Abstract
Elderly adults demonstrate increased propensity for breathing instability during sleep compared with younger adults, and this may contribute to increased prevalence of sleep-disordered breathing (SDB) in this population. Hence, in older adults with SDB, we examined whether addition of supplemental oxygen (O2) will stabilize breathing during sleep and alleviate SDB. We hypothesized that exposure to supplemental O2 during non-rapid eye movement (NREM) sleep will stabilize breathing and will alleviate SDB by reducing ventilatory chemoresponsiveness and by widening the carbon dioxide (CO2) reserve. We studied 10 older adults with mild-to-moderate SDB who were randomized to undergo noninvasive bilevel mechanical ventilation with exposure to room air or supplemental O2 (Oxy) to determine the CO2 reserve, apneic threshold (AT), and controller and plant gains. Supplemental O2 was introduced during sleep to achieve a steady-state O2 saturation ≥95% and fraction of inspired O2 at 40%–50%. The CO2 reserve increased significantly during Oxy versus room air (−4.2 ± 0.5 mmHg vs. −3.2 ± 0.5 mmHg, P = 0.03). Compared with room air, Oxy was associated with a significant decline in the controller gain (1.9 ± 0.4 L/min/mmHg vs. 2.5 ± 0.5 L/min/mmHg, P = 0.04), with reductions in the apnea-hypopnea index (11.8 ± 2.0/h vs. 24.4 ± 5.6/h, P = 0.006) and central apnea-hypopnea index (1.7 ± 0.6/h vs. 6.9 ± 3.9/h, P = 0.03). The AT and plant gain were unchanged. Thus, a reduced slope of CO2 response resulted in an increased CO2 reserve. In conclusion, supplemental O2 reduced SDB in older adults during NREM sleep via reduction in chemoresponsiveness and central respiratory events.
NEW & NOTEWORTHY This study demonstrates for the first time in elderly adults without heart disease that intervention with supplemental oxygen in the clinical range will ameliorate central apneas and hypopneas by decreasing the propensity to central apnea through decreased chemoreflex sensitivity, even in the absence of a reduction in the plant gain. Thus, the study provides physiological evidence for use of supplemental oxygen as therapy for mild-to-moderate SDB in this vulnerable population.
Keywords: apneic threshold, carbon dioxide reserve, central apnea, chemoresponsiveness, supplemental oxygen
INTRODUCTION
Aging is associated with increased propensity for central apneas during non-rapid eye movement (NREM) sleep (4, 21). Positive airway pressure (PAP) therapy for sleep apnea remains the primary therapeutic modality in the elderly population. Notably, pharmacological agents to treat sleep apnea, such as serotonergic agonists and antagonists and noradrenergic and cholinergic drugs (16), have yielded minimal or inconsistent response in human studies. This could be potentially attributed to differences in the pathophysiology of sleep apnea, especially in older adults. More recently, we have demonstrated that comparing young individuals with elderly adults without sleep apnea, the latter were more likely to develop hypocapnic central apnea owing to increased hypocapnic chemoresponsiveness during NREM sleep (8). Conversely, in healthy young adults without sleep apnea, we have demonstrated that sustained hyperoxia mitigated the propensity to hypocapnic central apnea (10), whereas administration of intermittent hypoxia precipitated breathing instability during NREM sleep (9). Thus, integrating these crucial pathophysiological underpinnings, the next logical step was to investigate the effect of exposure to supplemental oxygen on central apnea propensity in older adults with sleep-disordered breathing (SDB).
Specifically, our aim was to determine in older adults with mild-to-moderate SDB, whether intervention with supplemental oxygen (O2) will stabilize breathing during NREM sleep. We hypothesized that in older adults with SDB, supplemental O2 will stabilize breathing and alleviate SDB by reducing ventilatory chemoresponsiveness and by widening the carbon dioxide (CO2) reserve during NREM sleep. The overall rationale for our proposed research is that by targeting specific mechanisms of ventilatory control that predict breathing instability in the elderly, we will successfully alleviate breathing instability and sleep apnea in this population.
METHODS
Participants
The Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center approved the experimental protocols; informed written consent was obtained from all participants. All participants were free of daytime sleepiness and free from significant cardiovascular, pulmonary, neurological, or other medical disorders. Patients on certain medications including narcotics were excluded. Patients with body mass index (BMI) >34 kg/m2 were excluded to avoid the effects of morbid obesity on pulmonary mechanics and ventilatory control. Individuals with severe SDB on diagnostic polysomnography (PSG) study (apnea-hypopnea index, AHI > 20/h) were excluded, as they may have multiple complex mechanisms that contribute to sleep apnea and were referred for PAP therapy. We studied 14 elderly participants (age ≥ 60 yr) with mild-to-moderate SDB with AHI 5–20/h, of whom 10 individuals were able to complete the entire study protocol. SDB included both obstructive and central respiratory events (apneas and hypopneas). The remaining individuals either did not return for the study protocol after enrollment or could not maintain stable sleep with adequate ventilatory recordings during the experimental protocols. The latter individuals had similar baseline characteristics as the final study sample. All participants were naïve to PAP therapy.
Baseline Polysomnography Studies
To determine the eligibility for inclusion into the study protocol, all participants underwent overnight diagnostic clinical PSG studies performed as per usual standards, including measurements of electroencephalogram (EEG) (International 10–20 system), electrooculograms (EOG), submental and tibialis anterior electromyograms, pneumotachometer flow, and electrocardiogram; respiratory inductance plethysmography (RIP) to measure chest wall and abdominal movements; and pulse oximetry to measure oxygen saturation ().
Overview of the Study Protocol
After meeting eligibility criteria, participants underwent the study protocol using a within-subject, randomized crossover intervention study design. Simple randomization to room air or supplemental oxygen intervention was performed using a random table; the participants were blinded to the order of the condition. After stable stage N2 NREM sleep was attained, we studied the effect of O2 supplementation on breathing by adding O2 at 2 L/min (lpm) up to 6 lpm and (fraction of inspired O2) of ∼40%–50% to achieve a steady-state goal level of ≥ 95% during the study protocol. At the goal level of , the participants underwent noninvasive positive pressure mechanical ventilation [using bilevel positive airway pressure (PAP) device or BPPV] to determine the apneic threshold (AT) during NREM sleep. Thereafter, participants underwent PSG on supplemental O2 to measure the severity of SDB. A washout period of at least 2 days for crossover to the alternative intervention was deemed to be sufficient given that the effect of supplemental O2 would be limited to the duration of oxygen exposure itself (10). Sham bilevel positive pressure ventilation (BPPV) trials on room air and PSG on room air were replicated on separate nights. For the sham experiment, room air was bled into the breathing circuit from a medical room air tank at a flow rate similar to the flow rate with supplemental O2. We estimated that for an expected CO2 reserve difference of 1 mmHg with a standard deviation (SD) of 1 (i.e., effect size of 1), we would need 13 subjects in each arm for 90% power. With a smaller SD, we expected adequate power with a smaller sample size.
Breathing Circuit
During the nighttime experimental study protocols, each participant was connected to the breathing circuit via a nasal mask. An appropriate-sized, airtight silicone nasal mask (Respironics, Murrysville, PA) was glued to the face to prevent mask leaks. The mask was connected to a Plateau Exhalation Valve (Respironics, Pittsburgh, PA), via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected to the inspiratory line. Participants were restricted to nasal breathing by using a chin strap or placing tape over the mouth. During the noninvasive BPPV protocol, hyperventilation was achieved using a pressure support ventilator (bilevel positive airway pressure (PAP); Respironics ST) for which the minimum allowable continuous PAP was 4 cmH2O. This protocol has been standardized in our laboratory. Room air or supplemental O2 was introduced from external sources connected to the inspiratory line; gas tanks of medical room air and O2 were available for the protocols. During the O2 exposure (experimental arm termed here as: Oxy), participants breathed supplemental O2 from the gas source connected to the inspiratory line with a flow meter to keep O2 flow rate at 2–6 lpm and ∼40%–50% to keep ≥ 95%; whereas, during room air exposure, participants breathed only room air introduced into the inspiratory line from a separate source. To confirm the central etiology of apnea and to ascertain upper airway mechanics, we recorded supraglottic airway pressures (PSG) using a pressure transducer-tipped catheter (Millar Instruments, Houston, TX) (8–10), with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip for 2 cm after it disappeared behind the tongue. End-tidal carbon dioxide () and end-tidal oxygen () were measured using air sampled continuously from the nasal mask by infrared analyzers.
