Abstract
Introduction
Obesity worsens clinical outcomes of coronavirus disease 2019 (COVID-19). The aim of this study was to measure the association between history of bariatric surgery and the severity of COVID-19.
Methods
Data source included PubMed/MEDLINE, Scopus, Google Scholar, and pre-print servers between January and November 1, 2020. Literature was screened and selected to extract the relevant data. The two outcomes of this meta-analysis were the difference in mortality and hospitalization rates in patients with SARS-CoV-2 infection with and without history of bariatric surgery. Random-effect models were used to estimate the pooled effects.
Results
The systematic review yielded 3 retrospective studies on 9022 patients. The risk of mortality without previous bariatric surgery was 133 per 1000 cases and its risk with previous bariatric surgery was 33 per 1000 (summary OR 0.22, 95% CI 0.19–0.26). No heterogeneity was observed between the included studies (I2 = 0%, P = 0.98 for heterogeneity). In the pooled analysis, the hospitalization rate in patients without previous bariatric surgery was 412 per 1000 cases and its rate in patients with previous bariatric surgery was 164 per 1000 (summary OR 0.28, 95% CI 0.12–0.65). No heterogeneity was observed between the included studies (I2 = 0%, P = 0.71 for heterogeneity). There was a substantial risk of bias across the studies for confounding and selection bias.
Conclusion
Findings of this meta-analysis of observational studies suggest that prior bariatric surgery is associated with a lower rate of mortality and hospital admission in patients with obesity who become infected with SARS-CoV-2. Confirmation of these findings will require larger studies with better quality data.
Keywords: Bariatric surgery, Metabolic surgery, Coronavirus, COVID-19, SARS-CoV-2, Mortality, Obesity
Introduction
There is a large body of evidence indicating obesity, diabetes, hypertension, and related cardiovascular conditions worsen the clinical outcomes of coronavirus disease 2019 (COVID-19) including a higher rate of hospitalization, need for mechanical ventilation, and mortality [1–3]. There are several physiological explanations for poor clinical outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with severe obesity. Obesity is a pro-coagulant and pro-inflammatory state which may increase the risk of thrombosis and exaggerated cytokines and oxidative stress response in the appropriate setting. Furthermore, obesity and diabetes can impair the innate and adaptive immune response to infections. Many patients with obesity and metabolic disease suffer from different cardiovascular, pulmonary, renal, and liver diseases that can worsen the clinical outcomes of COVID-19. In addition, mechanical effects of excess fat on the function of the chest wall, diaphragm, and lung may contribute to the severity of clinical status of patients with obesity after developing COVID-19 pneumonia [1–8].
Bariatric and metabolic surgery, by providing substantial and sustained weight loss in most patients, has been shown to reverse the negative mechanical and physiological impacts of obesity on almost all body systems and is associated with improved survival and quality of life in patients with severe obesity [9–13].
Studies are emerging in the medical literature suggesting that a history of previous bariatric surgery may be protective against severe form of SARS-CoV-2 infection since patients’ health status significantly improves following bariatric surgery [3, 14]. The aim of this systematic review and meta-analysis is to present available evidence on the effects of bariatric surgery on the severity of SARS-CoV-2 infection in patients with severe obesity and to estimate the association between history of bariatric surgery and the severity of COVID-19.
Methods
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Review of Interventions and The Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15, 16].
Search Strategy
An electronic search was conducted in PubMed/MEDLINE, Scopus, Google Scholar, and pre-print servers including AAS Open Res, arXiv, bioRxiv, medRxiv, OSF Preprints, ChemRxiv, F1000Res, Gates Open Res, HRB Open Res, MNI Open Res, PeerJ Preprints, Preprints.org, Research Square, and Wellcome Open Res on November 1, 2020. The following search terms were used: (“COVID” OR “SARS-CoV-2” OR “coronavirus”) AND (“bariatric” OR “RYGB” OR “gastric bypass” OR “sleeve”). Titles and abstracts of the yielded studies were screened for relevancy. The full texts of the relevant papers were accessed for eligibility. Furthermore, the reference lists of the eligible papers were reviewed manually for additional citations.
Data Extraction and Risk of Bias Assessment
Data on the variables of interest were extracted from all primary and secondary sources into the customized study spreadsheet for future analysis. Corresponding authors of included studies were contacted to obtain additional data and clarify any questions about their study’s methods if needed.
