Abstract
Conventional one-sensor theory (one afferent fiber connects to a single sensor) categorizes the bronchopulmonary mechanosensors into the rapidly adapting receptors (RARs), slowly adapting receptors (SARs), or intermediate adapting receptors (IARs). RARs and SARs are known to sense the rate and magnitude of mechanical change, respectively; however, there is no agreement on what IARs sense. Some investigators believe that the three types of sensors are actually one group with similar but different properties and IARs operate within that group. Other investigators (majority) believe IARs overlap with the RARs and SARs and can be classified within them according to their characteristics. Clearly, there is no consensus on IARs function. Recently, a multiple-sensor theory has been advanced in which a sensory unit may contain many heterogeneous sensors, such as both RARs and SARs. There are no IARs. Intermediate adapting unit behavior results from coexistence of RARs and SARs. Therefore, the unit can sense both rate and magnitude of changes. The purpose of this review is to provide evidence that the multiple-sensor theory better explains sensory unit behavior.
Keywords: afferent, lung, receptor, sensory unit, vagus nerve
INTRODUCTION
Two doctrines are conventionally followed in the field of bronchopulmonary mechanosensory units and in their reflex functions: one-sensor theory and line-labeled theory (13). One-sensor theory posits that one afferent fiber is connected with a single sensor, either a rapidly adapting receptor (RAR), slowly adapting receptor (SAR), or intermediate adapting receptor (IAR). The sensor generates dynamic and static information during both lung inflation and deflation. In line-labeled theory, information from a given sensor travels in a particular afferent to specific parts of the central nervous system (CNS) to evoke reflex responses via efferent pathways. Thus, activation of one type of sensor produces a unique response. For example, in general, activation of RARs elicits excitatory effects on breathing (excitatory line), whereas activation of SARs induces inhibitory effects (inhibitory line). These two approaches have created a significant amount of confusion, especially regarding the IARs. With recent advances in morphological and physiological studies, the subcellular structure and function of a sensory unit can be explored. Based on new information, a multiple-sensor theory has been proposed (34a). However, most researchers maintain the conventional view. This review outlines multiple-sensor theory as it applies to the bronchopulmonary mechanosensors with a focus on IARs. It is the third article of “Spectrum of Myelinated Pulmonary Afferents” series, the first two being published in 2000 (36) and 2013 (20), respectively.
HISTORICAL ACCOUNT
Intermediate adapting receptors (IARs) were first described by Knowlton and Larrabee in 1946 (16). In their seminal paper, they studied 90 bronchopulmonary mechanosensors, dividing them according to adaptation rates (or adaptation index, AI) as rapidly adapting receptors (RARs; AI > 80%) and slowly adapting receptors (SARs; AI < 55%). Forty-eight receptors (53%) were slowly adapting and 35 receptors (39%) were rapidly adapting. The remaining seven (8%) showed adaptation rates in between and were named intermediate receptors. They state: “An examination of the original records for the characteristic patterns of discharge described … shows that each of these seven end-organs clearly belongs in one or the other of the two major groups.” (referring to RARs and SARs). In a classical paper, Widdicombe (in 1954) (33) examined 166 sensors, finding 82 SARs (49%), 46 RARs (28%), and 38 IARs (23%). He described: “it was not always possible to assign ‘intermediate’ endings to slowly or rapidly adapting classes.” A review article from Sant’Ambrogio and Widdicombe (in 2001) (28) states “The AI should be used only as an approximate guideline, and the AI of SARs may overlap with that found for RARs.” (Dr. Sant’Ambrogio believed only RARs and SARs exist; there are no IARs.) In the same issue of the journal, Widdicombe (32) in his own review stated: “… … certainly receptors with intermediate adaptation rates exist.” However, he did not comment on their functions. In 1986, Coleridge and Coleridge (7) wrote in their book chapter: “Included in this rapidly adapting category were receptors with an intermediate rate of adaptation that were distinguished from slowly adapting stretch receptors by their irregular discharge. … … Receptors showing pronounced adaptation cannot be distinguished from rapidly adapting receptors by their adaptation rate alone, but they can be identified as slowly adapting stretch receptors by the remarkable regularity of inter-spike interval characterizing their stead state discharge.” They believed that the sensors will be classified according to their final discharge patterns. The majority of investigators accepted this view. However, in 1999, Paintal and Ashima (25) reported that “the two types of pulmonary stretch receptors had many properties in common and could therefore be regarded as one group.” With such an approach, IARs were included in the same group. In the past, I classified IARs as their own group (41) because they have different behavior from that of RARs and SARs. I interpreted them as the middle part of a spectrum between the extremities of typical RARs and SARs (36).1 IARs are also found in non-mammalian animal lungs, such as the frog. In 1969, McKean (21) identified three overlapping groups of mechanosensors and named them as rate receptors, proportional receptors, and rate plus proportional receptors. These sensors correspond to RARs, SARs, and IARs, respectively. If we define IARs as sensors that detect both rate and magnitude of the stimulus, then the majority of the sensors (50%) are IARs (21). Such a sensor had been reported as early as in 1933 (see Fig. 7 in Ref. 1).
Furthermore, IARs are also reported in lungfish (see Fig. 3A in Ref. 8), although the sensor was classified as an SAR (AI is ∼60% calculated from the figure). Clearly, understanding IARs is utterly important for fully understanding airway mechanoreceptors.
ISSUES FOR ESTABLISHING IARs AS A SPECIFIC TYPE OF SENSORS
With one-sensor and line-labeled theories, if RARs sense rate of change, SARs sense amplitude of change, and IARs sense both rate and amplitude of change, then IARs are redundant with RARs and SARs. Questions arise. Where do IARs project to in the CNS? What is their reflex function? Are they excitatory like RARs, or inhibitory like SARs, or both (which will be impossible due to functionally canceling each other out)? It is only possible if IARs project to different areas than RARs and SARs and cause different functions. Then why do we need two sets of mechanosensory systems to detect the same mechanical variables while exerting different functions? This is probably why the majority of investigators only accept two types of sensors (RARs and SARs) and abandoned IARs. Widdicombe (33) tried to study IARs and their function and found that these sensors, like RARs and SARs, may also respond to lung deflation. He proposed several potential reflex functions. However, it is very difficult to establish a reflex function in the face of an overlapping sensory property with RARs and SARs. That is probably why at the late stage of his career Widdicombe specifically mentioned the existence of IARs without describing any of their potential roles (see historical account) (32).
