Abstract
Sutures, staples, clips and skin closure strips are used as the gold standard to close wounds after an injury. In spite of being the present standard of care, the utilization of these conventional methods is precarious amid complicated and sensitive surgeries such as vascular anastomosis, ocular surgeries, nerve repair, or due to the high-risk components included. Tissue adhesives function as an interface to connect the surfaces of wound edges and prevent them from separation. They are fluid or semi-fluid mixtures that can be easily used to seal any wound of any morphology – uniform or irregular. As such, they provide alternatives to new and novel platforms for wound closure methods. In this review, we offer a background on the improvement of distinctive tissue adhesives focusing on the chemistry of some of these products that have been a commercial success from the clinical application perspective. This review is aimed to provide a guide toward innovation of tissue bioadhesive materials and their associated biomedical applications.
Keywords: Sutures, Bioadhesives, Anastomosis, Tissue repair, Wound closure, Injury
Graphical abstract
Introduction
Surgical process or accidents can lead to tissue injury and require innovative efforts to accelerate hemostasis. Nowadays, sutures, staples, clips, or skin closure strips are used as the gold standard to enable wound closure [1]. Sutures are the ‘go to’ choice to close any injury wounds or tissues due to their wide flexibility and mechanical features and are thus used on a variety of wounds or surgical procedures. For example, while non-absorbable sutures provide mechanical support to close a superficial wound, absorbable sutures allow the suturing of deeper wounds without the need for removal after the wound is healed, as is the norm with many surgical stitches [2]. However, the use of sutures requires a time-consuming surgical procedure with high precision and trained personnel to perform the process. Therefore, staples, clips, or skin closure strips are the new alternatives to wound closure materials [3]. The use of staples and clips can rapidly close the wound edges through a simple ‘click’, especially on skin laceration. Compared to sutures, the use of staples and clips results in a low infection rate and a short healing time [4]. On the contrary, they have some disadvantages, e.g. wounds without a meticulous closure can easily lead to scarring, in addition, their high tensile strength can cause patients to experience more pain during their removal after wound healing [5]. Similar to the use of staples and clips, skin closure strips are sometimes used as an alternative. These strips can be used as tapes and provide a suitable tensile strength for the target wound. These properties endow the strips with not only a fast and easy operation to close the wound but also significantly reduce the formation of scars [6].
However, the use of wound closure strips still poses some challenges such as utilizing for an only small wound and having no moisture resistance, and their limited use in terms of the types and severity of a wound. Therefore, the development and investigation of novel materials for wound closure are worth discussing.
Tissue adhesives offer functionality to connect the surfaces of different substrates and prevent these substrates from separation. Generally, adhesives are used to seal the surfaces with a uniform or an irregular shape between dissimilar surfaces [7]. This property endows adhesives with wide applications in interdisciplinary research fields. In the past decades, the adhesives for biomedical applications, called tissue adhesives, have attracted increasing attention from the research community for development and use to repair wounds in damaged tissues. Cyanoacrylates (CAs) were one of the earliest compounds used for simple wound closure applications by soldiers in the 1950s. However, these tissue adhesives elicited inflammatory response [8]. Subsequently, other tissue adhesives such as Eastman 910 and modified CA-based derivatives with their enhanced biocompatibility have been widely used since the 1960s [9, 10].
The advantages of tissue adhesives include easy and rapid use with no follow-ups for removal of any residual components as is the case with sutures or staples. Until now, many tissue adhesives synthetic and semi-synthetic in origin with biomimetic characteristics and good biocompatibility have been developed and applied to clinical use. However, some challenges in the form of poor mechanical strength, swelling, and low stability limited their further applications in the field [11]. Here, we report on mechanical features, functions, and applications of tissue adhesives developed from natural and synthetic polymers. The objective of this review is to provide critical and constructive analyses of the recent advances in the field to evaluate current situations with a particular focus on the material selection, production, and their application in tissue adhesives.
