Abstract
Elastin is a primary structural protein in the arterial wall that contributes to vascular mechanical properties and degrades with aging. Aging is associated with arterial stiffening and an increase in blood pressure. There is evidence that arterial aging follows different timelines with sex. Our objective was to investigate how elastin content affects arterial remodeling in male and female mice with aging. We used male and female wild-type (Eln+/+) and elastin heterozygous (Eln+/−) mice at 6, 12, and 24 mo of age and measured their blood pressure and arterial morphology, wall structure, protein content, circumferential stress, stretch ratio, and stiffness. Two arteries were used with varying contents of elastin: the left common carotid and ascending aorta. We show that Eln+/− arteries start at a different homeostatic set point for circumferential wall stress, stretch, and material stiffness but show similar increases with aging to Eln+/+ mice. With aging, structural stiffness is greatly increased, while material stiffness and circumferential stress are only slightly increased, highlighting the importance of maintaining these homeostatic values. Circumferential stretch shows the smallest change with age and may be important for controlling cellular phenotype. Independent sex differences are mostly associated with males being larger than females; however, many of the measured factors show age × sex and/or genotype × sex interactions, indicating that males and females follow different cardiovascular remodeling timelines with aging and are differentially affected by reduced elastin content.
NEW & NOTEWORTHY A comprehensive study on arterial mechanical behavior as a function of elastin content, aging, and sex in mice. Elastin haploinsufficient arteries start at a different homeostatic set point for mechanical parameters such as circumferential stress, stretch, and material stiffness. Structural stiffness of the arterial wall greatly increases with aging, as expected, but there are interactions between sex and aging for most of the mechanical parameters that are important to consider in future work.
Keywords: aging, arterial stiffness, elastin, hypertension, sex
INTRODUCTION
A major consequence of aging in the arterial system is increased stiffness of the central arteries, which is a significant risk factor for cardiovascular disease (CVD) (38). Arterial stiffness in humans is often measured by pulse wave velocity (PWV), which depends on the arterial elastic modulus (linearized material stiffness), wall thickness, and diameter under physiological loading conditions (21). Hence changes in PWV represent overall cardiovascular remodeling encompassing changes in arterial material properties, geometry, and physiological loading (blood pressure). In vitro experiments using animals allow separation of remodeling parameters to isolate individual factors that contribute to increases in arterial stiffness associated with hypertension (29), diastolic dysfunction (2), congestive heart failure (5), and coronary heart disease (18).
Passive material properties of the large arteries are determined mainly by the composition of structural extracellular matrix (ECM) proteins, elastin and collagen, in the wall. Elastin and collagen are produced and regulated during development and maturation to provide appropriate material properties, including a physiological elastic modulus value that is conserved across species (45, 49). Elastin gene expression is limited to late embryonic and early postnatal development (31), so elastin cannot be replaced in aging arteries. Elastin degrades with aging, leading to a decrease in the elastin to collagen ratio and increased arterial stiffness (22); however, detailed studies of how changes in ECM content occur together with changes in arterial geometry and blood pressure to affect arterial stiffness with aging are limited.
Elastin heterozygous (Eln+/−) mice have ∼60% of normal elastin levels, high blood pressure, and increased arterial stiffness (13). Despite these changes, they are able to maintain a physiological elastic modulus near wild-type (Eln+/+) values because of adjustments made to arterial geometry and ECM organization, including a smaller arterial diameter, thinner, but more numerous elastic laminae, and possible collagen fiber reorganization (6, 49, 50), Male Eln+/− mice have an altered vascular aging process characterized by no increases in arterial wall thickness with aging and minimal cardiac hypertrophy at 24 compared with 6 mo of age (41). As arterial stiffening with age has been attributed to decreases in the elastin to collagen ratio (22), we aimed to study how arterial remodeling with age progresses in a model that has altered ECM content, yet demonstrates adaptive remodeling that may prevent additional stiffening with age. Cardiovascular aging in female Eln+/− mice has not previously been investigated, despite evidence that sex is an important factor in arterial stiffening and associated CVD risk (40).
The goal of the current study was to evaluate cardiovascular aging in male and female Eln+/+ and Eln+/− mice, with a focus on independent factors that contribute to overall changes in arterial stiffness. We evaluated mice at 6, 12, and 24 mo of age, which correspond approximately to human ages of 30 (mature adult), 43 (middle aged), and 69 (old) years of aging (16). We measured blood pressure and arterial geometry, material properties, and ECM content as functions of aging, sex, and genotype. We included two different arterial locations with varying elastin to collagen ratios, the left or right common carotid (LCC or RCC) and ascending aorta (ASC), to determine how starting ECM composition affects remodeling with aging. Our results provide insight into how aging, sex, and elastin content individually and in concert affect cardiovascular remodeling with aging so that CVD management can be better informed in aging humans.
MATERIALS AND METHODS
Animals.
Male and female Eln+/+ mice and Eln+/− (36) mice in a C57BL/6J background at 6, 12, and 24 mo were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis. A total of 122 animals were used in this study. Animal numbers for each experiment are shown in the figure legends. Investigators were blinded to animal genotype during data collection and to age, sex, and genotype during data analysis.
Blood pressure.
Each mouse was weighed and then anesthetized with 1.5% isoflurane. They were placed on a feedback-controlled heating pad to maintain body temperature at 37°C. A solid-state catheter (1.2 Fr, Transonic) was inserted through the RCC into the ASC and pressure readings were allowed to stabilize for 5 min. Blood pressure was then measured for ∼10 min, and pressures were averaged over the recording period. Mice were euthanized by thoracotomy under anesthesia after the blood pressure measurement.
Mechanical testing.
Pictures for in situ length measurement of the LCC and ASC were taken. The heart was removed, washed, blotted, and weighed. Then, both arteries were harvested and pictures were taken for ex vivo length measurements. The LCC or ASC was mounted on custom stainless steel cannulae in a pressure myograph (110P, Danish Myotechnology) and secured by 6-0 silk suture at the in situ length. Each artery was submerged in physiological saline solution at 37°C (50). The artery was preconditioned by cyclic pressurization from 0 to 175 mmHg for three cycles (32). The artery was then inflated from 0 to 175 mmHg in 25 mmHg pressure steps at a rate of 2 mmHg/s and a hold time of 30 s. The pressure and outer diameter were recorded at 1 Hz.
Unloaded dimension measurements.
After mechanical testing, each artery was removed from the myograph, cut into rings with a thickness of ∼0.2 mm, and imaged for unloaded dimension measurements. The ring was then cut radially to measure the opening angle to quantify residual strain (7). The outline of each individual ring was traced in ImageJ software (National Institutes of Health) and fitted with an ellipse. The equivalent unloaded outer diameter, thickness, and cross-sectional area were determined assuming a constant area. The opening angle was measured using custom Matlab (Mathworks) scripts (50). The rings and remaining tissue from each artery were transferred to a dry microtube and stored at −80°C for protein quantification.
Protein quantification.