DILEMMA IN CATEGORIZATION OF SENSORS
Researchers who record electrical activities in airway mechanosensory units face a dilemma when categorizing them. For example, assessing AI when the lungs are inflated to a constant pressure results in a full range on a continuum of 0%–100%. Any cutoff line to identify RARs and SARs is arbitrary. By changing the cutoff line, the percentage of different types of sensors changes significantly.
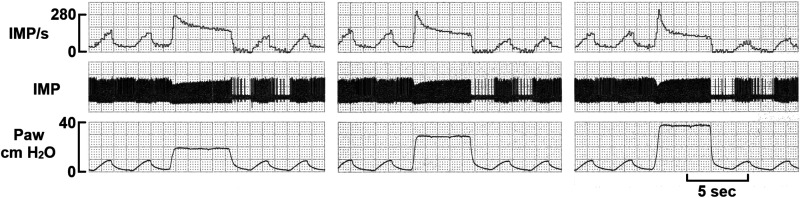
Furthermore, AI can be inflation pressure-dependent (Fig. 1). AI increases as inflation pressure increases. Thus, the unit will be classified into SAR at 20–30 cmH2O, but into IAR at 40 cmH2O. Nevertheless, according to Knowlton and Larrabee (16) and Coleridge and Coleridge (7), this unit is an SAR because it has very regular discharge during the maintained lung inflation. Some units are really difficult to be placed into one category or other (Fig. 2). This unit will be classified into a typical SAR, IAR, and RAR at low, medium, and high inflation pressures, respectively. Dr. Sant’Ambrogio believed this is an SAR based on the behavior at low inflation pressure (see discussion on Fig. 5 in Ref. 34a). It is clear that no matter how criteria are set, some units cannot be classified. To solve the problems in categorization based on sensory behavior, I proposed that myelinated pulmonary afferents are connected to heterogeneous group of sensors (one-sensor theory). Their behaviors are like a spectrum with the typical RARs and SARs at two ends of the spectrum (36). This approach can accommodate all types of mechanosensory behaviors, but the same questions remain regarding the functions of those sensors in the middle of the spectrum.
Fig. 1.
This rabbit mechanosensory unit demonstrates the adaptation rate is inflation pressure dependent. AIs for 20 (30%, 270/190, peak/2nd sec mean), 30 (45.2%, 310/170), 40 (56.3%, 320/140) cmH2O are different. The unit activity has two components when the lung inflated to 20, 30, and 40 cmH2O. At 20 cmH2O, the rapidly adapting component is small with a significant slowly adapting component. As the inflation pressure increases, the rapidly adapting component increased, whereas the slowly adapting component decreased. That is, the sustained activity of slowly adapting receptor (SAR) is highest at 20 cmH2O and lowest at 40 cmH2O, indicating a deactivation of SAR.
Fig. 2.
A sensory unit showing changes in discharge pattern during different lung inflation pressure. Note that higher inflation pressure produces a faster adaptation rate (AI). The lungs were sequentially inflated to 15 cmH2O (A, 75%), 12 cmH2O (B, 65%), and 7 cmH2O (C, 28%). Unit activity ceases after adaptation at 15 cmH2O. The activity after adaptation reached a plateau at 12 and 7 cmH2O. Clearly, this unit has an initial rapidly adapting receptor (RAR) component and a delayed slowly adapting receptor (SAR) component. Adapted from Fig. 5 in Ref. 36.
DEFINITION OF TERMINOLOGY
So far, the terms used are conventionally accepted, but some are erroneous. Moving forward, the following terminology is used. A sensory receptor (or sensor) is an encoder, which is the basic device that can generate action potentials (34a). Thus, RAR and SAR are single sensors. A sensory unit is a functional unit that transmits action potentials to the CNS. It consists of many sensors, which can be heterogeneous or homogeneous (multiple-sensor theory). A receptor structure is a morphological term where several sensors connected by a parent axon are observed under a microscope. A sensory unit may have more than one such structure (Fig. 3). The conventionally reported “RARs,” “SARs,” and “IARs” could be named as rapidly, slowly, and intermediate adapting units, respectively.
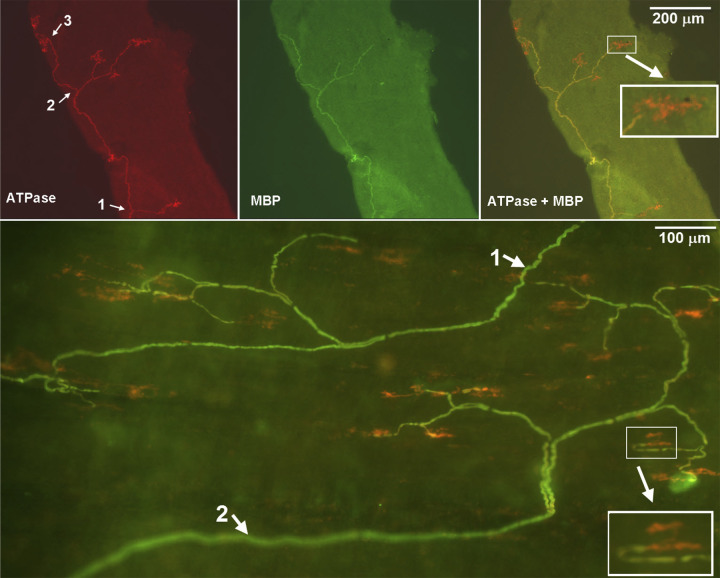
Fig. 3.
