Abstract
BACKGROUND
Previous studies have suggested that monitoring the levels of both hypnosis and antinociception could reduce periods of inadequate anaesthesia. However, the evidence regarding associated benefits of this monitoring is still limited.
OBJECTIVE
The primary objective of this study was to confirm that guidance of anaesthesia by depth of hypnosis and antinociception monitoring decreases the number of inadequate anaesthesia events in comparison with standard clinical practice.
DESIGN
A multicentre, single-blinded, randomised controlled trial.
SETTING
The study was conducted in four European University hospitals in four different countries between December 2013 and November 2016.
PATIENTS
The study population consisted of a total of 494 adult patients undergoing elective surgery requiring tracheal intubation.
INTERVENTIONS
The patients were allocated to one of two groups. The first group was treated using Entropy for depth of hypnosis and surgical pleth index to determine depth of antinociception (adequacy of anaesthesia group; AoA group). The second group was monitored using standard monitoring alone (control group). Anaesthesia was conducted with target-controlled infusions of propofol and remifentanil.
MAIN OUTCOME MEASURES
The primary outcome of the study was the number of total unwanted events for example signs of inadequately light or unintentionally deep anaesthesia.
RESULTS
Evidence of inadequate anaesthesia had an incidence of around 0.7 events per patient in both groups with no difference between groups (P = 0.519). In the AoA group, the overall consumption of propofol was significantly reduced (6.9 vs. 7.5 mg kg−1 h−1, P = 0.008) in comparison with the control group. The consumption of remifentanil was equal in both groups. The times to emergence [8.0 vs. 9.6 min (P = 0.005)] and full recovery in the postanaesthesia care unit (P = 0.043) were significantly shorter in the AoA group. No differences were seen in postoperative pain scores or in the use of analgesics.
CONCLUSION
In the current study, the guidance of total intravenous anaesthesia by Entropy and surgical pleth index in comparison with standard monitoring alone was not able to validate reduction of unwanted anaesthesia events. However, there was a reduction in the use of propofol, and shorter times for emergence and time spent in the postanaesthesia care unit.
TRIAL REGISTRATION
at ClinicalTrials.gov NCT01928875.
Introduction
General anaesthesia is considered to be a combination of three different components: hypnosis, immobility and antinociception.1 Traditionally, and still the clinical standard, depth of anaesthesia is guided by interpretation of responses from the autonomic nervous system, such as changes in blood pressure (BP), heart rate (HR) and/or movement of the patient. However, delivering adequate depth of anaesthesia and prevention of side effects remain challenging clinical issues.2–4 New anaesthesia monitoring variables have been developed within the last few decades to better evaluate, monitor and guide depth of hypnosis, immobility and antinociception during general anaesthesia. The term adequacy of anaesthesia (AoA) reflects the intention to guide anaesthesia individually, based on several distinct variables that reflect the depth of hypnosis, an optimal nociception/antinociception balance and the degree of motor block, thereby reducing unwanted memory recall, unwanted movement or unwanted haemodynamic responses and their consequences.
A threshold amount of hypnotic medication is needed to produce a state of unconsciousness to ensure that patients have no awareness of the procedure. However, defining the necessary amount of hypnosis in the clinical situation can be very difficult, as most anaesthetics have wide individual variability in producing and maintaining unconsciousness. The individual evaluation of pharmacodynamics and pharmacokinetics of anaesthetics is therefore challenging. Nevertheless, in the case of volatile anaesthetics, the end-tidal gas concentration may be used to guide delivery.2,3 In addition, monitoring variables based on the processed electroencephalogram (EEG) may add important information regarding the hypnotic drug effect, especially when intravenous anaesthetics such as propofol are used. Spectral entropy monitoring is an EEG-based variable which is claimed to indicate depth of hypnosis.3,5–8
With regard to titration of analgesics, measurement of sympathetic activation by nociception has been attempted. One of the developed variables is the surgical pleth index (SPI), which is based on the analysis of the photoplethysmographic waveform. It normalises the photoplethysmographic amplitude and pulse rate into an index number ranging from 0 to 100. This means that the SPI is high when noxious stimulation is high and the opioid concentration is inadequate. Conversely, the SPI is low when the opioid concentration is high or the stimulation is low.4 Several studies have shown that the SPI is reactive to nociception and analgesic opioid medication in various clinical scenarios.9–17 Further, there is increasing evidence from a previous pilot study and other recent publications that SPI can be used to guide opioid administration during anaesthesia, thereby providing beneficial effects.18–22 However, these studies are relatively small and mostly single-centred, and some of them report conflicting results.21,23
The aim of this randomised, controlled trial was to confirm that an anaesthesia guidance concept including monitoring of the depth of hypnosis and nociception/antinociception balance would be superior to standard clinical practice primarily with respect to the episodes of unwanted anaesthesia events and secondarily to anaesthetic drug consumption and time to emergence.
Methods
The current prospective postmarket, international, multicentre, single-blinded, randomised controlled trial was initiated in four European University hospitals [Kiel (Germany), Tampere (Finland), Amsterdam (the Netherlands) and Szeged (Hungary)] and registered at www.clinicaltrials.gov (NCT01928875). All monitoring devices were CE marked and were used according to the labelled indications. Each research team obtained written institutional review board and/or ethics committee approval before patient enrolment. Responsible ethics committee agreement was given by the Ethics Committee of the Medical Faculty of the University of Kiel (Chair Prof Mehdorn, protocol number A137/13) on 14 January 2014, the Tampere University Hospital Special Responsibility Area Regional Ethics Committee (Chair Prof Kivistö Kari, protocol number EC R13042) on 27 May 2013, the Human Investigation Review Board University of Szeged Albert Szent-György Clinical Centre (Chair Dr Wittmann Tibor, protocol number 01924/2014/OTIG) on 12 May 2015 and the Medical Ethics Committee University of Amsterdam (Chair Prof Levi, protocol number 2013_095, NL 4465.018.13) on 19 August 2013. Written informed consent was obtained from all patients before inclusion in the study. The study sponsor, GE Healthcare (Helsinki, Finland), provided the study equipment and was involved in planning, monitoring the conduct of the study and data collection and analysis. A medical writing team was composed, excluding the sponsor and was responsible for data interpretation and preparation of the article.
