Abstract
Perivascular adipose tissue (PVAT) modifies the contractile function of the vessel it surrounds (outside-in signaling). Little work points to the vessel actively affecting its surrounding PVAT. We hypothesized that inside-out arterial signaling to PVAT would be evidenced by the response of PVAT to changes in tangential vascular wall stress. Rats coarcted in the mid-thoracic aorta created PVAT tissues that would exemplify pressure-dependent changes (above vs. below coarctation); a sham rat was used as a control. Radiotelemetry revealed a ∼20 mmHg systolic pressure gradient across the coarctation 4 wk after surgery. Four measures (histochemical, adipocyte progenitor proliferation and differentiation, isometric tone, and bulk mRNA sequencing) were used to compare PVAT above versus below the ligature in sham and coarcted rats. Neither aortic collagen deposition in PVAT nor arterial media/radius ratio above coarctation was increased versus below segments. However, differentiated adipocytes derived from PVAT above the coarctation accumulated substantially less triglycerides versus those below; their relative proliferation rate as adipogenic precursors was not different. Functionally, the ability of PVAT to assist stress relaxation of isolated aorta was reduced in rings above versus below the coarctation. Transcriptomic analyses revealed that the coarctation resulted in more differentially expressed genes (DEGs) between PVAT above versus below when compared with sham samples from the same locations. A majority of DEGs were in PVAT below the coarctation and were enriched in neuronal/synaptic terms. These findings provide initial evidence that signaling from the vascular wall, as stimulated by a pressure change, influences the function and transcriptional profile of its PVAT.
NEW & NOTEWORTHY A mid-thoracic aorta coarcted rat was created to generate a stable pressure difference above versus below the coarctation ligature. This study determined that the PVAT around the thoracic aorta exposed to a higher pressure has a significantly reduced ability to assist stress relaxation versus that below the ligature and appears to retain the ability to be anticontractile. At the same time, the PVAT around the thoracic aorta exposed to higher pressure had a reduced adipogenic potential versus that below the ligature. Transcriptomics analyses indicated that PVAT below the coarctation showed the greatest number of DEGs with an increased profile of the synaptic neurotransmitter gene network.
Keywords: adipocyte, mechanotransduction, PVAT
INTRODUCTION
Perivascular adipose tissue (PVAT) dynamically modifies the contractile function of the blood vessel it surrounds. PVAT is best known for its ability to possess/secrete anticontractile factors (e.g., adiponectin), which are thought to be beneficial to the vessel (1, 3, 16, 27). This can be viewed as an outside-in form of communication between PVAT and the vessel. By contrast, little evidence points to inside-out signaling, i.e., the blood vessel actively affecting its surrounding PVAT. Does, for example, PVAT sense and respond to (changes in) pressure? Although many research groups identified that cardiovascular diseases impair PVAT physiology (3), to our knowledge, blood pressure has not been described as a specific factor to cause PVAT dysfunction. Here, we test the hypothesis that elevated blood pressure would modify the structure and function of PVAT.
To test this idea, we produced a coarctation of the descending thoracic aorta in the adult rat. The PVAT above the coarctation should be exposed to a higher pressure than that below the coarctation. Parallel investigation of these two sites allowed us to determine pressure-dependent changes in PVAT structure and function. This is, in part, because the animal serves as its own control relative to circulating factors that could also cause changes in PVAT structure and function. Importantly, in other animals, a ligature around the thoracic aorta was placed but not tightened (a sham control) such that pressure-independent differences in PVAT structure and function at the two different locations along the thoracic aorta could be determined. Any differences revealed between the upper and lower segments of the descending thoracic aorta from the sham animals would be intrinsic differences in these two tissue sites. As such, we use the best available model to isolate the variable of pressure (change) as one to modify PVAT structure and function.
Our hypothesis was tested by using four different experimental approaches: histochemical analysis of PVAT and vessel structure, analysis of stem cell replication and adipogenic potential, isometric contractility to quantify PVAT-assisted stress relaxation, and bulk mRNA sequencing to compare the differentially expressed genes (DEGs) that are pressure-dependent versus those that are pressure-independent. If our hypothesis was correct, then we should observe pressure-dependent changes that could include a gain in PVAT collagen deposition, loss of (potential) adipocytes, a reduced ability to assist vascular function [e.g., stress relaxation assistance (30) and/or anticontractile function], and a modified number of DEGs.
These studies allowed us to demonstrate that a blood pressure gradient induces transcriptomic changes in PVAT that are dependent on anatomical location in the thoracic aorta. Functionally, the PVAT exhibited a loss of adipogenic but not proliferative potential and a loss of PVAT-assisted stress relaxation. The DEGs in the coarcted samples were greater in number than those from the sham, with the greatest changes observed in PVAT below the coarctation, not above. Interestingly, these events occurred at a time/pressure at which the artery and PVAT had not remodeled structurally. Taken together, these results suggest that changes in the function of PVAT precede that of the artery in diseases with elevated arterial pressure.
METHODS
Materials.
Phenylephrine hydrochloride (PE) was purchased from Sigma Chemical (St. Louis, MO). Formalin (10%)-buffered phosphate solution was purchased from Fisher Scientific (Waltham, MA).
Animals.
Michigan State University Institutional Animal Care and Use Committee approved all protocols used in this study. MSU is an American Association for Accreditation of Laboratory Animal Care-accredited institution (A3955-01). Normal male Sprague-Dawley rats (Charles River, Indianapolis, IN) were used in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., 2011). Only male rats were used. All rats were housed in a temperature-controlled room (22°C) with 12-h:12-h light/dark cycles. Animals were given standard chow and distilled water ad libitum. The rats used were randomized to studies and experimental groups. Each n value represents data that came from one animal.
Coarctation of the rat thoracic aorta.
Subtotal descending thoracic aortic banding was performed in rats (∼300 g) under anesthesia with isoflurane (2% in oxygen) and artificial ventilation. The descending thoracic aorta was exposed through an intercostal incision between the fourth and fifth ribs. An 18-gauge blunt tip needle with the tip bent 90° was placed on the aorta. A uniform degree of constriction around the descending thoracic aorta was produced by tying a 5-0 surgical silk ligature tightly around the aorta and blunt tip needle. The blunt tip needle was then withdrawn from the ligature and the chest incision was closed. Sham-operated control animals underwent the same procedure, but the ligature on the aorta was not tightened. Animals received a preoperative dose of enrofloxacin (Baytril, 2.5 mg/kg, im), carprofen (Rimadyl, 5 mg/kg sc), and buprenorphine SR (buprenorphine sustained release 1.0 mg/kg sc). An image and a diagram of the ligature placed around the aorta in a sham or coarcted rat is shown in Fig. 1A.
Fig. 1.
