Abstract
In the Last decades, nanotechnology has provided novel and alternative methodologies and tools in the field of medical oncology, in order to tackle the issues regarding the control and treatment of cancer in modern society. In particular, the use of gold nanoparticles (AuNPs) in radiopharmaceutical development has provided various nanometric platforms for the delivery of medically relevant radioisotopes for SPECT/PET diagnosis and/or radionuclide therapy. In this review, we intend to provide insight on the methodologies used to obtain and characterize radiolabeled AuNPs while reporting relevant examples of AuNPs developed during the last decade for applications in nuclear imaging and/or radionuclide therapy, and highlighting the most significant preclinical studies and results.
Keywords: gold nanoparticles (AuNPs), nuclear imaging, radionuclide therapy, nanomedicine, nanotechnology
1. Introduction
1.1. General Considerations
During the last decades, progresses in cancer research has been remarkable and cancer survival has steadily improved along the years. Despite this progress, there is still the need of earlier and more precise diagnostics and better therapeutic outcomes, since cancer remains one of the leading causes of death worldwide. In fact, over 2.6 million people will be diagnosed with cancer in Europe during 2020, with over 1.2 million expected deaths, according to the European Cancer Information System (ECIS). In Europe, the most common types of cancer for men and women are prostate and breast cancers, respectively. However, considering both sexes, lung cancer shows the highest mortality rates and accounts for the highest number of cancer deaths in Europe (>20% of the total number of cancer deaths) [1].
The occurrence of different types of tumours and the multifactorial etiology of cancer makes cancer an extremely complex and heterogeneous disease, where every patient develops almost a unique expression of biomarkers. For this reason, the development of the so-called precision and personalized medicine is essential to achieve better diagnostic and therapeutic outcomes. The combination of nuclear medicine modalities with nanotechnology offers unique opportunities to achieve this goal by allowing the easy and convenient merge of a variety of diagnostic and therapeutic capabilities into a single agent, within a theranostic approach of cancer. This requires the design of radiolabeled nanoconstructs that can be tailored ideally to the needs of every patient by selecting the appropriate nanoparticle, targeting biomolecule and imaging or therapeutic radionuclide [2,3,4].
Nanoparticles can be obtained with a wide variety of different materials including inorganic compounds or organic polymers, among others. The use of various materials endows the nanoparticles with a variety of morphological and physico-chemical properties, which in many cases are relevant for biomedical applications [5]. Among the different classes of nanoparticles (NPs), gold nanoparticles (AuNPs) have gained high prominence in the biomedicine field. The success of AuNPs is due to their own physico-chemical properties that are suitable for different imaging or therapeutic uses, versatile structural modification, including easy functionalization of their surface with different chemical entities (e.g., chelators, targeting biomolecules or cytotoxic drugs), favourable biological half-life, low toxicity and biocompatibility [6].
The favourable features of AuNPs prompted the study of their radiolabelling with a plethora of imaging and therapeutic radionuclides. A significant part of these studies intended to contribute for the design of (nano)radiopharmaceuticals for imaging and therapy of cancer. However, many of them just used the radiolabel for a more straightforward assessment of the biodistribution and pharmacokinetics of the AuNPs or for image-guided delivery of cytotoxic anticancer drugs. This comprised also image-guided biodistribution and pharmacokinetisc studies of boron cage-containing AuNPs for boron neutron capture therapy (BNCT). Having this in consideration, this manuscript provides a comprehensive review on the more recent achievements reported for radiolabelled AuNPs as nanotools for imaging and therapy of cancer. In this introductory section, the more relevant characteristics of AuNPs for their use in biomedical applications are discussed and the properties of medical radionuclides and the capabilities of the different nuclear medicine modalities are presented.
1.2. Gold Nanoparticles for Biomedical Applications
Nanotechnology is a discipline of science and engineering that has led to innovative approaches in many areas of medicine based on the use of biocompatible nanoparticles. Its applications in the screening, diagnosis, and treatment of disease are collectively referred to as “nanomedicine”, an emerging field that has demonstrated great potential to revolutionize individual and population wide health in the future. It can be seen as a refinement of molecular medicine, integrating innovations in genomics and proteomics on the path to a more personalized medicine [7,8].
For biomedical applications, nanoparticles can be obtained with a wide variety of materials including inorganic compounds or organic polymers, among others. The use of different materials provides nanoparticles of different sizes and shapes with varied physico-chemical properties well-fitted for a specific use in biomedicine [9]. In this respect, it is important to have in mind the influence of surface and quantum effects that affect the chemical reactivity of nanosized materials, as well as their mechanical, optical, electric and magnetic properties [10,11].
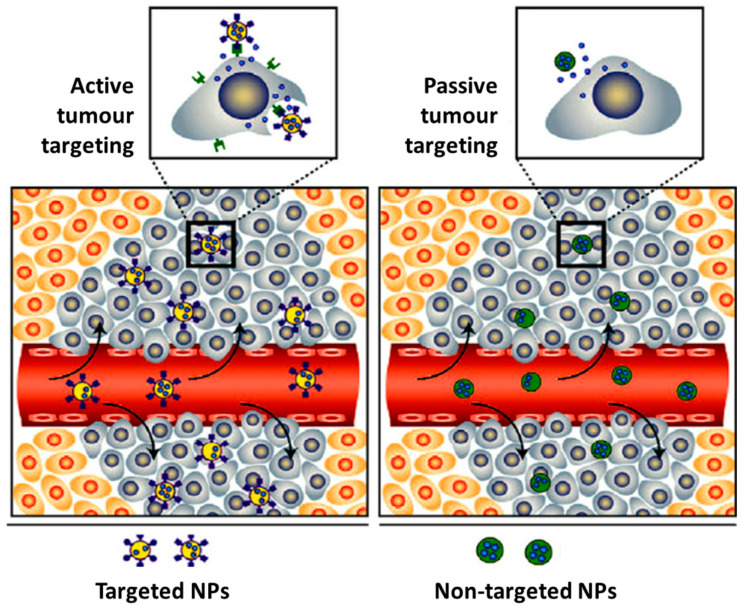
The biological fate and potential toxicity of nanoparticles are also crucial issues, which might restrict their use for medical applications. In fact, for some of them (e.g., quantum dots), their inherent toxicity is a potential drawback but for many others (e.g., iron oxide and AuNPs) toxicity issues are less relevant. Nanoparticle biodistribution can vary greatly depending on the type and size of the particle, as well as on their surface chemistry [12,13]. For imaging and/or therapy of cancer, the selective delivery of drugs or radionuclides into the tumour tissues is of paramount importance. For this purpose, nanoparticles offer unique advantages. In fact, many NPs undergo the enhanced permeability and retention (EPR) effect that is involved in the passive targeting of leaky tumour tissues. The EPR effect is a result of the leakiness of the newly forming blood vessels and poor lymphatic drainage in growing tumours. During the angiogenesis process, the endothelial cells from the blood vessel walls do not seal tightly against each other, leaving fenestrations of approximately 200–800 nm in diameter. These processes lead to a passive accumulation of nanoparticles in tumour tissues, as shown in Figure 1 [14]. On the other side, the versatile functionalization of the NPs surface with targeting biomolecules (e.g., a peptide or an antibody) allows the specific targeting of tumours through interaction with receptors overexpressed in the tumour cells or in the tumour microenvironment (Figure 1) [15,16,17].
Figure 1.
Illustration of the accumulation of nanoparticles in tumour tissues: passive vs active targeting. Adapted from Mahmoudi et al. (2011) [18].
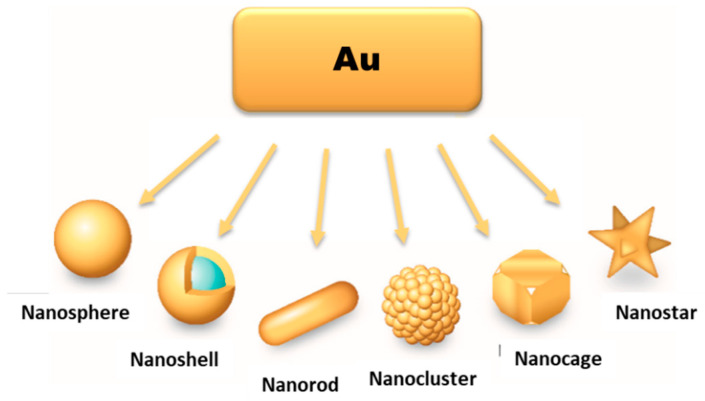
For biomedical applications, namely for cancer imaging and therapy, AuNPs offer the possibility of a versatile functionalization with targeting biomolecules for specific accumulation in tumour tissues, allowing more precise diagnostics and/or localized therapeutic effects. Moreover, there are currently available different methods to manipulate the size and shape of gold nanoparticles, spanning from shapes like nanospheres (or nanoshells), nanorods, nanocages to nanostars (Figure 2), to obtain AuNPs tailored to the different biomedical uses [19,20].
Figure 2.
Different types of AuNPs, according to their shape and morphology. Adapted from L. F. de Freitas et al. (2018) [21].
1.3. Nuclear Medicine Modalities and Medical Radionuclides
Nuclear medicine procedures involves the administration of radiolabeled drugs that are called radiopharmaceuticals, which are used for diagnostic or therapeutic applications depending on the physical properties of the labeling radionuclide.
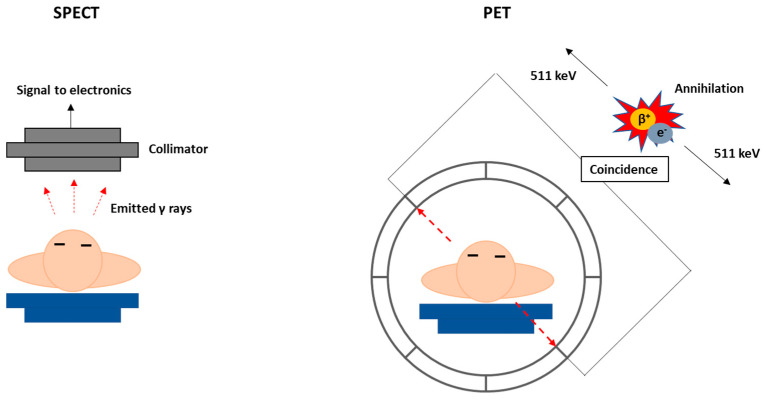
The two fundamental nuclear medicine imaging techniques are single-photon emission computed tomography (SPECT) and the positron emission tomography (PET) (Figure 3). Currently, SPECT and PET scans are essential for the diagnosis and follow-up of patients and can provide unique biological information, at molecular level, on healthy and pathological processes. By contrast, other imaging modalities, such as magnetic resonance imaging (MRI) or computed tomography (CT), only provide anatomical images or functional data. Nowadays, multimodal devices such as PET-CT, SPECT-CT or PET-MRI can combine in a synergic manner these techniques providing images with both quantitative functional information and high-resolution anatomic reference [22]. The high sensitivity of nuclear imaging techniques allows the detection of the photons emitted by the radiopharmaceuticals administered systemically, usually in an intravenous manner, to evaluate organ functionality and disease progression. Contrarily to the contrast agents used in other imaging techniques such as MRI or CT, the sub-nanomolar range dosage of radiopharmaceuticals does not induce any biochemical alteration in the system that is being imaged. Biochemical alterations always occur before anatomical changes. Therefore, PET and SPECT are more adequate for molecular imaging applications and earlier diagnostic of disease, when compared with classical CT, MRI or ultrasound (US) imaging. Nonetheless, it is important to notice that recent progresses in the development of more sensitive target-specific contrast agents for MRI or US imaging might render these techniques with higher translational potential for diagnostic molecular imaging [23]. However, nuclear imaging techniques offer the unique advantage to easily switching from a diagnostic radionuclide to a therapeutic one, using the same chemical entity, giving rise to an increasing number of clinical applications with theranostic radiopharmaceuticals, as detailed below.
Figure 3.
Schematic diagrams of SPECT (left) and PET (right) imaging.
Radionuclides useful for imaging emit either γ-photons or positrons and their optimal half-life is generally going from some minutes to few hours, as can be seen in Table 1. SPECT imaging involves the detection of γ-photons in a gamma camera placed outside the patient, which are emitted directly by the radionuclide with an energy typically in the range 100–250 keV. PET imaging is based on the detection of back-to-back 511 keV annihilation photons that result from the interaction of the positrons emitted by the radionuclide with electrons from the surrounding medium (Figure 3) [24,25].
Table 1.
Examples of Relevant Radionuclides for Imaging Use.
| Radionuclide | Half-Life | Mode of Decay (%) | Application |
|---|---|---|---|
| 11C | 20.3 min | β+ (100) | PET |
| 18F | 109.8 min | β+ (97) EC a (3) |
PET |
| 61Cu | 3.3 h | β+ (100) | PET |
| 62Cu | 9.7 min | β+ (100) | PET |
| 64Cu | 12.7 h | β− (40) β+ (19) EC (41) |
PET/Therapy |
| 67Ga | 3.27 d | EC (100) | SPECT |
| 68Ga | 67.8 min | β+ (90) EC (10) |
PET |
| 86Y | 14.7 h | β+ (33) EC (66) |
PET |
| 89Zr | 78.4 h | β+ (100) | PET |
| 99mTc | 6.0 h | IT b (100) | SPECT |
| 111In | 2.83 d | EC (100) | SPECT |
| 123I | 13.2 h | EC (100) | SPECT |
| 124I | 100.8 h | β+ (100) | PET |
a EC = Electron Capture; b IT = Isomeric Transition.
The radionuclides used in therapy are generally α or β− emitters. However, the cytotoxicity mediated by low-range Auger electrons, emitted by radionuclides undergoing electron capture (EC) and internal conversion (IC) decay processes, have also gained considerable attention when properly delivered to tumour cells [26]. All these radionuclides emit particulate radiation with different path-lengths and linear energy transfer (LET) values in soft tissues, allowing to choose the best suited for the specificity of the disease to target. Some of the most relevant radionuclides useful for therapy are presented in Table 2.
Table 2.
Examples of Relevant Radionuclides for Therapeutic Use.
| Radionuclide | Half-Life (h) | Mode of Decay (%) |
|---|---|---|
| 67Cu | 61.8 | β− (100) |
| 90Y | 64.1 | β− (100) |
| 131I | 192.0 | β− (100) |
| 153Sm | 46.3 | β− (100) |
| 166Ho | 26.8 | β− (100) |
| 177Lu | 161.0 | β− (100) |
| 186Re | 89.2 | β− (92) EC a (8) |
| 188Re | 17.0 | β− (100) |
| 198Au | 64.7 | β− (100) |
| 199Au | 75.3 | β− (100) |
| 211At | 7.2 | α (100) |
| 223Ra | 274.5 | α (100) |
| 225Ac | 238.1 | α (100) |
a EC = Electron Capture.
In current nuclear medicine practice, therapeutic approaches using radionuclides are still limited to the treatment of radiosensitive tumours, being generally preferred other strategies such as surgery, external radiotherapy or conventional chemotherapy for the treatment of solid malignancies. However, the possibility of integrating imaging and therapy make radiopharmaceuticals powerful tools for the development of more personalized approaches, especially in cancer theranostics. The term theranostics accounts for the almost unique opportunity that radiopharmaceuticals offer to develop more specific, individualized therapies and to combine diagnostic and therapeutic capabilities into a single agent. The same targeting biomolecule recognizing a particular molecular target, can be labelled either with a diagnostic or with a therapeutic radionuclide allowing the development of a patient-specific treatment [27]. For example, significant progresses have been reported recently for somatostatin analogs labelled with 68Ga for PET imaging or with 177Lu for peptide receptor radionuclide therapy (PRRT). These progresses led to the approval of the radiopharmaceuticals 68Ga-DOTATATE (NETSPOT®) and 177Lu-DOTATATE (LUTATHERA®) for clinical use in the diagnosis and treatment of neuroendocrine tumours (NETs) mediated by somatostatin receptor, both in Europe and in the USA [28].
