Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease, yet there are no disease-modifying treatments available and there is no cure. It is becoming apparent that metabolic and vascular conditions such as type 2 diabetes (T2D) and hypertension promote the development and accumulation of Alzheimer’s disease-related dementia pathologies. To this end, aerobic exercise, which is a common lifestyle intervention for both metabolic disease and hypertension, is shown to improve brain health during both healthy aging and dementia. However, noncompliance or other barriers to exercise response are common in exercise treatment paradigms. In addition, reduced intracellular proteostasis and mitochondrial function could contribute to the etiology of AD. Specifically, compromised chaperone systems [i.e., heat shock protein (HSP) systems] can contribute to protein aggregates (i.e., β-amyloid plaques and neurofibrillary tangles) and reduced mitochondrial quality control (i.e., mitophagy). Therefore, novel therapies that target whole body metabolism, the vasculature, and chaperone systems (like HSPs) are needed to effectively treat AD. This review focuses on the role of heat therapy in the treatment and prevention of AD. Heat therapy has been independently shown to reduce whole body insulin resistance, improve vascular function, activate interorgan cross talk via endocytic vesicles, and activate HSPs to improve mitochondrial function and proteostasis in a variety of tissues. Thus, heat therapy could offer immense clinical benefit to patients suffering from AD. Importantly, future studies in patients are needed to determine the safety and efficacy of heat therapy in preventing AD.
Keywords: Alzheimer’s disease, cognitive function, heat shock proteins, heat therapy, metabolism
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disease, affecting over 5 million Americans, with this number expected to balloon to nearly 14 million by 2050 (6). This devastating disease is characterized by worsening memory and social performance and declines in cognitive function (90, 117). Aerobic exercise, like walking and cycling, results in improved brain health during both healthy aging and in dementia models of AD (1, 2, 59). However, not all individuals benefit from exercise. Although controversial (84), this could be due to lack of compliance, nonoptimal intensity, duration or modality timing in relation to meals and effects on the related biomarker response, or an inability to complete exercise regimes due to comorbidity. For example, physical function and motor function continue to decline with age and AD, making it potentially difficult for individuals to exercise enough to receive the known benefits (13). For this reason, alternative therapies and treatments are needed for the decline in cognitive function that occurs with AD and other neurodegenerative diseases. One of these potential treatments is heat therapy.
Heat therapy, via hot water immersion or sauna bathing, has long been associated with tremendous health benefits (27). Recent studies have demonstrated the safety and efficacy of chronic heat therapy in cardiovascular and metabolic adaptations in young, heathy individuals (14) as well as in obese populations (36). In combination with the substantial evidence in preclinical rodent models demonstrating the benefits of heat therapy on vascular health, metabolic outcomes, and mitochondrial function (25, 35, 44, 48, 102), the benefits of heat therapy may also extend to the brain. Our increased understanding of the chaperone and cell signaling roles of heat shock proteins (HSPs) suggests that these highly conserved homeostatic proteins may mediate the beneficial effects of heat therapy. The purpose of this review is to highlight how heat therapy may mitigate the age-dependent declines in metabolism, vascular function, and mitochondrial quality that may be involved in AD etiology.
The Multifactorial Etiology of Alzheimer’s Disease Demands New Therapeutic Approaches
Between 2000 and 2017, deaths from AD have increased by 145% (6). In 2019, AD and other dementias cost the United States ∼$290 billion (6). By 2050, those costs could rise to high as $1.1 trillion (6). From a scientific standpoint, β-amyloid (Aβ) protein fragments, or plaques, accumulate outside of neurons and represent a primary brain change associated with AD (107, 122). Toxic amounts of Aβ may contribute to cell death by interfering with synaptic communication between neurons (122). Immune cells in the brain called microglia are activated to clear Aβ, but chronic and potentially neurodegenerative inflammation can occur when microglia fail to clear toxic Aβ (51, 107, 122).
Although the causative role of Aβ-containing senile plaques in the development of AD is controversial, the presence of these protein aggregates within the extracellular space of brain tissue is closely linked to synaptic nerve loss and progressive cognitive deficits (122). Although multiple phase-III trials have shown target engagement and clearance of Aβ, to date, they have failed to reduce primary outcomes of disease progression (77, 104). These findings have spurred studies examining other factors that likely play a role in AD etiology, such as reduced energy metabolism in the brain. Similarly, it has become increasingly clear that other factors, including an inactive/sedentary lifestyle, low aerobic capacity, and insulin resistance, also impact AD risk [reviewed in (86)]. This understanding highlights the need to pursue alternative metabolic and novel approaches for the prevention and treatment of AD. Moreover, these findings show that an increased focus on mitochondrial function during disease is warranted.
The benefits of heat therapy may occur as a result of heat shock protein (HSP) induction. HSPs are molecular chaperones that function as part of the heat stress response, a conserved mechanism for the body to mitigate cellular stress (16, 57), physical or bioenergetic, and maintain cellular function via regulation of protein folding and degradation under stress (96). HSPs specifically facilitate the folding of new proteins, the refolding of damaged proteins, targeted degradation of nonfunctional proteins/organelles, prevention of oxidative damage, intracellular signaling, and the import/export of proteins into/out of the mitochondria (35, 43, 52, 96, 121). Not surprisingly, changes in the HSP expression profile and cellular localization are linked to numerous disease states. Age-dependent decline in HSPs (characterized by their weight in kilodaltons) can leave neuronal cells open to proteotoxic insults and can increase the risk of AD development (55, 63). In turn, elevated expressions of various HSPs have been shown to improve protein homeostasis in other cells through noncell autonomous processes (114). In this review, we consider the ways in which activation of HSPs with heat therapy could positively impact pathways associated with cognitive decline.
