Abstract
Neural control of the heart is regulated by sympathetic and parasympathetic divisions of the autonomic nervous system, both opposing each other to maintain cardiac homeostasis via regulating heart rate, conduction velocity, force of contraction, and coronary blood flow. Sympathetic hyperactivity and diminished parasympathetic activity are the characteristic features of many cardiovascular disease states including hypertension, myocardial ischemia, and arrhythmias that result in heart failure. Restoring parasympathetic activity to the heart has recently been identified as the promising approach to treat such conditions. However, approaches that used vagal nerve stimulation have been shown to be unsuccessful in heart failure. This review focuses on novel chemogenetic approaches used to identify the cardioprotective nature of activating neural points along the vagal pathway (both central and peripheral) while being selectively therapeutic in heart failure and obstructive sleep apnea.
Keywords: chemogenetic, cholinergic, oxytocin, parasympathetic, vagus
INTRODUCTION
Neural control of the heart is regulated by sympathetic and parasympathetic divisions of the autonomic nervous system, both opposing each other to maintain cardiac homeostasis via regulating heart rate (HR), conduction velocity, force of contraction, and coronary blood flow (3). Sympathetic hyperactivity and diminished parasympathetic activity are the characteristic features of many cardiovascular disease states including hypertension, myocardial ischemia, and arrhythmias that result in heart failure (HF) (25). The current available treatment options are unfortunately insufficient for many patients and typically focus on reducing sympathetic activity (22). However, restoration of cardioprotective parasympathetic activity to the heart by vagal nerve stimulation has recently emerged as a promising new therapeutic approach to inhibit the progression of HF (13). Despite its cardioprotective nature in animal models of HF (13, 47), this treatment strategy had mixed results in humans (as reported in a recent clinical study NECTAR-HF). Limitations of this approach include nonselective activation of both sensory and motor vagal fibers, nonselective stimulation parameters, and ubiquitous activation of the entire vagus nerve (84). This review focuses on novel chemogenetic approaches that increase parasympathetic activity to the heart and their cardioprotective roles in cardiorespiratory diseases including HF and obstructive sleep apnea (OSA).
AUTONOMIC INNERVATION OF THE HEART
Parasympathetic division arising from the brainstem via the vagus nerve innervates the thoracic and abdominal organs including the autonomic cardiovascular, respiratory, gastrointestinal, immune, and endocrine organs. Parasympathetic vagal efferent fibers arising from preganglionic cardiac vagal neurons (CVNs) of the nucleus ambiguus and dorsal motor nucleus of the vagus (DMNX) of the brainstem project and synapse with the postganglionic intrinsic cardiac ganglionic (ICG) neurons located on the epicardial surface of the heart. The sympathetic preganglionic neurons involved in cardiac regulation lie in the intermediolateral cell column of gray matter in the upper thoracic (T1–T4) segments of the spinal cord and project their axons to sympathetic postganglionic neurons of intrathoracic extracardiac (middle and superior cervical ganglia, stellate, and mediastinal) ganglia and ICG neurons (3; Fig. 1).
Fig. 1.
Schematic showing the autonomic pathways from the brain to the heart. Parasympathetic cardiac vagal neurons (CVNs) of the brainstem receive synaptic projections from nucleus tractus solitarius (NTS) and paraventricular nucleus (PVN) of the hypothalamus. The intrinsic cardiac ganglia receive parasympathetic efferent inputs from the vagus nerve arising from the premotor CVNs of nucleus ambiguus and dorsal motor nucleus of the vagus (NA and DMNX) regions of brainstem. They also receive efferent inputs from the sympathetic postganglionic neurons located paravertebrally (T1–T4). Sensory afferent terminals arising from the heart have their neurons located in two regions; nodose ganglia and dorsal root ganglia. Afferent nerves related to nodose ganglia travel along with the vagus to the NTS of the brainstem. Whereas, afferents from dorsal root ganglia travel with sympathetic nerves to the spinal cord. ICG, postganglionic intracardiac ganglia; RVLM, presympathetic neurons of rostroventrolateral medulla; IML, postganglionic sympathetic neurons of spinal intermediolateral column.
ICG neurons, located within the fat pads of the heart, are the site of final parasympathetic pathway, sending axonal projections to discrete regions of the heart (3). ICG neurons form complex neural networks to regulate regional cardiac functions including coronary blood flow and myocardial perfusion (4). Ganglia located at the junction of the right superior vena cava and right atrium control sinoatrial node function, while those at the junction of the inferior vena cava and left atrium control atrio-ventricular node function (70). The evidence that mechanical (29) and chemical stimuli (73) and vagosympathetic nerve stimulation (28) alter the activity of ICG neurons suggests that ICG neurons receive inputs from sympathetic and parasympathetic efferent axons, as well as from sensory neurites of the ventricles (6). The intrinsic cardiac nervous system, therefore, controls cardiac function on a beat-to-beat basis, in the absence of input from the central nervous system (4).
Both parasympathetic and sympathetic nervous systems innervate sinoatrial node and antagonistically regulate HR (65). Activation of the sympathetic system is responsible for positive or increased chronotropism (rate of heartbeat), dromotropism (speed of conduction), and inotropism (force of contraction). In contrast, activation of the parasympathetic system causes negative inotropic, chronotropic, and dromotropic responses (4). The parasympathetic nervous system acts directly on the heart (sinoatrial node) to reduce the heart rate via inhibitory G-protein-coupled mechanisms (23) or indirectly by interacting with the sympathetic nervous system (58). At the cardiac neuroeffector junction, acetylcholine released from parasympathetic nerve terminals inhibits the release of noradrenaline (NE) by binding to the presynaptic muscarinic (M2) receptors on sympathetic terminals (76).
The normal resting HR (typically 60–80 beats/min) is determined by the dominance of parasympathetic activity over sympathetic activity (53). Recent optogenetic studies using transgenic mice demonstrate that photoactivation of parasympathetic right atrial intracardiac neurons results in cholinergic-mediated reduction in HR (55). Whereas, photostimulation of sympathetic fibers facilitates increases in HR and makes hearts more susceptible to arrhythmias (81).