Experimental Protocol
For each BPPV protocol, participants were asked to restrict sleep to a maximum of 5 h on the night preceding the study night during their usual circadian sleep time, so that the results obtained are independent of undefined circadian influences, respectively. Before the start of BPPV, for both arms of the study, all participants received zolpidem 10 mg to allow uninterrupted sleep. The BPPV trials were conducted during stable NREM sleep, that is, in stable stage N2 or stage N3 sleep. Hyperventilation using nasal BPPV was induced under conditions of Oxy or room air in random order in all participants. During exposure to supplement O2, BPPV was conducted, as described below in the section Mechanical Ventilation, with continuous flow of supplemental O2, where the (partial pressure of inspired O2) was continuously monitored to keep ≥ 95%, as noted earlier. During room air (sham) intervention, BPPV was conducted under room air conditions with a range of flow rates of room air similar to flow rates with supplemental O2.
Mechanical Ventilation
We used nasal noninvasive BPPV to induce hyperventilation. This methodology has been previously described (5, 8, 10, 34). BPPV was applied in the spontaneous-timed mode. In this mode, a backup respiratory rate was set at 10–12 breaths/min, or below the participant’s eupneic rate (thus, not in a “controlled” mode), to prevent neuromechanical inhibition of ventilatory motor output. During BPPV, hyperventilation was induced for a 3-min period and terminated during expiration by returning the expiratory positive airway pressure (EPAP) of 4–6 cmH2O. For each trial, the inspiratory PAP was increased gradually in 1- to 2-cmH2O increments, starting from 4 to 6 cmH2O at the beginning of each BPPV trial, while keeping expiratory PAP fixed at 4–6 cmH2O throughout the trial. BPPV was terminated after 3 min by returning the inspiratory PAP to the baseline expiratory PAP. The ensuing hypocapnia resulted in either a hypopnea or central apnea. An expiratory time (TE) ≥ 5 s after termination of BPPV with the absence of respiratory effort on the supraglottic catheter defined a central apnea. If a central apnea was not induced, additional hyperventilation trials were performed by increasing the inspiratory pressure in 1- to 2-cmH2O increments during successive trials until a central apnea was evident. At least 3 min of spontaneous breathing were allowed between each trial to allow ventilation and to return to baseline levels. At least three AT determinations were performed on each subject during stable NREM sleep to ensure reproducibility of observed results. The BPPV protocol was repeated on a separate night under the alternate condition, Oxy or room air, in the participants.
Measurements
EEG, EOG, and chin electromyograph (EMG) were recorded using the International 10–20 system of electrode placement as noted earlier. Inspiratory airflow and breath time were measured by a heated pneumotachometer (model 3700A, Hans Rudolph, Kansas City, MO) attached to an RSS 100 HR Research Pneumotach System. The tidal volume (VT) was obtained from the electronic integration of the flow signal on the pneumotach system. Supraglottic pressure was measured using a pressure transducer-tipped catheter as noted earlier. readings were obtained continuously by an infrared analyzer (model CD-3A, AEI Technologies, Pittsburgh, PA) from tubing placed in the nares via a port in the nasal mask. Arterial O2 saturation () was measured by a pulse oximeter (Biox 3700, Ohmeda) and also an oximeter placed on the ear lobe. was sampled continuously by an infrared analyzer (model CD-3A, AEI Technologies) via tubing attached to a port on the nasal mask. The signals were displayed on a polygraph recorder Somnostar, Vyaire and recorded using Powerlab data-acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
Data Analyses
PSG study.
Sleep efficiency was defined as total sleep time divided by the total time in bed during the PSG. Sleep staging (3) and scoring of EEG arousals (3) were completed using standard AASM defined criteria. PSG analysis followed AASM standards of scoring and definition for hypopnea (30% reduction in flow lasting a duration of at least 10 s, associated with 3% oxygen desaturation or an EEG arousal) (3). We scored central versus obstructive apneas (90% or greater reduction in flow lasting a duration of at least 10 s) as per AASM scoring rules (3). Thus, at least 90% decrease in pneumotachometer flow for at least 10 s with absence of effort on both thoracic and abdominal belts indicated a central apnea whereas at least 90% decrease in pneumotachometer flow with presence of effort on RIP indicated an obstructive apnea. AHI is defined as the number of apneas and hypopneas per hour (h) of sleep. The central apnea index (CAI) is the total number of central apneas (only) per hour of sleep as defined by the AASM (3). Obstructive apnea index (OAI) is the total number of obstructive apneas per hour of sleep. Hypopneas were differentiated into central and obstructive hypopneas as per AASM defined criteria (3). Thus, obstructive hypopnea is defined as the presence of flow limitation, snoring, and/or thoraco-abdominal paradox. The absence of all three criteria and reduced effort on the respiratory inductance belts indicates a central hypopnea. Central apnea-hypopnea index (CAHI) is the total number of central apneas and central hypopneas per hour of sleep. In addition, hypopnea indices (HI) (number of hypopneas only per hour of sleep) are scored using arousals alone, 3% oxygen desaturation alone, and with both arousal and 3% oxygen desaturation, to provide a detailed assessment of these indices to account for the presence of supplemental oxygen. Given that the experimental BPPV protocol was conducted in NREM sleep, the AHI, CAI, CAHI, and HI during NREM sleep alone were also calculated.
BPPV protocol.
The AT protocol using BPPV is a well-established technique in our laboratory (5, 8–10, 34) and is also noted here. We were careful to ensure that comparisons during BPPV protocol with room air and Oxy were performed in the same body position for each participant. During the control period, ten eupneic breaths recorded immediately before the onset of the BPPV were averaged. Likewise, during the BPPV period, the last five mechanically ventilated breaths before the return to baseline expiratory positive airway pressure were averaged. We limited our measurements to stable NREM sleep because central apneas are infrequent during rapid eye movement (REM) sleep as the central respiratory activity is elevated during REM sleep (31) under the control of inspiratory pontine neurons and breathing is not influenced by chemoreceptor afferents (30, 31). In addition, REM sleep comprises only a small portion of the total sleep time in participants with sleep apnea, the occurrence of REM sleep is unpredictable during these instrumented experiments and, in our physiology experiments, REM sleep stage is very infrequent. Moreover, sleep state and arousals may alter ventilation and upper airway caliber in the elderly population (22, 32). Hence, we have standardized our analysis protocols for stable N2 or N3 NREM sleep. Thus, we analyzed BPPV trials accompanied by a stable stage N2 or N3 sleep state for both Oxy and room air conditions. Ventilatory parameters/timing, and airway mechanics were measured as previously published (5, 7, 8, 10); inspiratory time (TI), total time of respiratory cycle (TTOT), frequency (f), VT, minute ventilation (VE), , , , and were calculated breath by breath during the normoxic control period, experimental exposure, and immediately postexposure during stable stage N2/N3 NREM sleep. Inspiratory resistance (RUA) was measured at a constant flow rate on the linear portion of the pressure-flow loop during inspiration of each eupneic breath (5, 7–10), during each control period, i.e., the control breaths before the start of each BPPV trial. Due to poor supraglottic pressure signals in two participants, inspiratory RUA could be analyzed in 8 out of 10 participants.
Definitions.