For each study, the risk of bias (critical, serious, moderate, or low risk of bias) was assessed on 7 domains using the ROBINS-I tool (Risk Of Bias In Non-randomized Studies—of interventions) [17, 18]. Within each domain, the risk of bias was independently assessed by the 2 authors.
Outcome Measures
The primary outcome of this meta-analysis was the difference in mortality rate in patients with SARS-CoV-2 infection with and without prior history of bariatric surgery. The secondary outcome was the difference in hospitalization rate in patients with SARS-CoV-2 infection with and without prior history of bariatric surgery.
Statistical Analysis
The pooled effect for mortality outcome was calculated based on raw numbers and the pooled effect for hospital admission was calculated based on the effect size from multivariate regressions. Forrest plots were generated and presented as the pooled effect results. Random-effect models were used to estimate the pooled effect with the Mantel-Haenszel method. The association between history of bariatric surgery and the study outcomes was quantified using the odds ratio (OR) and 95% confidence intervals (CIs). Statistical significance for the meta-analysis was set at P < 0.05.
Heterogeneity among study results was assessed using the I2 statistic, which is the percentage of variability in the effect sizes which is not caused by sampling error; small I2 statistics means low heterogeneity. Tau square statistics was also calculated, which is the inter-study variance in the meta-analysis, estimated from the random-effect model by using the Sidik-Jonkman method.
The summary of evidence table was created by using GRADEPro to assess the certainty of the evidence that bariatric surgery would be associated with a lower risk of mortality and hospitalization in patients with COVID-19 [19].
Results
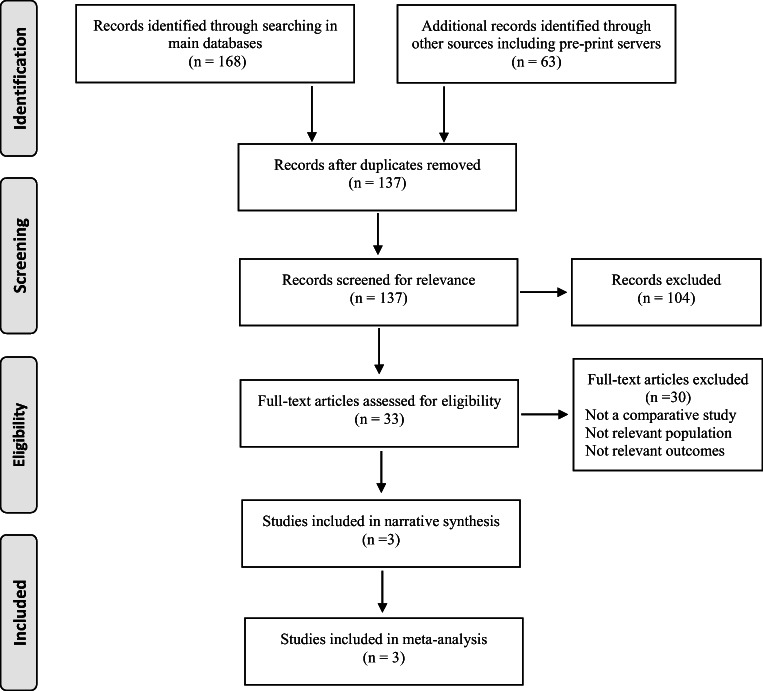
Our literature review yielded 3 retrospective studies [3, 20, 21] on 9022 unique patients including 590 patients who had a history of prior bariatric surgery (Fig. 1, Table 1).
Fig. 1.
Literature search to identify comparative studies on the role of bariatric surgery on clinical outcomes of COVID-19
Table 1.
