Abstract
Yield losses caused by pests, including aphids, can be substantial in cereals. Breeding for resistance against aphids is therefore desirable for enhancing the economic and environmental sustainability of cereal production. The aim of our study was to reveal the degree of antibiosis against Metopolophium dirhodum (Walker) (Homoptera: Aphididae), in four cultivars of spring wheat, Triticum aestivum L. (‘Alicia’, ‘Odeta’, ‘Libertina’, ‘Astrid’), and two cultivars of emmer, Triticum turgidum ssp. dicoccum (Schrank ex Schübler) Thell. (‘Rudico’, ‘Tapiruz’) (both Poales: Poaceae) under controlled laboratory conditions. Using age-stage, two-sex life table, we quantified responses of M. dirhodum to each cultivar and to project population growth. The spring wheat and emmer cultivars varied in their suitability to M. dirhodum. The cultivar most susceptible to M. dirhodum was the emmer cultivar ‘Rudico’; the projected population size of M. dirhodum on this cultivar was one order of magnitude larger than those on other cultivars. The most resistant cultivar was the spring wheat cultivar ‘Libertina’. Since emmer is commonly used as a gene source for breeding T. aestivum, we advocate that care be taken to avoid the transmission of genes responsible for suitability to aphids from emmer to T. aestivum.
Keywords: plant resistance, cultivar suitability, aphids, age-stage, two-sex life table, population projection
Wheat (Triticum aestivum L.) is one of the three major grain crops worldwide (Deutsch et al. 2018). It is infested by an array of insect pests that can cause significant yield reductions and increase costs of crop production (Foster et al. 2017), if the economic injury level is exceeded (Pedigo et al. 1986). Aphids (Homoptera: Aphididae) are one of the most important groups of cereal pests (van Emden and Harrington 2017). They typically exhibit rapid development and a high reproductive rate due to parthenogenetic reproduction and telescoping generations (Dixon 2012). Infestation by aphids negatively influences crop yield (Papp and Mesterházy 1996, Klueken et al. 2008), so the control of aphid populations is necessary. Aphids feed on phloem fluids by piercing their stylets through the plant tissues (Dixon 2012); thus, they are harmful not only due to the removal of the nutrients but also due to the release of salivary toxins into the plant, which cause necrosis of the tissues (Nicholson et al. 2012, Kettles and Kaloshian 2016). Furthermore, they can also be vectors of plant viruses, including the barley yellow dwarf virus, which may cause damage ranging from slight to rapid death of the whole plant (Brault et al. 2010, Dedryver et al. 2010, Stevens and Lacomme 2017). Yield losses caused by aphids can be substantial and can exceed 60% under massive infestation (Papp and Mesterházy 1993, Belay and Araya 2015).
Synthetic chemical pesticides are the most commonly employed means of controlling aphids in cereals (Dedryver et al. 2010, Dewar and Denholm 2017), but their use increases the costs of production and poses environmental risks (Biondi et al. 2012, Pisa et al. 2015, Özgökçe et al. 2018). The selection of pest-resistant cultivars may lead to reductions in pesticide use and provide economic benefits to the farmer, even if only a moderate level of resistance is achieved, which would allow reduced frequencies or dosages of pesticide applications (Smith 2005). Insect-resistant cultivars may represent an alternative and allow sustainable crop production because of the lack of health and environmental issues associated with their usage (Özgökçe and Atlıhan 2005).
Resistance to insect pests is manifested via multiple mechanisms. It is usually classified as antixenosis (plant attractiveness to herbivores), antibiosis (herbivore performance), and tolerance (compensation of the plant for herbivore feeding) (Smith 2005). Like other plants, cereals have natural mechanisms of resistance to pests, including traits such as a leaf morphology (Roberts and Foster 1983, Papp and Mesterházy 1993, He et al. 2011, Dixon 2012, Saska et al. 2020) and secondary plant metabolites (Niemeyer et al. 1992; Elek et al. 2013, 2014) that can be affected by aphid feeding (Chandrasekhar et al. 2018) and play important roles in the resistance against aphids.
Breeding for increased resistance against aphids is desirable for enhancing the economic and environmental sustainability of growing cereals. Screening cultivars and genetic lineages for resistance to aphids should be the first step of this process. Variability in resistance to aphids has been shown among different cultivars of a same host plant species, including cereals (Papp and Mesterházy 1996, Migui and Lamb 2004, Klueken et al. 2008, Hu et al. 2016, Girvin et al. 2017, Ajmal et al. 2018, Chandrasekhar et al. 2018). Ancient taxa of the genus Triticum, including einkorn (Triticum monococcum L.), durum wheat (Triticum turgidum (L.) ssp. durum (Desf.) Husn.), emmer (Triticum turgidum ssp. dicoccum (Schrank ex Schübler) Thell.), wild emmer (Triticum turgidum ssp. dicoccoides (Körn. ex Asch. & Graebner) Thell.), and spelt (Triticum aestivum (L.) ssp. spelta (L.) Thel..), provide a rich but underutilized source of genes for wheat breeding (Mesfin et al. 2000; Nevo 2001; Avni et al. 2014, 2017); however, knowledge of the resistance of these varieties to aphids is limited.