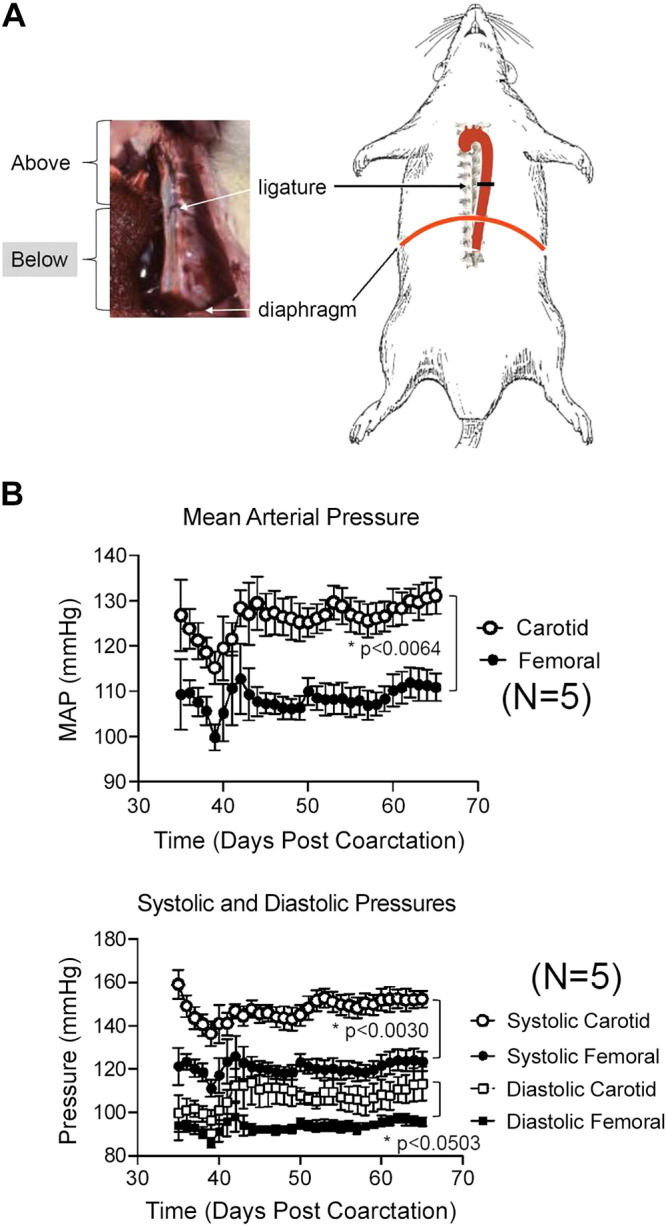
A: photograph and diagram of ligature placement on the descending thoracic aorta of the rat. B: radiotelemetric measures of mean (top) and systolic/diastolic (bottom) pressures in coarcted male Sprague–Dawley rats for 1 mo after implantation of a dual radiotelemeter (carotid for pressure above the ligature, femoral for pressure below). Points are means + SE for N number of individual rats. *Significant differences between bracketed groups as determined by mixed-effect analysis.
Blood pressure measurement.
Under isoflurane anesthesia (2% in oxygen), dual-channel radiotelemeter transmitters (HD-S21; Data Sciences International, MN) were implanted subcutaneously through a 1–1.5-cm incision in the left inguinal area. One catheter was introduced into the left femoral artery and advanced into the abdominal aorta below the renal artery (below coarctation). The second catheter was placed into the carotid (above coarctation). Animals received a preoperative dose of enrofloxacin (Baytril, 2.5 mg/kg im) and carprofen (Rimadyl, 5 mg/kg sc). After 5 days of postoperative recovery, baseline cardiovascular measurements were recorded for 60–90 days.
Aortic dissection and histology.
Before tissue removal, rats were given pentobarbital as a deep anesthetic (80 mg/kg ip). A bilateral pneumothorax was created before vessel dissection. The thoracic aorta was dissected from the aortic arch to the diaphragm with care taken to keep track of the orientation of the vessel. The thoracic aorta above the ligature, from just outside the ligature to the aortic arch, was considered “above.” The thoracic aorta from just below the ligature to the diaphragm was considered “below.” The tissue directly underneath the ligature was not used. Tissue dissection took place under a stereomicroscope and in a Silastic-coated dish filled with physiological salt solution (PSS) containing (in mM): 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 14.8 NaHCO3, 5.5 dextrose, 0.03 CaNa2EDTA, and 1.6 CaCl2 (pH 7.2). For creating rings of the aorta (∼5 mm wide) with and without PVAT, the vessel was guided onto a wire that was embedded into the dish silastic to allow for tissue revolution such that the vessel could be completely cleaned of PVAT. In this way, PVAT was removed for some experiments. The endothelium was intact in all experiments.
In some experiments, samples were placed in 10% formalin overnight, switched to 30% ethanol, and taken to Investigative Histopathology Services (https://humanpathology.natsci.msu.edu/) on the campus of MSU for paraffin embedding and section cutting to provide slides with 8–10-µm-thick sections. Slides were processed with Picrosirius Red or hematoxylin-eosin for collective determination of collagen, PVAT area and medial thickness/vessel radius, and adipocyte’s lipid droplet size distribution. Lipid droplet size was determined in PVAT regions with multilocular and unilocular adipocytes from >100 adjacent cells from five randomly selected fields per section using the Adiposoft plugin (v1.15) for ImageJ Fiji (v2.0.0). Frequency distribution was determined and analyzed using GraphPad Software (GraphPad, San Diego, CA). Investigators were blinded to the experimental group of the samples (6).
Immunohistochemistry.
Aorta was removed from above and below the coarctation from sham and coarcted animals, fixed in formalin, and preserved in paraffin. Slides of tissue (described above) were dewaxed, antigen unmasked, and blocked with goat blocker (S-1000 Vector, Burlingame, CA) for 1 h before incubating one section per slide at 4°C overnight with perilipin1 antibody [PLN1; (1:250) ab3526, Abcam, Cambridge, MA]. Alexafluor 488 goat anti-Rabbit (Life Technologies, A11008, Eugene, OR) was used on both sections for 1 h at room temperature as a secondary antibody. Images were taken on a Nikon SMZ18 stereomicroscope with a Lumencore LED lightsource using an Andor Zyla 4.2Plus camera and MicroManager software (v1.4.23). Sections were imaged such that paired tissues with or without primary, and from both above and below coarctation from the same animal, were acquired at the same settings and exposures (described later).
Adipogenic potential of PVAT progenitor cells.
PVAT dissected from the thoracic aorta was placed on ice in Krebs Ringer bicarbonate-buffered solution (KRBB) 2% BSA, minced, and digested in collagenase type I solution (1 mg/mL; Worthington Biochemical, Lakewood, NJ) as described (4). The digested tissue was filtered (40 μm) and then centrifuged at 4°C for 10 min at 300 g. Pellets containing the cells of the stromal vascular fraction (SVF) were resuspended in erythrocyte lysis buffer solution (Biolegend, San Diego, CA), incubated for 1 min at room temperature and then centrifuged at 4°C for 5 min at 300 g, and then resuspended in preadipocyte media.
PVAT cells with a high adipogenic potential (progenitor cells) were obtained by outgrowth of plastic-adherent cells from the SVF after two serial passages. Progenitor cells were plated at 50,000 cells/well in 24-well plates and 10,000 cells/well in 48-well plates for adipogenesis assays. The proliferation of progenitor cells was determined using a fluorescence-based DNA quantitation (QuantiFluor dsDNA System, Promega, Madison, WI). PVAT progenitor cells’ adipogenic capacity was evaluated after in vitro culturing of preadipocytes for 14 days in adipogenic media (4). Lipid accumulation was assessed using the AdipoRed assay (Lonza, Allendale, NJ). The expression of adipogenesis-related genes including Abhd5, Agpat2, Dgat1, Plin1, Pnpla2, and Pparg and brown adipocyte phenotype markers Ucp1 and Pgc1a was evaluated using RT-qPCR (4).
Isolated tissue bath measurement of isometric tone: stress relaxation and PE challenge.
Aortic rings with (+) or without (−) PVAT were mounted onto two L-shaped stainless-steel rings. The endothelium was left intact. Rings were mounted in warmed (37°C) and aerated (95% O2, 5% CO2) tissue baths (30 mL PSS) on Grass isometric transducers (FT03; Grass instruments, Quincy, MA) connected to a four-channel PowerLab (ADInstruments, Colorado Springs, CO). Sample type (+ or −PVAT; above or below coarctation) was randomized daily into one of four different baths. Care was taken to apply no tension on the tissue before the initiation of the experiment.
All rings started at a tension of 0 g. Aortic rings were challenged with a cumulative passive tension application of 0.25, 0.5 (0.25 g added), 1 (0.5 g added), 2 (1.0 g added), and 4 and 6 g (2 g each added). Tissues were allowed to relax to this stretch for 30 min; the tension achieved at this time was recorded. Tissues were then challenged with a maximum concentration of PE (10−5 M), the response allowed to plateau and recorded. Tissues were washed identically and repeatedly through 30 min to achieve a stable baseline. At this time, the next tension step was placed. Experiments were considered complete when the maximum 6 g of tension was achieved and PE tested at this point.
Bulk mRNA sequencing of thoracic aortic PVAT above and below coarctation.