1. Tissue adhesives: Basics and their characteristic properties
1.1. Adhesion versus Cohesion
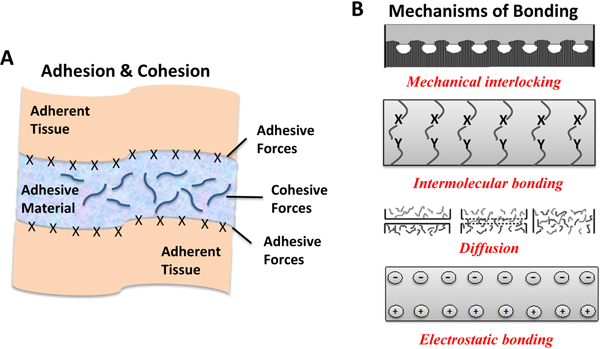
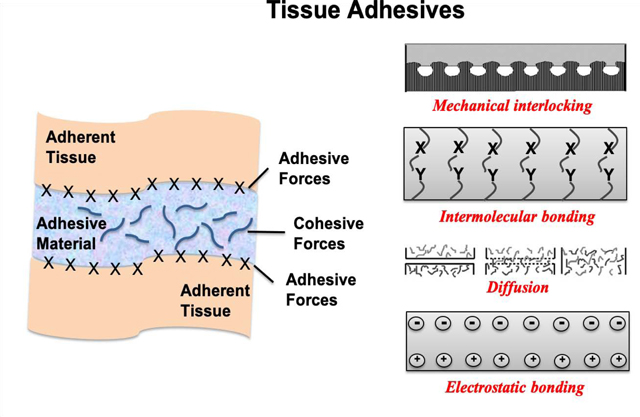
One of the most important features of an adhesive is to achieve strong interfacial bonds with the target tissue [12, 13]. The strength of these bonds can be explained by the balance of two different physical forces; adhesive and cohesive (Figure 1A). Adhesive force refers to molecular interactions at the interface of the adhering tissue substrate and adhesive materials. Cohesive force refers to intermolecular forces within the adhesive material to bear with shear stresses of external forces [13–15]. Principally, intimate contact of the adhesive material and the adhering surface and the penetration of the adhesive material into the adhering surface are two important steps for the adhesion process to happen successfully [16]. The bonding performance strongly depends on the effective contact area and wetting of the adherent by an adhesive material to provide an intimate contact [17]. High penetration ability leads to enhanced adhesion due to stronger electrostatic interactions, greater wetting, and adsorption with better mechanical locking. Moreover, crosslinking density is another parameter which results in higher cohesive strength. Since high cohesive forces can result in a strong interface, the choice of a tissue adhesive should be determined according to the target tissue [13, 15]. It is also worth noting that there are several other requirements to achieve a strong adhesion including a clean surface, adequate surface roughness, sufficient wetting, desired flow of adhesive material by maintaining required certain intermolecular, physicochemical and attractive forces, etc. [13, 17].
Figure 1.
A) Schematic representation of adhesion (attractive forces between adherent tissues and adhesive material) and cohesion (intermolecular strength of the material) forces, and B) Various mechanisms of bonding [22, 23]. Adapted with permission from ref. [22, 23], Copyright from 2004, The John Wiley & Sons, Inc.; Adapted with permission from ref. [22, 23], Copyright from 2002, The Kluwer Academic Publishers.
1.2. Requirements of an adhesive
To design a successful tissue adhesive, the material should achieve certain mechanical strength and adhesive properties of the wound area without any side effects. It should not delay the healing process and also it should not affect tissue function or movement. Moreover, the ideal adhesive material should be biocompatible, sterilizable, biodegradable, effective for wound healing, easy to prepare, easy to use, and cost-effective [18–21].
1.2.1. Adhesive and Cohesive Performance
An adhesive material firstly forms strong interfacial bonds between two detached tissue sections. It should also provide required cohesive strength and stand stable to achieve required support during the recovery period of the wound. The material should have proper flow characteristics to support easy application to the target area and also, it should be capable of solidifying rapidly even under mild physiological conditions to minimize bleeding and surgery time [12].
The method used for the solidification of an adhesive is a key parameter to achieve desired properties and mechanical strength. This process mostly includes chemical crosslinking using reactive chemicals, heat, mechanical fixing, or photo-crosslinking. The choice of method strongly depends on adhesive material and target application [24]. Notably, photopolymerization is widely utilized in the preparation of bioadhesives due to its rapid production rate, high chemical and mechanical stability with lower costs. Also, the light-curing process can occur without any solvent and at convenient temperatures [25]. However, this process often includes UV light activation in the presence of a photo-initiator. Recently, the use of visible light for covalent bonding in the presence of the photo-initiator reveals a safer alternative to UV crosslinking [26, 27]. Once the curing process occurs, adhesive material should be able to have strong tissue bonding and mechanical strength in compliance with the target tissue to successfully support the healing process. Particularly, the mechanical strength of the adhesive needs to be compatible with the target tissue to achieve proper load bearing which needs to be adapted for the soft tissue, muscle or bone. Finally, the material should ensure the required mechanical properties during the entire healing process and degrade in compliance with tissue recovery [12].