Total protein, crosslinked elastin, and collagen were determined using biochemical assays (32) in the ASC only, as we found that our assays were not sensitive enough to quantify protein levels in individual LCC samples, which have ∼20% of the protein content of ASCs (13). Total protein was determined with a ninhydrin assay (46) using an amino acid calibrator (012506C, Pickering Laboratories). For elastin, we adapted a competitive ELISA for desmosine to quantify crosslinked elastin (47). For collagen, we measured the content of hydroxyproline, a major component of collagen, through a reaction with chloramine T (27). We assumed that hydroxyproline constitutes 13.5% of collagen by mass (39). Concentrations were determined from standard curves (elastin, E60, Elastin Products; hydroxyproline, H54409, Sigma-Aldrich) and normalized by taking the ratios of each protein to the total protein content.
Histology.
The RCC was collected after blood pressure measurement, fixed in 10% formalin, transferred to 70% ETOH after 24–48 h, and then stored at stored at 4°C until further processing. For histology, the RCC was dehydrated in ethanol, embedded in paraffin, and sectioned. Verhoeff Van Gieson (VVG) staining was performed to visualize elastin and picrosirius red (PSR) staining was performed to visualize collagen. The stained sections were imaged using a Zeiss LSM 710 microscope at ×20 magnification.
Mechanical testing data analysis.
The in vivo axial stretch ratio (λz) was taken as the ratio of in situ to ex vivo length from the recorded images,
| (1) |
where l is the deformed in situ length and L is the undeformed ex vivo length. Circumferential stretch ratio (λθ) was calculated as
| (2) |
where D and d are the undeformed and deformed outer diameters with subscripts i and o corresponding to inner and outer surfaces, respectively. The calculated outer diameter at physiological pressure is defined by the empirical equation
| (3) |
where P is the physiological pressure, and a, b, c, and d are constants fit to the pressure-diameter data from the mechanical tests (17). We assumed incompressibility of the arterial wall (12), so the inner diameter can be calculated by
| (4) |
Mean physiological wall stress σθ in the circumferential direction is determined by
| (5) |
We calculated two measures of stiffness: Peterson’s modulus (Ep) and incremental Young’s modulus (Einc) (21). Ep is a common measure of structural stiffness and is defined as
| (6) |
where the subscripts sys and dias correspond to systolic and diastolic diameters and pressures.
Einc is a measure of material stiffness comparable to the physiological elastic modulus and is defined as
| (7) |
As we did not have blood pressure measurements and mechanical testing data for every mouse, we used average systolic and/or diastolic pressures for each group of matching age, sex, and genotype, along with individual artery dimensions at those pressures from the in vitro mechanical test data to calculate the physiological stress, stretch, and stiffness values. The circumferential stress and stretch ratio were calculated at the systolic pressures (Eqs. 2 and 5) and the structural and material stiffnesses incorporated both systolic and diastolic pressures (Eqs. 6 and 7).
Statistics.
Three-way ANOVA was performed to determine the effects of age, sex, genotype, and their interactions. An interaction between independent variables indicates that the effects of one variable (i.e., age) depend on another (i.e., sex). Post hoc analyses by the Tukey-Kramer test were also conducted to determine the effect of genotype within the same age and sex groups. All statistical analyses were performed with Prism (GraphPad, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
Heart weight is increased in aged mice and exacerbated by elastin haploinsufficiency.
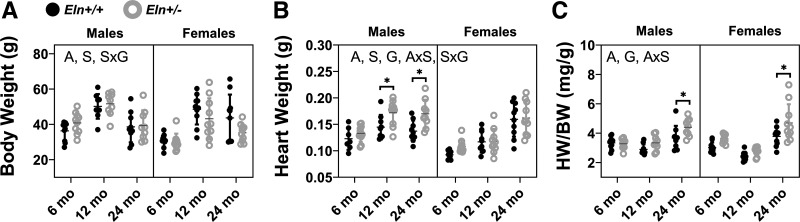
Mice gain body weight from 6 to 12 mo of age and lose body weight from 12 to 24 mo of age (Fig. 1A). Males weigh more than females (Fig. 1A). In contrast to the changes in body weight with aging, the heart continues to increase in weight throughout the aging process (Fig. 1B). Additionally, male hearts weigh more than female and Eln+/− hearts weigh more than Eln+/+ (Fig. 1B). Consequently, the normalized heart weight at 24 mo is significantly increased compared with the earlier ages in males and females and the increase is larger in Eln+/− mice than Eln+/+ (Fig. 1C). There are significant interactions between age and sex for the normalized heart weights suggesting that male and female mice follow different aging timelines (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.12558776).
Fig. 1.
Body weight (BW; A), heart weight (HW; B), and normalized heart weight (HW/BW; C) for male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–C, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with means ± SD are shown; n = 9–11 for each group.
Pulse pressure increases with aging and elastin haploinsufficiency.
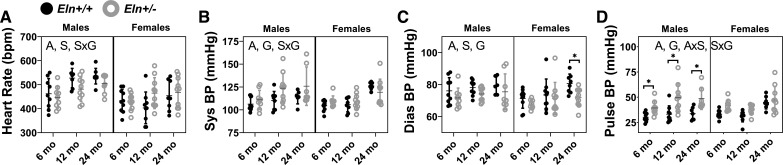
Heart rate is significantly affected by age and sex but not by genotype (Fig. 2A). There is a significant interaction between sex and genotype for heart rate (Supplemental Table S1). Systolic blood pressure is increased with aging and is higher in male Eln+/− compared with male Eln+/+ mice (Fig. 2B). By comparing the percent total variation results for the systolic pressure of 18% for age and 4% for genotype (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.12558797), it is apparent that age contributes 4.5 times more than genotype to the systolic pressure variation. Sex alone does not affect systolic pressure, but there is an interaction between sex and genotype suggesting that males and females respond differently to reduced elastin levels (Supplemental Table S1). Diastolic blood pressure is increased with aging, male sex, and Eln+/+ genotype (Fig. 2C). Pulse pressure is increased with age and Eln+/− genotype (Fig. 2D). Elastin haploinsufficiency contributes 2.4 times more to the pulse pressure difference than age (Supplemental Table S2). There are interactions between age and sex and between sex and genotype for pulse pressure suggesting that males and females follow different aging timelines and that they have different responses to elastin haploinsufficiency.
Fig. 2.
Heart rate (A), systolic blood pressure (Sys BP; B), diastolic blood pressure (Dias BP; C), and pulse blood pressure (Pulse BP; D) for male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–D, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with mean ± SD are shown; n = 6–10 for each group.
Arterial unloaded dimensions and residual strain have complex interactions between age, sex, and genotype.