The AT was defined as the end-tidal carbon dioxide (CO2) () that demarcated the central apnea closest to the eupneic . The CO2 reserve was defined as the change in between eupneic (control) and AT (Δ). In addition, “hypocapnic ventilatory response” or the controller gain was defined as the change in VE between control and hypopnea breaths and between control and the AT breath divided by the corresponding CO2 reserve, (ΔVE/Δ relationship), i.e., the slope of the ventilatory response. The controller gain was calculated for each eligible trial and then averaged for each individual. The plant gain was assessed as the eupneic steady-state divided by the corresponding VE expressed as a percentage. Plant gain was also calculated for each trial and averaged for each individual.
Statistical Analysis
The design was a simple crossover treatment study. The primary analysis for the intervention effect was determined using paired t tests (or a nonparametric test, if needed). Results are presented as means ± SE (standard error of mean), unless specified otherwise. Paired t tests were performed to compare all ventilatory parameters, including eupneic VE, eupneic , AT, CO2 reserve, and controller and plant gains, recorded during Oxy and room air exposures and to compare all PSG measures on supplemental oxygen and room air. When the normality test failed [TI, TI/TTOT, RUA, AHI, CAI, CAHI, OAI, NREM AHI, NREM CAHI, NREM CAI, hypopnea index (HI) with 3% desaturation, percent time with < 90%], a nonparametric test, the Wilcoxon signed-rank test, was used for comparison. Spearman’s correlation was used to assess correlations between AHI or CAHI and the physiological variables (CO2 reserve, AT, and controller and plant gains). A commercially available computer statistical package was used to analyze the data (SigmaPlot 14). The level of statistical significance was set at two-tailed P < 0.05.
RESULTS
Baseline data.
Fourteen older participants were included in the protocol. Ten participants (9 men and 1 woman) completed the study as per prescribed protocol, age = 63.8 ± 3.5 yr (age range = 60–72 yr), body mass index = 27.4 ± 2.1 kg/m2, and neck circumference = 38.8 ± 5.4 cm (means ± SD). Overnight baseline PSG data showed apnea-hypopnea index of 12.7 ± 7.2/h with the percent time with < 90% was 1.3% ± 1.7%. Of the remaining four participants, two could not maintain sleep during the BPPV protocols, one did not return for the protocols, and one could not maintain adequate mask seal during the trials, and this excluded group of patients was not different from the experimental group (n = 4, age = 66.5 ± 2.6 yr, BMI = 25.0 ± 3.8 kg/m2, neck circumference = 39.5 ± 3 cm, and AHI = 17.0 ± 6.7/h, P = ns, for all parameters).
PSG results.
Compared with room air exposure, Oxy was associated with significantly decreased AHI (room air vs. Oxy: 24.4 ± 5.6 vs. 11.8 ± 2.0/h, Table 1, Fig. 1A) and decreased HI scored with arousals (Fig. 1B) as well as with standard scoring methods (Table1). There was also decreased CAHI (room air vs. Oxy: 6.9 ± 3.9 vs. 1.7 ± 0.6/h, P = 0.03). Additional PSG analyses during NREM sleep revealed that the NREM AHI (Fig. 1C), NREM HI scored with arousal alone (Fig. 1D), and NREM CAHI (Fig. 1E, Table 1) were also significantly reduced. As expected, the nadir was higher on Oxy versus room air, whereas the percent time spent with < 90% was not significantly different (Table 1). With Oxy, the NREM AHI was reduced to <10/h in 7 out of 10 individuals and the NREM CAHI was reduced to <2/h in 8 out of 10 individuals. Comparing the two interventions, sleep efficiency and sleep stages were not different between room and Oxy (Table 1).
Table 1.
Grouped data for polysomnography respiratory and sleep variables on room air and oxygen, n = 10
| PSG Results | Room air | Oxygen | P Value |
|---|---|---|---|
| AHI, per hour | 24.4 ± 5.6 | 11.8 ± 2.0 | 0.006 |
| CAHI, per hour | 6.9 ± 3.9 | 1.7 ± 0.6 | 0.03 |
| CAI, per hour | 2.7 ± 2.0 | 0.4 ± 0.2 | ns |
| Obstructive hypopnea index, per hour | 14.0 ± 2.9 | 9.1 ± 2.0 | 0.07 |
| OAI, per hour | 3.5 ± 2.4 | 0.8 ± 0.4 | ns |
| HI with arousals alone, per hour | 16.8 ± 2.8 | 10.3 ± 1.9 | 0.03 |
| HI with 3% desaturation alone, per hour | 8.2 ± 2.4 | 0.9 ± 0.9 | 0.01 |
| NREM AHI, per hour | 20.9 ± 6.0 | 7.5 ± 1.1 | 0.01 |
| NREM CAHI, per hour | 7.8 ± 4.9 | 1.5 ± 0.5 | 0.01 |
| NREM CAI, per hour | 3.6 ± 3.1 | 0.4 ± 0.2 | ns |
| NREM HI, with arousals alone, per hour | 14.4 ± 2.7 | 6.6 ± 0.9 | 0.02 |
| NREM HI with 3% desaturation alone, per hour | 6.7 ± 2.4 | 0.5 ± 0.4 | 0.02 |
| REM AHI, per hour | 3.4 ± 0.8 | 4.4 ± 1.7 | ns |
| Nadir , % | 90.0 ± 1.1 | 96.3 ± 0.6 | <0.001 |
| Percentage time with < 90%, % | 1.2 ± 1.1 | 0 ± 0 | ns |
| Obstructive apnea length, s | 20.0 ± 2.1 (n = 4) | 34.0 ± 9.6 (n = 3) | ns |
| Central apnea length, s | 19.2 ± 5.4 (n = 5) | 19.4 ± 2.4 (n = 5) | ns |
| Sleep efficiency, % | 62.0 ± 5.0 | 66.4 ± 6.8 | ns |
| Stage N1 sleep, % | 29.7 ± 5.3 | 30.2 ± 5.1 | ns |
| Stage N3 sleep, % | 6.8 ± 3.0 | 4.3 ± 3.4 | ns |
| Stage REM sleep, % | 13.2 ± 4.1 | 16.4 ± 3.9 | ns |
Results are means ± SE. The differences between room air and supplemental O2 indices were significant even after removal of one outlier individual who had higher respiratory indices on room air (i.e., with n = 9, AHI: P = 0.02, NREM AHI: P = 0.03, NREM CAHI: P = 0.049). AHI, apnea-hypopnea index; CAI, central apnea index; CAHI, central apnea-hypopnea index; HI, hypopnea indices; NREM, non-rapid eye movement; OAI, obstructive apnea index; PSG, polysomnography; REM, rapid eye movement; , O2 saturation.
Fig. 1.