NEUROPHYSIOLOGY OF PARASYMPATHETIC PREMOTOR CARDIAC VAGAL NEURONS
Premotor cardiac vagal neurons (CVNs) are intrinsically silent, and hence, synaptic activation of these neurons is crucial in maintaining tonic ongoing parasympathetic activity to the heart, which dominates the autonomic balance and control of the HR (52). CVNs receive three major synaptic pathways, which are glutamatergic, GABAergic, and glycinergic in nature (77). CVNs are inhibited by inhibitory neurotransmission arising from two sources: inspiratory neurons of the brainstem and locus coeruleus. GABAergic neurotransmission to CVNs evoked by the activation of GABA neurons located close to nucleus tractus solitarius (NTS) is signatory of respiratory sinus arrhythmia (27, 77). Whereas, locus coeruleus noradrenergic neuron activation induces depression of CVN activity via polysynaptic activation of inhibitory neurotransmission and is critical in orchestrating autonomic nerve function to compensate increased alertness (80). One of the major monosynaptic glutamatergic synaptic pathways to CVNs originates from NTS that is essential in maintaining baroreceptor reflex (56). According to recent optogenetic and electrophysiological studies, a second powerful excitatory glutamatergic synaptic pathway to CVNs arises from oxytocin neurons of paraventricular nucleus of the hypothalamus (PVN) that coreleases oxytocin (OXT) (61).
Paraventricular nucleus, a heterogeneous nucleus of the hypothalamus, controls autonomic cardiovascular functions in normal and hypoxic stress conditions, via vasopressin and OXT neuronal activity. Recent work has shown that augmented vasopressin neuronal activity is involved in the generation of chronic intermittent hypoxia (CIH)-induced hypertension. While vasopressin neurons are sympathoexcitatory and their activation inhibits CVNs (78), OXT released from oxytocin neurons activates CVNs and is cardioprotective in nature. In addition to its classic effects on uterine contractions and milk ejection, OXT has been shown to buffer the effects of anxiety and stress on behavior and cardiovascular homeostasis. In unstressed human volunteers, intranasal administration of OXT restores autonomic balance by increasing parasympathetic and decreasing sympathetic cardiac control (57). OXT reduces stress-induced behavioral responses and anxiety (72) while preventing increases in HR, reductions in heart rate variability, and diminishment of vagal control of the heart induced by social isolation (32). Intracerebroventricular administration of OXT reduces the cardiac injury that occurs following episodes of ischemic reperfusion (54). OXT produces antihypertensive effects in spontaneously hypertensive rats and when administered in postnatal rats results in long-lasting reductions in blood pressure (BP) (38, 60).
CHEMOGENETIC APPROACH TO SELECTIVELY STIMULATE SYNAPTIC RELEASE FROM NEURONS
Chemogenetic approach utilizes designer receptors exclusively activated by designer drugs (DREADDs), engineered G-protein-coupled human muscarinic receptors that are selectively activated by its selective ligands (67). Excitatory and inhibitory DREADDs have been used to reversibly activate or inhibit, respectively, targeted neurons with selectivity often conferred by using the Cre recombination system. Excitatory DREADDs (hM3Dq) when activated by its ligand cause an increase in intracellular calcium levels via activation of phospholipase C, and ultimately result in burst firing of neurons (2). Activation of inhibitory DREADDs (hM4Di) inhibits neuronal activity via inhibition of adenylyl cyclase GPCR mechanism. DREADD expression is achieved in targeted areas in two ways, by injection of viral constructs carrying DREADD transgene a) into the tissue of interest using a promoter, or b) into transgenic animals expressing Cre recombinase in the tissue of interest (21). Both approaches use a double-floxed inverted open reading frame system that incorporates the DREADD gene in an antisense fashion, which is flipped into its functional orientation following the cleavage of Lox P sites for sense translation in tissue expressing the enzyme Cre recombinase.
Various ligands selective for DREADDs include clozapine N-oxide (CNO), Compound 21, JHU37152, and JHU37160. CNO itself does not cross the blood-brain barrier, but its metabolite clozapine penetrates the brain. Although CNO was shown to have a short plasma half-life (15 min to 2 h following intraperitoneal injection) (33), the biological effects induced by acute treatment of DREADDs expressing experimental animals ranged from 6 to 10 h (1, 33). A recently developed second-generation DREADD ligand 21 penetrates brain without converting to clozapine but exhibits lower in vivo potency than clozapine (43, 74). Other recently developed ligands including JHU37152 and JHU37160 exhibit high in vivo DREADD occupancy when used in lower concentrations in rodents and primates (9).
The use of Gq activating DREADDs is a widely adopted technology to selectively activate autonomic neurons involved in systems that regulate feeding, sleep-wake cycles, energy homeostasis, and other behaviors (46, 71). Chemogenetic activation of NTS neuronal population involved in appetite regulation (85) and astrocytes in dorsal vagal complex (49) causes decreases in food intake. Chemogenetic activation of hindbrain GABAergic NTS neurons increases systemic blood glucose concentrations and inhibits vagal motor activity in vivo (10). Likewise, hM3Dq DREADDs-mediated activation of noradrenergic locus coeruleus neurons restores memory function in Down syndrome-associated Alzheimer’s (26), and activation of dorsomedial hypothalamic leptin neurons promotes energy expenditure via promoting brown adipose tissue thermogenesis (66).