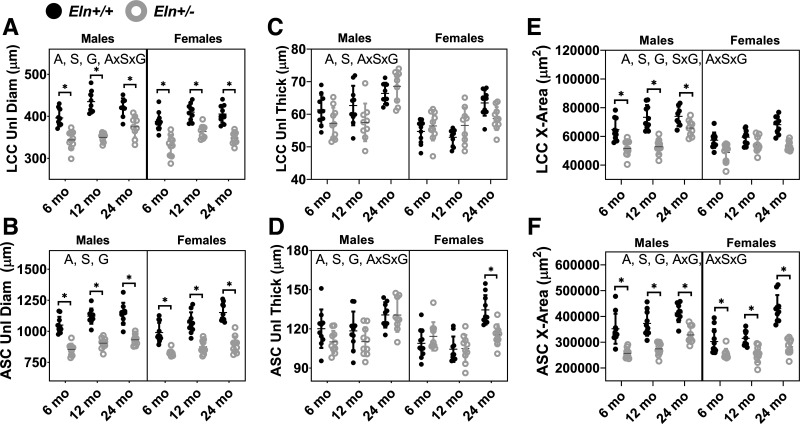
The unloaded outer diameters of the LCC and ASC increase with aging and are reduced in Eln+/− compared with Eln+/+ mice and in females compared with males (Fig. 3, A and B). The unloaded thicknesses of the LCC and ASC increase with aging and are lower in females than males (Fig. 3, C and D). The ASC has thinner walls in Eln+/− compared with Eln+/+ mice, but the LCC wall thickness does not depend on genotype (Supplemental Table S1). The unloaded cross-sectional area considers both diameter and thickness. The unloaded cross-sectional areas of the LCC and ASC increase with aging and are reduced in Eln+/− compared with Eln+/+ mice and in females compared with males (Fig. 3, E and F). There is a significant interaction between age and genotype for the ASC cross-sectional area (Supplemental Table S1), indicating that elastin haploinsufficiency affects the remodeling timeline with aging in this high-elastin content artery. For all of the unloaded dimensions, except the unloaded diameter of the ASC, there are significant interactions between the three independent variables demonstrating complex relationships between age, sex, and genotype that determine the arterial wall geometry.
Fig. 3.
Left common carotid (LCC) and ascending aorta (ASC) unloaded diameter (Unl Diam; A and B), unloaded thickness (Unl Thick; C and D), and unloaded cross-sectional area (X-Area; E and F) for male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–D, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with means ± SD are shown; n = 9–11 for each group.
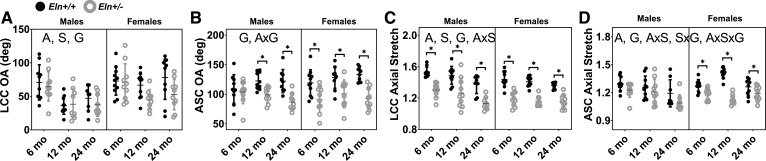
The opening angle, a measure of circumferential residual strain, decreases with aging and male sex in the LCC, but does not change with aging or sex in the ASC (Fig. 4, A and B). The opening angle is reduced in Eln+/− compared with Eln+/+ in both arteries. There is a significant interaction between age and genotype in the ASC, consistent with elastin haploinsufficiency altering the remodeling timeline with aging. The in vivo axial stretch ratios of the LCC and the ASC decrease with aging and in Eln+/− compared with Eln+/+ mice (Fig. 4, C and D). The axial stretch of the LCC, but not the ASC, depends on sex. For both arteries, there are interactions between age and sex for the axial stretch, consistent with males and females following different aging timelines (Supplemental Table S1). For the ASC axial stretch, there are also significant interactions between sex and genotype and between all three independent variables. Due to incompressibility of the arterial wall, a reduced axial stretch increases the calculated in vivo cross-sectional area. Therefore, the interactions between independent variables highlight the complex relationships determining in vivo arterial geometry in both the circumferential and axial directions.
Fig. 4.
Left common carotid (LCC) and ascending aorta (ASC) opening angle (OA) (A and B) and in vivo axial stretch ratio (C and D) for male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–D, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with means ± SD are shown; n = 9–11 for each group.
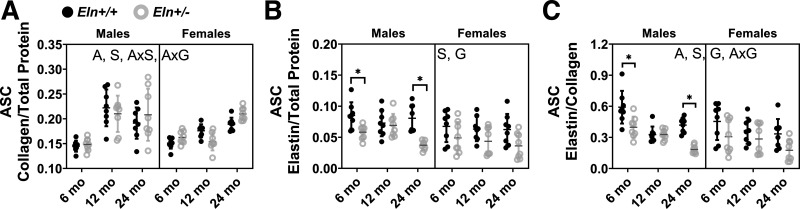
Elastin/collagen ratio in the ASC decreases with age.
Normalized collagen contents in the ASC increase with aging, male sex, and are not significantly affected by genotype (Fig. 5A). There is an interaction between age and sex and between age and genotype (Supplemental Table S1), indicating that sex and elastin haploinsufficiency alter collagen production during the aging process. Males have higher normalized elastin contents in the ASC than females (Fig. 5B), which may be important for differential arterial stiffening with sex. As expected, Eln+/− mice have decreased normalized elastin content compared with Eln+/+ mice. Normalized elastin content is unchanged with age, indicating little degradation of crosslinked elastin over the mouse life span. However, due to the increase in collagen content with aging, there is a significant decrease in elastin/collagen ratios with aging (Fig. 5C). Eln+/− mice have lower elastin/collagen ratios than Eln+/+, and females have lower elastin/collagen ratios than males. The interaction between age and genotype for elastin/collagen contents suggests that Eln+/+ and Eln+/− ASC follow a different aging timeline.
Fig. 5.
Protein quantification of the ascending aorta (ASC) for the ratio of collagen/total protein (A), elastin/total protein (B), and elastin/collagen (C) for male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters at the top of each panel indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with means ± SD are shown; n = 8 for each group.
Histological staining indicates no obvious structural changes in the elastic laminae with age.
Figure 6 shows representative VVG and PSR histological images for female RCCs. Male RCC images are similar. The elastic laminae in VVG stained Eln+/− arteries appear thinner and there are often four layers, rather than three across the wall as observed in Eln+/+ arteries. PSR images highlight the collagen staining directly adjacent to the elastic laminae. There are no obvious changes in elastic laminae structure or collagen organization in the RCC with aging.
Fig. 6.
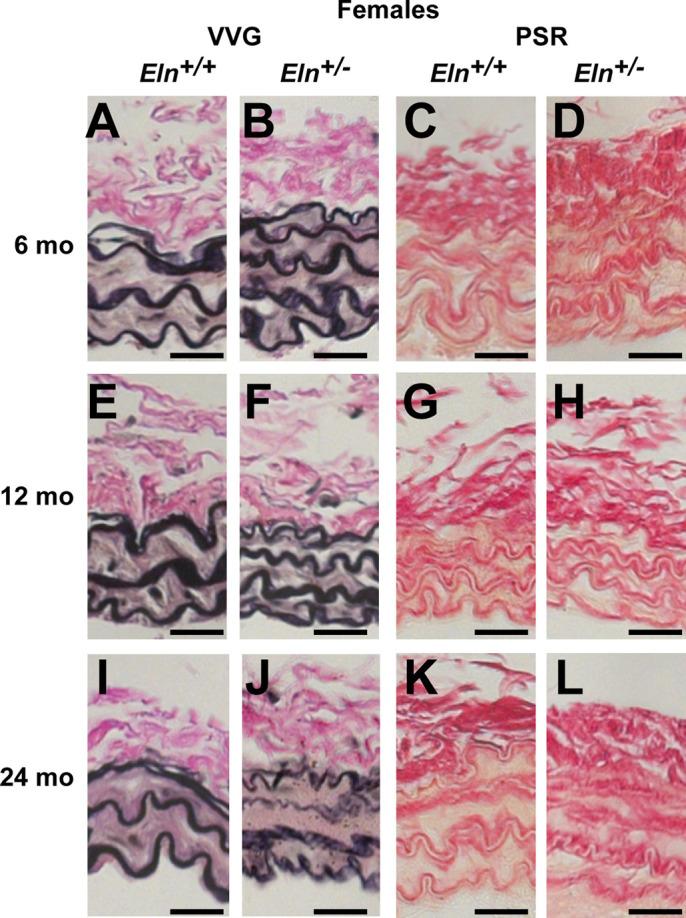
Representative female right common carotid (RCC) histology images stained with Verhoeff Van Gieson (VVG); A, B, E, F, I, and J) or picrosirius red (PSR; C, D, G, H, K, and L) for Eln+/+ (A, E, I, C, G, and K) and Eln+/− (B, F, J, D, H, and L) mice at 6 (A–D), 12 (E–H), and 24 (I–L) months of age. Images from male mice are similar; 5–7 images were examined per group. Scale bar = 20 µm.