1.2.2. Biocompatibility and biodegradation
Biocompatibility is one of the most important properties of bioadhesives. A bioadhesive material and its degradation products should be non-toxic, non-irritant, non-allergenic, and non-carcinogenic. It is well known that the curing process of an adhesive typically involves chemical crosslinking through reactive chemicals or photo-crosslinking. Also, adhesives contain excipients such as plasticizers, accelerators, and stabilizers, which can be toxic. Therefore, the release of reactive chemicals and the effect of other additives should be minimized through a proper choice of materials and pre-polymers [12, 13]. Additionally, an adhesive material should be biodegradable through hydrolysis or enzymatic degradation leaving behind no toxic byproducts. Notably, it must maintain its structural and functional integrity and remain stable for a required period of time to keep adherent tissues together until a substantial tissue regeneration has taken place. After complete recovery, the material should completely degrade without any intervention or leaving any toxic products behind.
In general, the degradation of applied material should start after 3 weeks from the application and complete after 2 months with consistency to the restorative and natural wound healing period [15, 28].
1.3. Mechanism of adhesion
Adhesion processes are complex. Understanding the mechanism of adhesion has significant relevance to understand tissue adhesives and their use in clinical settings. However, there is no single theory to explain all the mechanisms due to its complexity. The main mechanisms to better understand adhesion can be summarized as mechanical interlocking, intermolecular bonding, chain entanglement, diffusion, and electrostatic binding (Figure 1B) [29]. Adhesion generally occurs by either molecular interactions that can be physical or chemical in nature or by mechanical interlocking or both [15].
1.3.1. Intermolecular bonding
Intermolecular bonding is the principal mechanism of adhesion and arises from intermolecular forces between the adhesive and the adherent on the intimate contact surface [30]. These intermolecular forces include primary chemical forces such as ionic, covalent, and metallic bonds and secondary physical forces in the form of dipole-dipole interactions, London dispersion, and van der Waals forces. Intermolecular bonding can be formed by primary and secondary forces or their combination [31]. Herein, the increase of the applied force until bond formation can enhance contact intimacy and contact area which leads to a strong adhesion [32].
Primary bonds present stronger adhesion compared to secondary bonds. Since these bonds are obtained from interactions between a specific chemical group on the adhesive molecule and a responsive chemical group on the substrate, specific surface pretreatment is often needed to achieve stronger interaction. Herein, adhesive can be modified chemically by incorporating specific functional groups into its chemical structure to generate chemical bonds between the adhesive and the adherent. Moreover, the surface of the adherent can be pretreated by using coupling agents or other adhesion promotor molecules [22, 30, 33]. Particularly, covalent bond is the most common bond type among primary bonds. It mainly arises as an interaction between different functional groups such as activated ester, isocyanate, and aldehyde and primary amine of the chemical moieties on the surface of soft tissues through imine, Diels–Alder click chemistry or Schiff-base reaction [12, 28, 34].
Secondary forces also play a considerable role in adhesion. Notably, when the interface between the adhesive and the adherent presents a great number of available sites for secondary bonding, these bonds can provide enhanced strong adhesion [28, 33]. For instance, Gecko inspired adhesives provide adhesion by non-covalent interactions through van der Waals and capillary forces [34]. Since adhesion is related to physicochemical properties of surface, adhesion depends on physical properties of the adhesive surface and the response of adherent to this surface. In adhesion and physisorption, the van der Waals forces could arise from positively and negatively charged regions of the bonding molecules and keep together the surfaces of two materials owing to close proximity of their surfaces, as such the van der Waals interactions could become the main contributor to the adhesion [33, 35]. Additionally, London dispersion forces are formed because of the interactive forces between temporary multipoles in molecules without permanent polarity [36]. There are different approaches to define adhesion between adhesive and adherent such as electron donor and acceptor interactions. Molecules having donor and acceptor properties result in formation of molecular complex to achieve stronger adhesion. For instance, Lewis acid and base could be electron donor and acceptor, respectively. Hydrogen bond could be considered as donor and acceptor interaction [37]. Furthermore, wetting is strongly related to adhesion and in the adsorption theory, the formation of secondary forces is closely dependent to wetting of adherents by adhesives. To obtain effective wetting, the surface energy of the adherent should be higher than the surface energy of adhesive. Hence, surface pretreatment could be applied to enhance the surface energy of the adherent. It is known that wetting depends on mainly van der Waals forces, hydrogen bonding, and acid–base interactions [33].
1.3.2. Electrostatic bonding
The electrostatic bonding mechanism is based on electrostatic interaction between oppositely-charged adhesive and adherent surfaces through a transfer of electrons at the interface [24]. The strength of electrostatic interaction depends on the charge density which can be altered by modulating the ionic content of surrounding media of the adhesive. Nevertheless, it may need a long time to provide the required charge concentration in the presence of insulator components due to the slow nature of charge build-up and limitation of available electrons [12, 22]. Particularly, this mechanism is employed for incompatible materials such as a polymer, semiconductor, or a metal. The contribution of electrostatic interaction has been found to be low compared to chemical bonding [24, 38]. Meanwhile, it is also a possible mechanism for bioadhesion by sharing of electrons in some cases between the glycoproteins of the adherent and the bioadhesive material [16, 39]. For instance, the interaction between mucin and chitosan happens due to electrostatic forces additionally supported by hydrogen bonding and hydrophobic interactions [16].
