Abstract
A single session of leg heat therapy (HT) has been shown to elicit increases in leg blood flow and reduce blood pressure (BP) and the circulating levels of endothelin-1 (ET-1) in patients with symptomatic peripheral artery disease (PAD). We assessed whether 6 wk of supervised leg HT (3 times/wk) with water-circulating trousers perfused with water at 48°C improved 6-min walk distance in individuals with PAD compared with a sham treatment. Secondary outcomes included the assessment of leg vascular function, BP, quality of life, and serum ET-1 and nitrite plus nitrate (NOx) levels. Of 32 PAD patients randomized, 30 [age: 68 ± 8 yr; ankle-brachial index (ABI): 0.6 ± 0.1] completed the 3- and 6-wk follow-ups. Participants completed 98.7% of the treatment sessions. Compared with the sham treatment, exposure to HT did not improve 6-min walk distance, BP, popliteal artery reactive hyperemia, cutaneous microvascular reactivity, resting ABI, or serum NOx levels. The change from baseline to 6 wk in scores of the physical functioning subscale of the 36-item Short Form Health Survey was significantly higher in the HT group (control −6.9 ± 10 vs. HT 6.8 ± 15; 95% confidence interval: 2.5–24.3, P = 0.017). Similarly, the change in ET-1 levels after 6 wk was different between groups, with the HT group experiencing a 0.4 pg/mL decrease (95% confidence interval: −0.8–0.0, P = 0.03). These preliminary results indicate that leg HT may improve perceived physical function in symptomatic PAD patients. Additional, larger studies are needed to confirm these findings and determine the optimal treatment regimen for symptomatic PAD patients.
NEW & NOTEWORTHY This is the first sham-controlled study to investigate the effects of leg heat therapy (HT) on walking performance, vascular function, and quality of life in patients with peripheral artery disease (PAD). Adherence to HT was high, and the treatment was well tolerated. Our findings revealed that HT applied with water-circulating trousers evokes a clinically meaningful increase in perceived physical function and reduces the serum concentration of the potent vasoconstrictor endothelin-1 in patients with PAD.
Keywords: endothelin-1, heat therapy, intermittent claudication, peripheral artery disease, quality of life
INTRODUCTION
Lower extremity peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis that affects more than 236 million individuals worldwide (50). Approximately 1 in 10 individuals aged 70 yr and nearly 1 in 5 people older than 80 yr have PAD (50). As the prevalence of the primary risk factors for this condition, including obesity-associated diabetes and dyslipidemia, is expected to grow globally, the burden of PAD will continue to rise (17). Patients with PAD have worse quality of life than their healthy counterparts, in part because of the marked decline in physical functioning (12). Walking-induced ischemic pain may reduce daily physical activity levels and as a consequence accelerate mobility loss and functional decline in these patients (36). Effective management of leg symptoms in symptomatic patients is hampered largely by the lack of noninvasive, widely accessible treatment options. The cornerstone therapy to improve functional capacity in patients with PAD consists of supervised treadmill walking sessions (34). Unfortunately, the paucity of programs and other barriers including the inconvenience of travel to rehabilitation facilities make this option inaccessible to the vast majority of patients with this condition (11, 24).
Heat therapy (HT) has emerged as a practical treatment to improve cardiovascular health in young individuals (4) as well as patients with overt cardiovascular disease (25, 30, 41, 48, 49) and endocrine disorders (13, 14). The effects of HT, in the form of dry sauna and hot-water immersion, were previously reported in patients with PAD (2, 47, 51, 52). Tei and colleagues (52) first reported that dry sauna at 60°C for 10 wk (5 days/wk) improved leg pain, hemodynamics, and walking performance in patients with moderate to severe PAD. Recently, Akerman and coworkers (2) showed that HT via spa bathing combined with resistance band and callisthenic exercises for 12 wk (3–5 days/wk) improved walking distance and resting blood pressure in patients with PAD. Although encouraging, none of the aforementioned studies in patients with PAD included a sham-treated control group, and it is therefore difficult to exclude the possibility that the observed positive adaptations derived from a placebo effect.
The goal of the present study was to contrast and compare the effects of leg HT with a sham intervention in patients with symptomatic PAD. Leg HT was applied for 90 min, three times weekly for 6 wk, with customized water-circulating trousers perfused with water heated to 48°C. Using this modality, we previously demonstrated that exposure to a single HT session increases popliteal artery blood flow, lowers blood pressure, and reduces the levels of the potent endogenous vasoconstrictor endothelin-1 (ET-1) (40). In the sham-treated group, water at 33°C was circulated through the trousers. This regimen elevates skin temperature by ∼2°C but does not evoke measurable changes in core body temperature, heart rate, and blood pressure (40). In the present study, the primary outcome was a change from baseline to 6 wk in 6-min walk distance. We hypothesized, on the basis of the findings of studies with other HT modalities (2, 47, 52), that leg HT using tube-lined trousers would improve exercise capacity in people with PAD. Secondary outcomes included the change in health-related quality of life, blood pressure, conduit artery and cutaneous microvascular function, and systemic levels of ET-1 and nitrite plus nitrate (NOx, an index of nitric oxide bioavailability).
METHODS
Subjects.
Eligible patients were identified and contacted by Indiana Clinical and Translational Science Institute Research Network (ResNet) research assistants. After interest in participation was established, patients were contacted directly by the researchers. Additional study participants were obtained by direct physician referral from the Division of Vascular Surgery at Indiana University School of Medicine and the Richard L. Roudebush VA Medical Center. Thirty-two participants were randomized into the study. All patients had ankle-brachial index (ABI) values < 0.90 and claudication pain during exercise in one or both legs for >6 mo before enrolling in the study. Patients were excluded if their electronic medical record showed 1) a hemoglobin A1C value > 8.5% within 3 mo of screening, 2) exercise-limiting comorbidities (arthritis, heart failure, chronic obstructive pulmonary disease, etc.), 3) tissue loss, 4) prior amputation, 5) nonhealing wounds, 6) evidence of critical limb ischemia, 7) recent (<3 mo) infrainguinal revascularization (surgery or endovascular revascularization) or revascularization planned during the study period, 8) planned change in medical therapy, 9) active cancer, 10) chronic kidney disease [estimated glomerular filtration rate (eGFR) < 30], 11) HIV positive, active hepatitis B virus (HBV) or hepatitis C virus (HCV) disease, 12) peripheral neuropathy, numbness, or paresthesia in the legs, and 13) a body mass index (BMI) > 35. Patients with cardiovascular or other implants not compatible with magnetic resonance imaging (MRI) were allowed to participate in the study but were excluded from undergoing the phase-contrast MRI measurements. The protocol was approved by the Institutional Review Boards at Indiana University and at the Richard L. Roudebush VA Medical Center (no. 1601589496) and registered with the US Library of Medicine on clinicaltrials.gov (NCT02770547). Written informed consent was obtained, and all procedures adhere to the requirements of the US Federal Policy for the Protection of Human Subjects (45 CFR, Part 46) and support the general ethical principles of the Declaration of Helsinki.
Experimental design.