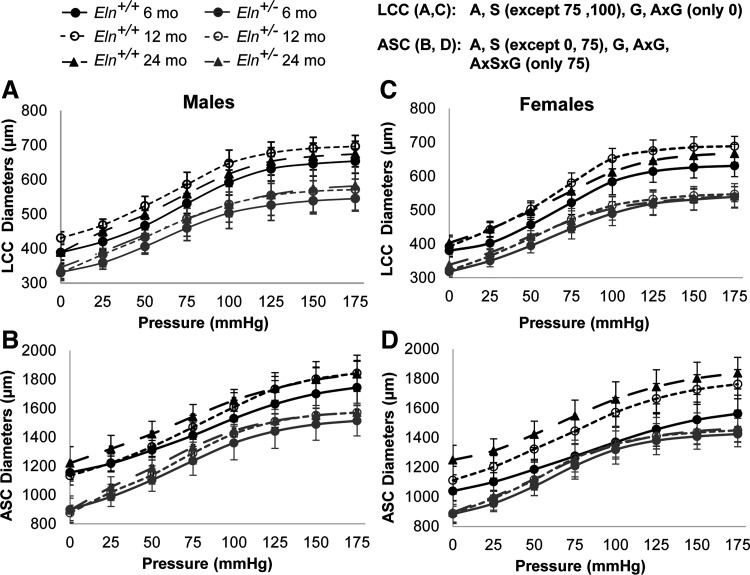
Arterial circumferential mechanical behavior is determined by age, sex, and elastin haploinsufficiency.
The outer diameters of the LCC and ASC are lower in Eln+/− compared with Eln+/+ mice at every applied pressure (Fig. 7). The outer diameters are increased with aging for both arteries at all applied pressures. Females have smaller outer diameters than males at most applied pressures for corresponding ages and genotypes. There is a significant interaction between age and genotype for the outer diameter of the LCC at 0 mmHg and for the ASC at all applied pressures (Supplemental Table S1), indicating that elastin haploinsufficiency affects the timeline of arterial remodeling with aging.
Fig. 7.
Mechanical testing data at the in vivo axial stretch show the outer diameter (µm) at each applied pressure for male (A and B) and female (C and D) Eln+/+ and Eln+/− left common carotid (LCC; A and C) and ascending aorta (ASC; B and D) at 6, 12, and 24 mo of age. The letters at top right indicate independent variables and their interactions with P < 0.05 by three-way ANOVA for each artery type at each pressure step. A, age; S, sex; G, genotype. The exact P values for each comparison are given in Supplemental Table S3 (see https://doi.org/10.6084/m9.figshare.12558818). Means ± SD shown; n = 8–11 for each group.
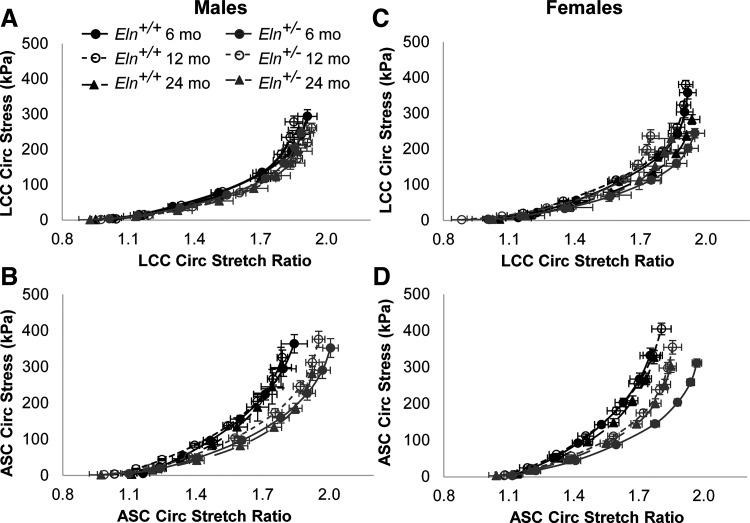
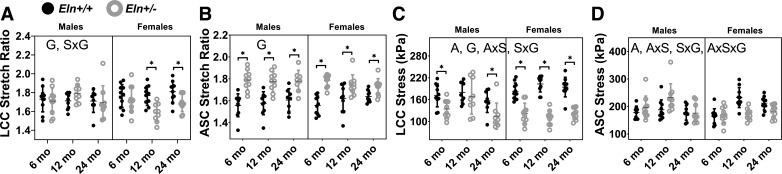
The circumferential stretch versus stress curves are qualitatively similar across groups for the LCC, but are shifted to the right for Eln+/− ASC compared with Eln+/+ and in females compared with males (Fig. 8). The physiological stretch and stress at the average systolic pressure for each age, sex, and genotype were calculated with individual artery dimensions from the in vitro mechanical tests to quantitatively compare values (Fig. 9). The physiological circumferential stretch ratio for the LCC is lower in Eln+/− mice compared with Eln+/+ (Fig. 9A), while the physiological circumferential stretch ratio for the ASC is higher in Eln+/− mice compared with Eln+/+ (Fig. 9B). The physiological circumferential stresses in the LCC and ASC are significantly affected by age, but there is no consistent trend in changes with age due to interactions between the independent variables (Fig. 9, C and D). The physiological stress in the LCC is decreased in Eln+/− mice compared with Eln+/+ but is not affected by genotype in the ASC suggesting that the ASC can maintain a homeostatic wall stress (19) despite reduced elastin levels. There are interactions between sex and genotype for the stretch and stress in the LCC and stress in the ASC (Supplemental Table S1). There are interactions between age and sex and between all three independent variables for the stress in the ASC, consistent with complex relationships that determine arterial remodeling behavior with age, sex, and genotype.
Fig. 8.
Circumferential stress versus circumferential stretch ratio for male (A and B) and female (C and D) Eln+/+ and Eln+/− left common carotid (LCC; A and C) and ascending aorta (ASC; B and D) at 6, 12, and 24 mo of age. Statistical comparisons are not shown as each artery has unique stress and stretch values based on the individual unloaded dimensions. Means ± SE shown; n = 8–11 for each group.
Fig. 9.
Left common carotid (LCC) and ascending aorta (ASC) circumferential stretch ratio (A and B) and circumferential stress (C and D) at the average systolic pressure for each group of male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–D, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with means ± SD are shown; n = 8–11 for each group.