As reviewed herein, AuNPs were evaluated in several instances as delivery systems for some of the medical radionuclides that are presented in Table 1 and Table 2. Part of the reported research work aimed at the design of innovative (nano)radiopharmaceuticals for imaging and therapy of cancer [29]. However, many of these studies dealt with AuNPs labeled with imaging radionuclides just to achieve a more straightforward evaluation of their in vivo biological fate and pharmacokinetics, profiting from the non-invasiveness and high sensitivity inherent to nuclear imaging modalities. For this purpose, it is of great importance that the incorporation of the radioisotope remains stable under in vivo conditions in order to exert properly its function. Otherwise, radioisotope biodistribution will no longer reflect that of the nanoparticles, meaning that the imaging data will not be useful to assess the fate of the nanoparticles.
2. Synthesis of Gold Nanoparticles
One of the most common methods of AuNP synthesis is by reduction of a gold precursor, generally the tetrachloroauric acid (HAuCl4), in the presence of a stabilizing agent (Figure 4a). In order to guarantee the reduction of the gold, strong to mild reducing agents are used, like NaBH4, hydrazine or citrate. In 1951, Turkevitch et al. developed one of the most conventional synthetic routes, still in use to this day, which consists on the reduction of Au(III) in HAuCl4 by citrate in water. It is known as the citrate reduction method, which allows the formation of citrate stabilized AuNPs and a controlled size of the particles by varying the citrate/gold ratio [30]. A few years later, in 1994, Brust et al. introduced a new procedure for the efficient synthesis of stable AuNPs with reduced dispersity and controlled size, which represented at the time an important breakthrough. This procedure is based on the use of thiolated ligands that strongly bind to gold due to the soft character of both Au and S. After addition of a reducing agent (NaBH4), the Au(III) is reduced to Au(I) and the AuNPs are formed [31]. This opened the opportunity to develop AuNPs using a great variety of thiolated ligands. This method allows the control of core nanoparticle size by shifting the ratio of thiol/Au in the reaction mixture; for instance, the use of larger thiol/Au ratios affords smaller core sizes with less polydispersity [32,33].
Figure 4.
Schematic synthesis of (a) AuNPs by the (1) Turkevitch and (2) Brust methodologies, (b) AuNRs by the seed-mediated method, and (c) (1) core and (2) hollow AuNSs.
In recent years there has been an increased interest on green methodologies for the synthesis of AuNPs, using alternative reducing agents to NaBH4 or hydrazine that are environmentally toxic. In this regard, Katti et al. have developed extensive work with phytochemical agents extracted from various biological media (e.g soybeans, tea leaves) [34,35,36]. It was demonstrated that these phytochemical agents performed the dual function of reducing the gold salt to form the AuNPs and at the same time provide a protein coating that can stabilize the nanoparticle structure [35,36].
As mentioned above, AuNPs can be obtained in various forms, including nanospheres, nanorods, nanoshells or nanocages. The synthetic methods described above are commonly used to obtain AuNPs in spherical amorphous form. The synthesis of AuNPs with a more complex shape requires alternative methodologies [6,21,37]. Gold nanorods (AuNRs) are commonly synthesized through the seed-mediated approach, which involves a two-step process where initially a seed solution is prepared with tetrachloroauric acid in the presence of a strong reducing agent (e.g., NaBH4) (Figure 4b). The seed solution is then added to a mixture of cetyltrimethylammonium bromide (CTAB), a mild reducing agent (e.g., ascorbic acid) and tetrachloroauric acid. The elongated ellipsoidal shape of the CTAB micelles permits the growth of the AuNPs of the seed solution in an elongated manner, in order to obtain a rod shape [38,39,40]. Some variations on this procedure include the addition of AgNO3 prior to the growth phase, which allows a better control of the shape and increase the yield of AuNRs [38].
Besides the seed-mediated method, other methodologies have also been reported in literature for the synthesis of AuNRs. The template method is based on the electrochemical deposition of Au within nanoporous template membranes, which can be of different materials (e.g., polycarbonate or alumina). Ag or Cu is added to the template membrane to form a conductive film that allows for the electrodeposition of Au and growth of the nanoparticles within the membrane nanopores. The nanorods are then recovered by selective dissolution of the template membrane and Ag or Cu film. The diameter of the AuNRs is dependent of the nanopore diameter of the membrane, while the length can be controlled by the amount of Au deposited [41,42].
Electrochemical methods for AuNR synthesis are usually based on the use of a dual electrode electrochemical cell. A gold layer is used as the anode and a platinum layer as cathode. Both electrodes are immersed in a surfactant solution composed of the cationic surfactant CTAB and a more hydrophobic cationic surfactant like tetradodecylammonium bromide (TCAB), which are responsible for the formation of the rod-shaped nanoparticles. During the process of controlled current electrolysis, the gold layer releases Au ions that migrate to the cathode where reduction occurs and the AuNRs are formed [43].
Gold nanoshells (AuNSs) can be of two types, namely solid or with a hollow core (Figure 4c). The synthesis of core-containing AuNSs is based on the use of a seed nanoparticle, which will form the core of the nanoshell. Then, the addition of tetrachloroauric acid in the presence of a reducing agent leads to the deposition of gold seeds on the surface of the core. SiO2 is one of the most commonly used cores. These silica nanoparticles have a capping agent on their surface, like 3-aminopropyltriethoxysilane (APTES), which provides NH2 groups that can link to the gold [44].
For the synthesis of hollow AuNSs, one approach is to use the silica core to synthesize the gold nanoshells as described above and then use HF to remove the SiO2 core. Another method is the template galvanic replacement of silver. This methodology is based on the higher standard reduction potential of the AuCl4−/Au pair when compared with that of the Ag+/Ag pair. Silver is oxidized into Ag+ when silver nanostructures and HAuCl4 are mixed in an aqueous medium. By optimizing the ratio between the silver nanoparticles and HAuCl4, silver atoms can diffuse into the gold shell (or sheath) to form a seamless, hollow nanostructure with its wall made of Au-Ag alloys [44,45]. The further increasing of the HAuCl4 present in the medium triggers a dealloying process that selectively removes silver atoms from the alloyed wall. This induces morphological reconstruction that leads to the formation of pinholes in the walls, and the nanoparticles acquire a cage like structure. This is one of the common methodologies for the synthesis of gold nanocages (AuNCs). Temperature also plays an important role in the replacement reaction because the solubility constant of AgCl and the diffusion coefficients of Ag and Au atoms are both strongly dependent on this parameter [45].
Due to the inherent difficulties in analyzing nanoscale materials, in comparison with molecular or bulk materials, the characterization of NPs requires particular analytical techniques and methodologies. It is common to recur to various characterization techniques, in a complementary manner, to obtain reliable information on the NPs structure and their physico-chemical properties. Besides the techniques summarized below, there are various other methodologies available nowadays for NP characterization. The use of a single one of these characterization techniques cannot provide all the required data for a proper assessment of the NP structure, hence it is necessary to take into consideration the technique’s strengths and weaknesses, depending on the nature of the NP [46,47].
Microscopy techniques, like transmission electron microscopy (TEM) or scanning electron microscopy (SEM), can provide information regarding the size and shape of the nanoparticles. On the other hand, the study of the hydrodynamic size distribution relies on techniques like dynamic light scattering (DLS) or nanoparticle tracking analyses (NTA), which can also provide information on the agglomeration state of the NPs in solution. Other commonly used techniques are zeta-potential measurements for surface charge determination and UV-Vis spectroscopy for characterization of optical properties, namely to determine the surface plasmon resonance wavelength that can be correlated with the size and shape of the nanoparticles. In the particular case of metallic NPs, X-ray-based techniques, like X-ray diffraction (XRD), are used to assess the crystalline structure and elemental composition [46,47].
3. Radiolabelling of Gold Nanoparticles
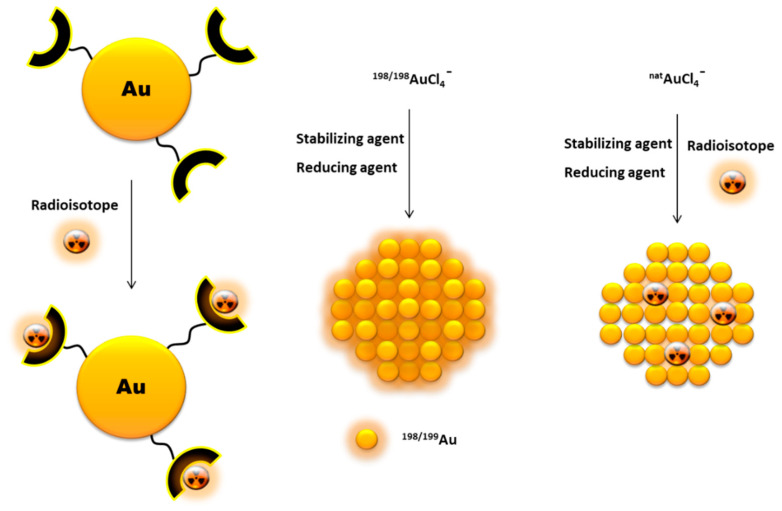
To pursue with a stable radiolabeling of AuNPs it is commonly required to perform their functionalization with suitable molecular entities, which will allow for the coordination/conjugation of the radioisotopes [48]. In this regard, there are different synthetic pathways available to functionalize AuNPs: (i) using bifunctional molecules that can act as a capping/stabilizing agent during the synthesis of the AuNPs and that can bind to the radioisotopes [49,50]; (ii) direct conjugation of amino/thiolated molecules to the surface of preformed AuNPs [51,52]; (iii) ligand exchange, in which some/all of the capping/stabilizing molecules on the AuNPs are exchanged with a different molecule with gold bonding capabilities [53]; and (iv) chemical modification of molecules already present in the AuNP structure [54,55].
Another way to incorporate radionuclides into the AuNP structure, without their further chemical functionalization, is by directly introducing the radioisotopes in the nanoparticle core (Figure 5). This is commonly achieved by using a 198/199Au precursor in the synthesis of the nanoparticles [56,57]. Alternatively, it has also been reported the neutron irradiation of non-radioactive AuNPs to originate 198/199Au-containing nanoparticles through neutron capture reactions (197Au(n,γ)198Au and 198Au(n,γ)199Au) [58].
Figure 5.
Schematic drawing of different pathways to incorporate radionuclides into AuNPs.
In some cases, it is possible to attach other radionuclides to the AuNPs without the need of extra chemical derivatization. This can be achieved by adsorption of the radionuclide to the AuNP surface, namely for 131I or 64Cu [59,60]. The incorporation of the radionuclide in the NPs core is another possibility, as reported by Liu et al. for 64Cu alloyed AuNPs modified with PEG. These 64Cu-labeled AuNPs were obtained starting from HAuCl4 and 64Cu(acac)2 and using oleylamine as reducing agent [61]. In the same way, Chen et al. have studied the integration of a 64Cu shell into PEG-stabilized AuNPs by reducing 64Cu(II) in the presence of hydrazine and polyacrylic acid [62].
4. Examples of Radiolabeled AuNPs for Nuclear Imaging Applications
As summarized in Table 3, many imaging radionuclides were used in the labeling of a variety of AuNPs to evaluate their biological fate in selected cellular and animal models, which included ex-vivo biodistribution studies or nuclear imaging scans (PET or SPECT). Part of this work also involved studies of image-guided drug delivery by AuNPs. In the next sub-sections, the most recent and relevant results are reviewed by the types of radionuclides used, i.e., radiohalogens vs. radiometals.
Table 3.
Examples of AuNPs labeled with imaging radionuclides and respective preclinical studies.