Although it has been suggested that the suitability of crops for pests increased with domestication (Sotherton and van Emden 1982; Wise et al. 2001; Lage et al. 2004; Chen et al. 2015a,b), this does not seem to always be the case for cereals. Analyzing fecundity of Rhopalosiphum maidis (Fitch) after 4 d of aphid infestation, Chandrasekhar et al. (2018) showed that wild emmer was more susceptible to this aphid than durum wheat (T. turgidum ssp. durum (Desf.) Husnot). Subsequent work using the same cultivars as in Chandrasekhar et al. (2018) and one bread wheat cultivar revealed increased fecundity of Rhopalosiphum padi (Linnaeus) under both of the tetraploid wheat genotypes than the bread wheat, indicating that the former were more susceptible to aphids (Batyrshina et al. 2020). Conversely, Migui and Lamb (2004) observed partial resistance to aphid infestation, as indicated by decreased fecundity of Sitobion avenae Fabricius, in a diploid einkorn compared with tetraploid and hexaploid species of Triticum. The resistance they observed was generally plant species-specific and antibiosis was negatively associated with ploidy level (Migui and Lamb 2003). Kazemi and van Emden (1992) proposed partial resistance of emmer compared with hexaploid UK varieties based on lowered fecundity of R. padi at ear emergence, although high susceptibility to aphid infestation was observed at the tillering stage. The only assessment of differences in antibiosis between modern and emmer wheat varieties to date, based on estimates of population growth of two aphid species, was conducted by Sotherton and van Emden (1982). Emmer exhibited some resistance properties and was of intermediate suitability for S. avenae and Metopolophium dirhodum (Walker) among the cultivars used. The results to date are not conclusive and are based on incomplete data on aphid performance.
In this study, we investigate the level of antibiosis to a serious aphid pests that attacks the leaves of most cereal species, M. dirhodum (Honek et al. 2018), among six cultivars of two species of the genus Triticum to identify aphid-resistant cultivars promising for future breeding. We apply age-stage, two-sex life table theory (Chi and Liu 1985, Chi 1988, Chi et al. 2020) to assess the population development of aphids. This approach has proven robust for evaluating aphid population dynamics (Saska et al. 2016), including the level of antibiosis among cereal cultivars (Saska et al. 2020).
Materials and Methods
Host Plant Cultivars
This experiment forms a part of a larger project aimed at evaluating selected commercial cultivars for future breeding. The selected cultivars are commercially used in the Czech Republic, and in further experiments, they will be subjected to various biotic and abiotic conditions. Four cultivars of T. aestivum (‘Alicia’, ‘Odeta’, ‘Libertina’, and ‘Astrid’) and two cultivars of T. turgidum subsp. dicoccum (‘Tapiruz’ and ‘Rudico’) were chosen as host plants (Supp Table 1 [online only]). The seed was not treated.
Plant Cultivation
Plants were prepared throughout the experiment using a standard protocol. Eight plastic 250-ml pots were employed for each cultivar. The experimental soil consisted of Alfisol and garden substrate in ratio 8:1. Ten seeds per pot were allowed germinate in a growth chamber under the controlled conditions of 16-h day (irradiation intensity of 450 μmol/m2/s) and 8-h night, and temperature of 20°C. The pots were covered with a transparent plastic foil for the first 2–3 d. Plants with fully developed second leaf (Zadoks 12) were used to initiate the experiment.
Aphids
A laboratory strain of M. dirhodum (accession ent019 from the collection at the Crop Research Institute, Prague, collected in Boh. centr., Prague-Ruzyně, 1994, H. Havlíčková lgt.) maintained on wheat in a greenhouse under a controlled temperature (20 ± 1°C) and a natural photoperiod, was used in this experiment.
Design of the Life Table Study
Life table data were collected using a group design (Chang et al. 2016), using eight replicated pots per cultivar. The experimental conditions inside the climatic chambers were constant temperature of 21°C and a long-day photoperiod (16:8 [L:D] h). Ten adult apterous aphids were allowed to produce nymphs overnight and removed the day after. Only 10 new-born aphids per pot were retained, one per plant. Host plants with aphids were isolated within a transparent polyethylene tube. The number of aphids and their instar were recorded every day. Molting to a new instar was determined by a present of exuviae (removed upon finding) and noticeable increase in body length, and by a presence of cauda in the case of adult females. Upon reaching adulthood, offspring were counted and removed daily. To facilitate handling and because of space limitation inside the climatic chambers, the aphids were transferred to new plants (prepared as described above) every seven days.