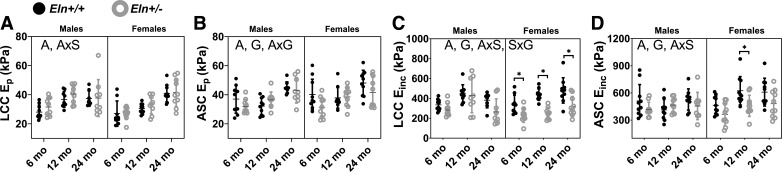
The structural stiffness (Ep) and material stiffness (Einc) were calculated at the average systolic and diastolic pressures for each age, sex, and genotype with individual arterial dimensions from the in vitro mechanical tests according to Eqs. 6 and 7 to quantitatively compare physiological stiffness values. Ep and Einc increase with aging, but are not affected by sex in both arteries (Fig. 10). Einc is decreased with elastin haploinsufficiency in both arteries and Ep is decreased with elastin haploinsufficiency in the ASC but not the LCC. Ep for both arteries and Einc for the ASC show significant interactions between age and sex, while Ep for the ASC has an interaction between age and genotype (Supplemental Table S1). Einc for the LCC has an interaction between sex and genotype.
Fig. 10.
Left common carotid (LCC) and ascending aorta (ASC) structural stiffness (Ep; A and B) and material stiffness (Einc; C and D) at the average systolic pressure for each group of male and female Eln+/+ and Eln+/− mice at 6, 12, and 24 mo of age. The letters in A–D, top, indicate independent variables and their interactions with P < 0.05 by three-way ANOVA. A, age; S, sex; G, genotype. *Significantly different between genotypes of the same age and sex by Tukey-Kramer post hoc test. Exact P values are given in Supplemental Table S1, and percent variation contributions are given in Supplemental Table S2. Independent data points with mean ± SD are shown; n = 8–11 for each group.
DISCUSSION
Individual effect of age.
The contribution of age to Ep and Einc in the LCC and ASC (Fig. 10) suggests aging-induced remodeling of the arterial wall that changes arterial stiffness. By comparing the percent total variation results for age with respect to Ep and Einc for each artery type (Supplemental Table S2), it is apparent that age contributes 2.7–4.1 times more to Epthan to Einc, demonstrating that aging affects structural stiffness more than material stiffness in arteries. This supports the concept that the physiological elastic modulus, Einc, is a critical design parameter for the large arteries that is maintained across species (45), despite genetic modifications to ECM content (6, 34), and to some extent during aging (3, 41). Age is the most important factor contributing to isolated systolic hypertension in our study (Supplemental Table S2). We found concomitant increases in structural stiffness (Fig. 10, A and B), systolic blood pressure (Fig. 2B), and heart weight (HW)/body weight (BW) (Fig. 1C) with aging, consistent with the proposed pathological feedback loop between arterial stiffening, hypertension, and cardiac disease (25). Normalized collagen protein contents (Fig. 5A) and wall thickness (Fig. 3, C and D) are increased with aging, providing a mechanistic explanation for the increases in structural stiffness.
In contrast to the stiffness measures, the circumferential stretch ratio (Fig. 9, A and B) is unaffected by age, indicating that circumferential stretch may be even more critical to maintain than the physiological elastic modulus. Smooth muscle cells (SMCs) show a parabolic length-tension relationship with peak active force generation depending on the circumferential arterial stretch ratio (9, 11). It may be that arterial circumferential stretch ratio is maintained with aging to optimize active force generation, but additional investigation of active mechanics is required. We focused here on passive mechanics of the aging arterial wall with differing elastin content. Aging accounts for 3–10% of the observed variation in circumferential stress (Supplemental Table S2). Our data suggest that passive circumferential stress is maintained within a small range of normal or homeostatic values (23) and that deviations from the passive stress set point may contribute to pathological vascular remodeling with aging (15).
Individual effect of sex.
The changes in body and heart weights (Fig. 1, A and B) and unloaded arterial dimensions (Fig. 3) are consistent with females being smaller overall compared with males. The opening angle (Fig. 4A) and in vivo axial stretch ratio (Fig. 4C) of the LCC are also affected by sex, indicating that residual strains in the LCC are different between males and females. Females have lower levels of normalized collagen and elastin (Fig. 5), indicating that arterial wall composition varies with sex. As arterial remodeling depends on relative changes in wall components, differences in the starting composition are an important consideration for future work. All other sex-related changes are interactive effects with age and/or genotype and may be linked to differences in wall composition.
Individual effect of genotype.
Increased HW and HW/BW (Fig. 1, B and C) suggest cardiac hypertrophy in Eln+/− mice, perhaps due to the increase in pulse pressure (Fig. 2D) which is most affected by genotype (Supplemental Table S2) and has been associated with increased heart failure in the elderly (5). Genotype also contributes the most to differences in axial stretch ratios (Fig. 4, C and D) and Einc (Fig. 10, C and D) in both the LCC and ASC (Supplemental Table S2). As axial stretch can modulate circumferential mechanical behavior (24), reduced axial stretch in Eln+/− arteries may represent one of the mechanisms by which circumferential mechanical parameters are maintained. The physiological circumferential stretch ratio (Fig. 9B) in the ASC is especially affected by genotype (Supplemental Table S2) and may be increased to maintain cardiac output in Eln+/− mice through a smaller outflow orifice (13, 35). Since circumferential stretch ratio is an important determinant of active SMC force generation (9, 11), it would be interesting to investigate active-length tension relationships in Eln+/+ and Eln+/− arteries. SMCs from elastin knockout (Eln−/−) mice have reduced expression of SMC contractile proteins and less actin stress fibers (30), so alteration in the SMC length-tension relationship with elastin amounts is possible. The unloaded cross-sectional areas (Fig. 3, E and F) of Eln+/− arteries are smaller, suggesting that the arterial wall scales with the available content of ECM building materials (48). There is less normalized elastin protein (Fig. 5B) in Eln+/− compared with Eln+/+ ASC. Although there are significant differences in the physiological elastic modulus, Einc, for the LCC and ASC with genotype (Fig. 10, C and D), the average difference between genotypes of comparable age and sex is maintained within 13–27% for the ASC, supporting previous data on a conserved physiological elastic modulus for the aorta in other mouse models of genetic elastic fiber defects (34) and in different species with varying elastin content (45).
Interactions between independent variables.
The interaction between age and sex for HW, HW/BW, and pulse pressure (Fig. 1, B and C, and Fig. 2D) suggests that cardiac remodeling follows a different timeline with aging in males and females. The interaction between age and sex for ASC and LCC in vivo axial stretch (Fig. 4, C and D), circumferential stress (Fig. 9, C and D), Einc (Fig. 10, C and D), LCC Ep (Fig. 10A), and ASC collagen content (Fig. 5A) suggests that arterial wall remodeling also follows a different timeline with aging in males and females. These data are important for understanding normal and pathological aging in males and females and their relationship to sex-related differences in arterial stiffening.
There are no interactions between age and genotype for cardiac- or LCC-related parameters. There are interactions between age and genotype for ASC unloaded cross-sectional area (Fig. 3F), opening angle (Fig. 4B), Ep (Fig. 10B), collagen content (Fig. 5A), and elastin/collagen ratio (Fig. 5C). These data indicate that elastin insufficiency alters the normal timeline of cardiovascular aging only in the ASC and it does so through changes in wall geometry and protein content that alter circumferential residual strains and structural stiffness. The changes in Eln+/− ASC are less pronounced than those in Eln+/+ ASC with aging, consistent with hypothesized developmental remodeling in the Eln+/− ASC (13) that may protect it from additional changes with aging (41). As the ASC has higher elastin content than the LCC (13), it is reasonable that this arterial segment is most affected by reduced elastin levels.