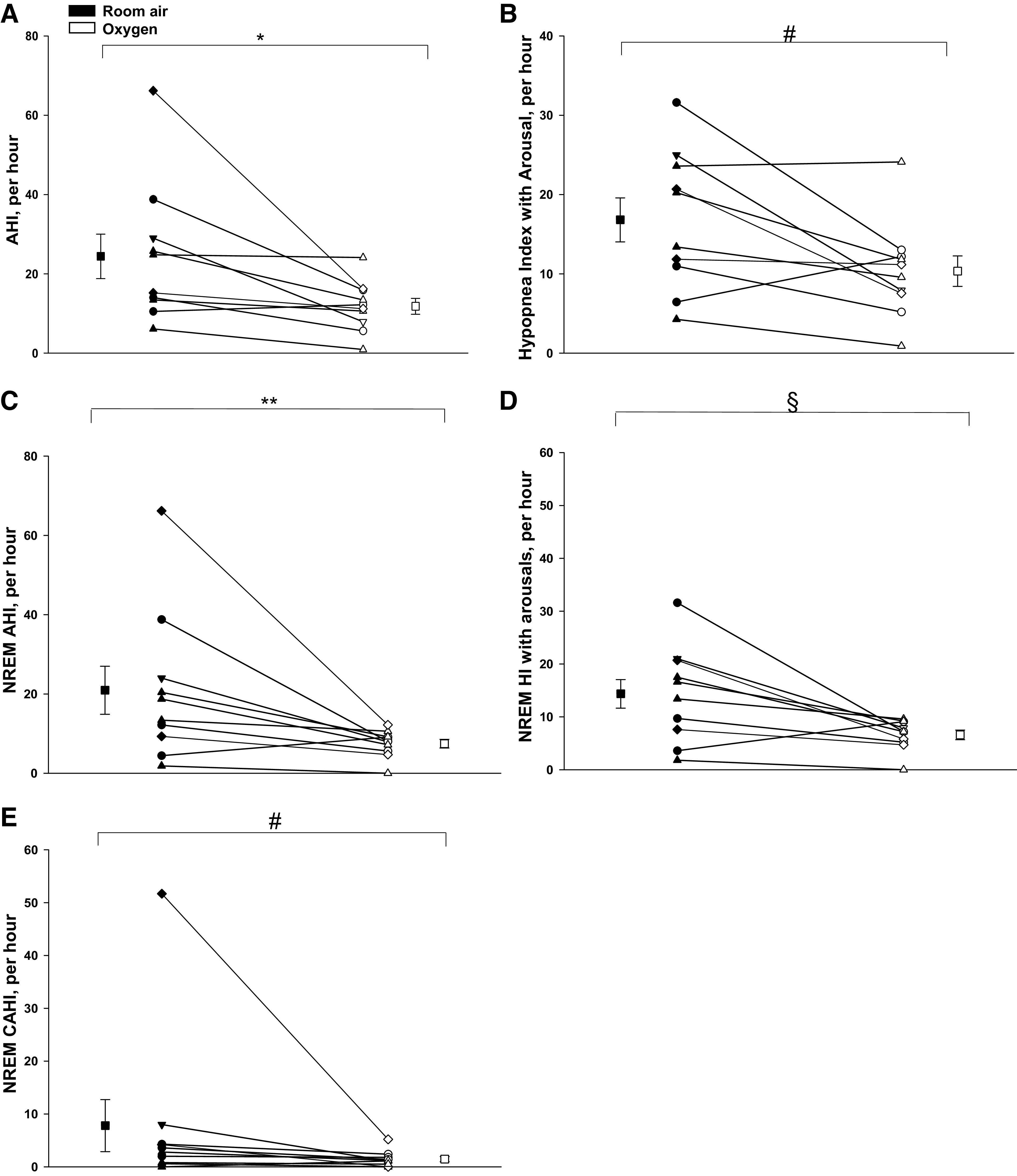
A: the apnea-hypopnea index (AHI) under conditions of room air and Oxy (supplemental oxygen). There was a significant decline in the mean AHI with Oxy vs. room air, *P = 0.006. B: the hypopnea index (HI) with arousals alone under conditions of room air and Oxy. There was a significant decline in the mean HI scored with arousals alone with Oxy versus room air, #P = 0.03. C: non-rapid eye movement (NREM) AHI under conditions of room air and Oxy. There was a significant decline in the NREM AHI during Oxy versus room air, **P = 0.01. D: NREM HI scored with arousals alone under conditions room air and Oxy, which declined significantly with Oxy versus room air, §P = 0.02. Individual and means (±SE) data are presented in all four figures, where black and white symbols represent room air and Oxy trials, respectively; h, hour. E: NREM central apnea-hypopnea index (CAHI) scored with arousals alone under conditions room air and Oxy, which declined significantly with Oxy versus room air, #P = 0.01 (also see Table 1). Individual and means (±SE) data are presented in all five figures, where black and white symbols represent room air and Oxy trials, respectively; h, hour.
Experimental BPPV trial results.
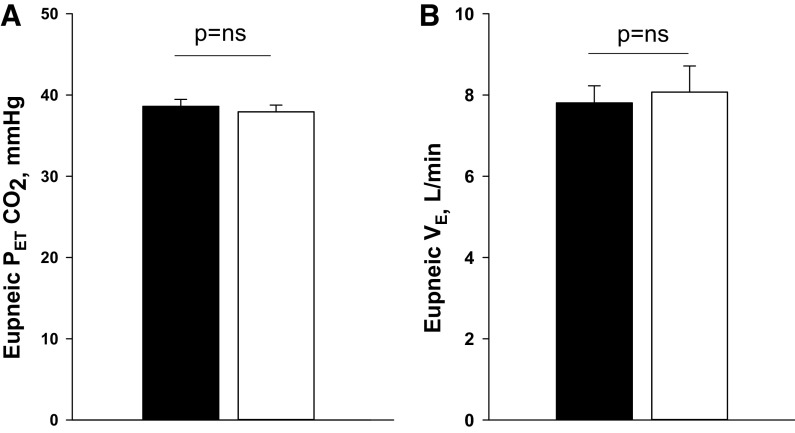
Comparing the two trial nights, eupneic VE (room air vs. Oxy: 7.8 ± 0.4 vs. 8.1 ± 0.6 L/min; P = ns) and eupneic (38.6 ± 0.9 vs. 37.9 ± 0.8 mmHg; P = ns) were similar, respectively (Fig. 2, A and B). Likewise, there was no difference in respiratory cycle timing between Oxy versus room air exposure (Table 2). However, the CO2 reserve was significantly larger with Oxy relative to room air (room air vs. Oxy: −3.2 ± 0.5 vs. −4.2 ± 0.5 mmHg; P = 0.03) (Fig. 3A). This was associated with a significant reduction in the hypocapnic ventilatory response (controller gain) under Oxy compared with room air (room air vs. Oxy: 2.5 ± 0.5 vs. 1.9 ± 0.4 L/min/ mmHg; P = 0.04, Fig. 4A), whereas the AT was similar during Oxy relative to room air exposure (room air vs. Oxy: 35.1 ± 1.3 vs. 34.1 ± 1.0 mmHg; P = ns) (Fig. 3B). Plant gain was also similar during the two conditions (5.2 ± 0.3 vs. 5.1 ± 0.3 mmHg/L/min, P = ns, Fig. 4B). A schematic summary figure demonstrates an increase in the CO2 reserve as a result of the decline in slope or controller gain with Oxy versus room air (Fig. 5). The eupneic inspiratory RUA during each control period was not significantly different between the two interventions (Table 2).
Fig. 2.
A: grouped data of the eupneic end-tidal carbon dioxide () under the two conditions, Oxy (oxygen) and room air. B: grouped data for the eupneic minute ventilation under the two conditions, Oxy and room air. There were no significant differences in either eupneic or eupneic minute ventilation under the two conditions. Black and white bars represent room air and Oxy trials, respectively.
Table 2.
Grouped data for timing, ventilation, and resistance during the control periods of all BPPV trials on room air and oxygen, n = 10
| Variables | Room air | Oxygen | P Value |
|---|---|---|---|
| TI, s | 2.2 ± 0.2 | 2.2 ± 0.2 | ns |
| TE, s | 2.1 ± 0.5 | 2.3 ± 0.2 | ns |
| TTOT, s | 4.3 ± 0.3 | 4.4 ± 0.2 | ns |
| TI/TTOT | 0.8 ± 0.3 | 0.7 ± 0.2 | ns |
| fR, breath/min | 14.4 ± 0.8 | 14.2 ± 0.8 | ns |
| VT, L | 0.6 ± 0.0 | 0.6 ± 0.0 | ns |
| VE, L/min | 7.8 ± 0.4 | 8.1 ± 0.6 | ns |
| , mmHg | 38.3 ± 0.9 | 37.9 ± 0.8 | ns |
| , mmHg | 97.2 ± 1.5 | 277.6 ± 7.8 | 0.004 |
| 0.48 ± 0.01 | 0.21 ± 0.00 | <0.001 | |
| , % | 95.7 ± 0.8 | 98.3 ± 0.3 | 0.002 |
| Eupneic inspiratory RUA (cmH2O/L/s (n = 8) | 5.4 ± 0.8 | 5.9 ± 0.9 | ns |
Results are means ± SE. TI, inspiratory time; TE, expiratory time; TTOT, total time of respiratory cycle; fR, respiratory frequency; VE, minute ventilation; , fraction of inspired O2; , end-tidal carbon dioxide; , end-tidal oxygen; RUA, inspiratory resistance; , arterial O2 saturation; TE, expiratory time; VT, tidal volume.