The Cre-Lox recombination system is used to achieve robust and highly selective expression of DREADDs in PVN OXT neurons. Expression of the enzyme Cre recombinase exclusively driven by the OXT promoter (AAV1-OXT-Cre) when coinjected with floxed excitatory DREADDs [AAV2-hSyn-DIOhM3D(Gq)-mcherry] elicits expression of DREADDs within PVN OXT neurons (61, 62; Fig. 2). Previous immunohistochemical work on hypothalamic slices demonstrates selective (83%) and robust expression of DREADDs in PVN OXT neurons (42).The excitatory pathway arising from PVN OXT neurons activates CVNs, reduces HR, and increases parasympathetic activity to the heart. Prior studies in animals using electrical stimulation demonstrate that activation of PVN facilitates reflex bradycardia that is suppressed by application of OXT receptor antagonist into the DMNX containing CVNs (68). The responses on activation of DREADDs in PVN OXT neurons were previously assessed both in vivo and in vitro. In vivo, acute DREADDs activation in telemetry-implanted conscious unrestrained rats reduces both HR and BP, mainly via increases in parasympathetic activity (30). In support of these findings, in vitro activation of DREADDs by exogenously applied CNO increases the firing activity of DREADDs expressing PVN OXT neurons that was long lasting (42). Likewise, optogenetic stimulation of PVN induces OXT receptor activation and mediates paired pulse facilitation in CVNs. High-frequency stimulation of PVN OXT fibers projecting to CVNs evokes long-lasting facilitation of glutamatergic neurotransmission in this pathway (62). Furthermore, optogenetic stimulation of this long-range PVN pathway activates oxytocin receptors on Chinese hamster ovarian (CHO) sniffer cells (expressing a genetically coded calcium indicator, R-GECO, and oxytocin receptors) that are sensitive to OXT release from PVN terminals within the DMNX (62; Fig. 2).
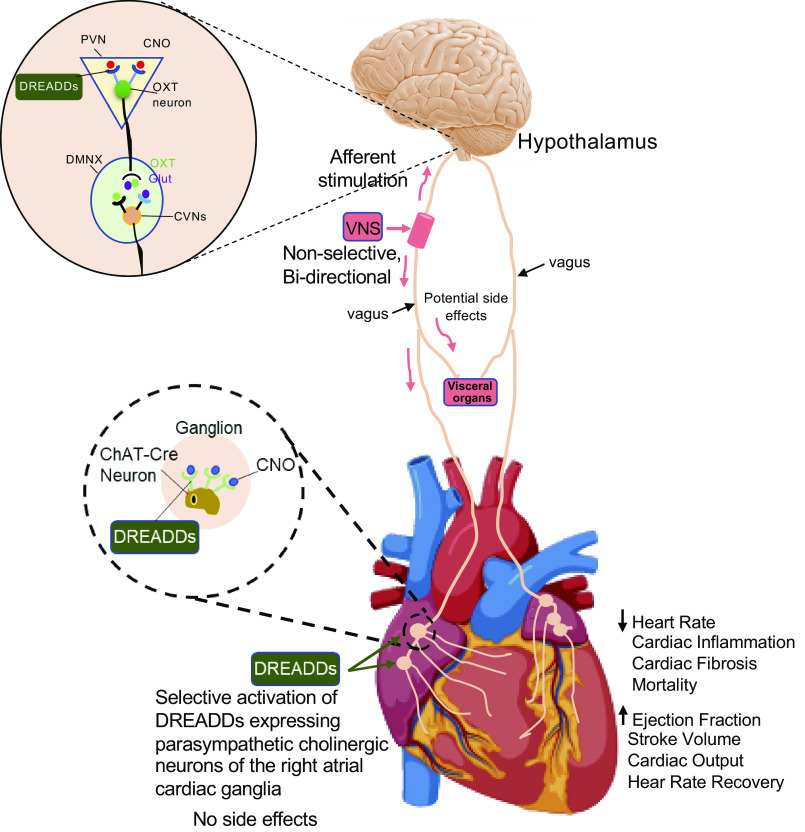
Fig. 2.
Schematic displaying the sites of intervention and chemogenetic approach to activate parasympathetic vagal pathway to the heart. Selective expression of designer receptors exclusively activated by designer drugs (DREADDs) within paraventricular nucleus oxytocin (PVN OXT) neurons or cardiac cholinergic (ChAT) neurons of the cardiac ganglia provides for cell-specific and chronic activation of those neurons by the designer drug, clozapine N-oxide (CNO). Chemogenetic activation of PVN OXT/ICG ChAT Cre neurons decreases heart rate, cardiac inflammation and fibrosis, mortality and increases cardiac functions i.e., ejection fraction, stroke volume, cardiac output, and heart rate recovery following peak effort capacity. This approach circumvents the off-target effects of electrical vagal nerve stimulation (VNS) and is a novel target of opportunity for potent and selective treatments that increase parasympathetic tone to the heart, with its many cardioprotective benefits.
Chemogenetic stimulation of ICG cholinergic neurons is achieved by using transgenic rats that only express Cre-recombinase in choline acetyl transferase (ChAT) neurons (Fig. 2). In combination with a viral microinjection of floxed DREADDs into the ICG neurons in these ChAT-Cre rats, ICG cholinergic parasympathetic activity to the heart is selectively increased in an animal model of chronic HF (18). Immunohistochemical studies in animals receiving pericardial DREADD injections in right atrial fat pads have shown coexpression of excitatory DREADDs in majority of Cre-expressing ChAT neurons, suggesting localization of DREADDs to cholinergic neurons of the intrinsic cardiac ganglia. Acute activation of ChAT Cre neurons expressing DREADDs by its ligand CNO results in robust reductions in BP (∼20 mmHg) and HR (∼100 bpm), suggesting a negative chronotropic effect of activation of parasympathetic ICG neurons (18).
OBSTRUCTIVE SLEEP APNEA
Obstructive sleep apnea (OSA) is a cardiorespiratory disease that affects ∼25%–30% of adult population in the Western world (64). Patients with OSA suffer from intermittent periods of hypoxia and hypercapnia during sleep that is accompanied by arterial oxygen desaturations and increases in arterial carbon dioxide levels due to repetitive collapses of the upper airway. OSA is an independent risk factor for the initiation and progression of neurological and cardiac disease resulting in the development of hypertension, arrhythmias, myocardial ischemia, stroke, and sudden death (11). Patients with OSA have increases in blood pressure, low heart rate variability, and reduced baroreflex sensitivity with chronic impairment in cardiac autonomic function, i.e., sympathetic hyperactivity and reduced parasympathetic activity. Chronic positive air pressure (CPAP) is the only therapy available to treat OSA that has been shown to be marginally effective in decreasing the increases in blood pressure by ∼2 mmHg (5). However, this device-based treatment suffers from disadvantages of being intrusive, poorly tolerated, and often discontinued. Other pharmacological and non-device-based approaches in OSA have been unsuccessful.