Included patients were men and women between 18 and 80 years of age, able to provide written informed consent and undergoing elective surgery demanding general anaesthesia with tracheal intubation, and with an expected procedure duration of at least 2 h. Exclusion criteria consisted of atrial fibrillation, more than five ventricular extrasystoles per minute at baseline or cardiac pacemaker in place; need for invasive BP monitoring during the study period; patients with haemodynamics considered ‘inadequate’ at baseline (Table 1); planned epidural anaesthesia/analgesia; surgery in the prone position; surgery with an expected high risk of significant blood loss (>500 ml); surgery requiring continuous infusion of a neuromuscular blocking agent; patients with BMI more than 35 kg m−2 due to incompatibility with target-controlled anaesthesia; and patients with chronic opioid abuse. Each hospital was scheduled to recruit 100 to 150 patients. In one site (Hungary), remifentanil was used under a special licence, however due to expiration of the licence the study was terminated, leaving a total recruitment of 494 patients.
Table 1.
Criteria for unwanted events
| Inadequate anaesthesia | ||
| Hypertension | MAP > 120% of baseline or MAP ≥ 100 mmHg | |
| Tachycardia | HR > 100 bpm | |
| Somatic arousal | Coughing, chewing, grimacing, breathing against ventilator | |
| Somatic response | Gross movement of extremities | |
| Hypotension | MAP < 80% of baseline or MAP < 60 mmHg | |
| Bradycardia | HR < 80% of baseline or HR < 45 bpm | |
Hypotension or bradycardia. The duration of the change needed to be clinically relevant to be counted. For heart rate, duration must be more than 30 s, and for noninvasive blood pressure, two consecutive measurements (3-min pause between measurements). HR, heart rate; MAP, mean arterial pressure. Adapted from18.
All patients received a screening interview for detection of inclusion and exclusion criteria, and informed consent was obtained from eligible patients before inclusion in the study.
After arrival in the operating theatre or anaesthesia induction room, a peripheral venous line and standard anaesthesia monitoring [five lead ECG, noninvasive BP (NIBP) and SpO2 (CARESCAPE B650, GE Healthcare)] was applied, and baseline values were obtained and checked for exclusion criteria. Before the start of anaesthesia, the randomisation was performed by opening a numbered sealed envelope, but the result was not conveyed to the patient. The envelopes were prerandomised, blinded and sealed into five sets for the five designated study sites. The number of patients in both groups was equal in each set of envelopes.
In the AoA group, patients received monitoring of Entropy via forehead electrodes, monitoring of SPI (contralateral to the NIBP arm) and monitoring of neuromuscular transmission via ulnar nerve stimulation if necessary (NMT module; ipsilateral to NIBP arm) (all GE Healthcare). In control group patients, the Entropy and SPI values were removed from the monitor screen. All documented variables were collected using a 3-min interval and at defined time points including: loss of consciousness (LOC), intubation, start of surgery, maximal surgical stimulation, end of surgery and emergence from anaesthesia.
In all patients, anaesthesia was induced via target-controlled infusion pumps using propofol [Marsh pharmacokinetic model, estimated effect-site target concentration (Ceprop) of 4 μg ml−1] and remifentanil (Minto pharmacokinetic model, Ceremi of 4 ng ml−1). LOC was defined as the time when the patient did not follow verbal commands during induction of anaesthesia. Administration of a neuromuscular blocking agent and tracheal intubation were performed according to the local standard clinical practice at the study site.
In the AoA group, the propofol infusion was adjusted to keep state entropy between 40 and 60, unless other clinical demands necessitated different values. The infusion of remifentanil was not adjusted until the start of surgery, unless required by clinical indication (e.g. movement or hypotension). After the start of surgery, Ceprop was adjusted using 0.5 μg ml−1 steps to keep state entropy between 40 and 60, and Ceremi was adjusted using 1.0 ng ml−1 steps to maintain SPI values in the predefined range of 20 to 50. Absolute lower and upper limits were 2 to 10 μg ml−1 for Ceprop and 2 to 15 ng ml−1 for Ceremi. Deviation of the SPI or Entropy values outside the predefined range for more than 10 min was considered a protocol deviation if no further action was taken to adjust the propofol and remifentanil infusions. Clinicians were allowed to make other changes to infusion rates based on clinical grounds.
In the control group, anaesthetics were adjusted (within safety limits) according to standard clinical practice, therefore there was no standardised guiding protocol. During maintenance of anaesthesia, all patients were assessed for signs of inadequate anaesthesia, somatic arousal, somatic response, hypotension and bradycardia as defined in the pilot study by Chen et al.18 (Table 1).
Fifteen minutes before the expected end of surgery, the infusion target concentration of propofol was reduced in all patients to facilitate emergence. In the AoA group, this was controlled allowing a state entropy value of more than 60 but less than 65. The remifentanil remained unchanged during this period in both groups. If required, any remaining effect of the neuromuscular blocking agent was reversed in both groups. Propofol and remifentanil were finally stopped at the end of surgery (defined by the surgeon), and postoperative analgesia was given according to standard operating procedures of the study site. Emergence from anaesthesia was assessed by measuring the time interval between the end of anaesthetic administration and eye-opening in response to verbal command.