Fig. 3.
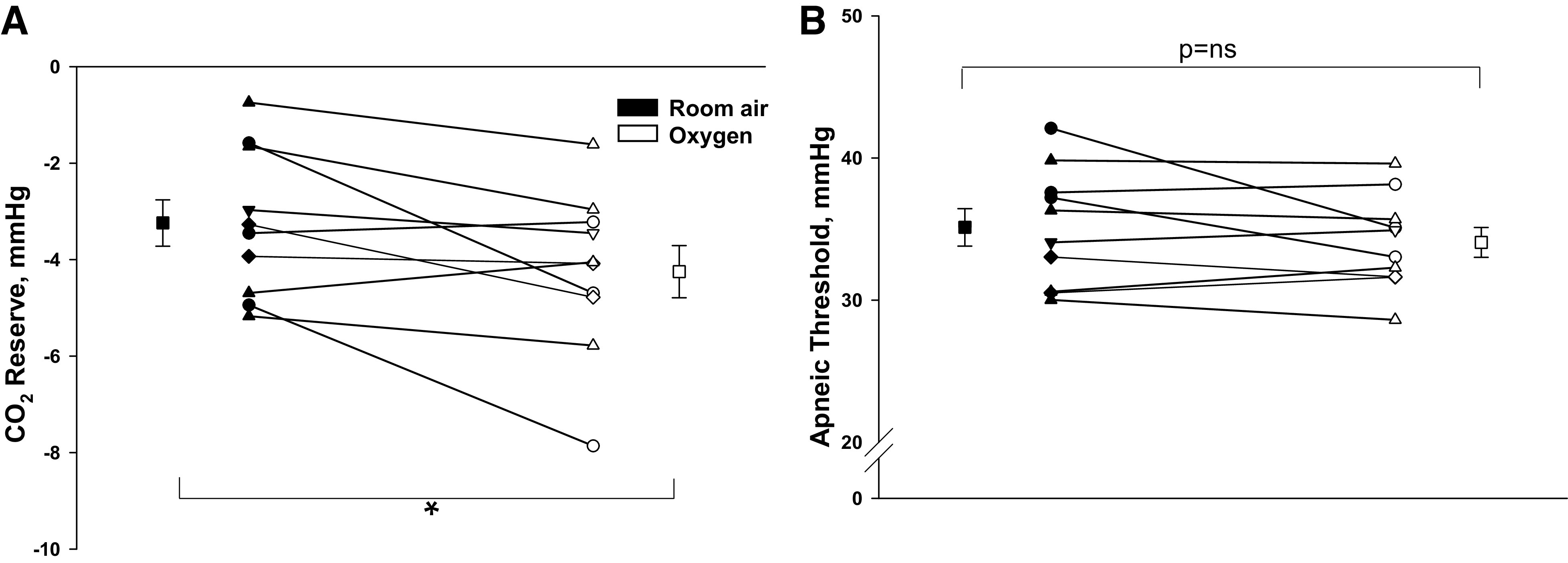
A: the CO2 reserve under conditions of Oxy and room air. The CO2 reserve was significantly increased (more negative) during Oxy versus room air exposure, *P = 0.03. B: the apneic threshold (AT) demonstrates no significant difference during Oxy vs. room air exposure. Individual and means (±SE) data are presented, where black and white symbols bars represent room air and Oxy trials, respectively.
Fig. 4.
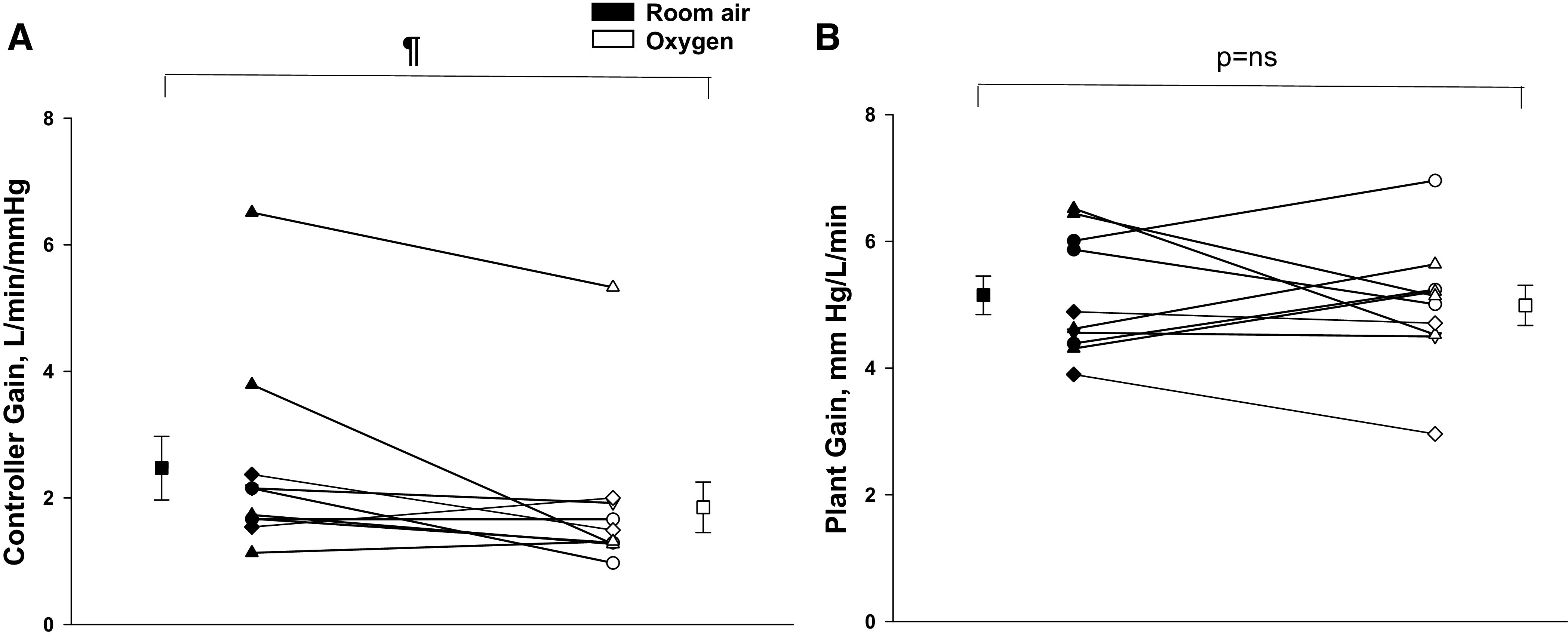
A: the controller gain or hypocapnic ventilatory response under the two conditions of Oxy and room air. The major finding was a significant decline in the controller gain or hypocapnic ventilatory response during Oxy vs. room air, ¶P = 0.04. B: the plant gain under the two conditions: no significant difference in plant gain during Oxy versus room air exposure. Individual and means (±SE) data are presented, where black and white symbols represent room air and Oxy trials, respectively.
Fig. 5.