Parasympathetic dysfunction in OSA.
Animals exposed to chronic intermittent hypoxia-hypercapnia (CIHH) have been a widely accepted model to simulate OSA in humans (14, 45). Decreased baroreflex control of heart rate is an important characteristic feature of OSA. CIH decreases central mediation of baroreflex bradycardia while augmenting the baroreceptor afferent function and the HR responses to electrical stimulation of the vagus nerve (48). CIH also induces a reduction in the number of glutamatergic immunoreactive neurons and NMDA receptor expression in the nucleus ambiguus that contributes to reduced HR responses to glutamatergic receptor activation (82, 83). These studies suggest that reduction in baroreflex control of HR with CIH occurs due to an impairment within the brainstem but not in sensory or efferent premotor fibers.
Of the few studies that identified mechanisms responsible for reduced parasympathetic activity, a recent study identified the changes in blood pressure, heart rate and synaptic neurotransmission to CVNs evoked by acute hypoxia-hypercapnia (H-H) and CIHH (19). In vivo studies in telemetry instrumented rats exposed to CIHH for 4 wk show increases in systolic and diastolic pressures to hypertensive levels and blunt heart rate responses to acute H-H. CIHH also blunts bradycardia evoked by acute H-H and abolishes acute H-H-evoked inhibition of GABAergic transmission while enhancing glycinergic transmission to CVNs in the nucleus ambiguus. According to in vitro whole cell patch-clamp studies, CIHH inhibits the activity of CVNs by reducing spontaneous GABA and glycinergic inhibitory synaptic neurotransmission while increasing glutamatergic excitatory synaptic input to CVNs in the DMNX and nucleus ambiguus (NA) (19).
CIHH inhibits the excitatory glutamatergic postsynaptic currents and paired pulse facilitation in CVNs evoked by optogenetic stimulation of PVN fibers, suggesting impaired presynaptic transmitter release mechanisms from PVN synaptic terminals due to CIHH (17). Under post-acute H-H conditions, an increase in the amplitude of glutamatergic postsynaptic currents in CVNs evoked by stimulation of PVN fibers is enhanced to activate CVNs and provide a cardioprotective role in recovery. However, in animals exposed to CIHH, inhibition of glutamatergic currents in CVNs occurs during acute H-H without any changes post-acute H-H (17). CIHH also inhibits the release of oxytocin in response to the photoactivation of PVN channel rhodopsin (ChR2) fibers projecting to CVNs (42). All these changes in synaptic neurotransmission will likely inhibit CVNs to diminish vagal outflow to the heart and depress baroreflex control of the heart rate, hallmarks of OSA.
PVN oxytocin neuron activation in OSA.
Activation of PVN oxytocin network to CVNs in both animals and humans has been shown to be cardioprotective in OSA (40, 42). Chemogenetic activation of PVN oxytocin neurons in an animal model of sleep apnea restores diminished oxytocin release from PVN synaptic terminals in CVNs and prevents hypertension evoked by CIHH (42). Other studies have reported elevated oxytocin concentrations in cerebrospinal fluid in response to oxytocin administration in rats (59), suggesting that the long-lasting decreases in BP due to oxytocin may be mediated by the central nervous system. These suggestions are further corroborated by the findings from chemogenetic PVN oxytocin neuron stimulation that causes oxytocin release to activate CVNs and reduce BP and HR (30).
In line with the animal studies, intranasal oxytocin treatment in patients with OSA restores cardiorespiratory homeostasis and reduces clinically relevant adverse events associated with OSA. Nocturnal administration of intranasal oxytocin (40 IU) in patients with OSA improves parasympathetic indices, i.e., increases heart rate variability, total sleep time, and post-polysomnogram sleep assessment score (an index of sleep satisfaction). Additionally, a reduction in the frequency of hypopneas (partial airway occlusion), duration of both apneas (total airway obstruction) and hypopneas, and percentage of apnea hypopnea events accompanied with an arousal are also evident upon oxytocin treatment in patients with OSA (40). Intranasal oxytocin administration in patients with OSA also decreases the duration of obstructive events, as well as the oxygen desaturations and incidence of bradycardia that are associated with these events while increasing respiratory rate during nonobstructive periods. Oxytocin administration can benefit patients with obstructive sleep apnea by reducing the duration and adverse consequences of obstructive events (41).
HEART FAILURE
Parasympathetic dysfunction and beneficial effects of PVN oxytocin neuron activation in HF.
Heart failure (HF) is a widespread and devastating cardiovascular disease affecting nearly 23 million people worldwide and contributing to 270,000 deaths a year in the United States (7). In the initial stages of HF, compensatory responses to improve cardiac output, including neurohormonal activation, are beneficial to maintain cardiovascular homeostasis. However, a chronic shift in autonomic balance results in hemodynamic stress and deleterious effects on the heart (44). Impaired parasympathetic control of the heart is a characteristic of HF that is a powerful independent negative prognostic predictor of arrhythmia and disease progression (63). An extensive range of studies exist in literature that focus on studying sympathetic activity in HF. In fact, the current therapies available target sympathetic activity in HF including adrenergic beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and antiarrhythmics (24). However, restoring parasympathetic activity to the heart has recently emerged as a promising target to inhibit sympathetic activity and disease progression in HF (13). Vagal nerve stimulation, a recent device-based therapy implemented in patients with HF to increase parasympathetic activity, was unsuccessful due to its nonselective nature of activating both vagal afferents and efferents (84). Several other forms of neural modulation therapies to restore autonomic balance in HF that are under investigation include electrical activation of carotid baroreceptor reflex, device-based inhibition of carotid chemoreceptors, and destruction of renal afferent nerves (24).