Clinicians responsible for postoperative care of the patients were blinded to group assignment. In the postanaesthesia care unit (PACU), the modified Aldrete Score for fitness for discharge, postoperative nausea and vomiting (PONV) and pain measured by a visual analogue scale (VAS) were recorded. Postoperative analgesia and medication for PONV were given according to standard operating procedures of the study site. Patients stayed in the PACU for at least 1 h. Entries were recorded on the modified Aldrete score form when the patient was connected to the PACU monitor, and at 30 and 60 min. If the patient was observed in the PACU for longer than 1 h, entries were made on the modified Aldrete score form every 30 min until discharge.
On the first postoperative day, all patients were asked by a blinded investigator using a modified Brice questionnaire if they had any memory or awareness during anaesthesia (intra-operative awareness) and the level of satisfaction with the whole procedure using a 0 to 100 scale (0, the worst satisfaction; 100, the greatest satisfaction) was recorded.
Statistical analysis
The primary objective was to study whether using Entropy and SPI guidance of anaesthetics during total intravenous anaesthesia would decrease the episodes of inadequate anaesthesia events18 in comparison with standard clinical practice. Further, we analysed whether using Entropy and SPI reduced consumption of anaesthetics, decreased time to emergence from anaesthesia or influenced postoperative side effects, for example inadequate pain control, nausea or vomiting. The numbers of dose adjustments of propofol and remifentanil were also recorded.
We planned to enrol 500 patients, sufficient to detect a 24% decrease in the episodes of inadequate anaesthesia [80% power, significance level of 0.05 (two-sided)]. A previous pilot study showed an 85% decrease in the number of unwanted events in only 80 subjects.18 However, as our purpose was to demonstrate the effect at different sites and patient populations, the selection of the number of subjects was not based on power analysis, but on having a reasonable number of sites and subjects to warrant generalisation. Initially, five hospitals in four different countries agreed to take part in this study by enrolling 100 subjects each (equal number of 50 AoA group and 50 control group subjects). However, one hospital dropped out due to the prolonged duration of the ethical approval process and the study was performed in the four remaining hospitals. The 100 subjects initially assigned to the fifth study site were divided between the two hospitals with the fastest recruitment rate. No interim analysis was planned or conducted.
To describe and illustrate the trend of variables used for anaesthesia guidance throughout the procedures, plots of Entropy vs. SPI (balance view) were drawn for different time-points during anaesthesia, and the percentage within the target range was calculated.
The data were analysed using SAS Enterprise Guide 7.1 software (SAS Institute Inc., Cary, North Carolina; USA). The demographic values are presented as mean ± SD for continuous data, median [IQR] or number (%). The comparability of the values is shown as standard mean difference. For outcome measures, the data are presented as mean (95% confidence intervals). In the figures, when a 5-min interval is presented, the nearest value from 3-min measurements is used. The statistical tests used were Wilcoxon rank sum test (age, height, weight, BMI), t test (baseline BP and HR, duration of surgery, duration of anaesthesia, time of emergence, propofol, remifentanil), Wilcoxon sign rank test (unwanted events), χ2 test (type of surgery), Kruskal–Wallis test (VAS) and Fisher's Exact test (all others). The recovery from anaesthesia and postoperative care are presented using Kaplan–Meier logistic survival analysis, and the P value is based on the test of equality over strata based on the Log-rank χ2. The limit of statistical significance was defined after Bonferroni correction (PB) using a nominal value P less than 0.05 as a base for calculation. The intra-operative and postoperative values were corrected separately.
Results
The study was conducted between December 2013 and November 2016. A total of 494 patients were included in the analysis. Patients were allocated randomly into one of two groups, the AoA group (n=246) or the control group (n=248). A flow chart is shown in Fig. 1.
Fig. 1.
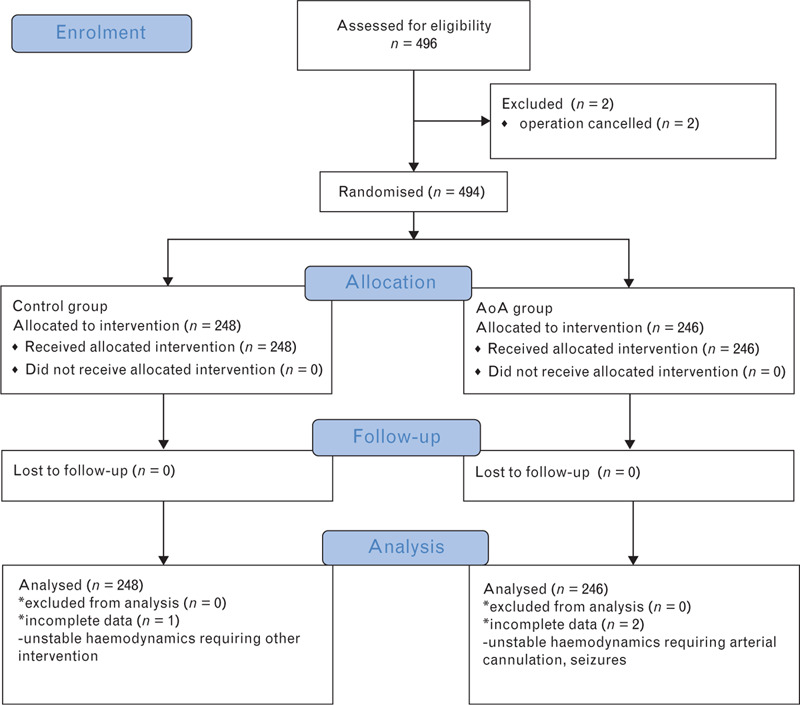
Consort flow chart detailing patient enrolment.
The mean age of all patients was 48.1 (range 19 to 78) years. Operative procedures were gynaecological (52%), ear nose and throat (30%) and other procedures (18%). Patient characteristics or operations did not differ between groups. A detailed description of demographics is shown in Table 2.
Table 2.