A separate cohort of 6 coarcted and 5 sham rats was made for this experiment, all experiencing 4 wk of coarctation before PVAT removal. RNA was extracted from thoracic aortic PVAT dissected from above and below the coarctation using the Quick RNA MiniPrep kit (Cat. No. R1054; Zymo Research, Irving, CA) that includes a DNase step to remove genomic DNA. Purity, concentration, and integrity of mRNA were evaluated using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE) and an Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA). All samples had a 260:280 nm ratio between 1.9 and 2.1 and RNA integrity number ≥ 7.5. Samples were sent to Novogene Corporation Inc. (Sacramento, CA) for analysis and reporting of RNA-seq quantification. Novogene performed quality control for the RNA isolation that occurred at Michigan State University. Comparative libraries were constructed, library quality control was certified, and sequencing was conducted. Analysis of bulk mRNA sequencing was carried out as described below.
Data and statistical analyses.
For each end point, the N value represents the number of individual animals used. Data, as line graphs or in bar graphs with scattered points, are reported as means ± SE. Most graphs were created in GraphPad Prism v8.0, whereas gene networks and functional associations were created within their respective programs. Mean arterial pressure (MAP) and heart rate (HR) were measured for 10 s every 10 min throughout the duration of the study and are reported as 24-h averages. Histological sections were quantified for the PVAT area by drawing a region of interest (ROI) around the PVAT, excluding the medial layers inward, and converting the pixels2 to µm2. Medial thickness was measured as the average distance of 4 points across the medial layer: thinnest, thickest, and two average lengths. The vessel radius was determined by filling in the lumen with an auto shape filler that calculated the average diameter of the shape and dividing that by 2. Picrosirius red staining was quantified with ImageJ 2.0.0 (24) after splitting the RGB images into red, green, and blue channels and then adjusting a single time the threshold for all the images. The collagen signal was measured in the red channel in a blinded fashion and is reported as a percentage (%) of the total PVAT area. Fluorescent immunohistochemical images of PLN1 staining were quantified on ImageJ 2.0.0. To do so, an ROI, which included only PVAT (as a whole), was the same size on images with and without the primary antibody. Threshold was set to baseline on sections without the primary antibody present to account for autofluorescence. Any signal observed in the no primary sections was subtracted from the signal of the image with primary antibody. This measure provided the signal as a percentage of the ROI area [signal (% area)].
The relative proliferation of adipocyte progenitors was calculated with measures taken at 8, 48, and 96 h after seeding and is reported as nanograms DNA/volume of cell suspension. Triacylglyceride (TAG) accumulation in adipocyte progenitors above and below the (sham) coarctation is reported as AdipoRed relative fluorescence units. Representative images of AdipoRed stained cells were captured on a normal bright-field microscope. Stress relaxation of isolated aorta in tissue baths was quantified by measuring the cumulative final tension arrived at after each tension application.
For bulk mRNA sequencing, samples were transcriptomic-sequenced via Illumina platform by Novogene. After quality control, the paired-end clean reads were mapped onto reference genome using STAR (v2.5). HTSeq (v0.6.1) was used to count the read numbers of each gene. The gene read counts matrix was further fed into DESeq2 (v1.24.0) to identify the differentially expressed genes (DEGs). These DEGs were further analyzed based on the Gene Ontology database using David (v6.8) (8), g.Profiler (2019 update) (23). Network analyses were performed in Cytoscape (25) using BinGO (v3.0.4) (17), String (v1.5.0) (5), ClusterMaker2 (v1.3.1) (19), AutoAnnotate (v1.3.2) (12), Wordcloud (v3.1.3) (20), and EnrichmentMap (v3.2.1) (18). For data analysis in R, the following libraries were used: tidyverse (v1.3.0), pheatmap (v1.0.12), Rattus.norvegicus (v1.3.1), RColorBrewer (v1.1–2), and wordcloud (v2.6). The statistical power of bulk RNA sequencing data was calculated using RNASeqSampleSize R package (34). For comparisons of the above and below samples, 91% of the DEGs from coarctation samples and 91% of the DEGs from sham samples have a statistical power larger than 80%.
Graph Pad Prism (v8.0) and JMP PRO software (v14.0) were used for most statistical analyses. For statistical tests, equality of variances was determined before statistical test conduction. Mixed effective analysis was used to determine differences in blood pressure over time. When two groups were compared, an unpaired Student’s t test was used. When three groups were compared, a one-way ANOVA followed by Tukey’s post hoc was used as long as variances were homogeneous as determined by the Brown Forsythe test. A P value of <0.05 was considered statistically significant. For transcriptome analyses, the resulting P values were adjusted using the Benjamin and Hochberg’s approach for controlling the false discovery rate (FDR). Genes with an adjusted P value <0.05 found by DESeq2 were assigned as differentially expressed.
RESULTS
Mid-thoracic aorta coarctation created a stable pressure gradient.
Initial studies (not shown) allowed us to determine that rats could survive and grow normally for at least 2 mo after this surgery. Fig. 1A depicts the placement of the ligature that was used for both the coarcted (ligature tightened) and sham (ligature lax) animals. Fig. 1B summarizes the mean arterial pressure (top) and individual systolic and diastolic pressures above (carotid) and below (femoral) the ligature beginning at ∼5 wk after surgery (bottom). Pressures were taken while animals were conscious through radiotelemetry. The coarctation created an ∼20 mmHg mean arterial pressure difference across the ligature that was stable for at least 1 mo. The difference in systolic pressure between the coarcted versus sham rat was +25 ± 6 mmHg and that in diastolic pressure was +14 ± 6 mmHg. The coarcted rat was thus used as one in which the PVAT above versus below the ligature was exposed to a pressure difference, whereas the parallel tissues from the sham rat were used to determine pressure-independent differences.
Elevated pressure did not modify PVAT or artery structurally.
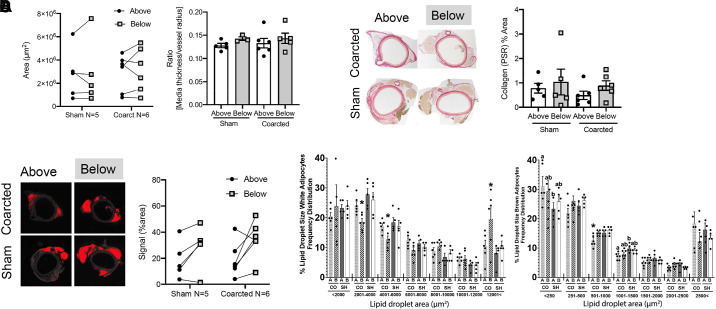
Sections immediately above and below the ligature were removed for histological processing to obtain measures of: 1) total PVAT area, 2) arterial media thickness/lumen radius, 3) PVAT collagen deposition, and 4) PLN1 total staining as an index of lipid droplets/adipocytes. These measures were done in sections that came from the same rats. The total area (Fig. 2A, left) and mass (Supplemental Fig. S1, see https://doi.org/10.6084/m9.figshare.12275000) of PVAT in sections above and below the ligature were not modified in the coarcted animal versus sham, indicating that PVAT as a whole was not gained or lost because of the surgery or coarctation. The arterial medial thickness/radius ratio was not different in sections from above versus below the ligature in sham rats (Fig. 2A, right). This ratio was also not elevated between the above and below sections of the coarcted rats or when the same experimental groups were compared between sham and coarcted. Such a change would be considered an arterial response to pressure. A lack of structural change to elevated pressure was also supported by a lack of increase in Picosirius red (PSR) staining in the sections above the coarctation ligature versus below (Fig. 2B left and quantified on the right). Additionally, there appears to be no loss of adipocytes with exposure to a higher pressure over 4 wk. PLN1 staining in coarcted above sections did not have a lower staining signal versus the sham above tissue (image in Fig. 2C, left). However, PLN1-positive staining was generally lower in the above sections of sham (18.69% ± 2.83%) and coarcted (19.00% ± 2.24%) versus the below areas (sham = 28.94% ± 3.35% and coarcted = 34.66% ± 2.40%; Fig. 2C). Coarctation induced only minor alterations in the lipid droplet size distribution in PVAT. White adipocytes below the coarctation had an increased number of large lipid droplets (>12,001 µm2) and fewer in the 2,001–6,000 µm2 range than above the coarctation and to sham animals above and below (Fig. 2D, left). As for brown adipocytes, coarctation reduced the number of midsize (1,001–1,500 µm2) and increased that of smaller (<250 µm2) lipid droplets below the coarctation compared with above and sham animals in both anatomical locations (Fig. 2D, right). Overall, structural changes in response to elevated pressure in both the tunica media and PVAT were not significant.