Altered brainstem and hypothalamic synaptic neurotransmission to CVNs contributes to parasympathetic dysfunction in HF with systolic dysfunction. Recent findings from an animal model of chronic HF show that pressure overload-induced left ventricular hypertrophy due to transverse aortic constriction (TAC) inhibits the excitation of premotor CVNs of the brainstem by reducing spontaneous glutamatergic neurotransmission and enhancing spontaneous inhibitory GABAergic neurotransmission to CVNs (15). Consistent with these findings, chronic HF also inhibits corresponding oxytocin release from PVN glutamatergic pathway to CVNs that is restored by the chronic chemogenetic activation of PVN oxytocin neurons (20).
Selective activation of excitatory DREADDs in PVN OXT neurons improves cardiac anatomy and function in HF animals (20, 30; Fig. 2). Although increases in echocardiographic Doppler aortic flow velocities (measured immediately next to constriction) were evident early from 2-wk post-TAC, reductions in cardiac functions including fractional shortening, ejection fraction, stroke volume, and cardiac output only occurred later from 6-wk post-TAC animals. However, interestingly, long-term activation of PVN oxytocin neurons mitigates the deleterious effects of TAC on cardiac function for 12 wk. Similarly, chronic chemogenetic activation of PVN oxytocin neurons also mitigates chronic HF-evoked reductions in heart rate recovery after peak exercise effort, a clinical index of cardiac autonomic tone. These beneficial effects of PVN oxytocin neuron activation on cardiac function also extend to significantly reduce the mortality in diseased animals (20). HF animals treated with chronic activation of PVN OXT neurons show blunted increases in heart weight, left ventricular (LV) wall thickness, and LV hypertrophy (30). In addition, chronic PVN OXT neuron activation also mitigates detrimental changes in cardiac function (assessed ex vivo) induced by HF, i.e., depressed LV developed pressure (LVDP), reduced coronary flow rate, and blunted velocity of both cardiac contraction and relaxation (20, 30).
Beneficial effects of ICG ChAT neuron activation in HF.
ICG system dysfunction has been implicated in arrhythmias, myocardial ischemia, and HF (36).
An attenuation in neurotransmission within ICG neurons (8) and altered membrane properties of ICG neurons (75) contributes to withdrawal of parasympathetic activity to the heart in HF. Low-intensity electrical stimulation of the right atrial ganglionic plexus protects against ventricular arrhythmogenesis in canines that have an acute myocardial infarction (35, 79). Electrical stimulation of the ICG neurons is nonselective and would stimulate both parasympathetic and many other populations of neurons including noncholinergic nerve inputs releasing nitric oxide, substance P, vasointestinal peptide, and sympathetic, sensory neurons that often oppose parasympathetic activation (39).
Chemogenetic activation of ChAT ICG neurons prevents cardiac dysfunctions due to chronic HF (18). Longitudinal echocardiographic studies in untreated TAC rats reveal LV hypertrophy and significant reductions in cardiac fractional shortening, ejection fraction, stroke volume, and cardiac output that were blunted by selective activation of ICG ChAT neurons using excitatory DREADDs (hM3Dq) for 3 wk. Chemogenetic activation of ICG ChAT neurons also improves both low heart rate recovery following peak effort capacity and survival rates seen in untreated TAC animals. Such measures of low heart rate recovery in TAC animals is most likely indicative of withdrawal of parasympathetic tone, as the maximum HR due to peak exercise remains unchanged among all the animal groups studied. Furthermore, lack of effect of ICG ChAT neuron activation on TAC-induced increases in PR interval (suggestive of atrioventricular node block) suggests that mechanisms involving changes in conduction through atrio-ventricular node are unlikely to contribute to the cardioprotective action of ICG ChAT neuron activation (18).
Mechanisms underlying cardioprotective actions of PVN oxytocin or ICG neuron activation in HF.
The cardioprotective actions of PVN OXT neuron activation and intranasal OXT are parasympathetic mediated. PVN OXT neuron activation-induced reductions in BP and HR were significantly blocked by muscarinic cholinergic receptor antagonist, atropine, suggesting parasympathetic activation (30). Intranasal oxytocin increases parasympathetic activity by an increase in the high-frequency component of heart rate variability (HRV) and short-term variability in Poincare plots of R-R interval. Frequency domain and nonlinear HRV analyses are used as an indirect measure of cardiac autonomic balance. Total HRV power was also significantly increased with oxytocin treatment, indicating increases in parasympathetic activity to the heart (42).
PVN OXT neuron activation improves cardiac functions in HF via anti-inflammatory and antifibrotic mechanisms. The vagus nerve via “the cholinergic anti-inflammatory pathway” inhibits the release of cytokines and attenuates systemic inflammatory responses. HF is associated with increased myocardial expression of proinflammatory mediators that results in increased morbidity and mortality. PVN oxytocin neuron activation also improves systolic cardiac function in TAC-induced HF by reducing the protein expression of inflammatory marker interleukin-1β (IL-1β) in the left ventricle (20, 30). The other mechanisms include attenuation of the expression of myocardial collagen III and fibrosis that are elevated in HF animals (30). Moreover, OXT mediates cardioprotective effect not by acting peripherally but by increasing parasympathetic activity to the heart, as plasma oxytocin levels remained unaltered between untreated and treated HF groups (30).