Characteristics of patients
| Control group, N=248 | AoA group, N=246 | SMD | |
| Age (years) | 48 ± 16 | 48 ± 15 | 0.021 |
| Height (cm) | 168 ± 9 | 169 ± 10 | −0.057 |
| Weight (kg) | 72 ± 14 | 73 ± 15 | −0.018 |
| BMI (kg m−2) | 25 ± 4 | 25 ± 4 | 0.033 |
| Baseline | |||
| SBP (mmHg) | 121 ± 12 | 121 ± 11 | 0.019 |
| DBP (mmHg) | 70 ± 8 | 70 ± 7 | −0.069 |
| Heart rate (bpm) | 73 ± 12 | 73 ± 11 | −0.050 |
| Duration of anaesthesia (min) | 142 ± 59 | 140 ± 61 | 0.023 |
| Sex | |||
| Female | 199 (80.2) | 189 (76.9) | |
| Male | 49 (19.7) | 57 (23.2) | |
| Type of surgery | |||
| Ear nose throat | 74 (29.8) | 75 (30.4) | |
| Gastrointestinal | 9 (3.6) | 6 (2.4) | |
| Gynaecological | 130 (52.4) | 122 (49.6) | |
| Maxillofacial | 15 (6.0) | 22 (8.9) | |
| Minor trauma | 5 (2.0) | 0 | |
| Orthopaedic | 4 (1.6) | 6 (2.4) | |
| Other | 11 (4.4) | 15 (6.1) | |
Duration of anaesthesia is calculated from intubation to discontinuation of anaesthetic. Values are mean ± SD, standardised mean difference (SMD) or number (%). AoA, adequacy of anaesthesia.
The number of unwanted events was similar in both groups. Detailed data are shown in Table 3.
Table 3.
Number of unwanted events during surgery
| Event | Control group, N=248 | AoA group, N=246 | P |
| Inadequate anaesthesia | 175 (0.71) | 183 (0.74) | 0.519 |
| Hypertension | 98 (0.40) | 119 (0.48) | 0.316 |
| Tachycardia | 15 (0.06) | 8 (0.03) | 0.433 |
| Movements | 62 (0.25) | 56 (0.23) | 0.493 |
| Somatic arousal | 48 (0.19) | 39 (0.16) | 0.652 |
| Somatic response | 14 (0.06) | 17 (0.07) | 0.497 |
| Hypotension | 292 (1.17) | 341 (1.38) | 0.543 |
| Bradycardia | 190 (0.77) | 229 (0.93) | 0.675 |
| Total unwanted events | 657 (2.65) | 753 (3.01) | 0.392 |
Values are total number of events (average number of events per patient). (Limit of statistical significance PB < 0.006). AoA, adequacy of anaesthesia.
The mean (95% CI) total consumption of propofol was smaller in the AoA group than in the control group: 6.9 (6.6 to 7.2) vs. 7.5 (7.4 to 8.0) mg kg−1 h−1 (P = 0.008, PB < 0.01). For remifentanil, no difference in consumption was detected: 0.2 (0.20 to 0.22) vs. 0.2 (0.19 to 0.22) (P = 0.617, PB < 0.01) (Fig. 3).
Fig. 3.
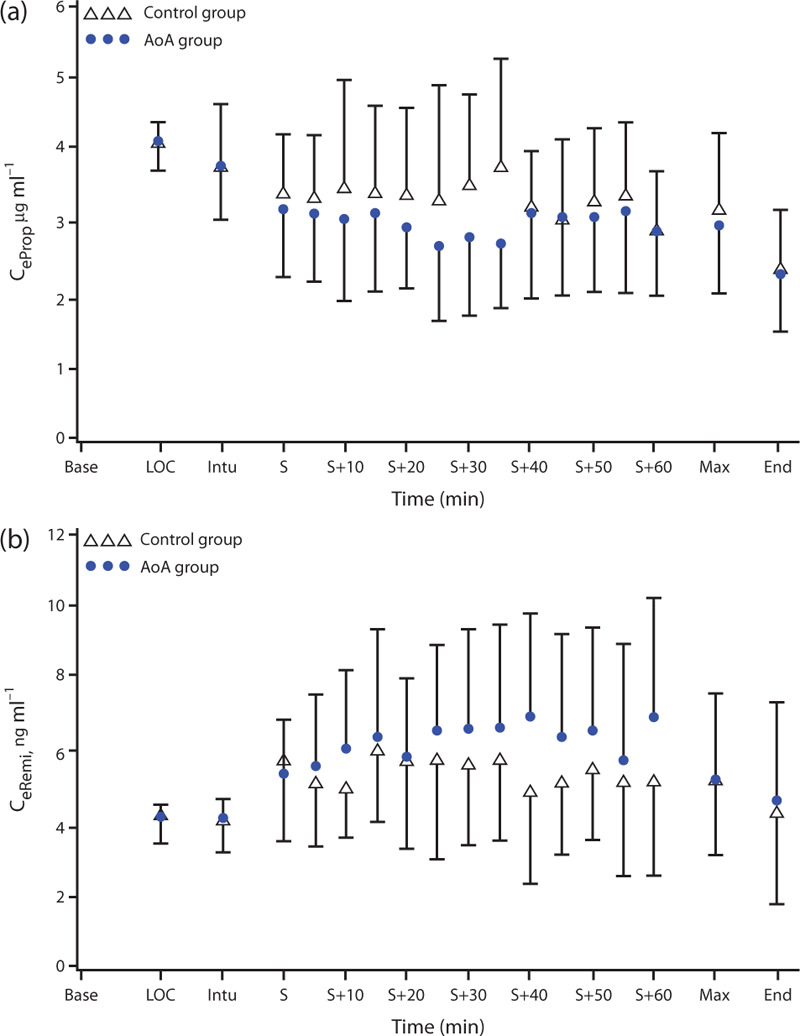
Time course of estimated effect-site concentrations (mean ± SD) of propofol (a, Ceprop) and remifentanil (b, Ceremi). +, minutes after start of surgery; AoA, adequacy of anaesthesia; Base, baseline; End, end of surgery; Intu, intubation; LOC, loss of consciousness; Max, maximal surgical stimulation; S, start of surgery.
