Abstract
Arsenic and trichloroethene (TCE) are among the most prevalent groundwater contaminants in the United States. Co-contamination of these two compounds has been detected at 63% of current TCE-contaminated National Priorities List sites. When in-situ TCE reductive dechlorination is stimulated by the addition of fermentable substrates to generate a reducing environment, the presence of arsenic can be problematic due to the potential for increased mobilization and toxicity caused by the reduction of arsenate (As(V)) to arsenite (As(III)). This study assesses the effects of arsenic exposure on the TCE-dechlorinating activities of Dehalococcoides mccartyi strain 195. Our results indicate that 9.1 μM As(III) caused a 50% decrease in D. mccartyi cell growth. While As(V) concentrations up to 200 μM did not initially impact TCE dechlorination, inhibition was observed in cultures amended with 200 μM As(V) and 100 μM As(V) in 12 and 17 days, respectively, corresponding with accumulation of As(III). Transcriptomic and metabolomic analyses were performed to evaluate cellular responses to both As(V) and As(III) stress. Amendment of amino acids enhanced arsenic tolerance of D. mccartyi. Results from this study improve our understanding of potential inhibitions of D. mccartyi metabolism caused by arsenic and can inform design of bioremediation strategies at co-contaminated sites.
Keywords: TCE, arsenic, dechlorination, Dehalococcoides, inhibition, transcriptomics, metabolomics
Graphical Abstract
Introduction
Trichloroethene (TCE) is a chlorinated organic that has been widely used as an industrial solvent for decades. Due to improper disposal, storage, and handling practices, TCE and its daughter-products of environmental transformation, i.e., dichloroethene (DCE) and vinyl chloride (VC) are now ubiquitous groundwater contaminants of environmental and public health concern.1 While numerous microorganisms have demonstrated the ability to reduce TCE, only Dehalococcoides mccartyi is known to convert TCE to the benign product ethene via reductive dehalogenation.2-7 Bioremediation of TCE by reductive dehalogenation using D. mccartyi strains has been well characterized, but there are substantial knowledge gaps regarding the dechlorination of TCE under environmentally-relevant conditions.
It is rare that chlorinated solvents are the only contaminants of concern at a contaminated site. For example, arsenic, one of the most frequent co-contaminants, has been reported at approximately 63% of current TCE-contaminated National Priorities List sites.8 Arsenic originates from both anthropogenic and geogenic sources 9-12 and is present as a background constituent in many drinking water sources across the United States, even when not identified as a contaminant.13 Arsenic is of particular interest as a co-contaminant in TCE-contaminated sites due to the critical effects of oxidation state on arsenic toxicity and bioavailability.14-15
The toxicity mechanism for arsenate (As(V)) is the inhibition of phosphate-related functions due to the physical and chemical similarities between the two oxyanions.16 The physical similarity to phosphate causes As(V) to enter cells through phosphate transporters. Detoxification of As(V) in bacteria is achieved through reduction of As(V) to As(III) and subsequent export from the cell,17 and D. mccartyi strains have been reported to contain the arsenic toxicity genes required for this process.18-19 Arsenite (As(III)) has been shown to cause toxicity to cells by binding to protein sulfhydryl groups, thereby inhibiting enzymatic catalysis.20-21 Previous studies also reported that, while As(III) has a higher affinity for sulfhydryl groups, As(V) also interacts with them, but at much higher concentrations.22
There is a consensus in the literature that the solubilization of arsenic is most common during perturbed redox conditions (i.e. oxidative to reductive) which is typical during in-situ reductive dehalogenation when fermentable substrates are amended to generate a reducing environment.10,14,23 The effect of increasing aqueous arsenic concentrations on the organisms responsible for in-situ reductive dehalogenation is unknown. However, past research has demonstrated that exposing TCE-reducing isolates and communities to various stressors (e.g. pH changes, sulfide accumulation) often results in the accumulation of the more toxic intermediates, DCE and VC.24-26
In this study, we investigated the effects of arsenic contamination on the dechlorination activity and gene expression in an axenic D. mccartyi strain 195 culture (Dhc195) in laboratory microcosms. To achieve this objective, we integrated results from physiological analyses, cell growth quantification, transcriptomic studies, and extracellular metabolomic analyses after perturbation by As(III) or As(V).
Methods and Materials
Reagents
TCE, dichloroethene, vinyl chloride, and ethene (99.6%, ACS reagent) were obtained from Acros organics (Geel, Belgium) or Sigma Aldrich (USA). Sodium arsenate and sodium arsenite solutions were prepared from solid sodium arsenate dibasic heptahydrate and sodium (meta) arsenite, respectively, obtained from Sigma Aldrich (USA). All other chemicals used were of reagent grade quality or higher.
Culture and growth conditions
Dhc195 was cultivated in defined mineral salts medium with an H2-CO2 (80:20 vol/vol) headspace as previously described.7,27 Dhc195 was grown with 5 mM acetate as a carbon source, TCE as electron acceptor in amendments of 77 μmol (with the number of amendments varying per experiment), H2 provided in the headspace as electron donor, and 0.5 mL vitamin solution28 containing 20 mg/L vitamin B12 (final concentration 100 μg/L) as additional nutrients. All cultures were incubated without light or agitation at 34°C for the duration of the experiment.
To study the effect of arsenic on the TCE-dechlorination activity, Dhc195 was grown with concentrations of As(V) and As(III) varying from 0 μM to 200 μM amended at the time of inoculation. Subsequent amendments of TCE were added to the cultures once TCE and cis-DCE were depleted. Experimental conditions were compared against positive biotic controls containing no arsenic. Abiotic controls with and without arsenic amendment showed neither TCE dechlorination or arsenic reduction (data not shown). All experiments were performed in triplicate.
Minimum inhibitory concentration assay
To calculate the minimum inhibitory concentration (MIC) of As(III), Dhc195 was grown with concentrations of As(III) ranging from 5 μM to 30 μM As(III). As(III) was amended 24 hours after inoculation. Cell samples were harvested every 24 hours for 5 days. The MIC was defined as the concentration that caused a 50% decrease in the exponential growth rate as compared to the control condition and calculated as previously described.29 To calculate the MIC for Dhc195 + folate and Dhc195 + amino acids, the above procedure was followed by amendment of folate (400 μg/L) or a 20-amino acid solution (20 mg/L each) at the time of inoculation, respectively.