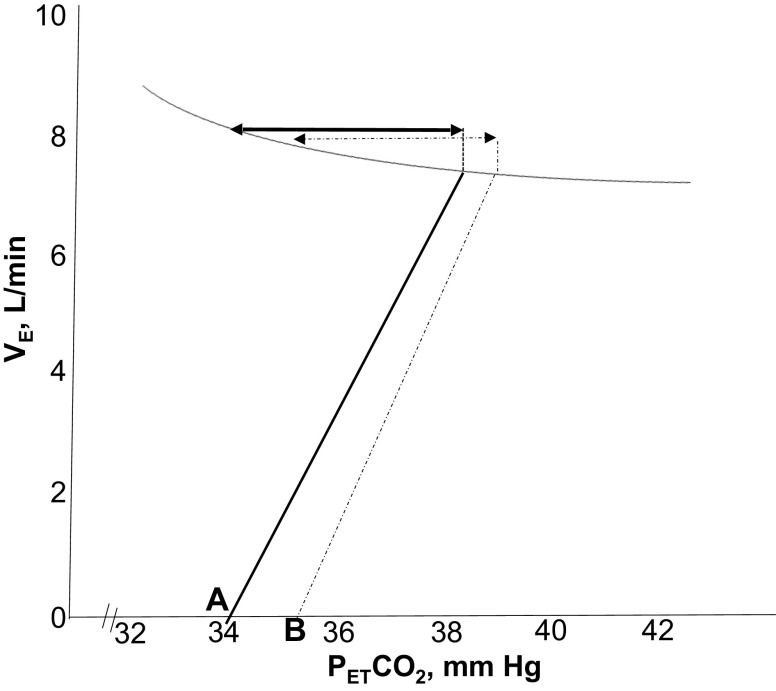
Schematic presentation of the relationship between minute ventilation (VE) and end-tidal carbon dioxide () along a representative isometabolic curve. Note a decrease in the slope of VE/ with supplemental O2 (solid line) compared with room air (dashed line), confirming a decline in the hypocapnic ventilatory responsiveness or controller gain upon exposure to supplemental O2. The two-sided arrows indicate the CO2 reserve during the two exposure conditions: a greater magnitude of CO2 reserve with supplemental O2 (solid arrow) compared with room air exposure (dashed arrow) was observed. Points A and B represent the apneic threshold (AT) with supplemental O2 and room air exposure, respectively.
Overall, there were significant correlations between the magnitude of CO2 reserve and AHI (r = 0.5, P = 0.03, n = 20) and between the AT and AHI (r = −0.5, P = 0.02, n = 20). Although there was no correlation between the AHI and either the controller gain (r = 0.2, P = ns) or plant gain, the CAHI and the NREM CAHI index tended to correlate with the controller gain, r = 0.4, P = 0.1 and r = 0.5, P = 0.2 (n = 20), respectively, indicating that central SDB was related to the magnitude of ventilatory chemoresponsiveness. In conclusion, the results demonstrate that compared with room air, in older adults exposed to hyperoxia with supplemental O2, a reduced slope of CO2 response resulted in increased CO2 reserve or increased breathing stability with decreased central SDB.
DISCUSSION
The mechanistic effects of oxygen supplementation in older adults with SDB may inform the development of pathophysiology defined personalized therapies for sleep apnea in the elderly. Our study demonstrated the following novel and significant findings: 1) oxygen supplementation in older adults with mild-to-moderate SDB was associated with decreased propensity to central apneas and hypopneas during NREM sleep as evidenced by widening of the CO2 reserve; 2) there was decreased AHI mainly through reduction in CAHI; and 3) decreased central apnea propensity was attributable to decreased hypocapnic chemoresponsiveness (controller gain) without a change in the plant gain, i.e., a reduced slope of CO2 response increased the CO2 reserve.
The results demonstrate, for the first time, to our knowledge, that for older adults who had a combination of both central and obstructive events, supplemental oxygen in the clinical range can effectively reduce SDB by eliminating the central hypopneas and central apneas while partly reducing the obstructive events. Although prior studies have examined the physiological effects of supplemental oxygen, they focused on predominantly obstructive sleep apnea (OSA) and, in fact, excluded individuals with predominant central respiratory events/central sleep apnea (CSA) (17, 36, 41, 44). Unlike these studies, our study aimed to examine the entire spectrum of central and obstructive events, as elderly populations have a higher prevalence (4) and an increased propensity of central respiratory events (8). Thus, our study is novel, as it identifies a new subpopulation of patients who could potentially benefit from oxygen therapy. Moreover, our study rigorously performed careful measurements of end-tidal CO2 that in turn delineated the components of loop gain that were altered with supplemental oxygen. Partitioning the components of the loop gain allows us to precisely identify the determinants of ventilatory control stability due to oxygen during sleep.
Physiologic Rationale for Using Supplemental Oxygen
We have previously shown increased hypoxic ventilatory response and response to brief hyperoxia in older versus young adults as potential markers of increased peripheral chemoresponsiveness and increase in slope of chemoresponsiveness during sleep in elderly adults (7, 8). Accordingly, reducing chemoresponsiveness with supplemental O2 could promote breathing stability in this older age-group. Supplemental O2 as a treatment for central apnea has been studied in multiple clinical studies of heart failure with Hunter–Cheyne–Stokes breathing (18, 23, 29, 37) or due to other causes (18). In the current study in older adults, oxygen nearly eliminated central apneas and hypopneas while also tending to reduce the number of obstructive hypopneas and the small number of obstructive apneas (Table 1). Likewise, we have previously demonstrated in a clinical series that supplemental O2 to keep > 93%, when added to PAP, eliminated residual apneas and hypopneas (6) in veterans with both central and obstructive sleep apnea. Supplemental O2 also partially reduced AHI in younger-middle-aged OSA patients with high loop gain (36, 41). However, prior studies examining the effects of hyperoxia on various phenotypic traits excluded patients with CSA while including patients receiving PAP therapy (17, 36, 41). PAP therapy (for at least 3 mo) reduces ventilatory chemoresponsiveness (35) and could have potentially masked the true effect of oxygen on loop gain in these studies (35). In contrast, the present study was performed in older adults with both central and obstructive SDB who were naïve to PAP and demonstrates that oxygen can reduce SDB by nearly eliminating the central apneas and central hypopneas while partly reducing the obstructive events during NREM sleep. Our findings confirmed the hypothesis that supplemental O2 would ameliorate central apnea/hypopnea by decreasing the propensity to central apnea through decreased chemoreflex sensitivity. Moreover, oxygen also tended to reduce obstructive respiratory events similar to aforementioned studies.
The findings of our present study are slightly different from our previous work in younger healthy adults that demonstrated hyperoxia could decrease both the chemoreflex sensitivity/controller gain and the plant gain (10). The major methodological difference from our prior study is that the present study used clinically relevant but lower of supplemental O2 (targeting oxyhemoglobin saturation > 95%), whereas the prior study used a higher range of (70%–80%). Therefore, the physiological effect of oxygen likely was dampening of ventilatory chemoresponsiveness, whereas higher levels of supplemental O2 were a ventilatory stimulant, resulting in increased minute ventilation and decreased eupneic . Thus, decreased chemoreflex sensitivity may be sufficient to mitigate the propensity to central apnea-central hypopnea in individuals with high chemoreceptor sensitivity during sleep, such as in older adults (8).
AT Determination and Mechanisms of Effect
The AT obtained via noninvasive pressure support is an objective, precise, and reproducible index that serves as a robust marker of susceptibility to apnea (14). This protocol has been described by Skatrud and Dempsey (38) and used extensively by our group (5, 8, 10, 34). The AT during NREM sleep is useful in determining the susceptibility to apnea because a small CO2 reserve means high propensity to hypocapnic central apnea, such that a slight increase in ventilation would precipitate an apnea. Conversely, a large CO2 reserve means that the individual is less susceptible to central apneas. Our findings are similar to previous studies with sustained hyperoxia in healthy young adults (10) and following transient hyperoxia exposure (42).
Peripheral versus central chemoreceptor effect?
