Abstract
The gut microbiome and intestinal dysfunction have emerged as potential contributors to the development of cardiovascular disease (CVD). Alterations in gut microbiome are well documented in hypertension, atherosclerosis, and heart failure and have been investigated as a therapeutic target. However, a perhaps underappreciated but related role for intestinal barrier function has become evident. Increased intestinal permeability is observed in patients and mouse models of CVD. This increased intestinal permeability can enhance systemic inflammation, alter gut immune function, and has been demonstrated as predictive of adverse cardiovascular outcomes. The goal of this review is to examine the evidence supporting a role for intestinal barrier function in cardiovascular disease and its prospect as a novel therapeutic target. We outline key studies that have investigated intestinal permeability in hypertension, coronary artery disease, atherosclerosis, heart failure, and myocardial infarction. We highlight the central mechanisms involved in the breakdown of barrier function and look at emerging evidence for restored barrier function as a contributor to promising treatment strategies such as short chain fatty acid, probiotic, and renin angiotensin system-targeted therapeutics. Recent studies of more selective targeting of the intestinal barrier to improve disease outcomes are also examined. We suggest that although current data supporting a contribution of intestinal permeability to CVD pathogenesis are largely associative, it appears to be a promising avenue for further investigation. Additional studies of the mechanisms of barrier restoration in CVD and testing of intestinal barrier-targeted compounds will be required to confirm their potential as a new class of CVD therapeutic.
Keywords: atherosclerosis, cardiovascular disease, gut microbiome, hypertension, intestinal permeability
INTRODUCTION
Evidence for an important role of the gut microbiome (GM) and intestinal dysfunction in the development of cardiovascular disease (CVD) is accumulating. Dysbiosis, an imbalance of microbial populations, is observed in patients with hypertension, atherosclerosis, and heart failure (18, 23, 33). Experimental models reveal important interactions between microbiota, the intestinal barrier, and immune function in these and other diseases. Although research has been predominantly focused on manipulating the microbiome to improve cardiovascular outcomes, a contribution of intestinal barrier function is evident and perhaps underappreciated. Increased intestinal permeability is observed in CVD and associated with enhanced systemic inflammation, potentially via leakage of bacterial components into the circulation and altered immune interactions in the gut. Importantly, defects in barrier function are often reversed with effective therapies, both those targeting the gut, such as probiotic or short chain fatty acid (SCFA) treatment (8, 15), and common therapies such as renin angiotensin system (RAS)-targeting drugs (48, 49). An interesting question raised by studies assessing host-microbial interactions is the nature of the relationship between the intestinal barrier and GM. It remains unresolved whether increased permeability precedes dysbiosis or is caused by it. While this may depend on the disease context, there is evidence to suggest that processes increasing intestinal permeability begin before the development of dysbiosis and hypertension (41). Mechanistic understanding of how specific microbiota contribute to the development of cardiovascular diseases remains somewhat lacking and makes selective targeting a challenge. Considering the potential for increased intestinal permeability to be upstream of detectable changes in microbiome and common to many disease states, enhancement of intestinal barrier function is emerging as an attractive therapeutic strategy. Herein we review current evidence for the role of intestinal permeability in the progression of cardiovascular diseases, the mechanisms associated with its dysfunction, and its potential as a therapeutic target.
MECHANISMS OF INTESTINAL BARRIER FUNCTION
Depicted in Fig. 1, the intestinal mucosa and epithelium act as a barrier between luminal contents and underlying tissue. Mucins, secreted from goblet cells, form the mucus layer, separating bacteria and large particles from the epithelial layer. The epithelium regulates paracellular transport of water and nutrients via a series of intercellular junctions, made up of tight junction, adherens junction, and desmosome proteins. Known as the apical junctional complex, these proteins work together to enable cell-cell communication and determine paracellular permeability. Forming a network at the most apical end of the epithelial cell, tight junction proteins (TJP) include occludin, claudin, zonula occludens, and junctional adhesion molecule families. These proteins are critical to epithelial barrier function, and their expression and distribution is altered in diseases of increased permeability (32). In more severe disease where epithelial damage and cell death occurs, complete loss of tight junctions allows transport of large molecules and whole bacteria. In conditions with increased leakage through these paracellular pathways, increased transcellular transport of luminal materials (transcytosis) is also common. Rather than utilizing TJPs, transcellular transport can occur through passive diffusion or endocytosis/exocytosis pathways and can also be dysregulated in disease states.