Other mechanisms underlying the benefits of PVN OXT/ICG ChAT neuron activation may include preservation of connexin 43, restoration of the expression of nerve growth factor (NGF) within ICG neurons, or increased neuropeptide release-induced vasodilation. Atrial ganglionated plexus stimulation has been shown to protect against ischemia reperfusion-induced ventricular arrhythmias via preserving connexin 43, a gap junction component critical in maintaining mechanical and electrical stability of cardiomyocytes (12, 79). Chronic HF is associated with reduced expression of nerve growth factor within ICG neurons that contributes to reduced vagal-sympatho neural connections and attenuated parasympathetic inhibition of sympathetic function (34). Restoration of parasympathetic cardiac neuronal activity in HF may blunt such detrimental loss of vagal-sympathetic nerve connections and promote inhibition of sympathetic activity. Neuropeptides including nitric oxide (NO) and vasointestinal peptide (VIP) are potent vasodilators that are co-released with acetylcholine from parasympathetic nerve fibers and control cardiac function. Previous studies in dogs with coronary microembolization-induced HF reported downregulation of the mRNA and protein expression of endothelial nitric oxide synthase (NOS) and an upregulation of neuronal NOS in LV myocardium. However, electrical vagal nerve stimulation therapy normalizes the expression of all forms of NOS (69). Similar to NO, VIP has also been found to be cardioprotective during acute experimental myocardial ischemia (16, 37). Clearly, future studies are warranted to identify any or all these mechanisms to determine cardioprotective role of activating PVN OXT neurons and ICG ChAT neurons in HF.
CONCLUSIONS, LIMITATIONS AND FUTURE DIRECTIONS
The use of DREADD technology has proven advantageous in probing neural circuit function by modulating neural activity transiently and repeatedly over days with spatial and phenotypic selectivity and producing naturalistic effects on brain activity >due to its noninvasiveness. However, its synthetic ligand CNO is not pharmacologically inert and warrants certain limitations. CNO, when used at concentrations required to activate DREADDs, can bind to non-DREADD receptors, undergoes reverse metabolism to clozapine that can the cross blood-brain barrier and produce numerous physiological and behavioral effects (31, 50, 51). Also, distribution of CNO and its metabolites is not equivalent between the circulatory system and the brain (31). This emphasizes the need for including proper controls in studies using DREADDs to control off-target effects of CNO.
Our studies suggest that parasympathetic nervous activation plays a defensive role in the pathogenesis of heart failure and OSA, and increases in parasympathetic activity to the heart could be an effective clinical target in treating heart diseases. In fact, the current clinical trials using intranasal oxytocin that can activate PVN oxytocin neurons and receptors within the brain are in progress and have been shown to reduce detrimental cardiac outcomes in a small population study suffering from OSA (ClinicalTrials.gov NCT03148899). It is clear from the animal studies that there is depressed OXT release in both HF and OSA, and hence, replacing OXT to normal levels is less likely to cause unwanted side effects. Vagal nerve stimulation that nonselectively stimulates parasympathetic pathways innervating organs other than heart is more likely to induce side effects. Whereas, intranasal oxytocin administration benefits from being noninvasive and selective bidirectional vagal efferent activation and has a potential for fewer side effects than other interventions. Likewise, stimulation of more downstream and selective peripheral parasympathetic cholinergic efferent neural networks can be powerful and beneficial in the settings of cardiorespiratory diseases (Fig. 2).
Neuromodulation-based approaches that target select points of neural hierarchy for cardiac control offer unique opportunities to positively affect therapeutic outcomes via improved efficacy of cardiac reflex control. Certainly, future studies investigating the mechanisms underlying the parasympathetic activation due to chemogenetic activation of OXT/ICG neurons and its benefits on cardiovascular system are necessary. Also, the studies in this review were limited to pressure overload-induced HF and HF with reduced ejection fraction. Future studies investigating the cardioprotective benefits of PVN OXT/ICG ChAT neuron activation in HF with preserved ejection fraction and acute coronary syndrome are needed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-137279.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
J.D. drafted, edited and revised; and approved final version of manuscript.
REFERENCES
- 1.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, Mcnamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39, 2009. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168, 2007. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 4.Armour JA. Physiology of the intrinsic cardiac nervous system. Heart Rhythm 8: 739, 2011. doi: 10.1016/j.hrthm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 50: 417–423, 2007. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 6.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ. et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 8.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99: 2958–2963, 1999. doi: 10.1161/01.CIR.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 9.Bonaventura J, Eldridge MAG, Hu F, Gomez JL, Sanchez-Soto M, Abramyan AM, Lam S, Boehm MA, Ruiz C, Farrell MR, Moreno A, Galal Faress IM, Andersen N, Lin JY, Moaddel R, Morris PJ, Shi L, Sibley DR, Mahler SV, Nabavi S, Pomper MG, Bonci A, Horti AG, Richmond BJ, Michaelides M. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat Commun 10: 4627, 2019. doi: 10.1038/s41467-019-12236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boychuk CR, Smith KC, Peterson LE, Boychuk JA, Butler CR, Derera ID, Mccarthy JJ, Smith BN. A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation. Sci Rep 9: 2722, 2019. doi: 10.1038/s41598-019-39490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373: 82–93, 2009. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 12.Brandenburger T, Huhn R, Galas A, Pannen BH, Keitel V, Barthel F, Bauer I, Heinen A. Remote ischemic preconditioning preserves Connexin 43 phosphorylation in the rat heart in vivo. J Transl Med 12: 228, 2014. doi: 10.1186/s12967-014-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley U, Shivkumar K, Ardell JL. Autonomic regulation therapy in heart failure. Curr Heart Fail Rep 12: 284–293, 2015. doi: 10.1007/s11897-015-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campen MJ, Shimoda LA, O’donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 15.Cauley E, Wang X, Dyavanapalli J, Sun K, Garrott K, Kuzmiak-Glancy S, Kay MW, Mendelowitz D. Neurotransmission to parasympathetic cardiac vagal neurons in the brain stem is altered with left ventricular hypertrophy-induced heart failure. Am J Physiol Heart Circ Physiol 309: H1281–H1287, 2015. doi: 10.1152/ajpheart.00445.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das DK, Kalfin R, Maulik N, Engelman RM. Coordinated role of vasoactive intestinal peptide and nitric oxide in cardioprotection. Ann N Y Acad Sci 865: 297–308, 1998. doi: 10.1111/j.1749-6632.1998.tb11190.x. [DOI] [PubMed] [Google Scholar]