Analysis
The age-stage, two-sex life table (Chi and Liu 1985, Chi 1988) was used for the analysis of the life table in the computer program TWOSEX-MSChart (Chi 2020b), taking into account stage differentiation, the variable developmental rate among individuals and sex to accurately describe the development, survival, and reproduction of insect populations (Chi et al. 2020). This method is suitable also for parthenogenetic species (Huang and Chi 2012, Tuan et al. 2016). The group-based life table data were adapted into individual-based life tables (Saemi et al. 2017), based on which the life table and population parameters were calculated (Chi and Liu 1985; Chi 1988; Chi and Su 2006; Tuan et al. 2014a,b; Chang et al. 2016). The SEs of the parameters were estimated using bootstraps with 100,000 re-samplings (Polat-Akköprü et al. 2015). Between-cultivar comparisons of all parameters were then made using a bootstrap paired test (Efron and Tibshirani 1993, Mou et al. 2015). The program TIMING-MSChart (Chi 2020a) was used to project the population growth, and 95% CIs of the projected population size after 35 d were estimated following Huang et al. (2018).
Results
Aphid Life Table
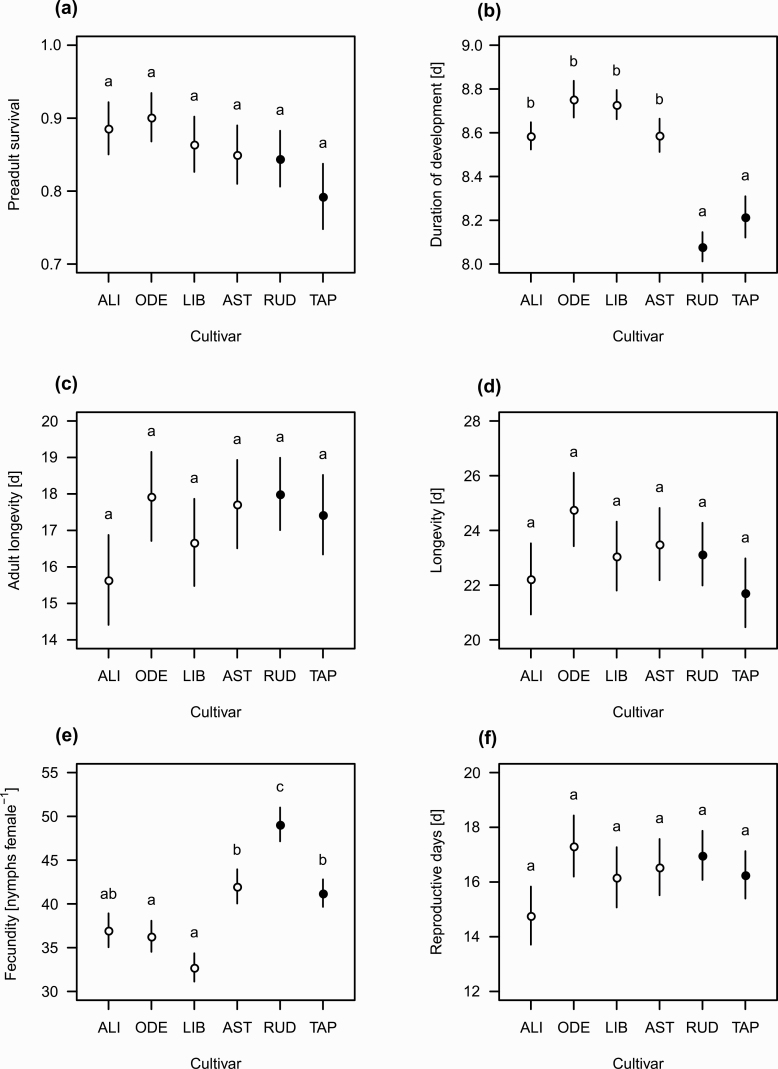
The spring wheat and emmer cultivars varied in their suitability for M. dirhodum. Mean survival of the nymphs from the initial cohorts ranged between 79 and 90%, but the differences among cultivars were not significant (Fig. 1a). Because of variable duration of development, nymphal stage overlapped with the adult stage, as shown by the age-stage-specific survival rate (sxj) values (Supp Fig. 1 [online only]). The maximum difference in the mean duration of nymphal development between any two cultivars was 0.67 d, and aphids developed significantly faster on the two emmer cultivars than on the four cultivars of spring wheat (Fig. 1b). Adult longevity and total longevity did not significantly differ among the cultivars (Fig. 1c and d).
Fig. 1.