Fig. 2.
A, left: area of PVAT between sham vs. coarcted male Sprague–Dawley rats from above to below the ligature. Right: quantitation of medial thickness and arterial radius, reported as a ratio, for rings from above and below the ligature both in sham (left: two bars) and coarcted (right: two bars) rats. Bars are means + SE for the number of animals depicted by each of the symbols scattered around the means. Two sham and two coarcted rats had incomplete ring structures, which made the measures unobtainable. B, left: image of picrosirius red (PSR) staining in thoracic aortic sections immediately above and below the coarctation, taken from male rats a month after surgery. Representative of seven different animals. Right: quantitation of PSR staining in thoracic aortic PVAT above (open bar) and below (gray bar) the ligature; individual symbols are values for 5–6 male rats. C, left: perilipin1 (PLN1) staining of aorta from coarcted and sham rats from above or below the ligature. Red represents a signal greater than the baseline threshold determined by the corresponding no primary tissue section. Representative of five rats. Right: quantification of the PLN1 signal as a percent of PVAT area from sham vs. coarcted animals from above and below the ligature. D: distribution of lipid droplet areas in white (left) and brown (right) PVAT above (A) and below (B) the ligature in coarcted (CO, n = 6) and sham (SH, n = 5) male Sprague–Dawley rats. Bars with different letters or * differ significantly (ab, *P < 0.05). PVAT, perivascular adipose tissue.
Elevated pressure reduced the adipogenic potential of cells in PVAT.
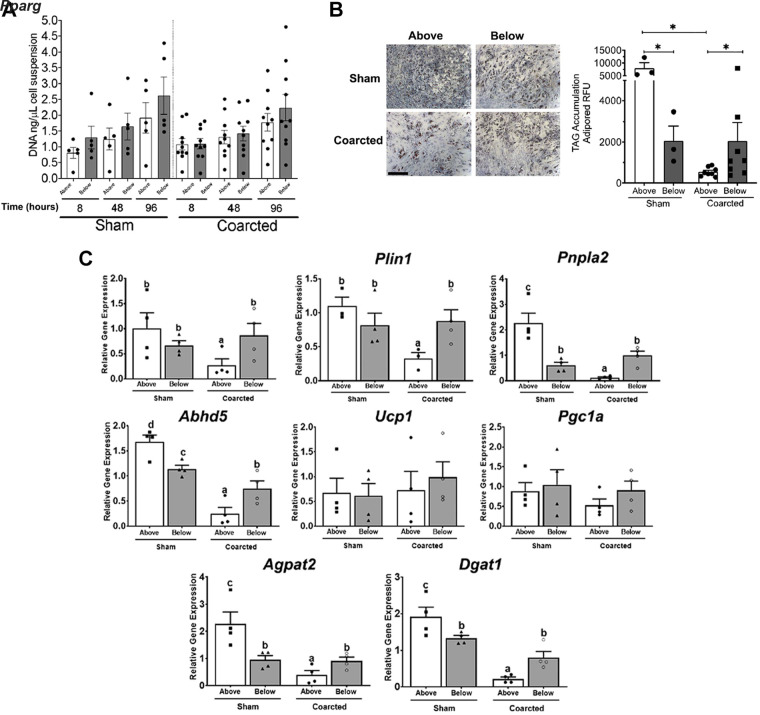
A separate cohort of coarcted and sham rats was created. Four weeks after surgery, the PVAT from above and below the coarctation was removed. The SVF was separated and adipogenic precursor cells (APs) were obtained for measurement of two end points: overall proliferation potential and adipogenic potential. The overall proliferative potential of the APs above and below the coarctation was not different whether the comparison was done within the sham or coarcted samples (Fig. 3A). Thus, neither pressure-independent nor pressure-dependent changes were observed in this measure. However, there was a pressure-dependent difference in the adipogenic potential of cells isolated from the upper (above) versus lower (below) thoracic aortic PVAT. This was evidenced by the greater synthesis of TAG in the above versus below sections from sham rats (left bars, Fig. 3B, bottom). Importantly, the placement of a pressure difference between these two aortic sections reduced the adipogenic potential of the APs above the coarctation (images in Fig. 3B, top, quantified in Fig. 3B, bottom). Differences in the adipogenic responses were reflected in higher expression of the master regulator of adipogenesis Pparg and adipocyte differentiation markers Plin1, Pnpla2 (encoding adipose triglyceride lipase), and Abhd5 (Pnpla2 activator) in sham above compared with below and in cells from coarcted animals (Fig. 3C). Differentiated adipocytes from sham above also had increased expression of the genes encoding the triglyceride synthesis regulators Agpat2 and Dgat1. Expression of brown adipocyte phenotype markers Ucp1 and Pgc1a was detected in cells from above and below the ligature in sham and coarcted animals (Fig. 3C).
Fig. 3.
A: proliferation of PVAT adipocyte progenitor cells (AP) above (open bar) and below (gray bar) the coarctation from sham (left, n = 4) and coarcted (right, n = 10) male Sprague–Dawley rats at 8, 48, and 96 h of culture. Points are values for individual rats around bars representing means + SE. B, top: representative images of AdipoRed staining in AP from above (left) and below (right) the coarctation in sham and coarcted male Sprague–Dawley rats after 14 days of culture in adipogenic media. Bottom: quantitation of TAG (triglyceride accumulation as adipored RFU) in sham (left, n = 3) or coarcted (right, n = 8) AP from aortic PVAT that was above (open bar) or below (gray bar) the ligature. Points are values for individual rats around bars representing means + SE. *P < 0.05, for one-way ANOVA followed by Tukey’s multiple comparisons. C: gene expression of adipogenic markers by RT qPCR in sham (left, n = 4) or coarcted (right, n = 4) AP from aortic PVAT that was above (open bar) or below (gray bar) the ligature after 14 days of culture in adipogenic media. Values are relative mRNA abundance after normalization with the reference gene Rps29. Significant differences are indicated by letters a, b, c, and d (P < 0.05). PVAT, perivascular adipose tissue; RFU, .
Elevated pressure reduced the ability of PVAT to assist stress relaxation.
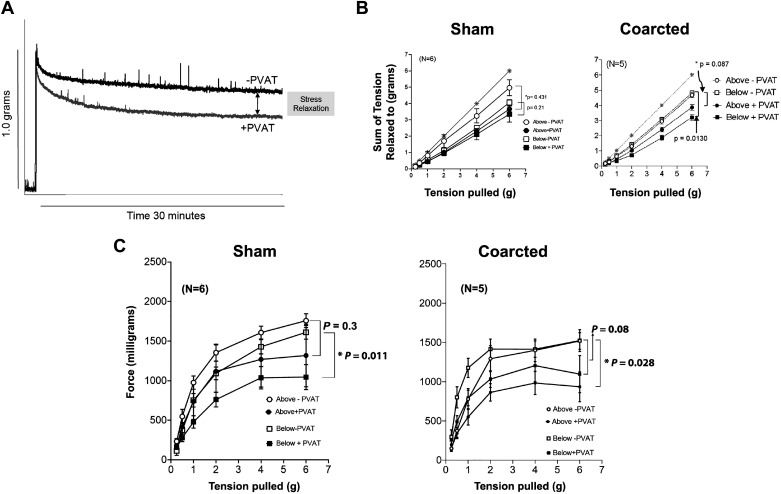
PVAT assists in vascular stress relaxation, the loss of force over time that occurs after a stretch is imposed on an isolated vessel (30). As depicted in representative tracings in Fig. 4A, the presence of (healthy) PVAT reduced the tension of the stretched aorta over time when compared with an aortic ring without PVAT (Fig. 4B); this is PVAT-assisted stress relaxation. Two rings above and below the ligature were randomized to the + or − PVAT group and mounted in isolated tissue baths. A cumulative application of tension (from 0.25 to 6 g) resulted in a loss of tension that was greater in tissues +PVAT versus −PVAT.