PERIPHERAL METABOLIC DYSFUNCTION AND ALZHEIMER’S DISEASE
Insulin signaling affects a variety of vital cellular processes within the brain, including Aβ trafficking and release, tau phosphorylation, long-term potentiation, and cell survival; such mechanisms may underscore the increased risk for neurodegeneration conferred by insulin resistance (29, 85). Research in animal models has supported the concept that type 2 diabetes (T2D) promotes the development and accumulation of Alzheimer’s disease-related dementia pathologies, such as Aβ plaques, tau phosphorylation and neurofibrillary lesions (66), and α-synuclein lesions (103). Common comorbidities of systemic insulin resistance in T2D—such as hyperglycemia, advanced glycation end products, oxidatively damaged proteins/lipids, inflammation, dyslipidemia, atherosclerosis, microvascular disease, renal failure, and hypertension (105)—all have their own complex effects on brain function through a variety of mechanisms independent of insulin signaling (61). In addition, systemic insulin resistance or high circulating level of insulin has been shown to impact the function of the blood-brain barrier by downregulating endothelial insulin receptors and decreasing permeability of the blood-brain barrier to insulin (101). This change in permeability could lead to decreased brain insulin levels and decreased insulin-facilitated neural and glial activity (54).
We have shown that insulin sensitivity—measured by the gold standard assessment, the hyperinsulinemic-euglycemic clamp—is impaired in patients with AD versus cognitively healthy older adults (87). The skeletal muscle is the site of 80–85% of glucose disposal during hyperinsulinemic clamp conditions (31), suggesting that these deficits are due to impaired skeletal muscle metabolism. Skeletal muscle mitochondrial content and respiratory capacity play a critical role in driving whole body aerobic capacity (69, 116), and low aerobic capacity is also a risk factor for AD.
Decades of research indicates that mitochondria from subjects with AD differ from mitochondria from subjects without dementia or cognitive dysfunction (113). Mitochondria appear to mediate the pathology associated with AD. Although it is difficult to assess cerebral mitochondrial function directly, there is evidence that mitochondrial function is also compromised systemically in cognitively impaired subjects (82). Individuals with mild cognitive impairment (MCI) or AD exhibit decreased blood platelet cytochrome oxidase activity and lower mitochondrial respiratory rates in cytoplasmic hybrid lines generated with mitochondrial DNA (mtDNA) from patients with AD compared with those generated with mtDNA from cognitively healthy older adults (108). Mitochondria-produced H2O2 emission [otherwise termed reactive oxygen species (ROS)] enhances Aβ production, which can be deposited within mitochondria as a proteostatic measure, but results in mitochondrial deficits (95, 108). Mitochondrial dysfunction in skeletal muscle has also been heavily studied as a primary cause of whole body insulin resistance (41, 72, 127), a recognized risk factor for AD (9, 24, 60, 74, 80, 93, 97, 100, 110, 119, 123, 124). However, the role of skeletal muscle mitochondrial function in AD remains to be elucidated.
Further supporting the role of mitochondrial function in AD, mice transgenic for the human apolipoprotein epsilon 4 (APOE4) gene, the primary genetic risk factor for AD, exhibit blunted mitochondrial respiratory capacity and reduced electron transport complex content in neurons (22). Moreover, both the triple transgenic AD mouse model [which harbors mutations for presenillin1 (PS1), amyloid precursor protein (APP), and tau] (92) and a double transgenic AD mouse model (which harbors mutations for PS1 and APP) (106) display similar mitochondrial respiratory deficits in the skeletal muscle (83, 106). As PS1 and APP genes are linked to familial AD, whereas APOE4 is linked to the much more common sporadic form of AD, teasing apart the genetic relationship between mitochondrial function and PS1 or APP is of great clinical relevance.
Mitochondrial quality control is regulated by the processes of mitochondrial biogenesis and mitophagy (91). Mitophagy involves the targeting of damaged or superfluous mitochondria to the lysosomes wherein the mitochondrial constituents are degraded and/or recycled (39, 94). Importantly, mitophagy plays a critical role in neuronal function and neuronal survival through the maintenance of a healthy mitochondrial pool and the inhibition of neuronal death (38, 39). Moreover, mitophagy via chaperone-mediated autophagy (CMA) using the cochaperones such as the heat shock cognate 71-kDa protein (HSC70) and the E3 ubiquitin ligase, C-terminus of HSC70-interacting protein (CHIP), is required for neuronal preconditioning to bioenergetic stress (79), and defective CMA is thought to contribute to neurodegenerative disorders (62). Despite this evidence, the role of mitophagy in AD progression is unclear. Using postmortem human AD brain samples, AD can be induced in pluripotent stem cell (iPSC)-derived neurons and transgenic animal models of AD; thus, mitophagy has been directly associated with AD pathology (20). Furthermore, the restoration of mitophagy ameliorates memory loss in both Caenorhabditis elegans and two mouse models of AD through the inhibition of Aβ plaques and p-tau (37). It is plausible that a defect in mitophagy induces the accumulation of dysfunctional mitochondria, thereby promoting AD pathology and memory loss, and suggests that it is a target for potential therapy (37).
