vascular permeability is also indirectly modulated by leukocyte-EC interactions. Neutrophil rolling and adhesion on microvascular endothelium increase permeability via intracellular cell adhesion molecule-1 (ICAM-1)-mediated signaling (130). Although constitutive PKC predominantly regulates ICAM-1-dependent permeability under physiological conditions, inducible Src activity plays a major role in ICAM-1 signaling during inflammation (130). Interestingly, many of these signaling pathways have been linked to EVs (25).
EV FUNCTION IN VASCULAR PERMEABILITY DURING INFLAMMATION
Circulating EVs are mainly derived from megakaryocytes and platelets, red blood cells, endothelial cells, and leukocytes, of which platelet EVs form the majority under normal conditions. EV production and cargo composition are altered during inflammation (117). For example, vascular inflammation is accompanied by increased ICAM-1+, VCAM-1+, and E-selectin+, and decreased endothelial protein C receptor (EPCR)+ EVs (25). In sepsis, plasma levels of total EVs and subsets derived from platelets, leukocytes, and endothelium are increased (117, 128, 153). In chronic vascular diseases like atherosclerosis, increased numbers of EVs derived from platelet, endothelium, and leukocyte origin are detected in the plasma (37). More importantly, EVs exert a detrimental role in promoting inflammation and microvascular permeability. Table 3 lists the EV cargo proteins and RNAs that participate in the regulation vascular inflammation and permeability. The role of EVs as pro-inflammatory mediators is further supported by the finding that blocking exosome generation with GW4869 inhibited cytokine production and attenuated systemic inflammatory injury in septic mice (42). In diabetic mice, plasma EVs laden with IgG are able to activate the classical complement pathway and promote microvascular leakage in the retina (53, 54). Similarly, patients with metabolic syndrome have a higher level of circulating EVs; when injected to normal mice, these EVs cause impaired endothelium-dependent vasorelaxation and decreased eNOS expression (3). Hence, EVs are thought to play a self-perpetuating role in augmenting inflammation in both acute and chronic systemic inflammatory diseases.
Table 3.
EV cargo regulating inflammation and endothelial junction permeability
| Origin | EV Cargo | Known Function | Ref. |
|---|---|---|---|
| Platelets | IL-1b | Increase permeability by activating inflammasome pathway in ECs | (51) |
| CCL5; P-selectin; sCD40L | Leukocyte recruitment and activation | (9, 92) | |
| Endothelial cells | c-Src; | Tyrosine phosphorylation of EC junction and cytoskeletal proteins to increase permeability | |
| ICAM-1; VCAM-1 | Increase leukocyte-EC adhesion | (25) | |
| Neutrophils | Annexin 1; | Improve EC permeability | (33) |
| MPO; PAF | Oxidative damage, inflammation, and thrombosis | (113, 149) | |
| Monocytes | IL-1b; tissue factor | Increase EC permeability and thrombosis | (118) |
| Microglia | IL-1b; miR-155 | Increase neuroinflammation | (73) |
| Nef | Increase BBB permeability by decreasing ZO-1 | (118) | |
| Red blood cells | miR-451a-Ago2 (RBC infected with malaria parasite) | Increase EC permeability by downregulating caveolin-1 and activating transcription factor 2 | (84) |
| Cancer cells | miR-25-3p (colon cancer) | Downregulate TJ proteins, alter VEGFR2 expression | (160) |
| miR103a (hepatoma) | Decrease VE-cadherin, p120 catenin, and ZO-1 expression in EC junctions | (43) | |
| miR-181c (brain metastatic breast cancer) | Disrupt actin cytoskeleton and increase EC permeability | (138) | |
| Semaphorin 3A; VEGF-A (glioblastoma) | Increase brain vascular permeability | (140, 141) |
EC, endothelial cell; EV, extracellular vesicle; ICAM-1, intracellular cell adhesion molecule-1; MPO, myeloperoxidase; PAF, platelet activating factor; RBC, red blood cell; TJ, tight junction; VEGF-A, vascular endothelial growth factor A.
Although many studies demonstrate a detrimental role of EVs in vascular inflammation, controversy exists. There is evidence suggesting that septic EVs protect vascular reactivity, and EV-leukocyte conjugates negatively correlate with organ dysfunction and mortality in sepsis (106, 127). Nevertheless, EVs not only serve as markers of cell stress or activation but also actively participate in pathophysiological responses to stress (Fig. 3). In the following section, we provide a detailed discussion about circulating EVs of different cell origins, centering on their roles in regulating vascular inflammation and permeability.
Fig. 3.
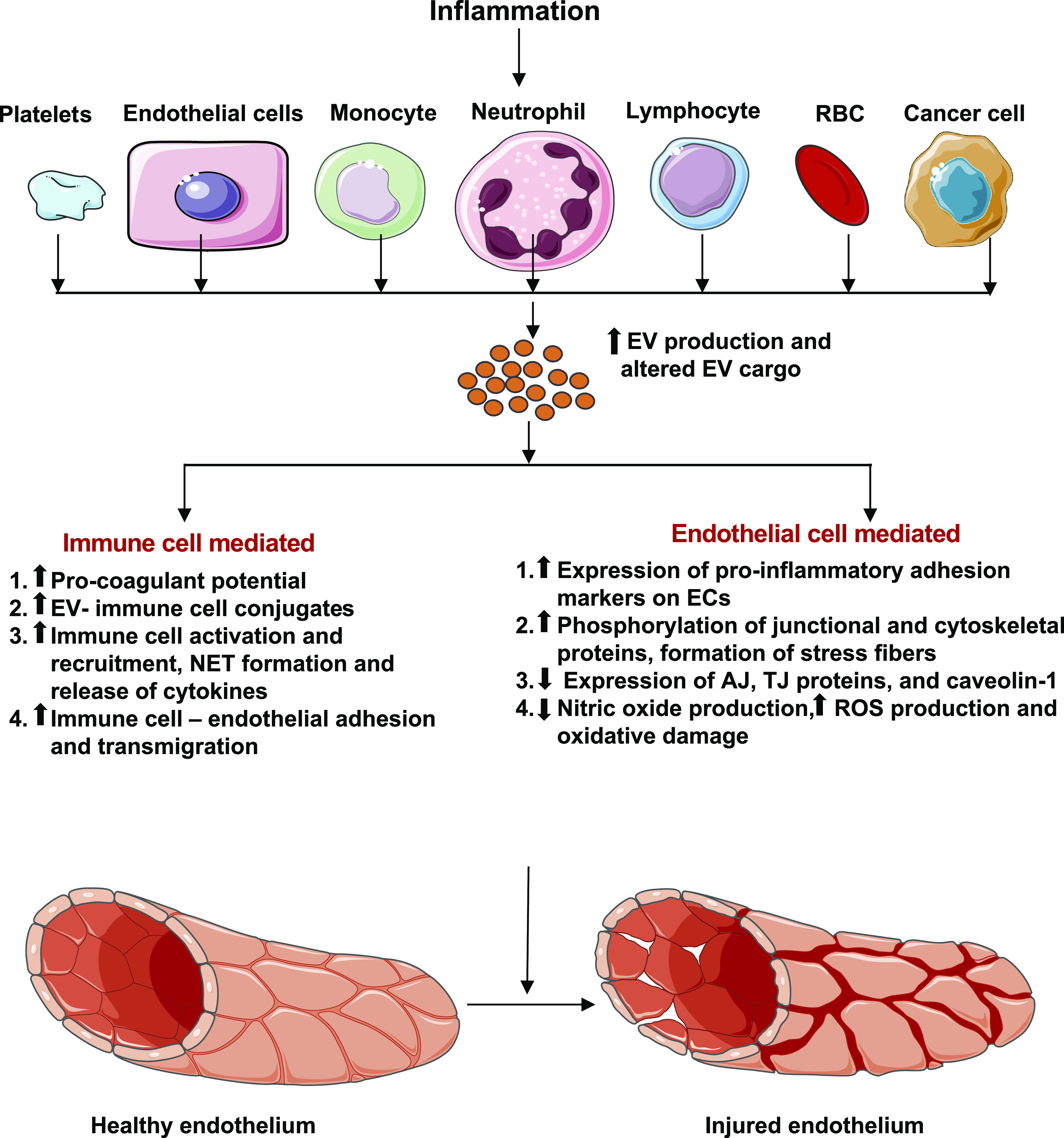
Inflammation alters the circulating extracellular vesicle (EV) profile that interacts with the vasculature to cause endothelial injury. Inflammation-associated EVs increase thrombogenicity, immune cell activation, adhesion, and transmigration through the endothelium. EVs also cause direct injury to the endothelium by increasing expression of pro-inflammatory adhesion molecules, modulating adherens junction (AJ), tight junction (TJ), caveolar, and cytoskeletal proteins through their protein and miRNA cargos and disturbing nitric oxide homeostasis. Some images of cells or organelles were obtained from Smart-Servier Medical Art (https://smart.servier.com).
