Abstract
A case is presented on a 40-year-old male with chronic lead poisoning with loss of consciousness, rhabdomyolysis, and acute renal failure after occupational exposure. Physical examination revealed generalized atrophy, tenderness, and swelling in the right limb and a decreased proximal muscle strength in the lower limb. A severe acute polyradiculoneuropathy in lower limbs documented by electromyography. All paraclinical tests were normal except increased blood lead level (75 μg/dl) and blue line in gum of the teeth. The patient was treated with penicillamine (500 mg q8 h) and pyridoxine (50 mg daily) for 8 months, only accessible drug in Iran. Force of patient's muscles in the proximal part of the lower limb was improved, and also the blood lead level reached to normal range. This is the first patient with rhabdomyolysis and muscle necrosis induced by lead poisoning.
Keywords: Case report, lead poisoning, muscle, necrosis, penicillamine, rhabdomyolysis
Introduction
Lead (Pb) as a heavy metal could disturb human health.[1] At present, the main sources of Pb exposure are restricted to an occupational setting.[2] According to occupational lead exposure is as an important health issue in Iran, the main employees are in higher risk of lead toxicity. The workers who work in these factories can be easily exposed to the dusts or fumes of lead.[3] In 2009, Keramati et al.[4] implemented an evaluation for blood lead level (BLL) on 105 workers in a car battery manufacturer in Mashhad, Iran. The mean BLL level of workers was 32.2 ± 13.7 μg/d, therefore in this study all workers suffered from lead poisoning.
Lead toxicity can usually happen due of chronic exposure.[1] Industrial workplaces are the main source for chronic lead exposure, which manifested mostly with neurological and psychiatric problem.[5] Previously has been reported that a significant lessening of sensory responses and cognitive impairment can occur in men workers with BLL ≥40 μg/100 ml.[6] Peripheral motor neuropathy that mostly involves distal upper limbs and reduced velocity of peripheral nerve conduction has also been presented in chronic lead toxicity.[7]
However, proximal muscle weakness is infrequent, and it can be usually presented with sudden interruption and massive lead exposure,[8] whereas promotion of rhabdomyolysis and consequently muscle necrosis by heavy metal except lead has been already described.[9] However, a relationship between toxic heavy metal accumulation and muscle necrosis is rarely reported.[10]
After a widespread literature search, clinical articles reporting the presence of rhabdomyolysis, muscle necrosis, and neuropathy in patients with lead poisoning were not found, and this is the first report where both conditions are documented in one patient. According to this point, early diagnosis, careful evaluation, and instant treatment are very imperative.[11] Therefore, it is necessary to be encounter with the rare presentation of chronic lead toxicity.
Case Report
A 40-year-old male with loss of consciousness was referred to neurology ward (AL-Zahra Hospital) after controlling for the elevation of creatine phosphokinase (CPK) (4050 U/L) and creatine (Cr) levels (4.9 mg/dL) due to rhabdomyolysis and acute renal failure in nephrology department. Hemodialysis approach was performed for him. In neurology service, he complained paresthesia in the upper limb, myalgia, cramp, and weakness in the lower limb without fasciculation. He worked in a battery manufacturing company and had no past medical and drug history or drug abuser.
On physical examination, tenderness and swelling in the right limb were detected. Given neurologic examination, the patient could not walk without aid, and generalized atrophy was seen without fasciculation. Force of muscle in upper limb both in proximal and distal was 5/5, in lower limb of the proximal part in the right side 1/5 and in left side 3/5, and in distal part was 0/5. Deep tendon reflex (DTR) was +2 in upper limb, +1 in left knee, 0 in left Achilles, and 0 in right knee and Achilles. Babinski was negative; stock sensory loss, abnormal position, and vibration sense in lower limb were detected.