Fig. 4.
A: representative tracing of stress relaxation in the isolated rat thoracic aorta as assisted by PVAT. PVAT = tissues without PVAT; +PVAT = tissues with PVAT intact around aorta. B: cumulative tension achieved with each stretch step vs. no relaxation (gray starred line) comparing responses of tissues from above and below the ligature in sham (left) and coarcted (right) male Sprague–Dawley rats. Points represent means ± SE for the number of animals in parentheses. *Significant differences in maximums as determined by a Student’s t test (P < 0.05). C: the response of vessels stretched in B to a maximum concentration of PE (10 5 M) at each stretch step from sham (left) and coarcted (right) male rats. Points represent means ± SE for the number of animals in parentheses. PVAT, perivascular adipose tissue.
All vessels (+ or −PVAT) showed some degree of stress relaxation, as their cumulative tension curves were below the starred line that indicated no relaxation). In tissues from sham rats, the presence of PVAT assisting greatest relaxation was the greatest in those rings from above versus below the ligature, as estimated by the final maximum of the + and −PVAT responses (Fig. 4B, left). In contrast, the reverse occurred in tissues from the coarctated rats. Here, the magnitude of PVAT-assisted stress relaxation was significantly reduced in the rings above the coarctation when compared with that below (Fig. 4B, right). The maximums of final tensions relaxed to (at 6 g) were compared statistically. Quantitatively, the presence of PVAT reduced the maximum achieved in aortic rings from below (+PVAT = 1,369 ± 52 mg vs. −PVAT = 1,745 ± 36 mg; P = 0.013) but not above the coarctation (+PVAT = 1,467 ± 82 mg vs. −PVAT = 1,734 ± 69; P = 0.086).
A better-known function of PVAT is to be anticontractile. In tissues from the sham rat below the ligature, the presence of PVAT reduced the contraction to a maximum concentration of the α adrenergic agonist PE. This was also the case in the tissues above the coarctation, but this was not statistically significant (Fig. 4C, left). Again, the reverse occurred in considering the tissues from the coarcted rat. Here, the elevated pressure experienced by the aortic rings above the coarctation did not cause a loss of PVAT-assisted reduction in active contraction (Fig. 4C, right). Thoracic aorta from above the coarctation without PVAT had a greater magnitude of maximum contraction than with PVAT (maximum: above −PVAT = 1,519 ± 106 mg vs. above +PVAT = 935 ± 190 mg; P = 0.0279). This same comparison was not significant in aorta below the coarctation (maximum: below −PVAT = 1,236 ± 212 mg; below +PVAT = 1,097 ± 237 mg; P = 0.34). These data support that the anticontractile nature of PVAT was retained and potentially improved in sections above the coarctation, consistent with the finding of the coarcted sections having a normal mature adipocyte population.
Coarctation affects the intrinsic gene expression pattern above and below the ligature.
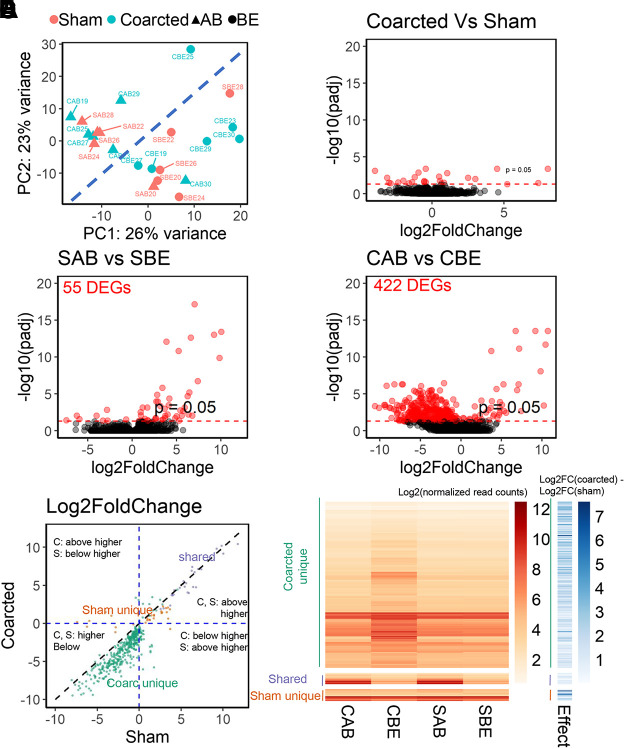
To determine the effect of coarctation on transcriptomic profiles of thoracic aortic PVAT, we performed next-generation sequencing (RNA-seq). Principal components analysis (PCA) showed that, contrary to our expectation, the transcriptomic profiles of samples segregated more strongly according to the anatomical location (above or below the ligature) than experimental groups (Fig. 5A). We further analyzed the RNA-seq data at the gene level. In agreement with the PCA result, few DEGs were identified when comparing gene expressions in coarcted and sham samples without regard to the anatomical location (Fig. 5B). Comparison of above and below ligature samples identified 3 and 2 DEGs, respectively (Supplemental Figs. S2, A and B). Comparing all samples from coarcted and sham rats identified 20 DEGs (Fig. 5B).
Fig. 5.
A: principal components analysis of the transcriptomics data. B: few DEGs (differentially expressed genes) are identified comparing coarcted and sham rats. Coarctation (D) induces more DEGs than in the sham rats (C). Red dots are statistically significant DEGs. E: comparison of the Log2fold change of the three DEG sets: sham unique, shared, and coarcted unique. The expressions (F) and coarctation effect index (shaded blue bars) of the three DEG sets in both sham and coarcted rats. CAB, coarcted above; CBE, coarcted below; SAB, sham above; SBE, sham below. The coarctation effect index defined as Log2FC (coarcted) − Log2FC (sham). The expression (numbers) is log transformed read counts. PVAT tissues are from coarcted (n = 6) and sham (n = 5) male Sprague–Dawley rats. PVAT, perivascular adipose tissue.
In contrast, comparing all samples (coarcted and sham) above versus below ligature, we identified 348 DEGs (Supplemental Fig. S2C). When comparing samples above versus below ligature in sham rats, we identified 55 DEGs with 47 of them highly expressed above the ligature (positive log2FC; Fig. 5C). These DEGs from sham rats included genes for the cardiac ryanodine receptor (ryr2), α-actin cardiac muscle 1 (actc1), tropomyosin 1 (tpm1), phospholamban (pln), potassium channel subunits kcnj5, kcnk2, and kcnj3), and cardiac troponin (tnnt2), to name a few. There was an effect of coarctation on the anatomical gene expression pattern. The number of DEGs increased from 55 in sham rats to 422 in coarcted rats (Fig. 5D). Most of the DEGs in coarcted rats (378 of 422) were highly expressed below the ligature (negative Log2FC).
The DEG sets in sham and coarcted rats overlapped with each other (Supplemental Fig. S2D). To separate the effects of pressure on DEGs, we gathered them into three groups: shared DEGs, sham unique DEGs, and coarcted unique DEGs. Shared DEGs are pressure-independent because they occur whether or not pressure had been changed. The sham unique and coarcted unique DEGs groups are pressure-dependent because coarctation effected a pressure difference. All the shared DEGs were highly expressed above the ligature (Fig. 5E, purple dots). The sham unique DEGs were highly expressed both above and below the ligature (Fig. 5E, orange dots). The Log2FC of the sham unique DEGs above and below the ligature were smaller (i.e., aggregated at the intercept shaded lines) than those of the shared DEGs and coarcted unique DEGs [Fig. 5E, compare the location of orange dots to that of purple (shared) and green (coarcted unique)]. The coarcted unique DEGs was the largest group. Most of coarcted unique DEGs were highly expressed below the ligature (Fig. 5E, green dots).