PLATELET-DERIVED EVs
Platelet EVs show both pro-inflammatory and anti-inflammatory effects via interactions with endothelial cells and leukocytes. They carry cytokines like interleukin 1 beta (IL-1b), IL-6, and tumor necrosis factor alpha (TNFa), which stimulate neutrophil adhesion to endothelium and transendothelial migration (19, 83, 155). In particular, P-selectin and soluble CD40 ligand (sCD40L) carried by platelet EVs bind to P-selectin glycoprotein ligand-1 and CD40 expressed on leukocyte surface, respectively, causing leukocyte activation (9). Platelet EVs increase monocyte-EC binding by upregulating CD11a/b and CD14 on monocytes (14) and delivering c-c-motif chemokine ligand 5 (CCL5) to ECs (92). They also prolong neutrophils’ effect on tissue injury by reducing neutrophil apoptosis (20). With respect to their roles in vascular function, IL-1b-rich platelet EVs increase vascular permeability by activating EC inflammasome pathway (51). Recent mechanistic investigation reveals their effects to cause aortic endothelial injury and increase glomerular endothelial permeability by damaging glycocalyx, decreasing nitric oxide production, and increasing reactive oxygen species (ROS) production, via a mechanism dependent on mammalian target of rapamycin complex 1 (mTORC1) (145). In contrast to these pro-inflammatory actions, however, platelet EVs have been shown to exert anti-inflammatory effects, as they decrease c-c-motif chemokine ligand 4, TNFa, and colony-stimulating factor-1 release from macrophages (74), reduce TNF-a and IL-8 secretion from plasmacytoid dendritic cells (24), and inhibit IL-17 and interferon gamma production by regulatory T cells (24). In addition, they attenuate thrombin-induced endothelial barrier dysfunction in vitro and vascular endothelial growth factor (VEGF)-induced microvascular hyperpermeability in vivo (100).
ENDOTHELIAL-DERIVED EVs
Different inflammatory stimuli promote the production of endothelial-derived EVs (EC-EVs) with different compositions (25). In vitro, EC-EVs can be induced by TNFa, IL-1b, thrombin, lipopolysaccharide (LPS), C-reactive protein, and plasminogen activator-1 (52). In addition, laminar shear stress, high glucose conditions, and hypoxia are strong inducers of EC-EV generation (52). Several studies have demonstrated that EC-EVs bind leukocytes and contribute to their activities in inflammatory disorders (52, 125). Our recent study (25) shows that EC-EVs upregulate CD11b expression on neutrophil surface and induce formation of neutrophil extracellular traps (NETs). As citrullinated histone H3 (cit-H3) comprises the major protein content of NETs, increased circulating level of cit-H3 is associated with vascular injury (95). We have identified its direct effects to cause endothelial paracellular leakage (94).
In addition to activating leukocytes, EC-EVs can directly target the vascular endothelium. For example, endothelial cell monolayers exposed to EC-EVs, in the absence of leukocytes, display adherens junction discontinuity coupled with paracellular hyperpermeability (25). At the molecular level, EC-EVs promote phosphorylation of myosin light chain and VE-cadherin, which triggers a cytoskeleton contractile response characterized by cortactin translocation from cell periphery to center and actin stress fiber formation. Further mechanistic studies revealed that these effects involved c-Src signaling transferred by EV cargo, as c-Src-depleted EVs failed to cause barrier disruption (25). In line with this finding, EC-EVs have recently been shown to regulate blood-brain barrier (BBB) permeability (99, 123). In neuroinflammatory diseases (e.g., multiple sclerosis), brain-derived EC-EVs are increased, which bear markers that are specific to brain ECs (112). EVs derived from LPS-stimulated brain microvascular EC (BMEC) promote angiogenesis and/or vascular leakage by upregulating VEGF expression in pericytes (157). EC-EVs also contribute to the pathogenesis of cerebral malaria complicated by BBB injury (31).
LEUKOCYTE-DERIVED EVs
All subtypes of white blood cells can produce EVs, with the neutrophil being the main source especially during acute inflammation. Neutrophil-derived EVs are capable of targeting endothelial cells and increasing their production of IL-6, monocyte chemoattractant protein 1, and tissue factor (TF) (97, 98). They also cause oxidative injury in ECs by transferring cargo containing myeloperoxidase (113). Their effects to reduce TJ protein expression and to increase junction permeability have been observed in human cerebral ECs (6). In response to endotoxin stimulation, neutrophils release EVs that are rich in platelet-activating factor, which is known to promote inflammation and coagulation (149).
On the other hand, some studies show that neutrophil EVs exhibit beneficial effects. For example, extravasating neutrophils deposited CD18+ EVs that are barrier protective (81). Neutrophil EVs carry anti-inflammatory molecules, such as annexin 1 which is capable of reducing neutrophil-EC adhesion in vitro and neutrophil infiltration in vivo (33). Neutrophil EVs also reduce the release of pro-inflammatory cytokines and enhance the release of anti-inflammatory transforming growth beta 1 from macrophages (45) and natural killer cells (114).