| Radioisotope | Type of AuNPs/Size/Coating (Radiolabeling Approach) | Imaging Application/Study | References |
|---|---|---|---|
| 18F | Spherical AuNPs/12 nm/LPFFD (18F-fluorobenzoate conjugation) | Biodistribution studies and in vivo PET imaging in healthy mice. | [63,64,65] |
| 64Cu | AuNSs/120 nm/cyclic-RGD (Chelator-based) | PET imaging and thermoablation treatment in HCT116 human colorectal cancer xenograft mice. | [66] |
| Spherical AuNPs/9.4 nm/PEG (64Cu/Au alloying) | Biodistribution and in vivo PET imaging in rats bearing EMT-6 breast cancer. | [61] | |
| AuNSs/44.7 nm/doxorubicin, lipiodol (chelator-based) | Biodistribution and chemotherapeutic drug delivery studies, laser induced thermal treatment and in vivo PET imaging in hepatic VX2 tumours in mice. | [67,68] | |
| AuNCs/35 nm/PEG, α-MSH (chelator-based) | Biodistribution studies and PET/CT imaging in vivo in B16/F10 melanoma mouse model. | [69] | |
| Spherical AuNPs/3 nm/PEG, bombesin, LUG, NIR dye SIDAG (chelator-based) | In vitro radiotoxicity studies in PC3 and LNCaP cell lines. Biodistribution studies and PET/CT imaging in healthy mice. | [70] | |
| Nanoclusters/4.2 nm/AMD3100 (chelator-based) | Biodistribution studies and PET/CT imaging in 4T1 mouse orthotopic breast cancer mouse model with lung metastases. | [71] | |
| Spherical, hexapodal and rod shaped AuNPs/10, 30, 80 nm/PEG, cyclic RGD (64Cu epitaxial growth on NP surface) | Biodistribution studies and in vivo PET imaging in U87MG glioblastoma xenograft mice. | [62] | |
| Tripod shaped AuNPs/25, 35 nm/DAPTA (64Cu-doped NPs) | In vivo PET imaging and image-guided photothermal treatment in 4T1-TNBC xenograft mice. | [72] | |
| AuNCs/30.4, 54.4 nm/PEG (chelator-based) | Biodistribution and in vivo PET imaging in EMT-6 murine breast cancer mouse model. | [73] | |
| AuNSs/120 nm/PEG (chelator-based) | In vivo PET imaging of 64Cu-NS-RGDfKS pharmacodynamics in nude rats xenografted with head and neck squamous cell carcinoma (HNSCC) | [74] | |
| 67Ga | Spherical AuNPs/4 nm/bombesin, DOTA (chelator-based) | In vitro radiotoxicity studies in PC3 cells. Biodistribution studies in PC3 xenograft mice. | [49,75] |
| 68Ga | Spherical AuNPs/42.5 nm/NOC, TOC (chelator-based) | In vitro binding kinetics studies in human colon cancer cell line (HT-29) and AR42J cell line of acinar pancreatic rat. | [76] |
| Spherical AuNPs/3 nm/PEG, bombesin, LUG, NIR dye SIDAG (chelator-based) | Ex vivo biodistribution studies and in vivo fluorescence imaging in LNCaP tumour bearing mice. | [77] | |
| 99mTc | Dendrimer-entraped spherical AuNPs/1.6 nm (Au core), 291.2 nm (dendrimer)/PAMAM (chelator-based) | Biodistribution studies in xenograft mice tumours with HeLa cells. | [78] |
| Dendrimer-entraped spherical AuNPs/2–6 nm (Au core), 127–139 nm (dendrimer)/PEG, cyclic RGD (chelator-based) | Ex vivo biodistribution studies in albino mice. In vivo Micro-SPECT/CT imaging, in albino mice and nude mice bearing C6 xenografted tumours. Therapeutic efficacy studies in C6 xenografted mice. | [79,80,81,82] | |
| Spherical AuNPs/5 nm/cyclic RGD (chelator-based) | Scintigraphy imaging in xenografted mice harboring 4T1 metastasis breast cancer. | [83] | |
| Spherical AuNPs/16.7 nm/Resveratrol (chelator-based) | In vivo biodistribution studies in HT 29 tumour bearing rats. | [84] | |
| PEI-entraped spherical AuNPs/3.3 nm (Au core)/PEG, fluorescein isothiocyanate, alkoxyphenyl acylsulfonamide (chelator-based) | In vitro CT and SPECT imaging of fribrosarcoma HT1080 cells. | [85] | |
| Spherical AuNPs/30.2 nm/Annexin V (chelator-based) | SPECT/CT imaging of mice with high fat diet-induced atherosclerosis. | [86] | |
| Spherical AuNPs/58.9 nm/doxorubicin, EGCG (chelator-based) | In vitro cytotoxicity studies in breast carcinoma MCF-7 and hepatocellular carcinoma HepG-2 cell lines. Biodistribution studies in Ehrlich ascites carcinoma tumour bearing albino mice. | [87] | |
| Spherical AuNPs/10 nm/gallic, doxorubicin (chelator-based) | In vitro anti-proliferative activity studies in MCF7 cell lines. Biodistribution studies in Ehrlich ascites carcinoma tumour bearing albino mice. | [88,89] | |
| 111In | Spherical AuNPs/10 nm/MMP9 (chelator-based) | In vivo SPECT/CT imaging in nude mice bearing bilateral tumours (A431 with high MMP9 expression and 4T1Luc with low MMP9 expression). | [90] |
| Spherical AuNPs/14 nm/EGF (chelator-based) | Internalization and radiotoxicity studies in MDA-MB-468 and MCF-7 cells. | [91] | |
| Spherical AuNPs/30 nm/trastuzumab (chelator-based) | Micro-SPECT/CT imaging in MDA-MB-361 human breast cancer xenograft mice. | [92,93] | |
| 124I | Spherical AuNPs/5, 10, 20 nm/oligotyrosine (124I-embeded NPs) | Dendritic cell and macrophages labeling in vivo for PET imaging detection of Sentinel Lymph Nodes. | [94,95,96] |
| Crushed Au Shell-covered spherical AuNPs/0.25 nm/ poly(N-vinyl-2-pyrrolidone (chloramine T oxidation combined with 124I-embeded NPs) | PET/CT imaging in 4T1 and CT26 tumour bearing mice and photothermal therapy in CT26 tumour bearing mice. | [97,98] | |
| 125I | Spherical and rod shaped AuNPs/56 nm/cyclic RGD (NP adsorption) | Biodistribution studies and SPECT/CT imaging in H1299 tumour bearing mice. | [99] |
| Spherical AuNPs/10 nm/MMP9 (chelator-based) | In vivo SPECT/CT imaging in nude mice bearing bilateral tumours (A431 with high MMP9 expression and 4T1Luc with low MMP9 expression). | [90] | |
| Spherical AuNPs/5, 10, 20 nm/ ogotyrosine (chloramine T oxidation combined with 125I-embeded NPs) | Dendritic cell and macrophages labeling in vivo for SPECT/PET imaging detection of Sentinel Lymph Nodes. | [94,95,96] | |
| 199Au | Spherical AuNPs/5, 18 nm/DAPTA (199Au-doped NPs) | Biodistribution studies and SPECt/CT imaging in 4T1 tumour bearing mice. | [100] |
| Amorphous/25–85 nm/PEG, folic acid, human immunoglobulin, Bharglob, M3-monoclonal antibody (199Au NP synthesis) | In vivo biodsitribution studies in healthy mice. | [101] | |
| Spherical AuNPs/not applicable (199AuNPs) | Assessment of dose distribution in human prostate cancer using Monte-Carlo simulations. | [102] |
4.1. Radiohalogens
4.1.1. Fluorine-18 (18F)
Fluorine-18 (18F) is the most widely used positron emitter in clinical PET imaging [103,104]. Conversely, there are only few examples of gold nanoparticles radiolabeled with 18F aiming at their assessment as PET probes. Kogan and co-authors were the pioneers in the biological evaluation of gold nanoparticles radiolabeled with 18F. In 2012, they attached covalently [18F]-fluorobenzoate to gold nanoparticles. The nanoparticles were functionalized with the Cys-Leu-Pro-Phe-Phe-Asp (CLPFFD) peptide, which has potential use in the treatment of Alzheimer disease by removing the toxic β-amyloid aggregates formed, and with the Cys-Lys (CK) peptide, which allows conjugation of the N-succinimidyl-4-[18F]-fluorobenzoate ([18F]-SFB) through the reaction of the amine of the side chain of the amino acid K with the carbonyl function present in the [18F]-SFB [104]. Biodistribution studies, performed two hours after intravenous administration of the resulting 18F-labeled nanoconjugate in rats, have shown high accumulation of radioactivity in the bladder and urine due to the peptide-associated pharmacokinetics. Lungs, liver, intestine, kidneys, blood are also target organs, being observed the lowest uptake of radioconjugate in the pancreas and brain [63]. Aiming to overcome the small uptake of radiolabeled AuNPs in the brain, Schirrmacher et al. assessed the properties of new gold nanoparticles bearing a maleimide group, partially hydrolyzed and non-hydrolyzed, and the prosthetic silicon-fluorine group 18F-SiFA-SH [64]. Brain images obtained by in vivo micro PET scans of normal rats, at 2 h after intravenous injection of the 18F-labeled AuNPs, revealed a higher brain uptake of the partially hydrolyzed form (0.13% ID/g) relatively to the non-hydrolyzed congeners (0.07% ID/g). As proof-of-concept, the authors functionalized these partially hydrolyzed radio-gold nanoparticles with a cysteine derivative of the octreotate peptide TATE, which has a high appetency for the somatostatin receptors present in several endocrine tumours. MicroPET biodistribution studies showed that the target-specific AuNPs have a similar brain uptake as the starting radio-nanogold platform partially hydrolyzed [64,65].
4.1.2. Iodine-124 (124I)
Iodine-124 is a rather-long lived positron emitter (T1/2 = 4.18 d) that is very suitable for the radiolabeling of compounds with long circulation times and/or slow excretion rates, as is often the case of AuNPs [105]. Lee and co-authors developed AuNPs functionalized with tannic acid (TA-AuNPs), which were radiolabeled with 124I and subsequently surrounded by a protective shell of Au to obtain the final NPs (124I-TA-Au@AuNP). 124I-TA-Au@AuNP was evaluated as a PET probe to label dendritic cells (DCs) and visualize their migration to lymphoid organs [105]. DCs can recognize several types of tumour-specific or associated antigens and induce anti-tumour immune reactions [106]. In vivo PET/CT images of mice, subcutaneously injected into the footpad with bone marrow-derived DCs (BMDCs) labeled with 124I-TA-Au@AuNPs, showed that the cells predominantly migrate to the draining lymph nodes. When the mice were pre-conditioned with tumour necrosis factor alpha (TNF-α), the 124I-TA-Au@AuNPs-BMDCs could be detected in the popliteal lymph nodes, after 15 h injection and until 96 h post injection [106]. The same authors have also performed studies with radionuclide-embedded AuNPs, carrying DNA (124I-RIe-AuNPs), PEG (124I-PEG-RIe-AuNPs) and polypeptides (124I-Poly-Y-RIe-AuNPs) [106]. The in vivo PET/CT and Cerenkov luminescence imaging (CLI) images obtained after injection of RIe-AuNPs into the foot pad of mice showed a highly selective migration of the labeled DCs to draining popliteal lymph nodes (DPLNs). Additionally, the combined in vivo PET/CLI images obtained for rats administered with 124I-PEG-RIe-AuNPs demonstrated that the AuNPs are effectively captured by the sentinel lymph nodes [107]. The CLI in vivo images also showed strong optical signals in lung, liver and spleen, with image quality equivalent to that of PET/CT images. Ex-vivo biodistribution studies have confirmed the migration of DCs to DPLNs, when in general optical imaging cannot detect the migration of DCs to deep tissues [106]. RIe-AuNPs have also demonstrated capabilities to monitor macrophage migration and, therefore, to follow-up the therapeutic effects of anti-inflammatory agents in vivo by PET imaging [95]. Studies with Poly-Y-RIe-AuNPs have shown that this platform not only allows selective screening of migration from DCs to lymphoid organs, but also promotes maturation of DCs with production of significant amounts of cytokines, such as TNFα and IL-6, in the spleen and lymphatic drainage nodes. PEG-RIe-AuNPs were evaluated as imaging probes for the detection of sentinel lymph nodes. The combined PET/CLI in vivo images performed on rats clearly demonstrated that PEG-RIe-AuNPs are effectively captured by the sentinel lymph nodes [96].
The same authors have also developed crushed gold shell radioactive nanoballs (124I-Au@AuCBs) and assessed their theranostic potential in photothermal therapy, based on a macrophage-mediated delivery of the NPs to the tumour tissues. The authors demonstrated the capability of 124I-Au@AuCBs to enhance photodynamic therapy in colon cancer bearing mice, when administered intratumourally [97,99]. The authors have also designed pegylated 124I-Au@AuCBs, which have been evaluated for multimodal (PET/CLI) in vivo detection of sentinel lymph nodes. The lymph nodes could be detected in mice following subcutaneous injection into the footpad, and its accumulation persisted until 24 h post injection. However, the utility of the platform as a lymphatic tracer is hampered by its unexpected in vivo toxicity [96,98].
4.1.3. Iodine-125 (125I)
Iodine-125 emits gamma rays followed by an average of 21 Auger electrons per decay with very low energies (0.050–0.500 keV). 125I is used clinically for brachytherapy, namely as 125I-seeds to treat prostate cancer, and has been thoroughly investigated at preclinical level for Auger therapy of cancer [26]. 125I is not the best suited radionuclide for imaging applications but it can be used for biodistribution studies and even for SPECT imaging scans. For this reason, 125I has been used in several instances to radiolabel AuNPs and to image their biodistribution in animal models [108]. For instance, Zhang and co-workers have recently reported on cisplatin-loaded and 125I-labeled gold nanoparticles (RGD-125IPt-AuNPs and RGD-125IPt-AuNRs) carrying an arginine-glycine-aspartic acid (RGD) peptide analog. These RGD-containing gold NPs were evaluated for their tumour accumulation and chemo-radiotherapy efficacy in mice xenografts. In vitro studies, performed on the human derived αvβ3 positive H1299 cells, have shown that both types of nanoparticles exhibit high affinity and specificity for αvβ3. However, SPECT/CT imaging of H1299 tumour xenograft nude mice, intravenously injected with the 125I-labeled AuNPs, demonstrated that tumour accumulation of the rod-shaped RGD-125IPt-AuNRs was significantly higher than that of the spherical RGD-125IPt-AuNPs, at each time point. However, no significant difference was observed for the distribution patterns of these two types of probes in the other major organs, such as the liver and spleen [99].
4.2. Radiometals
4.2.1. Copper-64
Copper-64 (64Cu) is one of the most widely used PET radioisotopes for nanoparticle labeling. Accordingly, several types of 64Cu-gold nanoparticles have been reported for the development of cancer theranostic tools based on PET imaging. The 64Cu radiolabeling was achieved either through chelator-free or through chelator-based strategies [109].
Using the chelator free approach, Xie et al. have studied the 64Cu-radiolabeling of gold nanoshells functionalized with a RGD peptide derivative (64Cu-NS-RGDfKs). The biodistribution and tumour specificity of the 64Cu-NSs were assessed by PET-CT imaging of live nude rats xenografted with head and neck squamous cell carcinoma (HNSCC). The images showed that the integrin-targeted 64Cu-NS-RGDfKs have a higher concentration in the tumour than the non-targeted 64Cu-NS-PEG, although they have similar biodistribution trends. Post-mortem biodistribution analyses by measurement of radioactivity (64Cu) and NAA (gold content), 46 h after intravenous injection, confirmed the improved tumour accumulation of the targeted NSs. In addition, the usefulness of the NS-RGDfKs as a photothermal therapeutic enhancer agent was confirmed in treatments conducted in nude mice xenografts with subcutaneous HCT116 human colorectal cancer [66].
Liu and co-authors developed spheric AuNPs radiolabelled with 64Cu, in which the radioisotope was incorporated directly into the structure of the AuNP core. These nanoparticles are very stable and constitute a good platform for oncology PET imaging, as shown by in vivo studies in rats bearing EMT-6 breast carcinoma. The studies showed that the tumour can be clearly visualized by these 64Cu-containing AuNPs with a definition similar to that obtained with 18F-2-deoxyglucose (18F-FDG), at 1 h p.i. [61]. Subsequently, other 64Cu-containing AuNPs with different shapes and sizes have been synthesized by a similar methodology. In particular, PEG modified gold nanorods (Au NR) and decorated with a RGD peptide analog, with UV absorption around 808, were radiolabeled with 64Cu. The resulting radioactive AuNRs (RGD-[64Cu]Au NR808) showed good potential for cancer theranostics, namely for PET image-guided photothermal therapy [62]. In fact, in vivo PET imaging studies performed in U87MG tumour xenograft rats, injected intravenously with RGD-[64Cu]Au NR808, clearly showed the accumulation of the NPs in the liver (21.7% ID/g, 45 h p.i.), spleen and tumour (7.6% ID/g, 45 h p.i.), at early and late post-injection times. Quantitative ROI analysis showed that the maximum tumour uptake of these radiolabeled AuNR was reached at 24 h post injection (8.37% ID/g), which stayed above 7% ID/g even after 45 h of administration. Xenograft rats irradiated with laser after injection with RGD-[64Cu]Au NR808 showed a remarkable decrease in tumour growth after two days of treatment. In addition, it was observed an insignificant tumour recurrence after 8 days of combined treatment. On the opposite, a clear growth of the tumour was observed in rats submitted only to laser treatment [62].
Within the chelator-based strategy, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetra-acetic acid (DOTA) derivatives are the most common choice for the 64Cu-labeling of AuNPs. Hollow gold nanospheres containing, or not, a RDG peptide derivative were radiolabeled with 64Cu via a thioctic acid-PEG-DOTA derivative. In order to enhance the uptake in liver tumours, the HAuNSs without the RGD peptide were coated with iodized oil (lipiodol). The tumoural uptake of the resulting NPs (64Cu-PEG-HAuNS-lipiodol) was evaluated in rabbits bearing hepatic VX2 tumours, after intravenous (i.v.) and hepatic intra-arterial (i.a.) injections and using PET/CT imaging. These studies showed that the retention of the HAuNSs is highly dependent on the route of administration, being the highest tumoural uptake achieved with i.a. Moreover, 64Cu-PEG-HAuNS-lipiodol presented a tumour uptake almost 4 times superior than the observed for the congeners without lipiodol and 2.5 times superior than the HAuNSs decorated with a RGD peptide. No significant difference was observed for the tumoural uptake of 64Cu-PEG-HAuNS and 64Cu-RGD-PEG-HAuNS administered intravenously [68].
The group of Tam et al. also reported on DOTA-containing AuNSs radiolabeled with 64Cu and on their evaluation in ablative treatments of rabbits with hepatic VX2 tumours [68]. The rabbits were subjected to different ablative treatments: nanoembolization (NE) alone and in combination with radiofrequency ablation (RFA+NE), irreversible electroporation (IRE+ NE) and laser induced thermal therapy (LITT+NE). NE was performed with 64Cu-DOTA-hollow-gold nanoparticles loaded with doxorubicin, which is the chemotherapeutic agent most frequently used in hepatic cancer therapy. PET images, obtained 1 and 18 h after each treatment, showed a great dependence on the location and accumulation of radionanoparticles with time and with the ablative energy applied in the treatment. The IRE + NE treatment resulted in the deposition of nanoparticles in and around the tumoural liver cells, enhancing the possibility to determine a more precise ablation zone by PET imaging [67].
Liu and co-authors evaluated the pharmacokinetics and tumour uptake of gold nanocages 64Cu-DOTA-PEG-AuNCs (30 and 55 nm) using in vivo PET/CT imaging. In normal male C57BL/6 mice, the 30 nm-64Cu-DOTA-PEG-AuNCs showed the best in vivo profile, with high blood, lung and heart retention and reduced reticuloendothelial system (RES) uptake. The biodistribution profile obtained for these AuNCs in nude mice bearing EMT-6 breast cancer is analogous. The tumour uptake quickly increases overtime (2.68 ± 0.12% ID/g; 7.2 ± 0.9% ID/g; 7.9 ± 1.1% ID/g, at 1 h; 4 h and 24 h, respectively) and it is almost four times superior to that observed for the 55 nm-64Cu-DOTA-PEG-AuNCs. The authors claimed that this tumour retention over time is particularly important for longitudinal and repeated photothermal cancer treatments. In addition, due to the relatively fast blood clearance (blood uptake higher than 20% ID/g at 1h and less than 3% ID/g at 24 h), the ratios tumour/muscle and tumour/blood also increase considerably overtime. All these findings prompted the authors to consider these nanoplatforms as a robust tool for further research studies in cancer theranostics [73].