The stabilizing effects of supplemental O2 may be due to peripheral and/or central chemoreceptor effects. The immediate effect is suppressing peripheral chemoreceptor activity, and hence decreasing the response of the carotid bodies to hypoxia or hypocapnia. Exposure to supplemental O2 in our study of older adults resulted in decreased hypocapnic ventilatory chemoresponsiveness, consistent with dampening of peripheral chemoreceptor activity by supplemental O2. The Dejours’ effect, described as reduction in tidal volume following exposure to brief hyperoxia, and a marker of peripheral chemoresponsiveness (13), may have been activated at the start of the experiment; however, our study protocol was not designed to assess this physiologic parameter. Dahan et al. (11) have shown that central CO2 sensitivity was 15% smaller with hyperoxia than with normoxia condition. However, the partition of ventilatory responsiveness to peripheral and central may be arbitrary. Evidence in a canine model suggests significant interdependence between peripheral and central chemoreceptors, and, in fact, the gain of the central chemoreceptors seems to be very dependent on the peripheral chemoreceptor activity (39). In addition, a small study in humans demonstrated a transient reduction in CO2 chemoreceptor sensitivity following bilateral carotid body resection. This finding corroborates findings from several animal studies demonstrating a synergistic (hyperadditive) interaction between the peripheral and central chemoreceptors (12). Thus, both chemoreceptor systems are likely to be involved. Whether the peripheral-central interaction was additive, hyperadditive, or hypoadditive in older adults cannot be determined from our model.
Absence of decreased plant gain.
As mentioned, our current findings are slightly different from our own observations in young healthy adults without SDB (10), with reductions in both plant gain and controller gains, whereas in the present study, the controller gain was reduced without any change in the plant gain with hyperoxia. Although acute hyperoxia is inhibitory, sustained hyperoxia is potentially a central nervous system ventilatory stimulant when the carotid body is intact (15, 20), by increasing cerebral Pco2, either through the Haldane effect by displacing CO2 into the cerebrospinal fluid, or cerebral vasoconstriction, either via direct effect of hyperoxia or a consequence of arterial hypocapnia. The net effect is reduced CO2 washout, elevated brain tissue Pco2, increased H+ in the medulla (16), and hence increased ventilatory motor output. However, in the present study, we did not find evidence for hyperventilation (and, thereby, plant gain was unchanged) (Table 2), suggesting that central ventilatory stimulation may not be present in older adults with SDB. Whether the lower level of in the present study contributed to the lack of hyperventilation will need to be determined from similar patient populations using higher levels of hyperoxia. We also speculate that the absence of hyperventilation (and, thereby, unchanged plant gain) in the present study in older adults with SDB may be attributed to differences in chemoreceptor gains with aging (8), as well as due to age-related changes in cerebrovascular responsiveness (24) and SDB-related reduction in cerebrovascular responsiveness (28) in older adults with SDB, given that these factors could potentially alter central/peripheral chemoreceptor interactions in response to oxygen. In other words, chronic intermittent hypoxia (CIH) consequential to underlying chronic SDB in our elderly patient population could potentially upregulate the carotid chemoreceptor centers preferentially (hyperadditive effect) and obscure the central stimulatory effects of oxygen on the central oxygen sensors or chemoreceptors. Whether CIH exposure due to SDB in our population of older adults had a hyperadditive effect on central/peripheral chemoreceptors interactions needs to be examined. The contributions of cerebrovascular CO2 responsiveness and CIH to this chemoreceptor relationship, and thereby on loop gain and its components, will need to be explored in future studies.
Clinical Implications
Aging is associated with increased prevalence of sleep apnea, especially central apneas (4). Moreover, sleep apnea in older adults is associated with major cardiovascular complications and increased mortality (1). A large prospective cohort study in 939 elderly adults confirmed that untreated sleep apnea posed a significantly higher risk of cardiovascular death: hazard ratio for mortality was 2.25 for untreated severe sleep apnea (26). Therefore, treatment of sleep apnea is recommended in older adults. Positive airway pressure therapy (PAP) is the first-line therapy for SDB. Unfortunately, adherence to PAP remains suboptimal, leading to limited effectiveness (40), and importantly, PAP adherence decreases as age increases (27). The clinical implication of our study findings is significant, in that it demonstrates that elderly populations with SDB, without heart disease, can potentially benefit from using supplemental oxygen as therapy for mild-to-moderate SDB. Supplemental oxygen can eliminate central apneas and hypopneas and reduce obstructive respiratory events. The observed pathophysiological mechanisms provide impetus for a future randomized controlled trial comparing oxygen to PAP and/or to conservative therapy, as the next logical step, to determine the clinical effects of oxygen alone in elderly adults with mild-to-moderate SDB. Potentially, supplemental O2 could emerge as a novel stand-alone or adjunctive therapeutic intervention in older adults with mild-to-moderate sleep disordered breathing.
Methodological Considerations
The experiments may have not been completed on a single night owing to inadequate stable NREM sleep. The level of hyperoxia achieved may not have been adequate. However, we perfected the techniques during our prior study and have demonstrated that these techniques can be executed without arousal if care is taken to deliver the gases during expiration using a gas blender (10). Our protocol did not determine whether the dose and duration of O2 supplementation determine the degree of ventilatory stability and its relationship with SDB severity. Our protocol did not allow us to determine whether the reduction in chemoresponsiveness involved the peripheral or central chemoreceptors or both, as noted earlier. While the controller gain tended to correlate with the CAHI, the relationship did not reach statistical significance. This may be related to the fact that our study was only powered to examine differences in AHI/AT/CO2 reserve between groups and not for individual correlations, which will likely require a large sample size in future studies.
We executed utmost care to ensure the experiments are conducted during stable stage N2 sleep. Despite our best efforts, arousals did occur, but trials with arousals or unstable sleep were discarded and the trials repeated. We and other investigators have used low-dose zolpidem to ensure stable sleep state when determining the AT to allow completion of the experimental protocol with an adequate number of BPPV trials during stable sleep (8, 43). We have used zolpidem in prior studies in older adults with no evidence of effect on chemoresponsiveness (7, 8). Published studies from other laboratories also did not find that zolpidem influences ventilatory parameters during sleep (2, 25). Initially, we did attempt to complete the experimental protocol without zolpidem; however, after the first few patients consistently failed to maintain sleep during the AT protocols with BPPV, only then we resorted to adding zolpidem. Zolpidem is a nonbenzodiazepine GABA receptor agonist. Studies measuring the effect of zolpidem on respiratory control demonstrate that zolpidem does not alter the minute ventilation, oxygen saturation, or chemoreflex function. For example, zolpidem did not impair nocturnal respiratory and sleep architecture, pulmonary function tests, occlusion pressure, or chemoresponsiveness in young, middle-aged, and older adults (19). In addition, in healthy elderly adults, zolpidem at 10 mg did not increase the incidence of sleep apnea or sleep-related hypoxia compared with placebo (33). Hence, given the established lack of effect of zolpidem on ventilation, nocturnal oxygenation, sleep apnea severity, or ventilatory control breathing during wake and sleep, we believe that zolpidem did not influence the CO2 reserve or on hypocapnic ventilatory responsiveness during sleep in older adults in our study. In case zolpidem did have an effect on the ventilatory response, the same dose of the drug was used in both arms of the study at a similar dose and would have had similar effects. Future studies should include an additional night of recording on hyperoxia in the absence of zolpidem as well as include measures of sleep quality and quality of life with oxygen exposure. Our intervention with oxygen was for only one night. Whether multiple nights or a higher dose of supplemental O2 would be more effective needs to be studied further. Finally, we did not record transcutaneous CO2 or end-tidal CO2 during the PSGs, and although we did not see evidence of hypoventilation (based on eupneic readings) as the study progressed during the night, future experiments with longer durations or higher doses of oxygen will need to record this parameter even during the PSG recordings to determine the possibility of hyperoxia-related hypoventilation during sleep.
In summary, we have shown that modest levels of supplemental O2 during sleep will increase breathing stability to mitigate central SDB in older adults with reduction in ventilatory chemoresponsiveness.