DNA extraction and cell enumeration
A 1.5 ml aliquot was sampled from each replicate for DNA extraction, and cells were pelleted by centrifugation (21,000g x 10 minutes at 4°C). DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, USA) according to the manufacturer instructions for Gram-positive bacteria. Cell enumeration was performed using qPCR with SYBR-green based detection agents. The qPCR primers used were strain-specific, targeting the reductive dehalogenase tceA gene as previously described.30
Differential gene expression assay
To elucidate gene expression changes resulting from arsenic perturbation, two arsenic-amended conditions (10 μM As(III) and 100 μM As(V)) and a positive control containing no arsenic were analyzed. Arsenic was amended during exponential growth of the cells when approximately 40% of the second dose of TCE had been consumed. Cells were collected approximately 5 hours after amendment of arsenic. Each filter containing cells from one serum bottle was placed in a 2 mL microcentrifuge tube and frozen with liquid nitrogen and stored at −80°C until processing. Cells from 24 serum bottles for each analyzed condition were collected via vacuum filtration (filter pore size 0.2 μm). RNA collection and purification were performed as previously described.31
Microarray data processing and analysis using the Affymetrix (USA) GeneChip microarray for measuring differential gene expression was applied as previously described.32 The chip contains 4,744 probe sets representing more than 98% of open reading frames from four published Dehalococcoides mccartyi genomes (strains 195, BAV1, VS, and CBDB1). cDNA was synthesized from 10 μg RNA. Each cDNA sample was fragmented, labeled, and hybridized to an array. The procedures were performed according to the protocols established in section 3 of the Affymetrix GeneChip Expression Analysis Technical Manual (http://www.affymetrix.com). Microarray data analysis methods were performed using the “affy” Bioconductor package as previously described.33-36 Probe set hybridization signal intensities were calculated using the “mas5” function from the “affy” package. Statistical significance of individual differentially expressed transcripts is defined as fold-change > 2, p-value < 0.05, and average signal intensity of either control or experimental condition > 200. Significance of differential expression of metabolic pathways was determined using the Wilcoxon rank-sum test as previously described.37
Extracellular metabolomics analysis
For extracellular metabolomic analysis, the same three conditions were studied as in the differential gene expression assay (10 μM As(III), 100 μM As(V), and positive control). Samples were taken at two timepoints during the experiment (when approximately 45% and 95% of the second TCE dose had been consumed, corresponding to 25 and 48 hours after amendment of the second TCE dose), at which point 1 mL liquid medium was withdrawn from the culture and cells were removed via centrifugation (21,000g x 10 minutes at room temperature ~23°C) and stored at −80°C until analysis. The first timepoint (approximately 45% TCE consumed) corresponds to the transcriptomic sample timepoint which was taken 5 hours after 40% of TCE was consumed.
Targeted and untargeted metabolomic data was acquired using gas chromatography time-of-flight mass spectrometry (GC-TOF-MS), processed, and normalized at the West Coast Metabolomics Center (University of California, Davis, CA, USA) as previously described.38 Statistical significance for this analysis was defined as changes exhibiting fold-change > 2, p-value < 0.05, and average signal intensity of either control or experimental condition > 500.
Untargeted Metabolite Identification
In-silico determination of candidate metabolites was performed with the workflow in Supplemental Figure S1. Briefly, we subtracted one and two trimethylsilyl (TMS) moieties from the mass spectrum and found matching m/z ions from the one and two TMS subtraction. The matched m/z ions and abundances were compared against two different mass spectrum databases – MassBank of North America (MoNA; http://mona.fiehnlab.ucdavis.edu/) and CFM-ID (http://cfmid.wishartlab.com)39 –to search for potential candidate metabolites. The workflow was validated with target metabolites (e.g. threonine) treating them as unknowns.
Calibration standards for candidate metabolites were prepared from HPLC grade crystalline solid. Candidate metabolites were quantified using liquid chromatography/mass spectrometry (LC-MS/MS) using a 1260 Infinity II LC (Agilent Technologies, USA) and 6460 Triple Quad MS (Agilent Technologies, USA) equipped with an HILIC Plus 3.5 μm (4.6 mm x 100 mm) column (Agilent Technologies, USA). The mobile phase was 50% 10 mM ammonium acetate in LCMS grade water and 50% 10 mM ammonium acetate in LCMS grade methanol and was run isocratically. The analyses were conducted at a flow rate of 0.4 mL/min. The injection volume was 25 μL. Time of data acquisition was 4 minutes per sample. The MS source parameters were as follows: Gas temperature (325 °C), sheath gas temperature (350 °C), gas flow (9 L min−1), sheath gas flow (9 L min−1), nebulizer (25 psi), nebulizer gas (nitrogen), capillary voltage (ESI+mode 3.5 kV; ESI-mode 3kV). Multiple reaction monitoring transitions for the candidate metabolites are shown in Supplemental Table S1.
Analytical Methods
Chlorinated ethenes and ethene were measured by injecting 100 μL headspace gas from the cell cultures on an Agilent 7890A gas chromatograph equipped with a flame ionization detector and a 30-m J&W capillary column with an inside diameter of 0.32-mm (Agilent Technologies, USA). A gradient temperature program method was used as previously described.40
Measurement of soluble As(III) and As(V) was performed using an Agilent 1260 Infinity high-performance liquid chromatograph (HPLC) (Agilent Technologies, USA) equipped with an Aminex HPX-87H Ion Exclusion Column (300mm x 7.8mm, BIO-RAD, USA) coupled to a Hamilton PRP-X300 Reversed-Phase Column (250mm x 4.1mm, Hamilton Co., USA) and an Agilent 7700 series inductively coupled plasma mass spectrometer (ICP-MS) (Agilent Technologies, USA) as previously described.41 Arsenous acid, arsenic acid, monomethylarsonate, dimethylarsinate, and arsenobetaine are quantifiable using the applied method.41 Samples consisting of 0.5 mL liquid medium were collected at three timepoints during the experiment (day 0, day 12, and day 17) from both As(III)- and As(V)-amended cultures. Liquid medium samples were filtered using 0.2 μM HPLC-grade syringe filters (Pall Life Sciences, England) and diluted to obtain analytical concentrations within standard solution calibration.
Results and Discussion
Inhibition by arsenic of TCE-dechlorination activity and cell growth of Dhc195
The effects of As(V) and As(III) on growth and TCE dechlorination of Dhc195 were evaluated in concentrations ranging from 5 μM to 200 μM and 5 μM to 30 μM, respectively. The presence of As(V) did not initially inhibit Dhc195 growth. Rates of TCE dechlorination in cultures amended with 100 μM and 200 μM As(V) were not significantly different from the control containing no As(V) during consumption of the first two amendments of TCE (Figure 1A). After the third amendment of TCE on day 10, a decrease in the dechlorination rate of Dhc195 was observed in cultures amended with 200 μM As(V), and 38% of TCE (27.1 ± 6.0 μmol TCE/bottle) from the third amendment remained at the end of the experiment on day 17. After the fourth amendment of TCE on day 12 in cultures amended with 100 μM As(V), the dechlorination rate of Dhc195 decreased, and 36% of TCE (27.1 ± 12.1 μmol TCE/bottle) remained at the end of the experiment (day 17). There were no significant differences in final cell number of Dhc195 in cultures containing 100 μM or 200 μM As(V) as compared to the control (Supplemental Figure S2). The similarities in cell number are not surprising, as it has been previously reported that most growth in Dhc195 microcosms occurs during consumption of the first TCE amendment – the exponential growth rate of Dhc195 for the first amendment of TCE was 0.21/day and decreased to 0.05/day during amendments 2 through 4.34 This indicates that most cell growth would have occurred during consumption of the first dose of TCE before As(III) accumulated and disrupted cellular function.
Figure 1:
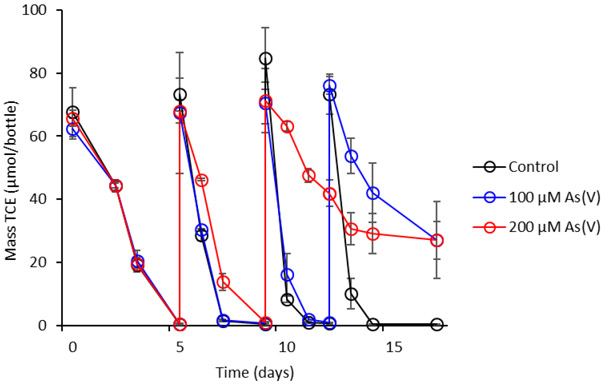
TCE consumption by Dhc195 amended with 0, 100, and 200 μM As(V). Additional TCE was amended on days 5, 10, and 13. Error bars represent one standard deviation of experimental triplicates.