Potential Benefits of Heat Therapy on Peripheral Metabolism
Growing evidence from preclinical and clinical studies suggests that the heat shock response and/or HSPs could play an important role in preventing insulin resistance and the development of T2D (8). HSP function is tightly coupled to insulin resistance and T2D (44). Specifically, HSP expression declines with T2D in humans, and rodents have increased susceptibility to insulin resistance when the gene for HSP72 is knocked out (25, 35). Conversely, HSP induction via transgenic overexpression, pharmacologic intervention, or heat protects against diet-induced obesity and insulin resistance in rodent studies (25, 48, 102). It is hypothesized that this association is due in part to the anti-inflammatory/antiapoptotic signaling roles of HSPs during disease-induced stress (44). For instance, T2D and insulin resistance increase oxidative stress, instigating c-Jun and NF-κB activation—ultimately increasing inflammation and inhibiting a critical component of the insulin signaling pathway, insulin receptor substrate-1 (IRS-1) (53, 68). HSP72 and HSP25 are shown to reduce c-Jun and NF-κB activity, respectively (44), thus relieving repression on the insulin signaling cascade to allow for proper substrate utilization. Importantly, it remains unknown whether induction of HSPs via heat therapy can modulate systemic metabolism or restore glucose/insulin homeostasis in AD.
Mitochondrial dysfunction contributes to the development of metabolic disease (111) and may be a likely target for heat therapy. Our laboratory and others have demonstrated that heat treatment improved skeletal muscle mitochondrial function by improving fatty acid oxidation (48), increasing mitochondrial enzyme activity (23, 48, 115), and increasing mitochondrial biogenesis (76). One way in which induction of the heat shock response may improve mitochondrial function could be through regulation of mitophagy or the targeted degradation of mitochondria through autophagy (35). Evidence suggests that mice lacking HSP72 in skeletal muscle have decreased mitophagy as well as enlarged, dysmorphic mitochondria with reduced respiratory capacity (35). Importantly, mitochondrial dysfunction associated with the lack of HSP72 extends beyond the skeletal muscle and occurs in the liver (7). Thus, it is possible that the activation of HSP72 may improve mitochondrial quality by enhancing the degradation of dysfunctional mitochondria via mitophagy.
Mitophagy could alternatively occur through chaperone-mediated autophagy or CMA, which both use HSC70 and various cochaperones, such as the E3 ubiquitin ligase CHIP, for ubiquitination of organelles/proteins for autophagic or lysosomal removal, respectively (5, 62). Specifically, HSC70 recognizes and binds to the pentapeptide KFERQ-like motifs on target cytosolic or membrane proteins, allows for ubiquitination from its binding partner CHIP, and targets them for degradation via movement to the autophagosome or lysosome upon bioenergetic stress (microautophagy and endosomal microautophagy can also occur) (28, 34). Importantly, ∼45–47% of the human proteome contains the pentapeptide KFERQ-like motifs, and deficiency in CMA contributes to disease states like neurodegenerative disorders (5, 67, 79). This pathway is gaining much interest, as it is reduced with age and appears to heavily regulate whole cell metabolic function and proteostasis. However, the relationship between CMA, proteasomal activity of ubiquitinated substrates, and mitochondrial biogenesis remains ill-defined. Moreover, it is unknown whether heat therapy activates neuronal CMA and can provide positive AD-related outcomes.
HYPERTENSION AND COGNITIVE DECLINE
Cardiovascular disease is a risk factor for both AD and vascular dementia, a form of cognitive decline resulting from small- or large-vessel cerebrovascular disease (64, 125). Together, these conditions account for most dementia cases worldwide (47, 99). Reductions in cerebral blood flow and alterations to the blood-brain barrier have been associated with AD (4, 30, 112), and reductions in regional blood flow are associated with cognitive decline and mild cognitive impairment with AD (73). In addition, more recent studies demonstrate a relationship between cerebrovascular health and AD neuropathological burden even in healthy older adults, suggesting a potential early role for vascular function in the development of neurodegenerative disease (78, 109). Hypertension, which is prevalent in one-third of adults and two-thirds of adults over the age of 65, may play an important role in the development of cognitive decline, AD, and vascular dementia. Given that hypertension is a modifiable risk factor, this makes it a potentially important mechanism for the prevention of age-related cognitive disorders.
Elevated systolic blood pressure is associated with smaller regional and total brain volume as well as decreased brain volume over time (40, 45, 46, 75, 89). Brains of individuals with chronic hypertension demonstrate increased β-amyloid, atrophy, and neurofibrillary tangles and evidence of decreased brain glucose metabolism (10, 98). Vascular remodeling as a result of hypertension is also thought to play a significant role in the development of cognitive dysfunction. Increased arterial stiffness can lead to increased arterial pulse wave velocity and pulse pressure, resulting in endothelial dysfunction. Endothelial dysfunction can also occur as a result of chronic decreases in cerebral blood flow with hypertension. Decreased cerebral blood flow can also result in unmet metabolic demand in vulnerable regions of the brain. Cerebral hypometabolism, a marker of reduced energy metabolism, is one of the earliest biomarkers of AD (12, 88). Brain hypometabolism occurs first in regions of the brain that are normally highly metabolic (19, 49). All these factors could contribute to the etiology of AD, causing increased research emphasis on the role of hypertension in AD progression and prevention.
Potential Benefits of Heat Therapy on Hypertension and Vascular Dysfunction
Prior research demonstrates that acute heating, either by sauna or water immersion, results in increased cardiac output and a redistribution of blood flow to the periphery (14). Shifts in blood flow with heat favor a beneficial shear pattern that enhances vascular remodeling and endothelial function (14, 18, 118). In murine models, 30-day heat acclimation affords protection from ischemia/reperfusion (I/R) injury such that cardiac myocytes are better able to survive I/R stress (81). In humans, acute hot tub use appears to temporarily protect tissue from I/R stress (15), but this effect has not been examined in a chronic heat intervention.