The three groups (coarcted unique, shared, and sham unique) were quantitatively compared as a heat map as shown in Fig. 5F. Compared with sham samples (SAB = sham above and SBE = sham below), gene expressions were upregulated below the ligature in coarcted rats (CBE = coarcted below). To quantify the pressure-dependency of these DEGs, we defined the coarctation effect index as the difference of log2FC between coarcted and sham rats (blue shaded bar, Fig. 5F). Coarctation affected sham unique and coarcted unique DEGs more than the shared DEGs (Fig. 5F, compare lighter blue of shared DEGs with darker blues of coarcted and sham unique DEGs).
Gene set enrichment analysis reveals potential mechanisms of the coarctation effects.
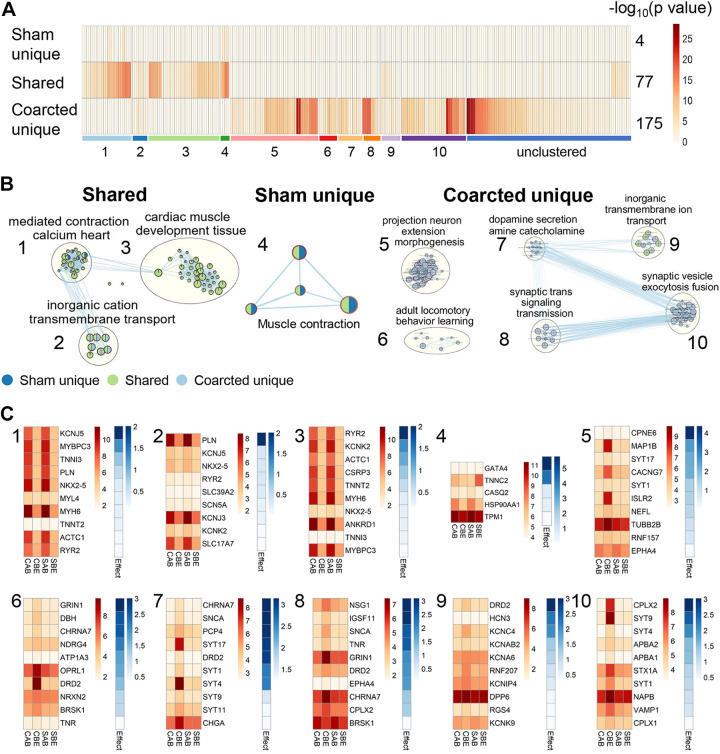
To identify functional differences, the three DEG groups were analyzed for enrichment by Gene Ontology (GO) Biological Process terms. Two hundred and forty-one (241) unique terms were enriched in the three groups (Fig. 6A). The sham unique, shared, and coarcted unique DEG sets were enriched in 4, 77, and 175 GO terms, respectively. The P values of GO terms enriched in the shared and coarcted unique DEG sets were smaller than those in the sham unique DEG set, indicated by the deeper colors in Fig. 6A. These enriched GO terms were further clustered into groups (Fig. 6B). In each cluster (numbered 1–10), the ten genes with the highest coarctation effect index are highlighted in Fig. 6C.
Fig. 6.
The three DEG sets enriched ten clusters of Gene Ontology Biological Process (GO) terms. A: enriched GO terms in the three DEG sets were color-coded by −log10 (P value) and ten coherent clusters (1–10) were identified. The number of enriched terms for each DEG set is labeled at the right side of the bar. The order of ten clusters are aligned with that in B. The order of terms in each cluster is ranked based on the Euclidian distance of the P value. B: clustered networks of enriched GO terms for each DEG set. Each node is a GO term and it is color-coded as the DEG sets enriched in this term. Blue, green, and light blue colors represent the sham unique, shared, and coarcted unique DEG sets, respectively. C: representative DEGs having the highest coarctation effect index value in each cluster. The numbers at upper left corners of each plot are aligned with those in B. CAB, coarcted above; CBE, coarcted below; SAB, sham above; SBE, sham below. Effect: coarctation effective index. PVAT tissues are from coarcted (n = 6) and sham (n = 5) male Sprague–Dawley rats. DEG, differentially expressed gene; PVAT, perivascular adipose tissue.
The shared DEGs were enriched in three clusters: “calcium-mediated contraction heart” (cluster 1), “inorganic cation transmembrane transport” (cluster 2), and “cardiac muscle development tissue” (cluster 3) (Fig. 6B, far left). Furthermore, some DEGs (for example, ryr2, myh6, and actc1) enriched in the inorganic cation transmembrane transport (cluster 2) were also a component of clusters 1 and 3. For these shared DEGs, individual genes were more highly expressed above the ligature in both sham and coarcted samples (1–3, Fig. 6C). These GO terms represent pressure-independent differences between above and below the ligature given that these genes had similar expression patterns in both the sham and coarcted samples.
We next report sham unique DEGs (Fig. 6B, middle). The four GO terms enriched in the sham unique DEG set were all related to “muscle contraction” (cluster 4). These four terms were also enriched in the shared DEG set in cluster 1. Four out of the 28 sham unique DEGs were enriched in these four GO terms (Fig. 6C, 4). For example, TNNC2 regulates the muscle contraction via intracellular calcium signals. It was expressed above the ligature in coarcted samples (CAB), but highly expressed below the ligature in sham samples (SBE).
Six clusters were enriched in the coarcted unique DEG set. Four of these clusters involve synaptic signaling and neurotransmission. The inorganic transmembrane transportation cluster was enriched in both shared and coarcted unique DEG sets. In the coarcted DEG set, however, all ion transportation cluster enriched genes were synaptic related genes (Supplemental Fig. S3). The ten DEGs with the highest coarctation effect index in each cluster were significantly upregulated in the below-ligature samples from coarcted rats (Fig. 6C, clusters 5–10). Representative genes in each cluster included cluster 5: microtubule-associated protein 1B (map1B), calcium voltage-gated channel subunit gamma 7 (cacng7), immunoglobulin superfamily containing leucine-rich repeat protein 2 (islr2); cluster 6: dopamine receptor D2 (drd2) and opioid-related nociceptin receptor 1 (oprl1), cluster 7: synaptotagmins 4 and 17 (syt4, syt17); cluster 8: cholinergic receptor nicotinic alpha7 subunit (chrna7) and glutamate [NMDA] receptor subunit zeta-1 (grin1); cluster 9: potassium voltage-gated channel subfamily C member 4 (kcnc4); and cluster 10: vesicle-associated membrane protein 1 (vamp1), beta-soluble NSF attachment protein (napb), syntaxin 1A (stx1A), synaptotagmin 9 (syt9), and complexin2 (cplx2).
DISCUSSION
The goal of this study was to determine if (thoracic aortic) PVAT responded to an elevated pressure with a change in structure and biological function, evidence of mechanotransduction. To remove circulating substances as a confounding factor for comparing the impact of higher versus lower pressure on PVAT, we created the mid-thoracic aortic-coarcted rat. Our model is similar to the one created by Xu et al. (31), in which measures were also made 4 wk after coarctation. We observed a similar pressure difference across the coarctation as they did (∼20 mmHg in anesthetized, catheterized rats), but improved upon this measure by using newly available dual pressure telemeters that allowed us to obtain chronic blood pressure measures from conscious rats.
In the present study, our coarcted model was paired with a sham rat for two reasons. First, we needed to control for the surgery and ligature placement that was likely accompanied by inflammation that in-and-of-itself could affect the outcomes measured. Second, we did not know, going into this study, if there were intrinsic differences in the structure and/or function the upper (above) versus lower (below) PVAT from the thoracic aorta. This is relevant because the different depots of PVAT around the thoracic aorta (ventral vs. the two lateral strips) originate from embryologically distinct cells (33). This report by Ye et al. did not suggest that lower versus upper PVAT also originated from different cells but caused us to consider that not all thoracic aortic PVAT is the same. By using the sham and coarcted rats in parallel, we gained significant and valuable pieces of knowledge about heretofore undescribed vascular pressure-driven effects on PVAT. We consider here first those differences that were independent of the pressure difference.