A schematic of the experimental protocol is depicted in Fig. 1. Participants were assigned, with a randomized, balanced design, to undergo either HT or a sham treatment 3 times/wk, for a total of 18 sessions across the 6 wk. Participants were informed that there were two different categories of HT, “low-heat” and “high-heat,” and that both might be beneficial for claudication symptoms. Participants were asked to report to the laboratory in a fasted state (>8 h postprandial), refrain from exercise (24 h) and smoking (>4 h), and take their usual prescription medications before the experimental visits. All visits took place in the morning in a temperature-controlled room (22–24°C). On visit 1, participants were asked about their health history and were asked to complete the 36-item Short Form Health Survey version 2 (SF-36v2; Optum, Eden Prairie, MN) and the Vascular Quality of Life Questionnaire (VascuQoL). Participants were then familiarized with the 6-min walk test. Visit 2 was conducted at least 72 h after visit 1. Upon arrival at the imaging research facility, participants rested in the supine position for 15 min. Blood pressure was then measured with an automated device, and the participant was transported to the scanning room for the assessment of postocclusive reactive hyperemia in the popliteal artery using phase-contrast MRI. After completion of the MRI assessment, participants were escorted to the Clinical Research Center to undergo the remaining experimental tests. After 10 min of quiet rest in the supine position, blood pressure measurements were taken in duplicate in the arms and ankles for the calculation of the ABI. The time period between the reactive hyperemia test and the resting ABI assessment was ∼30 min. Next, blood samples were collected from a vein in the antecubital space. Participants were then instrumented with skin heaters and laser-Doppler flowmetry probes for the assessment of leg cutaneous microvascular reactivity. This test lasted 70 min, and blood pressure was measured every 5 min throughout the test with an automated device. Finally, patients were escorted to an adjacent hallway and completed the 6-min walk test. Experimental visits 3 and 4, which were similar to visit 2, were conducted after 3 and 6 wk of treatment, respectively. Treatment sessions ceased at least 48 h before the experimental sessions to ensure that the chronic rather than the transient acute effects of HT were being assessed.
Fig. 1.
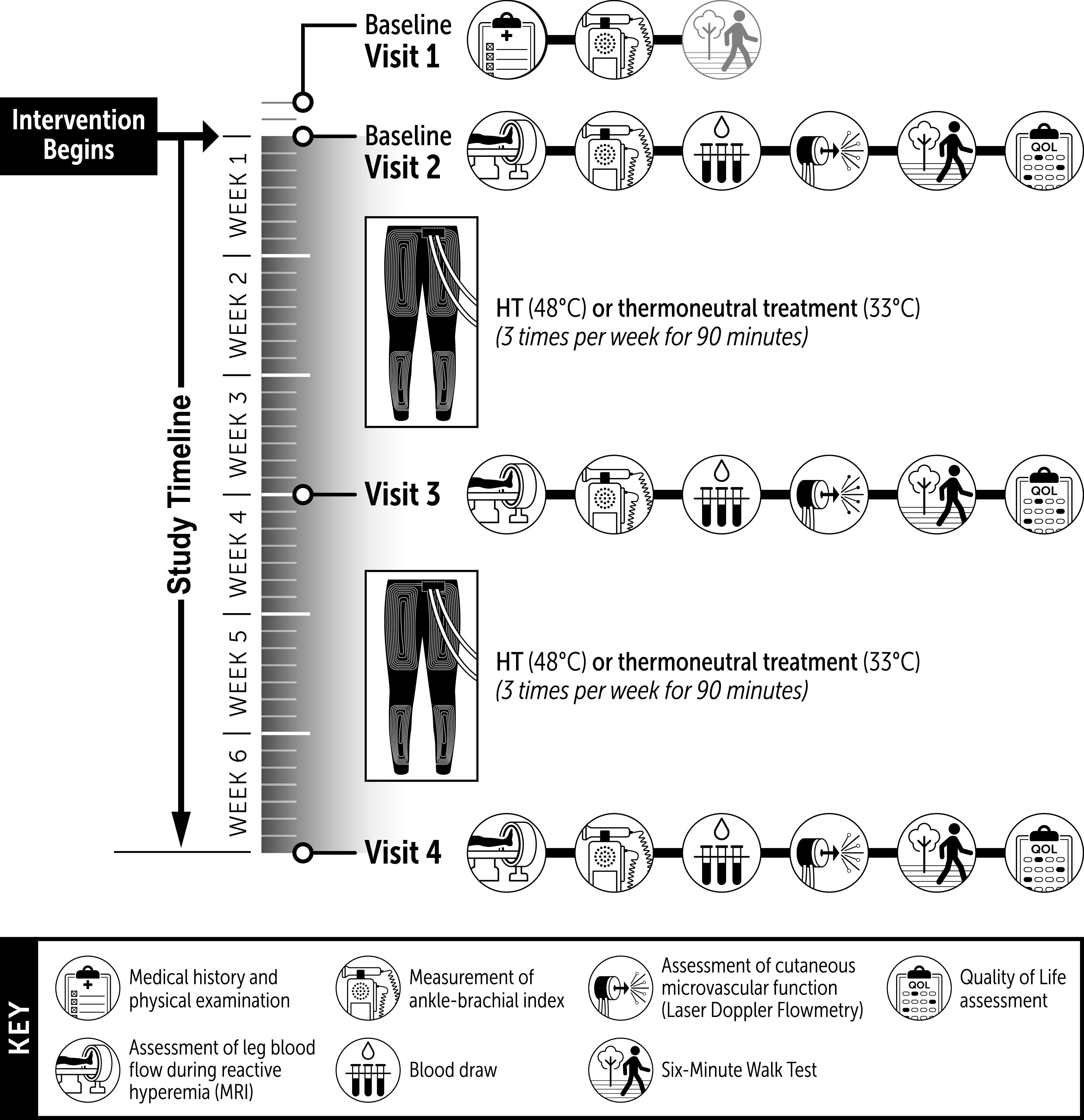
Schematic of the experimental protocol.
Intervention.
Participants were asked to put on the water-circulating trousers (Med-Eng, Ottawa, ON, Canada) and sit in a semirecumbent reclining chair. Water at 48°C (HT) or 33°C (control) was circulated through the garment for 90 min with a heated bath circulator (SAHARA S21; ThermoFisher Scientific, United States). An automatic oscillometric sphygmomanometer (Carescape Dinamap V100; GE Healthcare, United States) was used to measure blood pressure every 15 min throughout the treatment. Tympanic temperature was also recorded in a subset of patients (control n = 12, HT n = 11) with a digital thermometer (Braun ThermoScan PRO 6000; Welch Allyn, United States). Thermal comfort scores were assessed with a 9-point ISO categorical scale every 15 min during the treatment. The scale ranges from −4 (“very cold”) to +4 (“very hot) (16).
Six-Minute walk test.
The 6-min walk test was performed on a 30-m-long flat, straight corridor following the American Thoracic Society guidelines (2a). The length of the corridor was marked every 10 ft, and the turnaround points were marked with a cone. Patients were instructed to walk as far as possible for 6 min and were encouraged with standardized phrases every minute throughout the test. Two tests were completed at baseline to confirm reproducibility of results. The best of two tests was defined as the baseline walk distance (5).
Quality of life.
The Medical Outcomes Study 36-Item Short Form Health Survey (SF-36v2) was used to assess health-related quality of life (HRQoL). Prior studies revealed that the physical function subscale of the SF-36v2 Health Survey is the most impaired subscale in patients with symptomatic PAD (26). The SF-36v2 Health Survey was scored with proprietary software (Health Outcomes; Optum, United States). Disease-specific health-related quality of life was assessed with the Vascular Quality of Life (VascuQoL) questionnaire, a PAD-specific HRQoL instrument (37). The total VASCUQOL score is the average score of questions answered and ranges from 1 (worst QOL) to 7 (best QOL) (38).
Reactive hyperemia.
Postocclusive reactive hyperemia in the popliteal artery was assessed using phase-contrast MRI as described previously (40). MRI scanning was performed on a Siemens 3-T Magnetom Prisma scanner (Siemens AG Healthcare Sector, Erlangen, Germany). A transmit/receive knee coil was placed around the knee of the most symptomatic leg, defined as the leg in which the participant self-described as having the most severe claudication pain. A cuff (CC22; Hokanson, United States) was snugly wrapped around the upper thigh and connected to a commercially available air source (Hokanson AG101; Hokanson, United States) coupled to a rapid cuff inflator (Hokanson E20; Hokanson, United States). After baseline data acquisition, the cuff was inflated to suprasystolic values (75 mmHg above brachial systolic blood pressure (SBP), as assessed before scanning). Phase-contrast imaging was performed during cuff inflation to ensure that total arterial occlusion of the upper leg was achieved. After 5 min of occlusion, the cuff was deflated and postocclusion reactive hyperemia was monitored for 10 min.