Modification of arsenic species by Dhc195 was monitored during inhibition experiments. In cultures amended with 100 μM As(V), 23% of As(V) was reduced to As(III) by day 17, with all observed reduction of As(V) occurring between day 12 and day 17 (Figure 2A). In cultures amended with 200 μM As(V), 19% of total As was reduced to As(III) by day 12 and 54% of total As was reduced by day 17 (Figure 2B). These observed As(V) reduction rates were significantly greater than those observed in abiotic controls (Figure 2C) as well as published abiotic As(V) reduction rates under similar conditions,42 indicating the generation of As(III) was due to the detoxification functions of Dhc195.
Figure 2.
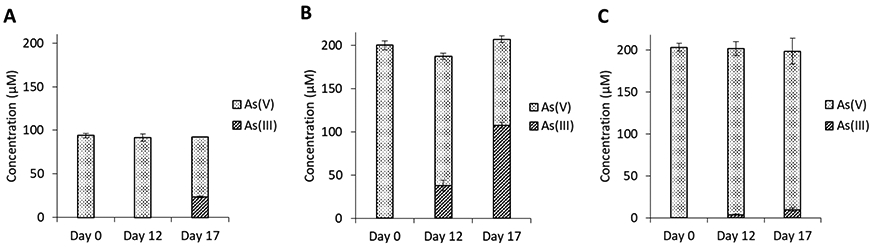
Concentrations of aqueous As(III) and As(V) on days 0, 12, and 17 in Dhc195 cultures amended with (A) 100 μM As(V), (B) 200 μM As(V) and (C) abiotic control amended with 200 μM As(V). Error bars represent one standard deviation of experimental triplicates.
The genomes of D. mccartyi strains contain a suite of arsenic resistance genes, including arsR, arsB, arsC, and arsM (DET1005, 0908, 0143, 1420), encoding the functions of reducing As(V) to As(III), arsenic methylation, and transporting As(III) out of the cell as pathways of arsenic detoxification.18 Arsenic “detoxification” was observed here via reduction of As(V) to As(III) by Dhc195, as Dhc195 does not carry the genes required for dissimilatory As(V) reduction, arrA and arrB.18,43 Even though the amended As(V) concentrations did not initially affect metabolic function of Dhc195, the so-called detoxification mechanism caused accumulation of the more toxic As(III), which inhibited TCE dechlorination.
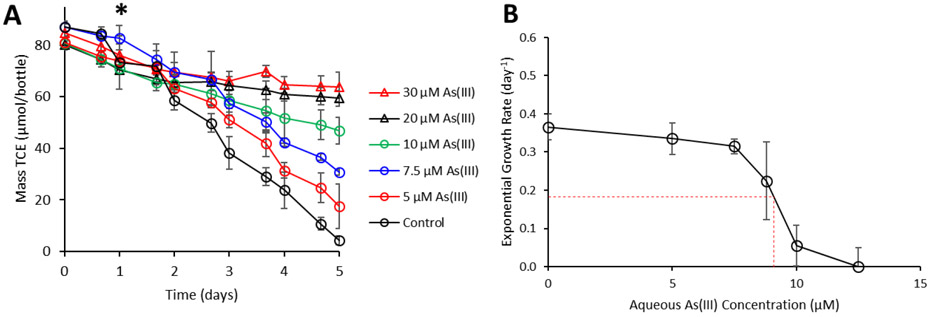
Physiological experiments were performed to quantify the impacts of As(III) on Dhc195. Because of more potent toxic properties of As(III) as compared to As(V) in a variety of organisms,16,44-45 concentrations of As(III) ranging from 5 to 30 μM were applied. In the positive control samples, complete reduction of 87.1 ± 2.3 μmol TCE occurred within five days (Figure 3A). In comparison, cultures containing 5 μM, 7.5 μM, 10 μM, 20 μM, and 30 μM As(III) only reduced 78% (63.4 ± 8.7 μmol), 65% (56.2 ± 2.9 μmol), 42% (33.9 ± 7.8 μmol), 26% (20.7 ± 4.6 μmol), and 21% (18.0 ± 9.3 μmol), of TCE respectively. To determine the MIC of As(III) for Dhc195, cell numbers were quantified every 24 hours over a 5-day experiment under various As(III) concentrations. The MIC for Dhc195 was calculated to be 9.1 μM As(III) (Figure 3B).
Figure 3.
A) TCE consumption (μmol/bottle) by Dhc195 amended with a range of As(III) concentrations (0-30 μM). The asterisk denotes the time at which As(III) was amended to the cultures. B) Exponential growth rates (per day) of Dhc195 amended with increasing As(III). The red dashed line indicates the MIC of As(III) for Dhc195. Error bars represent one standard deviation of experimental triplicates. The absence of error bars indicates bars are smaller than the marker.
In experiments when 200 μM As(V) was amended (rather than As(III)), Dhc195 was able to continue to dechlorinate TCE even when generated aqueous As(III) concentrations reached 38 μM, suggesting that Dhc195 was able to develop a higher As(III) tolerance under gradual accumulation as compared to pulse input. In cultures amended directly with As(III), no change in the oxidation state of arsenic was observed (Supplemental Figure S3).
Differential gene expression resulting from arsenic inhibition in Dhc195
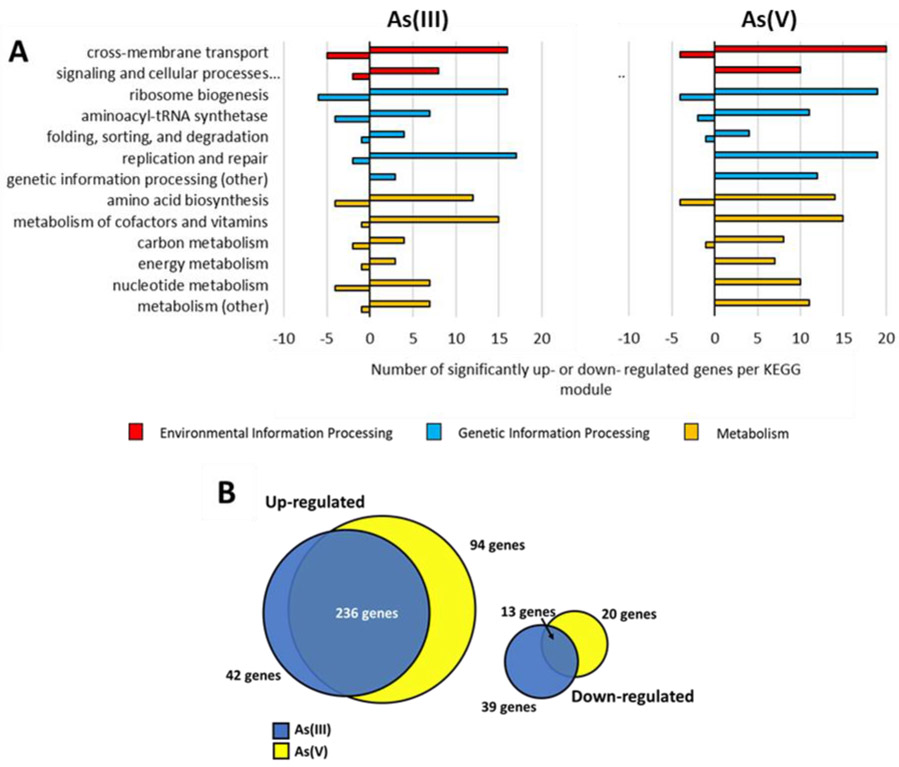
Transcriptomic analysis was performed to identify genes with significant changes in expression in both As(III)- and As(V)-amended cultures as compared to the positive controls. In 10 μM As(III)-amended cultures, 52 transcripts were down-regulated and 278 transcripts were up-regulated. In cultures amended with 100 μM As(V), 33 transcripts were down-regulated and 332 transcripts were significantly up-regulated. The differentially expressed genes were categorized by module based on their annotation in the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Figure 4). Genes not categorized in the KEGG system or identified as hypothetical proteins were not included in Figure 4. Not surprisingly, exposure to As(III) resulted in more down regulated genes (Figure 4A) compared to As(V) exposure, which mostly resulted in upregulation of genes (Figure 4B). There is more overlap in upregulated genes than in downregulated genes in the different treatments (Figure 4B). A table of significantly up- and down- regulated genes after amendment of 10 μM As(III) and 100 μM As(V) relevant to the discussion can be found in Supplemental Tables S2 and S3, respectively.