Pressure-independent differences.
We interpreted differences in experimental outcomes between PVAT samples above versus below the ligature from sham rats to be pressure-independent differences. To our surprise, we discovered several differences. Two are particularly worth discussion. First, the adipogenic potential of AP cells from above versus below was markedly different, with higher potential observed in those cells closer to the heart. This finding may reflect the patterns of PVAT distribution along the thoracic aorta with larger PVAT depots of white adipocytes in the vicinity of the heart. Second, the transcriptomic data showed that all the shared DEGs (genes differentially expressed in both sham and coarcted samples) were more highly expressed above the ligature. The shared DEGs were enriched with processes related to muscle contraction, cardiac muscle development, morphogenesis terms, and ion transportation. The enriched genes related to ion transport from shared DEGs are also enriched in other clusters, for example PLN, KCNJ5, NKX2–5, and RYR2 (clusters 1, 2, and 3 in Fig. 6C). We were careful to ensure that the PVAT above the ligature was not contaminated by the cardiac tissue, one possible explanation for an enrichment in cardiac-related genes. These results reveal new physiological patterns in PVAT along the aorta, supporting a more cardiac genotype near the heart. The potential physiological impact of this is as yet unknown.
Pressure-dependent differences.
We have interpreted differences in experimental outcomes between PVAT samples above versus below the ligature from coarcted rats to be pressure-dependent differences. Two experimental measures directly support that PVAT was responsive to the change in pressure created by the coarctation. First, PVAT assistance of stress relaxation in rings exposed to a higher pressure was reduced to 45.8% of that of the rings exposed to a lower pressure. At this same time, there was also no discernable difference in PSR staining or medial hypertrophy, suggesting that tissue remodeling had not occurred. The relative lack of medial hypertrophy was also observed by Xu et al. (31). Though a higher collagen content (PSR) in PVAT above ligature was not observed in samples above the ligature from the coarcted rat, we recognize that PSR is but one measure of collagen and does not include different types of collagen, orientation of collagen, etc. It is also possible that pressure difference was not sustained for long enough and/or to a large enough magnitude to stimulate medial or PVAT remodeling.
A second measure is that adipocyte progenitor (AP) cells had a reduced adipogenic potential without changes in its proliferation capacity in PVAT samples above versus below the coarctation. These adipocyte progenitors are stem cells in the process of committing to an adipogenic fate, but still have the potency for fibroblastic, osteogenic, or chondrogenic lineages (2, 7). Differences in pressure induced by the coarctation may have decreased the number of adipocyte progenitor cells committed to become adipocytes. This finding aligns with results from cyclical stretch studies that used adipocyte progenitors from gonadal, inguinal, and bone marrow adipose sites reporting lower lipid accumulation compared with cells unexposed to mechanical stimuli (9, 14, 22, 32). In contrast, static or noncyclical stretch promotes lipid accumulation (13, 26). Thus, this raises the question of specific mechanisms of mechanotransduction carried out by PVAT where its adipocyte progenitor cells respond by altering its adipogenic potential in response to increased cyclical stretch, and this is of significant interest for future work.
The sham and coarcted unique DEG sets together reveal tentative pressure-related changes in PVAT that could potentially regulate smooth muscle tissue indirectly via synaptic functions. Coarctation reduced the differences of sham unique DEGs expression comparing above and below the ligatures (Fig. 5). Only four of the sham unique DEGs were enriched in cluster 1 (Fig. 6C, cluster 4). The other 24 sham unique DEGs are not significantly correlated with GO terms. The interactions among these 24 genes deserve careful study to reveal new biology of the PVAT and smooth muscle tissue interactions. Besides directly regulating smooth muscle tissue via transmembrane ion signals (Fig. 6C, clusters 1 and 2), coarctation may drive PVAT to initiate new mechanisms of regulation. The coarcted unique DEG set was the largest DEG set (395 genes), enriched with more than seventy percent (73%) of totally enriched GO terms, most (four out of six clusters) of which were related to synaptic functions.
Most coarcted unique DEGs were highly expressed in samples below the ligature (Figs. 5, D–F and Fig. 6C). Compared with the pattern in sham rats, a new physiological pattern of gene transcription emerged in the coarcted rat. Though not confirmed at the protein level, many of the genes discovered as elevated in the PVAT below the ligature in coarcted rats are involved in the process of synaptic neurotransmission (21) and have been found in presynaptic terminals (28). These genes were represented throughout the clusters and include, but are not limited to, dopamine-β-hydroxylase (dbh), neurotrophic receptor tyrosine kinase 1 (ntrk1), cholinergic receptor nicotinic alpha 3 subunit (chrna3), dopamine D2 receptor (drd2), tyrosine hydroxylase (th), acetylcholinesterase (ache), and synaptotagmin17 (syt17). We could not find reports about these genes or their translated protein function in PVAT though, for example, nonneuronal forms of synaptotagmin have been found in nonPVAT adipose tissue (10). What benefit would be achieved by upregulating expression of these particular suite of genes is unknown, especially given the sparse evidence that PVAT is innervated.
Limitations.
We acknowledge limitations to this shared work. First, we have pooled the ventral and two lateral strips of thoracic aortic PVAT for bulk mRNA sequencing experiments. These geographically distinct PVAT strips form from developmentally different origins (33), so any distinct differences between lateral versus ventral strips would be lost in our experiment as would any particular cellular difference. Second, the majority of adipocytes in the rat thoracic aorta are of the brown phenotype. Therefore, the adipogenic responses of their progenitors evaluated in this study may differ from those in the abdominal aorta and other large conduit vessels. Third, our sham animal is not a naïve animal in that it also underwent surgery and recovery. The argument could be made that a rat without any intervention should serve as another control. However, this was not necessary in this study because we needed to have the appropriate baseline measures to compare an animal in which pressure differences were experienced; this makes our sham ligature rat ideal. We fully appreciate that our discovery of DEGs between the thoracic aortic PVAT sections nearer the heart versus diaphragm raise the interesting and important questions of whether these differences would be observed in a naïve rat, and whether these differences have a functional consequence. Fourth, this study did not evaluate endothelial responses to pressure changes and how these are modified by anatomical location; therefore, the possibility of a mechanosensitive role of the vascular endothelium in the thoracic aorta remains to be elucidated. These questions are, however, beyond the intent of this study. Finally, the bulk mRNA sequencing was done at 4 wk after coarctation with a limited sample size. Although structural and blood pressure changes are stable, it could be that changes in the mRNA signature of PVAT would precede our measured changes, and thus our current experiment would have missed these differences.
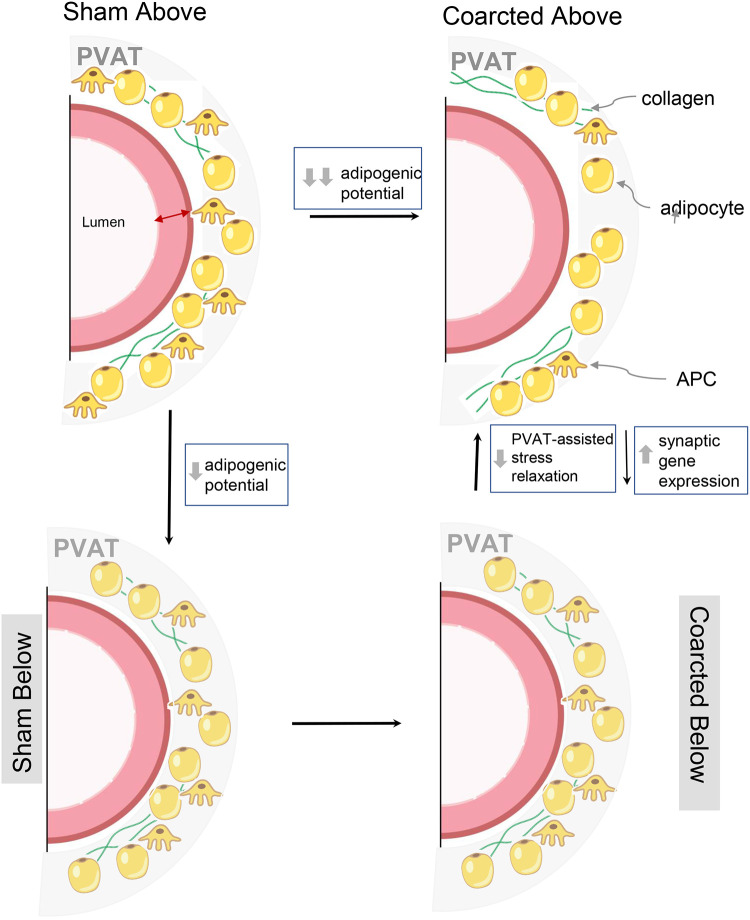
To conclude, Fig. 7 (graphical abstract) depicts a synthesis of the work done within this study. The coarcted animal allowed us to discover that differences in arterial pressure led to differences in aortic PVAT function (adipogenic potential and stress relaxation) and gene expression. These findings add to a growing consensus that (thoracic aortic) PVAT must be considered an essential, integral, and dynamic part of the vessel (11, 15, 29). The heretofore unsuspected mechanosensitivity of PVAT shown in this study provides a novel pathway for physiologically important regulatory crosstalk between PVAT and underlying vascular segments.