The life table and population growth parameters for Metopolophium dirhodum reared on four cultivars of Triticum aestivum (open circles: Alicia [ALI], Odeta [ODE], Libertina [LIB], Astrid [AST]) and two cultivars of Triticum turgidum subsp. dicoccum (closed circles: Rudico [RUD], Tapiruz [TAP]). Vertical bars represent s.e. estimated with 100,000 bootstrap resamplings. Cultivars assigned with the same letters were significantly not different from each other (the paired bootstrap test). The values ± SE of all these parameters are presented in Supp Table 2 (online only).
Fecundity differed by 16.34 nymphs per female between the cultivars most and least resistant to aphids (Fig. 1e), being highest on Rudico and lowest on Libertina, but the duration of the reproductive period did not differ significantly among the cultivars (Fig. 1f). The age-specific survival rates (lx), age-specific fecundities (mx), net maternities (lxmx), and age-stage-specific life expectancies (exj) are shown for particular cultivars in Supp Figs. 2 and 3 (online only). The age-stage reproductive value (vxj) was highest at a similar age for all cultivars, with emmer cv. Rudico reaching higher values than the others (Supp Fig. 4 [online only]).
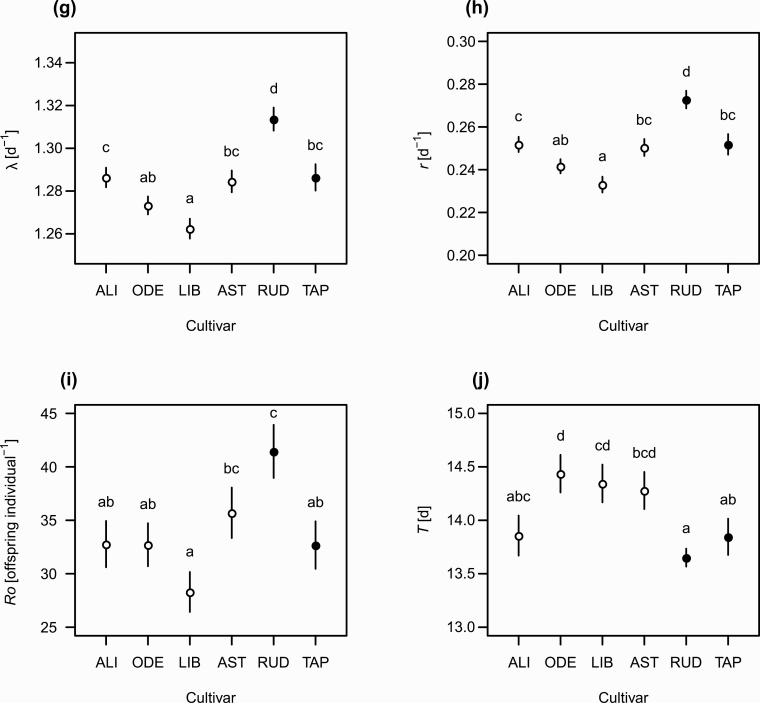
Population growth of M. dirhodum was rapid on all host cultivars, as shown by the values of the finite rate of increase, λ (Fig. 1g), and intrinsic rate of increase, r (Fig. 1h). Both parameters were significantly highest for Rudico. The other emmer cultivar was similar to those of the T. aestivum cultivars. The net reproductive rate, R0 (Fig. 1i), significantly differed among cultivars, being highest on Rudico (41.44 nymphs per female) and lowest on Libertina (28.3 nymphs per female). The mean generation time (T) significantly differed among cultivars (Fig. 1j), being shortest on Rudico and longest on Odeta.
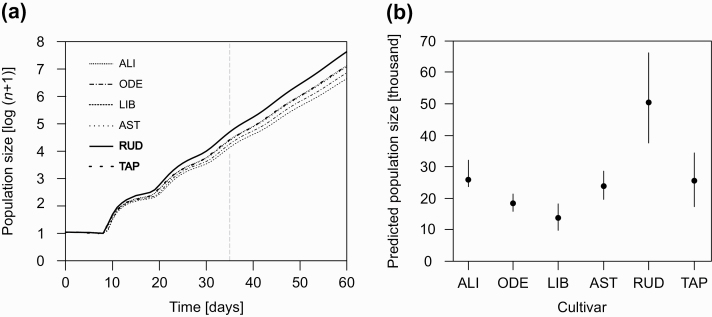
Variable resistance resulted in prominent cultivar differences in the population size projected for 35 d (Fig. 2a). The population size was projected to be considerably larger for the least resistant cultivar (Rudico) and ca. 10 times greater for this cultivar than for the most resistant cultivar (Libertina). The projected population size was approximately 50.5 thousands individuals for Rudico, 25.6 thousands for Tapiruz, 26 thousands for Alicia, 23.8 thousands for Astrid, 18.4 thousands for Odeta, and only 13.7 thousands for Libertina (Fig. 2b). The population growth curves, on a logarithmic scale, approach linearity after ca. 35 d (Fig. 2a), suggesting that the populations would approach a stable age-stage distribution in the case of unlimited resources.