Fig. 1.
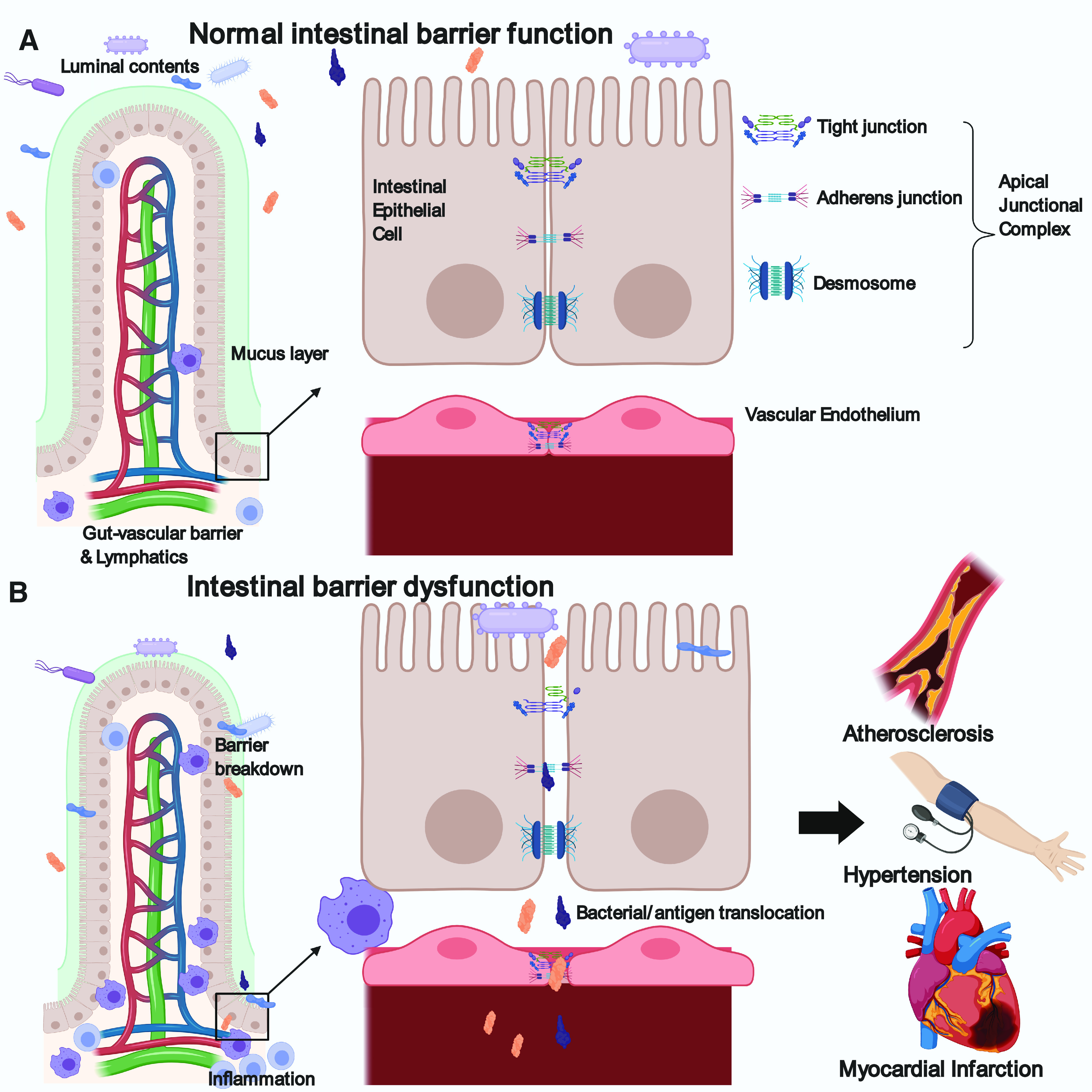
Mechanisms of intestinal barrier function. A: normal intestinal barrier function in a healthy subject. LHS, a thick layer of mucus creates a barrier between the intestinal epithelium and the luminal contents. Each villus has its own vasculature, further regulating entry of intestinal contents into circulation (gut-vascular barrier). Some immune cells are present and contribute to homeostasis. RHS, enlarged view of epithelial cells demonstrating the structure of the apical junctional complex made up of the tight junction, adherens junction and desmosome. These protein complexes work together to regulate epithelial permeability. Below the epithelium, the vascular endothelium also contains tight and adherens junctions that regulate vascular permeability. B: intestinal barrier dysfunction occurs through multiple mechanisms including reduced mucus thickness, increased inflammation, and reduced epithelial and endothelial tight junction protein expression and function. This leads to the translocation of intestinal bacteria and antigens into circulation, leading to systemic inflammation. These processes have been shown to occur during the development of cardiovascular diseases such as atherosclerosis, hypertension and myocardial infarction. Figure was created with BioRender.com and published under license.
Inflammatory and redox signaling can influence permeability pathways and is evident in CVD. A cyclic process appears to occur whereby inflammation contributes to intestinal dysfunction, which in turn leads to systemic activation of inflammatory pathways such as toll like receptors and the inflammasome (13, 29). Intestinal Th17 expansion is also reported with increased permeability and has proinflammatory effects at distant tissues (28). Activation of these pathways and subsequent cytokine production can lead to further barrier dysfunction (2). Oxidative stress can also reduce TJP function and cause epithelial damage (21, 34). Transcellular pathways can also be stimulated by proinflammatory cytokines, most notably TNFα and IFNγ, both commonly upregulated in cardiovascular diseases (30).
While the intestinal epithelium plays an important role in dictating permeability, access of bacteria to systemic circulation is a two-way street and the endothelial barrier also has a functional contribution. Known as the gut-vascular barrier (GVB), the endothelium has its own tight and adherens junctions. It can be investigated in vivo using FITC-dextran intravenous injection and examining leakage into the intestine (43); the reverse of experiments used to examine gut epithelial permeability. It is interesting to speculate that endothelial dysfunction, which occurs early in CVD, may occur within the