Figure 4.
Number of significantly up- and down-regulated genes in Dhc195 organized by KEGG module in cultures amended with A) 10 μM As(III) and 100 μM As(V). Genes that were identified to code for hypothetical proteins and genes not identified in the KEGG database are not shown. Positive numbers denote genes up-regulated while negative numbers denote genes down-regulated. The colors of the bars indicate the KEGG structural complex category of each module B) Graphical representation of quantification of up- and down- regulated genes that were found to be shared and distinct between the As(III)- and As(V)-amended conditions.
The reductive dehalogenase gene responsible for dechlorination of TCE to VC in Dhc195, tceA (DET 0078-0079), did not exhibit significant differential expression in response to As(III) or As(V). The arsR family transcriptional regulation gene (DET 1005) exhibited significant up-regulation in both As(III)- and As(V)-containing cultures. However, neither the cytoplasmic arsenate reductase arsC (DET0143) or the arsenite efflux transporter genes (DET0908) exhibited differential expression at the time of sample collection, suggesting it may take more time after exposure to arsenic for these genes to be exhibit differential expression. It is also possible that this transcriptional regulator is induced by the presence of arsenic but has a different role in genetic regulation for Dhc195 arsC repression, as arsR has been previously characterized as a regulator which represses arsC expression unless arsenic is present to inhibit the repressor and induce arsC expression.46 It is important to note that the arsC enzyme is usually expressed at low levels due to the leaky ars operon promoter,47 and was expressed in the positive control, As(III)-amended, and As(V)-amended conditions even though no differential expression was observed. The arsenite methyltransferase arsM (DET1420) was up-regulated in both As(III)- and As(V)-amended cultures, but no production of methylated arsenic compounds were observed. It is possible that this gene is mis-annotated in the Dhc195 genome and the substrate for this methyltransferase is not arsenic.
Both As(III)- and As(V)-containing compounds have been shown to promote DNA mutagenesis in bacteria.48 Due to this, and general stress response, it is unsurprising that genes involved in DNA repair and replication, translation, and nucleotide metabolism were found to be upregulated in this study (Figure 4). Genes involved in membrane transport may be upregulated to serve multiple purposes, including export of potential stress-signal molecules and import of necessary biomolecules for assimilation from the environment. Genes involved in folate biosynthesis were observed to be upregulated under As(III)- (DET0016, 1603-1604) and As(V)- (DET0016, 1603, 1605) amended conditions. Biosynthesis of arginine (det00220 per KEGG pathway database) was observed to be upregulated in both As(III)- and As(V)-containing cultures (p-value = 0.035 and p-value = 0.044, respectively), and the valine, leucine, and isoleucine biosynthesis pathway (det00290) was observed to be upregulated in As(III)-containing cultures (p-value = 0.047). The prolyl-tRNA, isoleucyl-tRNA, and tryptophanyl-tRNA synthetases (DET0368, 1038, 1343) were also found to be upregulated in both As(III)- and As(V)-containing cultures, while histidyl-tRNA and tyrosyl-tRNA synthetases were found to be down-regulated under both conditions.
Significant changes in the abundance of aminoacylated-tRNA molecules were also identified by microarray analysis. In cultures amended with As(V), glutamyl-tRNA and prolyl-tRNA were found in significantly higher abundance as compared to the control. In culture amended with As(III), glycyl-tRNA and threonyl-tRNA were found in significantly higher abundance as compared to the control. In both As(III)- and As(V)-amended cultures, arginyl-tRNA, alanyl-tRNA, histidyl-tRNA, methionyl-tRNA, and seryl-tRNA were found in significantly lower abundance as compared to the control. In As(V) containing cultures, asparaginyl-tRNA was also found in lower concentrations. Virtually all aminoacyl-tRNA synthetase enzymes that have been biochemically characterized contain sulfhydryl groups, and previous studies have identified tRNA synthetases as enzymes that can be affected by sulfhydryl group inhibitors.49-51 The results of this study, showing higher abundances for some aminoacyl-tRNA molecules in the presence of arsenic and lower concentrations for others, suggest that arsenic has greater impacts on some aminoacyl-tRNA synthetases than others in Dhc195. Numerous aminoacyl-tRNA synthetases in other bacteria have been identified as resistant to inhibition by sulfhydryl-inhibiting agents, including the lysyl-tRNA synthetase from Escherichia coli and the methionyl-tRNA synthetase from Sarcina lutea.52-53 Biochemical characterization of tRNA synthetases in Dhc195 is required to draw further conclusions regarding the sensitivity of certain synthetases to sulfhydryl group inhibitors.
Of the differentially expressed genes observed herein, 236 up-regulated genes and 13 down-regulated genes were shared between As(III)- and As(V)- amended cultures (Figure 4B), suggesting certain cellular responses in the presence of 10 μM As(III) and 100 μM As(V) in Dhc195 are shared while others are specific to As(III) or As(V). It is possible that the observed similarities in genetic response to As(III) and As(V) are a result of small amounts of cytoplasmic reduction of As(V) to As(III) by Dhc195 (lower than the limit of detection) and subsequent presence of As(III) since arsC is always slightly expressed. Additionally, both As(III) and As(V) have been shown to induce heat-shock response proteins such as dnaJ, dnaK, and grpE in bacteria Leuconostoc esenteroides, Pseudomonas aeruginosa, and Klebsiella pneumoniae.54-56 However, these stress-response genes were not upregulated in Dhc195 under As(III) or As(V) stress in this study (DET1399-1400, 1411). Arsenic is also known to interact with sulfur-containing enzymes directly, which may impact multiple cellular processes.20-22 Iron-sulfur clusters are common in bacterial proteins, and a variety of proteins in Dhc195 contain iron-sulfur units, including reductive dehalogenases4 and formate dehydrogenase (DET0112),57 the latter of which was upregulated under As(III)-amended conditions. Since As(III) and As(V) are otherwise known to act via different modes of toxicity, many of these shared gene expression changes may be related to general stress response in Dhc195.
In As(V)-amended cultures, many of the up-regulated genes distinct from As(III)-amended cultures coded for proteins known to interact with phosphate groups, including kinases (DET0049, 0405, 0744) and phosphatases (DET0713, 0797). As(V) has been previously shown to act as a competitive inhibitor with phosphatases,17 and the upregulation of kinases may be a transcriptional response to the tendency of arsenate to form ADP-arsenate complexes, inhibiting formation of ATP.57-59 Other genes upregulated solely in As(V)-amended cultures included genes for potassium uptake (DET0026-0027, 0029).