Monocyte-derived EVs are known to induce endothelial inflammation in many ways. EVs produced from THP-1 (monocytic cell line) or primary monocytes bind to ECs, causing ERK1/2 phosphorylation and increasing the expression of ICAM-1, VCAM-1, and E-selectin in ECs via the nuclear factor-κB (NF-κB) pathway (147), or they transfer cargo carrying IL-1b and components of the inflammasome pathway. Blocking NLR family pyrin domain-containing 3 (NLRP3) in THP1 cells, or IL-1b receptor on ECs, mitigated the pro-inflammatory effects of monocytic EVs (147). Additionally, monocyte EVs increased nitrative stress via enhanced production of peroxynitrites (88). They also promote thrombogenicity by upregulating tissue factor, exposing phosphatidylserine, and downregulating anticoagulant tissue factor pathway inhibitor and thrombomodulin in ECs (5). This is evident in patients with meningococcal sepsis who display TF+ monocytic EVs coupled with severe disseminated intravascular coagulation (108). In vitro treatment of monocytes with LPS or interferon alpha produced EVs with unique miRNA signatures, which interacted with human brain microvascular ECs to increase inflammatory signaling and monocyte chemotaxis (34). Despite their well-recognized pro-inflammatory nature, however, monocytic EVs do not seem to be a potent barrier-damaging factor in human brain EC monolayers (150).
Compared with neutrophils and monocytes, lymphocyte EVs are relatively less well studied in the context of vascular inflammation and permeability. It is plausible that T cell-derived EVs play a crucial role in the chronic stage of inflammation, autoimmune disease, and cancer, based on the studies showing their effects to decrease nitric oxide (NO) production and increase ROS production in ECs; both contribute to impaired vasorelaxation responses (86, 105).
Microglia, the tissue-resident macrophages in the central nervous system, release EVs that regulate the blood-brain barrier and cerebral microcirculation in various neuroinflammatory diseases. Extracellular ATP, a common damage-associated molecular pattern (DAMP) associated with sterile inflammation and tissue injury, is known to increase the release of microglia EVs that are rich in IL-1b and GAPDH (18, 133). Microglia-derived EVs also play a complex role in the pathogenesis of neuroinflammation after traumatic brain injury (TBI). Although they are generally known as promoters of neuroinflammation after TBI (73), a study shows that microglia EVs containing miR-124-3p inhibit neuronal inflammation and promote neurite growth (56).
During infectious conditions, microglia release pro-inflammatory EVs in response to bacterial and viral pathogens. In particular, LPS stimulates the production of microglia EVs that are rich in IL-1b and miR-155 conferring pro-inflammatory effects (73). Microglia transfected with the HIV auxiliary protein Nef, release EVs that can disrupt the BBB by downregulating an important TJ protein, ZO-1. Nef+ microglia EVs also promote neuroinflammation by increasing production of proinflammatory cytokines like IL-6, IL-8, and IL-12 (118). EVs isolated from plasma of LPS-injected mice, when stereotactically introduced to the brain of healthy mice, are preferentially internalized by microglia as opposed to other neuronal cells (163). Thus, microglia play an important role in brain-blood crosstalk during inflammation through EVs.
RBC-DERIVED EVs
Red blood cells are a significant source of circulating EVs under physiological conditions, and their levels are increased in malaria (13), hematological disorders, systemic inflammation, and after prolonged blood storage (4, 8, 77, 117). RBCs infected with the malaria parasite Plasmodium falciparum show increased production of EVs, which carry functional miR-451a-Ago2 complexes capable of increasing endothelial permeability by downregulating caveolin-1 and activating transcription factor 2 (84). RBC-EVs also carry heme and iron that can cause oxidative damage to phospholipids on the cell membrane (21) and scavenge nitric oxide, resulting in impaired vascular function (38). RBC-EVs from stored blood are procoagulant in vitro, as they are rich in phosphatidylserine and can induce tissue factor upregulation and activate platelets (7). RBC-EVs have also been implicated in neutrophil priming and CD11b upregulation for enhanced adhesiveness to vascular endothelium (16, 22).
TUMOR-DERIVED EVs
EVs released by cancer cells circulate in the blood; they increase endothelial permeability and promote angiogenesis, thus facilitating tumor progression. A recent study indicates that metastatic tumor-derived EVs increase EC activity and permeability through exosomal miRNAs (120). Another study shows that colon cancer cells produce EVs that can increase vascular permeability by altering the expression of vascular endothelial growth factor receptor 2 (VEGFR-2) and tight junction proteins ZO-1, occludin, and claudin-5, through exosomal miR-25-3p (160). Likewise, hepatoma cells secrete EVs containing miR103a that increases vascular permeability by downregulating VE-cadherin, p120 catenin, and ZO-1 expression in EC junctions (43). Moreover, glioblastoma-secreted EVs containing protein semaphorin 3A increase brain vascular permeability, an effect attenuated by blocking semaphorin 3A or by inhibiting its receptor neuropilin-1 (140). These EVs also carry VEGF-A that increases the permeability of brain ECs (141). In addition, brain metastatic cancer cells produce EVs containing miR-181c, which is shown to disrupt actin cytoskeletal dynamics leading to increased BBB permeability (138).
CLINICAL AND TRANSLATIONAL PERSPECTIVE
Biomarker role of EVs.
Distinct EV signatures have been identified as potential biomarkers for cardiovascular diseases (28). For example, increased cystatin C, serpin F2, and CD14 protein levels in EVs are associated with an elevated risk for adverse vascular events and mortality in patients with atherothrombotic disease (64). In patients with diabetes, reduced miRNA-126-3p and miRNA-26 in circulating EVs correlate with prevalence of cardiovascular complications (110). Moreover, increased blood level of CD31+/annexin V+ EC-EVs is considered an independent predictor of adverse cardiovascular outcomes in subjects with stable coronary artery disease (124, 151). In patients with ultrasound evidence of subclinical atherosclerosis, increased CD11a+ leukocytic EVs are detected in their blood (26). Although the diagnostic potential of these EV markers is quite promising, more in-depth and large-scale analyses are required to establish their validity and clinical utility.
Therapeutic potential of EVs.
EVs are highly biocompatible because of low toxicity and immunogenicity. They are stable in the circulation and can easily be taken up by tissues, making them excellent therapeutic candidates for multiple diseases (131). Currently, engineering stem cell EVs represents an attractive field. With respect to the treatment of diseases associated with vascular barrier dysfunction, mesenteric stem cell EVs (MSC-EVs) have shown promise in attenuating pulmonary vascular permeability and lung injury in hemorrhagic shock (116). In a model of ex vivo human lung perfusion, MSC-EVs improved alveolar fluid clearance, decreased lung edema, and improved respiratory as well as hemodynamic function (46). Likewise, EVs from endothelial progenitor cells (EPCs) carrying miR-126-5p/3p increased the survival rate in mice after septic challenge and suppressed lung vascular leakage by increasing expression of TJ proteins (164). In addition, engineered EVs are being designed as part of drug delivery system or vectors to deliver specific miRNAs, peptides, antibodies, or synthetic compounds (132). These techniques involve multiple procedures using viral (adenoviral/retroviral) or nonviral (incubation, lipofection, sonication, electroporation) approaches (132).
The use of engineered EVs as nanotherapeutics for combating systemic inflammatory diseases is still at its stages of infancy. Several approaches have been proposed to modify EV surface and cargo for improved targeting of inflamed tissues. For example, integrins αLβ2 and α4β1 expressed on leukocyte EVs can sense and traffic toward ICAM-1 and VCAM-1 on inflamed endothelium (159). This property of integrin-bearing EVs can be used to deliver EV-based therapeutics. In addition to native proteins, other synthetic approaches to engineer EV surface and cargo by adding antibodies, peptides, RNAi, small molecules, and aptamers have been used to achieve tissue-specific targeting. For example, Tian et al. (137) used click chemistry-based modification of EVs to decorate EV surface with αvβ3 integrin-specific RGD peptides for targeting the ischemic brain in a stroke model. For ischemic heart diseases, myocardium-targeting peptides introduced on EV surface by fusion with lamp2 protein have been used (66, 148). ICAM-1 and VCAM-1 binding synthetic peptides are available; however, when conjugated to EVs, their ability and specificity to target inflamed tissues have yet to be confirmed (107, 162).