For quantitative flow measurements, a two-dimensional gradient-echo technique was employed with the following acquisition parameters: repetition time (TR) 36.4 ms, echo time (TE) 3.59 ms, flip angle 20°, field of view (FOV) 200 mm, matrix size 256, and reconstructed voxel dimensions of 0.8 × 0.8 × 10.0 mm. A single slice was prescribed perpendicular to a straight section of the popliteal artery. Fifty dynamic phases were acquired to obtain flow waveforms over the cardiac cycle with a maximum range of flow velocity encoding of 100 cm/s. A pulse oximeter on the index finger was used for peripheral gating with the minimum trigger delay. A quantitative flow analysis package (QFlow; Medis, Leiden, The Netherlands) was used to analyze phase-contrast flow data. A region of interest covering the entire visible cross section of the artery was manually drawn every 10 frames, and then software interpolation created arterial lumen tracings for the remaining frames. Each series was manually checked in duplicate to ensure consistency of interpolation. Flow parameters included velocities averaged across the respective vessel lumen and corresponding flow volumes (53, 54).
Ankle-brachial index.
Cuffs (SC10, SC12; Hokanson, United States) were wrapped around the ankles and the upper arms. Systolic pressures were measured sequentially in the right posterior tibial artery, right dorsalis pedis artery, right brachial artery, left posterior tibial artery, left dorsalis pedis artery, and left brachial artery with a handheld 5-MHz Doppler ultrasound (Lumeon; McKesson, United States). Pressures were taken at each site in duplicate. The ABI of each leg was calculated by dividing the higher of the dorsalis pedis pressure or posterior tibial pressure by the higher of the right or left arm blood pressure (1).
Cutaneous microvascular reactivity.
Leg cutaneous hyperemia in response to rapid local skin heating to 39°C was assessed as described by Choi and colleagues (7). Briefly, two local skin heaters (SH02 Skin Heater/Temperature Monitor; Moor Instruments, Axminster, UK) were affixed with dual-sided stickers to the proximal portion of the lower leg, ∼3 in. below the patella on the dermal surface overlying the tibialis anterior muscle. Single-point laser-Doppler flowmetry probes (Moor Instruments, Axminster, UK) were positioned in the center of each local heater. Red blood cell flux, an index of skin blood flow, was recorded for 10 min with skin temperature held constant at 33°C. Next, local skin temperature at each site was raised to 39°C at a rate of 0.1°C/s and maintained for 40 min (7). Finally, local skin temperature was raised to 43.0°C at a rate of 0.1°C/s and maintained at this level for 20 min. Red blood cell flux and the temperature of the skin heaters were recorded at 40 Hz with a data acquisition system (PowerLab and LabChart; ADInstruments, United States), and the last 2 min of every 5-min bin was averaged for the entire protocol. An automated device (Tango+; Suntech Medical, United States) was used to measure SBP and diastolic blood pressure (DBP) every 5 min. Mean arterial pressure (MAP) was calculated as DBP plus one-third pulse pressure (i.e., the difference between SBP and DBP). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by MAP and was normalized as a percentage of maximal vasodilation (%CVCmax). There were no observable or statistical differences in %CVCmax between the two laser-Doppler sites within subjects for a given trial, so values from each site were averaged. At the end of the session, the heating probes were traced with a permanent marker and covered with a protective film (Tegaderm; 3M, United States). Participants were asked to preserve the marks to facilitate consistent placement of the probes in subsequent visits.
Analysis of blood markers.
Samples were drawn into tubes containing sodium heparin or serum separator tubes (SST) (BD Vacutainer; BD, Ontario, Canada). One tube was transported to the Indiana University Health Pathology Laboratory for the assessment of a routine basic metabolic panel. Serum samples were allowed to clot at room temperature for 30 min before centrifugation at 1,100 g for 10 min (ST16; Thermo Scientific, United States). Samples were then aliquoted and immediately placed into a −80°C freezer. Serum ET-1 concentrations were measured in duplicate with a commercially available enzyme-linked immunosorbent assay kit (DET100, Endothelin-1 Quantikine ELISA Kit; R&D Systems, United States). Total NOx was assessed via high-performance liquid chromatography (HPLC) (ENO-30; Eicom USA, United States) by injecting 10 μL of serum and then colorimetrically determining the concentration by comparison to a nitrite (NO2)/nitrate (NO3) standard injection series as described previously (8).
Sample size calculation.
An a priori power calculation indicated that n = 16 subjects per group would have 80% power to detect a clinically meaningful change of 50 m in the 6-min walk distance after 6 wk of treatment with a two-sided, two-sample t test (α = 0.05) and assuming a standard deviation of change of 48.7, estimated from Table 3 in Gardner et al. (21). Controlling for baseline with analysis of covariance (ANCOVA) results in improved power.
Statistical analysis.
All analyses were performed with SAS v9.4, and results are expressed as means ± standard deviation (SD). Two-sample t tests, Wilcoxon rank sum test, chi-square tests, or Fisher’s exact tests were used to compare demographic and clinical characteristics between the two groups. Linear mixed models were fit (using PROC MIXED) to the raw values of MAP and tympanic temperature during the treatment sessions, with effects for group, time, session, and all two- and three-way interactions using an unstructured correlation for session and AR(1) structure for time. If any interactions with session were significant, individual ANCOVAs were performed for sessions 1, 9, and 18. The raw MAP and tympanic temperature data were also separately averaged across the 18 sessions and then reanalyzed by ANCOVA. ANCOVA was used to test whether the 3- and 6-wk means were different between the HT and control groups for all the outcomes with baseline as a covariate. When a significant interaction of time and group was detected, a Sidak adjustment was used to adjust the group-effect P value at each time point, and the Sidak-adjusted 95% confidence interval (CI) was reported for the group effect at each time point. If the interaction was not significant, it was removed and the main effects for time and group tested, and 95% CI for group effect was reported. Since the 3-wk assessment was included to inform us about the time course of eventual changes in performance but our a priori power calculation did not include the 3-wk assessment, for key outcomes we also tested the group effect at weeks 3 and 6 separately, even if there were no group × time interaction. The intraclass correlation coefficient (ICC) estimates for the 6-min walk distance and ABI were calculated based on a single-measurement, absolute-agreement, two-way mixed-effects model. The measures from the two laser-Doppler flowmeter probes were compared with linear-mixed models.
RESULTS
Subject characteristics.
A total of 345 patients were contacted regarding the study. Fifty-eight participants were deemed ineligible, and 255 refused to participate. The remaining 32 patients were randomly allocated to receive HT (n = 16) or the control treatment (n = 16). One participant in the control group had recurrent hypertensive episodes that required a change in the medication regimen. As a result, this individual was withdrawn from the study after 12 treatment sessions. One female patient in the HT group had minor skin irritation and some blistering on the back of the right thigh, which was apparent after completion of the fifth HT session. Although the patient recovered promptly after interruption of the treatment, this individual was also withdrawn from the study. All others completed the treatment sessions and both the 3- and 6-wk follow-ups. Demographic and clinical characteristics for those participants that completed all follow-ups are presented in Table 1. There were no baseline differences between groups. All but 2 patients were current or former smokers, and 10 patients were diabetic. The treatment was well tolerated, with participants in the HT group reporting, on average, perceived thermal comfort scores ranging between +2 (“warm”) and +3 (“hot”) on a 9-point bipolar feeling scale. Participants completed 98.7% (532/540 sessions) of the required sessions.
Table 1.