A double staining approach to illustrate slowly adapting receptor (SAR) sensory structures identified in rabbit airways. Na+-K+-ATPase stains all structures in the sensory unit (red), whereas myelin basic protein (MBP) stains the myelin sheath (green) and shows yellow (costaining) in the composite figure (top-right and bottom parts). Clearly, the axon is demyelinated before it reaches the end formation. Thus, the receptor can be identified (pure red portions without costain with MBP). Top (small airway, 300 µm in diameter): the parent axon of the sensory structure is running from the bottom up. It gives off three branches, indicated by white arrows 1, 2, and 3 in the top left. Its first branch is at the bottom of the figure, the second one in the middle part, and the third one at the upper part. Six receptors can be identified in this microscopic view (1 in the first branch, 3 in the second, and 2 in the third). They are showing red on double stain. Bottom (trachea): two parent axons (one starts at top-right and one at low left, indicated by white arrows 1 and 2) can be identified. The top-right sensory structure (1) has 9 receptors and the bottom-left one (2) has 13 receptors. Insets are enlarged to illustrate the sensory receptors. Adapted from Fig. 1 in Ref. 19 with permission.
PROCESS OF FORMING A NEW THEORY
The inability to understand the bronchopulmonary mechanosensory system is partly due to scant morphological information about the sensors. Receptor morphologies have been typically hand-drawn (31). Therefore, we decided to do morphological studies using confocal microscopy. By injecting a bidirectional neural tracer into the nodose ganglia to anterogradely label receptor structures in the lung, afferent activities in SAR units were recorded (30). The receptive field in the lung was then identified and dissected in piecemeal until isolated. Then the isolated tissue was morphologically examined to reveal the SAR structure (see Fig. 7 in Ref. 34a). Unfortunately, the success rate for good staining is extremely low. The tracer takes 5–7 days to be transported to the receptor sites. In addition, isolating a receptive field requires tedious work. Only one or two fields can be obtained in one day and might not be stained. Thus, many trials are required to obtain a physiologically identified, well-stained receptor structure. Therefore, we decided to develop a new histochemical staining approach to identify receptor structures. After extensive screening, an ATPase α3 subunit antibody to label receptor structures was identified. Combining histochemical labeling with confocal microscopy produces high-quality, unbiased images of receptor structures (31). We can now effectively isolate a physiologically studied SAR unit first and characterize its morphology (37, 40).
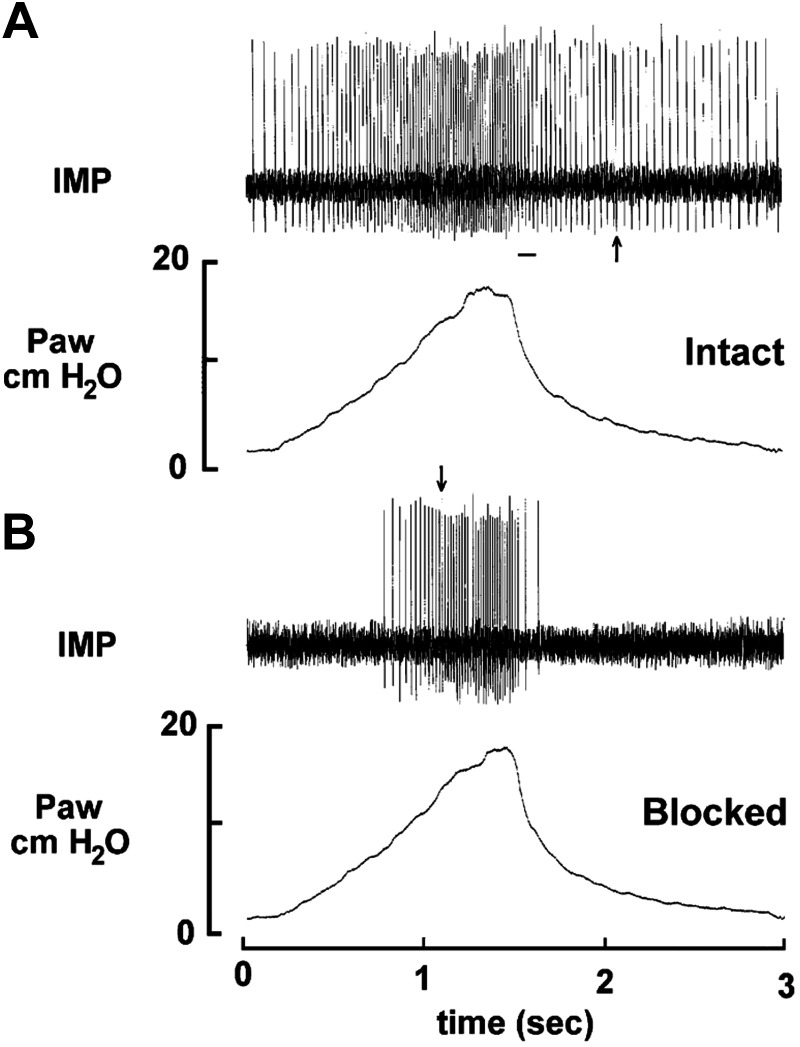
During the process of receptor isolation, we found that a sensory unit may contain multiple receptive fields. To test whether a receptive field is an encoder, or a contributor for generating action potentials, we recorded afferent activities from an SAR unit with multiple receptive fields and examined their discharges before and after blocking one of the fields with lidocaine. Blocking a field may not decrease the maximum discharge frequency during a ventilator cycle, indicating the remaining field is an encoder that can independently generate action potentials (Fig. 4). According to the discharge pattern, SAR units can be further classified as low-threshold (tonic), which fire throughout the respiratory cycle, and high-threshold (phasic), which fire only during the inspiratory phase. According to the responses to lung inflation, “SARs” could also be classified as type I (activity plateaus at inflation pressures above 10 cmH2O) and type II (activity increases linearly up to 30 cmH2O) (22). Since different discharge patterns were thought to generate different reflex effects (line-labeled theory), the coexistence of tonic and phasic SARs (Fig. 4) and type I and type II SARs (12) in a single unit challenged the established theory.
Fig. 4.