1.3.3. Mechanical interlocking
Mechanical interlocking is one of the oldest adhesion theories [40]. It involves the penetration of the adhesive materials into pores and irregularities of the surface of the adherent. Herein, the trapped air on the interface is replaced with the adhesive and is followed by adherence of the material into microscopic roughness of the surface to provide complete binding. Therefore, controlling the surface topography by surface modification is vital to achieving the desired roughness and adhesion property [24, 41]. For instance, Yang et. al., inspired by endoparasite Pomphorhynchus laevis, developed swellable microneedle adhesive (including a poly(styrene)-block-poly(acrylic acid) with a swellable tip and non-swellable polystyrene core) providing mechanical interlocking with the tissue substrate [42]. Additionally, favorable wetting of the adherent by adhesive and rheological characteristics of adhesive are also important for adhesion strength in addition to the roughness, porosity, and disorders of the surface [33, 43]. For example, a decrease in the wetting of adherents can cause poor adhesion because of a decrease in contact area [23]. Also, for strong adhesion, the adhesive material can fill into pores and surface disorders in a suitable time. Adhesives with low viscosity can achieve faster and effective penetration into the cavities, which in turn results in better adhesive strength [43]. Since increased adhesion by mechanical interlocking is mostly seen by enhanced interfacial area, strong adherence can be also presented between smooth surfaces and adhesives. Amalgam, filler for pretreated teeth cavities, can be an example of mechanical interlocking [23, 33, 43].
1.3.4. Diffusion
The interdiffusion of polymer networks at the intimate contact surface across the adhesive interface also affects the adhesion [24, 38]. To achieve diffusion of a polymer chain, the adhesive and adherent surface should be compatible with each other and polymer chains of both should have favorable mobility [23]. This mechanism is also affected by concentration, molecular weight, chain length, temperature, and glass transition temperature since they directly influence the mobility of polymer networks. The strength of adhesion highly depends on the contact time besides the mentioned factors [16, 23, 44]. As an example, in mucoadhesive systems, the diffusion mechanism offers the diffusion of polymer chains into the glycoprotein network as a function of time. The main parameters that affect this interaction are diffusion coefficient, molecular weight of the polymers, their chain mobility, crosslinking density, topological properties, and temperature at which the binding happens [16].
2. Tissue adhesives based on natural polymers
Adhesives such as fibrin glue, CAs and gelatin formaldehyde/glutaraldehyde (FA/GA) glues have been confirmed for clinical use. While these polymers have garnered considerable attention, they have certain constraints such as low bonding under humid conditions and poor cytocompatibility [45–47]. Polysaccharides, polypeptides, and proteins are rich in amine, hydroxyl, or carboxylic acid functional groups. Bonding interactions due to these groups with different chemical groups of the tissue surface is generally accelerated through chemically activating them using N-hydroxysuccinimide (NHS) or through the imine formation [1]. Through proper understanding of chemistry, various steps have been taken to make these adhesives a great choice for wound closures. In the following subsections, we will discuss some of the adhesives derived from natural sources and how they were modified to enhance their cytocompatibility, lower immunogenicity, and tune degradation profiles.