EV-based RNAi therapeutics, such as EVs loaded with miR-155 inhibitors, miR-15a mimics, or Myd88 siRNA, have shown promise as anti-inflammatory therapies to reduce LPS-induced acute lung injury (161). In a mouse study, EVs with designer mRNAs were delivered through implantation of engineered cells in the brain, which were shown to successfully reduce neuroinflammation (68).
Other EV nanotherapeutics have used the anti-inflammatory mechanisms intrinsic to cells. Some notable studies include injecting EVs loaded with IκBα, an inhibitor of transcription factor NFκB, which helped reduce mortality and inflammation in septic mice (27). Similarly, EVs loaded with catalase, an anti-oxidant enzyme, reduced oxidative stress, improved neuronal activity and attenuated neuroinflammation in a mouse model of Parkinson’s disease (166). In addition, neutrophil-derived EVs loaded with resolvin D2, and stem cell EVs loaded with curcumin, reduced inflammation and restored the BBB in ischemia-reperfusion-induced brain injury models (39, 62). In a recent study, decoy EVs expressing cytokine receptors lacking the signaling domain were generated and proved effective in neutralizing pro-inflammatory cytokines in an in vitro model of inflammation (40). Drugs like dexamethasone and piceatannol have been synthesized into macrophage/neutrophil-derived EVs, which successfully reduced endothelial inflammation in the kidney and lungs (44, 134). It must be noted that most of these approaches have been tested at the preclinical stages and their clinical utility, specificity, and safety need further validation (131).
Potential role of EVs in the pathogenesis and treatment of COVID-19.
Our understanding of COVID-19 pathophysiology has been rapidly evolving. A current view emphasizes its vasculitis nature, characterized by microcirculatory dysfunction, barrier leakage, and coagulopathy (2). Severe cases present with cytokine storm and advance to acute respiratory distress syndrome (ARDS) culminating in multiple organ failure (55, 144). EVs likely participate in the pathophysiological response of pulmonary and systemic circulation to viral infection. Previous studies show that severe acute respiratory syndrome-related coronavirus (SARS-Cov), responsible for the SARS outbreak in 2003 and sharing a high degree of amino acid homology with the novel SARS-Cov, enters host cells through endocytosis and is processed in the endosomal pathway (146). Given that exosome biogenesis involves the endosomal pathway, we speculate that EVs released by infected host cells might carry viral proteins or nucleic acids as part of their cargo (Fig. 4A). This is supported by the fact that many other viruses, such as paramyxoviruses, hepatitis C virus, rhabdoviruses, filoviruses, herpes viruses, and hepatitis B virus, use the ESCRT pathway to promote their release (93). These viral components assembled in EVs can act as pathogen-associated molecular patterns (PAMPs) which interact with pattern recognition receptors (PRRs), such as Toll-like receptors, in host cells, thereby activating downstream inflammatory processes (Fig. 4A).
Fig. 4.
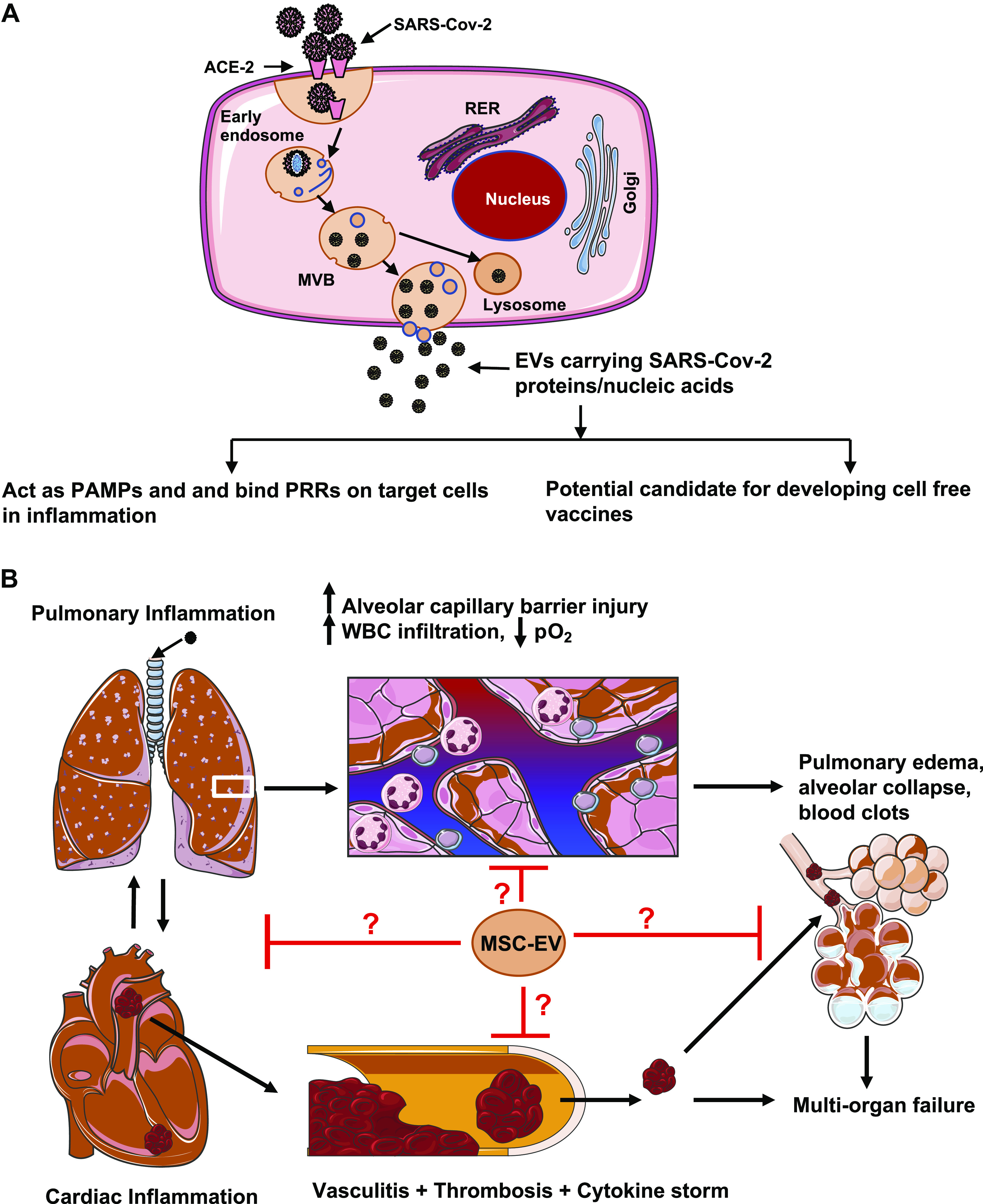
A: severe acute respiratory syndrome-related coronavirus 2 (SARS-Cov-2)-infected cells can release extracellular vesicles (EVs) expressing viral antigens that may play a role in inflammation and act as candidates for cell-free vaccines. B: potential role of mesenchymal stem cell-derived EVs (MSC-EVs) in mitigating inflammatory injury of the cardiovascular and the respiratory system in COVID-19. ACE-2, angiotensin-converting enzyme 2. Some images of cells or organelles were obtained from Smart-Servier Medical Art (https://smart.servier.com).