Characteristics of 3 studies included in the meta-analysis
| Study location | Population | Study type | Data source | Analysis | Bariatric surgery, n | Non-surgery group, n | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Hospital admission | Mortality | Total | Hospital admission | Mortality | |||||
| Cleveland Clinic [3] | Severe obesity | Retrospective | Clinical | Propensity matching and multivariate regression | 33 | 6a | 0 | 330 | 139 | 8 |
| University of Minnesota [20] | NAFLD | Retrospective | Clinical | Multivariate regression | 16 | 4b | 0 | 357 | 144 | 14 |
| France [21] | Inpatients with obesity | Retrospective | Administrative | Multivariate regression | 541 | NA | 19 c | 7745 | NA | 1098 |
Reported odds ratio (OR) form multivariate regression in each original publication:
aOR 0.31 [95% CI 0.11–0.88], P = 0.028
bOR 0.22 [95% CI 0.05–0.98], P < 0.05
cOR 0.50 [95% CI 0.31–0.80], P = 0.0039
Cleveland Clinic Study
Among 4365 patients who tested positive for SARS-CoV-2 between March 8, 2020, and July 22, 2020, in the Cleveland Clinic Health System, 33 patients were identified who had a prior history of bariatric surgery. The surgical patients were propensity matched 1:10 to nonsurgical patients to assemble a cohort of control patients (n = 330) with a body mass index (BMI) ≥ 40 kg/m2 at the time of SARS-CoV-2 testing. The primary endpoint was the rate of hospital admission. In the univariate analysis, 6 (18.2%) patients in the bariatric surgery group and 139 (42.1%) patients in the control group were admitted to the hospital (P = 0.013). In the multivariate analysis, prior history of bariatric surgery was associated with a lower hospital admission rate compared with control patients with obesity (OR 0.31 [95% CI 0.11–0.88], P = 0.028). While none of the 4 exploratory outcomes occurred in the bariatric surgery group, 43 (13.0%) patients in the control group required ICU admission (P = 0.021), 22 (6.7%) required mechanical ventilation, 5 (1.5%) required dialysis, and 8 (2.4%) patients died [3].
In the surgical group, the median interval between the bariatric surgery and the positive viral test was 46 months (interquartile range, 29–85). Body mass index of patients decreased from 49.1 ± 8.8 kg/m2 at the time of bariatric surgery to 37.2 ± 7.1 kg/m2 at the time of SARS-CoV-2 test (paired difference of 12.6 kg/m2, P < 0.001). Nine (27.3%) patients had type 2 diabetes at the time of bariatric surgery including 4 patients on insulin therapy. At the time of the SARS-CoV-2-positive test, diabetes was in remission in 7 patients. Similarly, of the 21 (63.6%) patients who had hypertension at the time of bariatric surgery, 9 patients were not on antihypertensive medications at the time of a positive viral test. In patients with a history of hypertension, the median number of antihypertensive medications decreased from 2 at the time of bariatric surgery to 1 at the time of positive viral test (P = 0.001) [3].
University of Minnesota Study
This retrospective analysis of electronic medical record data of patients with a positive SARS-CoV-2 test from March 1, 2020, to August 25, 2020, showed that a history of nonalcoholic fatty liver disease (NAFLD) was associated with increased odds of hospital admission after SARS-CoV-2 infection. Among 373 patients with NAFLD, a subgroup of patients who had undergone bariatric surgery (n = 16) had significantly decreased odds of hospitalization (OR 0.22 [95% CI 0.05–0.98], P < 0.05) [20].
Study from France
This is a retrospective population-based study based on administrative data of all inpatient cases identified by the French National Health Insurance database. In total, 8286 patients with a diagnosis of obesity were admitted for SARS-CoV-2 infection between January 1, 2020, and May 15, 2020. In total, 541 had a history of bariatric surgery between 2010 and 2019. The need for an invasive mechanical ventilation and death occurred in 7% and 3.5% in the bariatric surgery group versus 15% and 14.2% in the non-bariatric surgery group, respectively (both P < 0.0001). The logistic regression analysis showed that prior bariatric surgery was associated with a lower risk of invasive mechanical ventilation (OR 0.67 [95% CI 0.48–0.95], P = 0.025) and mortality (OR 0.50 [95% CI 0.31–0.80], P = 0.004) [21].
Mortality Outcome
All 3 studies reported the mortality outcome [3, 20, 21]. The risk of mortality without previous bariatric surgery was 133 per 1000 cases and its risk with previous bariatric surgery was 33 per 1000 (summary OR 0.22, 95% CI 0.19–0.26, based on the random-effects meta-analysis). No heterogeneity was observed between the included studies (I2 = 0%, P = 0.98 for heterogeneity) (Fig. 2, Table 2).
Fig. 2.
Meta-analysis of association between history of prior bariatric surgery with mortality in patients with COVID-19. Data are presented as the odds ratios and 95% confidence interval (error bars). The area of the shaded squares is proportional to the study weight and the shaded diamonds represent pooled odds ratios and 95% confidence intervals
Table 2.