GVB in diseases such as atherosclerosis and hypertension. To date, few studies have specifically looked at GVB function in CVD with experiments directed toward the epithelial barrier. A recent diabetes study demonstrated GVB dysfunction and intestinal pathology in a type 1 diabetes (T1D) mouse model and increased gut epithelial permeability and GVB permeability in T1D and T2D patients (11). Whether these processes occur in other cardiovascular diseases remains to be investigated.
INTESTINAL BARRIER DYSFUNCTION IN CARDIOVASCULAR DISEASE
Increased intestinal permeability has been reported in multiple human and animal studies of CVD. It is generally identified using a combination of circulating markers and functional tests. The most commonly used marker is serum lipopolysaccharide (LPS or endotoxin). Increased serum LPS can indicate bacterial translocation from gut to circulation and has been observed in hypertension (23), chronic heart failure (CHF) (39), myocardial infarction (MI) (9), and atherosclerosis (46). Markers such as circulating anti-LPS antibodies (40), soluble LPS receptor (sCD14), LPS-binding protein (LBP) (6, 7), and d-lactate [a product released abundantly by gastrointestinal (GI) microbiota] (53) are also used to indicate endotoxemia. Additionally, diamine oxidase, intestinal fatty acid binding protein (I-FABP), and zonulin are derived from the intestinal epithelium and associated with increased intestinal permeability when found at high circulating concentrations (26, 51, 52). Sugar excretion tests such as lactulose/mannitol ratio are also valuable and involve oral administration of differently sized sugars that are subsequently measured in urine (20, 33). In a similar vein, animal studies commonly use FITC-dextran assays where fluorescently labeled dextrans of various sizes are given orally and measured in serum. While the lactulose/mannitol ratio is mostly selective for increased small intestine permeability (44), most of these in vivo methods do not distinguish between different sites along the intestine. Therefore, findings from these experiments demonstrate a general state of increased intestinal permeability without identifying specific sites of dysfunction. Finally, altered TJP expression or reduced mucus layer, assessed either by molecular methods or immunohistochemical staining, further indicate barrier dysfunction. Although there are inherent limitations associated with each of these tests, discussed extensively in several reviews (14, 44, 45), when used in combination and replicated among multiple studies, the data are compelling. Recent studies performed in the settings of heart failure, MI, atherosclerosis, coronary artery disease (CAD), and hypertension are outlined below and summarized in Table 1.