The interaction between sex and genotype for BW (Fig. 1A), HW (Fig. 1B), heart rate (Fig. 2A), systolic pressure (Fig. 2B), pulse pressure (Fig. 2D), LCC cross-sectional area (Fig. 3E), LCC circumferential stretch ratio (Fig. 9A), LCC Einc (Fig. 10C), LCC and ASC circumferential stress (Fig. 9, C and D), and ASC in vivo axial stretch ratio (Fig. 4D) suggests that the effects of elastin haploinsufficiency differ between males and females for these parameters. As these parameters are crucial determinants in the relationship between hypertension and arterial stiffness and/or for mechanoadaptation, it will be important to further investigate sex differences in cardiovascular diseases associated with elastin insufficiency, such as Williams Syndrome (33, 44).
Three-way interactions between the independent variables do not affect cardiac remodeling (Fig. 1 B and C), but other measured parameters for the LCC and ASC are affected that determine overall cardiovascular mechanics and functionality. Examples include unloaded thicknesses (Fig. 3, C and D) and cross-sectional areas of both LCC and ASC (Fig. 3, E and F), which follow different remodeling timelines for each group. The results highlight the complicated interactions among age, sex, and elastin content in arterial mechanics.
Comparison to previous studies on arterial mechanics in aging mice.
Pezet et al. (41) compared cardiovascular function and ASC mechanics in Eln+/+ and Eln+/− male mice at ages 6 and 24 mo. Pezet et al. included aortic reactivity studies that we did not, but they did not include female mice, examine other arteries beyond the ASC, or measure circumferential and axial residual strains. Similar to Pezet et al., we saw an increase in HW/BW with aging and genotype (Fig. 1C) and an increase in blood pressure with Eln+/− genotype (Fig. 2B). Pezet et al. did not see an increase in blood pressure with aging, but they only measured mean pressure with a fluid-filled catheter. Similar to Pezet et al., we found no changes in elastin content (Fig. 5B) but observed an increase in collagen content with aging (Fig. 5A). Pezet et al. showed that some cardiovascular alterations that appear with age in Eln+/+ mice are already present in young Eln+/− mice, including cardiac hypertrophy and increased arterial stiffness, while Eln+/− mice are protected from changes characteristic of Eln+/+ aging, including significant aortic wall thickening. They suggested that Eln+/− mice live in a different state of cardiovascular physiology than Eln+/+ mice and that this difference in initial conditions established during embryogenesis determines the way that arteries remodel with age. This concept is consistent with our suggestion that Eln+/− arteries have a different mechanical homeostatic set point compared with Eln+/+ arteries due to available ECM building materials and that developmental adaptation in the Eln+/− ASC may protect it from aging-related changes.
Ferruzzi et al. (15) compared cardiovascular function and mechanics of five different arterial segments in male wild-type mice at ages 5 and 25 mo. Ferruzzi et al. included axial mechanical data that we did not, but they did not include females or any other genotypes beyond wild-type mice in their study. From histological sections, Ferruzzi et al. measured a decrease in elastin area fraction and an increase in collagen area fraction. From biochemical protein quantification, we measured an increase in collagen content (Fig. 5A), but no changes in crosslinked elastin content in the ASC (Fig. 5B). Our histological results in the RCC (Fig. 6) indicated no obvious structural changes with aging. As in our study, Ferruzzi et al. found aging-associated wall thickening (Fig. 3, C and D) and a decrease in the in vivo axial stretch for both ASC and LCC (Fig. 4, C and D), although they did not find aging-associated increases in the outer diameter for either artery. Similar to our study, they found a decrease in circumferential stress with aging in wild-type males. Interestingly, we found the opposite change in circumferential stress for wild-type females (Fig. 9, C and D), which were not included in their study. Although Einc was significantly affected by age and age × sex interactions in our study, we did not see a consistent decrease in Einc (Fig. 10, C and D) with aging in the wild-type male ASC or LCC as Ferruzzi et al. reported. Differences in the absolute values and the trends in Einc with aging in different arteries may be due to the different calculations used for material stiffness, the physiological blood pressure, or variations in the measured in vivo axial stretch ratios. We used age-, sex-, and genotype-specific systolic blood pressure for each group, while Ferruzzi et al. used an average systolic blood pressure of 120 mmHg. In general, Ferruzzi et al. concluded that mouse arteries show compromised mechanical homeostasis through their inability to maintain circumferential stress and material stiffness with aging that may be important for understanding cardiovascular function in aging humans. Our results are generally consistent with their conclusions and add further details on how elastin haploinsuficiency and sex affect arterial mechanical homeostasis in aging.
Brankovic et al. (3) compared LCC mechanics in male mice at 6, 12, and 24 mo of age with and without matrix metalloproteinase-12, a major arterial elastase. Brankovic et al. did not examine any other arterial segments, female mice, or changes in wall component content and microstructure. Unlike our study, they found no differences in unloaded dimensions with aging in the LCC (Fig. 3, A, C, and E). Brankovic et al. measured unloaded dimensions with the LCC mounted on the test system rather than from cut rings, which could account for some differences. Similar to our study (Fig. 4C) and despite differences in the way the in vivo axial stretch was determined, Brankovic et al. found that the in vivo axial stretch decreases with aging in the LCC. They found that the in vivo axial stretch is increased in Mmp12−/− arteries, consistent with more intact elastin being present due to the absence of a major elastase. Similar to our study (Fig. 10C), Brankovic et al. found an increase in the circumferential material stiffness in the LCC with aging. Our data are consistent with their conclusion that axial stretch is used to mitigate aging associated increases in arterial stiffness.
Implications for mechanical homeostasis.
It has been known for over a century that arteries remodel in response to hemodynamic loads (8). Fung postulated that the remodeling is a response of the cells within the living tissue to maintain a homeostatic stress state (19). Stress-mediated remodeling has been used successfully to explain arterial changes during development and disease (23, 49). Ferruzzi et al. (15) argued that an inability to maintain the homeostatic stress state may be one of the major consequences of arterial aging in mice. Due to the interactions between age, sex, and genotype, female Eln+/+ ASC have the largest change in physiological circumferential stress (Fig. 9D), with a 29% increase from 6 to 24 mo of age (compared with an 8% increase for female Eln+/−, 1% decrease for male Eln+/+, and 10% decrease for male Eln+/−), indicating that wild-type female mice, in particular, may experience significant deviations from mechanical homeostasis.
It should be noted that the average circumferential wall stress is not necessarily the circumferential stress experienced by the SMCs within the wall. It is generally assumed that the ECM carries a large proportion of the mechanical stress in the large, elastic arteries. Hence, as SMCs respond to the changes in stress during aging, the process will depend on 1) the relative amounts of stress on the SMCs and the ECM, and 2) changes to the ECM, such as age-related degeneration, that alter the stress balance. There is a need for new measurement techniques capable of determining stresses in different wall components to better understand the remodeling stimuli. The arterial wall is often modeled as a constrained mixture (26) where all of the components (SMCs, elastin, and collagen) undergo the same stretch, but the stress is additive. In this case, the average circumferential wall stretch is equal to the circumferential SMC stretch. We found the circumferential wall stretch ratios for the ASC and LCC between 6 and 24 mo of age generally vary by less than 10% from the average values for each genotype in males and females (Fig. 9), indicating that circumferential SMC stretch may be a critical parameter controlling homeostatic arterial remodeling in aging.