The mean (SD) time to eye-opening was significantly shorter in the AoA group: 8.0 (7.4 to 8.4) vs. 9.6 (9.0 to 10.2) in the control group (P = 0.005, PB < 0.01; Fig. 2).
Fig. 2.
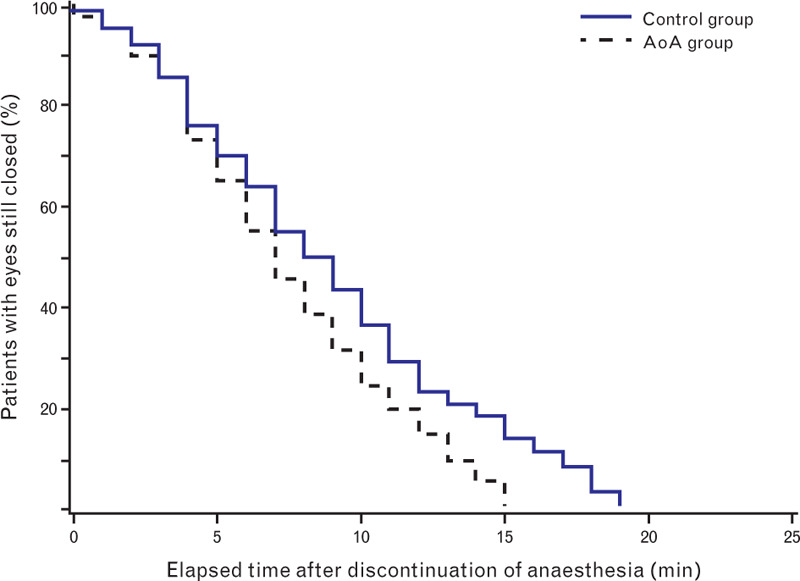
Emergence time to eye-opening, calculated from discontinuation of anaesthetic administration to eye-opening, described as a Kaplan–Meier curve. The log-rank P value comparing the adequacy of anaesthesia group and control group is 0.0016. AoA, adequacy of anaesthesia.
The characteristics of postoperative treatment are shown in Table 4. The control group had slightly more unstable haemodynamics on arrival in PACU, but without statistical significance (P = 0.025, PB < 0.01). Furthermore, a faster full recovery in the AoA group was observed during the first 2-h stay in PACU (cumulative log-rank P value = 0.043, Supplemental Fig. 1). Otherwise, there were no significant differences between groups. In the 24-h postoperative survey, one patient in the control group reported recall of events during surgery, and one patient in the AoA group reported pain during surgery.
Table 4.
Characteristics of postoperative treatment
| Time at PACU | Assessment | Control group, N=248 | AoA group, N=246 | P |
| Arrival at PACU | Activity level | 0.088 | ||
| Does not move extremities | 4.5% (11/242) | 1.7% (4/239) | ||
| Moves two extremities | 11.2% (27/242) | 7.9% (19/239) | ||
| Able to move four extremities voluntarily/on command | 84.3% (204/242) | 90.4% (216/239) | ||
| Ventilation | 0.590 | |||
| Dyspnoea with shallow breathing | 14.0% (34/242) | 12.1% (29/239) | ||
| Spontaneous, unlaboured ventilation, coughs freely | 86.0% (208/242) | 87.9% (210/239) | ||
| Circulation | 0.025 | |||
| BP > ±50% pre-anaesthetic level | 0.4% (1/242) | 1.3% (3/239) | ||
| BP ± 2 to 50% pre-anaesthetic level | 16.5% (40/242) | 9.2% (22/239) | ||
| BP < ±20% pre-anaesthetic level | 83.1% (201/242) | 89.5% (214/239) | ||
| Consciousness | 0.107 | |||
| Not responding | 4.5% (11/242) | 2.5% (6/239) | ||
| Aroused upon calling | 47.5% (115/242) | 40.6% (97/239) | ||
| Fully awake | 47.9% (116/242) | 56.9% (136/239) | ||
| Oxygen saturation | 0.926 | |||
| <90% with O2 inhalation | 0.4% (1/242) | 0.4% (1/238) | ||
| Requires O2 inhalation to maintain level >90% | 58.7% (142/242) | 57.6% (137/238) | ||
| Maintains level >90% on room air | 40.9% (99/242) | 42.0% (100/238) | ||
| PONV, yes | 3.4% (8/235) | 2.6% (6/234) | 0.787 | |
| VAS, median [IQR] | 3.0 [5.0] | 4.0 [4.0] | 0.163 | |
| 1 h after arrival in PACU | Activity level | 0.118 | ||
| Does not move extremities | 0.4% (1/234) | 0.0% (0/226) | ||
| Moves two extremities | 0.0% (0/234) | 1.3% (3/226) | ||
| Able to move four extremities voluntarily/on command | 99.6% (233/234) | 98.7% (223/226) | ||
| Ventilation | 0.473 | |||
| Dyspnoea with shallow breathing | 4.7% (11/234) | 3.1% (7/226) | ||
| Spontaneous, unlaboured ventilation, coughs freely | 95.3% (223/234) | 96.9% (219/226) | ||
| Circulation | 0.156 | |||
| BP ± 20 to 50% pre-anaesthetic level | 11.5% (27/234) | 7.5% (17/226) | ||
| BP < ±20% pre-anaesthetic level | 88.5% (207/234) | 92.5% (209/226) | ||
| Consciousness | 0.340 | |||
| Aroused upon calling | 20.5% (48/234) | 16.8% (38/226) | ||
| Fully awake | 79.5% (186/234) | 83.2% (188/226) | ||
| Oxygen saturation | 0.562 | |||
| Requires O2 inhalation to maintain level >90% | 38.5% (90/234) | 35.6% (80/225) | ||
| Maintains level >90% on room air | 61.5% (144/234) | 64.4% (145/225) | ||
| PONV, yes | 3.9% (9/232) | 3.6% (8/224) | 1.000 | |
| VAS, median [IQR] | 4.0 [3.0] | 3.0 [3.0] | 0.932 |
Data are presented as percentage (number of patients/total number analysed) or score number. P values are presented without Bonferroni correction (Limit of statistical significance PB < 0.003). AoA, adequacy of anaesthesia; PACU, postanaesthesia care unit; PONV, postoperative nausea and vomiting; VAS, visual analogue scale.