Recording of a single slowly adapting receptor (SAR) unit in a single ventilator cycle. This unit has two receptive fields identified. A: control, the unit activity represents a low-threshold pattern; and (B) after blocking one field with lidocaine. The unit represents a high-threshold pattern. The bar in (A) indicates where unit activity switches between encoders. The arrow in (B) indicates where discharge of a high-threshold encoder is about to exceed the low-threshold encoder. Drawing two vertical lines at the arrow in (B) and at the beginning of the bar in (A) divides figure (A) into three sections. The activity during deflation (first and third sections) is determined by the low threshold encoder (with a low peak discharge frequency), whereas the activity during inflation (second section) is determined by the high-threshold encoder. Thus, the pacemaker switches back and forth between the two encoders within a ventilator cycle. Adapted from Fig. 3 in Ref. 39 with permission.
To identify whether sensory responses to lung inflation and deflation are from single or different types of sensors, we examined units with bidirectional responses and multiple receptive fields and found that the injection of lidocaine into a field may selectively block an inflation or deflation response (see Fig. 5 in Ref. 20). In addition, a single sensory unit may contain both RARs and SARs, because the injection of lidocaine into a field blocked the rapidly adapting component, leaving the slowly adapting component intact (see Fig. 6 in Ref. 20). These results indicate that a single axon can connect to many different types of sensors with different modalities. In morphology, a parent axon can be fed by many receptor structures. Thus, a new multiple-sensor theory becomes necessary and has been proposed (34a).
THE MULTIPLE-SENSOR THEORY AND THE DENOTATION OF UNITS
Multiple-sensor theory posits that a single myelinated vagal afferent fiber connects to a sensory unit consisting of multiple sensors. There are two main types of sensors: 1) inflation-activated sensors, which can be divided into RARs and SARs; and 2) deflation-activated receptors (DARs) that also can be rapidly adapting (dRARs) or slowly adapting (dSARs). Each type detects a particular force. A unit containing all four types of sensors is denoted RARs-SARs/dRARs-dSARs and responds to both lung inflation and deflation, with intermediate adaptation behavior (Fig. 5). Sensors before the slash respond to lung inflation and after the slash respond to lung deflation. Plurals are used in sensors because there are many same-type sensors in each unit. The unit in Fig. 1 is denoted RARs-SARs/, because it contains both RARs and SARs. All the reported IARs, most SARs, and a few RARs belong to such a unit. Here nothing after the slash denotes the response to deflation is unknown (either not tested or not reported). A unit not responding to deflation would be denoted RARs-SARs/0. Here the 0 means no DARs. Figure 2 is a RARs-SARs/DARs unit, because it was identified as an “RAR-like SAR,” which by definition responded to lung deflation. However, whether the deflation response was rapidly or slowly adapting had not been determined. A unit containing many homogeneous SARs or RARs only (denoted as SARs/0 or RARs/0) will be the conventionally defined typical “SAR” and nontypical “RAR,” because a typical “SAR” does not respond to lung deflation, but an “RAR” will. A typical “RAR” should be a RARs/DARs unit. Here DARs are dRARs-dSARs or dSARs, but not dRARs, because dRARs cannot be responsible for Hering–Breuer deflation reflex (34), which was believed to be the major function of “RARs”. Similarly, a conventional “pure deflation receptor” is denoted as a 0/DARs unit (DARs could be dRARs or dSARs, or dRARs-dSARs). A variety of dynamic and static sensory behaviors can be generated in the unit depending on the number and characteristic of the operating sensors.
Fig. 5.
A rabbit mechanosensory unit responded to both lung inflation and deflation with an intermediate adaptation rate, i.e., both had an initial rapidly adapting component and a delayed slowly adapting component. A, C, E: lung inflation (30 cmH2O) and B, D, F: lung deflation (−4 cmH2O). A and B: the precontrols. C and D: after one receptive field was blocked by injection of 2% lidocaine (10 µL). Please note that the inflation response is unaffected, the slowly adapting component to lung deflation is completely blocked, and the rapidly adapting component is greatly attenuated, suggesting that inflation and deflation activities come from different encoders. E and F: postcontrols, i.e., after recovery from the anesthetics.
APPLICATION AND INTERPRETATION BY THE MULTIPLE-SENSOR THEORY
During the review process, reviewers raised many important issues that have been asked repeatedly by the science community. These points need to be addressed directly and clearly. Therefore, I took the question-answer format.
Question 1.
You state that the traditional one-sensor theory is that one afferent fiber connects to one sensor. Wouldn’t it be more accurate to say that the one-sensor theory states that one afferent fiber connects to one type of sensor?
Answer 1.
Conventionally, people believe that in airway mechanosensory units (such as SAR units), one afferent fiber connects to a single sensor, i.e., a single spike-generating zone, to produce regular activity (27). We have found that a SAR unit may house many SARs, i.e., connecting to multiple homogenous sensors (39). This concept is easily accepted because, although it violates single-sensor theory, it may not violate line-labeled theory if phasic and tonic SARs, and type I and type II SARs are believed to have the same function. With study progression, we found a single parent axon may terminate into multiple heterogeneous sensors that respond to different mechanical stimuli. This is a real challenge to our established doctrines and incomprehensible to many investigators. Thus, it is the focus of this review.
Question 2.
Typically, SARs have regular action potentials in response to sustained mechanical stimuli, whereas RARs have an irregular pattern to the same stimuli. How do these two sensors combine together to form an intermediate unit? In addition, RARs often will have a cardiovascular modulation, which is superimposed in the sensory activity if the sensors are located near the heart and big vessels.
Answer 2.
After the combination of RARs and SARs, the unit creates intermediate behavior with a rapidly adapting component at first and a slowly adapting component later, i.e., it gives a regular discharge pattern during maintained stimulation (Figs. 1 and 2, B and C), unless the SAR is deactivated, giving it an irregular pattern (Fig. 2A). The details regarding how these two sensors interact in the unit are dealt with in other review articles (10, 34a). Regarding cardiovascular modulation, SAR’s behavior is the same. As Coleridge and Coleridge (6) stated: “Many slowly adapting stretch receptors in the normal lung display a pulsatile cardiac modulation of discharge frequency during expiration, perhaps because their location makes them susceptible to adventitious stimulation by pulsations in the heart or great vessels as bronchial wall tension decreases….” Cardiovascular modulation is more easily identified in RARs because they have low background activity.