The increase in tolerance to As(III) after gradual accumulation as opposed to a pulse input observed in the physiological experiments in this study may be explained by the high number of differentially expressed genes shared between As(III)- and As(V)-amended cultures. It is possible that by the time As(III) began to accumulate to toxic levels in the cell, many of the required stress-response genes had been up-regulated and the resulting cellular changes had already taken effect. This response may illustrate a more environmentally relevant system more closely than a pulse input of As(III). However, the kinetics of As(V) reduction in an environmental system could potentially be much faster than observed herein, as As(V)-respiring bacteria are common in subsurface environments.60
Extracellular metabolite profile of D. mccartyi in the presence of arsenic
Both targeted and untargeted metabolomic analyses were applied to culture supernatant samples taken at 2 timepoints in cultures amended with no arsenic (positive control), 10 μM As(III) and 100 μM As(V). Timepoint 1 represents samples taken at the same time as the transcriptomic analysis and timepoint 2 represents samples taken 24 hours after transcriptomic analysis, when 95% of TCE had been consumed. Significant changes in metabolite abundance resulting from amendment of As(III) and As(V) were identified at both timepoints (Figure 5).
Figure 5:
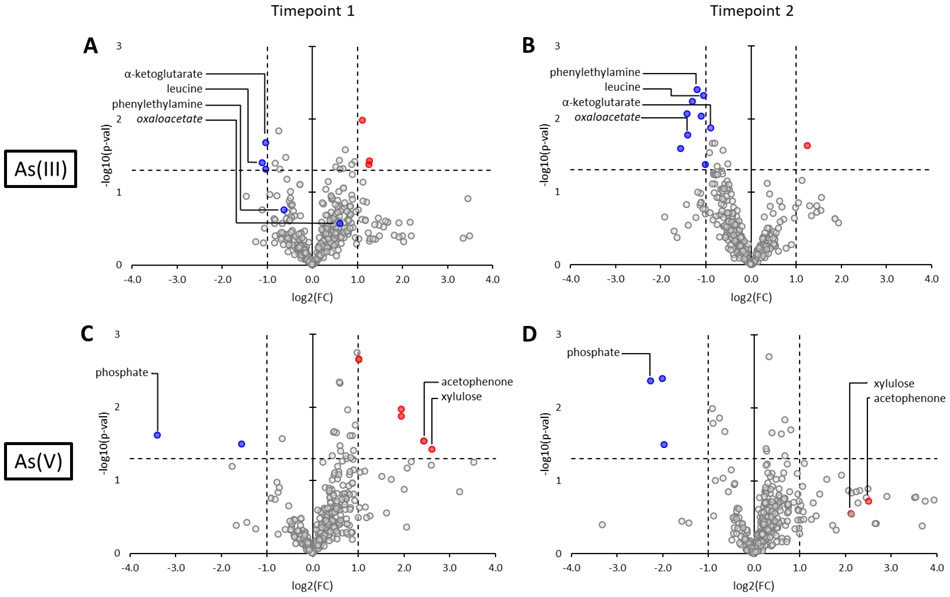
Volcano plots showing changes in metabolite abundance in the extracellular metabolite pool in Dhc195 amended with As(III) at timepoints A) 1 and B) 2 and Dhc195 amended with As(V) at timepoints C) 1 and D) 2 with log base 2 of the fold change as (arsenic/control) on the x-axis and negative log base 10 of the p-value on the y-axis. The horizontal and vertical dotted lines indicate the cutoffs for statistical significance. The colored markers indicate statistically significant metabolites that were present in higher (red) or lower (blue) abundances after arsenic amendment. Metabolites that were statistically significant under only one timepoint are colored in both timepoint figures for reference. Significant targeted metabolites are labeled and significant untargeted metabolites that were identified and experimentally validated are labeled in italics. Colored markers without labels denote untargeted metabolites that could not be identified.
In cultures amended with As(III) at timepoint 1 (Figure 5A), α-ketoglutarate and leucine were found to be present in lower abundance as compared to the control. Ketoglutarate is an intermediate in the central carbon assimilation pathway of Dhc195 and a precursor to glutamate.61 At timepoint 2 (Figure 5B), 88% of all observed changes were decreases in abundance including all but one significant change. Leucine and phenylethylamine were observed to be significantly diminished in the supernatant. One untargeted metabolite significantly diminished in the supernatant under As(III)-stress was identified to be oxaloacetate, an intermediate in the tricarboxylic acid cycle and a precursor to glutamate, aspartate, and homocysteine in Dhc195.62 Tyrosine, isoleucine, fumaric acid, and malic acid were also observed to be decreased, while not statistically significant. The decrease in extracellular metabolites from timepoint 1 to 2 suggests that Dhc195 imported many metabolites from the environment in concordance with the up-regulation of ABC transporters and an ion channel (DET0224, 0795, 0814, 0817, 0851, 0856, 1179-1180, 1491, 1579) observed at timepoint 1. The observed decrease could also be the result of decreased export of certain metabolites by Dhc195, as five genes related to membrane transport were down-regulated in As(III)-amended cultures (DET0034, 0138, 0784, 1125, 1493). The transcriptomic analysis at timepoint 1 identified the tyrosine, isoleucine, and leucine biosynthesis pathways as significantly upregulated, indicating Dhc195 utilized extracellular import as well as upregulated biosynthesis to obtain these amino acids in the presence of As(III). Additionally, malic acid and fumaric acid are both precursors to multiple amino acids in the biosynthesis pathway of Dhc195,61 and phenylethylamine is an intermediate in phenylalanine metabolism.
At timepoint 1 in cultures amended with As(V) (Figure 5C), extracellular phosphate was found to be in lower abundance whereas xylulose, an intermediate in pentose interconversions,63 and acetophenone, an intermediate in phenylalanine biotransformation,64 were observed to increase in abundance. In As(V)-amended cultures at timepoint 2 (Figure 5D), phosphate and shikimic acid (a folate precursor) were observed to be diminished in the supernatant, however the change in shikimic acid was not found to be statistically significant. As(V) is known to interact with phosphate-related cellular processes, which would induce Dhc195 to increase import of phosphate in the presence of As(V). This increased importation results in higher uptake of As(V) as well, because the transporter responsible for phosphate also transports As(V) with varying specificity,65-66 perpetuating the cytoplasmic reduction of As(V) to As(III) in Dhc195 and leading to As(III) toxicity. As As(III) accumulates, Dhc195 exhibits the gene regulation and import of amino acids and carbon pathway intermediates observed herein to counteract As(III) toxicity.
Only ten of the common amino acids were detected using this method (alanine, cysteine, glycine, isoleucine, leucine, methionine, proline, serine, threonine, tyrosine, and valine). Arginine was not reported using this method, as it is broken down into ornithine during sample analysis. The remaining nine (asparagine, aspartate, glutamate, glutamine, histidine, lysine, phenylalanine, serine, and tryptophan) were present in too low abundance for detection in the supernatant. It has been previously asserted that Dhc195 almost exclusively synthesizes lysine, aspartate, and glutamine when these amino acids are provided in excess in growth medium of laboratory culture.67 These amino acids were not found to be present in the extracellular metabolite profile in this study, consistent with their uptake by Dhc195 and the selectivity of ABC-transporters in Dhc195,67-68 supporting the hypothesis that Dhc195 does possess selective ABC-transporters for amino acids from the environment in response to arsenic stress. Based on these results, amino acids, anabolism intermediates, and folate may be important to Dhc195 under arsenic stress. A comprehensive table of changes in targeted and identified untargeted metabolites after amendment of 10 μM As(III) and 100 μM As(V) can be found in Supplemental Tables S4 and S5, respectively.