Fig. 2.
The population projections (log10[n+1]) of Metopolophium dirhodum reared on four cultivars of Triticum aestivum (Alicia [ALI], Odeta [ODE], Libertina [LIB], Astrid [AST]) and two cultivars of Triticum turgidum subsp. dicoccum (Rudico [RUD], Tapiruz [TAP]), based on age-stage, two-sex life table theory. (a) The predicted course of population growth for particular cultivars. The relationships for d ≥ 35, after the stable age-stage distribution was reached, and projected population size for the individual cultivars. Alicia: (log10[n+1]) = 0.5803 + 0.1088d; Odeta: (log10[n+1]) = 0.602 + 0.1040d; Libertina: (log10[n+1]) = 0.612 + 0.1003d; Astrid: (log10[n+1]) = 0.5747 + 0.1081d; Rudico: (log10[n+1]) = 0.5528 + 0.1179d; Tapiruz: (log10[n+1]) = 0.5643 + 0.1090d. (b) Projected population size ± 95% CIs after 35 d.
Discussion
Our experiment demonstrated variable degrees of antibiosis of young spring wheat and emmer cultivars to M. dirhodum, consistent with the outcomes of previous studies. Of the cultivars included in our study, spring wheat Libertina was the least susceptible for aphid development and reproduction, while the emmer cultivar Rudico was the most susceptible one.
The projected population size of M. dirhodum after 35 d was two to three times larger on the emmer cultivar Rudico than on the other cultivars. The high intrinsic rate of increase on this cultivar was caused by the very short developmental time and high fecundity of the aphids. The other emmer cultivar used, Tapiruz, yielded a projected population size comparable to those of the susceptible spring wheat cultivars. Our results are supported by those of Kazemi and van Emden (1992) and Migui and Lamb (2003, 2004) and indicate that some emmer cultivars, at least during early phases of growth, can be highly susceptible to other species of aphids. Other studies found that emmer was more resistant to aphids than modern wheat cultivars (Sotherton and van Emden 1982); however, as these studies were based on incomplete data, their results are not fully comparable with ours. Our study is the first study to follow the entire life table of an aphid on emmer.
However, care is warranted in drawing conclusions for several reasons. Different levels of antibiosis can be exhibited against a particular aphid species, as shown for Sitobion avenae (Fabricius) and M. dirhodum (Sotherton and van Emden 1982), where differences in antibiosis between cultivars were generally smaller for M. dirhodum than for other species studied. Susceptibility to aphids also varies with the growth stage of the crop (Kazemi and van Emden 1992, Klueken et al. 2008), so differences among studies can result from study differences in the phenological stage of the plant. In a comparison of ancient and modern varieties, the level of antibiosis in ancient varieties did not differ among the growth stages, whereas modern varieties showed a decreased level of antibiosis at ear emergence (Sotherton and van Emden 1982). The use of combinations of different cultivars of host plants in the field can significantly reduce aphid occurrence (Ninkovic et al. 2002). To determine whether a crop cultivar is resistant to aphids, testing is required under changing temperature and humidity conditions similar to those in the field (Atlıhan et al. 2017) and during different seasons in field conditions (Hu et al. 2016). Such testing is important because interannual variability in antibiosis has been reported (Hu et al. 2016). Assessment of plant tolerance to aphids is also important (Havlíčková 1997, Hu et al. 2016).
In conclusion, among the studied cultivars, the emmer cultivar Rudico was the most susceptible to aphids. The most promising resistance source appears to be the spring wheat cultivar Libertina, which was significantly more unfavorable for aphids than the other cultivars. Although more resistant cultivars may not assure that the pest populations will be always kept below the economic injury levels, it at least provides a less favorable environment for the aphid pests, with respect to the population growth rate (Atlıhan et al. 2017). Emmer is a rich genetic resource for wheat improvement that has been extensively studied (Nevo 2001) but might have weak potential to resist pests, especially during the early growth stage (Sotherton and van Emden 1982, Chandrasekhar et al. 2018, Batyrshina et al. 2020; this study). We therefore advocate that great care be taken to avoid the transmission of genes responsible for suitability to aphids from emmer, as the gene source, to T. aestivum.
Supplementary Material
Acknowledgments
This study was supported by the National Agency for Agricultural Research (NAZV), project QK1910041 (J.S., P.S.) and Czech Science Foundation (GAČR), project 18-13174J (H.P.). We thank Helena Uhlířová and Hana Smutná for their laboratory assistance, and Nela Gloríková for preparing the life table figures.