Question 3.
SARs are believed to have fiber conduction velocities in Aα or Aβ, whereas RARs most often in Aδ. Although admittedly, there is overlap, a difference in mean conduction velocities of the respective fibers is demonstrable. Those investigators who have studied conduction velocities are well aware of the adaptation rates. How do these data fit the multiple-sensor theory?
Answer 3.
In one-sensor theory, RARs connect with small fibers, whereas SARs connect to large fibers. Each type varies significantly, that is, fiber sizes and conduction velocities overlap. With multiple-sensor theory, conduction velocity is measured in the parent axon. The axon diameter results from the convergence of consecutive levels of daughter axons and determines the conduction velocity. With more receptor convergence, there is thicker axon diameter and faster conduction velocity. Since conduction velocity measurement is highly technical, the comparison between RARs and SARs should be based on data coming from the same research group under the same study conditions. Through careful review, I found four credible investigative groups in the field. Their reported conduction velocities of both SARs and RARs are detailed in Table 1.
Table 1.
Comparison of conduction velocities of SARs and RARs
| SARs |
RARs |
||||
|---|---|---|---|---|---|
| Range, m/s (n) | Mean, m/s | Range, m/s (n) | Mean, m/s | Species | Reference |
| 8–35* (10) | 20.2 | 8–12* (4) | 9.1 | Cat | Knowlton and Larrabee, 1946 (16) |
| 14–59 (57) | 36 ± 1.3 | 16–37 (10) | 25 ± 2.2 | Cat | Paintal, 1953 (24) |
| 5–68.5 (100) | 32.3 ± 11.8 | 12–40 (41) | 23.3 ± 7.1 | Dog | Sampson and Vidruk, 1975 (26) |
| 17–66 (27) | 32.0 ± 2.0 | 11–52 (29) | 31.9 ± 1.7 | Dog | Jonzon et al., 1988 (15) |
RARs, rapidly adapting receptors; SARS, slowly adapting receptors. For a mean of 9 m/s, the highest possible conduction velocity of RARs is 12 m/s (if the individual ones were 8, 8, 8, and 12 m/s). The lowest possible conduction velocity of SARs is 8 m/s. n in the parentheses refers to the number of afferents tested.
This is assumed according to the text.
Personally, I do not agree with the statement that SARs belong to the Aα or Aβ group, while RARs belong to Aδ. Jonzon et al. (15) demonstrated that the conduction velocities of SARs and RARs completely overlap in dogs. The only report showing clear differences in conduction velocity is by Knowlton and Larrabee (16). They stated: “The values for 4 rapidly adapting and 10 slowly adapting fibers ranged from 8 to 35 m/s. The conduction velocities of all the fibers from rapidly adapting receptors were less than 15 m/s (average 9.1 m/s), while fibers from 7 of the 10 slow adaptors conducted faster than 15 m/s (average 20.2 m/s for all 10). This suggests a difference between the conduction velocities of fibers from the two kinds of receptors, but not enough data were obtained to be sure that the difference was real.” Clearly, the sample is too small. As a matter of fact, SARs and RARs have significant overlap in their conduction velocities, although mean conduction velocity is faster in SARs than in RARs. By looking at their reported figures [Fig. 4 by Paintal (24) and Fig. 3 by Sampson and Vidruk (26)], the RAR conduction velocities completely overlap with SARs, but occupy the lower end. RAR fiber sizes seem to be the same as those of small SARs. However, some SARs have larger afferents. One possibility is that, like pure RAR units, pure SAR units have thin axons, whereas RAR + SAR units have large axons, due to more convergence. For example, assume a sensory region houses three sets of airway sensors (RAR, SAR, dRAR, and dSAR as one set), giving a total of 12 sensors. A unit can be any combination of the 12 sensors. If the unit contains either 1, 2, or 3 SARs, it is a pure SAR unit; similarly, 1, 2, and 3 RARs give a pure RAR unit; any combination of SARs and RARs makes an intermediate adapting unit. By random selection, statistically, intermediate adapting units (usually identified as SARs in the literature) will have larger fibers than pure SARs and RARs, because of more convergences. In the Coleridge laboratory (Jonzon), RARs are identified by deflation first. Therefore, most, if not all, identified RAR units also contain dSARs and dRARs and have thick fibers due to more convergence. This may explain the same conduction velocities in both SAR and RAR units in the report (15). Another potential explanation for SARs having faster conduction velocity than RARs could be the inclusion of high-threshold Aδ receptors (HTARs; having slower conduction velocities) in the RARs (35). The conventional belief is that there are only three types of airway sensors (SARs, RARs, and C-fibers). Therefore, any sensors, if not SARs and C-fibers, are classified as RARs. Furthermore, the AIs of HTARs often fall into rapidly adapting range. Thus, HTARs were often classified into RARs. However, RARs are mechanosensors and should be activated vigorously during hyperinflation. Mechanical sensitivity is one of the major criteria to differentiate RARs from HTARs (18).
Question 4.
Is the one sensor-one fiber the overwhelming norm, or is it an exception? Such issues must be addressed before accepting the statement of the second last sentence of the conclusion.
Answer 4.
An afferent fiber connects to many mechanosensors. For example, receptor structures average 9.6 ± 0.6 sensors in the large airway and 3.6 ± 0.3 sensors in the peripheral airway (19). As mentioned in DEFINITION OF TERMINOLOGY, several receptor structures form a sensory unit. Furthermore, a receptive field may contain several receptor structures and a unit may have many receptive fields (39). Therefore, there are many sensors in a sensory unit.
Question 5.
What contributing variables such as the receptor location could account for the behaviors of the intermediate adapting units? It will be ideal to compare these receptors with mechanoreceptors in other tissues.
Answer 5.