Table 1.
Summary of recent CVD studies where intestinal barrier dysfunction was investigated
| Disease | Observed Intestinal Barrier Defects | Treatment Effects on CV and Intestinal Function |
|---|---|---|
| Heart failure and MI |
|
|
| Atherosclerosis/CAD |
|
|
| Hypertension |
|
|
CVD, cardiovascular disease; LBP, LPS-binding protein; IMT, intima-media thickening; ART, anti-retroviral therapy; CV, cardiovascular; CHF, chronic heart failure; MI, myocardial infarction; OSA, obstructive sleep apnea; CAD, coronary artery disease; CMT, cecal microbiome transfer; BP, blood pressure; HSD, high-salt diet; WD, Western diet; PMB, polymyxin B; PWV, pulse wave velocity; LV, left ventricular; SHR, spontaneously hypertensive rats; CRP, C-reactive protein; STEMI, ST-elevation myocardial infarction.
Heart failure and myocardial infarction.
Studies linking intestinal dysfunction and heart failure date back many years with associations between intestinal diseases such as inflammatory bowel disease and increased risk. Dysfunctional mucosal barrier (5) and increased small and large intestine permeability were observed in early investigations of CHF establishing a role for intestinal barrier function specifically (38). It is proposed that a vicious cycle of intestinal dysfunction leading to myocardial and microcirculatory dysfunction and further intestinal dysfunction may occur (37). Whether vascular or intestinal dysfunction is the initiating factor in these processes remains a source of debate. Sandek et al. have made a major contribution to this field over recent years reporting reduced intestinal blood flow, increased GI symptoms, increased serum LPS, and increased anti-LPS IgA levels in multiple studies of CHF patients (37–40). Most compelling is the observation that increased intestinal permeability correlated with right atrial pressure and circulating inflammatory markers (33). Much work has also been done in the setting of MI. A 2018 study by Zhou et al. (53) shows increased serum markers of permeability in ST-elevation myocardial infarction (STEMI) patients. Akin to the finding correlating permeability with heart function in CHF, in this study, LPS and d-lactate levels were predictive of major adverse cardiovascular events within 3 yr post-STEMI (53). These observations are complemented by findings in mouse models of MI demonstrating intestinal barrier dysfunction (7, 53). Furthermore, deficits in intestinal barrier function are observed in young acute MI patients (9).
Atherosclerosis and coronary artery disease.
Accumulating evidence demonstrates increased intestinal permeability in CAD and atherosclerosis. Interestingly, the presence of intestine-derived bacteria has been reported in human atherosclerotic plaques (24, 26) suggesting a role for bacterial translocation in atherogenesis. Furthermore, comparisons between CAD and non-CAD as well as diabetic vs non diabetic CAD patients reveal increased intestinal permeability with disease (26, 36). Correlations between the extent of carotid intima-media thickening and the permeability markers FABP2 and zonulin are also observed in two recent studies examining CVD and HIV+ antiretroviral therapy patient cohorts, respectively (10, 51). Thus, a role for intestinal barrier dysfunction in early stages of disease is evident. Although human data are limited, preclinical studies shed some light on the mechanisms leading to increased intestinal permeability in atherosclerosis. Reduced TJP expression is reported in commonly used mouse models of atherosclerosis expression (3, 16), and a recent study implicates hyperlipidemia as an initiating factor. High lipid levels are shown to reduce the expression of intestinal alkaline phosphatase, which normally protects the intestinal barrier by detoxifying LPS. This increased epithelial cell exposure to LPS can in turn reduce TJP expression and function (16). Additionally, in vitro experiments suggest that a hyperlipidemic environment can increase macrophage sensitivity to NF-κB activation by LPS, requiring lower levels of LPS to induce inflammation and therefore increasing macrophage infiltration to the artery wall (16).