HSPs have demonstrated roles in cardiovascular protection. HSP25 can downregulate an early step in the formation of atherosclerotic plaques (26), whereas HSP72 has been shown to inhibit angiotensin II and decrease vascular smooth muscle hypertrophy (129). In addition, HSP90 plays an important role in NO synthase stability (11). Future studies are needed to demonstrate the effects of chronic heat therapy on vascular function and hypertension in AD patients as well as to determine HSP-driven adaptations that may occur in this population.
Heat-Mediated Extracellular Vesicle Organ Cross Talk May Benefit the Brain
One possible mechanism by which heat therapy may benefit the brain is by facilitating the delivery of molecular mediators within extracellular vesicles (EVs; exosomes and microvesicles). Although it has been shown that exercise increases brain HSP content (17), HT-induced increases in neuronal HSP content in humans remain unknown. EVs, which are shown to carry molecules/proteins across the blood-brain barrier (21), are likely an additional mechanism to increase the neuronal HSP content. EVs are bilayer-phospholipid enclosed vesicles that carry protein and mi-RNA cargo throughout circulation, whose contents will ultimately be delivered into the cytoplasm of target cells due to their hydrophobic membrane (21). EVs have been shown to act as key regulators of nerve regeneration, synaptic function, and behavior (32, 126). Importantly, when directly injected into the brain, EVs can effectively eliminate protein aggregates like Aβ (126). Despite this, very little is known about how EV content or biological function change in the context of aging, AD, or exercise training or with chronic heat. However, it has been established that HSPs can travel in EVs, and recent studies showed that their expression was elevated following acute exercise (42, 120). In addition to their roles in mediating inflammation, interacting with the insulin signaling pathway, and modulating mitochondrial quality control, HSPs are essential for the maintenance of protein structure and stability in most tissues, including neurons. In this way, EVs and their HSP cargo could provide a mechanism for interorgan cross talk—specifically regarding stress sensing, metabolic function, and proteostasis.
In the context of the brain and neuronal cells, EVs are postulated to remove and discard unwanted proteins, RNAs, and lipids via microglia intercellular-dependent mechanisms (32). A recent study characterized the proteome of brain-derived EVs from control and AD cohorts (33). Not surprisingly, the AD EVs contained more phosphorylated tau cargo, and pathway analysis showed gene enrichment for APP signaling, Prion disease ontology, and stress-activated p38 MAPK cascade (33). These novel findings demonstrated that despite their biophysiological similarities, there were significant differences in the protein signatures of EVs derived from AD as compared with control brains. Although previous studies have considered the role of EVs in AD pathology and as disease biomarkers, we are proposing a conceptually novel mechanism, whereby the autophagosomal capacity of EVs and the cargo they contain can be targeted for the prevention and treatment of AD. Specifically, the EV system could be leveraged for both proteostatic and metabolic maintenance (HSPs as cargo from other tissues) and degradation via microglial EV formation and export of aggregates (i.e., p-tau). In this way, we would be restoring the normal interorgan cross talk that may be aberrant in AD.
Current Evidence if Heat Therapy Benefits in Humans
A growing number of research studies are examining the benefits of repeated heat bouts in health and disease. Research from Hooper (56) in 1999 first examined the potential effects of hot water immersion on blood glucose regulation. With significant reductions in blood glucose and hemoglobin A1C after 3 wk of heat therapy, these findings were attributed to increased blood flow and glucose clearance. However, Minson and colleagues (14) only recently demonstrated the efficacy of repeated bouts of hot water immersion on cardiovascular outcomes. They found that 8 wk of heat therapy resulted in improved endothelial function, arterial stiffness, wall thickness, and blood pressure in young, healthy individuals. A more recent study from the Minson laboratory demonstrated that 30 sessions of hot water immersion over 8–10 wk were effective at reducing metabolic risk in obese women with polycystic ovarian syndrome (36). Like Hooper’s initial hot tub study, Minson et al. showed that repeated heat therapy resulted in significant reductions in fasting glucose and improved glucose clearance following an oral glucose tolerance test. Importantly, these findings collectively demonstrate the validity of chronic heat therapy as a clinical treatment to improve glucose metabolism in obese and/or insulin-resistant individuals, both known risk factors for AD. However, it remains to be tested whether improved glucose control and improved insulin sensitivity could impact cognitive decline in individuals with mild cognitive impairment or AD.
Despite lack of data available in AD cohorts, a recent study did demonstrate the safety and adherence of heat therapy in aged individuals. In this 12-wk study, heat therapy via hot water immersion and supervised exercise both improved walking distance and resting blood pressure in patients with peripheral arterial disease (PAD) (3). Like the work of Minson et al. in younger healthy cohorts, this study demonstrates that heat therapy improves functional ability and cardiovascular outcomes in aged individuals (mean age of heat group = 76 ± 8 yr). Importantly, adherence to heat therapy was excellent and the heat was well tolerated in this population.
Although many of the studies cited in this review use hot water immersion as the primary modality of heat therapy, sauna therapy has also been shown to have an impact on cardiovascular health. Two weeks of 60°C far-infrared sauna 6 days/week significantly improved endothelial function in men with elevated cardiovascular risk (58) and in men with congestive heart failure (65). Evidence from large prospective studies also indicate that increased frequency and duration of heat (sauna) exposure reduce cardiovascular morbidity risk of incident hypertension (128) and mortality (70). Importantly, a population-based prospective cohort study of 2,315 healthy men aged 42–60 yr at baseline demonstrated that moderate-to-high frequency of sauna bathing (2–3 times/week and 4–7 times/week, respectively) was associated with lowered risks of dementia and AD (71). These data support the rationale for conducting larger-scale hot water immersion therapy studies in AD.