Summary of findings: association of prior bariatric surgery with mortality and hospitalization in patients with COVID-19
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk without bariatric surgery history | Risk with previous bariatric surgery | ||||
| Mortality | 133 per 1000 | 33 per 1000 (28 to 38) | OR 0.22 (0.19 to 0.26) | 9022 (3 observational studies) |
⨁⨁⨁◯ Moderate |
| Hospital Admission | 412 per 1000 | 164 per 1000 (78 to 313) | OR 0.28 (0.12 to 0.65) | 736 (2 observational studies) |
⨁⨁⨁◯ Moderate |
GRADE Working Group grades of evidence [19]:
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
CI, confidence interval; OR, odds ratio
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Hospital Admission Outcome
The Cleveland Clinic [3] and the University of Minnesota [20] studies independently reported a lower risk of hospitalization in patients with prior bariatric surgery after contracting SARS-CoV-2 infection. In the pooled analysis, the hospitalization rate in patients without previous bariatric surgery was 412 per 1000 cases and its rate in patients with previous bariatric surgery was 164 per 1000 (summary OR 0.28, 95% CI 0.12–0.65, based on the random-effects meta-analysis). No heterogeneity was observed between the included studies (I2 = 0%, P = 0.71 for heterogeneity) (Fig. 3, Table 2).
Fig. 3.
Meta-analysis of association between history of prior bariatric surgery with hospitalization in patients with COVID-19. Data are presented as the odds ratios and 95% confidence interval (error bars). The area of the shaded squares is proportional to the study weight and the shaded diamonds represent pooled odds ratios and 95% confidence intervals
Quality Assessment of Included Studies
The quality of the included studies was examined using the domain-based risk of bias assessment tool. There was a substantial risk of bias across the studies for confounding and selection bias (Fig. 4).
Fig. 4.
Risk of bias in 7 domains for 3 included studies based on the ROBINS-I tool
Discussion
The findings of this systematic review and meta-analysis of 3 observational studies on 9022 patients (including 590 patients who had history of prior bariatric surgery) indicate that history of bariatric surgery is associated with lower rates of hospitalization and mortality in patients with obesity after contracting SARS-CoV-2 infection.
A large meta-analysis of 75 studies has shown that obesity is a major risk factor for both contracting the SARS-CoV-2 infection and having poor clinical outcomes. Specifically, in the pooled analysis, obesity is associated with 46% greater risk of testing positive for SARS-CoV-2, 113% greater risk of hospitalization, 74% greater risk of being placed in the ICU, 66% greater risk of the need for invasive mechanical ventilation, and more importantly 48% greater risk of mortality in patients with COVID-19 [1]. The current meta-analysis suggests that prior history of bariatric surgery may mitigate the expected severity of COVID-19 in patients with obesity. In patients who had prior bariatric surgery, the odds of hospitalization was 72% lower than that in the control group (41% vs 16% favoring bariatric surgery group). Furthermore, the odds of mortality after contracting SARS-CoV-2 infection was 78% lower compared with that in the control group (13% vs 3% favoring bariatric surgery group).
Bariatric surgery leads to substantial and sustained weight loss and improves the metabolic profile of patients including hyperglycemia, hypertension, and dyslipidemia. Risk of cardiovascular disease, chronic kidney disease, fatty liver disease, and a certain type of cancer decreases after surgery. Eventually, bariatric surgery improves the quality of life and is associated with survival benefits in patients with severe obesity [9–13]. Furthermore, it has been shown that surgically induced weight loss can positively affect the immune system, level of cytokines, and inflammatory markers [22, 23]. Findings of the current study suggest that patients with obesity and metabolic disease become healthier following bariatric surgery and may be able to fight better with SARS-CoV-2 infection. If these findings are confirmed in future mechanistic and clinic studies, improved clinical outcomes of COVID-19 can be added to the long list of health benefits of bariatric surgery [3].