In vivo PET studies with 64Cu(-DOTA)-gold nanocages incorporating α-melanocyte-stimulating hormone (α-MSH) peptide (64Cu-AuNCS-PEG-MSH) enabled the very adequate imaging of tumours in mice bearing B16/F10 melanoma, at 24 h post injection. The tumoural uptake of these nanoparticles is related to the concentration of the α-MSH peptide present in their surface to target the melanocortin 1 receptor (MC1R). Maximum tumour uptake of these MC1R-targeting gold nanocages varied from 7.43 + 0.55% ID/g to 7.52 + 0.40% ID/g, at 24 to 48 h post injection. Nevertheless, studies to improve the biodistribution profile and reduce the inherent toxicity of 64Cu-AuNCS-PEG-MSH are desirable in order to drastically reduce the liver and the spleen uptake (approximately 2.5 and 12 times superior to the tumour uptake, respectively) [69].
DOTA-based complexes with several metals, namely Cu, have a high thermodynamic stability [110]. However, there are some evidences that DOTA is not the ideal chelator for 64Cu with possible in vivo release of the radiometal and concomitant accumulation in liver [70,111,112,113]. On the other hand, Cu-NOTA complexes (NOTA = 1,4,7-triazacyclononane-1,4,7-triacetic acid) also have similarly high thermodynamic stability in solution, but in general show superior kinetic stability in vivo compared to their Cu-DOTA counterparts. The same is verified for some other metals, like in the case of Ga [70,110,114,115].
Taking this into consideration, Pretze and co-authors focused on AuNPs functionalized with the NOTA derivative NODAGA, aiming to obtain AuNPs more stable in vivo and suitable for dual imaging of prostate cancer using near-infrared (NIR) fluorescence and PET. Towards this goal, these authors assessed the pharmacokinetics of PEGylated AuNPs carrying NODAGA as the chelating agent for complexation of 64Cu and decorated with a NIR dye (SIDAG). To recognize prostate cancer cells, the AuNPs were further functionalized with a bombesin (BBN) peptide analog ([7,8,9,10,11,12,13,14] BBN) or with a Lys-Urea-Glu (LUG) motif for the targeting of the gastrin releasing peptide receptor (GRPr) or the prostate-specific membrane antigen (PSMA), respectively. In vitro assays were performed for 64Cu-AuNP-BBN and 64Cu-AuNP-LUG to assess their acute and long-term toxicity in PC3 and LNCaP cancer cell lines, due to the action of the β− radiation. After 24 h of incubation, the toxicity induced by the nanoparticles was higher in the LNCaP than in the PC3 cell line. This result was somewhat unexpected for 64Cu-AuNP-BBN, since the LNCaP cell line does not express the gastrin releasing peptide (GRP) receptor that is recognized by BBN derivatives. However, after four days of incubation, 64Cu-AuNP-BNN displayed higher toxicity in PC3 cells while 64Cu-AuNP-LUG had higher toxicity in the LNCaP cell line, as expected. Ex vivo biodistribution studies performed in healthy male SHO mouse with co-injection of 64Cu-AuNP-LUG and AuNP-NIR-LUG showed similar uptake of the fluorescent and radioactive AuNPs in the different organs, 25 h after injection. However, the biodistribution profile of 64Cu-AuNP-NIR-LUG in male athymic nude mice showed radioactivity uptake in brain, spleen, and pancreas lower than the uptake in the same organs measured based on the respective fluorescence intensities. The authors attributed this discrepancy to the possible release of the NIR dye in vivo [70].
Other less common types of chelators have also been explored for the 64Cu-labeling of AuNPs. The bicyclam plerixafor (AMD3100) chelator was used to stabilize a gold nanocluster (64CuAuNCs−AMD3100) that showed high and improved stability. AMD3100 is a CXCR4 antagonist approved for the mobilization of hematopoietic stem cells in lymphoma and multiple myeloma patients, under the trademark Plerixafor. 64CuAuNCs−AMD3100 was evaluated as a PET radioprobe to detect in vivo the expression of the chemokine receptor CXCR4 in a 4T1 mouse orthotopic breast cancer model with lung metastases, through PET imaging. It was found a strong correlation between the CXCR4 receptor levels in the tumour and the quantitative tumoural uptake of 64CuAuNCs−AMD3100. Moreover, competitive receptor blocking studies confirmed a tumour accumulation mediated by the CXCR4 receptors. Taking together all these findings, the authors claimed that these radio nanoclusters showed a good potential in translational research for the first early cancer and metastasis diagnosis. However, these excellent results were not translated into later phases of primary and metastatic breast cancer. Some improvements still need to be made so that 64CuAuNCs—AMD3100 extend its potential usefulness for the diagnosis of breast cancer and its metastasis, in all stages of the disease [71].
4.2.2. Gallium-67/Gallium-68
67Ga is a gamma emitter suitable for SPECT imaging while 68Ga is an emerging PET radionuclide (Table 1). For this reason, the evaluation of 67/68Ga compounds as medical diagnostic probes, carried out over the past few decades, has been a very active field of research. Contrastingly, the evaluation of nanoparticles labeled with these radioisotopes has been much less intense, namely when compared with 64Cu [49,116,117].
As mentioned above, Pretze et al. have evaluated 64Cu-AuNP-BBN and 64Cu-AuNP-LUG as new nanotools for the theranostic of prostate cancer. In the same work, these authors have also extended their studies to the 68Ga-labelled congeners. It was observed that 68GaAuNPBBN has a strong internalization in prostate cancer PC3 cells, within 3–5 h of incubation, being mainly concentrated in the cytoplasmic fraction. Blockade experiments performed in PC3 and in LNCaP cell lines with monomeric BBN (7–14) showed a significant reduction in the cellular internalization of 68GaAuNPBBN. Analogous results were observed in blockade experiments with LUG for 68GaAuNPLUG in LNCaP cells. These findings led the authors to conclude that the cellular uptake of these nanoparticles involves, at least in part, a receptor-specific mechanism [77].
Silva and co-workers have studied spherical AuNPs stabilized with thiolated derivatives of DOTA or DTPA (diethylenetriaminepentaacetic acid), proceeding with their 67Ga labeling and their preclinical evaluation in cellular and animal models of prostate cancer. Initial in vitro studies indicated that the DOTA-containing AuNPs display a higher capability to maintain the radiometal coordination than the DTPA congeners, in the presence of various media or biological substrates [49]. The AuNP-DOTA nanoparticles were decorated with BBN analogs, covalently appended by a unidentate cysteine or a bidentate thioctic group to form the nanoconstructs CBBN-AuNP-TDOTA and BBN-AuNP-TDOTA, respectively. Competitive binding assays in prostate cancer PC3 cells showed that both nanoconstructs have a high affinity towards the GRPr; however, there was a significant contrast in the cell internalization behavior of the two radiolabeled nanoconstructs in the same cell line. BBN-AuNP-TDOTA-67Ga showed a very high and rapid internalization in cells (almost 25% of the applied radioactivity after 15 min of incubation) with a relatively slow efflux overtime (≈ 15% after 3 h of incubation). The internalization of CBBN-AuNP-TDOTA-67Ga was only about 2%, and remained almost constant during 3 h. These results did not translate to the in vivo performance of these 67Ga-labeled nanoparticles. In fact, their biodistribution profile in BALB/c nude mice bearing human prostate PC3 xenografts was relatively similar, namely in which concerns the uptake in the organs that overexpress GRP receptors: moderate tumour uptake and low pancreas uptake for both NPs. These results discard, to some extent, that the tumoural uptake mechanism of these nanoparticles in vivo is through an active targeting mediated by GRPr. Eventually, other factors, such as EPR and the protein corona effect, might play prominent role in the in vivo transport of these BBN-containing nanoparticles. Additionally, the administration route also plays an important role on the pharmacokinetic profile of the nanoparticles. After intraperitoneal administration, a lower retention of the radioactive NPs in the RES organs (liver, spleen and lung) is observed, as well as a greater absorption in the pancreas that is accompanied however by a lower tumour uptake. Blocking experiments were done for BBN-AuNP-TDOTA-67Ga using the intraperitoneal administration route and after previous treatment of the tumour-bearing mice with free BBN. It was observed a significant decrease (≈34%) of the pancreas uptake but no alteration was observed in the tumour accumulation. These results suggest that the uptake of BBN-AuNP-TDOTA-67Ga in the pancreas is possibly mediated by GRPr, while in the case of the tumour uptake, the contribution of the EPR effect seems to be dominant [49].
To further expand the theranostic capabilities of these BBN-AuNP-TDOTA platforms, the authors have also studied their loading with gadolinium aiming to obtain new tools for multimodal SPECT/MRI imaging. Relaxometric studies showed that the Gd-containing AuNPs display contrast properties for MRI T1 and/or T2 relaxometry. Furthermore, radiosensitization studies showed that these AuNPs induce radiotoxic effects in prostate cancer PC3 cells, upon incubation of the cells with the NPs and exposure to a dose of 2 Gy (γ-photons, 1530 keV). These effects were slightly enhanced by the presence of the Gd in the AuNPs. Biodistribution studies were performed for Gd-BBN-AuNP-TDOTA-67Ga in PC3-xenograft Balb/c mice after intravenous and intraperitoneal administration of the NPs. The obtained biodistribution pattern is in perfect agreement with that observed for the same AuNPs without Gd. In addition, it was observed a very low uptake in the main organs and a high tumour retention (96.5 ± 26.0% and 76.8 ± 23.3% ID/g at 1 and 24 h after injection, respectively) following the intratumoural administration of the NPs [75].
Niculae et al. have recently evaluated the added value of using gold radionanoplatforms to enhance the intracellular retention of 68Ga in tumour cells with respect to the use of the congener radiocomplexes carrying the somatostatin analogs Tyr(3)-octreotide (TOC) and NaI(3)-octreotide (NOC) or a neurotensin (NT) analog. Thus, 68Ga-DOTA-TOC, 68Ga-DOTA-NOC and 68Ga-DOTA-NT were conjugated to AuNPs and evaluated in vitro in human colon cancer cell line (HT-29). 68Ga-AuNPDOTA-NOC and 68Ga-AuNPDOTA-TOC provide a 35% and 50% improvement relatively to 68Ga-DOTA-NOC and 68Ga-DOTA-TOC respectively, approximately 40 min after the incubation in HT-29 cells. However, it was found that the gain conferred by 68Ga-AuNPDOTA-NT relative to 68Ga-DOTA-NT was only approximately 10%, 20 min after incubation in HT-29 cells [76].
4.2.3. Technetium-99m
The emission of favorable low energy γ-rays (140 keV), suitable half-life (6.02 h), easy and economical availability of the 99Mo/99mTc generators, justify why 99mTc remains the most widely used SPECT imaging radionuclide in clinics [118]. In the last years, several multifunctional low-generation dendrimer-entrapped gold nanoparticles (DENPs) radiolabeled with 99mTc have been developed and reported in literature. Shen and co-authors were the first to evaluate in vitro and in vivo gold NPs functionalized with low-generation poly(amidoamine) dendrimers (PAMAM) as nanoprobes for dual SPECT/CT imaging. For that purpose, the NPs were modified with folic acid (FA) as a targeting vector and with a DTPA chelator, which were covalently attached to the PAMAM dendrimer. The resulting dendrimer/Au nanoparticles were radiolabeled with 99mTc, showing high colloidal and radiochemical stability and absence of toxicity in HeLa cells, up to concentrations of the order of 400 nM. Studies in HeLa-HFAR cells, that overexpress folic acid receptors, confirmed the specific uptake of the nanoparticles functionalized with folic acid. However, the study of the biodistribution of the 99mTc-labeled dendrimer/AuNPs in a murine HeLa xenograft tumour model showed significantly higher uptake in spleen, lung, liver and kidney than in the tumour [78]. Related 99mTc-dendrimer-nanoplatforms, with acetylated or hydroxylated terminal dendrimers, exhibited good properties for the detection of sentinel lymph node by dual SPECT/CT imaging. On the other hand, it has been shown that 99mTc-dendrimer-AuNPs functionalized with a CXCR4 ligand (FC131 peptide) can specifically target glioma and other types of cancer that overexpress CXCR4 receptors, for use in SPECT/CT dual bioimaging [80]. 99mTc-AuNP-DENPs were also evaluated as SPECT radioprobes for the detection of apoptosis, being proved in vitro that 99mTc AuNP-DENPs decorated with the duramycin peptide have a high propensity for targeted imaging of apoptotic C6 cancer cells.
99mTc-AuNP-DENPs decorated with a RGD analog peptide showed a favorable profile for targeted SPECT/CT imaging of αvβ3 integrin overexpressing tumours [79,80,81,82]. The capability of pegylated 99mTc-labeled AuNPs decorated with a RGD peptide to effectively target αvβ3 integrin receptors had previously been documented. The preclinical evaluation of these RGD-containing AuNPs showed that the nanoparticles have a high uptake in the lung metastases (14% of the injected dose at 60 min after intravenous injection) of a 4T1 mouse model of breast cancer [83].
Dhawan and co-workers have studied 99mTc-labeled NPs for the non-invasive detection of colon cancer by SPECT imaging. For this purpose, they have conjugated 3,5,4′-trihydroxytrans-stilbene (resveratrol, Res) to the AuNPs in order to increase their selectivity towards colon cancer cells. The accumulation and retention of 99mTc-Res-AuNP in HT 29 colon cancer cells was significantly higher than the congener non-targeted 99mTc-AuNPs. Biodistribution studies performed in rats with colon cancer confirmed that 99mTc-Res-AuNP have an higher uptake ratio colon tumour/normal colon than the non-targeted 99mTc-AuNPs, which leads to an improved tumour to background contrast [84].
Recently, Shi et al. have developed 99mTc-labeled polyethylenimine (PEI)-entrapped AuNPs, functionalized with PEG and alkoxyphenyl acylsulfonamide (APAS) groups (APAS-99mTc-AuPENs). Due to their negatively charged sulfamine groups and positively charged ammonium groups, APAS units are neutral at physiological pH (pH 7.4) and are positively charged at more acidic pH. The authors have considered that this feature could improve the cellular retention of the nanoparticles in cancer cells, which have a mild acid microenvironment. This reasoning was corroborated by the results of in vitro studies performed with the fibrosarcoma HT1080 cell line. It has been observed a higher concentration of radioactivity in the cells treated with APAS-99mTc-AuPENs, at pH 6, when compared with the cells treated with the NPs not functionalized with APAS [85]. Shi and co-workers also developed 99mTc-AuNPs functionalized with Annexin V for in vivo targeting of apoptotic macrophages, which are abundant in atherosclerosis plaques. In vitro studies performed on macrophages (RAW264.7) with apoptosis induction and in vivo studies conducted on high-fat diet fed ApoE−/− mice demonstrated the suitability of these nanoparticles to target specifically arteriosclerotic plaques containing apoptotic macrophages [86].
Sakr et al. have investigated 99mTc-labeled AuNPs conjugated with gallic acid and loaded with doxorubicin (99mTc-gallic-AuNPs-DOX) for image-guided drug delivery. The non-labeled AuNPs display suitable in vitro stability in saline and in rat serum for 3 days. Biodistribution studies of the 99mTc-labeled nanoparticles, performed in female albino Swiss mice having Ehrlich ascites carcinoma, showed a considerable tumour uptake of 22.45% ID/g after 2 h of intravenous injection. Furthermore, 99mTc-gallic-AuNPs-DOX displayed a nearly 80% tumour retention upon intratumoural injection, at least for 2 h after administration [87,88,89].
Silva et al. have studied AuNPs stabilized with a dithiolated DTPA (DTDTPA), previously developed by Roux and co-workers, as potential glutathione-responsive drug delivery systems. The AuNP-DTDTPA were labeled with the [99mTc(CO)3(H2O)3]+ precursor and the resulting radiolabeled NPs were studied in vitro in the presence of glutathione (GSH). The results pinpointed that GSH promotes the cleavage of the disulfide bonds of the polymeric DTDTPA coating, which can be exploited for GSH-mediated delivery of drugs attached at the DTDTPA framework [119,120,121].