References Cited
- Ajmal, M S, Iqbal J, Qayyum M A, Saleem M A, Tayyab M, and Sajjad M. 2018. Preferential influence of wheat varieties (Triticum aestivum L.) on population build-up of aphid (Homoptera: Aphididae) and its natural enemies. J. Entomol. Zool. Stud. 6: 609–612. [Google Scholar]
- Atlıhan, R, Kasap İ, Özgökçe M S, Polat-Akköprü E, and Chi H. 2017. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol. 110: 1890–1898. [DOI] [PubMed] [Google Scholar]
- Avni, R, Nave M, Eilam T, Sela H, Alekperov C, Peleg Z, Dvorak J, Korol A, and Distelfeld A. 2014. Ultra-dense genetic map of durum wheat × wild emmer wheat developed using the 90K iSelect SNP genotyping assay. Mol. Breed. 34: 1549–1562. [Google Scholar]
- Avni, R, Nave M, Barad O, Baruch K, Twardziok S O, Gundlach H, Hale I, Mascher M, Spannagl M, Wiebe K, et al. 2017. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science. 357: 93–97. [DOI] [PubMed] [Google Scholar]
- Batyrshina, Z S, Yaakov B, Shavit R, Singh A, and Tzin V. 2020. Comparative transcriptomic and metabolic analysis of wild and domesticated wheat genotypes reveals differences in chemical and physical defense responses against aphids. BMC Plant Biol. 20: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay, T, and Araya A. 2015. Grain and biomass yield reduction due to Russian wheat aphid on bread wheat in northern Ethiopia. Afr. Crop Sci. J. 23: 197–202. [Google Scholar]
- Biondi, A, Mommaerts V, Smagghe G, Viñuela E, Zappalà L, and Desneux N. 2012. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 68: 1523–1536. [DOI] [PubMed] [Google Scholar]
- Brault, V, Uzest M, Monsion B, Jacquot E, and Blanc S. 2010. Aphids as transport devices for plant viruses. C. R. Biol. 333: 524–538. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar, K, Shavit R, Distelfeld A, Christensen S A, and Tzin V. 2018. Exploring the metabolic variation between domesticated and wild tetraploid wheat genotypes in response to corn leaf aphid infestation. Plant Signal. Behav. 13: e1486148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C, Huang C-Y, Dai S-M, Atlıhan R, and Chi H. 2016. Genetically engineered ricin suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on demographic analysis of group-reared life table. J. Econ. Entomol. 109: 987–992. [DOI] [PubMed] [Google Scholar]
- Chen, Y H, Gols R, and Benrey B. 2015a. Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 60: 35–58. [DOI] [PubMed] [Google Scholar]
- Chen, Y H, Gols R, Stratton C A, Brevik K A, and Benrey B. 2015b. Complex tritrophic interactions in response to crop domestication: predictions from the wild. Entomol. Exp. Appl. 157: 40–59. [Google Scholar]
- Chi, H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17: 26–34. [Google Scholar]
- Chi, H. 2020a. TIMING-MSChart: a computer program for the population projection based on age-stage, two-sex life table. National Chung Hsing University, Taichung, Taiwan: (http://140.120.197.173/Ecology/) [Google Scholar]
- Chi, H. 2020b. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. National Chung Hsing University, Taichung, Taiwan: (http://140.120.197.173/Ecology/) [Google Scholar]
- Chi, H and Liu H. 1985. Two new methods for the study of insect population ecology. Bull Inst Zool, Acad. Sinica. 24: 225–240. [Google Scholar]
- Chi, H, and Su H Y. 2006. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35(1): 10–21. [Google Scholar]
- Chi, H, You M, Atlihan R, Smith C L, Kavousi A, Özgökçe M S, Güncan A, Tuan S-J, Fu J-W, Xu Y-Y, et al. 2020. Age-stage, two-sex life table: an introduction to theory, data analysis, and application. Entomol. Gen. 40: 103–124. [Google Scholar]
- Dedryver, C A, Le Ralec A, and Fabre F. 2010. The conflicting relationships between aphids and men: a review of aphid damage and control strategies. C. R. Biol. 333: 539–553. [DOI] [PubMed] [Google Scholar]
- Deutsch, C A, Tewksbury J J, Tigchelaar M, Battisti D S, Merrill S C, Huey R B, and Naylor R L. 2018. Increase in crop losses to insect pests in a warming climate. Science. 361: 916–919. [DOI] [PubMed] [Google Scholar]
- Dewar, A M, and Denholm I. 2017. Chemical control, pp. 398–425. Invan Emden H F and Harrington R. (eds.), Aphids as crop pests, 2nd edn. CABI, Wallingford, United Kingdom. [Google Scholar]