Finally, a recent study by Hafen et al. (50) demonstrated the first evidence that mitochondrial adaptation can occur in human skeletal muscle in response to repeated exposures to mild heat stress. These investigators used local, deep-tissue heating of the vastus lateralis via pulsed shortwave diathermy in young men and women for 2 h (6 consecutive days). Increases in HSP72 and HSP90 corresponded with increased maximal coupled and uncoupled respiratory capacity. Although these data are encouraging, they also highlight the need for new studies in additional populations and with other heat treatment modalities, as there are surely differences in whole body versus tissue-specific heating. Importantly, one modality may better impact whole body metabolic outcomes and increase patient ease-of-treatment/compliance.
Considerations for Future Work Examining Heat Therapy in Humans
An important consideration regarding published HT literature is that the core temperature used differs between animal and human studies. Animal studies have typically been performed between 41°C and 42°C (7, 48, 102), whereas human studies have typically settled around 38.5°C (14, 25). Mechanistic outcomes in human studies have thus far also been limited; for instance, it remains unclear as to whether there are increases in local HSP content versus translocated HSP content via EV. This is a potential focus of future studies. Negative effects of heat treatment in our studies have been mild and limited to anecdotal reports of dehydration and headache, which resolved within 24 h post treatment. No deleterious events have been observed beyond 24 h.
Summary
AD is the most common neurodegenerative disease, yet there are no disease-modifying treatments available and there is no cure. We believe that heat therapy can be of tremendous clinical benefit to patients with AD (Fig. 1). Specifically, we and others have shown that heat therapy prevents obesity and insulin resistance and restores target blood glucose and insulin levels—all risk factors associated with AD. Moreover, it is well established that heat therapy increases blood flow and vascular compliance, in addition to potentially increasing interorgan cross talk via EV transport/formation. Finally, we propose that HSPs induced via heat therapy are critical for proteostasis (protein aggregate degradation), mitochondrial function (mitophagy, mitochondrial respiratory capacity, and mitochondrial health), cross talk (stress sensing in distant organs such as the brain), and general cell health (inhibition of c-Jun and NF-κB signaling). Overall, emerging research indicates that heat and HSP induction show immense therapeutic potential in nearly all diseases with an inflammatory, proteostatic, and/or metabolic component—making heat therapy a logical and important research focus for the prevention of chronic disease.
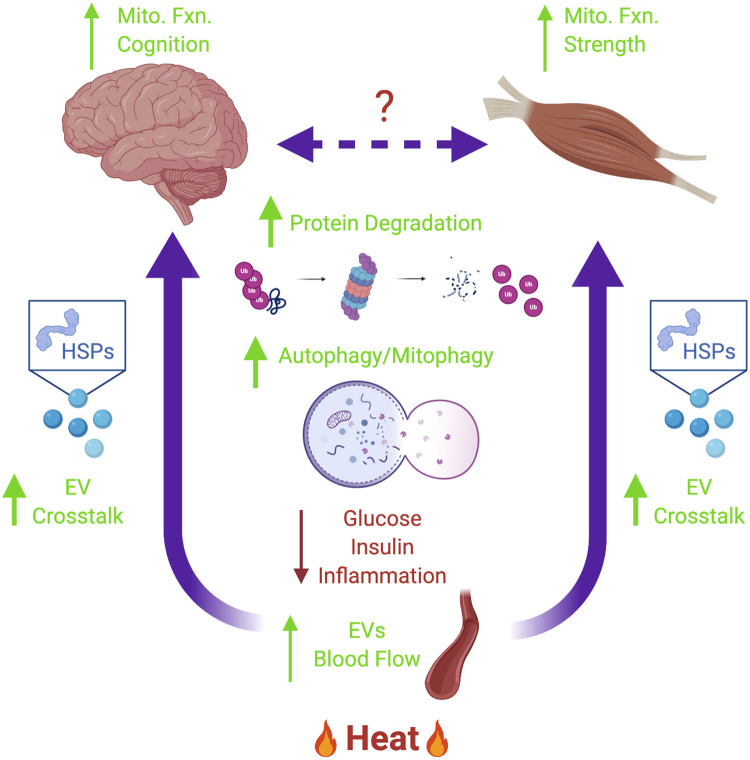
Fig. 1.
The role of heat therapy in preventing AD. Heat therapy increases peripheral blood flow and may increase interorgan cross talk via endocytic vesicle (EV) formation and transport—although interorgan cross talk remains ill-defined. EVs may contain heat shock proteins (HSPs) that can improve mitochondrial function (increase mitophagy/respiratory function), reduce inflammation, and restore proteostasis [increase aggregate degradation via chaperone-mediated autophagy (CMA), chaperone-assisted selective autophagy (CSA), or the proteasome). Combined, these effects can improve whole body metabolic homeostasis (reducing blood glucose and insulin) and improve tissue-specific outcomes such as cognitive function or strength. AD, Alzheimer’s disease.
GRANTS
Research support was provided by the Madison and Lila Self Graduate Fellowship (to A. T. Von Schulze). Work performed by A. T. Von Schulze, F. Deng, and P. C. Geiger was supported by the National Institutes of Health Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under Grant number P20 GM103418, the University of Kansas Alzheimer’s Disease Center P30 AG035982, and the National Institute on Aging AG066488.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.C.G. conceived and designed research; A.T.V.S. prepared figures; A.T.V.S. and P.C.G. drafted manuscript; A.T.V.S., F.D., J.K.M., and P.C.G. edited and revised manuscript; A.T.V.S., F.D., J.K.M., and P.C.G. approved final version of manuscript.