Demographic and clinical characteristics
| All Subjects (n = 30*) | Control (n = 15) | HT (n = 15) | P Value | |
|---|---|---|---|---|
| Age, yr | 68.9 (8.3) | 69.0 (7.8) | 68.7 (9.0) | 0.93 |
| Height, cm | 174.3 (6.8) | 173.5 (7.1) | 175.2 (6.7) | 0.47 |
| Weight, kg | 83.5 (15.7) | 81.4 (15.8) | 85.7 (15.8) | 0.47 |
| ABI—most affected leg | 0.7 (0.1) | 0.6 (0.1) | 0.7 (0.1) | 0.16 |
| ABI—the other leg | 0.8 (0.2) | 0.8 (0.2) | 0.9 (0.2) | 0.09 |
| BMI, kg/m2 | 27.4 (4.11) | 26.9 (4.2) | 27.8 (4.1) | 0.57 |
| Sex, n (%) | 0.59 | |||
| Male | 26 (86.7) | 12 (80.0) | 14 (93.3) | |
| Female | 4 (13.3) | 3 (20.0) | 1 (6.7) | |
| Most symptomatic leg, n (%) | 0.46 | |||
| Left | 12 (40.0) | 5 (33.3) | 7 (46.7) | |
| Right | 18 (60.0) | 10 (66.7) | 8 (53.3) | |
| Stents, n (%) | 0.88 | |||
| No | 17 (58.6) | 8 (57.1) | 9 (60.0) | |
| Yes | 12 (41.4) | 6 (42.9) | 6 (40.0) | |
| Smoking status, n (%) | 0.41 | |||
| Never smoked | 2 (6.7) | 2 (13.3) | 0 (0.0) | |
| Current smoker | 13 (43.3) | 7 (46.7) | 6 (40.0) | |
| Past smoker | 15 (50.0) | 6 (40.0) | 9 (60.0) | |
| Diabetes, n (%) | 0.12 | |||
| No | 20 (66.7) | 12 (80.0) | 8 (53.3) | |
| Yes | 10 (33.3) | 3 (20.0) | 7 (46.7) | |
| Race, n (%) | 0.65 | |||
| Black/African American | 6 (20.0) | 2 (13.3) | 4 (26.7) | |
| White | 24 (80.0) | 13 (86.7) | 11 (73.3) | |
| Medications, n (%) | ||||
| Beta blocker | 10 (33.3) | 5 (33.3) | 5 (33.3) | |
| ACE inhibitor | 7 (23.3) | 4 (26.6) | 3 (20.0) | |
| Statin | 23 (76.7) | 13 (86.6) | 10 (66.6) | |
| Cilostazol | 5 (16.7) | 4 (26.6) | 2 (13.3) | |
| Calcium channel blocker | 12 (40.0) | 7 (46.6) | 5 (33.3) | |
| Diuretic | 6 (20.0) | 2 (13.3) | 4 (26.6) | |
| Insulin | 4 (13.3) | 1 (6.6) | 3 (20.0) | |
| Antiplatelet agent | 22 (73.3) | 10 (66.6) | 12 (80.0) | |
| Angiotensin II receptor antagonist | 8 (26.7) | 4 (26.6) | 4 (26.6) | |
| Other | 4 (13.3) | 1 (6.7) | 3 (20.0) |
Values are mean (SD) or n (%) when indicated and associated P values. ABI, ankle-brachial index; ACE, angiotensin-converting enzyme; BMI, body mass index; HT, heat therapy.
Missing values for height, weight, BMI (n = 1); distance walked before pain begins (n = 5); stents (n = 1); ABI—most affected leg (n = 1).
Changes in blood pressure and temperature during treatment sessions.
A three-way interaction between group, time, and session was noted for MAP (P = 0.0219), indicating that the group × time effect was different for different treatment sessions. To further explore the effects of treatment session on MAP responses, we compared the changes in MAP between groups at the beginning (session 1), middle (session 9), and end (session 18) of the treatment (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.12887804.v1). A significant group × interaction was observed for the changes in MAP at session 9 (P = 0.0196), but no group differences were detected in subsequent post hoc testing. Despite the nonsignificant main effect of group on these three particular sessions (sessions 1, 9, and 18), the changes in MAP averaged across the 18 treatment sessions were greater (P < 0.05) in the HT group compared with the control group starting at 45 min and persisting until the end of the treatment (Fig. 2A). A two-way interaction between group and session was noted for tympanic temperature (P = 0.008). Subsequent analysis revealed a significant group × time interaction at sessions 9 (P = 0.005) and 18 (P = 0.001), with the HT group displaying higher temperatures at 45–60 min (Supplemental Fig. S1). When averaged across the 18 treatment sessions, the changes in temperature were greater (P < 0.05) in the HT group compared with the control group starting at 30 min and persisting until the end of the treatment (Fig. 2B).
Fig. 2.
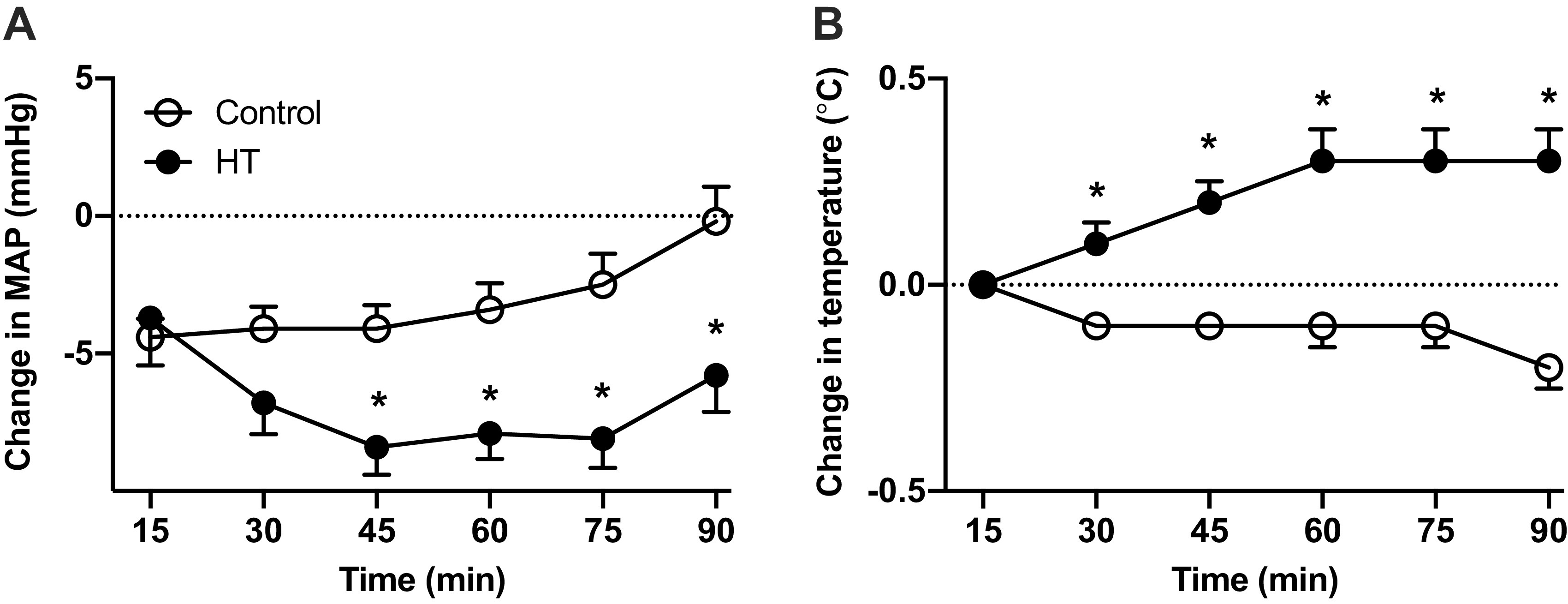
Average changes from baseline (time 0) in mean arterial pressure (MAP; A) and tympanic temperature (B) during exposure to 90 min of heat therapy (HT, n = 15) or the sham treatment (control, n = 15). Participants were asked to complete 18 sessions (3 times/wk) over 6 wk. The graphs display the average values across all 18 sessions. Data are means ± SD. Data were analyzed by analysis of covariance (ANCOVA) with baseline as a covariate. The Sidak adjustment was used to adjust the group effect P value at each time point. *Difference between groups (P < 0.05).
Six-Minute walk test.