This is an excellent question. However, at this stage, technically, we will be unable to answer the question until we know exactly where the receptor is located, not only the location of the generation of the bronchus but also which layer of the bronchus, and how it is situated and connected with its adjacent tissues. Undoubtedly, any mechanical changes in the lung, or in the linkage between the receptor and its vicinity structures, will affect sensory behavior. Thus, any endogenous and exogenous factors that may alter viscoelastic properties of the lung tissues can impact on the sensors’ behavior. In addition, a sensor located at a bifurcation or in the nonbranching portion of an airway may behave differently. Sensors may also be influenced by its nearby structures, such as large vessels or the heart. Multiple-sensor theory does not alter conventional interpretation of mechanosensory behavior, but rather describes the final input to the CNS results from integration of all the sensors in the unit. RARs and SARs coexist in many other organs, such as in the skin, tendon organ, and muscle spindle (3, 5). Furthermore, both RARs and SARs are found to share an axon in the muscle spindle (4, 14).
Question 6.
If the multiple-sensor theory is valid, then the intermediate unit terminates in a discrete area of the nucleus tractus solitarius (NTS), which carries both dynamic and static information. This would make interpretation of the fiber activity more complicated than if fibers of SARs and RARs synapse on different neurons in the respiratory centers.
Answer 6.
I fully understand the reviewer’s statement. This is an issue raised in the issues for establishing iars as a specific type of sensors and is a major hurdle to accept the multiple-sensor theory. How does the CNS decode? I had no answer to this question until accepting the multiple-sensor theory. In one-sensory theory, we only need one type of decoder to decipher the incoming sensory unit information. In multiple-sensor theory, the units are encoded by four different types of sensors. Therefore, one type of decoder cannot effectively complete its work. Four different types of decoders may be needed to decipher the incoming information (see section 10 of review article in Ref. 34a). Although there is no known answer, there are many potential decoding mechanisms. For example, at the terminating region, different types of decoders may coexist; each may extract specifically encoded information (dynamic vs. static, inflation vs. deflation, etc.) (34a). Alternatively, the sensory unit may have multiple synapses on different NTS regions, each region having a specific type of decoder to extract information. In multiple-sensor theory, the unit is an integrator and the afferent transmits heterogeneous signals for decoding. In single-sensor theory, the unit is a transducer and the afferent transmits homogeneous signals. Since in multiple-sensor theory information is decoded by different types of decoders separately, the complexity level is the same as SARs and RARs synapsing on different neurons in the respiratory center. However, multiple-sensor theory solves problems that single-sensor theory does not. For example, if RARs (dynamic signal) stimulate breathing and SARs (static signal) inhibit it, what are the effects of intermediate units during lung inflation with constant pressure? Then, using local anesthetics to block the rapidly adapting component, what kind of effect does this unit produce? In multiple-sensor theory, the unit produces early excitatory with sustained inhibitory effects; after anesthetics, it causes inhibition only. How does the one-sensor approach answer this scenario?
One-sensor theory classifies RARs-SARs/ units (Figs. 1 and 2), the same as the SARs/ units, and fails to explain the function of the rapidly adapting component is in these units. Clearly, the unit in Fig. 2 cannot participate in the Hering–Breuer inflation reflex during lung inflation at 15 cmH2O. It needs to be emphasized that the discharge pattern is an important information cue. A sensory unit integrates heterogeneous sensors to give a variety of discharge patterns, which are decoded in the CNS. Thus, by accepting the multiple-sensor theory, researchers have to take a new look at the decoding mechanism in the CNS. The discharge pattern describes the nature of mechanical stimuli, whereas the discharge frequency indicates the intensity.
Question 7.
What reflex responses does the intermediate unit produce?
Answer 7.
Again, multiple-sensor theory does not alter conventional interpretations of the reflex effects of mechanosensors insofar as they pass logical tests. RARs and SARs in intermediate adapting units function the same as other RARs and SARs. They work together. For example, large lung inflation stimulates breathing through RARs operating in different types of units (RARs/0; RARs/DARs; RARs-SARs/0; RARs-SARs/DARs). Similarly, static lung inflation stimulates SARs and causes Hering–Breuer inflation reflex. These SARs include all SARs (in SARs/0; SARs/DARs; RARs-SARs/0; RARs-SARs/DARs units). On the other hand, a conventional belief that activation of “RARs” by lung deflation stimulates breathing does not withstand the logical test. According to the multiple-sensor theory, RARs only respond to lung inflation, not deflation (34). Therefore, the contributing sensors for the deflation-induced reflex should be revised accordingly to DARs. Simply, we can view the reflex effects from intermediate adapting units as the same reflex generated by simultaneous activation of SAR and RAR units. RARs (and so SARs) in different types of units may terminate at the same type of the neurons or may even at the same neurons for decoding and further integration to generate reflex responses.
Question 8.
Species differences among mammals should be discussed regarding different categories in bronchopulmonary mechanosensors. For example, in rats, there exists purely deflationary SARs but very few RARs. In addition, the relative population of the intermediate adapting units also needs discussion. Knowlton and Larrabee identified that 8% sensors were IARs, does this imply only 8% of sensors are heterogeneous RARs-SARs/ units?
Answer 8.
Regarding species differences, my personal experience is that bronchopulmonary sensors in the rat are similar to large animals, except there are more identifiable DARs. The reason is unknown. One explanation is rats may have lower threshold for DARs than larger animals. Therefore, under normal breathing conditions, these DARs are active (34). Personally, I found RARs in the rat. This was stated in one of our studies in the mouse (41). This is also in agreement with the other investigators (17, 29). The idea that rats lack RARs is from a pioneer study on airway sensors in rats (2). In that study, the investigators still found RARs, but only less numerous than in other animal species. For intermediate adaptation units, they are certainly found in small animals, including mice (41). The 8% is derived by arbitrary AIs for SARs and RARs (see DILEMMA IN CATEGORIZATION OF SENSORS). Actually, the majority of reported SAR units belong to this category. If we define the intermediate adapting unit as possessing both dynamic and static properties, it accounts for 50% of inflation-activated mechanosensor population in the frog. In mammals, airway mechanosensory units with an AI between 20% and 80% may fall into this category, i.e., the majority identified “SARs” are RARs-SARs/ units (Please also see Answer 9).
Question 9.
What is the relationship between the intermediate adapting unit and conventionally identified SARs?