REFERENCES
- 1.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 25: 4217–4221, 2005. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86: 876–884, 2011. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316: H1495–H1506, 2019. doi: 10.1152/ajpheart.00151.2019. [DOI] [PubMed] [Google Scholar]
- 4.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 10: 642–653, 2013. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 5.Alfaro IE, Albornoz A, Molina A, Moreno J, Cordero K, Criollo A, Budini M. Chaperone mediated autophagy in the crosstalk of neurodegenerative diseases and metabolic disorders. Front Endocrinol (Lausanne) 9: 778, 2019. doi: 10.3389/fendo.2018.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15: 321–387, 2019. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 7.Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, Thyfault JP, Geiger PC. Heat shock protein 72 regulates hepatic lipid accumulation. Am J Physiol Regul Integr Comp Physiol 315: R696–R707, 2018. doi: 10.1152/ajpregu.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond B Biol Sci 373: 20160529, 2018. doi: 10.1098/rstb.2016.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61: 661–666, 2004. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 10.Ashby EL, Miners JS, Kehoe PG, Love S. Effects of hypertension and anti-hypertensive treatment on amyloid-β (Aβ) plaque load and Aβ-synthesizing and Aβ-degrading enzymes in frontal cortex. J Alzheimers Dis 50: 1191–1203, 2016. doi: 10.3233/JAD-150831. [DOI] [PubMed] [Google Scholar]
- 11.Averna M, Stifanese R, De Tullio R, Passalacqua M, Salamino F, Pontremoli S, Melloni E. Functional role of HSP90 complexes with endothelial nitric-oxide synthase (eNOS) and calpain on nitric oxide generation in endothelial cells. J Biol Chem 283: 29069–29076, 2008. doi: 10.1074/jbc.M803638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailly M, Ribeiro MJ, Vercouillie J, Hommet C, Gissot V, Camus V, Guilloteau D. 18F-FDG and 18F-florbetapir PET in clinical practice: regional analysis in mild cognitive impairment and Alzheimer disease. Clin Nucl Med 40: e111–e116, 2015. doi: 10.1097/RLU.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 13.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 66: 1339–1344, 2009. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunt VE, Jeckell AT, Ely BR, Howard MJ, Thijssen DH, Minson CT. Acute hot water immersion is protective against impaired vascular function following forearm ischemia-reperfusion in young healthy humans. Am J Physiol Regul Integr Comp Physiol 311: R1060–R1067, 2016. doi: 10.1152/ajpregu.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review. Gerontology 55: 550–558, 2009. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol 284: R520–R530, 2003. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- 18.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 19.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583, 2006. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 20.Chakravorty A, Jetto CT, Manjithaya R. Dysfunctional mitochondria and mitophagy as drivers of Alzheimer’s disease pathogenesis. Front Aging Neurosci 11: 311, 2019. doi: 10.3389/fnagi.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX, Zimak J, Ségaliny A, Riazifar M, Pham V, Digman MA, Pone EJ, Zhao W. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9: 509–529, 2016. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HK, Ji ZS, Dodson SE, Miranda RD, Rosenblum CI, Reynolds IJ, Freedman SB, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J Biol Chem 286: 5215–5221, 2011. doi: 10.1074/jbc.M110.151084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HW, Chen SC, Tsai JL, Yang RC. Previous hyperthermic treatment increases mitochondria oxidative enzyme activity and exercise capacity in rats. Kaohsiung J Med Sci 15: 572–580, 1999. [PubMed] [Google Scholar]
- 24.Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 31: 424–430, 2011. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly EM, Kelly CJ, Chen G, O’grady T, Kay E, Leahy A, Bouchier-Hayes DJ. Pharmacological induction of HSP27 attenuates intimal hyperplasia in vivo. Eur J Vasc Endovasc Surg 25: 40–47, 2003. doi: 10.1053/ejvs.2002.1793. [DOI] [PubMed] [Google Scholar]
- 27.Crinnion WJ Sauna as a valuable clinical tool for cardiovascular, autoimmune, toxicant- induced and other chronic health problems. Altern Med Rev 16: 215–225, 2011. [PubMed] [Google Scholar]
- 28.Cuervo AM Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21: 142–150, 2010. doi: 10.1016/j.tem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Monte SM Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 4: 1582–1605, 2012. doi: 10.2741/e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Torre JC Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3: 184–190, 2004. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLeo AM, Ikezu T. Extracellular vesicle biology in Alzheimer’s disease and related tauopathy. J Neuroimmune Pharmacol 13: 292–308, 2018. doi: 10.1007/s11481-017-9768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLeo AM, Sethi M, Muraoka S, Ruan Z, Ikezu S, Golantla S, Gendelman HE, Zaia J, Ikezu T. O2–01–02: Characterization of human Alzheimer’s disease brain-derived exosomes. Alzheimers Dement 14, 7S_Part_11: P608, 2018. doi: 10.1016/j.jalz.2018.06.2640. [DOI] [Google Scholar]
- 34.Dice JF Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci 15: 305–309, 1990. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 35.Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, Soleymani T, Daraei P, Sitz D, Vergnes L, Wanagat J, Reue K, Febbraio MA, Hevener AL. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 63: 1488–1505, 2014. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Needham KW, Comrada LN, Minson CT. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 317: E172–E182, 2019. doi: 10.1152/ajpendo.00549.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22: 401–412, 2019. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, Iser WB, Wollman BN, Morevati M, Li J, Kerr JS, Lu Q, Waltz TB, Tian J, Sinclair DA, Mattson MP, Nilsen H, Bohr VA. NAD+ Replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab 24: 566–581, 2016. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157: 882–896, 2014. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol 254: 713–721, 2007. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 41.Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 23: 142–153, 2012. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers E-M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles 4: 28239, 2015. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frydman J Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70: 603–647, 2001. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 44.Geiger PC, Gupte AA. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev 39: 34–42, 2011. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage 31: 754–765, 2006. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glodzik L, Mosconi L, Tsui W, de Santi S, Zinkowski R, Pirraglia E, Rich KE, McHugh P, Li Y, Williams S, Ali F, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Alzheimer’s disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol Aging 33: 1215–1227, 2012. doi: 10.1016/j.neurobiolaging.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694, 2001. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 50.Hafen PS, Preece CN, Sorensen JR, Hancock CR, Hyldahl RD. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol (1985) 125: 1447–1455, 2018. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- 51.Hands S, Sinadinos C, Wyttenbach A. Polyglutamine gene function and dysfunction in the ageing brain. Biochim Biophys Acta 1779: 507–521, 2008. doi: 10.1016/j.bbagrm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature 475: 324–332, 2011. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 53.Hemi R, Paz K, Wertheim N, Karasik A, Zick Y, Kanety H. Transactivation of ErbB2 and ErbB3 by tumor necrosis factor-alpha and anisomycin leads to impaired insulin signaling through serine/threonine phosphorylation of IRS proteins. J Biol Chem 277: 8961–8969, 2002. doi: 10.1074/jbc.M109391200. [DOI] [PubMed] [Google Scholar]
- 54.Heni M, Schöpfer P, Peter A, Sartorius T, Fritsche A, Synofzik M, Häring HU, Maetzler W, Hennige AM. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol 51: 679–681, 2014. doi: 10.1007/s00592-013-0546-y. [DOI] [PubMed] [Google Scholar]
- 55.Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13: 477–491, 2017. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 56.Hooper PL Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341: 924–925, 1999. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 57.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300: 1142–1145, 2003. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 58.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. doi: 10.1016/S0735-1097(01)01467-X. [DOI] [PubMed] [Google Scholar]
- 59.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer’s disease. Neurobiol Dis 57: 47–55, 2013. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53: 474–481, 2004. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 61.Kang S, Lee YH, Lee JE. Metabolism-centric overview of the pathogenesis of Alzheimer’s disease. Yonsei Med J 58: 479–488, 2017. doi: 10.3349/ymj.2017.58.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19: 365–381, 2018. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med 21: 1406–1415, 2015. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 64.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disorder 2: 241–260, 2009. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/S0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 66.Kimura N Diabetes mellitus induces Alzheimer’s disease pathology: histopathological evidence from animal models. Int J Mol Sci 17: 503, 2016. doi: 10.3390/ijms17040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirchner P, Bourdenx M, Madrigal-Matute J, Tiano S, Diaz A, Bartholdy BA, Will B, Cuervo AM. Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLoS Biol 17: e3000301, 2019. doi: 10.1371/journal.pbio.3000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271: 24313–24316, 1996. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 69.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr 89: 467S–471S, 2009. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 71.Laukkanen T, Kunutsor S, Kauhanen J, Laukkanen JA. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing 46: 245–249, 2017. doi: 10.1093/ageing/afw212. [DOI] [PubMed] [Google Scholar]
- 72.Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell 20: 1533–1544, 2009. doi: 10.1091/mbc.e08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leeuwis AE, Benedictus MR, Kuijer JPA, Binnewijzend MAA, Hooghiemstra AM, Verfaillie SCJ, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement 13: 531–540, 2017. doi: 10.1016/j.jalz.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145: 301–308, 1997. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 75.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 54: 2659–2671, 2011. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol (1985) 112: 354–361, 2012. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, Klunk WE, Mathis CA, Salloway S, Fox NC, Hill DL, Les AS, Collins P, Gregg KM, Di J, Lu Y, Tudor IC, Wyman BT, Booth K, Broome S, Yuen E, Grundman M, Brashear HR; Bapineuzumab 301 and 302 Clinical Trial Investigators . Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 85: 692–700, 2015. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Perdomo SJ, Ward J, Vidoni ED, Sisante JF, Kirkendoll K, Burns JM, Billinger SA. Vascular health is associated with amyloid-β in cognitively normal older adults. J Alzheimers Dis 70: 467–475, 2019. doi: 10.3233/JAD-181268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lizama BN, Palubinsky AM, Raveendran VA, Moore AM, Federspiel JD, Codreanu SG, Liebler DC, McLaughlin B. Neuronal preconditioning requires the mitophagic activity of C-terminus of HSC70-interacting protein. J Neurosci 38: 6825–6840, 2018. doi: 10.1523/JNEUROSCI.0699-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 64: 570–575, 2007. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 81.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 23: 79–88, 2005. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- 82.Maruszak A, Żekanowski C. Mitochondrial dysfunction and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 35: 320–330, 2011. doi: 10.1016/j.pnpbp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Monteiro-Cardoso VF, Castro M, Oliveira MM, Moreira PI, Peixoto F, Videira RA. Age-dependent biochemical dysfunction in skeletal muscle of triple-transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res 12: 100–115, 2015. doi: 10.2174/1567205012666150204124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montero D, Lundby C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training. J Physiol 595: 3377–3387, 2017. doi: 10.1113/JP273480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morris JK, Burns JM. Insulin: an emerging treatment for Alzheimer’s disease dementia? Curr Neurol Neurosci Rep 12: 520–527, 2012. doi: 10.1007/s11910-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris JK, Honea RA, Vidoni ED, Swerdlow RH, Burns JM. Is Alzheimer’s disease a systemic disease? Biochim Biophys Acta 1842: 1340–1349, 2014. doi: 10.1016/j.bbadis.