Laboratory data showed CPK (1315 unit/L), lactate dehydrogenase (LDH) (1550 unit/L), increased levels of liver enzymes (aspartate aminotransferase: 75 unit/L, alanine aminotransferase; 88 unit/L), gamma-glutamyl transferase level (152 unit/L and minimally elevated blood urea nitrogen (41 mg/dL), Cr (1.8 mg/dL), and thyroid-stimulating hormone (21.4 mu/l). Vasculitis test and ganglioside antibodies panel, cerebrospinal fluid (CSF) analysis, Brucella antibodies, hepatitis B surface antigen, hepatitis C virus Ab, and HIV Ab were all normal. Moreover, the urine toxin screen test for morphine was positive (only one time) and in second and third time was negative. BLL (6 months before this symptom) was 75 μg/dL, and it was rechecked again after hemodialysis in hospital, and the BLL in three separate times was 30, 10, and 12 μg/dL. In addition, mild polychromasia, hypochrome, and anisocytosis in blood smear were reported. CBC analysis showed that elevated level of leukocytosis (white blood cell: 15,800/mm3 and anemia (red blood cell: 3.50Mil/mm3, Hb: 10.6 g/dL, Hct32.6%) was reported.
Electrophysiological test revealed a severe acute-subacute polyradiculoneuropathy with nonirritative proximal myopathy in lower limbs, without any evidence of compression neuropathy pattern. Magnetic resonance imaging (MRI) showed hyperintensity in the anterior and posterior compartment in both thighs (right > left) and also in paraspinal muscles in short-tau inversion recovery [Figure 1]. Thereafter, magnetic resonance neurography (MRN) was ordered, which showed multilobulated collection in the right gluteus muscle and right femur neck osteomyelitis; however, lumbosacral nerve roots were normal [Figure 2]. Normal Doppler sonography of limb and abdomen and pelvic sonography for rule out of deep venous thrombosis were performed which no evidence was found.
Figure 1.
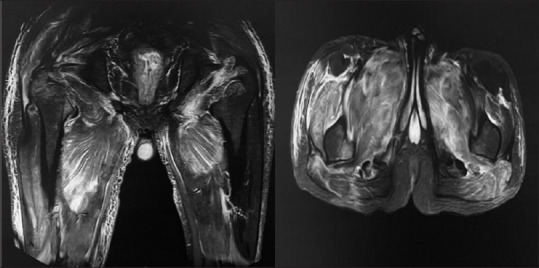
Magnetic resonance imaging with hyper intensity in anterior and posterior compartment in both hip and thigh (right > left) in short tau inversion recovery
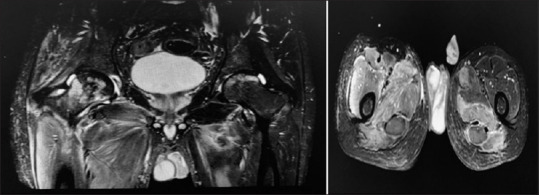
Figure 2.
Magnetic resonance neurography with multi located collection in right gluteus muscle and right femur neck osteomyelitis
First, with suspicious of autoimmune neuropathy, treatment was started with intravenous immunoglobulin (IVIG), but due to lack of efficacy and having allergy to drug, it was stopped. After, the first evaluation, with suspicious of nondiabetic polyradiculoneuropathy, the patient was treated with corticosteroids and due to MRN findings was referred to infectious service. After taking antibiotics without efficacy, corticosteroid regimen was stopped, and the patient was discharged.
Three months later, he was admitted again in our neurology ward. On neurological examination, he was wheelchair-bound, like before generalized atrophy was seen but without fasciculation. Force of muscles decreased as 3 months ago, and DTR was absent in both upper and lower limbs. Again, vasculitis test and CSF analysis were normal. The normal level of myositis-specific antibodies panel was measured. Erythrocyte sedimentation rate, C-reactive protein, and tuberculosis antibodies were normal. Hemoglobin levels were measured 12, 11, 10.3, and 9.7 g/dL in different times.
In new electromyography finding, a severe subacute lumbosacral polyradiculoneuropathy with proximal myopathy in the lower limb and paraspinal muscle was reported. Plasma exchanged was started without efficacy. In second lumbosacral MRI with Gadolinium enhancement of paraspinal muscles was reported [Figure 3]. In MRI of hip and thigh, hyperintensity in proximal muscles, particularly in the right side with avascular necrosis in the right femur neck, was noticed [Figure 4]. In addition, hyperintensity in anterior and posterior muscles in leg MRI was presented [Figure 5]. With these results, it was decided to take the patient to muscle biopsy, confirming the diagnosis. In muscle biopsy, severe necrosis was displayed, and in left sural nerve biopsy, a mild wall thickening in vessels without infiltration with no evidence of vasculitis was shown [Figure 6].