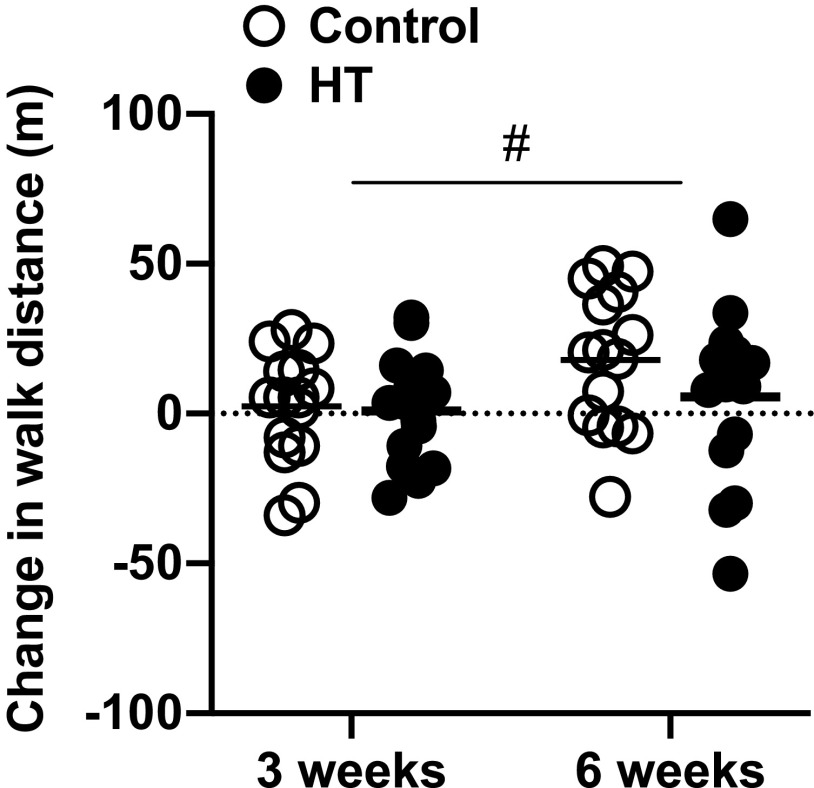
Test-retest reliability of the 6-min walk test at baseline was excellent (ICC = 0.96). Baseline walk distances were 386 ± 85 m in the control group and 414 ± 109 m in the HT group. Figure 3 depicts the change from baseline in 6-min walk distances after 3 and 6 wk of treatment. Small improvements in both groups were noted after 3 wk [control 2 ± 19 m, HT 0.8 ± 18 m; group effect estimate (95% confidence interval): −1.5 (−15.6 to 12.6)] and 6 wk [control 18 ± 23 m, HT 6 ± 29 m; group effect estimate (95% confidence interval): −12.3 (−32.3 to 7.8)]. There was no group × time interaction (P = 0.14) and no main effects of group (P = 0.80), but there was a significant effect of time (P = 0.009).
Fig. 3.
Individual and group mean changes from baseline in walking distance on the 6-min walk test after 3 and 6 wk of heat therapy (HT, n = 15) or the sham treatment (control, n = 15). Data were analyzed by analysis of covariance (ANCOVA) with baseline as a covariate. #Significant effect of time (P = 0.009).
Quality of life.
The responses to quality of life data questionnaires were obtained from 14 patients in the control group and 14 patients in the HT group. One outlier in the control group was identified and removed because of the change in scores being ∼2.5 standard deviations above the mean. The changes in the scores for the physical function subscale (PF) of the SF-36v2 questionnaire are shown in Fig. 4. In the HT group, the average PF scores increased by 3.6 ± 12.4 and 6.8 ± 15.5 points after 3 and 6 wk of treatment, respectively. Conversely, the scores declined by 2.2 ± 17.4 points after 3 wk and by 6.9 ± 10.3 points at 6 wk in the control group. The ANCOVA analysis comparing the 3- and 6-wk means between the HT and control groups revealed no group × time interaction (P = 0.11) but a trend for a main effect of group (P = 0.051). Subsequent testing for the main effect of group at 6 wk revealed a statistically significant difference between groups [group effect estimate (95% confidence interval): 13.4 (2.5–24.3), P = 0.018]. There was no significant group effect at week 3. There were no group differences in any of the other subscales of the SF-36 survey (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.12630482.v1) or the scores from the VascuQol questionnaires (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.12630491.v1).
Fig. 4.
Individual and group mean changes from baseline in the scores for the physical functioning (PF) subscale of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36v2) after 3 and 6 wk of heat therapy (HT, n = 14) or the sham treatment (control, n = 14). Data were analyzed by analysis of covariance (ANCOVA) with baseline as a covariate. *Difference between groups (P < 0.05).
Reactive hyperemia.
A total of 25 participants completed the baseline phase-contrast MRI assessment, and 23 participants completed all three assessments. Technical issues, particularly with peripheral gating, prevented the analysis of data from 11 participants. Therefore, the final analysis included data from nine participants in the control group and nine participants in the HT group. One representative example of changes in peak flow in the popliteal artery at baseline, during occlusion, and after cuff release is shown in Supplemental Fig. S2 (see https://doi.org/10.6084/m9.figshare.12619649.v1). Peak blood flow (Supplemental Fig. S2) as well as other indexes of flow and velocity (Supplemental Table S3; see https://doi.org/10.6084/m9.figshare.12630512.v1) were unaltered in both groups after the interventions.
Cutaneous microvascular reactivity.
A representative response from one subject to rapid local heating to 39°C at a rate of 0.1°C/s is shown in Supplemental Fig. S3 (see https://doi.org/10.6084/m9.figshare.12619700.v1). Cutaneous vascular conductance rose progressively, reaching a plateau by ∼30–40 min into heating. On average, CVC at the end of the 39°C heating period was ∼55% of the maximal value obtained after 20 min of heating at 43°C. There was no group × time interaction (P = 0.55) and no main effects of group (P = 0.32) or time (P = 0.89) for the plateau CVC (Supplemental Fig. S3).
Blood pressure.
BP was measured on the right arm every 5 min for 70 min during the assessment of cutaneous microvascular reactivity. Supplemental Fig. S4 (see https://doi.org/10.6084/m9.figshare.12619724.v1) displays the changes from baseline to 3 and 6 wk of treatment of both systolic and diastolic pressures across the 70-min assessment. A significant group × time interaction was observed for the changes in SBP from baseline to 6 wk (P = 0.0042), with significant group differences at 20 min (control 9.7 ± 11.9 vs. HT −6.0 ± 12.7, P = 0.0178). Although a significant group × time interaction was also observed for the changes in DBP (P = 0.0168), post hoc testing did not detect statistically significant group differences throughout the 70-min assessment. The averages of all 15 repeated measurements of SBP and DBP at baseline were 149 ± 12 mmHg and 81 ± 10 mmHg, respectively, in the control group and 154 ± 12 mmHg and 82 ± 9 mmHg in the HT group. The changes in average SBP and DBP after exposure to 6 wk of control and HT are shown in Supplemental Fig. S5 (see https://doi.org/10.6084/m9.figshare.12619739.v1). There were no group differences in the change of either SBP or DBP.
Ankle-brachial index.
Test-retest reliability of ABI was excellent (ICC = 0.90 for right leg and 0.91 for left leg). At baseline, the ABI in the most affected leg was 0.6 ± 0.1 in the control group and 0.7 ± 0.1 in the HT group. There was no group × time interaction (P = 0.84) and no main effects of group (P = 0.75) or time (P = 0.94) for the most affected leg. Results were similar for the least affected leg (Supplemental Table S4; see https://doi.org/10.6084/m9.figshare.12630527.v1).
Blood biomarkers.