Developing effective vaccines is crucial to combating COVID-19. As a natural means of cell-to-cell and tissue-to-tissue communication, EVs provide an efficient vehicle for presentation and distribution of antigens through the circulation for them to reach distal organs. The presence of adhesion molecules and integrins on exosome surface ensures specific binding and uptake by host cells. Exosomal vaccines containing the S protein of SARS coronaviruses have been shown to induce high levels of neutralizing antibodies (72). We believe that similarly designed exosomes can be used as a viable cell-free vaccine platform for SARS-Cov-2 (Fig. 4A).
Mesenchymal stem cells have been extensively used in regenerative medicine to attenuate acute lung injury and facilitate tissue healing or recovery (78). Currently, there are many ongoing clinical trials worldwide testing the effect of MSC therapies against ARDS in patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04288102, NCT03042143, NCT04355728, NCT04333368). However, as cell-based therapies are associated with an increased risk of tumor development, immune rejection, and microvessel obstruction, MSC-EVs may become a preferred therapeutic option because of lower allogenic immune rejection, easier preservation, and higher stability compared with MSCs. Currently, MSC-EVs have gained significant interest as therapeutics in cardiovascular regenerative medicine and have shown promise in mitigating ischemic heart injury and LPS-induced acute lung injury in preclinical models (10, 165). Given the commonality of cardiovascular and pulmonary responses to respiratory infection, MSC-EVs might represent a novel therapeutic strategy to mitigate COVID-19-associated organ injury (Fig. 4B).
Future perspectives.
The future of EV research lies in the translational studies that test and validate their clinical applications in disease diagnosis as biomarkers, as well as in disease treatment as therapeutics or drug vectors. In the past few years, there has been tremendous progress in this field, evidenced by the surge in clinical trials involving exosome-based interventions, although many roadblocks need to be overcome before such EV-based approaches can be widely used in clinical settings. For example, there must be consensus in standardizing protocols for EV isolation and purification from human samples or preclinical models. Within this context, combining two different techniques of EV isolation (e.g., ultrafiltration or ultracentrifugation followed by size exclusion chromatography) to increase efficiency and purity is gaining popularity (17, 101). Another unmet challenge lies in EV quality and quantity with respect to large-scale production of reproducible batches of clinical grade EVs for therapeutics. In addition, the route of EV administration to achieve a consistent and desired therapeutic outcome needs to be optimized. Nevertheless, the future of applied EV research looks very promising.
Conclusion.
EV production from blood cells and/or endothelium is increased during acute and chronic cardiovascular diseases, and they carry distinct molecular signatures and serve as unique biomarkers for disease diagnosis. Functionally, circulating EVs induce vascular inflammation by promoting thrombosis, immune cell activation, leukocyte/platelet adhesion, and transmigration across the endothelium. They also directly impair vascular barrier function by altering endothelial junction and cytoskeleton dynamics via transfer of cargo containing permeability signaling molecules. Although many studies suggest EVs as inflammatory mediators, there is ample evidence supporting their anti-inflammatory and barrier-protective function, especially in the processes associated with inflammation resolution and tissue repair. The pleiotropic effects of EVs depend on the physiological state of their parent cells, cargo profile, and pathological conditions that promote their release. Further molecular insights into EV biogenesis, cargo loading mechanisms, and functional consequences of EV-targeting tissues will help us to better understand their enigmatic role in the regulation of various cardiovascular diseases. Moreover, as promising biomarkers and vaccine delivery vehicles, EVs and their engineered products may significantly contribute to the development of novel diagnostics and therapeutics for vascular injury in inflammatory or infectious diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R35 HL150732 and GM097270 (to S. Y. Yuan) and HL120954 (to M. H. Wu) and Department of Veterans Affairs Grants 101BX000799 and IK6BX004210 (to M. H. Wu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.W. and S.Y.Y. conceived and designed research; V.C. prepared figures; V.C. drafted manuscript; X.Y., Y.M., M.H.W., and S.Y.Y. edited and revised manuscript; V.C., X.Y., Y.M., M.H.W., and S.Y.Y. approved final version of manuscript.
References
- 1.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36: 301–312, 2016. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 173: 1210–1219, 2008. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aharon A, Rebibo-Sabbah A, Tzoran I, Levin C. Extracellular vesicles in hematological disorders. Rambam Maimonides Med J 5: e0032, 2014. doi: 10.5041/RMMJ.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 100: 878–885, 2008. doi: 10.1160/TH07-11-0691. [DOI] [PubMed] [Google Scholar]
- 6.Ajikumar A, Long MB, Heath PR, Wharton SB, Ince PG, Ridger VC, Simpson JE. Neutrophil-derived microvesicle induced dysfunction of brain microvascular endothelial cells in vitro. Int J Mol Sci 20: 5227, 2019. doi: 10.3390/ijms20205227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aleshnick M, Foley JH, Keating FK, Butenas S. Procoagulant activity in stored units of red blood cells. Biochem Biophys Res Commun 474: 680–685, 2016. doi: 10.1016/j.bbrc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Almizraq RJ, Holovati JL, Acker JP. Characteristics of extracellular vesicles in red blood concentrates change with storage time and blood manufacturing method. Transfus Med Hemother 45: 185–193, 2018. doi: 10.1159/000486137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol 54: 669–677, 2009. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 10.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJ, Skog J, Maguire CA. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol 115: 343–351, 2013. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awojoodu AO, Keegan PM, Lane AR, Zhang Y, Lynch KR, Platt MO, Botchwey EA. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood 124: 1941–1950, 2014. doi: 10.1182/blood-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babatunde KA, Yesodha Subramanian B, Ahouidi AD, Martinez Murillo P, Walch M, Mantel PY. Role of extracellular vesicles in cellular cross talk in malaria. Front Immunol 11: 22, 2020. doi: 10.3389/fimmu.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry OP, Praticò D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 102: 136–144, 1998. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beard RS Jr, Yang X, Meegan JE, Overstreet JW, Yang CG, Elliott JA, Reynolds JJ, Cha BJ, Pivetti CD, Mitchell DA, Wu MH, Deschenes RJ, Yuan SY. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat Commun 7: 12823, 2016. doi: 10.1038/ncomms12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg 214: 648–655, 2012. doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, Wouters EFM, Savelkoul PH, Lopez-Iglesias C, Koenen RR, Rohde GGU, Stassen FRM. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep 7: 15297, 2017. doi: 10.1038/s41598-017-15717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174: 7268–7277, 2005. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 19.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327: 580–583, 2010. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunetti M, Martelli N, Manarini S, Mascetra N, Musiani P, Cerletti C, Aiello FB, Evangelista V. Polymorphonuclear leukocyte apoptosis is inhibited by platelet-released mediators, role of TGFbeta-1. Thromb Haemost 84: 478–483, 2000. doi: 10.1055/s-0037-1614048. [DOI] [PubMed] [Google Scholar]