MIC experiments with amendment of folate or amino acids
After transcriptomic and metabolomic analyses, folate and amino acids were identified as metabolites of interest that may increase the arsenic tolerance of Dhc195 in arsenic-amended cultures. As(III) MIC experiments in Dhc195 were subsequently performed with amendment of 400 μg/L folate and with a 20-amino acid solution (20 mg/L each). Exponential growth rates of Dhc195 with increasing As(III) were compared with exponential growth rates of Dhc195 + folate and Dhc195 + amino acids (Figure 6). The estimated MIC of As(III) for Dhc195 + folate and Dhc195 + amino acids were 6.8 μM As(III) and 12.2 μM As(III), respectively.
Figure 6.
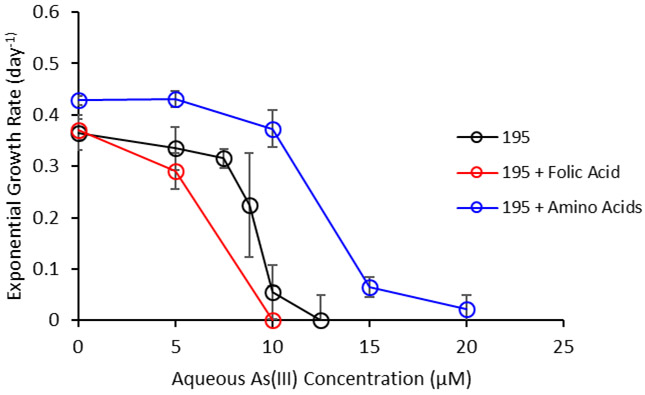
Exponential growth rate (day−1) with increasing As(III) concentrations for Dhc195, Dhc195 amended with 400 μg/L folic acid, and Dhc195 amended with amino acids (20 mg/L each of the 20 common amino acids). The MICs for Dhc195, Dhc195 + folate, and Dhc195 + amino acids were 9.1, 6.8, and 12.2 μM As(III), respectively. Error bars represent one standard deviation of experimental triplicates.
Results indicate that while amendment of folate did not enhance the tolerance of Dhc195 for As(III), that amendment of the 20-amino acid solution achieved this. Zhuang et al. demonstrated the uptake and utilization of exogenous amino acids by Dhc195 under regular growth conditions.67 Dhc195 was observed to exhibit higher growth rates with the amendment of exogenous amino acids, which is verified in this study (Figure 6 – timepoint: day 0). This metabolomic analysis along with the transcriptomic analysis indicates Dhc195 uptakes or retains amino acids and their biosynthesis intermediates to combat arsenic toxicity, likely due to inhibition of enzymatic catalysis by sulfhydryl-containing enzymes.
Folate is an important biomolecule, impacting DNA stability and serving as a precursor to DNA, RNA, and various amino acids including methionine.69-70 Numerous studies have observed enhanced expression of folate biosynthesis genes, including folE, resulting from various cell stressors (e.g. temperature stress and antibiotic stress)71 Waller et al. demonstrated a folate requirement, most likely tetrahydrofolate, in the catalytic activity of ygfZ a tRNA-modifying enzyme involved in the assembly or repair of iron-sulfur clusters.72 Waller et al. also observed a correlation between inactivation of folate biosynthesis genes and lowered activity of tRNA modification enzyme miaB72 The upregulation of both folate biosynthesis and miaB, an iron-sulfur cluster protein,73 in Dhc195 in response to arsenic stress observed in this study suggests that Dhc195 may produce more folate to support repair of iron-sulfur clusters inhibited by arsenic interaction with sulfide-containing enzymes, including aminoacyl-tRNA synthetases. However, amendment of folate to Dhc195 cultures did not increase resistance to As(III) toxicity as measured by growth rate. It is possible that Dhc195 does not have a folate transport system, as none is annotated in the genome.18 Further studies are required to elucidate the importance of the folate biosynthesis pathway in Dhc195 under arsenic stress.
This study has demonstrated that the presence of As(III) or As(V) in a groundwater aquifer could lead to inhibition of TCE dechlorination by Dhc195. Based on these results, engineered bioremediation strategies, such as retarding As(V) reduction rates and/or providing the TCE-dechlorinating community with exogenous amino acids could promote robust TCE dechlorination at arsenic contaminated sites. Future studies should build upon the complexity of the system (e.g. analysis of multi-member consortia) to develop a comprehensive understanding of the impacts of co-contaminants on TCE reductive dechlorination.
Supplementary Material
Acknowledgements
This work was funded by NIEHS grant P42ES004705. Thank you to the West Coast Metabolomics Center for metabolomics services, Jessican Counihan and Daniel Nomura for help and insight into preliminary metabolomics discussions, and Shan Yi, Ray Keren, Aidan Cecchetti, and Scott Miller for manuscript edits.
Footnotes
Supporting Information. Supporting information file includes the workflow for untargeted metabolite identification, cell enumeration for As(V)-amended cultures, arsenic speciation of As(III)-amended cultures, and tabular results of transcriptomic and targeted metabolomic analyses.
References
- 1.Hopple JA, Delzer GC, and Kingsbury JA (2009). Anthropogenic organic compounds in source water of selected community water systems that use groundwater. 2002-05: U.S. Geological Survey Scientific Investigations Report 2009-5200. 74 p. [Google Scholar]
- 2.Freedman DL and Gossett JM (1989). Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl. Environ. Microbiol 55:9 2144–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maymó-Gatell X, Chien Y, Gossett JM, & Zinder SH (1997). Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science, 276(5318), 1568–1571. doi: 10.1126/science.276.5318.1568 [DOI] [PubMed] [Google Scholar]
- 4.Holliger C, Wohlfarth G, Diekert G (1999) Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev 22:383–398 [Google Scholar]
- 5.He J, Sung Y, Dollhopf ME, Fathepure BZ, Tiedje JM, Loffler FE (2002) Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environmental Science & Technology. 36(18): 3945–3952 [DOI] [PubMed] [Google Scholar]
- 6.Cupples AM, Spormann AM, McCarty PL. (2003). Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl. Environ. Microbiol 69: 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Ritalahti KM, Yang K, Koenigsberg SS, Loffler FE (2003a). Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature. 424:62–65. 10.1038/nature01717 [DOI] [PubMed] [Google Scholar]
- 8.United States, Environmental Protection Agency, (2017). Superfund: National Priorities List (NPL). Retrieved August 10, 2017, from https://www.epa.gov/superfund/superfund-national-priorities-list-npl [Google Scholar]
- 9.Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000). Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Applied Geochemistry. 15:4 403–413. [Google Scholar]
- 10.Smedley PL, & Kinniburgh DG (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5): 517–568 doi: 10.1016/S0883-2927(02)0001f8-5. [DOI] [Google Scholar]
- 11.Bissen M and Frimmel FH (2003) Arsenic – a Review. Part I: Occurrence, Toxicity, Speciation, Mobility. CLEAN – Soil, Air, Water. 31:1 10.1002/aheh.200390025 [DOI] [Google Scholar]
- 12.Zhu Y-G, Yoshinaga M, Zhao F-J, Rosen BP (2014) Earth abides arsenic biotransformations. Annual Review of Earth and Planetary Sciences. 42:443–467. 10.1146/annurev-earth-060313-054942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSimone LA, McMahon PB, and Rosen MR (2015). The quality of our Nation’s waters: Water quality in principal aquifers of the United States, 1991-2010 (Circular 1360). Reston, VA: United States Geological Survey. [Google Scholar]