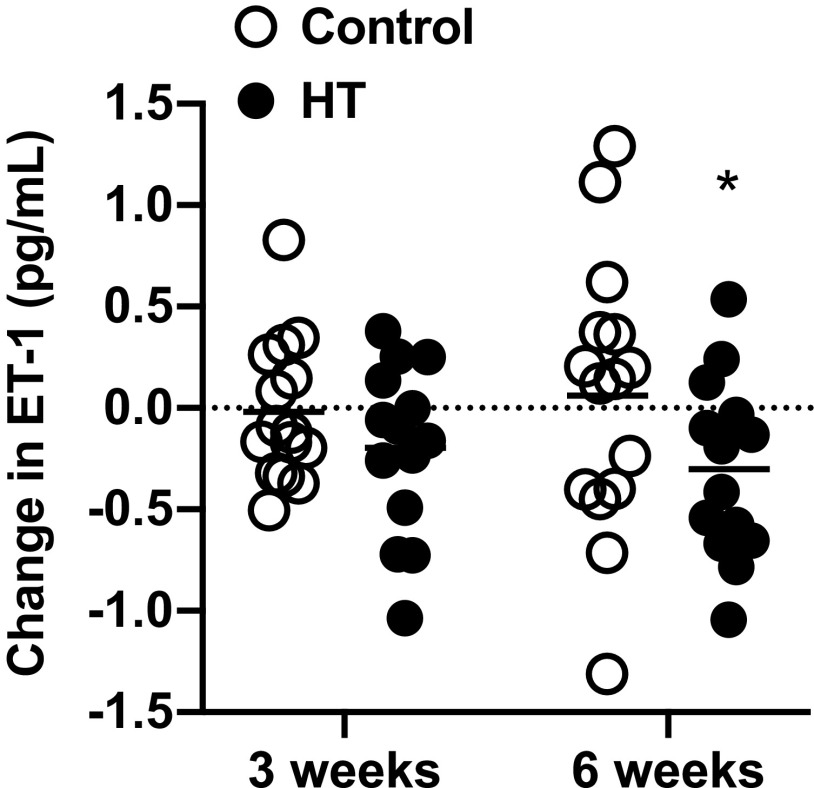
Serum ET-1 concentrations were 2.6 ± 0.7 pg/mL in the control group and 2.3 ± 0.5 pg/mL in the HT group at baseline. As shown in Fig. 5, average serum ET-1 concentration rose by ∼6.5% relative to baseline after 6 wk of treatment in the control group, whereas a 13% reduction was noted in the HT group. The ANCOVA analysis comparing the 3- and 6-wk means between the HT and control groups revealed no group × time interaction (P = 0.24) but a trend for a main effect of treatment (P = 0.053). Subsequent testing for the main effects of treatment at 6 wk revealed a statistically significant difference between groups [group effect estimate (95% confidence interval): −0.4 (−0.8–0.0), P = 0.03]. There was no significant group effect at week 3. The serum NOx concentrations at baseline were 64.3 ± 66.3 μmol/L in the control group and 42.2 ± 25.7 μmol/L in the HT group. There were no differences between groups for the changes in NOx concentrations following 3 and 6 wk of treatment (Supplemental Fig. S6; see https://doi.org/10.6084/m9.figshare.12619757.v1).
Fig. 5.
Individual and group means for the changes from baseline in serum concentrations of endothelin-1 (ET-1) after 3 and 6 wk of heat therapy (HT, n = 14) or the sham treatment (control, n = 14). Data were analyzed by analysis of covariance (ANCOVA) with baseline as a covariate. *Difference between groups (P < 0.05).
DISCUSSION
The primary findings of the present study were that 6 wk of supervised leg HT improved perceived physical functioning but had no measurable impact on 6-min walk distance and vascular reactivity in patients with symptomatic PAD. These findings are incongruous with earlier reports of improved leg hemodynamics (52) and exercise capacity (2, 47) after sauna or hot tub therapy in PAD patients. Of importance, a sham-treated group was not included in these previous studies, and it is thus unclear whether the reported benefits derive from the treatment or are due to a placebo effect. The present study is unique because we compared the effects of leg HT against a sham treatment that produces small increases in leg skin temperature. We observed that whereas perceived physical function tended to decline over time in the group exposed to the sham device, individuals treated with leg HT reported a progressive, clinically meaningful improvement in quality of life during the 6-wk intervention. These novel findings imply that leg HT may be a useful adjunctive therapy to restore perceived well-being in symptomatic PAD patients.
Repeated exposure to heat stress in the form of hot tub therapy or sauna bathing has been shown to evoke robust increases in both conduit artery and microvascular endothelial function in young individuals (4, 23, 39) as well as in patients with cardiovascular risk factors (25) and chronic heart failure (30). Serial assessments of vascular function throughout the HT interventions in these previous studies revealed that improvements in vascular function are evident after as little as 2 wk of treatment (4, 39) and are fully manifested within 6–8 wk (4). Based upon these previous findings, the length of the intervention here was 6 wk, with vascular assessments performed after 3 and 6 wk of treatment. We anticipated that palpable improvements in vascular reactivity would occur within 3 wk, thereby enabling a greater oxygen delivery capacity to the calf muscles and a consequent increase in exercise capacity. Contrary to these predictions, HT had no measurable impact on conduit artery and cutaneous vascular function and on walking performance on the 6-min walk test.
The discrepancies between our findings and the aforementioned studies may stem from several important differences in study design and methods. First, most previous studies employed HT modalities that produced marked increases in core body temperature (38°C–38.5°C) during the treatment sessions (4, 39). The tube-lined trousers used here elicit local leg heating and small changes in core temperature (Fig. 2). Conceivably, a greater thermal strain may be necessary to evoke appreciable changes in vascular function in patients with symptomatic PAD. Second, it is possible that the treatment frequency and length of the intervention used in the present study (3 sessions/wk) were not optimal for patients with severe vascular disease. Along these lines, studies reporting clinical improvements in patients with heart failure and cardiovascular risk following repeated sauna bathing required participants to undergo the treatment 5 days/wk (25, 30). Furthermore, the length of previous studies in PAD patients ranged from 10 to 12 wk of treatment (2, 52). Third, the vast majority of the previous studies (4, 23, 25, 30, 39) examined vascular function in the arm through the assessment of flow-mediated dilation (FMD) of the brachial artery. In contrast, we focused on the legs because 1) the degree of vascular impairment is greater in the legs than in the arms in PAD patients (45) and 2) leg peak hyperemic blood flow relates to peak exercise performance in these patients (44). Intriguingly, contrary to brachial artery FMD (35), lower extremity reactive hyperemic blood flow does not consistently improve after exercise training in PAD patients (42), underscoring the notion that the lower extremity vasculature may be less responsive to treatment interventions in these individuals.
This is the first sham-controlled study to examine the impact of HT on 6-min walk distance in patients with PAD. It is noteworthy that the changes in walk distance varied considerably among participants (Fig. 2). On average, 6-min walk distance improved by 18 m in the control group and by 5 m in the HT group after 6 wk of treatment. Albeit not clinically meaningful (46), these subtle improvements may be placebo related and attest to the critical importance of incorporating a sham-treated group to account for psychosocial factors that promote placebo effects (18). This is particularly important for clinical trials involving medical devices because there is evidence of an enhanced placebo effect compared with placebo pills (29). Water at 33°C was selected as the sham treatment in the present study because it is perceived as slightly warm but it is not sufficient to provoke changes in core body temperature and the consequent hemodynamic adjustments (40). Nonetheless, it is important to highlight that the sham thermal treatment does not induce the same sensation as the active treatment and may unblind patients and change their expectations toward the efficacy of the treatment.
One consistent finding among prior studies is a permanent reduction in BP, particularly SBP, following repeated exposure to HT. The magnitude of the reduction in SBP varies considerably (from −4 to −10 mmHg), likely reflecting differences in the experimental protocol, HT modality, and the clinical characteristics of the target population (2, 15, 25, 30). The BP measuring method in these previous studies consisted of averaging two or three readings obtained manually or with an automated device. There is little consensus on the optimal number of readings for accurate BP measurement, but it is clear that averaging multiple measurements may reduce the inherent variability of BP over short periods (43). In a study of 444 hypertensive individuals taking antihypertensive medication, at least five BP measurements were needed to be 80% certain whether SBP was <140 mmHg or not (43). Along these lines, one strength of the present study is that we performed a total of 15 BP measurements during the three experimental visits. After 6 wk of treatment, the average difference between groups in the change in SBP from baseline was ∼4 mmHg, but the variability across the 70-min interval was high (Supplemental Fig. S3). For instance, at 20 min the groups differed by nearly 16 mmHg (P = 0.01), whereas no differences (0.07 mmHg) were noted at 35 min (P = 0.96). These results suggest that although HT may possibly lower SBP in patients with PAD, the results are not uniform across multiple measurements. Future studies should incorporate other BP measurement strategies, including home-based and 24-h ambulatory monitoring, to confirm whether the BP-lowering action of HT holds true in unsupervised settings. The substantial and durable hypotensive effect observed across all 18 treatment sessions (Fig. 2) signifies that within a 24-h period BP was lowered for a significant amount of time. It is plausible that these repeated transient changes in BP may confer cardioprotective effects even in the absence of marked changes in resting BP.