Hypertension.
Many studies have shown a connection between GM and hypertension (27, 50), and anti-hypertensive effects of probiotics have been reported in patients (12, 22). Although the majority of these studies look at general gut pathology but not intestinal permeability per se, recent evidence suggests permeability changes contribute to these mechanisms (23, 41, 49). The research group led by Raizada and colleagues provided the first evidence for increased intestinal permeability in hypertensive patients reporting increased circulating I-FABP, LPS, and zonulin (23). In their additional studies of hypertensive rats and mice, reductions in TJP expression were observed before the development of hypertension or intestinal pathology (41). In these models of hypertension, reduced mesenteric blood flow and elevated splanchic sympathetic nerve drive from the paraventricular nucleus (PVN) contributed to the breakdown of epithelial barrier function whereby noradrenaline release and altered blood flow lead to reduced TJP expression and enhanced inflammation (41). Further studies demonstrated that ANG II infusion leads to PVN microglial activation which increases SNS signals to both the gut and bone marrow (42). In the bone marrow, this enhanced SNS drive initiates Th17 T cell release into circulation, contributing to and potentially initiating systemic inflammation (1, 42). Thus hypertension and the RAS appear to regulate intestinal permeability and systemic inflammation at multiple levels.
Other research groups have replicated findings for increased permeability in models of hypertension. In high-salt diet (HSD)-induced hypertension, increased serum FITC-dextran and reduced TJP expression were observed (17, 52). In a Western diet (WD) model, increased LBP and sCD14 correlated with aortic stiffening [pulse wave velocity (PWV)] and endothelial dysfunction (6). Finally, in a study of obstructive sleep apnea (OSA)-induced hypertensive rats, increased serum FITC-dextran and reduced mucus thickness was reported (15).
CVD risk factors, intestinal permeability, and microbiome.
It should be acknowledged that patient studies are mostly correlative with demonstration of a causal mechanism for intestinal permeability currently lacking. However, in support of a role for intestinal barrier dysfunction in the early stages of CVD, many CVD risk factors are associated with intestinal barrier dysfunction. Both high-fat and high-salt diets are associated with alterations in gut integrity and inflammation (35, 47). Furthermore, patients with hyperglycemia, insulin resistance and obesity have increased intestinal permeability (25). It remains under debate whether increased permeability at the onset of disease occurs through diet-induced dysbiosis or conversely whether these factors may promote epithelial damage leading to barrier disruption before dysbiosis.
CURRENT THERAPEUTIC STRATEGIES THAT IMPROVE “LEAKY GUT”
Key preclinical studies of intestinal barrier function and CVD report improvements in barrier function following effective treatments (Table 1). This occurs not only for microbiome- or gut-targeted therapies but also typical cardiovascular therapeutics. The latter is particularly evident in the setting of hypertension. The RAS has long been utilized as an effective drug target for hypertension and in three different rodent studies, RAS-targeted drugs restored barrier function while reducing blood pressure and improving cardiac function (41, 48, 49). How these treatments restore intestinal pathology remains to be fully elucidated but the findings suggest an important role for gut integrity.