- 21.Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, Loufrani L, Grimaud L, Lambry JC, Charue D, Kiger L, Renard JM, Larroque C, Le Clésiau H, Tedgui A, Bruneval P, Barja-Fidalgo C, Alexandrou A, Tharaux PL, Boulanger CM, Blanc-Brude OP. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 125: 3805–3814, 2015. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apheresis Sci 38: 117–125, 2008. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 9: 1486, 2018. doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceroi A, Delettre FA, Marotel C, Gauthier T, Asgarova A, Biichlé S, Duperrier A, Mourey G, Perruche S, Lagrost L, Masson D, Saas P. The anti-inflammatory effects of platelet-derived microparticles in human plasmacytoid dendritic cells involve liver X receptor activation. Haematologica 101: e72–e76, 2016. doi: 10.3324/haematol.2015.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee V, Yang X, Ma Y, Cha B, Meegan JE, Wu M, Yuan SY. Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc Res 116: 1525–1598 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chironi G, Simon A, Hugel B, Del Pino M, Gariepy J, Freyssinet JM, Tedgui A. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol 26: 2775–2780, 2006. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, Kim S, Kim NH, Cho ES, In Yook J, Yoo TH, Song E, Kim P, Shin EC, Chung K, Choi K, Choi C. Exosome-based delivery of super-repressor IkappaBalpha relieves sepsis-associated organ damage and mortality. Sci Adv 6: eaaz6980, 2020. doi: 10.1126/sciadv.aaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang JW. Extracellular vesicles in cardiovascular diseases: alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int J Mol Sci 20: 3272, 2019. doi: 10.3390/ijms20133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark MR. Flippin’ lipids. Nat Immunol 12: 373–375, 2011. doi: 10.1038/ni.2024. [DOI] [PubMed] [Google Scholar]
- 30.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 31.Combes V, Taylor TE, Juhan-Vague I, Mège JL, Mwenechanya J, Tembo M, Grau GE, Molyneux ME. Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. JAMA 291: 2542–2544, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mäger I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R. Methodological guidelines to study extracellular vesicles. Circ Res 120: 1632–1648, 2017. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 33.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 112: 2512–2519, 2008. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 34.Dalvi P, Sun B, Tang N, Pulliam L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci Rep 7: 9954, 2017. doi: 10.1038/s41598-017-10449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta SK, Abdel-Monem H, Niravath P, Le A, Bellera RV, Langlois K, Nagata S, Rumbaut RE, Thiagarajan P. Lactadherin and clearance of platelet-derived microvesicles. Blood 113: 1332–1339, 2009. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 37.Deng W, Tang T, Hou Y, Zeng Q, Wang Y, Fan W, Qu S. Extracellular vesicles in atherosclerosis. Clin Chim Acta 495: 109–117, 2019. doi: 10.1016/j.cca.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 38.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476, 2011. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano 13: 1272–1283, 2019. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duong N, Curley K, Brown A, Campanelli A, Do MA, Levy D, Tantry A, Marriott G, Lu B. Decoy exosomes as a novel biologic reagent to antagonize inflammation. Int J Nanomedicine 14: 3413–3425, 2019. doi: 10.2147/IJN.S196975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516, 2007. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan GC. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta 1852: 2362–2371, 2015. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang JH, Zhang ZJ, Shang LR, Luo YW, Lin YF, Yuan Y, Zhuang SM. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 68: 1459–1475, 2018. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 44.Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 135: 62–73, 2017. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104: 2543–2548, 2004. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 46.Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant 15: 2404–2412, 2015. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greening DW, Simpson RJ. Understanding extracellular vesicle diversity - current status. Expert Rev Proteomics 15: 887–910, 2018. doi: 10.1080/14789450.2018.1537788. [DOI] [PubMed] [Google Scholar]
- 48.Guequén A, Carrasco R, Zamorano P, Rebolledo L, Burboa P, Sarmiento J, Boric MP, Korayem A, Durán WN, Sánchez FA. S-nitrosylation regulates VE-cadherin phosphorylation and internalization in microvascular permeability. Am J Physiol Heart Circ Physiol 310: H1039–H1044, 2016. doi: 10.1152/ajpheart.00063.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Happonen KE, Tran S, Mörgelin M, Prince R, Calzavarini S, Angelillo-Scherrer A, Dahlbäck B. The Gas6-Axl protein interaction mediates endothelial uptake of platelet microparticles. J Biol Chem 291: 10586–10601, 2016. doi: 10.1074/jbc.M115.699058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778: 660–669, 2008. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 122: 3405–3414, 2013. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W. Endothelial extracellular vesicles-promises and challenges. Front Physiol 8: 275, 2017. doi: 10.3389/fphys.2017.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Fisher KP, Hammer SS, Busik JV. Extracellular vesicle-induced classical complement activation leads to retinal endothelial cell damage via mac deposition. Int J Mol Sci 21: 1693, 2020. doi: 10.3390/ijms21051693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes 67: 1639–1649, 2018. doi: 10.2337/db17-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H, Zhang J, Lei P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32: 512–528, 2018. doi: 10.1096/fj.201700673r. [DOI] [PubMed] [Google Scholar]
- 57.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, and Coffey RJ. Reassessment of exosome composition. Cell 177: 428–445, 2019. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 59.Johnstone RM, Ahn J. A common mechanism may be involved in the selective loss of plasma membrane functions during reticulocyte maturation. Biomed Biochim Acta 49: S70–S75, 1990. [PubMed] [Google Scholar]
- 60.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 74: 1844–1851, 1989. doi: 10.1182/blood.V74.5.1844.1844. [DOI] [PubMed] [Google Scholar]
- 61.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol 147: 27–36, 1991. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 62.Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 79: 360–369, 2016. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalra H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10: e1001450, 2012. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanhai DA, Visseren FL, van der Graaf Y, Schoneveld AH, Catanzariti LM, Timmers L, Kappelle LJ, Uiterwaal CS, Lim SK, Sze SK, Pasterkamp G, de Kleijn DP, Group SS; SMART Study Group . Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int J Cardiol 168: 2358–2363, 2013. doi: 10.1016/j.ijcard.2013.01.231. [DOI] [PubMed] [Google Scholar]
- 65.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 428: 688–692, 2016. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, Yun N, Mun D, Kang JY, Lee SH, Park H, Park H, Joung B. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Biophys Res Commun 499: 803–808, 2018. doi: 10.1016/j.bbrc.2018.03.227. [DOI] [PubMed] [Google Scholar]
- 67.Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, Coimbra R. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J 32: 97–110, 2018. doi: 10.1096/fj.201700488r. [DOI] [PubMed] [Google Scholar]
- 68.Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, Ausländer S, Tan KR, Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 9: 1305, 2018. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res Int 2018: 8545347, 2018. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koritzinsky EH, Street JM, Star RA, Yuen PS. Quantification of exosomes. J Cell Physiol 232: 1587–1590, 2017. doi: 10.1002/jcp.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kronstein R, Seebach J, Grossklaus S, Minten C, Engelhardt B, Drab M, Liebner S, Arsenijevic Y, Taha AA, Afanasieva T, Schnittler HJ. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc Res 93: 130–140, 2012. doi: 10.1093/cvr/cvr256. [DOI] [PubMed] [Google Scholar]