Figure 3.
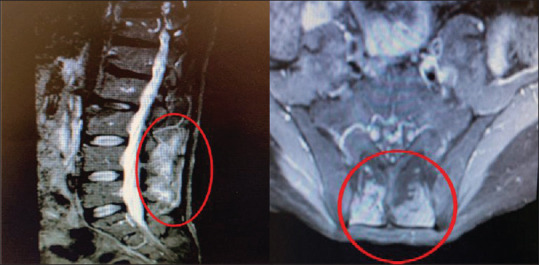
Magnetic resonance imaging with Gadolinium with enhancement of paraspinal muscles in second visit
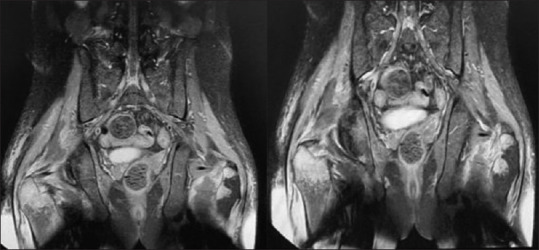
Figure 4.
Magnetic resonance imaging in hip, thigh with hyperintensity in proximal muscles particularly in right side with avascular necrosis in right femur neck in second visit
Figure 5.
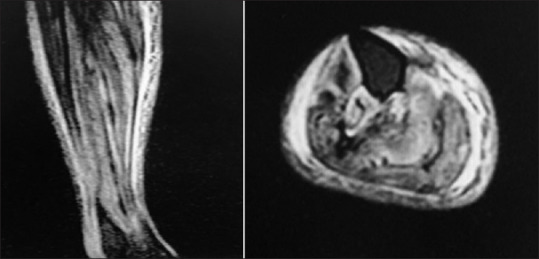
Magnetic resonance imaging in leg with hyperintensity in anterior and posterior muscles in leg
Figure 6.
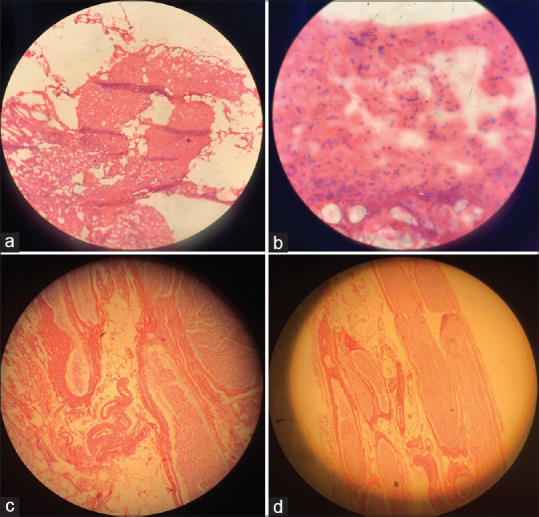
(a) Muscle biopsy right vastus lateralis with severe necrosis (a and b) and left sural nerve biopsy with mild wall thickening in vessels without infiltration (c and d)
All paraclinical tests for acute polyradiculoneuropathy such as ganglioside antibodies panel, CSF analysis, and vasculitis test and also for necrotizing myopathy such as myositis-specific antibodies panel were performed, and we did not find any etiology except increased BLL (30 μg/dl). In retrograde examination, blue line in gum of the teeth was detected [Figure 7], and the patient was referred to poisoning specialist, and penicillamine (500 mg q8h) with pyridoxine (50 mg daily) for 8 months was started for him. Penicillamine is the only accessible drug for lead intoxication in Iran. After treatment, the BLL reached to 20 μg/d. In follow-up examination, the force of muscles in the proximal part of the lower limb was mildly improved (+3/5 in both sides), but the force of distal muscles was not improved (0/5). Finally, the patient was advised to monthly follow-up of BLL and neurologic exams.
Figure 7.