The reduction in serum concentrations of ET-1 following 6 wk of HT aligns closely with our previous observations (40). The importance of this finding is evident when considering that 1) ET-1 promotes hypertension, vascular and cardiac hypertrophy, fibrosis, and atherosclerosis (10); 2) the systemic ET-1 concentrations are markedly elevated in PAD (9); and 3) ET-1 levels predict adverse outcomes in patients with chronic heart failure (22) and all-cause mortality in the general population (27, 56). Nonetheless, it is unknown whether the observed 13% reduction in serum ET-1 is clinically meaningful for PAD patients. Also, serum levels of ET-1 do not necessarily reflect the overall ET-1 production because this factor is secreted abluminally, toward the vascular smooth muscle, and is cleared by ETB receptors (10). It remains to be determined whether the HT-induced reduction in serum ET-1 is the result of reduced production, accelerated clearance, or a combination of both.
Despite the absence of changes in exercise capacity and vascular function, supervised HT for 6 wk had a moderate impact on health-related quality of life. The scores for the physical function subscale (PF), the most significantly impaired among all subscales of the short-form SF-36 questionnaire in PAD patients (26), improved by 3.6 and 6.8 points after 3 and 6 wk of treatment in the group exposed to HT, whereas a progressive decline was observed in the control group (−2.2 at week 3 and −6.9 at week 6). Gardner and colleagues recently reported that the minimal clinically important differences for small, moderate, and large changes in the PF score following 3 mo of supervised exercise in symptomatic PAD patients were 3, 9, and 14 points (19). On the basis of these estimates, it appears that as little as 3 wk of HT evokes small, albeit clinically significant changes in quality of life in claudicants. Furthermore, the improvements attained after 6 wk of HT are nearly equivalent to the changes observed after 12 wk of supervised exercise, the gold standard treatment regimen for symptomatic PAD (19). Nonetheless, as the assessment of quality of life was a secondary outcome, additional, adequately powered studies are warranted to confirm these seminal findings.
The improvement in health-related quality of life in the HT group occurred despite the lack of changes in 6-min walk distances. This is not surprising given the well-documented weak relationship between objective measures of physical function and quality of life in this patient population (3, 6, 26). In fact, the strongest predictors of the PF scores in PAD patients include the perceived ability to walk fast, climb stairs, and perform certain activities of daily living, including bathing (20). The mechanisms by which HT improves self-reported quality of life are unclear, but it is worth noting that we previously reported increases in muscle strength following repeated heat stress in a preclinical model of PAD (32) as well as in young individuals (31). It is thus tempting to speculate that HT may enhance muscle strength in PAD patients and in turn restore the capacity to execute functional tasks and improve perceived physical function.
Limitations.
Participants underwent the assessment of several outcomes during the experimental visits, and it is possible that the order in which the tests were conducted influenced the results of those tests. For instance, it is conceivable that thigh occlusion and reactive hyperemia during phase-contrast imaging altered the responses of subsequent tests, including the measurement of cutaneous microvascular reactivity and the 6-min walk test. Nonetheless, it is important to note that all participants underwent the exact same protocol, and it is therefore expected that the ordering effect was similar between groups. Second, the circulating levels of nitric oxide metabolites are heavily influenced by diet (55) as well as other factors, such as the use of mouthwash (28). The large variability in serum NOx levels reported here may thus derive by the lack of control for these important factors. Third, we have previously shown that a single HT session reduces serum levels of ET-1 in claudicants (40), and it is unclear how long this beneficial effect persists. Although the experimental outcomes were assessed at least 48 h after the last treatment session, it is impossible to exclude the possibility that the observed reduction in ET-1 concentrations partially reflect a residual effect of treatment. Finally, we did not measure BP during the assessment of leg reactive hyperemia. This is an important limitation because eventual changes in BP due to discomfort or pain during thigh cuff occlusion or other factors can directly alter leg blood flow.
Summary and clinical implications.
In summary, we report that leg HT via water-circulating trousers was well tolerated and elicited important improvements in perceived physical function in patients with symptomatic PAD. The excellent adherence to the prescribed regimen in the present study raises the prospect of favorable uptake and compliance with this new modality in unsupervised settings. Indeed, water-circulating garments combined with a portable pump might be an ideal tool for delivering HT in patients with symptomatic PAD, because this strategy is amenable for home use, which eliminates the need for patients to travel to a clinical facility to receive the treatment. In addition, water-circulating garments are practical for patients with multiple comorbidities who cannot undergo standard exercise regimens and are amenable for combination with other established approaches employed for PAD treatment. Thus, given its accessibility, tolerability, and ease of use, HT via water-perfused garments has the potential for rapid translation and application in the clinical setting. Nonetheless, additional studies are necessary to determine the optimal leg HT regimen and to compare the effectiveness of local versus whole body HT modalities in PAD patients.
GRANTS
Support for this work was provided by the American Heart Association (16SDH27600003), the NIH (1R21 AG-053687-01A1), the Indiana Clinical and Translational Science Institute, and the Showalter Trust Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.M., B.J.W., R.L.M., and B.T.R. conceived and designed research; J.C.M., C.L., and B.T.R. performed experiments; J.C.M., S.M.P., Y.H., and B.T.R. analyzed data; J.C.M., C.L., S.M.P., Y.H., B.J.W., R.L.M., and B.T.R. interpreted results of experiments; J.C.M., S.M.P., Y.H., and B.T.R. prepared figures; J.C.M. and B.T.R. drafted manuscript; J.C.M., C.L., S.M.P., Y.H., B.J.W., R.L.M., and B.T.R. edited and revised manuscript; J.C.M., C.L., S.M.P., Y.H., B.J.W., R.L.M., and B.T.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Maryanne Bowyer-Cherry and Janet Klein for invaluable assistance with the supervision of heat therapy sessions and Leigh A. Mott for providing regulatory support.