2.1. Fibrin-based tissue adhesives
Fibrin based tissue adhesives were first introduced in the 1940s. The glue was unprocessed and comprised of fibrinogen and thrombin [48]. In 1995, Alving et al. summarized various fibrin compositions, their implementations, negative responses or uses of them, fresh feasible applications as well as the need for controlled clinical efficacy studies. In Europe, fibrin glues have been a step further than those in the United States, in which antifibrinolytic agents like aprotinine and epsilon-aminocaproic acid have been used as their compositions [1], although the effectiveness of the use of these antifibrinolytic agents was not evident [49]. The formation of coagulation in fibrin glue, as explained by Martinowitz and Saltz, is like the final phase in physiological adhesion. Fibrin sealants consist mainly of two main parts: factor XIII fibrinogen and Ca2+ thrombin. Thrombin splits off fibrinopeptide A and B from α and β chains, commonly, to constitute a fibrin monomer. The constituted monomer substantially connects to an unstable clot through a hydrogen bond. Factor XIII (FXIII) is a thrombin-activated fibrin steadying factor that is used to build factor FXIIIa with Ca2+. Factor FXIIIa performs in the formation of amide bonds among glutamine and lysines, leading to insoluble clot-resistant proteolytic cleavages, onto the fibrin monomer or the ambiguous clot. The cross-linking requires insertion into the α-chain of fibrin of plasmin inhibitors such as plasmin α2 (α2-PI), α2-macroglobulin and plasminogen activator 2 Inhibitors (PAI-2). FXIII performs on other adherents such as fibronectin, thrombospondin, vitronectin and Willebrand factor, as well. Clot formation involves several cross-linkage steps; for instance, at the wound site, fibrin joins with collagen and bonding glycoproteins. At the same time, interconnections generate between the bonding collagen-based glycoproteins and other tissue proteins. All links at this injury place and the existence of plasmin inhibitors are the cumulative consequence of the creation of a fibrinolysis resistant solid adhesive insoluble clot [50]. A comparison of adhesives showed that they vary in fibrinogen and thrombin, the origin of thrombin and the process utilized for deactivation of viruses, in the concentration of their principal components. In turn, the mechanical force of the clot of fibrin depends on its concentration of fibrinogens and is often used as a measure of the quality of the adhesive. For the achievement of fast weathering, adequate adhesion and mechanical characteristics and optimum concentration of the two parts are therefore needed [1, 49, 51]. The adhesive power of the fibrin relies upon the substrate, glue structure, process of preparing of fibrinogen, the presence of water, and fat or collagen with its set time [1, 52].
Autologous fibrin sealants have been created for patient-specific application as the plasma is received from the same patient for which the sealant is to be used [1]. Fibrin glue is resorbable and biocompatible and does not result in necrosis, fibrosis or swelling of the tissue. The degradation time for fibrin glue depends on several factors and can last for several days to months [46]. Despite its ease of use, fibrin glue still carries the risk of contamination. Pre-treatments such as pasteurization, two-phase heat therapy with steam, dilution of solvent, dry thermal therapy, nanofiltration, plaster, pH treatment and chromatographic steps are used to disinfect it of any viral or bacterial contamination. Spotnitz has collected a comprehensive overview of the structure and relatively latest treatments of fibrin glue as a hemostat, sealant or adhesive [46].
2.2. Collagen-based tissue adhesives
Collagen is the major element of the extracellular matrix (ECM) and is thus necessitated in the initial stage of wound healing process generating granulation tissue after blood clotting [53]. The inherent pathway of the clot formation cascade is thereby activated by collagen-based materials. Collagen is biocompatible owing to its mammalian origin. Blood and coagulation components are adsorbed into the fibers of collagen and captured in the cross-pillars, thus are efficiently adhering to the wound [54]. These sealants are comparably similar to the fibrin-based sealants in the mechanism of adhesion. A dose-dependent decrease in human plasma coagulation by activating the collagen bound factor XII is caused by collagen type-I. The activation of glycoprotein-VI stimulates plasma thrombin production and improves the impacts of platelets on the healing of wounds. Nevertheless, collagen-based hemostats can swell with compression of the tissue. Recently collagen-based sealants joined the marketplace, and the regulatory authorities from many parts of the world have approved a few of these products. CoStasis® surgical hemostat is a spray liquid and can be applied to open wounds. CoStasis® is actively used in the field of vascular surgery for the sealing of cerebrospinal fluid leaks [55]. FloSeal®, initially created by Fusion Technologies Inc. are employing a CoStasis-like mixture. Baxter’s FloSeal® matrix utilizes human thrombin and bovine gelatin[56]. Pahacel® Absorbable Hemostat is a wet-absorbable collagen sponge and can be used on bleeding surfaces. It is used to help stop and control capillary, venous, and minor arterial bleeding when ligation or other standard techniques of control are not practical or effective. It has been used in surgical procedures [57, 58]. Helistat® Absorbable Collagen Hemostatic Sponge is collagen produced from the profound bovine flexor tendon. The tendon is considered to be one of the purest collagen sources which are easily available in commercial quantities. Generations of the Helistat® Absorbable Collagen Hemostats were approved long-time ago [59, 60]. The collagen-based hemostatic sponge of Avitene® (Davol, Inc.) is another commercial product. It is additionally accessible in collagen hemostat microfibrillar sheets. It was first introduced in 1976, and since then, more development work has improved the product [53, 61, 62]. In comparison to their fibrin counterparts, collagen-based sealants have a lower chance of infection. They are also relatively cheaper. New crosslinkers were investigated to enhance the adhesion strength of the tissue. The new citric acid crosslinker was used by Taguchi et al to boost tissue adhesion. Its adhesion strength was eleven times higher than a fibrin sealant, and it provided great consistencies and resorbability [63].
Up to date, different collagen sealants have been successfully introduced into the market and used in clinical settings, but more study is needed to better investigate their advantages for sealing surgery cuts [64].