- 72.Kuate S, Cinatl J, Doerr HW, Uberla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 362: 26–37, 2007. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14: 47, 2017. doi: 10.1186/s12974-017-0819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laffont B, Corduan A, Rousseau M, Duchez AC, Lee CH, Boilard E, Provost P. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost 115: 311–323, 2016. doi: 10.1160/th15-05-0389. [DOI] [PubMed] [Google Scholar]
- 75.Lane RE, Korbie D, Trau M, Hill MM. Purification protocols for extracellular vesicles. Methods Mol Biol 1660: 111–130, 2017. doi: 10.1007/978-1-4939-7253-1_10. [DOI] [PubMed] [Google Scholar]
- 76.Laulagnier K, Vincent-Schneider H, Hamdi S, Subra C, Lankar D, Record M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol Dis 35: 116–121, 2005. doi: 10.1016/j.bcmd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Leal JKF, Adjobo-Hermans MJW, Bosman GJCGM. Red blood cell homeostasis: mechanisms and effects of microvesicle generation in health and disease. Front Physiol 9: 703, 2018. doi: 10.3389/fphys.2018.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29: 913–919, 2011. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31: 4740–4749, 2012. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics 7: 789–804, 2017. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim K, Sumagin R, Hyun YM. Extravasating neutrophil-derived microparticles preserve vascular barrier function in inflamed tissue. Immune Netw 13: 102–106, 2013. doi: 10.4110/in.2013.13.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linares R, Tan S, Gounou C, Brisson AR. Imaging and quantification of extracellular vesicles by transmission electron microscopy. Methods Mol Biol 1545: 43–54, 2017. doi: 10.1007/978-1-4939-6728-5_4. [DOI] [PubMed] [Google Scholar]
- 83.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol 154: 485–490, 2001. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mantel PY, Hjelmqvist D, Walch M, Kharoubi-Hess S, Nilsson S, Ravel D, Ribeiro M, Grüring C, Ma S, Padmanabhan P, Trachtenberg A, Ankarklev J, Brancucci NM, Huttenhower C, Duraisingh MT, Ghiran I, Kuo WP, Filgueira L, Martinelli R, Marti M. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun 7: 12727, 2016. doi: 10.1038/ncomms12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Margolis L, Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol 17: e3000363, 2019. doi: 10.1371/journal.pbio.3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin S, Tesse A, Hugel B, Martínez MC, Morel O, Freyssinet JM, Andriantsitohaina R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 109: 1653–1659, 2004. doi: 10.1161/01.CIR.0000124065.31211.6E. [DOI] [PubMed] [Google Scholar]
- 87.Mastoridis S, Bertolino GM, Whitehouse G, Dazzi F, Sanchez-Fueyo A, Martinez-Llordella M. Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front Immunol 9: 1583, 2018. doi: 10.3389/fimmu.2018.01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mastronardi ML, Mostefai HA, Soleti R, Agouni A, Martínez MC, Andriantsitohaina R. Microparticles from apoptotic monocytes enhance nitrosative stress in human endothelial cells. Fundam Clin Pharmacol 25: 653–660, 2011. doi: 10.1111/j.1472-8206.2010.00898.x. [DOI] [PubMed] [Google Scholar]
- 89.Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles 6: 1286095, 2017. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 91.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40, D1: D1241–D1244, 2012. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol 25: 1512–1518, 2005. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 93.Meckes DG., Jr Exosomal communication goes viral. J Virol 89: 5200–5203, 2015. doi: 10.1128/JVI.02470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meegan JE, Yang X, Beard RS Jr, Jannaway M, Chatterjee V, Taylor-Clark TE, Yuan SY. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun 503: 1498–1502, 2018. doi: 10.1016/j.bbrc.2018.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meegan JE, Yang X, Coleman DC, Jannaway M, Yuan SY. Neutrophil-mediated vascular barrier injury: role of neutrophil extracellular traps. Microcirculation 24: e12352, 2017. doi: 10.1111/micc.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol 28: R435–R444, 2018. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 97.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol 161: 4382–4387, 1998. [PubMed] [Google Scholar]
- 98.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem 274: 23111–23118, 1999. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 99.Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Horstman LL, Ahn YS. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 56: 1319–1324, 2001. doi: 10.1212/WNL.56.10.1319. [DOI] [PubMed] [Google Scholar]
- 100.Miyazawa B, Trivedi A, Togarrati PP, Potter D, Baimukanova G, Vivona L, Lin M, Lopez E, Callcut R, Srivastava AK, Kornblith LZ, Fields AT, Schreiber MA, Wade CE, Holcomb JB, Pati S. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J Trauma Acute Care Surg 86: 931–942, 2019. doi: 10.1097/TA.0000000000002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine (Lond) 13: 2061–2065, 2017. doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol 1660: 25–32, 2017. doi: 10.1007/978-1-4939-7253-1_3. [DOI] [PubMed] [Google Scholar]
- 103.Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, Rosner A, Demberg T, Watson DC, Karpova TS, Freeman GJ, DeKruyff RH, Pavlakis GN, Terabe M, Robert-Guroff M, Berzofsky JA, Jones JC. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep 7: 1878, 2017. doi: 10.1038/s41598-017-01731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 31: 15–26, 2011. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 105.Mostefai HA, Agouni A, Carusio N, Mastronardi ML, Heymes C, Henrion D, Andriantsitohaina R, Martinez MC. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J Immunol 180: 5028–5035, 2008. doi: 10.4049/jimmunol.180.7.5028. [DOI] [PubMed] [Google Scholar]
- 106.Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med 178: 1148–1155, 2008. doi: 10.1164/rccm.200712-1835OC. [DOI] [PubMed] [Google Scholar]
- 107.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114: 1504–1511, 2006. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 108.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 95: 930–935, 2000. doi: 10.1182/blood.V95.3.930.003k46_930_935. [DOI] [PubMed] [Google Scholar]
- 109.Nizamudeen Z, Markus R, Lodge R, Parmenter C, Platt M, Chakrabarti L, Sottile V. Rapid and accurate analysis of stem cell-derived extracellular vesicles with super resolution microscopy and live imaging. Biochim Biophys Acta Mol Cell Res 1865: 1891–1900, 2018. doi: 10.1016/j.bbamcr.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol Aspects Med 60: 81–91, 2018. doi: 10.1016/j.mam.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Park-Windhol C, D’Amore PA. Disorders of vascular permeability. Annu Rev Pathol 11: 251–281, 2016. doi: 10.1146/annurev-pathol-012615-044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paul D, Baena V, Ge S, Jiang X, Jellison ER, Kiprono T, Agalliu D, Pachter JS. Appearance of claudin-5+ leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles. J Neuroinflammation 13: 292, 2016. doi: 10.1186/s12974-016-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pitanga TN, de Aragão França L, Rocha VC, Meirelles T, Borges VM, Gonçalves MS, Pontes-de-Carvalho LC, Noronha-Dutra AA, dos-Santos WL. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol 15: 21, 2014. doi: 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pliyev BK, Kalintseva MV, Abdulaeva SV, Yarygin KN, Savchenko VG. Neutrophil microparticles modulate cytokine production by natural killer cells. Cytokine 65: 126–129, 2014. doi: 10.1016/j.cyto.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, Ilkovics L, Hampl A, Crha I, Jandakova E, Minar L, Weinberger V, Bryja V. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 4: 25530, 2015. doi: 10.3402/jev.v4.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, Srivastava AK, Trivedi A, Matthay M, Holcomb JB, Schreiber MA, Pati S. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg 84: 245–256, 2018. doi: 10.1097/TA.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raeven P, Zipperle J, Drechsler S. Extracellular vesicles as markers and mediators in sepsis. Theranostics 8: 3348–3365, 2018. doi: 10.7150/thno.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U, Pilakka-Kanthikeel S, Nair MP. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neurovirol 22: 129–139, 2016. doi: 10.1007/s13365-015-0397-0. [DOI] [PubMed] [Google Scholar]