REFERENCES
- 1.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 126: 2890–2909, 2012. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 2.Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316: H1495–H1506, 2019. doi: 10.1152/ajpheart.00151.2019. [DOI] [PubMed] [Google Scholar]
- 2a.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117, 2002. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 3.Breek JC, Hamming JF, De Vries J, van Berge Henegouwen DP, van Heck GL. The impact of walking impairment, cardiovascular risk factors, and comorbidity on quality of life in patients with intermittent claudication. J Vasc Surg 36: 94–99, 2002. doi: 10.1067/mva.2002.124369. [DOI] [PubMed] [Google Scholar]
- 4.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra D, Wise RA, Kulkarni HS, Benzo RP, Criner G, Make B, Slivka WA, Ries AL, Reilly JJ, Martinez FJ, Sciurba FC; NETT Research Group . Optimizing the 6-min walk test as a measure of exercise capacity in COPD. Chest 142: 1545–1552, 2012. doi: 10.1378/chest.11-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetter IC, Dolan P, Spark JI, Scott DJ, Kester RC. Correlating clinical indicators of lower-limb ischaemia with quality of life. Cardiovasc Surg 5: 361–366, 1997. doi: 10.1016/S0967-2109(97)00011-2. [DOI] [PubMed] [Google Scholar]
- 7.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail 8: 914–920, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Haro Miralles J, Gónzalez AF, Varela Casariego C, García FA. Onset of peripheral arterial disease: role of endothelin in endothelial dysfunction. Interact Cardiovasc Thorac Surg 10: 760–765, 2010. doi: 10.1510/icvts.2009.227967. [DOI] [PubMed] [Google Scholar]
- 10.Dhaun N, Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol 16: 491–502, 2019. doi: 10.1038/s41569-019-0176-3. [DOI] [PubMed] [Google Scholar]
- 11.Dua A, Gologorsky R, Savage D, Rens N, Gandhi N, Brooke B, Corriere M, Jackson E, Aalami O. National assessment of availability, awareness, and utilization of supervised exercise therapy for peripheral artery disease patients with intermittent claudication. J Vasc Surg 71: 1702–1707, 2020. doi: 10.1016/j.jvs.2019.08.238. [DOI] [PubMed] [Google Scholar]
- 12.Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract 54: 826–831, 2004. [PMC free article] [PubMed] [Google Scholar]
- 13.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Needham KW, Comrada LN, Minson CT. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 317: E172–E182, 2019. doi: 10.1152/ajpendo.00549.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbri K Assessment of the influence of the thermal environment using subjective judgement scales In: Indoor Thermal Comfort Perception. Cham, Switzerland: Springer, 2015, p. 127–147. doi: 10.1007/978-3-319-18651-1_5. [DOI] [Google Scholar]
- 17.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 14: 156–170, 2017. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 18.Fregni F, Imamura M, Chien HF, Lew HL, Boggio P, Kaptchuk TJ, Riberto M, Hsing WT, Battistella LR, Furlan A; International placebo symposium working group . Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil 89: 160–172, 2010. doi: 10.1097/PHM.0b013e3181bc0bbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med 23: 349–357, 2018. doi: 10.1177/1358863X18762599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner AW, Montgomery PS, Wang M, Xu C. Predictors of health-related quality of life in patients with symptomatic peripheral artery disease. J Vasc Surg 68: 1126–1134, 2018. doi: 10.1016/j.jvs.2017.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 3: e001107, 2014. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb SS, Harris K, Todd J, Estis J, Christenson RH, Torres V, Whittaker K, Rebuck H, Wawrzyniak A, Krantz DS. Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem 48: 292–296, 2015. doi: 10.1016/j.clinbiochem.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg 34: 280–289, 2016. doi: 10.1016/j.avsg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. doi: 10.1016/S0735-1097(01)01467-X. [DOI] [PubMed] [Google Scholar]
- 26.Izquierdo-Porrera AM, Gardner AW, Bradham DD, Montgomery PS, Sorkin JD, Powell CC, Katzel LI. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. J Vasc Surg 41: 625–630, 2005. doi: 10.1016/j.jvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Jankowich MD, Wu WC, Choudhary G. Association of elevated plasma endothelin-1 levels with pulmonary hypertension, mortality, and heart failure in African American individuals: The Jackson Heart Study. JAMA Cardiol 1: 461–469, 2016. doi: 10.1001/jamacardio.2016.0962. [DOI] [PubMed] [Google Scholar]
- 28.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93–100, 2013. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 53: 786–792, 2000. doi: 10.1016/S0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 30.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/S0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 31.Kim K, Reid BA, Casey CA, Bender BE, Ro B, Song Q, Trewin AJ, Petersen AC, Kuang S, Gavin TP, Roseguini BT. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J Appl Physiol (1985) 128: 483–492, 2020. doi: 10.1152/japplphysiol.00701.2019. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Reid BA, Ro B, Casey CA, Song Q, Kuang S, Roseguini BT. Heat therapy improves soleus muscle force in a model of ischemia-induced muscle damage. J Appl Physiol (1985) 127: 215–228, 2019. doi: 10.1152/japplphysiol.00115.2019. [DOI] [PubMed] [Google Scholar]
- 34.McDermott MM Exercise training for intermittent claudication. J Vasc Surg 66: 1612–1620, 2017. doi: 10.1016/j.jvs.2017.05.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui MH. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 301: 165–174, 2009. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA 292: 453–461, 2004. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 37.Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur J Vasc Endovasc Surg 31: 46–52, 2006. doi: 10.1016/j.ejvs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg 33: 679–687, 2001. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 39.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 40.Neff D, Kuhlenhoelter AM, Lin C, Wong BJ, Motaganahalli RL, Roseguini BT. Thermotherapy reduces blood pressure and circulating endothelin-1 concentration and enhances leg blood flow in patients with symptomatic peripheral artery disease. Am J Physiol Regul Integr Comp Physiol 311: R392–R400, 2016. doi: 10.1152/ajpregu.00147.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. doi: 10.1016/j.amjcard.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Parmenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on haemodynamics in intermittent claudication: a systematic review of randomized controlled trials. Sports Med 40: 433–447, 2010. doi: 10.2165/11531330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med 154: 781–788, 2011. doi: 10.7326/0003-4819-154-12-201106210-00005. [DOI] [PubMed] [Google Scholar]
- 44.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol (1985) 111: 81–86, 2011. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanada H, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sueda T. Vascular function in patients with lower extremity peripheral arterial disease: a comparison of functions in upper and lower extremities. Atherosclerosis 178: 179–185, 2005. doi: 10.1016/j.atherosclerosis.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Sandberg A, Cider Å, Jivegård L, Nordanstig J, Wittboldt S, Bäck M. Test-retest reliability, agreement, and minimal detectable change in the 6-minute walk test in patients with intermittent claudication. J Vasc Surg 71: 197–203, 2020. doi: 10.1016/j.jvs.2019.02.056. [DOI] [PubMed] [Google Scholar]
- 47.Shinsato T, Miyata M, Kubozono T, Ikeda Y, Fujita S, Kuwahata S, Akasaki Y, Hamasaki S, Fujiwara H, Tei C. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol 56: 361–366, 2010. doi: 10.1016/j.jjcc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Sobajima M, Nozawa T, Fukui Y, Ihori H, Ohori T, Fujii N, Inoue H. Waon therapy improves quality of life as well as cardiac function and exercise capacity in patients with chronic heart failure. Int Heart J 56: 203–208, 2015. doi: 10.1536/ihj.14-266. [DOI] [PubMed] [Google Scholar]
- 49.Sobajima M, Nozawa T, Ihori H, Shida T, Ohori T, Suzuki T, Matsuki A, Yasumura S, Inoue H. Repeated sauna therapy improves myocardial perfusion in patients with chronically occluded coronary artery-related ischemia. Int J Cardiol 167: 237–243, 2013. doi: 10.1016/j.ijcard.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 50.Song P, Rudan D, Zhu Y, Fowkes FJ, Rahimi K, Fowkes FG, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 7: e1020–e1030, 2019. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 51.Tei C, Shinsato T, Kihara T, Miyata M. Successful thermal therapy for end-stage peripheral artery disease. J Cardiol 47: 163–164, 2006. [PubMed] [Google Scholar]
- 52.Tei C, Shinsato T, Miyata M, Kihara T, Hamasaki S. Waon therapy improves peripheral arterial disease. J Am Coll Cardiol 50: 2169–2171, 2007. doi: 10.1016/j.jacc.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Versluis B, Leiner T, Nelemans PJ, Wildberger JE, Schurink GW, Backes WH. Magnetic resonance imaging-based monitoring of collateral artery development in patients with intermittent claudication during supervised exercise therapy. J Vasc Surg 58: 1236–1243, 2013. doi: 10.1016/j.jvs.2012.11.136. [DOI] [PubMed] [Google Scholar]
- 54.Versluis B, Nelemans PJ, Wildberger JE, Schurink GW, Leiner T, Backes WH. Magnetic resonance imaging-derived arterial peak flow in peripheral arterial disease: towards a standardized measurement. Eur J Vasc Endovasc Surg 48: 185–192, 2014. doi: 10.1016/j.ejvs.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Brown MA, Tarn SH, Chan MC, Whitworth JA. Effects of diet on measurement of nitric oxide metabolites. Clin Exp Pharmacol Physiol 24: 418–420, 1997. doi: 10.1111/j.1440-1681.1997.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 56.Yokoi K, Adachi H, Hirai Y, Enomoto M, Fukami A, Ogata K, Tsukagawa E, Kasahara A, Imaizumi T. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population: the Tanushimaru study. Circ J 76: 2779–2784, 2012. doi: 10.1253/circj.CJ-12-0469. [DOI] [PubMed] [Google Scholar]