GRANTS
This study was supported by the US Department of Veterans Affairs: Merit Review Award # CX001201 (Federal grant funding for S. Chowdhuri) and by NIH R01 HL 130552 (grant funding to M.S. Badr).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R., M.S.B., and S.C. conceived and designed research; R.R. and S.C. performed experiments; R.R., M.S.B., A.A., and S.C. analyzed data; R.R., M.S.B., A.A., and S.C. interpreted results of experiments; R.R., M.S.B., and S.C. prepared figures; R.R., M.S.B., and S.C. drafted manuscript; R.R., M.S.B., A.A., and S.C. edited and revised manuscript; R.R., M.S.B., A.A., and S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank K. Ross, A. Qadir,, K. Bakkila, R. Williams, K. Chugh, M. Nusrat, and S. Pranathiageswaran for their excellent technical support.
REFERENCES
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep 14: 486–495, 1991. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med 153: 1864–1869, 1996. doi: 10.1164/ajrccm.153.6.8665047. [DOI] [PubMed] [Google Scholar]
- 3.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Ward SLD, Tangredi MM; American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 8: 597–619, 2012. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. I. Prevalence and severity. Am J Respir Crit Care Med 157: 144–148, 1998. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhuri S, Bascom A, Mohan D, Diamond MP, Badr MS. Testosterone conversion blockade increases breathing stability in healthy men during NREM sleep. Sleep (Basel) 36: 1793–1798, 2013. doi: 10.5665/sleep.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhuri S, Ghabsha A, Sinha P, Kadri M, Narula S, Badr MS. Treatment of central sleep apnea in U.S. veterans. J Clin Sleep Med 8: 555–563, 2012. doi: 10.5664/jcsm.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhuri S, Pranathiageswaran S, Franco-Elizondo R, Jayakar A, Hosni A, Nair A, Badr MS. Effect of age on long-term facilitation and chemosensitivity during NREM sleep. J Appl Physiol (1985) 119: 1088–1096, 2015. doi: 10.1152/japplphysiol.00030.2015. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhuri S, Pranathiageswaran S, Loomis-King H, Salloum A, Badr MS. Aging is associated with increased propensity for central apnea during NREM sleep. J Appl Physiol (1985) 124: 83–90, 2018. doi: 10.1152/japplphysiol.00125.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 108: 369–377, 2010. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol (1985) 109: 1378–1383, 2010. doi: 10.1152/japplphysiol.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahan A, DeGoede J, Berkenbosch A, Olievier IC. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol 428: 485–499, 1990. doi: 10.1113/jphysiol.1990.sp018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4: e239, 2007. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejours P Control of respiration by arterial chemoreceptors. Ann N Y Acad Sci 109: 682–695, 1963. doi: 10.1111/j.1749-6632.1963.tb13497.x. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey JA Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey JA, Smith CA. Update on chemoreception: influence on cardiorespiratory regulation and pathophysiology. Clin Chest Med 40: 269–283, 2019. doi: 10.1016/j.ccm.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, Malhotra A, Wellman A. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol 592: 4523–4535, 2014. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin KA, Sahlin C, Lundgren R, Eriksson P. Reversal of central sleep apnea with oxygen. Chest 111: 163–169, 1997. doi: 10.1378/chest.111.1.163. [DOI] [PubMed] [Google Scholar]
- 19.Girault C, Muir JF, Mihaltan F, Borderies P, De La Giclais B, Verdure A, Samson-Dollfus D. Effects of repeated administration of zolpidem on sleep, diurnal and nocturnal respiratory function, vigilance, and physical performance in patients with COPD. Chest 110: 1203–1211, 1996. doi: 10.1378/chest.110.5.1203. [DOI] [PubMed] [Google Scholar]
- 20.Gourine AV, Funk GD. On the existence of a central respiratory oxygen sensor. J Appl Physiol (1985) 123: 1344–1349, 2017. doi: 10.1152/japplphysiol.00194.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoch CC, Reynolds CF III, Monk TH, Buysse DJ, Yeager AL, Houck PR, Kupfer DJ. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep 13: 502–511, 1990. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 22.Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. J Appl Physiol (1985) 74: 2198–2204, 1993. doi: 10.1152/jappl.1993.74.5.2198. [DOI] [PubMed] [Google Scholar]
- 23.Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep 22: 1101–1106, 1999. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 24.Klein T, Bailey TG, Wollseiffen P, Schneider S, Askew CD. The effect of age on cerebral blood flow responses during repeated and sustained stand to sit transitions. Physiol Rep 8: e14421, 2020. doi: 10.14814/phy2.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillard D, Thiercelin JF, Fuseau E, Rosenzweig P, Attali P. Effects of zolpidem versus diazepam and placebo on breathing control parameters in healthy human subjects. Int J Clin Pharmacol Res 12: 27–35, 1992. [PubMed] [Google Scholar]
- 26.Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, Durán-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 186: 909–916, 2012. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Garcia MA, Valero-Sánchez I, Reyes-Nuñez N, Oscullo G, Garcia-Ortega A, Gómez-Olivas JD, Campos-Rodriguez F. Continuous positive airway pressure adherence declines with age in elderly obstructive sleep apnoea patients. ERJ Open Res 5: 00178-2018, 2019. doi: 10.1183/23120541.00178-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan BJ, Reichmuth KJ, Peppard PE, Finn L, Barczi SR, Young T, Nieto FJ. Effects of sleep-disordered breathing on cerebrovascular regulation: a population-based study. Am J Respir Crit Care Med 182: 1445–1452, 2010. doi: 10.1164/rccm.201002-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakao YM, Ueshima K, Yasuno S, Sasayama S. Effects of nocturnal oxygen therapy in patients with chronic heart failure and central sleep apnea: CHF-HOT study. Heart Vessels 31: 165–172, 2016. doi: 10.1007/s00380-014-0592-6. [DOI] [PubMed] [Google Scholar]
- 30.Orem J Neuronal mechanisms of respiration in REM sleep. Sleep 3: 251–267, 1980. doi: 10.1093/sleep/3.3-4.251. [DOI] [PubMed] [Google Scholar]
- 31.Orem J, Montplaisir J, Dement WC. Changes in the activity of respiratory neurons during sleep. Brain Res 82: 309–315, 1974. doi: 10.1016/0006-8993(74)90611-8. [DOI] [PubMed] [Google Scholar]
- 32.Pack AI, Silage DA, Millman RP, Knight H, Shore ET, Chung DC. Spectral analysis of ventilation in elderly subjects awake and asleep. J Appl Physiol (1985) 64: 1257–1267, 1988. doi: 10.1152/jappl.1988.64.3.1257. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes SP, Parry P, Hanning CD. A comparison of the effects of zolpidem and placebo on respiration and oxygen saturation during sleep in the healthy elderly. Br J Clin Pharmacol 30: 817–824, 1990. doi: 10.1111/j.1365-2125.1990.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 29: 95–103, 2006. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 35.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181: 189–193, 2010. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sands SA, Edwards BA, Terrill PI, Butler JP, Owens RL, Taranto-Montemurro L, Azarbarzin A, Marques M, Hess LB, Smales ET, de Melo CM, White DP, Malhotra A, Wellman A. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J 52: 1800674, 2018. doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H; CHF-HOT Study Group . Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J 70: 1–7, 2006. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 38.Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol 55: 813–822, 1983. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- 39.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol 173: 288–297, 2010. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunwoo BY, Light M, Malhotra A. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology 25: 363–371, 2020. doi: 10.1111/resp.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 162: 144–151, 2008. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol (1985) 100: 171–177, 2006. doi: 10.1152/japplphysiol.00440.2005. [DOI] [PubMed] [Google Scholar]
- 43.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245–1250, 2002. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 44.Xie A, Teodorescu M, Pegelow DF, Teodorescu MC, Gong Y, Fedie JE, Dempsey JA. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol (1985) 115: 22–33, 2013. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]