Studies targeting the microbiome have utilized various methods to either deplete it (nonabsorbable antibiotics), enhance beneficial populations or metabolites (prebiotics, probiotics, SCFAs), or replace it with a “healthy” microbiome (fecal transplantation). Although in its early stages in application to humans, data from patient studies show promise for the use of probiotics. Both antiatherogenic and antihypertensive effects have been observed, although the effects are strain specific (22, 31). Although there is limited information on barrier function in these investigations, experimental models indicate restored barrier function with probiotic treatments in MI (4) and hypertension (15). Additionally, although it has a multitude of mechanisms of action, treatment with lactulose has pre- and probiotic effects and improved barrier function, inflammation, and blood pressure in HSD-induced hypertension (52). The SCFA butyrate was also effective in improving intestinal and cardiovascular parameters in ANG II-induced hypertension and in the ApoE−/− mouse model of atherosclerosis (19, 23). Antibiotics elicited improvements in both barrier function and disease end points in models of MI, atherosclerosis, and hypertension (6, 16, 17, 53). Taken together, these findings suggest that intestinal barrier function contributes to both development and improvement of CVD pathogenesis. However, this was not supported by a cecal microbiome transfer (CMT) study where control to obese CMT failed to improve barrier function, despite effectively reducing infarct size in a mouse model of MI (7). These findings suggest that while microbial transfer can worsen cardiac disease and barrier function, restoration of barrier function may not be required for improvement of all cardiovascular end points. The authors suggest that the lack of barrier improvement following CMT was due to loss of Akkermansia muciniphila, a bacterium that promotes barrier function, in the transfer. Nonetheless, no improvement in aortic pulse wave velocity was observed suggesting a role of barrier function on this end point. More studies are required to tease out these mechanisms.
To date there have been minimal therapeutics specifically designed to target barrier function; however, findings in mouse models of atherosclerosis highlight its potential. Recently, lubiprostone, a ClC-2 chloride channel activator, was evaluated as a drug candidate for treatment of “leaky gut.” It was shown to reduce lactulose/mannitol ratio in a small healthy patient cohort with NSAID-induced intestinal barrier dysfunction (20). Lubiprostone was subsequently shown to improve barrier function and increase ZO-1 and occludin expression in WD-fed ApoE−/− mice. It also reduced lesion formation and perivascular adipose tissue inflammation indicating the potential for leaky gut-targeted therapies to treat atherosclerosis (3). Further evidence for a causative effect of barrier function on atherosclerosis is provided by a study of FABP2−/− mice in which elimination of this intestinal protein prevents barrier dysfunction and reduces atherosclerosis (51). Finally, curcumin treatment reduced both permeability and atherosclerosis in WD-fed LDLR−/− mice (16). Curcumin is thought to have anti-inflammatory and antioxidant effects and poor oral bioavailability meaning it exerts its actions primarily in the gut. In this study, curcumin also prevented LPS-mediated increased permeability of epithelial monolayers suggesting a direct effect on intestinal epithelial permeability.
CONCLUSIONS AND FUTURE DIRECTIONS
Although not all studies of the GM in CVD have investigated barrier function, those that do invariably demonstrate it to be impaired. In the settings of MI, heart failure, atherosclerosis, and hypertension, there is increasingly convincing evidence that intestinal barrier dysfunction contributes to disease. Multiple studies demonstrate intestinal permeability to be predictive of adverse outcomes, and experimental models show improvements in barrier function associated with both GM- and RAS-targeted therapies. To date, data for a role of the intestinal barrier in CVD are largely associative and thus whether compounds targeting the intestinal barrier could be a new class of CVD therapeutics remains to be seen. Although it appears promising, further studies targeting intestinal barrier function in CVD are required to confirm its utility as a therapeutic target.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-131414 and UL1-TR-002378.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.V.L. prepared figures; C.V.L. drafted manuscript; C.V.L. and W.R.T. edited and revised manuscript; C.V.L. and W.R.T. approved final version of manuscript.