Answer 9.
According to the conventional definition, any sensory unit that contains SARs (having a regular discharge during maintained lung inflation) will be classified into SAR units. Therefore, by a random selection (Please see Answer 3) most identified SARs are actually RARs-SARs/ units. This explains the adaptation rate of SAR units. As Sant’Ambrogio stated: “When a given lung inflation is introduced and maintained, SARs characteristically show a long-lasting discharge with an immediate rapid decline that slows progressively into a sustained firing. This behavior describes the slowly adapting properties of these endings. … … In addition to responding to static pressure, most SARs respond to its rate of change.” (27). In a study, Pack et al. (23) stated: “…at low lung volumes the pulmonary stretch receptor is primarily a proportional receptor. …. at higher lung volumes the receptor behaves increasingly as a rate receptor. Although the mechanism producing this response is unknown….” We now know that many reported “SARs” are RARs-SARs/ units. The SARs usually have lower activation thresholds than RARs. Thus, as the lung volume (or inflation pressure) increases, RAR behavior becomes more and more apparent (Figs. 1 and 2). This phenomenon is also demonstrated in Adrian’s paper (see Fig. 7 in Ref. 1), in which the rapidly adapting component is much more prominent at the inflation volume of 230 mL than at lower volumes.
Multiple-sensor theory explains intermediate adapting behavior as well as other dynamic and static sensory unit properties, such as sensor deactivation and creeping. Figure 2 illustrates deactivation as lung inflation pressure is increased (Fig. 2A). SAR activities disappeared due to deactivation (12). Creeping is a common, long-neglected phenomenon where unit activity increases during lung inflation under constant pressure (9, 38). Both deactivation and creeping result from encoder switching (or pacemaker switching) within the unit, supporting the multiple-sensor theory. Encoder switching is also clearly demonstrated between a low-threshold and a high-threshold SARs in a SARs unit (Fig. 4); between a RAR and SAR in RARs-SARs/DARs units (Fig. 2); and among four different types of sensors in a RARs-SARs/dRARs-dSARs unit (Fig. 5). These phenomena cannot be explained by one-sensor theory.
CONCLUSION
Bronchopulmonary myelinated mechanosensory units display a spectrum of behaviors responding to lung inflation and deflation. Multiple-sensor theory—rather than single-sensor theory—better explains these behaviors. Multiple-sensor theory is based on sensory units composed of different types of sensors and having no IARs as such. Intermediate adaption results from the interaction between RARs and SARs. With many questions left unanswered by single-sensor theory, it is time to adopt the new theory and refute conventional doctrines. Accordingly, new hypotheses can be formulated as to how the CNS decodes sensory information, and to elucidate reflex functions of myelinated pulmonary afferents.
Perspectives and significance.
Bronchopulmonary mechanosensors provide mechanical information of the lung to the respiratory centers to regulate breathing. Thus, understanding how these sensors operate is crucial. However, after nearly a century of intensive investigation, our view on whether intermediate adapting receptors exist is still debatable. Current multiple-sensor theory describes no IARs and intermediate adapting behavior results from the coexistence of RARs and SARs in the same sensory unit. It supports that the sensory unit is not merely a transducer but a processor that integrates heterogeneous information. This new insight challenges our view on how the central nervous system decodes the sensory information from the respiratory system. Hopefully, it will facilitate the research of decoding system in the respiratory center. Furthermore, the integration through interaction of heterogeneous sensors in the peripheral sensory neuron provides an excellent model for understanding the integration at the CNS, because processes common to both are likely conserved.
GRANTS
This review was supported by a Veterans Affairs Merit Review Award PULM-024-17S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
J.Y. drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
Footnotes
I now believe multiple-sensor theory. In this theory, there are no IARs and an intermediate adapting behavior results from coexistence of RARs and SARs.
REFERENCES
- 1.Adrian ED. Afferent impulses in the vagus and their effect on respiration. J Physiol 79: 332–358, 1933. doi: 10.1113/jphysiol.1933.sp003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? J Physiol 464: 681–698, 1993. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess PR, Perl ER. Cutaneous mechanoreceptors and nociceptors In: Handbook of Sensory Physiology. II. Somatosensory System, edited by Iggo A. New York: Springer-Verlag, 1973, p. 29–38. [Google Scholar]
- 4.Carr RW, Morgan DL, Proske U. Impulse initiation in the mammalian muscle spindle during combined fusimotor stimulation and succinyl choline infusion. J Neurophysiol 75: 1703–1713, 1996. doi: 10.1152/jn.1996.75.4.1703. [DOI] [PubMed] [Google Scholar]
- 5.Catton WT. Mechanoreceptor function. Physiol Rev 50: 297–318, 1970. doi: 10.1152/physrev.1970.50.3.297. [DOI] [PubMed] [Google Scholar]
- 6.Coleridge HM, Coleridge JCG. Afferent innervation of lungs, airways and pulmonary artery In: Reflex Control of the Circulation, edited by Zucker IH, Gilmore JP. Boca Raton, Ann Arbor, Boston: CRC Press, 1991, p. 579–607. [Google Scholar]
- 7.Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs In: Handbook of Physiology. The Respiratory System. Bethesda, MD: American Physiological Society, 1986, p. 395–430. [Google Scholar]
- 8.DeLaney RG, Laurent P, Galante R, Pack AI, Fishman AP. Pulmonary mechanoreceptors in the dipnoi lungfish Protopterus and Lepidosiren. Am J Physiol Regul Integr Comp Physiol 244: R418–R428, 1983. doi: 10.1152/ajpregu.1983.244.3.R418. [DOI] [PubMed] [Google Scholar]