The mean (95% CI) number of dose adjustments was higher in the AoA group than in the control group for both propofol [4.3 (3.9 to 4.7) vs. 3.3 (3.0 to 3.6)] (P < 0.001) and remifentanil [7.0 (6.2 to 7.8) vs. 4.1 (3.7 to 4.4)] (P < 0.001), respectively.
The mean ± SD percent of the time the guided variables were within the defined range during surgery were 45 ± 33% for state entropy and 75 ± 20% for SPI. Both values together were within the target range for 34 ± 29% of the time. State entropy and SPI values were low most of the time during surgery. This can be visualised in the ‘balance view’ plots displaying the target of state entropy and SPI in the AoA group. In the figure, a wide random cloud distribution is visualised at LOC, whereas, at incision, the measurements were much more consolidated at the lower left quadrant (Supplemental Fig. S2).
Protocol deviations due to the lower threshold of 2 μg ml−1 for propofol limited further reduction of infusion rates in 20 cases. Similarly, for remifentanil, the low threshold was reached four times. On six occasions, protocol deviations were based on clinical decisions by the attending anaesthesiologist.
Discussion
In this international, multicentre, single-blinded, randomised, controlled trial of unselected adults, anaesthesia guidance with Entropy and SPI did not decrease the episodes of unwanted intra-operative anaesthesia events compared with standard monitoring alone. However, AoA guidance enabled reduction of propofol consumption, shorter emergence from anaesthesia and shorter recovery time in the PACU. We detected a higher number of dose adjustments in the AoA group. There was no significant difference in consumption of remifentanil or PACU characteristics. To our knowledge, the current study represents today's largest available trial testing anaesthesia guidance based on both depth of anaesthesia and antinociception monitoring.
Our results were unexpected, because a single-centre pilot study reported substantial effects regarding the reduction of unwanted events in the AoA group among ENT patients.18 The pilot study reported a marked reduction in the number of hypertensive, hypotensive, bradycardic and movement events. Similar findings were made in polytrauma patients; although the number of movements was not reported in that study, haemodynamics were more stable in patients guided by depth of anaesthesia monitors despite reduction in fentanyl consumption.24 A third trial by Bergmann et al.20 did not report any major effect of SPI monitoring on unwanted events during surgery, but the consumption of remifentanil and propofol, as well as the time to eye opening, was reduced in this outpatient population. In all these studies, the levels of Entropy or Bispectral Index were kept well within predetermined target ranges. This is in contrast to our results, where a mean of only 45% of the values reached the predefined ranges. Our results emphasise the real-world challenges of multicentre clinical studies with unselected patients; despite planned protocols, the clinician's view of optimal anaesthesia can make it difficult to obey the protocol. For example, adjusting the delivery of propofol to maintain Entropy values below 40 helps to conduct smoother and uneventful anaesthesia. Therefore, although SPI reflects the nociception/antinociception balance, that changes constantly in response to surgical stimuli, the potential benefits gained with such monitoring may be overlooked due to otherwise deep anaesthesia states.
Patient movements may be considered the most obvious sign of inadequate anaesthesia. A recent meta-analysis reported an advantage of antinociception guidance with respect to movement events.25 In the present trial, we could not detect a difference in movement events, either regarding minor arousal (e.g. chewing or breathing against the ventilator) or in more pronounced movement responses. Overall, studies report a large variation in the number of episodes of movement during anaesthesia. Whereas Martinez et al.26 reported movements in more than 50% of patients during light sedation for colonoscopy and a reduction of movements in the monitoring group by using the cardiovascular reflex index, a trial by Bergmann et al.20 did not report any movements in orthopaedic outpatient anaesthesia using SPI monitoring. In the present trial, the number of movement episodes was low and ranged from 0.1 to 0.5 movements per patient between sites. No difference between groups was detected at any of the study sites. The small number of movements is probably due partly to the observed deep hypnotic levels of anaesthesia, demonstrated as low entropy readings, and also partly to the use of neuromuscular blockade, which was allowed according to local standards. The results are also in line with a previous study during sevoflurane/sufentanil anaesthesia.23
One additional possible explanation for the similarity in the movement and other unwanted events between the groups could lie in the study protocol. We decided to use a fixed SPI target range in the AoA group instead of an additional inclusion of changes in value (Δ). In two studies, ΔSPI was shown to be a better predictor of movement than SPI alone.13,16 The SPI is typically a balance index, where both nociceptive and pharmacodynamics of anaesthetics and fluids have an impact on its value. SPI increases in response to noxious stimulation, and decreases if the stimulus stops. In other words, a change in value, even within target limits, should be interpreted in close relation to surgical stimulation and knowledge of confounders such as use of parasympatholytics.27
With respect to the guidance of the hypnosis component in the AoA group, we failed to reach the Entropy target during a relatively long period of time. Even though we found reduced propofol consumption in the AoA group, there was a high number of low Entropy periods, probably caused by two major factors. It is likely that the protocol and its implementation allowed too slow a reaction to deepening anaesthesia and thus overdosing of anaesthetic (i.e. propofol). First, protocol deviations were documented whenever values remained outside the predefined range for longer than 10 min. In many cases, the anaesthesia team reduced the propofol infusion due to lower Entropy values, but these reactions were too slow and unable to cause an adequate increase of Entropy values. Therefore, despite the fact that in the AoA group there were more dose adjustments than in the control group, a more persistent protocol to reach the target range would have been needed. Second, the lower safety limit of propofol plasma concentration prevented a further dose reduction in a number of patients. Although Entropy was frequently too low, SPI was kept more accurately within the target range. It has been shown previously that the SPI should be reliable regardless of the level of Entropy.9 Because the study protocol included dosing safety limits for anaesthetics, investigators may have been reluctant to lighten anaesthesia further when these were reached. Consequently, deep anaesthesia reduces the likelihood for movement and other reactions recorded as unwanted events, possibly influencing the results of our study. Comparison would be more valuable by using a stricter protocol in both groups, especially by avoiding too deep anaesthesia in the control group. On the contrary, the study did not aim to record state entropy and SPI values in the control group, which might have given further insight into the conduct of anaesthesia between groups. Because of an electronic data record in site 003, we are able to present those data only from 141 patients.