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris JK, Vidoni ED, Mahnken JD, Montgomery RN, Johnson DK, Thyfault JP, Burns JM. Cognitively impaired elderly exhibit insulin resistance and no memory improvement with infused insulin. Neurobiol Aging 39: 19–24, 2016. doi: 10.1016/j.neurobiolaging.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mosconi L Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging 32: 486–510, 2005. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 89.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 26: 1636–1641, 2008. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- 90.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71: 362–381, 2012. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ni H-M, Williams JA, Ding W-X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol 4: 6–13, 2015. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39: 409–421, 2003. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 93.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53: 1937–1942, 1999. doi: 10.1212/WNL.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 94.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521: 525–528, 2015. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 95.Parker WD Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology 40: 1302–1303, 1990. doi: 10.1212/WNL.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 96.Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27: 437–496, 1993. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 97.Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 51: 1256–1262, 2002. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 98.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia Aging Study. Neurobiol Aging 21: 57–62, 2000. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 99.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9: 63–75.e2, 2013. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 67: 505–512, 2010. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 101.Rhea EM, Banks WA. Role of the blood-brain barrier in central nervous system insulin resistance. Front Neurosci 13: 521, 2019. doi: 10.3389/fnins.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoin CS, White KS, Wilson DR, Meers GM, Koch LG, Britton SL, Thyfault JP, Geiger PC. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 65: 3341–3351, 2016. doi: 10.2337/db16-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rotermund C, Truckenmüller FM, Schell H, Kahle PJ. Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice. J Neurochem 131: 848–858, 2014. doi: 10.1111/jnc.12813. [DOI] [PubMed] [Google Scholar]
- 104.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR; Bapineuzumab 301 and 302 Clinical Trial Investigators . Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370: 322–333, 2014. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schram MT, Sep SJS, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RMA, Stehouwer CDA. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol 29: 439–451, 2014. doi: 10.1007/s10654-014-9889-0. [DOI] [PubMed] [Google Scholar]
- 106.Schuh RA, Jackson KC, Schlappal AE, Spangenburg EE, Ward CW, Park JH, Dugger N, Shi GL, Fishman PS. Mitochondrial oxygen consumption deficits in skeletal muscle isolated from an Alzheimer’s disease-relevant murine model. BMC Neurosci 15: 24, 2014. doi: 10.1186/1471-2202-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29: 15–32, 2001. doi: 10.1016/S0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 108.Silva DF, Selfridge JE, Lu J, e L, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet 22: 3931–3946, 2013. doi: 10.1093/hmg/ddt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sisante JV, Vidoni ED, Kirkendoll K, Ward J, Liu Y, Kwapiszeski S, Maletsky R, Burns JM, Billinger SA. Blunted cerebrovascular response is associated with elevated beta-amyloid. J Cereb Blood Flow Metab 39: 89–96, 2019. doi: 10.1177/0271678X17732449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med 16: 93–112, 1999. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 111.Su Z, Nie Y, Huang X, Zhu Y, Feng B, Tang L, Zheng G. Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol 10: 1193, 2019. doi: 10.3389/fphar.2019.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21: 1318–1331, 2018. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Swerdlow RH Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis 62: 1403–1416, 2018. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci USA 112: E2497–E2506, 2015. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tamura Y, Kitaoka Y, Matsunaga Y, Hoshino D, Hatta H. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J Physiol 593: 2707–2720, 2015. doi: 10.1113/JP270093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol 586: 55–63, 2008. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ten L, Borson S, Kiyak HA, Yamagishi M. Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer’s disease. J Am Geriatr Soc 37: 109–116, 1989. doi: 10.1111/j.1532-5415.1989.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 118.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol 79: 205–221, 2006. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27: 237–251.e4, 2018. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 121.Willmund F, del Alamo M, Pechmann S, Chen T, Albanèse V, Dammer EB, Peng J, Frydman J. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152: 196–209, 2013. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J 27: 336–349, 2008. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 58: 71–77, 2009. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 63: 658–663, 2004. doi: 10.1212/01.WNL.0000134666.64593.BA. [DOI] [PubMed] [Google Scholar]
- 125.Ying H, Jianping C, Jianqing Y, Shanquan Z. Cognitive variations among vascular dementia subtypes caused by small-, large-, or mixed-vessel disease. Arch Med Sci 12: 747–753, 2016. doi: 10.5114/aoms.2016.60962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem 289: 24488–24498, 2014. doi: 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuzefovych LV, Solodushko VA, Wilson GL, Rachek LI. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology 153: 92–100, 2012. doi: 10.1210/en.2011-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zaccardi F, Laukkanen T, Willeit P, Kunutsor SK, Kauhanen J, Laukkanen JA. Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens 30: 1120–1125, 2017. doi: 10.1093/ajh/hpx102. [DOI] [PubMed] [Google Scholar]
- 129.Zheng Y, Im CN, Seo JS. Inhibitory effect of Hsp70 on angiotensin II-induced vascular smooth muscle cell hypertrophy. Exp Mol Med 38: 509–518, 2006. doi: 10.1038/emm.2006.60. [DOI] [PubMed] [Google Scholar]