This study is the first meta-analysis reporting the possible protective effects of bariatric surgery in patients with COVID-19 infection. Since this study was conducted based on the data originated from the early phase of the COVID-19 pandemic, there are several limitations. Due to the small number of studies and lack of high-quality data, any strong conclusion on the protective effects of bariatric surgery after contracting SARS-CoV-2 infection in patients with obesity seems premature. At the time of conducting this meta-analysis, the study from the University of Minnesota [20] was available in pre-print version and its peer-review process had not been completed yet. Although the amount of heterogeneity across the 3 studies was small, there was a substantial risk of bias in quality metrics including the risk of confounding and selection bias across the included studies. Furthermore, there is always a risk of selective reporting or of publication bias in these types of studies. The definitions and reporting of other outcomes indicating the severity of COVID-19 such as the need for supplemental oxygen or mechanical ventilation were not consistent across the studies and therefore, a meta-analysis for these endpoints was not conducted. In the analysis of mortality outcome, the study from France contributed to 98.9% of the weight, although there was zero inconsistency between the effects of prior bariatric surgery on mortality estimated by the included studies. Considering these limitations, the findings of this meta-analysis should be interpreted with caution and considered hypothesis-generating.
In conclusion, the findings of this meta-analysis of observational studies suggest that prior bariatric surgery is associated with a lower rate of mortality and hospital admission in patients with obesity who become infected with SARS-CoV-2. Confirmation of these findings will require larger studies with better quality data.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval Statement
For this type of study, formal consent or ethical approval is not required.
Informed Consent Statement
Informed consent does not apply to this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain A, Mahawar K, Xia Z, et al. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;S1871-403X(20):30550–0. 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Retracted]
- 3.Aminian A, Fathalizadeh A, Tu C, et al. Association of prior metabolic and bariatric surgery with severity of COVID-19 in patients with obesity. Surg Obes Relat Dis Epub ahead of print. 2021; 10.1016/j.soard.2020.10.026. [DOI] [PMC free article] [PubMed]
- 4.Caci G, Albini A, Malerba M, et al. COVID-19 and obesity: dangerous liaisons. J Clin Med. 2020;9(8):E2511. [DOI] [PMC free article] [PubMed]
- 5.Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med (Lond). 2020;20(4):e109–e113. doi: 10.7861/clinmed.2020-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):dgaa346. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rottoli M, Bernante P, Belvedere A, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. 2020;1:EJE-20–0541.R2. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safari S, Keyvani H, Alamdari NM, et al. Abdominal surgery in patients with COVID-19: detection of SARS-CoV-2 in abdominal and adipose tissues. Ann Surg. 2020;272:e253–e256. doi: 10.1097/SLA.0000000000004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. 2020;383(16):1535–1543. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminian A, Nissen SE. Success (but unfinished) story of metabolic surgery. Diabetes Care. 2020;43(6):1175–1177. doi: 10.2337/dci20-0006. [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169(5):300–310. doi: 10.7326/M17-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322(13):1271–1282. doi: 10.1001/jama.2019.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uccelli M, Cesana GC, Ciccarese F, et al. COVID-19 and obesity: postoperative risk in patients who have undergone bariatric surgery. preliminary report from high volume center in Italy (Lombardy) Obes Surg. 2020;30(12):5119–5122. doi: 10.1007/s11695-020-04792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: The Cochrane Collaboration, 2011.; 2011.
- 16.Peters JP, Hooft L, Grolman W, et al. Reporting quality of systematic reviews and meta-analyses of otorhinolaryngologic articles based on the PRISMA statement. PLoS One. 2015;10:e0136540. [DOI] [PMC free article] [PubMed]
- 17.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020; 10.1002/jrsm.1411. [DOI] [PubMed]
- 19.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). Available from gradepro.org.
- 20.Bramante C, Tignanelli CJ, Dutta N, et al. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv [Preprint]. 2020 Sep 2:2020.09.01.20185850. 10.1101/2020.09.01.20185850.
- 21.Iannelli A, Bouam S, Schneck AS, et al. The impact of previous history of bariatric surgery on outcome of COVID-19. A Nationwide Medico-Administrative French Study. Obes Surg. 2020:1–9. 10.1007/s11695-020-05120-z. [DOI] [PMC free article] [PubMed]
- 22.Bhatt DL, Aminian A, Kashyap SR, et al. Cardiovascular biomarkers after metabolic surgery versus medical therapy for diabetes. J Am Coll Cardiol. 2019;74(2):261–263. doi: 10.1016/j.jacc.2019.04.058. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Zhang J, Liu W, Chen X, Liu Z, Zhou Z. Improvements in humoral immune function and glucolipid metabolism after laparoscopic sleeve gastrectomy in patients with obesity. Surg Obes Relat Dis. 2019;15(9):1455–1463. doi: 10.1016/j.soard.2019.05.021. [DOI] [PubMed] [Google Scholar]