Fig. 7.
Rendering/synthesis of data presented within this manuscript. Grey shade, perivascular adipose tissue total area; APC, adipocyte progenitor cell.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant HL70687 (P01) and MSU Research and Innovation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A.C., Y.Y., H.G., G.D.F., and S.W.W. conceived and designed research; G.A.C., E.D.F., H.G., G.D.F., and S.W.W. performed experiments; G.A.C., Y.Y., E.D.F., H.G., G.D.F., and S.W.W. analyzed data; G.A.C., Y.Y., and S.W.W. interpreted results of experiments; G.A.C., Y.Y., E.D.F., and S.W.W. prepared figures; G.A.C. and S.W.W. drafted manuscript; G.A.C., Y.Y., E.D.F., H.G., S.B., G.D.F., and S.W.W. edited and revised manuscript; G.A.C., Y.Y., E.D.F., H.G., S.B., G.D.F., and S.W.W. approved final version of manuscript;
REFERENCES
- 1.Agabiti-Rosei C, Paini A, De Ciuceis C, Withers S, Greenstein A, Heagerty AM, Rizzoni D. Modulation of vascular reactivity by perivascular adipose tissue (PVAT). Curr Hypertens Rep 20: 44, 2018. doi: 10.1007/s11906-018-0835-5. [DOI] [PubMed] [Google Scholar]
- 2.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 53: 227–246, 2012. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CK, Bakar HA, Gollasch M, Huang Y. Perivascular adipose tissue: the sixth man of the cardiovascular system. Cardiovasc Drugs Ther 32: 481–502, 2018. doi: 10.1007/s10557-018-6820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contreras GA, Thelen K, Ayala-Lopez N, Watts SW. The distribution and adipogenic potential of perivascular adipose tissue adipocyte progenitors is dependent on sexual dimorphism and vessel location. Physiol Rep 4: e12993, 2016. doi: 10.14814/phy2.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: Network analysis and visualization of proteomics data. J Proteome Res 18: 623–632, 2019. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grobbel MR, Shavik SM, Darios E, Watts SW, Lee LC, Roccabianca S. Contribution of left ventricular residual stress by myocytes and collagen: existence of inter-constituent mechanical interaction. Biomech Model Mechanobiol 17: 985–999, 2018. doi: 10.1007/s10237-018-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 15: 230–239, 2012. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 9.Huang SC, Wu TC, Yu HC, Chen MR, Liu CM, Chiang WS, Lin KM. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol 11: 18, 2010. doi: 10.1186/1471-2121-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson AW, Birnbaum MJ. Identification of a nonneuronal isoform of synaptotagmin. Proc Natl Acad Sci USA 92: 5895–5899, 1995. doi: 10.1073/pnas.92.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Peruski B, Hunley C, Kwon S, Baek S. Influence of surrounding tissues on biomechanics of aortic wall. Int J Exp Comput Biomech 2: 105–117, 2013. doi: 10.1504/IJECB.2013.056516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucera M, Isserlin R, Arkhangorodsky A, Bader GD. AutoAnnotate: A cytoscape app for summarizing networks with semantic annotations. F1000 Res 5: 1717, 2016. doi: 10.12688/f1000research.9090.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy A, Enzer S, Shoham N, Zaretsky U, Gefen A. Large, but not small sustained tensile strains stimulate adipogenesis in culture. Ann Biomed Eng 40: 1052–1060, 2012. doi: 10.1007/s10439-011-0496-x. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y, Yu B. Mechanical stretch inhibits mesenchymal stem cell adipogenic differentiation through TGFβ1/Smad2 signaling. J Biomech 48: 3656–3671, 2015. doi: 10.1016/j.jbiomech.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Dang C, Garcia M, Gregersen H, Kassab GS. Surrounding tissues affect the passive mechanics of the vessel wall: theory and experiment. Am J Physiol Heart Circ Physiol 293: H3290–H3300, 2007. doi: 10.1152/ajpheart.00666.2007. [DOI] [PubMed] [Google Scholar]
- 16.Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 17.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449, 2005. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 18.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 5: e13984, 2010. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, Bader GD, Ferrin TE. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics 12: 436, 2011. doi: 10.1186/1471-2105-12-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oesper L, Merico D, Isserlin R, Bader GD. WordCloud: a Cytoscape plugin to create a visual semantic summary of networks. Source Code Biol Med 6: 7, 2011. doi: 10.1186/1751-0473-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padmanabhan P, Bademosi AT, Kasula R, Lauwers E, Verstreken P, Meunier FA. Need for speed: Super-resolving the dynamic nanoclustering of syntaxin-1 at exocytic fusion sites. Neuropharmacology 169: 107554, 2020. doi: 10.1016/j.neuropharm.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Paul NE, Denecke B, Kim BS, Dreser A, Bernhagen J, Pallua N. The effect of mechanical stress on the proliferation, adipogenic differentiation and gene expression of human adipose-derived stem cells. J Tissue Eng Regen Med 12: 276–284, 2018. doi: 10.1002/term.2411. [DOI] [PubMed] [Google Scholar]
- 23.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47, W1: W191–W198, 2019. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev 82: 518–529, 2015. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoham N, Gottlieb R, Sharabani-Yosef O, Zaretsky U, Benayahu D, Gefen A. Static mechanical stretching accelerates lipid production in 3T3-L1 adipocytes by activating the MEK signaling pathway. Am J Physiol Cell Physiol 302: C429–C441, 2012. doi: 10.1152/ajpcell.00167.2011. [DOI] [PubMed] [Google Scholar]
- 27.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 28.Südhof TC. The presynaptic active zone. Neuron 75: 11–25, 2012. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res 105: 906–911, 2009. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 30.Watts SW, Flood ED, Garver H, Fink GD, Roccabianca S. A new function for perivascular adipose tissue (PVAT): assistance of arterial stress relaxation. Sci Rep 10: 1807, 2020. doi: 10.1038/s41598-020-58368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Lee S, Singh TM, Sho E, Li X, Sho M, Masuda H, Zarins CK. Molecular mechanisms of aortic wall remodeling in response to hypertension. J Vasc Surg 33: 570–578, 2001. doi: 10.1067/mva.2001.112231. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Cai X, Wang J, Tang H, Yuan Q, Gong P, Lin Y. Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif 45: 158–166, 2012. doi: 10.1111/j.1365-2184.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Ruan CC, Fu M, Xu L, Chen D, Zhu M, Zhu D, Gao P. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell Mol Life Sci 76: 777–789, 2019. doi: 10.1007/s00018-018-2970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao S, Li CI, Guo Y, Sheng Q, Shyr Y. RnaSeqSampleSize: real data based sample size estimation for RNA sequencing. BMC Bioinformatics 19: 191, 2018. doi: 10.1186/s12859-018-2191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]