- 17.Dergacheva O, Dyavanapalli J, Pinol RA, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension 64: 597–603, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyavanapalli J, Hora AJ, Escobar JB, Schloen JR, Dwyer MKR, Rodriguez J, Spurney CF, Kay MW, Mendelowitz D. Chemogenetic activation of intracardiac cholinergic neurons improves cardiac function in pressure overload induced heart failure. Am J Physiol Heart Circ Physiol 319: H3–H12, 2020. doi: 10.1152/ajpheart.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol 592: 2799–2811, 2014. doi: 10.1113/jphysiol.2014.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyavanapalli J, Rodriguez J, Rocha Dos Santos C, Escobar JB, Dwyer MK, Schloen J, Lee KM, Wolaver W, Wang X, Dergacheva O, Michelini LC, Schunke KJ, Spurney CF, Kay MW, Mendelowitz D. Activation of oxytocin neurons improves cardiac function in a pressure-overload model of heart failure. JACC Basic Transl Sci 5: 484–497, 2020. doi: 10.1016/j.jacbts.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, Lee HM, Sciaky N, Simmons A, Nonneman RJ, Huang XP, Hufeisen SJ, Guettier JM, Moy SS, Wess J, Caron MG, Calakos N, Roth BL. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38: 854–862, 2013. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felder RB, Yu Y, Zhang ZH, Wei SG. Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther 86: 216–220, 2009. doi: 10.1038/clpt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming JW, Wisler PL, Watanabe AM. Signal transduction by G proteins in cardiac tissues. Circulation 85: 420–433, 1992. doi: 10.1161/01.CIR.85.2.420. [DOI] [PubMed] [Google Scholar]
- 24.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36: 1974–1982, 2015. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 26.Fortress AM, Hamlett ED, Vazey EM, Aston-Jones G, Cass WA, Boger HA, Granholm AC. Designer receptors enhance memory in a mouse model of Down syndrome. J Neurosci 35: 1343–1353, 2015. doi: 10.1523/JNEUROSCI.2658-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol 101: 1755–1760, 2009. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. Am J Physiol 255: H789–H800, 1988. doi: 10.1152/ajpheart.1988.255.4.H789. [DOI] [PubMed] [Google Scholar]
- 29.Galoyan A, Srapionian R, Arora RC, Armour JA. Responsiveness of intrinsic cardiac neurons to left atrial and hypothalamic cardioactive peptides. Auton Neurosci 92: 11–20, 2001. doi: 10.1016/S1566-0702(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 30.Garrott K, Dyavanapalli J, Cauley E, Dwyer MK, Kuzmiak-Glancy S, Wang X, Mendelowitz D, Kay MW. Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure. Cardiovasc Res 113: 1318–1328, 2017. doi: 10.1093/cvr/cvx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507, 2017. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grippo AJ, Trahanas DM, Zimmerman RR II, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34: 1542–1553, 2009. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guettier JM, Gautam D, Scarselli M, Ruiz De Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA 106: 19197–19202, 2009. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan W, Smith PG. Decreased adrenoceptor stimulation in heart failure rats reduces NGF expression by cardiac parasympathetic neurons. Auton Neurosci 181: 13–20, 2014. doi: 10.1016/j.autneu.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He B, Lu Z, He W, Wu L, Huang B, Yu L, Cui B, Hu X, Jiang H. Effects of low-intensity atrial ganglionated plexi stimulation on ventricular electrophysiology and arrhythmogenesis. Auton Neurosci 174: 54–60, 2013. doi: 10.1016/j.autneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 36.He B, Lu Z, Jiang H. Atrial ganglionated plexi stimulation may be an effective therapeutic tool for the treatment of heart failure. Med Hypotheses 81: 905–907, 2013. doi: 10.1016/j.mehy.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res 49: 27–37, 2001. doi: 10.1016/S0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 38.Holst S, Uvnas-Moberg K, Petersson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Auton Neurosci 99: 85–90, 2002. doi: 10.1016/S1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 39.Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, Marbach J, Kimbro S, Andrade DC, Jain A, Capozzi E, Mele K, Del Rio R, Kay MW, Mendelowitz D. Benefits of oxytocin administration in obstructive sleep apnea. Am J Physiol Lung Cell Mol Physiol 313: L825–L833, 2017. doi: 10.1152/ajplung.00206.2017. [DOI] [PubMed] [Google Scholar]
- 41.Jain V, Kimbro S, Kowalik G, Milojevic I, Maritza Dowling N, Hunley AL, Hauser K, Andrade DC, Del Rio R, Kay MW, Mendelowitz D. Intranasal oxytocin increases respiratory rate and reduces obstructive event duration and oxygen desaturation in obstructive sleep apnea patients: a randomized double blinded placebo controlled study. Sleep Med 74: 242–247, 2020. doi: 10.1016/j.sleep.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Jameson H, Bateman R, Byrne P, Dyavanapalli J, Wang X, Jain V, Mendelowitz D. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 310: H1549–H1557, 2016. doi: 10.1152/ajpheart.00808.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jendryka M, Palchaudhuri M, Ursu D, Van Der Veen B, Liss B, Katzel D, Nissen W, Pekcec A. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9: 4522, 2019. doi: 10.1038/s41598-019-41088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein HU, Ferrari GM. Vagus nerve stimulation: A new approach to reduce heart failure. Cardiol J 17: 638–644, 2010. [PubMed] [Google Scholar]
- 45.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 48.Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 293: H997–H1006, 2007. doi: 10.1152/ajpheart.01124.2006. [DOI] [PubMed] [Google Scholar]
- 49.Macdonald AJ, Holmes FE, Beall C, Pickering AE, Ellacott KLJ. Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia 68: 1241–1254, 2020. doi: 10.1002/glia.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maclaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro 3: ENEURO.0219-16.2016, 2016. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manvich DF, Webster KA, Foster SL, Farrell MS, Ritchie JC, Porter JH, Weinshenker D. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci Rep 8: 3840, 2018. doi: 10.1038/s41598-018-22116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol 271: H2609–H2614, 1996. doi: 10.1152/ajpheart.1996.271.6.H2609. [DOI] [PubMed] [Google Scholar]