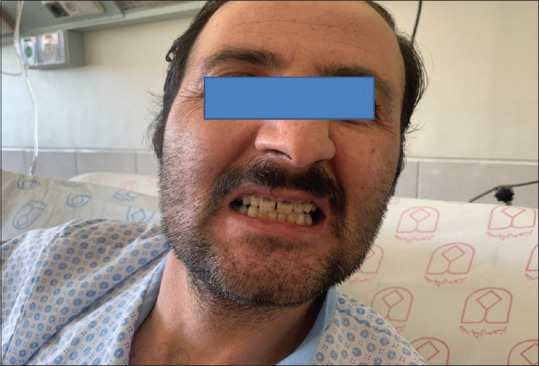
Facial image of patient showing blue line on gums
Discussion
Lead poisoning can present a broad range in its clinical manifestation and a variable intensity, mainly impressing on the blood levels of this metal. A case of concomitance of rhabdomyolysis, muscle necrosis, and neuropathy in patient with lead poisoning is presented. At the first admission, the patient had normal serum lead levels due to hemodialysis, and also we missed the diagnosis, and due to the symptoms with suspicious autoimmune problem, treatment was started with IVIG. Then, after no response to treatment, we evaluated all causes of acute neuropathy with rhabdomyolysis and concluded that lead-induced intoxication is a probable etiology.
Peripheral nervous system has been involved in adults with the reduced velocity of peripheral nerve conduction, weakness of the extensor muscles, myalgia, and muscle atrophy following lead poisoning.[12] However, proximal muscle weakness is an uncommon problem, and Srisuma et al. represented patient in severe lead poisoning from retained bullet fragments with proximal muscle weakness following abrupt and excessive lead exposure.[13] Also in anemic condition like our patient's condition due to more dissolving of lead in plasma, it could better penetrate to the target organs and cause more complications.[14]
Nevertheless, in our case, we observed necrotizing myopathy due to rhabdomyolysis in the lower limb with a positive toxin screen test. Two reasons could justify the worsening of this symptoms of lead poisoning in this case. First, he was probably addicted with lead-contaminated opium, and he has been consumed during the entire treatment period. Second, he worked in a battery manufacturing company.
Previously, a case series has been reported quadriplegia induced by lead contaminated opium.[15,16] It was suggested that, in chronic toxicity, a mild sensory and autonomic polyneuropathy was attributed to a direct neurotoxic effect. However, in our case, for ruling out of other causes of rhabdomyolysis, according to having an only one positive morphine test, we guess that the patient had consumed oral opiate and may be induced some parts of his problem. However, he did not report of addiction history and we could not confirm positive morphine test due to negative second and third morphine test. False positive results have been reported for opioids for a variety of reasons, including the use of many medications, foods and in patients at risk for lactic acidosis, as well as interference caused by lactate dehydrogenase and lactate.[17] it seems that urine sample containing high level of creatinine can encourage a “false-positive” result for opiate test,[18] we guess that, in the first report, we had a false-positive result, and following dialysis and decrease level of urine creatinine, negative urine morphine test was reported.
In the second issue, previous studies have been indicated that exposed workers with elevated lead concentrations did not display any clinical neuropathic abnormalities, regardless of mild electrophysiological abnormalities.[7] Most manifestations in industrial workers have been reported as motor deficiency and cognitive problems.[19] It seems that the potential effect of lead toxicity into the peripheral nervous system was considerably higher in our case than in those reported previously. Indeed, this difference could be explained with disregard for personal hygiene by workers and lack of wearing appropriate respiratory protection, gloves, and mask.
As mentioned, despite an extensive searching, no association or coexistence between peripheral neuropathy and muscle necrosis was found in patients with lead poisoning. Only one case reported myoglobinuria following gasoline sniffing.[20] In addition, proximal muscle weakness and polymyositis have been reported following occupational poisoning.[21] We conclude that, according to this point that, chronic lead toxicity for Iranian population due to existence sources of lead pollution in air, waters, soil, food should be concerned[22], hence in a patient with lead poisoning and myopathy symptoms, the differential diagnosis must be broad, and rhabdomyolysis and muscular necrosis should be considered.