- 14.Masscheleyn PH, Delaune RD, and Patrick WH Jr. (1991). Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol. 25 (8), 1414–1419 doi: 10.1021/es00020a008. [DOI] [Google Scholar]
- 15.Violante A, Cozzolino V, Perelomov L, Caporale AG, and Pigna M (2010). Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr 10(3): 268–292 [Google Scholar]
- 16.Hughes MF (2002). Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 133(1) 1–16. doi: 10.1016/S0378-4274(02)00084-X [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay R, Rosen BP, Phung LT, and Silver S (2002). Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiology Reviews. 26(3): 311–325. doi: 10.1111/j.1574-6976.2002.tb00617.x [DOI] [PubMed] [Google Scholar]
- 18.Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, Ward NL, Nelson WC, Deboy RT, Khouri HM, Kolonay JF, Dodson RJ, Daugherty SC, Brinkac LM, Sullivan SA, Madupu R, Nelson KT, Kang KH, Impraim M, Tran K, Robinson JM, Forberger HA, Fraser CM, Zinder SH, Heidelberg JF (2005). Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105–108. [DOI] [PubMed] [Google Scholar]
- 19.Pöritz M, Goris T, Wubet T, Tarkka MT, Buscot F, Nijenhuis I, Lechner U, and Adrian L (2013) Genome sequences of two dehalogenation specialists – Dehalococcoides mccartyi strains BTF08 and DCMB5 enriched from the highly polluted Bitterfield region. FEMS Microbiol. Lett 343(2): 101–104. [DOI] [PubMed] [Google Scholar]
- 20.Knowles FC and Benson AA (1983). The biochemistry of arsenic. Trends Biochem. Sci 8, 178–180. [Google Scholar]
- 21.Klemperer NS and Pickart CM (1989). Arsenite inhibits two steps in the ubiquitin-dependent proteolytic pathway. J. Biol. Chem 264, 19245–19252. [PubMed] [Google Scholar]
- 22.Winski SL and Carter DE. 1995. Interaction of rat red blood cell sulfydryls with arsenate and arsenite. Journal of Toxicology and Environmental Health, 46(3): 379–397 doi:/ 10.1080/15287399509532043 [DOI] [PubMed] [Google Scholar]
- 23.Bhatt P, Kumar MS, Mudliar S, & Chakrabarti T (2007). Biodegradation of chlorinated Compounds—A review. Critical Reviews in Environmental Science and Technology, 37(2), 165–198. doi: 10.1080/10643380600776130 [DOI] [Google Scholar]
- 24.Löffler FE, Ritalahti KM, and Zinder SH. (2013). Dehalococcoides and reductive dechlorination of chlorinated solvents Bioaugmentation for Groundwater Remediation, Stroo HF, Leeson A, and Ward CH, Editors. p. 39–88. [Google Scholar]
- 25.Mao X, Polasko A, and Alvarez-Cohen L (2017). Effects of sulfate reduction on trichloroethene dechlorination by Dehalococcoides-containing microbial communities. Appl Environ Microbiol. 83(8): e03384–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Capiro NL, Yan J, Marcet TF, Pennell KD, Loffler FE (2017). Resilience and recovery of Dehalococcoides mccartyi following low pH exposure. FEMS Microbiology Ecology. 93(12):fix130. doi: 10.1093/femsec/fix130 [DOI] [PubMed] [Google Scholar]
- 27.He J, Ritalahti KM, Aiello MR, Loffler FE (2003b). Complete detoxification of vinyl chloride by an anaerobic enrichment culture and identification of the reductively dechlorinating populations as a Dehalococcoides species. Appl. Environ. Microbiol 69(2): 996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolin EA, Wolin MJ, Wolfe RS (1963). Formation of methane by bacterial extracts. J. Biol. Chem 238:2882–2886 [PubMed] [Google Scholar]
- 29.Mukhopadhyay A, He Z, Alm EJ, Arkin AP, Baidoo EE, Borglin SC, Chen W, Hazen TC, He Q, Holman H-Y, Huang K, Huang R, Joyner DC, Katz N, Keller M, Oeller P, Redding A, Sun J, Wall J, Wei J, Yang Z, Yen H-C, Zhou J, and Keasling JD (2006). Salt stress in Desulfovibrio vulgaris Hildenborough: an integrated genomics approach. J. Bacteriol 188(11): 4068–4078. doi: 10.1128/JB.01921-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DR, Lee PKH, Holmes VF, Alvarez-Cohen L (2005). An internal reference technique for accurately quantifying specific mRNAs by realtime PCR with application to the tceA reductive dehalogenase gene. Appl. Environ. Microbiol 71:3866–3871. 10.1128/AEM.71.7.3866-3871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao X, Stenuit B, Polasko A, Alvarez-Cohen. (2015). Efficient metabolic exchange and electron transfer within a syntrophic trichloroethene-degrading coculture of Dehalococcoides mccartyi 195 and Syntrophomonas wolfei. Appl Environ Microbiol 81:2015–2024. 10.1128/AEM.03464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee PK, Cheng D, Hu P, West KA, Dick GJ, Brodie EL, Andersen GL, Zinder SH, He J, and Alvarez-Cohen L (2011). Comparative genomics of two newly isolated Dehalococcoides strains and an enrichment using a genus microarray. ISME J. 5(6): p. 1014–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier L, Cope L, Bolstad BM, Irizarry RA (2004). affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics, 20(3), 307–315. ISSN 1367-4803, doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DR, Brodie EL, Hubbard AE, Andersen GL, Zinder SH, and Alvarez-Cohen L (2008). Temporal transcriptomic microarray analysis of “Dehalococcoides ethenogenes” strain 195 during the transition into stationary phase. Appl Environ Microbiol 74(9): 2864–2872. doi: 10.1128/AEM.02208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Men Y, Fed H, VerBerkmoes NC, Shah MB, Johnson DR, Lee PKH, West KA, Zinder SH, Anderson GL, Alvarez-Cohen L (2011) Sustainable syntrophic growth of Dehalococcoides ethenogenes strain 195 with Desulfovibrio vulgaris Hildenborough and Methanobacterium congolense: global transcriptomic and proteomic analyses. ISME J. 6:410–421. 10.1038/ismej.2011.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West KA, Lee PKH, Johnson DR, Zinder SH, Alvarez-Cohen L (2013). Global gene expression of Dehalococcoides within a robust dynamic TCE-dechlorinating community under conditions of periodic substrate supply. Biotechnology and Bioengineering. 110(5):1333–1341. 10.1002/bit.24819 [DOI] [PubMed] [Google Scholar]
- 37.Barry WT, Nobel AB, and Wright FA (2005). Significance analysis of function categories in gene expression studies: a structured permutation approach. Bioinformatics. 21:1943–1949. [DOI] [PubMed] [Google Scholar]
- 38.Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S, and Nikolau B (2008). Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J. 53(4): 691–704 [DOI] [PubMed] [Google Scholar]
- 39.Allen F, Pon A, Wilson M, Greiner R, and Wishart D (2014). CFM-ID: a web server for annotation, spectrum prediction and metabolite identification from tandem mass spectra. Nucleic Acids Res 12:W94–W99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Men Y, Lee PKH, Harding KC, and Alvarez-Cohen L (2013). Characterization for four TCE-dechlorinating microbial enrichments grown with different cobalamin stress and methanogenic conditions. Appl Microbiol Biotechnol. 97(14): 6439–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter W; Davidowski L; Neubaur K; Di Bussolo J (2003). Speciation of five arsenic compounds in urine by HPLC/ICP-MS, Application Note; Perkin Elmer, Inc.: Waltham, MA. [Google Scholar]