REFERENCES
- 1.Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, Denson H, Khoshbouei H, Baekey DM, Raizada MK, Zubcevic J. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317: H279–H289, 2019. doi: 10.1152/ajpheart.00510.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14: 2765–2778, 2009. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa K, Ishigami T, Nakai-Sugiyama M, Chen L, Doi H, Kino T, Minegishi S, Saigoh-Teranaka S, Sasaki-Nakashima R, Hibi K, Kimura K, Tamura K. Lubiprostone as a potential therapeutic agent to improve intestinal permeability and prevent the development of atherosclerosis in apolipoprotein E-deficient mice. PLoS One 14: e0218096, 2019. doi: 10.1371/journal.pone.0218096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arseneault-Bréard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, Rousseau G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr 107: 1793–1799, 2012. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 5.Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol 125: 240–245, 2008. doi: 10.1016/j.ijcard.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 6.Battson ML, Lee DM, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am J Physiol Endocrinol Metab 314: E468–E477, 2018. doi: 10.1152/ajpendo.00187.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battson ML, Lee DM, Li Puma LC, Ecton KE, Thomas KN, Febvre HP, Chicco AJ, Weir TL, Gentile CL. Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity. Am J Physiol Heart Circ Physiol 317: H1210–H1220, 2019. doi: 10.1152/ajpheart.00346.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bultman SJ. Bacterial butyrate prevents atherosclerosis. Nat Microbiol 3: 1332–1333, 2018. [Erratum in Nat Microbiol 4: 375, 2019]. doi: 10.1038/s41564-018-0299-z. [DOI] [PubMed] [Google Scholar]
- 9.Carrera-Bastos P, Picazo Ó, Fontes-Villalba M, Pareja-Galeano H, Lindeberg S, Martínez-Selles M, Lucia A, Emanuele E. Serum zonulin and endotoxin levels in exceptional longevity versus precocious myocardial infarction. Aging Dis 9: 317–321, 2018. doi: 10.14336/AD.2017.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirajlal-Fargo S, Albar Z, Bowman E, Labbato D, Sattar A, Karungi C, Longenecker CT, Nazzinda R, Funderburg N, Kityo C, Musiime V, McComsey GA. Subclinical vascular disease in children with HIV in uganda is associated with intestinal barrier dysfunction. Clin Infect Dis ciz1141, 2019. doi: 10.1093/cid/ciz1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Y, Prasad R, Feng D, Beli E, Li Calzi S, Longhini AL, Lamendella R, Floyd JL, Dupont M, Noothi SK, Sreejit G, Athmanathan B, Wright J, Jensen AR, Oudit GY, Markel TA, Nagareddy PR, Obukhov AG, Grant MB. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res 125: 969–988, 2019. doi: 10.1161/CIRCRESAHA.119.315743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ejtahed HS, Ardeshirlarijani E, Tabatabaei-Malazy O, Hoseini-Tavassol Z, Hasani-Ranjbar S, Soroush AR, Larijani B. Effect of probiotic foods and supplements on blood pressure: a systematic review of meta-analyses studies of controlled trials. J Diabetes Metab Disord 19: 617–623, 2020. doi: 10.1007/s40200-020-00525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One 14: e0218384, 2019. doi: 10.1371/journal.pone.0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil 28: 957–965, 2016. doi: 10.1111/nmo.12871. [DOI] [PubMed] [Google Scholar]
- 15.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72: 1141–1150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS One 9: e108577, 2014. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Luo H, Wang J, Tang W, Lu J, Wu S, Xiong Z, Yang G, Chen Z, Lan T, Zhou H, Nie J, Jiang Y, Chen P. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp Mol Med 49: e370, 2017. doi: 10.1038/emm.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8: 845, 2017. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Bäckhed F, Lusis AJ, Rey FE. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol 3: 1461–1471, 2018. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato T, Honda Y, Kurita Y, Iwasaki A, Sato T, Kessoku T, Uchiyama S, Ogawa Y, Ohkubo H, Higurashi T, Yamanaka T, Usuda H, Wada K, Nakajima A. Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: A prospective randomized pilot study in healthy volunteers. PLoS One 12: e0175626, 2017. doi: 10.1371/journal.pone.0175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, Fields JZ. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut 52: 720–728, 2003. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64: 897–903, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 132: 701–718, 2018. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 108, Suppl 1: 4592–4598, 2011. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leech B, McIntyre E, Steel A, Sibbritt D. Risk factors associated with intestinal permeability in an adult population: a systematic review. Int J Clin Pract 73: e13385, 2019. doi: 10.1111/ijcp.13385. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Gao M, Zhang W, Chen C, Zhou F, Hu Z, Zeng C. Zonulin regulates intestinal permeability and facilitates enteric bacteria permeation in coronary artery disease. Sci Rep 6: 29142, 2016. doi: 10.1038/srep29142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126: 2049–2063, 2016. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol 9: 1082, 2018. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 3: 247–259, 2010. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 31.O’Morain VL, Ramji DP. The Potential of Probiotics in the Prevention and Treatment of Atherosclerosis. Mol Nutr Food Res 64: e1900797, 2020. doi: 10.1002/mnfr.201900797. [DOI] [PubMed] [Google Scholar]
- 32.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 4: 220–227, 2016. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002. doi: 10.1042/bj20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr 11: 77–91, 2020. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Alcoholado L, Castellano-Castillo D, Jordán-Martínez L, Moreno-Indias I, Cardila-Cruz P, Elena D, Muñoz-Garcia AJ, Queipo-Ortuño MI, Jimenez-Navarro M. Role of gut microbiota on cardio-metabolic parameters and immunity in coronary artery disease patients with and without type-2 diabetes mellitus. Front Microbiol 8: 1936, 2017. doi: 10.3389/fmicb.2017.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandek A, Anker SD, von Haehling S. The gut and intestinal bacteria in chronic heart failure. Curr Drug Metab 10: 22–28, 2009. doi: 10.2174/138920009787048374. [DOI] [PubMed] [Google Scholar]
- 38.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569, 2007. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, Kalra PR, Buhner S, Herrmann R, Springer J, Doehner W, von Haehling S, Anker SD, Rauchhaus M. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol 157: 80–85, 2012. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 64: 1092–1102, 2014. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 41.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res 120: 312–323, 2017. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C, Raizada MK. Microglial cells impact gut microbiota and gut pathology in angiotensin ii-induced hypertension. Circ Res 124: 727–736, 2019. doi: 10.1161/CIRCRESAHA.118.313882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350: 830–834, 2015. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 44.Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med 19: 12–24, 2013. [PubMed] [Google Scholar]
- 45.Volynets V, Reichold A, Bárdos G, Rings A, Bleich A, Bischoff SC. Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ mice. Dig Dis Sci 61: 737–746, 2016. doi: 10.1007/s10620-015-3935-y. [DOI] [PubMed] [Google Scholar]
- 46.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol 34: 1975–1981, 1999. doi: 10.1016/S0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 47.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu D, Tang X, Ding L, Cui J, Wang P, Du X, Yin J, Wang W, Chen Y, Zhang T. Candesartan attenuates hypertension-associated pathophysiological alterations in the gut. Biomed Pharmacother 116: 109040, 2019. doi: 10.1016/j.biopha.2019.109040. [DOI] [PubMed] [Google Scholar]
- 49.Yang T, Aquino V, Lobaton GO, Li H, Colon-Perez L, Goel R, Qi Y, Zubcevic J, Febo M, Richards EM, Pepine CJ, Raizada MK. Sustained captopril-induced reduction in blood pressure is associated with alterations in gut-brain axis in the spontaneously hypertensive rat. J Am Heart Assoc 8: e010721, 2019. doi: 10.1161/JAHA.118.010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Wang F, Wang J, Wang Y, Fang Y. Intestinal fatty acid-binding protein mediates atherosclerotic progress through increasing intestinal inflammation and permeability. J Cell Mol Med 24: 5205–5212, 2020. doi: 10.1111/jcmm.15173. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Zhang Z, Zhao J, Tian C, Chen X, Li H, Wei X, Lin W, Zheng N, Jiang A, Feng R, Yuan J, Zhao X. Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol Nutr Food Res 63: e1800941, 2019. doi: 10.1002/mnfr.201800941. [DOI] [PubMed] [Google Scholar]
- 53.Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, Li J, Liu X, Liu J, Guo Z, Cai W, Ma Y, Ren D, Miao J, Chen S, Zhang Z, Chen J, Zhong J, Liu W, Zou M, Li Y, Cai J. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 6: 66, 2018. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