- Dixon, A F G. 2012. Aphid ecology. Springer Science & Business Media, New York. [Google Scholar]
- Efron, B, and Tibshirani R J. 1993. An introduction to the bootstrap. Chapman and Hall, N ew York [Google Scholar]
- Elek, H, Smart L, Martin J, Ahmad S, Gordon-Weeks R, Welham S, Nadasy M, Pickett J A, and Werner C P. 2013. The potential of hydroxamic acids in tetraploid and hexaploid wheat varieties as resistance factors against the bird-cherry oat aphid, Rhopalosiphum padi. Ann. Appl. Biol. 162: 100–109. [Google Scholar]
- Elek, H, Smart L, Ahmad S, Anda A, Werner C P, and Pickett J A. 2014. A comparison of the levels of hydroxamic acids in Aegilops speltoides and a hexaploid wheat and effects on Rhopalosiphum padi behaviour and fecundity. Acta Biol. Hung. 65: 38–46. [DOI] [PubMed] [Google Scholar]
- van Emden, H F and Harrington R. 2017. Aphids as crop pests, 2nd edn. CABI, Wallingford, United Kingdom. [Google Scholar]
- Foster, S P, Devine G, and Devonshire A E. 2017. Insecticide resistance, pp. 426–447. Invan Emden H F and Harrington R. (eds.), Aphids as crop pests, 2nd edn. CABI, Wallingford, United Kingdom. [Google Scholar]
- Girvin, J, Whitworth R J, Rojas L M A, and Smith C M. 2017. Resistance of select winter wheat (Triticum aestivum) cultivars to Rhopalosiphum padi (Hemiptera: Aphididae). J. Econ. Entomol. 110: 1886–1889. [DOI] [PubMed] [Google Scholar]
- Havlíčková, H. 1997. Differences in level of tolerance to cereal aphids in five winter wheat cultivars. Rostl. Výroba. 43: 593–596. [Google Scholar]
- He, J, Chen F, Chen S, Lv G, Deng Y, Fang W, Liu Z, Guan Z, and He C. 2011. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 168: 687–693. [DOI] [PubMed] [Google Scholar]
- Honek, A, Martinkova Z, Saska P, and Dixon A F G. 2018. Aphids (Homoptera: Aphididae) on winter wheat: predicting maximum abundance of Metopolophium dirhodum. J. Econ. Entomol. 111: 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X S, Liu Y J, Wang Y H, Wang Z, Yu X L, Wang B, Zhang G S, Liu X F, Hu Z Q, Zhao H Y, et al. 2016. Resistance of wheat accessions to the English grain aphid Sitobion avenae. PLoS One. 11: e0156158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y B, and Chi H. 2012. Age-stage, two-sexlife tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 19: 263–273. [Google Scholar]
- Huang, H W, Chi H, and Smith C L. 2018. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) Fed on Solanum photeinocarpum (Solanales: Solanaceae): with a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 111: 1–9. [DOI] [PubMed] [Google Scholar]
- Kazemi, M H, and van Emden H F. 1992. Partial antibiosis to Rhopalosiphum padi in wheat and some phytochemical correlations. Ann. Appl. Biol. 121: 1–9. [Google Scholar]
- Kettles, G J, and Kaloshian I. 2016. The potato aphid salivary effector Me47 is a Glutathione-S-Transferase involved in modifying plant responses to aphid infestation. Front. Plant Sci. 7: 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klueken, A M, Poehling H-M, and Hau B. 2008. Attractiveness and host suitability of winter wheat cultivars for cereal aphids (Homoptera: Aphididae). J. Plant Dis. Prot. 115: 114–121. [Google Scholar]
- Lage, J, Skovmand B, and Andersen S B. 2004. Field evaluation of emmer wheat-derived synthetic hexaploid wheat for resistance to Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 97: 1065–1070. [DOI] [PubMed] [Google Scholar]
- Mesfin, A, Frohberg R C, Khan K, and Olson T C. 2000. Increased grain protein content and its association with agronomic and end-use quality in two hard red spring wheat populations derived from Triticum turgidum L. var. dicoccoides. Euphytica. 116: 237–242. [Google Scholar]
- Migui, S M, and Lamb R J. 2003. Patterns of resistance to three cereal aphids among wheats in the genus Triticum (Poaceae). Bull. Entomol. Res. 93: 323–333. [DOI] [PubMed] [Google Scholar]
- Migui, S M, and Lamb R J. 2004. Seedling and adult plant resistance to Sitobion avenae (Hemiptera: Aphididae) in Triticum monococcum (Poaceae), an ancestor of wheat. Bull. Entomol. Res. 94: 35–46. [DOI] [PubMed] [Google Scholar]
- Mou, D F, Lee C C, Smith C L, and Chi H. 2015. Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J. Appl. Entomol. 139: 579–591. [Google Scholar]
- Nevo, E. 2001. Genetic resources of wild emmer, Triticum dicoccoides, for wheat improvement in the third Millennium. Isr. J. Plant Sci. 49: 77–92. [Google Scholar]