4.2.4. Indium-111
111In is a gamma emitter with a half-life of 2.8 d that is suitable for clinical SPECT imaging. In addition, it also emits Auger electrons that are potentially useful for targeted radionuclide therapy. In the last years, there have been only a few studies reported for AuNPs radiolabelled with 111In, seeking to demonstrate their potential interest for imaging and/or therapy [26,122]. These studies include the evaluation of the pharmacokinetics and biodistribution of AuNPs decorated with pMMP9 (pMMP9 = DTPA-Gly-Pro-Leu-Gly-Val-Arg-Gly-Lys-Gly-Tyr-Gly- Ahx-Cys-NH2), which is a matrix metalloproteinase-9 (MMP9) cleavable peptide. The pMMP9-containing AuNPs were radiolabeled simultaneously with 111In and 125I and were evaluated in tumour-bearing mice by in vivo SPECT imaging. At 4 h after intravenous injection, 111In was detected mainly in the blood while 125I was present in the thyroid, stomach and bladder. This result was attributed to the higher in vivo stability of the 111In-radiolabeled moiety if compared with the 125I-radiolabeled one. Two types of tumours with different MMP9 expression levels (high = A431; low = 4T1Luc) were implanted in nude mice to explore the ability of the nanoparticles to accumulate in tumours showing MMP9 activity. SPECT/CT images showed that the nanoparticles progressively accumulated in 4T1Luc tumours with low expression of MMP9, reaching 48 h upon intravenous injection a SUV value of 2.8 ± 0.11 (10.2 ± 0.33% ID/g), while a lower SUV of 1.75 ± 0.2 (6.23 ± 0.72% ID/g) was observed in the same period in the A431 tumours with high expression of MMP9. The difference in pharmacokinetics was assigned to the highest MMP9 level in the A431tumours that led to cleavage of the peptide radiolabeled with 111In and its clearance from the tumour [90].
AuNPs loaded with the epidermal growth factor (EGF) and radiolabeled with 111In (111In-EGF-AuNP) were evaluated in vitro using two breast cancer cell lines with different levels of EGFR expression. The 111In-labelled EGF-AuNPs presented significantly higher levels of uptake and more pronounced radiotoxicity in MDA-MB-468 cells compared with MCF-7 cells. This reflects the higher EGFR expression (100 times-fold) of MDA-MB-468 cells versus MCF-7 cells [91,123]. In another study, 111In-labeled AuNPs decorated with pegylated trastuzumab (trastuzumab-AuNP-111In) were evaluated for the targeting of HER2-positive breast cancer cells. Dark field and confocal fluorescence microscopy showed the perinuclear location of trastuzumab-AuNP-111In in SK-BR-3 cells having a high HER2 expression. Biodistribution studies of trastuzumab-AuNP-111In in mice bearing subcutaneous MDA-MB-361 xenografts have shown a low accumulation of the NPs in the tumour with a high liver uptake [92]. Nevertheless, the intratumoural injection of trastuzumab-AuNP-111In, using the same animal model, led to a significant reduction of the tumour mass over 70 days, without apparent toxicity in normal tissues [93].
4.2.5. Gold-198/199
As can be verified in Table 2, 198Au (T1/2 = 64.7 h) and 199Au (T1/2 = 75.3 h) are relatively long-lived β− emitters that are suitable for therapeutic use. In addition, both radionuclides emit also γ-photons that allow SPECT imaging studies. In the case of 199Au, 5 nm gold nanoparticles doped with 199Au decorated with D-Ala1-peptide (DAPTA) have been evaluated for in vivo target of the C-C chemokine receptor 5 (CCR5), overexpressed in triple negative breast cancer (TNBC). NanoSPECT/CT images obtained 24 h after intravenous injection of 199AuNP-DAPTA in a 4T1 TNBC orthotopic mouse model showed a heterogeneous pattern of penetration and retention within the tumour, in addition to high liver and spleen accumulation. The images are in full agreement with the results of biodistribution studies, which showed a tumour uptake of 7.13 ± 0.08% ID/g and a ratio tumour/muscle of 18.7 ± 1.69. All together these results led the authors to conclude that 199AuNP-DAPTA is a promising nanoplatform for the CCR5-targeted imaging of triple breast cancer [100].
Biodistribution studies of 199Au-labeled AuNPs decorated with a non-specific antibody (Bharglob) in normal rats showed that the accumulation of radioactivity occurs predominantly in stomach and organs of the RES system, at 24 h after injection. In an attempt to minimize the unfavorable pharmacokinetics observed, non-specific gammaglobulin was co-administered and a considerable decrease in the RES uptake was observed (about 50%) [101].
Loyalka and co-authors estimated the dose distribution delivered by 198/199Au-labeled AuNPs to the tumour sites, inside the human prostate, as well as to the surrounding normal tissues using the Monte-Carlo N-Particle code (MCNP-6.1.1 code). A simple geometric model of the tumour, prostate, bladder and rectum was constructed. MCNP simulations showed that the doses are deposited homogenously and mostly within the tumour and marginally in the bladder and rectum. However, the dose deposited by 198Au is significantly higher than the dose deposited by 199Au in the tumour region, as well as in normal tissues [102].
Katti et al. have reported the synthesis of radioactive 198Au-AuNPs functionalized with mangiferin (MGF) [56]. The specificity of MGF towards the laminin receptor promoted the accumulation of the AuNPs in prostate tumours (PC-3) induced in mice. Detailed in vivo therapeutic efficacy studies, through the intratumoural delivery of the AuNPs, showed retention of over 80% of the injected dose in tumours up to 24 h. By three weeks post treatment, tumour volumes of the treated group of animals showed an over 5 fold reduction as compared to the control saline group.
Chakravarty et al. have developed 198Au-AuNPs functionalized with a RGD peptide derivative and studied their suitability for melanoma cell targeting [124]. In vitro studies showed that the AuNPs bind to murine melanoma B16F10 cells with high affinity and specificity. Biodistribution studies of the AuNPs administered intravenously in melanoma tumour bearing C57BL/6 mice showed high uptake in the tumour within 4 h post-injection, with significant decrease at the same time point when co-injected with a blocking dose of the RGD peptide. Radiotherapy studies in melanoma tumour bearing mice showed significant regression of tumour growth without apparent body weight loss over the course of 15 days.
5. Examples of Radiolabeled AuNPs for Therapeutic Applications
Besides 198Au and 199Au, various other therapeutic radionuclides of the β− or α-emitting types were used to label AuNPs aiming to obtain enhanced therapeutic effects, namely within a theranostic approach of cancer. As resumed in Table 4 and reviewed below, part of these studies comprised also SPECT imaging experiments since some of these radionuclides also emit γ photons during their decay and, for this reason, are also suitable for in vivo imaging.
Table 4.
Examples of AuNPs labeled with therapeutic radionuclides and respective preclinical studies.
| Radioisotope | Type of AuNPs/Size/Coating (Radiolabeling Approach) | Application/Study | Refs. |
|---|---|---|---|
| 90Y | Spherical AuNPs-loaded nanoparticle depots/15 nm/PEG, polyglutamide (chelator-based) | Monte Carlo simulations of permanent seed implantation brachytherapy. | [125] |
| AuNRs/40 nm/PEG (chelator-based) | Biodistribution studies, combined radiotherapy and hyperthermia treatment in prostate DU145 xenograft mice. | [126] | |
| 131I | PEI-entraped spherical AuNPs/4.4 nm (AuNP core), 151 nm (PEI)/HPAO, PEG, CTX (chloramine T oxidation) | Targeted SPECT/CT imaging and radionuclide therapy in subcutaneous glioma tumour model in vivo. | [127] |
| AuNRs/93 nm/PEG, cyclic RGD (NP adsorption) | SPECT/CT imaging and biodistribution analyses in B16F10 and MCF7 tumour bearing mice | [59] | |
| 177Lu | Dendrimer-entraped spherical AuNPs/2.5 nm (AuNP core), 5.6 nm (dendrimer)/folate, bombesin (chelator-based) | Radiocytotocixity studies in T47D cells. Biodistribution studies and optical imaging in T47D xenograft mice. |
[128,129] |
| Spherical AuNPs/30 nm/orthopyridyl disulfide, PEG, panitumumab(chelator-based) |
Biodistribution/radiotoxicity studies and small-animal SPECT/CT imaging in MDA-MB-468 xenograft mice. | [130,131] | |
| AuNRs/15 nm/PEG, polyglutamide (chelator-based) | Monte Carlo simulations of permanent seed implantation brachytherapy. | [125] | |
| 198Au | Spherical AuNPs/12.5 nm/cyclic RGD (198Au NP synthesis) | Biodistribution and tumour regression studies in melanoma C57BL/6 tumour bearing mice. | [124] |
| Spherical AuNPs/35 nm/mangiferin (198Au NP synthesis) | Biodistribution and therapeutic efficacy studies in prostate PC3 xenograft mice. | [56] | |
| 211At | Spherical AuNPs/5 nm/PEG, trastuzumab (NP adsorption) | In vitro radiotoxicity studies in human ovarian cancer cell line SKOV-3 | [132] |
| 225Ac | Spherical AuNPs/2–3 nm/DOTAGA (chelator-based) | Biodistribution and therapeutic efficacy studies in glioblastoma multiform cell line U87 xenograft mice. | [133] |
5.1. Beta-Emitting Isotopes
5.1.1. Yttrium-90
Yttrium-90 (90Y) is a β− emitter decaying to 90Zr with a half-life of 64.6 h and with a decay energy of 2.28 MeV. It is a hard β− emitter and the emitted particles can penetrate tumour soft tissue to a length of 11 mm. For this reason, 90Y leads to important cross-fire effects and does not require its accumulation in every tumour cell to produce deleterious radiotoxic effects. However, it can kill non-targeted cells in the vicinity of the target tumours. Ghandehari et al. have reported on the use of AuNRs to increase hyperthermia in tumours and to enhance the radiotherapeutic effect of a 90Y-labeled N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer [126]. The macromolecular nature of HPMA allows it to passively target tumours through the EPR effect. Prostate tumour animal models were treated with a co-injection of PEGylated AuNRs and 90Y-labeled HPMA, and thereafter were submitted to laser treatment to induce localized hyperthermia. Results showed an increase in the uptake of radiolabeled copolymer in the hyperthermia treated prostate tumours, with no significant accumulation in non-targeted tissues. Additionally, the highest reduction in tumour growth was observed in the tumours submitted to hyperthermia and treated with 90Y-labeled HPMA copolymer conjugates. Althoughmost radioactivity accumulation was found in the tumours, the biodistribution studies also showed a significant uptake in the kidneys; however, the histological studies did not show any pronounced damage in the primary organs of the mice.
Reilly et al. have performed in vivo imaging and Monte Carlo simulations of nanoparticle depots (NPD) [134]. consisting of a porous calcium alginate platform loaded with AuNPs coated with PEG and polyglutamide, and functionalized with a DOTA derivative for radiolabeling with 111In, 90Y and 177Lu [125]. The studies were performed in a way to compare these NPDs with conventional permanent seed implantation (PSI) brachytherapy in mice bearing subcutaneous human breast cancer xenografts. For the simulated NPDs, 90Y delivered the most homogeneous dose distribution.
5.1.2. Iodine-131
Iodine-131 (131I) has a half-life of 8.02 d and emits β− particles and γ radiation. It decays in two steps to form the stable 131Xe, initially through beta decay (606 keV) followed rapidly after by gamma emission (364 keV). Lan et al. have reported on the synthesis of 131I-labelled AuNRs decorated with a cyclic RGD peptide derivative for integrin αvβ3 receptor targeting, which is responsible for tumour angiogenesis [59]. Results showed that the AuNPs were selectively taken up by the tumour in murine melanoma B16F10 cancer bearing mice mainly via integrin αvβ3-receptor mediated endocytosis, after intravenous administration. However, the biodistribution studies also showed higher uptakes in organs of the RES, such as liver, spleen and lungs, most likely due the large size of the AuNRs (93.4 nm, lenght). Additionally, when administered in breast cancer MCF7 tumour bearing mice, there was no significant uptake in the tumours, which was attributed to the low αvβ3 receptor expression in this cell line in comparison with B16F10.
Zhao et al. have studied polyethylenimine-entrapped AuNPs functionalized with a chlorotoxin (CTX) peptide and labeled with 131I for SPECT/CT imaging and radionuclide therapy of glioma [127]. CTX is a peptide capable of targeting various cancer cells including glioma, sarcoma and prostate, and capable of permeating the blood brain barrier (BBB) intact. The AuNPs were entrapped inside a polyethylenimine polymeric nanoparticle, functionalized with PEG, chlorotoxin, and 3-(4-hydroxyphenyl)propionic acid-OSu (HPAO). The presence of the HPAO allowed for a facile radiolabeling with 131I. After intravenous injection of the radiolabeled NPs in a subcutaneous glioma-bearing mice, it was possible to visualize through SPECT imaging a high tumour accumulation, with the highest uptake at 8 h p.i. The congener NPs, without the CTX, still displayed some significant tumour uptake at 8 h p.i., but it was less than half intensity when compared with the ones bearing peptide. These results also translated to the in vivo studies performed in orthotopic rat glioma models, where the CTX-containing NPs displayed a significant SPECT signal, which peaked at 8 h p.i., demonstrating the capability of these NPs to cross the BBB.
5.1.3. Lutetium-177
Lutetium-177 (177Lu) has a half-life of 6.7 d and undergoes β− decay, emitting β− particles (134 keV) but also γ radiation (208 keV). As mentioned in the introduction, 177Lu is a soft β− emitter with increasing clinical impact on PRRT of cancer. The emission of γ photons allows for the use of 177Lu in preclinical SPECT imaging.
Ferro-Flores et al. have developed a DOTA-dendrimer-folate-bombesin conjugate that was used to entrap AuNPs in the dendritic cavity (DenAuNP-folate-bombesin). The presence of the folate and bombesin was to improve affinity of the NPs to the folate receptor and gastrin releasing peptide receptor, respectively, which are overexpressed in certain types of breast cancer cells. The entrapped AuNPs provided photophysical properties to the whole nanoconjugate suitable for optical imaging. The final nanoconjugate was labeled with 177Lu, seeking for multimodal platforms suitable for breast cancer cell targeting [128,129]. The radiolabeled nanoconjugate showed specific uptake in breast cancer T47D cells and provided suitable optical images. Plasmonic–photothermal therapy studies in T47D cells incubated with DenAuNP-folate-bombesin showed a higher increase in medium temperature (46.8 °C), compared with the congeners without the entrapped AuNPs (39.1 °C), which consequently led to a more significant decrease in cell viability [132]. Moreover, preliminary in vivo studies showed quantitative tumour retention 96 h after intratumoural administration of the 177Lu-labeled DenAuNP-folate-bombesin in breast cancer T47D tumour bearing mice [128].
The group of Reilly et al. has studied the in vivo stability of AuNPs functionalized with different PEG derivatives containing DOTA for 177Lu labeling [131]. These PEG derivatives varied on their thiol group responsible for AuNP surface conjugation, including monothiol, dithiol and multithiol groups. Biological studies showed that the AuNPs containing the multi-thiol PEGs displayed the highest stability in vitro and the lowest liver uptake in vivo. The group also developed new AuNPs constructs functionalized with monothiolated PEG chains linked to DOTA and with panitumumab for epidermal growth factor receptor (EGFR) targeting [130]. These multifunctional AuNPs were labeled with 177Lu and underwent a preclinical study as nanoseeds for brachytherapy of locally advanced breast cancer.