In common with previous studies, our data show that depth of anaesthesia monitoring reduces emergence time in the operating theatre.2,3 A stricter anaesthesia adjustment toward lighter anaesthesia at the end of the procedure would probably translate to faster emergence. In our study, this was seen as a faster time to eye-opening as well as a faster recovery and fitness for discharge in the AoA group patients. These effects may be clinically of greatest interest in an anaesthesia environment with a high patient turnover.
In a recent meta-analysis, no benefit of depth of anaesthesia monitoring with regard to the episodes of awareness with recall was reported.28 Our study was not powered to investigate differences in episodes of awareness with recall.
The following limitations have to be considered when interpreting the present results. First, the durations that patients spent within the predefined state entropy range were relatively low (see above). Second, the number of unwanted events, especially movement, was very low in both groups. We included relatively young and healthy patients undergoing elective surgery, where the effects of anaesthesia guidance may be smaller than in patients with more comorbidities. Third, defining ranges for adequate HR and BP by using values recorded just prior to induction of anaesthesia may be problematic. In this situation, patients often suffer from anxiety and agitation, thus altering the pre-anaesthetic baselines, and the criteria may set the target level relatively high. Last, the uses of the Trendelenburg position or laparoscopic surgery with pneumoperitoneum were not recorded. This might have an effect on the SPI values due to increased venous return.29 Finally, the level of remifentanil administration was not changed between the induction and start of surgery which might have slightly increased the total consumption of remifentanil.
In conclusion, the current study could not confirm previous results for depth of anaesthesia and nociception monitoring and its use during total intravenous anaesthesia, as the number of unwanted intra-operative events was comparable between groups. There was a lower consumption of propofol and faster emergence times in the AoA group. Our findings may have been influenced by the study design with a low to moderate risk profile of operations and patients, along with low frequency of unwanted anaesthesia events. Therefore, the findings may not routinely be transferred to fragile patients and/or interventions/procedures with a very light (diagnostic) or even high-risk profile. Additional studies are necessary to find the optimal use and optimal patient groups for AoA monitoring.
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: Kiel (Germany): Markus Steinfath, Christina Schuldt, Angelika Gminder, Corinna Buchholz. Tampere (Finland): Atte Kukkurainen, Kaisa Malila, Susanna Mennander, Anne Mäyrä, MD (physician), Kati Rautaneva, MD (physician), Simo Varila (study nurse); Amsterdam (Netherlands): Markus W. Hollmann, MD (professor and chair of department), Marissa P.C. Gaanderse (study nurse); Szeged (Hungary): Idiko Laszlo (PhD student), Nandor Oveges (PhD student), Katalin Laszlo (study nurse), Domonkos Trasy (PhD student), Emese Csullog, MD (physician), Anna Molnar, MD (physician).
Financial support and sponsorship: this trial was supported by GE Healthcare, Helsinki, Finland, as sponsor.
Conflicts of interest: MG has received lecture fees from GE Healthcare. He further reports consultancies, honoraria and payments for lectures from Baxter, CSL Behring, Ferring, Fresenius Medical Care, Grünenthal. JAH has received lecture fees from GE Healthcare. BB received honoraria for consulting and giving lectures from GE Healthcare, Maquet, CSL Behring, Edwards Life Sciences, CNSystems, Orion Pharma, Baxter Healthcare, Abbvie, Grünenthal and CytoSorbents. AY-H received research funding from GE Healthcare; AY-H is a part of an advisory board for MSD Finland; AY-H received honoraria for giving lectures from Fresenius-Kabi, GE Healthcare, MSD and OneMed. BP received research funding from GE Healthcare, Edwards Lifesciences, Air Liquide France and NovoNordisk Netherlands; BP is part of an advisory board for Laboratoire Aguettant, France; BP received lecture fees from Abbvie, The Netherlands and Orion Pharma BVBA, The Netherlands, and research grants from SCA, ESA and ZonMW. ZM received honoraria for consulting and giving lectures from Pulsion-Maquet, ThermoFisher Scientific, Biotest and CytoSorbents. No other conflicts of interest relevant to this article.
Presentation: preliminary data were presented at the Euroanaesthesia 2018 congress, 3 June 2018, Copenhagen.
Footnotes
Matthias Gruenewald and Jarkko Harju contributed equally to the article.
Supplemental digital content is available for this article.