2.3. Gelatin-based tissue adhesives
Collagen is extracted from the skin, bones, cartilages, ligaments, etc. and through partial hydrolysis can be turned into gelatin. On hydration, gelatin constitutes a semi-solid colloidal gel. It is suitable for soft-tissue applications due to the capacity to form a gel in situ. Gelatin is classified into Type A or Type B, depending on the method of producing it. It is biologically resorbable but needs to be cross-linked to be physiologically stable. Commercial sealing agents utilize body proteins, like thrombin, to catalyze chemical gelatin, which constitutes bonds over specific amino acids inside the gelatin chain, efficiently sealing the gel assembly [65]. Gelatin is utilized in the form of a hemostatic mechanical material whereby direct pressure is implemented owing to blood flow until the inherent body coagulation system provides platelets to stick and boost coagulation. The granular characteristic of a gelatin matrix allows the substance to comply with abnormalities on the surface of the wound. Commercially available pork gelatin products are Gelfoam®, absorbable gelatin powder - Surgifoam®. Pfizer®, Baxter®, and Gelfoam® have created products with additional modifications for a particular use. They are accessible in different sets, depending on the need. One can use them in many different ways, whether with dry sponge or moisturizing with saline solution or topical purified thrombin. They are frequently used in saturated tubes. Surgifoam® is a sponge that can also be observed in a flowable SurgiFlo® matrix. SurgiFlo® necessitates a combination of the human thrombin catalyst “Thrombin-JMI” and the porcine gelatin sealant [66]. Once applied, the Surgifoam® gelatin sponge is fully absorbed in 4 to 6 weeks after implementation. Baxter Healthcare’s FloSeal® hemostatic matrix comprises of a gelatin matrix from bovine origin and thrombin element derived from a human [67]. It has advantages from mechanical strength and enzymatic degradation perspectives [68].
The fluidic nature of gelatin-based sealants provides a considerable benefit than other technologies, such as the ease of use for irregular operational failures [53]. New cross-linking strategies were attempted to enhance gelatin-based sealants’ adhesive strength. To chemically crosslink gelatin, aldehydes like glutaraldehyde (GA) have been used. For utilization in thoracic and common vascular cases, gelatin-resorcinol-formaldehyde/glutaraldehyde (GRFG) adhesives were developed. These adhesives are sometimes referred to as “French adhesives”, comprised of (1) combination of gelatin and resorcinol or (2) combinations of formaldehyde (FA) and GA as polymerizing agents. Also, cross-linking through dityrosine is well tolerated with low inflammations, excellent wound healing, and little to no lung, gastrointestinal and vascular abnormalities. To increase the power of gelatin adhesion, photo-activation with UV-visible light can be used. GRFG bond was described as early as 1966. The resorcin-FA shapes a cross-linked polymer in essential settings. Glues based on FA have solid introductory bonding, and GA-based glues provide improved cohesion in vivo [69]. Thus, FA and GA are also often used in the adhesive formulations for optimum compliance and durability. The incorporation of gelatin into the adhesive provides elasticity and degradability similar to the surrounding tissue. GRFG’s binding strength is equivalent to the CAs glue on dry substrates and considerably greater than that of a fibrous glue. GRFG has been used for sealing surgical wounds in hemostatic surgery, gastrointestinal surgery, thoracoscopic surgery and lung surgery [70]. Although the weathering characteristics and adequate adhesive characteristics have shown to be remarkable, carcinogenicity arising from the incorporation of aldehydes limits the clinical employment of some glues [71]. There were conflicting results from trials of GRFG-treated tissues from various groups [72]. The benefits of using GA to support tissue adhesion have been proved by Matsuda and co-workers [73]. A dual function of cross-linking GA with the amine groups on gelatin is the remarkable adherence power of those GA linked gelatin along with the relationships with tissue amine groups. The aldehyde content depends on temperature, pH, GA-treatment time and GA-concentration, thus improving the ligament strength with growing concentrations of aldehyde. A longer cross-linking time and less bonding adhesive force were discovered to be available with cross-linking gelatin-resorcin, water-soluble carbodiimide and genipin. Carbodiimide and genipin glue have been improved in their cytocompatibility, while gelatin-resorcin glue has been deemed not suitable for clinical purposes [74].