The preclinical studies of the panitumumab-containing AuNPs comprised the intratumoural administration of the 177Lu-labeled nanoconstructs in CD-1 athymicmice bearing subcutaneous MDA-MB-468 xenografts. It was observed a high radioactivity concentration in the tumour at 1 h post-injection, but with a significant decrease (2–3 fold) after 48 h of administration. However, when compared with the respective congeners without the EGFR-targeting panitumumab, the uptake in tumour was not significantly different; and in both cases, some accumulation in non-targeted organs like liver and spleen had increased about 3–4 fold between 1 to 48 h p.i. Dosimetry studies estimated that the tumour receives the highest dose, and the liver, spleen and pancreas are the non-targeted organs more exposed to radioactivity. Long-term treatment studies showed the inhibition of tumour growth in mice treated with both targeted and non-targeted 177Lu-labelled AuNPs, without toxicity in normal tissues. Additionally, non-labelled NPs did not display any visible tumour growth inhibition [130].
5.2. Alpha-Emitting Isotopes
5.2.1. Astatine-211
Astatine-211 (211At) has a half-life of 7.21 h and undergoes a branched decay: 41.8% decays to 207Bi (T1/2 = 32.9 y) with α emission (5.9 MeV); 58.2% decays by electron capture to 211Po (T1/2 = 516 ms) that then quickly decays to the stable 207Pb with α emission (7.5 MeV). Majkowska-Pilip et al. have reported on AuNPs modified with PEG chains and attached to the antibody trastuzumab. This antibody not only possesses chemotherapeutic properties but it also has affinity towards HER2 receptors, which are overexpressed in certain breast cancer cells [132]. The AuNPs were labeled with 211At by adsorption of the radionuclide to the nanoparticle surface by taking advantage of the high affinity of gold for heavy halogens. In vitro biological studies showed a higher affinity and cytotoxicity for the trastuzumab-containing AuNPs, compared with the ones without the antibody, towards HER2-overexpressing human ovarian SKOV-3 cells. Additionally, it was also verified that the trastuzumab-containing AuNPs were able to internalize into the cells and deposited near the nucleus.
5.2.2. Actinium-225
Actinium-225 (225Ac) is a radionuclide that decays to 221Fr with a T1/2 of 10.0 d and through α emission (5.94 MeV). Bouziotis et al. have reported on the synthesis of AuNPs functionalized with a thioctic acid-modified DOTAGA and radiolabeled with 225Ac. The resulting 225Ac-labeled AuNPs were evaluated as an injectable radiopharmaceutical form of brachytherapy for local radiation cancer treatment, using cellular and animal models of glioblastoma multiforme [133]. In vitro radiocytotoxicity studies in glioblastoma multiforme U87MG cells showed a significant cell death upon exposure to the radiolabeled AuNPs. Consistently, their intratumoural administration in U87MG tumour bearing mice resulted in the retardation of tumour growth, even with a low injected dose (1 kBq) per mice. However, while biodistribution studies showed the highest uptake of radioactivity in the tumours, some significant accumulation in non-targeted organs like liver, kidneys and spleen was observed.
5.3. Boron Neutron Capture Therapy (BNCT)
BNCT is a technique based on the nuclear reaction 10B(n, α)7Li that involves a neutron-capture process causing fission reactions that originate high-LET alpha particles (150 keV/μm) [135]. BNCT differs from the classical radionuclide therapy discussed previously, as the therapeutic radiation delivered by BNCT is triggered by the external neutron irradiation of the boron atoms that are accumulated by tumour cells. However, both methodologies are categorized as internal radionuclide therapies, and BNCT has been gaining increased interest in the last few years, with some examples reported in literature combining AuNPs with this technique.
Llop et al. recently synthesized AuNPs (core diameter of 19.2 ± 1.4 nm; hydrodynamic diameter of 37.8 ± 0.5 nm) functionalized with PEG and an anion-rich cobalt bis (dicarbollide), commonly known as COSAN, and evaluated its potential for BNCT in mouse model xenografts with human fibrosarcoma HT1080 cells [136]. These COSAN-containing NPs have been radiolabelled with 124I (in two different positions, the core and the shell) to allow tracking of their biodistribution pattern by PET imaging. The studies showed that the radiolabeled nanoparticles are stable in vivo, since no significant iodine accumulation was detected in the thyroid during the imaging studies (0–144 h). However, no significant uptake was found in the tumours. The maximum values obtained are below 0.5% ID cm−3, regardless of the labelling approach used (core vs shell labelling), and progressively decreased over time being almost undetectable at t > 72 h.
To improve the tumour uptake and retention of boron cage-containing AuNPs for BNCT, the same authors synthetized smaller gold nanoparticles (core size = 4.1 ± 1.5 nm, hydrodynamic diameter of 39.6 ± 0.8 nm) loaded with PEG, COSAN and functionalized with tetrazine (Tz) units [137]. To enable the in vivo screening of the biodistribution of AuNPs by positron emission tomography (PET), the nanoparticles were radiolabelled with [64Cu] CuCl2 (core labelling). PET biodistribution studies were conducted in cancer-xenograft bearing mouse model with HER2 positive BT-474 breast cancer cells, using both a simple and a pre-targeting strategy. For a pre-targeting approach, Trastuzumab, an antibody that selectively binds to HER2, was functionalized with trans-cyclooctene (TCO) ligand, to promote the in vivo click reaction with tetrazine present in the radiolabeled AuNPs and was administered intravenously 24 h before the AuNPs. PET-CT scans were performed ≈ 1, 6, 24, and 48 h. The accumulation in the tumours was clearly visualized by PET images. No added value was observed from the pre-targeting approach. Indeed, the maximum tumoural uptake was achieved at t = 24 h after AuNP injection, with values of 4.76 ± 1.85% ID cm−3 and 4.42 ± 2.08% ID cm−3 for pre-targeting and normal strategy, respectively.
6. Concluding Remarks
In this review, we have highlighted some of the most relevant AuNPs developed in the last few years for nuclear medicine applications, particularly for cancer treatment. To this date, AuNPs have provided interesting platforms for the delivery of radioisotopes to cancer cells and tissues, with the advantage of their low toxicity, biocompatibility and enhanced biological half-life. Despite these encouraging progresses, there is the need to optimize the efficacy of most radiolabeled AuNPs to obtain new nanotools with clinical usefulness. To fulfil this goal, still is necessary the development of novel AuNPs core structures and their controlled modification with different molecular entities to further improve their pharmacological profile. In this respect, one can profit from recent achievements on site-specific approaches to modify clinically relevant biomolecules and from technological advances in the production of innovative medical radionuclides.
Having in consideration the reported preclinical studies, radiolabeled AuNPs are not expected to be a valuable alternative to more conventional molecular radio-pharmaceuticals designed for systemic administration, such as radiolabeled peptides or antibodies that are already in clinical use for peptide receptor radionuclide therapy (PRRT) or radioimmunotherapy (RIT). In fact, a large majority of the tested radiolabeled AuNPs have shown a sub-optimal biodistribution with more or less prolonged accumulation in non-target organs, mainly the liver and spleen, which can lead to unfavorable radiation dosimetry. By contrast, we consider that the use of radiolabeled AuNPs in combination with topical administration, as a kind of “nanoseeds”, might open new avenues in cancer theranostics with minimization of detrimental side effects. This is particularly true when using therapeutic radionuclides that emit high LET and short-range particle radiation, as is the case of alpha or Auger emitters. In this respect, AuNPs are clearly advantageous over classical molecular radiopharmaceuticals. The NPs are expected to exhibit a higher retention in the tumors, following their intratumoral administration, when having the proper size, shape, charge and/or coating. Moreover, the development of multifunctional “nanoseeds” for the simultaneous delivery of radionuclides, cytotoxic drugs and/or radiosensitizers will allow combined chemo- and radiotherapy regimens with a better chance to surpass radio/chemoresistance processes. Hence, it is our conviction that AuNPs can play a role in future applications of nuclear medicine by providing unique combinations of imaging and therapy modalities to improve the diagnosis, treatment and management of cancer.
Abbreviations
| α | Alpha |
| α-MSH | α-Melanocyte-stimulating hormone |
| AuNCs | Gold nanocages |
| AuNP | Gold nanoparticle |
| AuNR | Gold nanorod |
| AuNS | Gold nanoshell |
| APTES | 3-Aminopropyltriethoxysilane |
| β | Beta |
| BBB | Blood brain barrier |
| BBN | Bombesin |
| BNCT | Boron neutron capture therapy |
| CLI | Cerenkov luminescence imaging |
| CT | Computed tomography |
| CTAB | Cetyltrimethylammonium bromide |
| CTX | Chlorotoxin |
| DAPTA | D-Ala1-peptide T-amide |
| DC | Dendritic cell |
| DLS | Dynamic light scattering |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOX | Doxorubicin |
| DPLNs | Draining popliteal lymph nodes |
| DTDTPA | Dithiolated DTPA |
| DTPA | Diethylene triamine pentaacetic acid |
| EC | Electron capture |
| ECIS. | European Cancer Information System |
| EGFr | Epidermal growth factor receptor |
| EPR | Enhanced permeability and retention |
| GRPr | Gastrin releasing peptide receptor |
| HNSCC | Head and neck squamous cell carcinoma |
| HPAO | 3-(4-Hydroxyphenyl)propionic acid-OSu |
| HPMA | N-(2-Hydroxypropyl) methacrylamide |
| HER | Human epidermal growth factor receptor |
| IT | Isomeric transition |
| LET | Linear energy transfer |
| LPFFD | Cys-Leu-Pro-Phe-Phe-Asp |
| MC1R | Melanocortin 1 receptor |
| MRI | Magnetic resonance imaging |
| NAA | Neutron activation analysis |
| NET | Neuroendocrine tumours |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| NODAGA | 1,4,7-Triazacyclononane-1-glutaric acid-4,7-acetic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| NP | Nanoparticle |
| NPD | Nanoparticle depots |
| NTA | Nanoparticle tracking analyses |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| p.i | Post-injection |
| PRRT | Peptide receptor radionuclide therapy |
| PSI | Permanent seed implantation |
| PSMA | Prostate-specific membrane antigen |
| RES | Reticuloendothelial system |
| RGD | Cyclic arginine-glycine-aspartic acid |
| RIe-AuNP | Radionuclide-embedded gold nanoparticles |
| ROI | Region of interest |
| SEM | Scanning electron microscopy |
| SFB | N-Succinimidyl-4-fluorobenzoate |
| SPECT | Single-photon emission cumputed tomography |
| SPR | Surface plasmon resonance |
| SUV | Standardized uptake value |
| TATE | Octreotate |
| TEM | Transmission electron microscopy |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumour necrosis factor alpha |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
Author Contributions
Writing—original draft preparation, F.S. and M.P.C.C.; writing—review and editing, all Authors. Funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fundação para a Ciência e Tecnologia (Project PTDC/MED-QUI/29649/2017).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Source: ECIS—European Cancer Information System. [(accessed on 10 October 2020)]; Available online: https://ecis.jrc.ec.europa.eu.
- 2.Jeon J. Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. Int. J. Mol. Sci. 2019;20:2323. doi: 10.3390/ijms20092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb J., Holland J.P. Advanced Methods for Radiolabeling Multimodality Nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018;59:382–389. doi: 10.2967/jnumed.116.187419. [DOI] [PubMed] [Google Scholar]
- 4.Koziorowski J., Stanciu A.E., Gomez-Vallejo V., Llop J. Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anti-Cancer Agents Med. Chem. 2017;17:333–354. doi: 10.2174/1871520616666160219162902. [DOI] [PubMed] [Google Scholar]
- 5.Raj S., Khurana S., Choudhari R., Kesari K.K., Kamal M.A., Garg N., Ruokolainen J., Das B.C., Kumar D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Maccora D., Dini V., Battocchio C., Fratoddi I., Cartoni A., Rotili D., Castagnola M., Faccini R., Bruno I., Scotognella T., et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019;9:3232. doi: 10.3390/app9163232. [DOI] [Google Scholar]
- 7.Yetisgin A.A., Cetinel S., Zuvin M., Kosar A., Kutlu O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daraee H., Eatemadi A., Abbasi E., Fekri Aval S., Kouhi M., Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:410–422. doi: 10.3109/21691401.2014.955107. [DOI] [PubMed] [Google Scholar]
- 9.Saboktakin M. The biological and biomedical naparticles—Synthesis and applications. Adv. Mater. Sci. 2017 doi: 10.15761/AMS.1000127. [DOI] [Google Scholar]
- 10.Delfi M., Ghomi M., Zarrabi A., Mohammadinejad R., Taraghdari Z.B., Ashrafizadeh M., Zare E.N., Agarwal T., Padil V.V.T., Mokhtari B., et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis. 2020;2:117–139. doi: 10.3390/prosthesis2020012. [DOI] [Google Scholar]
- 11.Thiruppathi R., Mishra S., Ganapathy M., Padmanabhan P., Gulyás B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2016;4:1600279. doi: 10.1002/advs.201600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zein R., Sharrouf W., Selting K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020;2020:5194780. doi: 10.1155/2020/5194780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y., van der Meel R., Chen X., Lammers T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajamanickam K. Multimodal Molecular Imaging Strategies using Functionalized Nano Probes. J. Nanotechnol. Res. 2019;1:119. doi: 10.26502/jnr.2688-85210010. [DOI] [Google Scholar]
- 16.Majumder J., Minko T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2020 doi: 10.1080/17425247.2021.1828339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lina L., Shuhe K., Chao S., Chufeng S., Zhong G., Jia L., Taofeng Z., Xingping L., Bin L. Multifunctional Nanoparticles in Precise Cancer Treatment: Considerations in Design and Functionalization of Nanocarriers. Curr. Top. Med. Chem. 2020;20:2427–2441. doi: 10.2174/1568026620666200825170030. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudi M., Sant S., Wang B., Laurent S., Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011;63:24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Farjadian F., Ghasemi A., Gohari O., Roointan A., Karimi M., Hamblin M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine. 2018;14:93–126. doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown T.D., Habibi N., Wu D., Lahann J., Mitragotri S. Effect of Nanoparticle Composition, Size, Shape, and Stiffness on Penetration Across the Blood–Brain Barrier. ACS Biomater. Sci. Eng. 2020;6:4916–4928. doi: 10.1021/acsbiomaterials.0c00743. [DOI] [PubMed] [Google Scholar]
- 21.Freitas de Freitas L., Varca G.H.C., Baptista J.G.S., Lugão A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials. 2018;8:939. doi: 10.3390/nano8110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z.Y., Wang S., Dong D., Wei J.W., Fang C., Zhou X.Z., Sun K., Li L.F., Li B., Wang M.Y., et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019;9:1303–1322. doi: 10.7150/thno.30309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M., Shu J. Multimodal Molecular Imaging: Current Status and Future Directions. Contrast Media Mol. Imaging. 2018;2018:1382183. doi: 10.1155/2018/1382183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks R., Hofman M. Is there still a role for SPECt CT in oncology in the PEt CT era? Nat. Rev. Clin. Oncol. 2012;9:712–720. doi: 10.1038/nrclinonc.2012.188. [DOI] [PubMed] [Google Scholar]
- 25.Goel S., England C.G., Chen F., Cai W.B. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017;113:157–176. doi: 10.1016/j.addr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku A., Facca V.J., Cai Z., Reilly R.M. Auger electrons for cancer therapy—A review. Ejnmmi Radiopharm. Chem. 2019;4:27. doi: 10.1186/s41181-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gharatape A., Salehi R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017;138:221–233. doi: 10.1016/j.ejmech.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 28.FDA.gov. [(accessed on 21 December 2020)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-digestive-tract-cancers.