Declaration of patient consent
The authors certify that they have obtained appropriate patient consent forms. In the form, the patient has given his consent for clinical information to be reported in the journal. He understood that his name will not be published and due efforts will be made to conceal his identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney.Int J Environ Res Public Health. 2019;16:274. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–22. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 3.Staudinger KC, Roth VS. Occupational lead poisoning. Am Fam Physician. 1998;57:719. [PubMed] [Google Scholar]
- 4.Keramati MR, Nemai Ghasemi M, Balali-Mood M. Correlation between iron deficiency and lead intoxication in the workers of a car battery manufacturer. J Birjand Univ Med Sci. 2009;16:51–8. [Google Scholar]
- 5.Mason LH, Mathews MJ, Han DY. Neuropsychiatric symptom assessments in toxic exposure. Psych Clin. 2013;36:201–8. doi: 10.1016/j.psc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Majchrzak M, Celiński R, Kowalska T, Sajewicz M. Fatal case of poisoning with a new cathinone derivative: α-propylaminopentiophenone (N-PP) Forensic Toxicol. 2018;36:525–33. doi: 10.1007/s11419-018-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubens O, Logina I, Kravale I, Eglîte M, Donaghy M. Peripheral neuropathy in chronic occupational inorganic lead exposure: A clinical and electrophysiological study. J Neurol Neurosurg Psychiatry. 2001;71:200–4. doi: 10.1136/jnnp.71.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson RM, Parry GJ. Neuropathies associated with excessive exposure to lead. Muscle Nerve. 2006;33:732–41. doi: 10.1002/mus.20510. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes PM, Davenport RJ. How to do it: Investigate exertional rhabdomyolysis (or not Pract neurol. 2019;19:43–8. doi: 10.1136/practneurol-2018-002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJ, de Visser M. Rhabdomyolysis: Review of the literature. Neuromuscular Disorders. 2014;24:651–9. doi: 10.1016/j.nmd.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Mason LH, Harp JP, Han DY. Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed Res Int. 2014:1–8. doi: 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu ML, Deng JF, Lin KP, Tsai WJ. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. Am J Med. 2013;126:451–4. doi: 10.1016/j.amjmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Srisuma S, Lavonas EJ, Wananukul W. Proximal muscle weakness in severe lead poisoning from retained bullet fragments. Clinical toxicology. 2015;53:586–7. doi: 10.3109/15563650.2015.1046182. [DOI] [PubMed] [Google Scholar]
- 14.Kazzi ZN. Critical care toxicology: Diagnosis and management of the critically poisoned patient. Ann Emerg Med. 2005;46:302–3. [Google Scholar]
- 15.Beigmohammadi MT, Aghdashi M, Najafi A, Mojtahedzadeh M, Karvandian K. Quadriplegia due to lead-contaminated opium--case report. Middle East J Anaesthesiol. 2008;19:1411–6. [PubMed] [Google Scholar]
- 16.Mesri M, Najari F, Baradaran Kayal I, Najari D. Hyper acute quadriplegia with chronic lead toxicity; a case report. Emergency. 2018;6:1–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Keary CJ, Wang Y, Moran JR, Zayas LV, Stern TA. Toxicologic testing for opiates: Understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14:51–8. doi: 10.4088/PCC.12f01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fucci N. EMIT dau: Methadone false positive with creatinine interference. Forensic Sci Int. 2005;148:81. doi: 10.1016/j.forsciint.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Gidlow D. Lead toxicity. Occupational Medicine. 2015;65:348–56. doi: 10.1093/occmed/kqv018. [DOI] [PubMed] [Google Scholar]
- 20.Moss M, Cooper P. Gasoline sniffing and lead poisoning. Acta Pharmacol Toxicol. 1986;59:48–51. doi: 10.1111/j.1600-0773.1986.tb02705.x. [DOI] [PubMed] [Google Scholar]
- 21.Becerra L, Colorado M, Molina J, Rivera A, Mesa M, Velásquez-Franco CJ, Muñoz-Grajales C. Co-existence of peripheral neuropathy secondary to lead poisoning and chronic polymyositis: Case report. Revista Colombiana de Reumatologí (English Edition) 2016;23:213–7. [Google Scholar]
- 22.Karrari P, Mehrpour O, Abdollahi M. A systematic review on status of lead pollution and toxicity in Iran; Guidance for preventive measures.DARU Journal of Pharmaceutical Sciences. 2012;20:20–37. doi: 10.1186/1560-8115-20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