- 42.Newman DK, Kennedy EK, Coates JD, Ahmann D, Ellis DJ, Lovley DR, Morel FMM (1997) Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch. Microbiol 168:380–388. [DOI] [PubMed] [Google Scholar]
- 43.Silver S and Phung LT (2005). Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol 71(2): 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson JF and Gavis J (1972). A review of the arsenic cycle in natural waters. Water Research. 6:1259–1274. [Google Scholar]
- 45.Collinet M-N. and Morin D (1990). Characterization of arsenopyrite oxidizing Thiobacillus. Tolerance to arsenite, arsenate, ferrous and ferric iron. Antonie va Leeuwenhoek. 57(4): 237–244. [DOI] [PubMed] [Google Scholar]
- 46.Wu J and Rosen BP (1991). The ArsR protein is a trans-acting regulatory protein. Molecular Microbiol. 5(6): 1331–1336. doi: 10.1111/j.1365-2958.1991.tb00779.x [DOI] [PubMed] [Google Scholar]
- 47.Stocker J, Balluch D, Gsell M, Harms H, Feliciano J, Daunert S, Malik KA, and Roelof van der Meer J (2003). Development of a set of simple bacterial biosensors for quantitative and rapid measurements of arsenite and arsenate in potable water. Environ Sci Technol. 37(20): 4743–4750. doi: 10.1021/es034258b. [DOI] [PubMed] [Google Scholar]
- 48.Nishioka H (1975). Mutagenic activities of metal compounds in bacteria. Mutat. Res 31:185–189. [DOI] [PubMed] [Google Scholar]
- 49.Bruton CJ and Hartley BS (1968). Sub-unit structure and specificity of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem. J 108(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iaccarino M and Berg P (1969) Requirement of sulfhydryl groups for the catalytic and tRNA recognition functions of isoleucyl-tRNA synthetase. J. Mol. Biol 42(12): 151–169. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence FJ (1970). Studies on methionyl transfer RNA synthetase from Escherichia coli K12. European Journal of Biochemistry, 15(3), 436–441. [DOI] [PubMed] [Google Scholar]
- 52.Stern R, DeLuca D, Mehler AH, McElroy WD (1966). Role of sulfhydryl troups in activating enzymes: Properties of Escherichia coli lysine-transfer ribonucleic acid synthetase. Biochemistry. 5(1):126–130. [PubMed] [Google Scholar]
- 53.Hahn GA and Brown JW (1967) Properties of a methionyl-tRNA synthetase from Sarcina lutea. Biochim. Biophys. Acta 146(1):264–271. [DOI] [PubMed] [Google Scholar]
- 54.Salotra P, Singh DK, Seal KP, Krishna N, Jaffe H, and Bhatnagar R (1995). Expression of DnaK and GroEL homologs in Leuconostoc esenteroides in response to heat shock, cold shock or chemical stress. FEMS Microbiology Letters. 131:57–62. [DOI] [PubMed] [Google Scholar]
- 55.Parvatiyar K, Alsabbagh EM, Ochsner UA, Stegemeyer MA, Smulian AG, Hwang SH, Jackson CR, McDermott TR, and Hassett DJ (2005). Global analysis of cellular factors and responses involved in Pseudomonas aeruginosa resistance to arsenite. J Bacteriol. 187(14): 4853–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daware V, Kesavan S, Patil R, Natu A, Kumar A, Kulkarni M, Gade W (2012). Effects of arsenite stress on growth and proteome of Klebsiella pneumoniae. J. Biotechnol 158:8–16. [DOI] [PubMed] [Google Scholar]
- 57.Ferry JG (1990). Formate dehydrogenase. FEMS Microbiol Rev 87: 377–382 [DOI] [PubMed] [Google Scholar]
- 58.TerWelle HF and Slater EC (1967). Uncoupling of respiratory-chain phosphorylation by arsenate. [DOI] [PubMed] [Google Scholar]
- 59.Gresser MJ (1981). ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J. Biological Chemistry 256: 5981–5983. [PubMed] [Google Scholar]
- 60.Macur RE, Jackson CR, Botero LM, Mcdermott TR, Inskeep WP (2004). Bacterial populations associated with the oxidation and reduction of arsenic in an unsaturated soil. Environ Sci Technol. 38(1): 104–111/ [DOI] [PubMed] [Google Scholar]
- 61.Tang YJ, Yi S, Zhuang W-Q, Zinder SH, Keasling JD, Alvarez-Cohen L (2009). Investigation of carbon metabolism in Dehalococcoides ethenogenes strain 195 by use of isotopomer and transcriptomic analyses. J. Bacteriol 191(16): 5224–5231. 10.1128/JB.00085-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhuang WQ, Yi S, Bill M, Brisson VL, Feng X, Men Y, Conrad ME, Tang YJ, and Alvarez-Cohen L (2014). Incomplete Wood-Ljungdahl pathway facilitates one-carbon metabolism in organohalide-respiring Dehalococcoides mccartyi. PNAS. 111(17):6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doelle HW (1969). Bacterial metabolism. Academic Press, New York London, 171–175. [Google Scholar]
- 64.Lapadatescu C, Gines C, Le Quere J-L, and Bonnarme P (2000). Novel scheme for biosynthesis of aryl metabolites from L-phenylalanine in the fungus Bjerkandera adusta. Appl Environ Microbil 66(4): 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dixon HBF (1997). The biochemical action of arsenic acids especially as phosphate analogues. Advances in Inorganic Chemistry. 44: 191–227. doi: 10.1016/S0898-8838(08)60131-2. [DOI] [Google Scholar]
- 66.Ravera S, Virkki LV, Murer H, Forster IC (2007). Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol 293: C606–C620. doi: 10.1152/ajpcell.00064.2007. [DOI] [PubMed] [Google Scholar]
- 67.Zhuang W-Q, Yi S, Feng X, Zinder SH, Tang YJ, Alvarez-Cohen L (2011). Selective utilization of exogenous amino acids by Dehalococcoides ethenogenes strain 195 and its effects on growth and dechlorination activity. Appl. Environ. Microbiol 77(21): 7797–7803. 10.1128/AEM.05676-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Islam AM, Edwards EA, Mahadevan R. (2010). Characterizing the metabolism of Dehalococcoides with a constraint-based model. PLoS Comput. Biol 6:e1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duthie SJ, Narayananm S, Brand GM, Pirie L, and Grant G (2002). Impact of folate deficiency on DNA stability. J Nutrition. 132(8): 2444S–2449S. 10.1093/jn/132.8.2444S [DOI] [PubMed] [Google Scholar]
- 70.Brosnan JT and Brosnan ME (2006). The sulfur-containing amino acids: An overview. J Nutr. 136(6): 1636S–1640S. [DOI] [PubMed] [Google Scholar]
- 71.Crapoulet N, Barbry P, Raoult D, Renesto P (2006). Global transcriptome analysis of Tropheryma whipplei in response to temperature stresses. J Bacteriol. 188(14): 5228–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waller JC, Alvarez S, Naponelli V, Lara-Nunez A, Blaby IK, Da Silva V, Ziemak MJ, Vickers TJ, Beverley SM, Edison AS, Rocca JR, Gregory JF III, de Crecy-Lagard V, Hanson AD (2010). A role for tetrahydrofolates in the metabolism of iron-sulfur clusters in all domains of life. PNAS. 107(23): 10412–10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierrel F, Bjork GH, Fontecave M, and Atta M (2002). Enzymatic modification of tRNAs: MiaB is an iron-sulfur protein. J Biol Chem 277:13367–13370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.