- 119.Russell AE, et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles 8: 1684862, 2019. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schillaci O, Fontana S, Monteleone F, Taverna S, Di Bella MA, Di Vizio D, Alessandro R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep 7: 4711, 2017. doi: 10.1038/s41598-017-05002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277: 40091–40098, 2002. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 122.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272–280, 2010. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheremata WA, Jy W, Delgado S, Minagar A, McLarty J, Ahn Y. Interferon-beta1a reduces plasma CD31+ endothelial microparticles (CD31+EMP) in multiple sclerosis. J Neuroinflammation 3: 23, 2006. doi: 10.1186/1742-2094-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sinning JM, Losch J, Walenta K, Böhm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32: 2034–2041, 2011. doi: 10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- 125.Słomka A, Urban SK, Lukacs-Kornek V, Żekanowska E, Kornek M. Large extracellular vesicles: Have we found the holy grail of inflammation? Front Immunol 9: 2723, 2018. doi: 10.3389/fimmu.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, Powis SJ. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 136: 192–197, 2012. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soriano AO, Jy W, Chirinos JA, Valdivia MA, Velasquez HS, Jimenez JJ, Horstman LL, Kett DH, Schein RM, Ahn YS. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit Care Med 33: 2540–2546, 2005. doi: 10.1097/01.CCM.0000186414.86162.03. [DOI] [PubMed] [Google Scholar]
- 128.Souza AC, Yuen PS, Star RA. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int 87: 1100–1108, 2015. doi: 10.1038/ki.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda) 34: 169–177, 2019. doi: 10.1152/physiol.00045.2018. [DOI] [PubMed] [Google Scholar]
- 130.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol 301: C804–C813, 2011. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Surman M, Drożdż A, Stępień E, Przybyło M. Extracellular vesicles as drug delivery systems - methods of production and potential therapeutic applications. Curr Pharm Des 25: 132–154, 2019. doi: 10.2174/1381612825666190306153318. [DOI] [PubMed] [Google Scholar]
- 132.Sutaria DS, Badawi M, Phelps MA, Schmittgen TD. Achieving the promise of therapeutic extracellular vesicles: the devil is in details of therapeutic loading. Pharm Res 34: 1053–1066, 2017. doi: 10.1007/s11095-017-2123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takenouchi T, Tsukimoto M, Iwamaru Y, Sugama S, Sekiyama K, Sato M, Kojima S, Hashimoto M, Kitani H. Extracellular ATP induces unconventional release of glyceraldehyde-3-phosphate dehydrogenase from microglial cells. Immunol Lett 167: 116–124, 2015. doi: 10.1016/j.imlet.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 134.Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, Wu M, Wang FM, Wen Y, Zhou LT, Ni HF, Chen PS, Gu N, Crowley SD, Liu BC. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 9: 4740–4755, 2019. doi: 10.7150/thno.33520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241, 2008. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 136.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569–579, 2002. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 137.Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150: 137–149, 2018. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 138.Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun 6: 6716, 2015. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645: 63–70, 1981. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 140.Treps L, Edmond S, Harford-Wright E, Galan-Moya EM, Schmitt A, Azzi S, Citerne A, Bidère N, Ricard D, Gavard J. Extracellular vesicle-transported Semaphorin 3A promotes vascular permeability in glioblastoma. Oncogene 35: 2615–2623, 2016. doi: 10.1038/onc.2015.317. [DOI] [PubMed] [Google Scholar]
- 141.Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles 6: 1359479, 2017. doi: 10.1080/20013078.2017.1359479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 143.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778: 794–809, 2008. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 144.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China: JAMA, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang GH, Ma KL, Zhang Y, Hu ZB, Liu L, Lu J, Chen PP, Lu CC, Ruan XZ, Liu BC. Platelet microparticles contribute to aortic vascular endothelial injury in diabetes via the mTORC1 pathway. Acta Pharmacol Sin 40: 468–476, 2019. doi: 10.1038/s41401-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 18: 290–301, 2008. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood 118: 2366–2374, 2011. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc 7: e008737, 2018. doi: 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RC, Zimmerman GA, McIntyre TM. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem 278: 33161–33168, 2003. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]
- 150.Wen B, Combes V, Bonhoure A, Weksler BB, Couraud PO, Grau GE. Endotoxin-induced monocytic microparticles have contrasting effects on endothelial inflammatory responses. PLoS One 9: e91597, 2014. doi: 10.1371/journal.pone.0091597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 26: 112–116, 2006. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 152.Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Woei-A-Jin FJ, De Kruif MD, Garcia Rodriguez P, Osanto S, Bertina RM. Microparticles expressing tissue factor are concurrently released with markers of inflammation and coagulation during human endotoxemia. J Thromb Haemost 10: 1185–1188, 2012. doi: 10.1111/j.1538-7836.2012.04733.x. [DOI] [PubMed] [Google Scholar]
- 154.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 155.Xie RF, Hu P, Wang ZC, Yang J, Yang YM, Gao L, Fan HH, Zhu YM. Platelet-derived microparticles induce polymorphonuclear leukocyte-mediated damage of human pulmonary microvascular endothelial cells. Transfusion 55: 1051–1057, 2015. doi: 10.1111/trf.12952. [DOI] [PubMed] [Google Scholar]
- 156.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 126: 1152–1162, 2016. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamamoto S, Niida S, Azuma E, Yanagibashi T, Muramatsu M, Huang TT, Sagara H, Higaki S, Ikutani M, Nagai Y, Takatsu K, Miyazaki K, Hamashima T, Mori H, Matsuda N, Ishii Y, Sasahara M. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci Rep 5: 8505, 2015. doi: 10.1038/srep08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J 12: 1600699, 2017. doi: 10.1002/biot.201600699. [DOI] [PubMed] [Google Scholar]
- 159.Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142: 1–12, 2017. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun 9: 5395, 2018. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther 26: 2119–2130, 2018. doi: 10.1016/j.ymthe.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle--peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem 19: 145–152, 2008. doi: 10.1021/bc700227z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng T, Pu J, Chen Y, Mao Y, Guo Z, Pan H, Zhang L, Zhang H, Sun B, Zhang B. Plasma exosomes spread and cluster around β-amyloid plaques in an animal model of Alzheimer’s disease. Front Aging Neurosci 9: 12, 2017. doi: 10.3389/fnagi.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Zingarelli B, Fan H. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care 23: 44, 2019. doi: 10.1186/s13054-019-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 32: 116–125, 2014. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19: 1769–1779, 2011. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17: 564–580, 2016. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]