- 53.Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- 54.Moghimian M, Faghihi M, Karimian SM, Imani A, Houshmand F, Azizi Y. Role of central oxytocin in stress-induced cardioprotection in ischemic-reperfused heart model. J Cardiol 61: 79–86, 2013. doi: 10.1016/j.jjcc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Moreno A, Endicott K, Skancke M, Dwyer MK, Brennan J, Efimov IR, Trachiotis G, Mendelowitz D, Kay MW. Sudden heart rate reduction upon optogenetic release of acetylcholine from cardiac parasympathetic neurons in perfused hearts. Front Physiol 10: 16, 2019. doi: 10.3389/fphys.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res 792: 277–282, 1998. doi: 10.1016/S0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- 57.Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol Psychol 86: 174–180, 2011. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Ondicova K, Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocr Regul 44: 69–75, 2010. doi: 10.4149/endo_2010_02_69. [DOI] [PubMed] [Google Scholar]
- 59.Petersson M. Cardiovascular effects of oxytocin. Prog Brain Res 139: 281–288, 2002. doi: 10.1016/S0079-6123(02)39024-1. [DOI] [PubMed] [Google Scholar]
- 60.Petersson M, Lundeberg T, Uvnas-Moberg K. Oxytocin decreases blood pressure in male but not in female spontaneously hypertensive rats. J Auton Nerv Syst 66: 15–18, 1997. doi: 10.1016/S0165-1838(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 61.Piñol RA, Bateman R, Mendelowitz D. Optogenetic approaches to characterize the long-range synaptic pathways from the hypothalamus to brain stem autonomic nuclei. J Neurosci Methods 210: 238–246, 2012. doi: 10.1016/j.jneumeth.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piñol RA, Jameson H, Popratiloff A, Lee NH, Mendelowitz D. Visualization of oxytocin release that mediates paired pulse facilitation in hypothalamic pathways to brainstem autonomic neurons. PLoS One 9: e112138, 2014. doi: 10.1371/journal.pone.0112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, Dicarlo LA, Ardell JL, Rector TS, Amurthur B, Kenknight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20: 808–816, 2014. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5: 136–143, 2008. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajendran PS, Challis RC, Fowlkes CC, Hanna P, Tompkins JD, Jordan MC, Hiyari S, Gabris-Weber BA, Greenbaum A, Chan KY, Deverman BE, Munzberg H, Ardell JL, Salama G, Gradinaru V, Shivkumar K. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun 10: 1944, 2019. doi: 10.1038/s41467-019-09770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwartzenburg C, Morrison CD, Derbenev AV, Zsombok A, Munzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab 3: 681–693, 2014. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev 63: 291–315, 2011. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers RC, Hermann GE. Hypothalamic paraventricular nucleus stimulation-induced gastric acid secretion and bradycardia suppressed by oxytocin antagonist. Peptides 7: 695–700, 1986. doi: 10.1016/0196-9781(86)90046-X. [DOI] [PubMed] [Google Scholar]
- 69.Sabbah HN. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med 78, Suppl 1: S24–S29, 2011. doi: 10.3949/ccjm.78.s1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sampaio KN, Mauad H, Spyer KM, Ford TW. Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp Physiol 88: 315–327, 2003. doi: 10.1113/eph8802525. [DOI] [PubMed] [Google Scholar]
- 71.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6: e20360, 2011. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron 76: 142–159, 2012. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 73.Thompson GW, Collier K, Ardell JL, Kember G, Armour JA. Functional interdependence of neurons in a single canine intrinsic cardiac ganglionated plexus. J Physiol 528: 561–571, 2000. doi: 10.1111/j.1469-7793.2000.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S, Jin J, Liu J, Xiong Y, Olsen RHJ, Diberto JF, Boyt KM, Pina MM, Pati D, Molloy C, Bundgaard C, Sexton PM, Kash TL, Krashes MJ, Christopoulos A, Roth BL, Tobin AB. DREADD agonist 21 is an effective agonist for muscarinic-based DREADDs in vitro and in vivo. ACS Pharmacol Transl Sci 1: 61–72, 2018. doi: 10.1021/acsptsci.8b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu H, Liu J, Zhang D, Zheng H, Patel KP, Cornish KG, Wang WZ, Muelleman RL, Li YL. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am J Physiol Cell Physiol 306: C132–C142, 2014. doi: 10.1152/ajpcell.00223.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vizi ES, Kobayashi O, Torocsik A, Kinjo M, Nagashima H, Manabe N, Goldiner PL, Potter PE, Foldes FF. Heterogeneity of presynaptic muscarinic receptors involved in modulation of transmitter release. Neuroscience 31: 259–267, 1989. doi: 10.1016/0306-4522(89)90048-1. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res 889: 78–83, 2001. doi: 10.1016/S0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Irnaten M, Venkatesan P, Evans C, Mendelowitz D. Arginine vasopressin enhances GABAergic inhibition of cardiac parasympathetic neurons in the nucleus ambiguus. Neuroscience 111: 699–705, 2002. doi: 10.1016/S0306-4522(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 79.Wang S, Li H, Yu L, Chen M, Wang Z, Huang B, Zhou L, Zhou X, Jiang H. Anti-arrhythmic effects of atrial ganglionated plexi stimulation is accompanied by preservation of connexin43 protein in ischemia-reperfusion canine model. Int J Clin Exp Med 8: 22098–22107, 2015. [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J Neurosci 34: 6182–6189, 2014. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wengrowski AM, Wang X, Tapa S, Posnack NG, Mendelowitz D, Kay MW. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc Res 105: 143–150, 2015. doi: 10.1093/cvr/cvu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am J Physiol Regul Integr Comp Physiol 296: R299–R308, 2009. doi: 10.1152/ajpregu.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan B, Soukhova-O’hare GK, Li L, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult Fischer 344 rats. Neuroscience 153: 709–720, 2008. doi: 10.1016/j.neuroscience.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 84.Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Castel MA, D’onofrio A, Solomon SD, Wold N, Ruble SB. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J 36: 425–433, 2015. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33: 3624–3632, 2013. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]