- 9.Farber JP, Fisher JT, Sant’Ambrogio G. Distribution and discharge properties of airway receptors in the opossum, Didelphis marsupialis. Am J Physiol Regul Integr Comp Physiol 245: R209–R214, 1983. doi: 10.1152/ajpregu.1983.245.2.R209. [DOI] [PubMed] [Google Scholar]
- 10.Grigg P. Biophysical studies of mechanoreceptors. J Appl Physiol (1985) 60: 1107–1115, 1986. doi: 10.1152/jappl.1986.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 12.Guardiola J, Proctor M, Li H, Punnakkattu R, Lin S, Yu J. Airway mechanoreceptor deactivation. J Appl Physiol (1985) 103: 600–607, 2007. doi: 10.1152/japplphysiol.01286.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hendry SH, Hsiao SS, Brown MC. Fundamentals of sensory systems In: Fundamental Neuroscience, edited by Squire LR, Bloom FE, Spitzer NC, Lac GA, Burlington DB. San Diego, CA; London: Elsevier, 2008, p. 535–548. [Google Scholar]
- 14.Hunt CC. Mammalian muscle spindle: peripheral mechanisms. Physiol Rev 70: 643–663, 1990. doi: 10.1152/physrev.1990.70.3.643. [DOI] [PubMed] [Google Scholar]
- 15.Jonzon A, Pisarri TE, Roberts AM, Coleridge JC, Coleridge HM. Attenuation of pulmonary afferent input by vagal cooling in dogs. Respir Physiol 72: 19–33, 1988. doi: 10.1016/0034-5687(88)90076-X. [DOI] [PubMed] [Google Scholar]
- 16.Knowlton GC, Larrabee MG. A unitary analysis of pulmonary volume receptors. Am J Physiol 147: 100–114, 1946. doi: 10.1152/ajplegacy.1946.147.1.100. [DOI] [PubMed] [Google Scholar]
- 17.Lai CJ, Kou YR. Stimulation of pulmonary rapidly adapting receptors by inhaled wood smoke in rats. J Physiol 508: 597–607, 1998. doi: 10.1111/j.1469-7793.1998.597bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Du L, Otmishi P, He Y, Guardiola J, Yu J. Opposite responses to lidocaine between intrapulmonary mechanical and chemical sensors. Am J Physiol Regul Integr Comp Physiol 297: R853–R858, 2009. doi: 10.1152/ajpregu.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Song N, Guardiola J, Roman J, Yu J. Slowly adapting sensory units have more receptors in large airways than in small airways in rabbits. Front Physiol 7: 588, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Yu J. Spectrum of myelinated pulmonary afferents (II). Am J Physiol Regul Integr Comp Physiol 305: R1059–R1064, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKean TA. A linear approximation of the transfer function of pulmonary mechanoreceptors of the frog. J Appl Physiol 27: 775–781, 1969. [DOI] [PubMed] [Google Scholar]
- 22.Miserocchi G, Sant’Ambrogio G. Responses of pulmonary stretch receptors to static pressure inflations. Respir Physiol 21: 77–85, 1974. [DOI] [PubMed] [Google Scholar]
- 23.Pack AI, Ogilvie MD, Davies RO, Galante RJ. Responses of pulmonary stretch receptors during ramp inflations of the lung. J Appl Physiol (1985) 61: 344–352, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Paintal AS. The conduction velocities of respiratory and cardiovascular afferent fibres in the vagus nerve. J Physiol 121: 341–359, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paintal AS, Ashima A. Respiratory function and the autonomic nervous system In: The Autonomic Nervous System, Part 1: Normal Functions. Handbook of Clinical Neurology, edited by Vinuelas PJ, Bruyn GW. Amsterdam: Elsevier, 1999, p. 573–593. [Google Scholar]
- 26.Sampson SR, Vidruk EH. Properties of ‘irritant’ receptors in canine lung. Respir Physiol 25: 9–22, 1975. [DOI] [PubMed] [Google Scholar]
- 27.Sant’Ambrogio G. Information arising from the tracheobronchial tree of mammals. Physiol Rev 62: 531–569, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Sant’Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol 125: 33–45, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Tsubone H. Characteristics of vagal afferent activity in rats: three types of pulmonary receptors responding to collapse, inflation, and deflation of the lung. Exp Neurol 92: 541–552, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Cheng Z, Zhang JW, Yu J. A novel approach to investigate pulmonary receptors. FASEB J 16: A453, 2002. [Google Scholar]
- 31.Wang YF, Yu J. Na(+)/K(+)-ATPase as a marker for detecting pulmonary sensory receptors. Sheng Li Xue Bao 54: 390–394, 2002. [PubMed] [Google Scholar]
- 32.Widdicombe J. Airway receptors. Respir Physiol 125: 3–15, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol 123: 71–104, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J. Deflation-activated receptors, not classical inflation-activated receptors, mediate the Hering-Breuer deflation reflex. J Appl Physiol (1985) 121: 1041–1046, 2016. [DOI] [PubMed] [Google Scholar]
- 34a.Yu J. Pulmonary mechanosensors deactivate when overexcited. Am J Respir Crit Care Med 148: 217–243, 2005. [Google Scholar]
- 35.Yu J. An overview of vagal airway receptors. Sheng Li Xue Bao 54: 451–459, 2002. [PubMed] [Google Scholar]
- 36.Yu J. Spectrum of myelinated pulmonary afferents. Am J Physiol Regul Integr Comp Physiol 279: R2142–R2148, 2000. doi: 10.1152/ajpregu.2000.279.6.R2142. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Wang YF, Zhang JW. Structure of slowly adapting pulmonary stretch receptors in the lung periphery. J Appl Physiol (1985) 95: 385–393, 2003. doi: 10.1152/japplphysiol.00137.2003. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Xu L. A paradoxical response of pulmonary slowly adapting receptor units during constant pressure lung inflation. FASEB J 34, Suppl 1: 1, 2020. doi: 10.1096/fasebj.2020.34.s1.02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Zhang J. A single pulmonary mechano-sensory unit possesses multiple encoders in rabbits. Neurosci Lett 362: 171–175, 2004. doi: 10.1016/j.neulet.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Zhang J, Wang Y, Fan F, Yu A. Neuroepithelial bodies not connected to pulmonary slowly adapting stretch receptors. Respir Physiol Neurobiol 144: 1–14, 2004. doi: 10.1016/j.resp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JW, Walker J, Yu J. Pulmonary vagal afferent activities in the mouse. J Investig Med 101: 986–992, 2002. [Google Scholar]