- 29.Aghanejad A., Omidi Y. Chapter 25—Radiolabeled Theranostics: Magnetic and Gold Hybrid Nanoparticles. In: Mohapatra S., Nguyen T.A., Nguyen-Tri P., editors. Noble Metal-Metal Oxide Hybrid Nanoparticles. Woodhead Publishing; Cambridge, UK: 2019. pp. 535–547. [DOI] [Google Scholar]
- 30.Turkavich J., Stevenson P.C., Hillier J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951;11:55. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 31.Brust M., Walker M., Bethell D., Schiffrin D.J., Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994:801–802. doi: 10.1039/C39940000801. [DOI] [Google Scholar]
- 32.Chen S.W. 4-hydroxythiophenol-protected gold nanoclusters in aqueous media. Langmuir. 1999;15:7551–7557. doi: 10.1021/la990398g. [DOI] [Google Scholar]
- 33.Chen S.W., Murray R.W. Arenethiolate monolayer-protected gold clusters. Langmuir. 1999;15:682–689. doi: 10.1021/la980817u. [DOI] [Google Scholar]
- 34.Khoobchandani M., Katti K.K., Karikachery A.R., Thipe V.C., Srisrimal D., Kumar D., Mohandoss D., Darshakumar R.D., Joshi C.M., Katti K.V. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine—Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020;15:181–197. doi: 10.2147/IJN.S219042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nune S.K., Chanda N., Shukla R., Katti K., Kulkarni R.R., Thilakavathy S., Mekapothula S., Kannan R., Katti K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009;19:2912–2920. doi: 10.1039/b822015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla R., Nune S.K., Chanda N., Katti K., Mekapothula S., Kulkami R.R., Welshons W.V., Kannan R., Katti K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanopartictes. Small. 2008;4:1425–1436. doi: 10.1002/smll.200800525. [DOI] [PubMed] [Google Scholar]
- 37.De Souza C.D., Nogueira B.R., Rostelato M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019;798:714–740. doi: 10.1016/j.jallcom.2019.05.153. [DOI] [Google Scholar]
- 38.Perez-Juste J., Pastoriza-Santos I., Liz-Marzan L.M., Mulvaney P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005;249:1870–1901. doi: 10.1016/j.ccr.2005.01.030. [DOI] [Google Scholar]
- 39.An L., Wang Y.Y., Tian Q.W., Yang S.P. Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy. Materials. 2017;10:1372. doi: 10.3390/ma10121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed W., Bhatti A.S., van Ruitenbeek J.M. Efficient seed-mediated method for the large-scale synthesis of Au nanorods. J. Nanoparticle Res. 2017;19:115. doi: 10.1007/s11051-017-3815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji C.X., Searson P.C. Synthesis and characterization of nanoporous gold nanowires. J. Phys. Chem. B. 2003;107:4494–4499. doi: 10.1021/jp0222200. [DOI] [Google Scholar]
- 42.Moon J.M., Wei A. Uniform gold nanorod arrays from polyethylenimine-coated alumina templates. J. Phys. Chem. B. 2005;109:23336–23341. doi: 10.1021/jp054405n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y.Y., Chang S.S., Lee C.L., Wang C.R.C. Gold nanorods: Electrochemical synthesis and optical properties. J. Phys. Chem. B. 1997;101:6661–6664. doi: 10.1021/jp971656q. [DOI] [Google Scholar]
- 44.Wang Y.C., Rheaume E., Lesage F., Kakkar A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules. 2018;23:2851. doi: 10.3390/molecules23112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y.G., Xia Y.N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004;126:3892–3901. doi: 10.1021/ja039734c. [DOI] [PubMed] [Google Scholar]
- 46.Mourdikoudis S., Pallares R.M., Thanh N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10:12871–12934. doi: 10.1039/C8NR02278J. [DOI] [PubMed] [Google Scholar]
- 47.Modena M.M., Rühle B., Burg T.P., Wuttke S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019;31:1901556. doi: 10.1002/adma.201901556. [DOI] [PubMed] [Google Scholar]
- 48.Tiwari P.M., Vig K., Dennis V.A., Singh S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials. 2011;1:31–63. doi: 10.3390/nano1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva F., Zambre A., Campello M.P.C., Gano L., Santos I., Ferraria A.M., Ferreira M.J., Singh A., Upendran A., Paulo A., et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016;27:1153–1164. doi: 10.1021/acs.bioconjchem.6b00102. [DOI] [PubMed] [Google Scholar]
- 50.Alric C., Taleb J., Le Duc G., Mandon C., Billotey C., Le Meur-Herland A., Brochard T., Vocanson F., Janier M., Perriat P., et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 51.Mendoza-Sanchez A.N., Ferro-Flores G., Ocampo-Garcia B.E., Morales-Avila E., Ramirez F.D., De Leon-Rodriguez L.M., Santos-Cuevas C.L., Medina L.A., Rojas-Calderon E.L., Camacho-Lopez M.A. Lys(3)-Bombesin Conjugated to Tc-99m-Labelled Gold Nanoparticles for In Vivo Gastrin Releasing Peptide-Receptor Imaging. J. Biomed. Nanotechnol. 2010;6:375–384. doi: 10.1166/jbn.2010.1132. [DOI] [PubMed] [Google Scholar]
- 52.Reznickova A., Slavikova N., Kolska Z., Kolarova K., Belinova T., Kalbacova M.H., Cieslar M., Svorcik V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloids Surf. A-Physicochem. Eng. Asp. 2019;560:26–34. doi: 10.1016/j.colsurfa.2018.09.083. [DOI] [Google Scholar]
- 53.Kluenker M., Mondeshki M., Tahir M.N., Tremel W. Monitoring Thiol-Ligand Exchange on Au Nanoparticle Surfaces. Langmuir. 2018;34:1700–1710. doi: 10.1021/acs.langmuir.7b04015. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J.J., Mou L., Jiang X.Y. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020;11:923–936. doi: 10.1039/C9SC06497D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farooq M.U., Novosad V., Rozhkova E.A., Wali H., Ali A., Fateh A.A., Neogi P.B., Neogi A., Wang Z.M. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018;8:2907. doi: 10.1038/s41598-018-21331-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Al-Yasiri A.Y., Khoobchandani M., Cutler C.S., Watkinson L., Carmack T., Smith C.J., Kuchuk M., Loyalka S.K., Lugao A.B., Katti K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-(198)AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017;46:14561–14571. doi: 10.1039/C7DT00383H. [DOI] [PubMed] [Google Scholar]
- 57.Chakravarty R., Chakraborty S., Guleria A., Shukla R., Kumar C., Nair K.V.V., Sarma H.D., Tyagi A.K., Dash A. Facile One-Pot Synthesis of Intrinsically Radiolabeled and Cyclic RGD Conjugated Au-199 Nanoparticles for Potential Use in Nanoscale Brachytherapy. Ind. Eng. Chem. Res. 2018;57:14337–14346. doi: 10.1021/acs.iecr.8b02526. [DOI] [Google Scholar]
- 58.Hosseini S.F., Sadeghi M., Aboudzadeh M.R., Mohseni M. Production and modeling of radioactive gold nanoparticles in Tehran research reactor. Appl. Radiat. Isot. 2016;118:361–365. doi: 10.1016/j.apradiso.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y.Y., Zhang Y.X., Yin L.L., Xia X.T., Hu F., Liu Q.Y., Qin C.X., Lan X.L. Synthesis and Bioevaluation of Iodine-131 Directly Labeled Cyclic RGD-PEGylated Gold Nanorods for Tumor-Targeted Imaging. Contrast Media Mol. Imaging. 2017 doi: 10.1155/2017/6081724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frellsen A.F., Hansen A.E., Jolck R.I., Kempen P.J., Severin G.W., Rasmussen P.H., Kjaer A., Jensen A.T.I., Andresen T.L. Mouse Positron Emission Tomography Study of the Biodistribution of Gold Nanoparticles with Different Surface Coatings Using Embedded Copper-64. ACS Nano. 2016;10:9887–9898. doi: 10.1021/acsnano.6b03144. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y.F., Sultan D., Detering L., Cho S.H., Sun G.R., Pierce R., Wooley K.L., Liu Y.J. Copper-64-Alloyed Gold Nanoparticles for Cancer Imaging: Improved Radiolabel Stability and Diagnostic Accuracy. Angew. Chem. Int. Ed. 2014;53:156–159. doi: 10.1002/anie.201308494. [DOI] [PubMed] [Google Scholar]
- 62.Sun X.L., Huang X.L., Yan X.F., Wang Y., Guo J.X., Jacobson O., Liu D.B., Szajek L.P., Zhu W.L., Niu G., et al. Chelator-Free Cu-64-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano. 2014;8:8438–8446. doi: 10.1021/nn502950t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrero S., Herance J.R., Rojas S., Mena J.F., Gispert J.D., Acosta G.A., Albericio F., Kogan M.J. Synthesis and In Vivo Evaluation of the Biodistribution of a F-18-Labeled Conjugate Gold-Nanoparticle-Peptide with Potential Biomedical Application. Bioconjugate Chem. 2012;23:399–408. doi: 10.1021/bc200362a. [DOI] [PubMed] [Google Scholar]
- 64.Zhu J., Chin J., Wangler C., Wangler B., Lennox R.B., Schirrmacher R. Rapid F-18-Labeling and Loading of PEGylated Gold Nanoparticles for In Vivo Applications. Bioconjugate Chem. 2014;25:1143–1150. doi: 10.1021/bc5001593. [DOI] [PubMed] [Google Scholar]
- 65.Farzin L., Sheibani S., Moassesi M.E., Shamsipur M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. Part A. 2019;107:251–285. doi: 10.1002/jbm.a.36550. [DOI] [PubMed] [Google Scholar]
- 66.Xie H.A., Diagaradjane P., Deorukhkar A.A., Goins B., Bao A., Phillips W.T., Wang Z., Schwartz J., Krishnan S. Integrin alpha(v)beta(3)-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int. J. Nanomed. 2011;6:259–269. doi: 10.2147/IJN.S15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tam A.L., Melancon M.P., Abdelsalam M., Figueira T.A., Dixon K., McWatters A., Zhou M., Huang Q., Mawlawi O., Dunner K., et al. Imaging Intratumoral Nanoparticle Uptake After Combining Nanoembolization with Various Ablative Therapies in Hepatic VX2 Rabbit Tumors. J. Biomed. Nanotechnol. 2016;12:296–307. doi: 10.1166/jbn.2016.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian M., Lu W., Zhang R., Xiong C.Y., Ensor J., Nazario J., Jackson J., Shaw C., Dixon K.A., Miller J., et al. Tumor Uptake of Hollow Gold Nanospheres After Intravenous and Intra-arterial Injection: PET/CT Study in a Rabbit VX2 Liver Cancer Model. Mol. Imaging Biol. 2013;15:614–624. doi: 10.1007/s11307-013-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y.F., Pang B., Detering L., Luehmann H., Yang M.X., Black K., Sultan D., Xia Y.N., Liu Y.J. Melanocortin I Receptor Targeted Imaging of Melanoma With Gold Nanocages and Positron Emission Tomography. Mol. Imaging. 2018;17 doi: 10.1177/1536012118775827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pretze M., van der Meulen N.P., Wangler C., Schibli R., Wangler B. Targeted Cu-64-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019;62:471–482. doi: 10.1002/jlcr.3736. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y., Detering L., Sultan D., Cooper M.L., You M., Cho S., Meier S.L., Luehmann H., Sun G., Rettig M., et al. Gold Nanoclusters Doped with (64)Cu for CXCR4 Positron Emission Tomography Imaging of Breast Cancer and Metastasis. ACS Nano. 2016;10:5959–5970. doi: 10.1021/acsnano.6b01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pang B., Zhao Y., Luehmann H., Yang X., Detering L., You M., Zhang C., Zhang L., Li Z.-Y., Ren Q., et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano. 2016;10:3121–3131. doi: 10.1021/acsnano.5b07968. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Liu Y., Luehmann H., Xia X., Brown P., Jarreau C., Welch M., Xia Y. Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano. 2012;6:5880–5888. doi: 10.1021/nn300464r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie H., Wang Z.J., Bao A., Goins B., Phillips W.T. In Vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int. J. Pharm. 2010;395:324–330. doi: 10.1016/j.ijpharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Silva F., Paulo A., Pallier A., Même S., Tóth É., Gano L., Marques F., Geraldes C.F.G.C., Castro M.M.C.A., Jurado A.S., et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials. 2020;13:513. doi: 10.3390/ma13030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chilug L.E., Leonte R.A., Barbinta Patrascu M.E., Ion A.C., Tuta C.S., Raicu A., Manda G., Niculae D. In Vitro binding kinetics study of gold nanoparticles functionalized with Ga-68-DOTA conjugated peptides. J. Radioanal. Nucl. Chem. 2017;311:1485–1493. doi: 10.1007/s10967-016-5075-z. [DOI] [Google Scholar]
- 77.Pretze M., Hien A., Rädle M., Schirrmacher R., Wängler C., Wängler B. Gastrin-Releasing Peptide Receptor- and Prostate-Specific Membrane Antigen-Specific Ultrasmall Gold Nanoparticles for Characterization and Diagnosis of Prostate Carcinoma via Fluorescence Imaging. Bioconjugate Chem. 2018;29:1525–1533. doi: 10.1021/acs.bioconjchem.8b00067. [DOI] [PubMed] [Google Scholar]
- 78.Li X., Xiong Z.G., Xu X.Y., Luo Y., Peng C., Shen M.W., Shi X.Y. Tc-99m-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors. ACS Appl. Mater. Interfaces. 2016;8:19883–19891. doi: 10.1021/acsami.6b04827. [DOI] [PubMed] [Google Scholar]
- 79.Wen S.H., Zhao L.Z., Zhao Q.H., Li D., Liu C.C., Yu Z.B., Shen M.W., Majoral J.P., Mignani S., Zhao J.H., et al. A promising dual mode SPECT/CT imaging platform based on Tc-99m-labeled multifunctional dendrimer-entrapped gold nanoparticles. J. Mater. Chem. B. 2017;5:3810–3815. doi: 10.1039/C7TB00543A. [DOI] [PubMed] [Google Scholar]
- 80.Li Y.J., Zhao L.Z., Xu X.Y., Sun N., Qiao W.L., Xing Y., Shen M.W., Zhu M.L., Shi X.Y., Zhao J.H. Design of Tc-99(m)-Labeled Low Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted Single Photon Emission Computed Tomography/Computed Tomography Imaging of Gliomas. J. Biomed. Nanotechnol. 2019;15:1201–1212. doi: 10.1166/jbn.2019.2760. [DOI] [PubMed] [Google Scholar]
- 81.Xing Y., Zhu J.Y., Zhao L.Z., Xiong Z.J., Li Y.J., Wu S., Chand G., Shi X.Y., Zhao J.H. SPECT/CT imaging of chemotherapy-induced tumor apoptosis using Tc-99m-labeled dendrimer-entrapped gold nanoparticles. Drug Deliv. 2018;25:1384–1393. doi: 10.1080/10717544.2018.1474968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X.Y., Zhao L.Z., Li X., Wang P., Zhao J.H., Shi X.Y., Shen M.W. Targeted tumor SPECT/CT dual mode imaging using multifunctional RGD-modified low generation dendrimer-entrapped gold nanoparticles. Biomater. Sci. 2017;5:2393–2397. doi: 10.1039/C7BM00826K. [DOI] [PubMed] [Google Scholar]
- 83.Peiris P.M., Deb P., Doolittle E., Doron G., Goldberg A., Govender P., Shah S., Rao S., Carbone S., Cotey T., et al. Vascular Targeting of a Gold Nanoparticle to Breast Cancer Metastasis. J. Pharm. Sci. 2015;104:2600–2610. doi: 10.1002/jps.24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamal R., Chadha V.D., Dhawan D.K. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles (Tc-99m-Res-AuNP) in colon cancer tissue. Nanomed. Nanotechnol. Biol. Med. 2018;14:1059–1071. doi: 10.1016/j.nano.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Zhu J.Y., Zhao L.Z., Yang J.X., Chen L., Shi J.H., Zhao J.H., Shi X.Y. Tc-99m-Labeled Polyethylenimine-Entrapped Gold Nanoparticles with pH-Responsive Charge Conversion Property for Enhanced Dual Mode SPECT/CT Imaging of Cancer Cells. Langmuir. 2019;35:13405–13412. doi: 10.1021/acs.langmuir.9b02617. [DOI] [PubMed] [Google Scholar]