- Nicholson, S J, Hartson S D, and Puterka G J. 2012. Proteomic analysis of secreted saliva from Russian wheat aphid (Diuraphis noxia Kurd.) biotypes that differ in virulence to wheat. J. Proteomics. 75: 2252–2268. [DOI] [PubMed] [Google Scholar]
- Niemeyer, H, Copaja S, and Barria B. 1992. The Triticeae as sources of hydroxamic acids, secondary metabolites in wheat conferring resistance against aphids. Hereditas. 116: 295–299. [Google Scholar]
- Ninkovic, V, Olsson U, and Pettersson J. 2002. Mixing barley cultivars affects aphid host plant acceptance in field experiments. Entomol. Exp. Appl. 102: 177–182. [Google Scholar]
- Özgökçe, M S, and Atlıhan R. 2005. Biological features and life table parameters of the mealy plum aphid Hyalopterus pruni on different apricot cultivars. Phytoparasitica. 33: 7–14. [Google Scholar]
- Özgökçe, M S, Chi H, Atlıhan R, and Kara H. 2018. Demography and population projection of Myzus persicae (Sulz.) (Hemiptera: Aphididae) on five pepper (Capsicum annuum L.) cultivars. Phytoparasitica. 46: 153–167. [Google Scholar]
- Papp, M, and Mesterházy Á. 1993. Resistance to bird cherry-oat aphid (Rhopalosiphum padi L.) in winter wheat varieties. Euphytica. 67: 49–57. [Google Scholar]
- Papp, M, and Mesterházy Á. 1996. Resistance of winter wheat to cereal leaf beetle (Coleoptera: Chrysomelidae) and bird cherry-oat aphid (Homoptera: Aphididae). J. Econ. Entomol. 89: 1649–1657. [Google Scholar]
- Pedigo, L P, Hutchins S H, and Higley L G. 1986. Economic injury levels in theory and practice. Ann. Rev. Entomol. 31: 341–368. [Google Scholar]
- Pisa, L W, Amaral-Rogers V, Belzunces L P, Bonmatin J M, Downs C A, Goulson D, Kreutzweiser D P, Krupke C, Liess M, McField M, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. Int. 22: 68–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat-Akköprü, E, Atlıhan R, Okut H, and Chi H. 2015. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 108: 378–387. [DOI] [PubMed] [Google Scholar]
- Roberts, J J, and Foster J E. 1983. Effect of leaf pubescence in wheat on the bird cherry oat aphid (Homoptera: Aphidae). J. Econ. Entomol. 76: 1320–1322. [Google Scholar]
- Saemi, S, Rahmani H, Kavosi A, and Chi H. 2017. Group-rearing did not affect the life table and predation rate of Phytoseiulus persimilis (Acari: Phytoseiidae) fed on Tetranychus urticae. Syst. Appl. Acarol. 22(10): 1698–1715. [Google Scholar]
- Saska, P, Skuhrovec J, Lukáš J, Chi H, Tuan S J, and Honěk A. 2016. Treatment by glyphosate-based herbicide alters life history parameters of the rose-grain aphid Metopolophium dirhodum. Sci. Rep. 6: 27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saska, P, Skuhrovec J, Tylová E, Platková H, Tuan S-J, Hsu Y-T, and Vítámvás P. 2020. Leaf structural traits rather than drought resistance determine aphid performance on spring wheat. J. Pest Sci. doi: 10.1007/s10340-020-01253-3 [DOI] [Google Scholar]
- Smith, C M. 2005. Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Sotherton, N W, and van Emden H F. 1982. Laboratory assessments of resistance to the aphids Sitobion avenae and Metopolophium dirhodum in three Triticum species and two modern wheat cultivars. Ann. Appl. Biol. 101: 99–107. [Google Scholar]
- Stevens, M, and Lacomme C H. 2017. Transmission of plant viruses, pp. 323–361. Invan Emden H F and Harrington R (eds.), Aphids as Crop Pests, 2nd edn. CABI, Wallingford, United Kingdom. [Google Scholar]
- Tuan, S J, Lee C C, and Chi H. 2014a. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 70: 805–813. [DOI] [PubMed] [Google Scholar]
- Tuan, S-J, Lee C-C, and Chi H. 2014b. Erratum: population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 70: 1936. [DOI] [PubMed] [Google Scholar]
- Tuan, S J, Lin Y H, Yang C M, Atlihan R, Saska P, and Chi H. 2016. Survival and reproductive strategies in two-spotted spider mites: demographic analysis of arrhenotokous parthenogenesis of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 109: 502–509. [DOI] [PubMed] [Google Scholar]
- Wise, I L, Lamb R J, and Smith M A H. 2001. Domestication of wheats (Gramineae) and their susceptibility to herbivory by Sitodiplosis mosellana (Diptera: Cecidomyiidae). Can. Entomol. 133: 255–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.