2.4. Polysaccharide-based tissue adhesives
Polysaccharide-based tissue adhesives composed of dextran, hyaluronic acid, chondroitin sulfate, and chitosan (CHI) are non-cytotoxic and advantageous in a clinical setting compared to a conventional tissue-adhesive [75]. Polysaccharides adhere to soft natural tissue surfaces and mucosal surfaces, rendering them a perfect substratum for adhesive conjugates [76]. The presence of such groups also permits the polymer structure to be specifically modified, which provide important functional properties, improving their delivery or targeting ability. Moreover, natural polysaccharides show a variation in their overall electrostatic properties, e.g. they can be neutral (e.g., cellulose, dextran), negative (e.g., hyaluronic acid, alginate, chondroitin sulfate), or positively charged (e.g., chitosan) [77–79]. Dextran is a glucose polymer in which the glycosidic links are primarily of the α-(1–6) form naturally occurring polysaccharides as an adhesive biomaterial [80]. Compared to other natural polysaccharides, naturally abundant, non-toxic, and strong water-soluble dextran is more sustainable as bioadhesive material. Dextran could also be chemically modified by crosslinking to impart adhesion properties to its hydrogels [81, 82]. It has been used for a long time in biological and biomedical fields, such as plasma expansion and drug and protein transportation in the bioadhesive field owing to its low tissue toxicity [83].
Chondroitin is a glucuronic acid and N-acetyl galactosamine polysaccharide. The polymer is present in soft tissues and can be modified chemically, e.g. chondroitin sulfate [84, 85]. Because this polysaccharide is already present in human tissues, it has high biocompatibility and is low in toxicity. As such it makes a good candidate as a tissue adhesive. Chondroitin sulfate is a proteoglycan present in many ECM tissues. It is typically present in cornea stroma on proteoglycan core proteins 2-chondroitin sulfate-dermatan: biglycan and decorin. As a result, keratocytes in the stroma can recognize, degrade, and reshape chondroitin-sulfate based adhesives [86]. Most of the scientific studies concentrate on advances in the biologic functions of chondroitin sulfate [87]. However, there are still plenty of challenges including batch-to-batch variation, quick degradation upon interaction with body fluids, and poor tunability. Systematic and in-depth studies, therefore, on the stable adhesion and multifunctional integration, such as self-healing, high viscoelasticity, and good biocompatibility are needed [88–90].
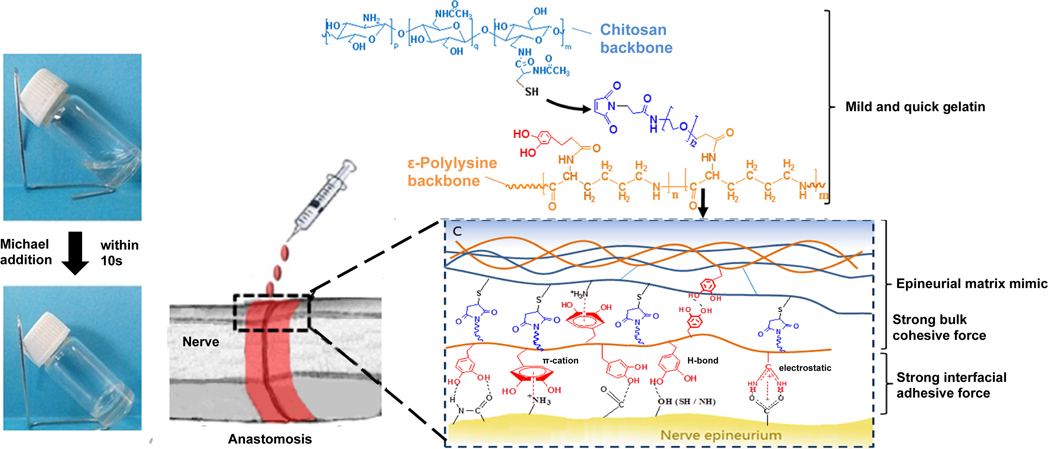
As an easy to obtain, biocompatible and biodegradable compound, CHI receives particular attention [91]. The chemical linkages between the chains are of great importance as they reflect intermolecular interactions and adhesion in the adhesive [92]. The cohesiveness of the chemical bonds between the chains must be taken into account, but inter-molecular interactions and adhesion inside the adhesive also affect the crosslinking connections. Ishihara and colleagues have created a photo-cross-linking CHI-based tissue adhesive, using photoactive azide groups [93, 94]. Upon UV illumination, the azide groups have been transformed into extremely reactive nitrene groups that, in turn, have responded to azo groups in the tissue or CHI amines, leading to tissue bonding and adhesive cross-relationships [95]. The use of UV light in connection with the presence of poisonous functional groups is harmful to the underlying tissue and requires comprehensive biocompatibility assessment [96]. In another work, Zhou and colleagues developed an adhesive hydrogel by using CHI and ε-polylysine for in situ forming nerve applications. In this study, gelation time, biocompatibility, mechanical property, and nerve bonding characteristic have been investigated. Moreover, to increase the binding strength, the glue was modified by catechol groups through a polylysine backbone (Figure 2). In an animal model of peripheral nerve, anastomosis was used for the efficacy of the resulted materials [97].