- 86.Li X., Wang C., Tan H., Cheng L.L., Liu G.B., Yang Y., Zhao Y.Z., Zhang Y.Q., Li Y.L., Zhan C.F., et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials. 2016;108:71–80. doi: 10.1016/j.biomaterials.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 87.Sakr T.M., Morsy S.A.G., Mahmoud N.A., Rashed H.M., El-Rehim H.A.A., Khoobchandani M., Katti K.K., Katti K.V. Preparation, Characterization, Cytotoxicity and Biological Evaluation of 99mTc-Doxorubicin-Epigallocatechingallate Functionalized Gold Nanoparticles as a New Genaration of Theranostic Radiopharmaceutical. Preprints. 2018;2018080331 doi: 10.20944/preprints201808.200331.v201801. [DOI] [Google Scholar]
- 88.Sakr T.M., El-Hashash M.A., El-Mohty A.A., Essa B.M. 99mTc-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl. Radiat. Isot. 2020;164 doi: 10.1016/j.apradiso.2020.109269. [DOI] [PubMed] [Google Scholar]
- 89.El-Ghareb W.I., Swidan M.M., Ibrahim I.T., El-Bary A.A., Tadros M.I., Sakr T.M. 99mTc-doxorubicin-loaded gallic acid-gold nanoparticles (99mTc-DOX-loaded GA-Au NPs) as a multifucntional theranostic agent. Int. J. Pharm. 2020;586:119514. doi: 10.1016/j.ijpharm.2020.119514. [DOI] [PubMed] [Google Scholar]
- 90.Black K.C.L., Akers W.J., Sudlow G., Xu B.G., Laforest R., Achilefu S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale. 2015;7:440–444. doi: 10.1039/C4NR05269B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song L., Falzone N., Vallis K.A. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. Int. J. Radiat. Biol. 2016;92:716–723. doi: 10.3109/09553002.2016.1145360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chattopadhyay N., Fonge H., Cai Z.L., Scollard D., Lechtman E., Done S.J., Pignol J.P., Reilly R.M. Role of Antibody-Mediated Tumor Targeting and Route of Administration in Nanoparticle Tumor Accumulation In Vivo. Mol. Pharm. 2012;9:2168–2179. doi: 10.1021/mp300016p. [DOI] [PubMed] [Google Scholar]
- 93.Cai Z.L., Chattopadhyay N., Yang K.Y., Kwon Y.L., Yook S., Pignol J.P., Reilly R.M. In-111-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl. Med. Biol. 2016;43:818–826. doi: 10.1016/j.nucmedbio.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Lee S.B., Ahn S.B., Lee S.W., Jeong S.Y., Ghilsuk Y., Ahn B.C., Kim E.M., Jeong H.J., Lee J., Lim D.K., et al. Radionuclide-embedded gold nanoparticles for enhanced dendritic cell-based cancer immunotherapy, sensitive and quantitative tracking of dendritic cells with PET and Cerenkov luminescence. NPG Asia Mater. 2016;8:e281. doi: 10.1038/am.2016.80. [DOI] [Google Scholar]
- 95.Lee S.B., Lee H.W., Singh T.D., Li Y., Kim S.K., Cho S.J., Lee S.-W., Jeong S.Y., Ahn B.-C., Choi S., et al. Visualization of Macrophage Recruitment to Inflammation Lesions using Highly Sensitive and Stable Radionuclide-Embedded Gold Nanoparticles as a Nuclear Bio-Imaging Platform. Theranostics. 2017;7:926–934. doi: 10.7150/thno.17131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee S.B., Lee Y.J., Cho S.J., Kim S.K., Lee S.W., Lee J., Lim D.K., Jeon Y.H. Antigen-Free Radionuclide-Embedded Gold Nanoparticles for Dendritic Cell Maturation, Tracking, and Strong Antitumor Immunity. Adv. Healthc. Mater. 2018;7:e1701369. doi: 10.1002/adhm.201701369. [DOI] [PubMed] [Google Scholar]
- 97.Lee S.B., Kumar D., Li Y., Lee I.-K., Cho S.J., Kim S.K., Lee S.-W., Jeong S.Y., Lee J., Jeon Y.H. PEGylated crushed gold shell-radiolabeled core nanoballs for in vivo tumor imaging with dual positron emission tomography and Cerenkov luminescent imaging. J. Nanobiotechnol. 2018;16:41. doi: 10.1186/s12951-018-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee S.B., Lee J.E., Cho S.J., Chin J., Kim S.K., Lee I.K., Lee S.W., Lee J., Jeon Y.H. Crushed Gold Shell Nanoparticles Labeled with Radioactive Iodine as a Theranostic Nanoplatform for Macrophage-Mediated Photothermal Therapy. Nano-Micro Lett. 2019;11:36. doi: 10.1007/s40820-019-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang L., Su H.L., Wang H.L., Li Q., Li X., Zhou C.Q., Xu J., Chai Y.M., Liang X.W., Xiong L.Q., et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics. 2019;9:1893–1908. doi: 10.7150/thno.30523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Y.F., Pang B., Luehmann H., Detering L., Yang X., Sultan D., Harpstrite S., Sharma V., Cutler C.S., Xia Y.N., et al. Gold Nanoparticles Doped with Au-199 Atoms and Their Use for Targeted Cancer Imaging by SPECT. Adv. Healthc. Mater. 2016;5:928–935. doi: 10.1002/adhm.201500992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garg P., Hazra D.K. Conjugation of Antibodies with Radiogold Nanoparticles, as an Effector Targeting Agents in Radiobioconjugate Cancer Therapy: Optimized Labeling and Biodistribution Results. Indian J. Nucl. Med. 2017;32:296–303. doi: 10.4103/ijnm.IJNM_80_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Yasiri A.Y., White N.E., Katti K.V., Loyalka S.K. Estimation of tumor and local tissue dose in gold nanoparticles radiotherapy for prostate cancer. Rep. Pract. Oncol. Radiother. 2019;24:288–293. doi: 10.1016/j.rpor.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang Y.-Y. An Overview of PET Radiopharmaceuticals in Clinical Use: Regulatory, Quality and Pharmacopeia Monographs of the United States and Europe. In: Shahzad A., Bashir S., editors. Nuclear Medicine Physics. IntechOpen; London, UK: 2018. [DOI] [Google Scholar]
- 104.Sivasubramanian M., Chuang Y.C., Chen N.T., Lo L.W. Seeing Better and Going Deeper in Cancer Nanotheranostics. Int. J. Mol. Sci. 2019;20:3490. doi: 10.3390/ijms20143490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koehler L., Gagnon K., McQuarrie S., Wuest F. Iodine-124: A promising positron emitter for organic PET chemistry. Molecules. 2010;15:2686–2718. doi: 10.3390/molecules15042686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S.B., Lee S.W., Jeong S.Y., Yoon G., Cho S.J., Kim S.K., Lee I.K., Ahn B.C., Lee J., Jeon Y.H. Engineering of Radioiodine-Labeled Gold Core Shell Nanoparticles As Efficient Nuclear Medicine Imaging Agents for Trafficking of Dendritic Cells. ACS Appl. Mater. Interfaces. 2017;9:8480–8489. doi: 10.1021/acsami.6b14800. [DOI] [PubMed] [Google Scholar]
- 107.Lee S.B., Yoon G., Lee S.W., Jeong S.Y., Ahn B.C., Lim D.K., Lee J., Jeon Y.H. Combined Positron Emission Tomography and Cerenkov Luminescence Imaging of Sentinel Lymph Nodes Using PEGylated Radionuclide-Embedded Gold Nanoparticles. Small. 2016;12:4894–4901. doi: 10.1002/smll.201601721. [DOI] [PubMed] [Google Scholar]
- 108.Pérez-Medina C., Teunissen A.J.P., Kluza E., Mulder W.J.M., van der Meel R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliv. Rev. 2020 doi: 10.1016/j.addr.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 109.Ge J.X., Zhang Q.Y., Zeng J.F., Gu Z., Gao M.Y. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials. 2020;228:119553. doi: 10.1016/j.biomaterials.2019.119553. [DOI] [PubMed] [Google Scholar]
- 110.Anderegg G., Arnaud-Neu F., Delgado R., Felcman J., Popov K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report) Pure Appl. Chem. 2005;77:1445–1495. doi: 10.1351/pac200577081445. [DOI] [Google Scholar]
- 111.Boswell C.A., Sun X.K., Niu W.J., Weisman G.R., Wong E.H., Rheingold A.L., Anderson C.J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 112.Jorgensen J.T., Persson M., Madsen J., Kjaer A. High tumor uptake of Cu-64: Implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol. 2013;40:345–350. doi: 10.1016/j.nucmedbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 113.Jalilian A.R., Osso J., Jr. The Current Status and Future of Theranostic Copper-64 Radiopharmaceuticals. Volume 25. Research Center for Nuclear Medicine, Tehran University of Medical Sciences; Tehran, Iran: 2017. pp. 1–10. [Google Scholar]
- 114.Kubicek V., Bohmova Z., Sevcikova R., Vanek J., Lubal P., Polakova Z., Michalicova R., Kotek J., Hermann P. NOTA Complexes with Copper(II) and Divalent Metal Ions: Kinetic and Thermodynamic Studies. Inorg. Chem. 2018;57:3061–3072. doi: 10.1021/acs.inorgchem.7b02929. [DOI] [PubMed] [Google Scholar]
- 115.Baranyai Z., Tircso G., Rosch F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020;2020:36–56. doi: 10.1002/ejic.201900706. [DOI] [Google Scholar]
- 116.Meisenheimer M., Saenko Y., Eppard E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. IntechOpen; London, UK: 2019. [DOI] [Google Scholar]
- 117.Zambre A., Silva F., Upendran A., Afrasiabi Z., Xin Y., Paulo A., Kannan R. Synthesis and characterization of functional multicomponent nanosized gallium chelated gold crystals. Chem. Commun. 2014;50:3281–3284. doi: 10.1039/C3CC47308B. [DOI] [PubMed] [Google Scholar]
- 118.Duatti A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2020 doi: 10.1016/j.nucmedbio.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 119.Debouttiere P.J., Roux S., Vocanson F., Billotey C., Beuf O., Favre-Reguillon A., Lin Y., Pellet-Rostaing S., Lamartine R., Perriat P., et al. Design of gold nanoparticles for magnetic resonance imaging. Adv. Funct. Mater. 2006;16:2330–2339. doi: 10.1002/adfm.200600242. [DOI] [Google Scholar]
- 120.Alric C., Miladi I., Kryza D., Taleb J., Lux F., Bazzi R., Billotey C., Janier M., Perriat P., Roux S., et al. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale. 2013;5:5930–5939. doi: 10.1039/c3nr00012e. [DOI] [PubMed] [Google Scholar]
- 121.Silva F., Gano L., Campello M.P.C., Marques R., Prudencio I., Zambre A., Upendran A., Paulo A., Kannan R. In vitro/in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable Tc-99m-label. Dalton Trans. 2017;46:14572–14583. doi: 10.1039/C7DT00864C. [DOI] [PubMed] [Google Scholar]
- 122.Psimadas D., Georgoulias P., Valotassiou V., Loudos G. Molecular Nanomedicine Towards Cancer: In-111-Labeled Nanoparticles. J. Pharm. Sci. 2012;101:2271–2280. doi: 10.1002/jps.23146. [DOI] [PubMed] [Google Scholar]
- 123.Reilly R.M., Kiarash R., Cameron R.G., Porlier N., Sandhu J., Hill R.P., Vallis K., Hendler A., Gariépy J. 111In-labeled EGF Is Selectively Radiotoxic to Human Breast Cancer Cells Overexpressing EGFR. J. Nucl. Med. 2000;41:429. [PubMed] [Google Scholar]
- 124.Chakravarty R., Chakraborty S., Guleria A., Kumar C., Kunwar A., Nair K.V.V., Sarma H.D., Dash A. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized Au-198 nanoparticles for targeted cancer therapy. Nucl. Med. Biol. 2019;72–73:1–10. doi: 10.1016/j.nucmedbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Lai P., Cai Z.L., Pignol J.P., Lechtman E., Mashouf S., Lu Y.J., Winnik M.A., Jaffray D.A., Reilly R.M. Monte Carlo simulation of radiation transport and dose deposition from locally released gold nanoparticles labeled with In-111, Lu-177 or Y-90 incorporated into tissue implantable depots. Phys. Med. Biol. 2017;62:8581–8599. doi: 10.1088/1361-6560/aa9106. [DOI] [PubMed] [Google Scholar]
- 126.Buckway B., Frazier N., Gormley A.J., Ray A., Ghandehari H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer-Y-90 conjugates in treatment of prostate tumors. Nucl. Med. Biol. 2014;41:282–289. doi: 10.1016/j.nucmedbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao L.Z., Li Y.J., Zhu J.Y., Sun N., Song N.N., Xing Y., Huang H., Zhao J.H. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019;17 doi: 10.1186/s12951-019-0462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mendoza-Nava H., Ferro-Flores G., Ramirez F.D., Ocampo-Garcia B., Santos-Cuevas C., Aranda-Lara L., Azorin-Vega E., Morales-Avila E., Isaac-Olive K. Lu-177-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomater. 2016 doi: 10.1155/2016/1039258. [DOI] [Google Scholar]
- 129.Mendoza-Nava H., Ferro-Flores G., Ramirez F.D., Ocampo-Garcia B., Santos-Cuevas C., Azorin-Vega E., Jimenez-Mancilla N., Luna-Gutierrez M., Isaac-Olive K. Fluorescent, Plasmonic, and Radiotherapeutic Properties of the Lu-177-Dendrimer-AuNP-Folate-Bombesin Nanoprobe Located Inside Cancer Cells. Mol. Imaging. 2017;16 doi: 10.1177/1536012117704768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yook S., Cai Z.L., Lu Y.J., Winnik M.A., Pignol J.P., Reilly R.M. Intratumorally Injected Lu-177-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. J. Nucl. Med. 2016;57:936–942. doi: 10.2967/jnumed.115.168906. [DOI] [PubMed] [Google Scholar]
- 131.Yook S., Lu Y.J., Jeong J.J., Cai Z.L., Tong L., Alwarda R., Pignol J.P., Winnik M.A., Reilly R.M. Stability and Biodistribution of Thiol-Functionalized and Lu-177-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules. 2016;17:1292–1302. doi: 10.1021/acs.biomac.5b01642. [DOI] [PubMed] [Google Scholar]
- 132.Dziawer L., Majkowska-Pilip A., Gawel D., Godlewska M., Pruszynski M., Jastrzebski J., Was B., Bilewicz A. Trastuzumab-Modified Gold Nanoparticles Labeled with At-211 as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials. 2019;9:632. doi: 10.3390/nano9040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salvanou E.A., Stellas D., Tsoukalas C., Mavroidi B., Paravatou-Petsotas M., Kalogeropoulos N., Xanthopoulos S., Denat F., Laurent G., Bazzi R., et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics. 2020;12:188. doi: 10.3390/pharmaceutics12020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai P., Lechtman E., Mashouf S., Pignol J.-P., Reilly R.M. Depot system for controlled release of gold nanoparticles with precise intratumoral placement by permanent brachytherapy seed implantation (PSI) techniques. Int. J. Pharm. 2016;515:729–739. doi: 10.1016/j.ijpharm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Dymova M.A., Taskaev S.Y., Richter V.A., Kuligina E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020;40:406–421. doi: 10.1002/cac2.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pulagam K.R., Gona K.B., Gómez-Vallejo V., Meijer J., Zilberfain C., Estrela-Lopis I., Baz Z., Cossío U., Llop J. Gold Nanoparticles as Boron Carriers for Boron Neutron Capture Therapy: Synthesis, Radiolabelling and In Vivo Evaluation. Molecules. 2019;24:3609. doi: 10.3390/molecules24193609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feiner I.V.J., Pulagam K.R., Gómez-Vallejo V., Zamacola K., Baz Z., Caffarel M.M., Lawrie C.H., Ruiz-de-Angulo A., Carril M., Llop J. Therapeutic Pretargeting with Gold Nanoparticles as Drug Candidates for Boron Neutron Capture Therapy. Part. Part. Syst. Charact. 2020 doi: 10.1002/ppsc.202000200. [DOI] [Google Scholar]