For small changes in circumferential stretch (i.e., over 1 cardiac cycle), the resulting change in stress will be directly related to the physiological elastic modulus (Einc). Therefore, even if the starting stresses differ, the change in stress for one cardiac cycle will be similar if Einc is maintained. Based on data from male Eln+/− mice, we previously hypothesized that Einc is an important parameter controlling homeostatic arterial remodeling (6, 49, 50). Our current data show that LCC and ASC Einc (Fig. 10, C and D) values change between −7 and +14% from 6 to 24 mo of age in male Eln+/+ and Eln+/− mice but change between +31 and +42% from 6 to 24 mo of age in female Eln+/+ and Eln+/− mice, again indicating an increase in deviation from mechanical homeostasis specific to female mice.
Implications for human aging and disease.
Arterial stiffness, as measured by PWV, increases with aging and blood pressure. PWV provides additional information about CVD risk beyond that associated with age or blood pressure alone, indicating that factors involved in arterial remodeling that alter PWV play a role in CVD (4, 42). There are conflicting data on the role of sex differences in PWV and the effects of aging. One study found negligible sex differences in PWV from 30 to 70 yr of age (42). AlGhatrif et al. (1) also found no significant sex differences in PWV over the full age spectrum, but they found a faster rate of PWV increase with aging in males compared with females between 40 and 90 yr of age. In contrast, Weng et al. (51) found that men have higher PWV in the 20- to 39- and 40- to 59-year-old age groups but that men and women had the same PWV in the 60- to 79-yr-old age groups, indicating a different timeline for arterial stiffening with aging. Consistent with AlGhatrif et al. (1) and Weng et al. (51), we found age × sex interactions for many factors associated with arterial stiffening in mice. It has been hypothesized that hormonal changes during menopause play a role in the different timelines of arterial stiffening with aging and sex in humans (40). Mice share many features and endocrine changes associated with natural reproductive senescence in humans, with an approximate onset of 8 mo of age. However, only ∼25% of aging female mice will naturally model the human menopausal transition (10), and we did not measure hormone levels in the current study. Additional animal studies are needed to better understand the implications of sex differences in arterial remodeling with age.
The increase in arterial stiffness with age is often attributed to the degradation of elastin over time and its replacement by stiffer collagen (22). Elastin has a half-life of ∼70 yr (43), consistent with the human life span but much longer than the mouse. The difference in life span is important to consider for aging studies. Although humans live ∼33 times longer than mice (assuming 78 yr and 28 mo average life spans, respectively), due to the higher heart rate in mice, humans only have approximately four times as many lifetime heart beats or loading cycles on the arterial walls (assuming 70 and 600 beats/min, respectively). Our biochemical results show no decrease in elastin content, indicating that elastin degradation is not occurring over the mouse life span. Our biochemical results show an increase in collagen content, indicating that collagen production is associated with aging and/or total loading cycles even when elastin is not degraded. Our histological results show no obvious changes in wall structure with age but are limited to qualitative evaluation that must be extended to determine changes in the compositional and structural organization of the ECM proteins in aged mice.
Individuals with Williams Syndrome, a disease caused by deletion of 26–28 genes, including elastin, on human chromosome 7, have increased PWV compared with matched controls and PWV increases with aging (33). These data are consistent with arterial remodeling observed in Eln+/− mice. Males are diagnosed with Williams Syndrome at a younger age than females and have more severe aortic stenosis and total CVD (44), indicating different arterial remodeling timelines with elastin content and sex, consistent with some of our mouse data. Eln+/− mice do not get focal narrowing of the aortic root, the characteristic cardiovascular phenotype of Williams Syndrome, and so have limitations for investigating more severe CVD associated with the human disease. Mice with SMC-specific inactivation of elastin have focal stenosis in the aortic arch but die at 2 wk of age from pressure-overload cardiac hypertrophy (37) and so cannot be used to investigate elastin contents and aging.
Limitations and future work.
The heart rate and blood pressure were measured under isoflurane anesthesia, so our values of heart rate and blood pressure were lower than those observed in conscious mice (28). Our comparisons between groups assumed that isoflurane has the same effect regardless of age, genotype, or sex, which may not be correct (20). We used the anesthetized blood pressures to determine the physiological stress, stretch, and stiffness values for the arteries. The deformed dimensions of the artery in an in vitro configuration at the measured in vivo axial stretch ratio, without the surrounding tissue and applied external forces present in vivo (14), were also used in those calculations. The calculations represent our best approximation of the in vivo state but not an exact replication. However, our methods allowed detailed mechanical characterization of the arterial wall that is not possible in vivo. Hormonal changes, in particular estrogen changes in aging females, were not measured in the current study. Additional work is needed to investigate how hormonal changes associated with reproductive senescence affect arterial mechanics. Our mechanical experiments were performed under passive conditions. Additional work is needed to understand the contribution of active SMC contraction/relaxation and ECM composition and organization to the observed changes in arterial mechanics. Lastly, new technologies are needed to measure the stresses and/or stretches experienced by individual components within the arterial wall that are driving the associated remodeling.
Conclusions
Our work provides comparative values for arterial mechanics and remodeling for young, to middle-aged, to old mice for males and females with varying elastin content. We demonstrate arterial remodeling that attempts to restore mechanical homeostasis with respect to circumferential wall stress, stretch, and material stiffness. Homeostatic stress and material stiffness are maintained to some degree with aging in male mice but are altered in female mice, indicating sex-dependent differences in the arterial remodeling process. Arterial remodeling will directly affect arterial stiffness, which is linked to sex-dependent CVD-risk in humans. Circumferential stretch is maintained at the most consistent value in all groups with aging, indicating the importance of keeping homeostatic arterial wall stretch and the associated SMC stretch within critical limits.
GRANTS
This work was partially supported by National Science Foundation Grant 1662434, National Institutes of Health Grant R01-HL-105314, and American Heart Association Grant 19TPA-34910047.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z.H., A.C., R.P.M., and J.E.W. conceived and designed research; J.Z.H., A.C., A.H.C., and M.C.S. performed experiments; J.Z.H., A.C., A.H.C., D.B.G., M.C.S., and J.E.W. analyzed data; J.Z.H., A.C., A.H.C., R.P.M., and J.E.W. interpreted results of experiments; J.Z.H., A.H.C., and J.E.W. prepared figures; J.Z.H. and J.E.W. drafted manuscript; J.Z.H., R.P.M., and J.E.W. edited and revised manuscript; J.Z.H., A.C., A.H.C., D.B.G., M.C.S., R.P.M., and J.E.W. approved final version of manuscript.