Published online 16 October 2020
References
- 1.Antognini JF, Carstens E. In vivo characterization of clinical anaesthesia and its components. Br J Anaesth 2002; 89:156–166. [DOI] [PubMed] [Google Scholar]
- 2.Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev 2014; 6:CD003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhabra A, Subramaniam R, Srivastava A, et al. Spectral entropy monitoring for adults and children undergoing general anaesthesia. Cochrane Database Syst Rev 2016; 3:CD010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huiku M, Uutela K, van Gils M, et al. Assessment of surgical stress during general anaesthesia. Br J Anaesth 2007; 98:447–455. [DOI] [PubMed] [Google Scholar]
- 5.Gruenewald M, Zhou J, Schloemerkemper N, et al. M-Entropy guidance vs. standard practice during propofol-remifentanil anaesthesia: a randomised controlled trial. Anaesthesia 2007; 62:1224–1229. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd J, Jones J, Frampton G, et al. Clinical effectiveness and cost-effectiveness of depth of anaesthesia monitoring (E-Entropy, Bispectral Index and Narcotrend): a systematic review and economic evaluation. Health Technol Assess 2013; 34:1–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vakkuri A, Yli-Hankala A, Talja P, et al. Time-frequency balanced spectral entropy as a measure of anesthetic drug effect in central nervous system during sevoflurane, propofol, and thiopental anaesthesia. Acta Anaesthesiol Scand 2004; 48:145–153. [DOI] [PubMed] [Google Scholar]
- 8.Vakkuri A, Yli-Hankala A, Sandin R, et al. Spectral entropy monitoring is associated with reduced propofol use and faster emergence in propofol-nitrous oxide-alfentanil anesthesia. Anesthesiology 2005; 103:274–279. [DOI] [PubMed] [Google Scholar]
- 9.Struys MMRF, Vanpeteghem C, Huiku M, et al. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br J Anaesth 2007; 99:359–367. [DOI] [PubMed] [Google Scholar]
- 10.Ahonen J, Jokela R, Uutela K, et al. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br J Anaesth 2007; 98:456–461. [DOI] [PubMed] [Google Scholar]
- 11.Wennervirta J, Hynynen M, Koivusalo A, et al. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol Scand 2008; 52:1038. [DOI] [PubMed] [Google Scholar]
- 12.Kallio H, Lindberg LI, Majander AS, et al. Measurement of surgical stress in anaesthetized children. Br J Anaesth 2008; 101:383–389. [DOI] [PubMed] [Google Scholar]
- 13.Gruenewald M, Meybohm P, Ilies C, et al. Influence of different remifentanil concentrations on the performance of the surgical stress index to detect a standardized painful stimulus during sevoflurane anaesthesia. Br J Anaesth 2009; 103:586–593. [DOI] [PubMed] [Google Scholar]
- 14.Paloheimo MP, Sahanne S, Uutela KH. Autonomic nervous system state: the effect of general anaesthesia and bilateral tonsillectomy after unilateral infiltration of lidocaine. Br J Anaesth 2010; 104:587–595. [DOI] [PubMed] [Google Scholar]
- 15.Mustola S, Parkkari T, Uutela K, et al. Performance of surgical stress index during sevoflurane-fentanyl and isoflurane-fentanyl anesthesia. Anesthesiol Res Pract 2010; 2010:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenewald M, Herz J, Schoenherr T, et al. Measurement of the nociceptive balance by analgesia nociception index (ANI) and surgical pleth index (SPI) during sevoflurane-remifentanil anaesthesia. Minerva Anestesiol 2015; 81:480–489. [PubMed] [Google Scholar]
- 17.Harju J, Kalliomaki ML, Leppikangas H, et al. Surgical pleth index in children younger than 24 months of age: a randomized double-blinded trial. Br J Anaesth 2016; 117:358–364. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Thee C, Gruenewald M, et al. Comparison of surgical stress index-guided analgesia with standard clinical practice during routine general anesthesia: a pilot study. Anesthesiology 2010; 112:1175–1183. [DOI] [PubMed] [Google Scholar]
- 19.Colombo R, Raimondi F, Corona A, et al. Comparison of the surgical pleth index with autonomic nervous system modulation on cardiac activity during general anaesthesia: a randomised cross-over study. Eur J Anaesthesiol 2014; 31:76–84. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann I, Göhner A, Crozier TA, et al. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth 2013; 110:622–628. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Lim B, Kim H, et al. Comparison of surgical pleth index-guided analgesia with conventional analgesia practices in children: a randomized controlled trial. Anesthesiology 2015; 122:1280–1287. [DOI] [PubMed] [Google Scholar]
- 22.Won YJ, Lim BG, Lee SH, et al. Comparison of relative oxycodone consumption in surgical pleth index-guided analgesia versus conventional analgesia during sevoflurane anesthesia: a randomized controlled trial. Medicine 2016; 95:e4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruenewald M, Willms S, Broch O, et al. Sufentanil administration guided by surgical pleth index vs standard practice during sevoflurane anaesthesia: a randomized controlled pilot study. Br J Anaesth 2014; 112:898–905. [DOI] [PubMed] [Google Scholar]
- 24.Rogobete AF, Sandesc D, Cradigati CA, et al. Implications of entropy and surgical pleth index-guided general anaesthesia on clinical outcomes in critically ill polytrauma patients. A prospective observational nonrandomized single centre study. J Clin Monit Comput 2018; 32:771–778. [DOI] [PubMed] [Google Scholar]
- 25.Gruenewald M, Dempfle A. Analgesia/nociception monitoring for opioid guidance: meta-analysis of randomized clinical trials. Minerva Anestesiol 2017; 83:200–213. [DOI] [PubMed] [Google Scholar]
- 26.Martinez JY, Wey PF, Lions C, et al. A beat-by-beat cardiovascular index, CARDEAN: a prospective randomized assessment of its utility for the reduction of movement during colonoscopy. Anesth Analg 2010; 110:765–772. [DOI] [PubMed] [Google Scholar]
- 27.Korhonen I, Yli-Hankala A. Photoplethysmography and nociception. Acta Anaesthesiol Scand 2009; 53:975–985. [DOI] [PubMed] [Google Scholar]
- 28.Messina AG, Ward MJ, Pace NL. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database Syst Rev 2016; 10:CD007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilies C, Ludwigs J, Gruenewald M, et al. The effect of posture and anaesthetic technique on the surgical pleth index. Anaesthesia 2012; 67:508–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