REFERENCES
- 1.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62: 934–941, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 50: 1570–1577, 2007. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Brankovic S, Hawthorne EA, Yu X, Zhang Y, Assoian RK. MMP12 preferentially attenuates axial stiffening of aging arteries. J Biomech Eng 141: 081004, 2019. doi: 10.1115/1.4043322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 54: 1328–1336, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 5.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA 281: 634–639, 1999. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JK, Stoilov I, Mecham RP, Wagenseil JE. A fiber-based constitutive model predicts changes in amount and organization of matrix proteins with development and disease in the mouse aorta. Biomech Model Mechanobiol 12: 497–510, 2013. doi: 10.1007/s10237-012-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuong CJ, Fung YC. On residual stresses in arteries. J Biomech Eng 108: 189–192, 1986. doi: 10.1115/1.3138600. [DOI] [PubMed] [Google Scholar]
- 8.Clark ER. Studies on the growth of blood-vessels in the tail of the frog larva - by observation and experiment on the living animal. Am J Anat 23: 37–88, 1918. doi: 10.1002/aja.1000230103. [DOI] [Google Scholar]
- 9.Cox RH. Influence of muscle length on series elasticity in arterial smooth muscle. Am J Physiol 234: C146–C154, 1978. doi: 10.1152/ajpcell.1978.234.5.C146. [DOI] [PubMed] [Google Scholar]
- 10.Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 153: 3571–3578, 2012. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrin PB. Influence of initial length on length-tension relationship of vascular smooth muscle. Am J Physiol 225: 664–670, 1973. doi: 10.1152/ajplegacy.1973.225.3.664. [DOI] [PubMed] [Google Scholar]
- 12.Faury G, Maher GM, Li DY, Keating MT, Mecham RP, Boyle WA. Relation between outer and luminal diameter in cannulated arteries. Am J Physiol Heart Circ Physiol 277: H1745–H1753, 1999. doi: 10.1152/ajpheart.1999.277.5.H1745. [DOI] [PubMed] [Google Scholar]
- 13.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferruzzi J, Di Achille P, Tellides G, Humphrey JD. Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PLoS One 13: e0201379, 2018. doi: 10.1371/journal.pone.0201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferruzzi J, Madziva D, Caulk AW, Tellides G, Humphrey JD. Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences. Biomech Model Mechanobiol 17: 1281–1295, 2018. doi: 10.1007/s10237-018-1026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flurkey K, Currer JM, Harrison DE. The mouse in aging research In: The Mouse in Biomedical Research, edited by Fox J, Barthold S, Divisson M, Newcomer C, Quimy F, Smith A. Amsterdam, The Netherlands: Elsevier, 2007, p. 637–672. [Google Scholar]
- 17.Fonck E, Prod’hom G, Roy S, Augsburger L, Rüfenacht DA, Stergiopulos N. Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol 292: H2754–H2763, 2007. doi: 10.1152/ajpheart.01108.2006. [DOI] [PubMed] [Google Scholar]
- 18.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 100: 354–360, 1999. doi: 10.1161/01.CIR.100.4.354. [DOI] [PubMed] [Google Scholar]
- 19.Fung YC. Mechanical properties and active remodeling of blood vessels In: Biomechanics: Mechanical Properties of Living tissues. New York: Springer-Verlag, 1993, chapt 8, p. 321–391. [Google Scholar]
- 20.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J 53: E55–E69, 2012. doi: 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Gosling RG, Budge MM. Terminology for describing the elastic behavior of arteries. Hypertension 41: 1180–1182, 2003. doi: 10.1161/01.HYP.0000072271.36866.2A. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald SE. Ageing of the conduit arteries. J Pathol 211: 157–172, 2007. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys 50: 53–78, 2008. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 24.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42: 1–8, 2009. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central artery stiffness in hypertension and aging: a problem with cause and consequence. Circ Res 118: 379–381, 2016. doi: 10.1161/CIRCRESAHA.115.307722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphrey JD, Rajagopal KR. A constrained mixture model for growth and remodeling of soft tissues. Math Models Methods Appl Sci 12: 407–430, 2002. doi: 10.1142/S0218202502001714. [DOI] [Google Scholar]
- 27.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem 112: 70–75, 1981. doi: 10.1016/0003-2697(81)90261-X. [DOI] [PubMed] [Google Scholar]
- 28.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287: H1618–H1624, 2004. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130: 411–423, 2003. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62: 153–188, 2004. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Staiculescu MC, Cocciolone AJ, Yanagisawa H, Mecham RP, Wagenseil JE. Crosslinked elastic fibers are necessary for low energy loss in the ascending aorta. J Biomech 61: 199–207, 2017. doi: 10.1016/j.jbiomech.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozel BA, Danback JR, Waxler JL, Knutsen RH, de Las Fuentes L, Reusz GS, Kis E, Bhatt AB, Pober BR. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension 63: 74–79, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le VP, Cheng JK, Kim J, Staiculescu MC, Ficker SW, Sheth SC, Bhayani SA, Mecham RP, Yanagisawa H, Wagenseil JE. Mechanical factors direct mouse aortic remodelling during early maturation. J R Soc Interface 12: 20141350, 2015. doi: 10.1098/rsif.2014.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le VP, Wagenseil JE. Echocardiographic characterization of postnatal development in mice with reduced arterial elasticity. Cardiovasc Eng Technol 3: 424–438, 2012. doi: 10.1007/s13239-012-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102: 1783–1787, 1998. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CJ, Staiculescu MC, Hawes JZ, Cocciolone AJ, Hunkins BM, Roth RA, Lin CY, Mecham RP, Wagenseil JE. Heterogeneous cellular contributions to elastic laminae formation in arterial wall development. Circ Res 125: 1006–1018, 2019. doi: 10.1161/CIRCRESAHA.119.315348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem 186: 549–556, 1950. [PubMed] [Google Scholar]
- 40.Ogola BO, Zimmerman MA, Clark GL, Abshire CM, Gentry KM, Miller KS, Lindsey SH. New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol 315: H1073–H1087, 2018. doi: 10.1152/ajpheart.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP, Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res 11: 97–112, 2008. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reference Values for Arterial Stiffness’ Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 31: 2338–2350, 2010. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rucker RB, Tinker D. Structure and metabolism of arterial elastin. Int Rev Exp Pathol 17: 1–47, 1977. [PubMed] [Google Scholar]
- 44.Sadler LS, Pober BR, Grandinetti A, Scheiber D, Fekete G, Sharma AN, Urbán Z. Differences by sex in cardiovascular disease in Williams syndrome. J Pediatr 139: 849–853, 2001. doi: 10.1067/mpd.2001.118889. [DOI] [PubMed] [Google Scholar]
- 45.Shadwick RE. Mechanical design in arteries. J Exp Biol 202: 3305–3313, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem 292: 125–129, 2001. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 47.Stoilov I, Starcher BC, Mecham RP, Broekelmann TJ. Measurement of elastin, collagen, and total protein levels in tissues. Methods Cell Biol 143: 133–146, 2018. doi: 10.1016/bs.mcb.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Wagenseil JE. Reduced amount or integrity of arterial elastic fibers alters allometric scaling exponents for aortic diameter, but not cardiac function in maturing mice. J Biomech Eng, 141: 044504, 2019. doi: 10.1115/1.4042766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 51.Weng C, Yuan H, Yang K, Tang X, Huang Z, Huang L, Chen W, Chen F, Chen Z, Yang P. Gender-specific association between the metabolic syndrome and arterial stiffness in 8,300 subjects. Am J Med Sci 346: 289–294, 2013. doi: 10.1097/MAJ.0b013e3182732e97. [DOI] [PubMed] [Google Scholar]