Figure 2.
Gelation mechanism of nerve adhesive hydrogel composed of CHI and ε-polylysine [97]. Adapted with permission from ref. [97], Copyright from 2016, The American Chemical Society.
While bioadhesives derived from natural sources offer many advantages in the form of low toxicity, excellent biocompatibility, and tunable enzymatic degradability, however, they have their limitations. They need some processing to be obtained from the biological source and hence are not cheap. The other major problem is the limitation with scalability and batch to batch variation. As such, some artificial or synthetic materials have been used to make medical-grade bio sealants. We discuss some of these in the next section.
3. Tissue adhesives based on synthetic polymers
The primary expectancies by using tissue adhesives can be summarized as cost-effectiveness, time efficiency, easy to perform and showing optimum cosmetic results. These parameters are important to evaluate the materials used as tissue adhesives. Synthetic tissue adhesives provide broad opportunities with their diverse properties and production methods. However, from early epoxy resin, polyurethane foam to phosphate-based bone cement and lactide-methacrylate-based platforms, the biggest problem with synthetic polymers is their degradability and release of toxic materials [98]. Here we discuss some of these.
3.1. Cyanoacrylate-based tissue adhesives
The first synthetic wound closure material was CA (cyanoacrylate) which was developed by a German chemist in 1949 and this tissue adhesive was clinically successfully used by a British plastic surgeon for the first time in 1959 [99]. Then n-butyl-2-cyanoacrylate was used for the first time to close skin incisions in Canada and Europe. In 1998, Octyl-2-cyanoacrylate (OCA) was approved by FDA (Dermabond) [100]. Properties of alkyl groups (-R) are very effective on the characteristics of overall polymer such as the length of alkyl group increases the polymerization rate of CA and this leads to a more flexible polymer with weak mechanical properties. On the other hand, longer CA chains cause slightly less tissue response due to slow release rate of toxic monomers [101]. It has five times higher breaking strength than general monofilament sutures and starts to function (polymerize) 10 seconds after application at room temperature [102]. For cutaneous applications, adhesiveness and continuity of the tissue adhesives are important primarily due to cosmetic reasons. For these applications, wound starts to re-epithelialize within 5–10 days, thus an adhesive of choice should be functional during this period. A newer formulation on OCA, high-viscosity OCA (HVOCA) remains on the wound site longer than the normal unmodified OCA due to its high viscosity and thus thicker texture [103]. There were no reports related to adverse effects or carcinogenicity of OCA [102] and a decade ago a study has shown that OCA lowers wound infection especially for gram-positive bacteria and inhibits bacterial growth around the site of surgery [104]. Since each material should be used according to its feasibility, OCA is suitable for only external use and there should be no gap or bleeding on the wound site, otherwise the adhesive can seep through and may block epithelialization. Despite these positive aspects, OCA is ten times more expensive than normal sutures. Furthermore, it cannot be used in high wound tension sites -tissue separations or locations on the surface of high muscular activity. In these areas, excessive tension should be decreased before application via subcutaneous sutures [105]. Early CAs were causing long-lasting inflammatory reactions in tissues, but by increasing the chain length of this polymer it can be less immunogenic. However, OCA is still not suitable for applications under the dermis [101].
To compare OCA with other wound closure and tissue adhesive materials, one can say that OCA decreases the operating room time [106], fewer reported infections than conventional sutures and better antimicrobial effect [101] with better long-term cosmetic outcome [107]. CAs are being studied to seal esophageal cancer related anastomotic leakages. Some studies have revealed that CA creates a mechanically strong adhesion to seal the leakage. However as pointed out, their internal use can lead to inflammation, tissue necrosis or infection. Thus, subjects of the experiment showed low collagen production at the site of application and decreased burst pressure [64]. CAs are becoming popular in dentistry and plastic surgery. However, their exothermic polymerization reaction, release of toxic monomers, low viscosity, and lack of flexibility for strong mechanical properties impose limitations on their use in a wide range of applications. On the contrary, scientists are looking for ways to render CA into a more bio-friendly state. To increase their biodegradability, more hydrophilic Cas isoforms such as methoxypropyl CAs [108] or different plasticizers and viscosity adjustments are made to be used with these adhesives to accommodate CAs for broader tissue applications.
3.2. Polyethylene glycol (PEG) and polyester-based tissue adhesives
PEG is a broadly used biomaterial and there are multiple adhesive products in the market which consist of PEG as their primary component. PEG is biocompatible and has highly tunable physical properties [109]. Even though it has low adhesion capability PEG-based tissue adhesives are used with other polymers to create layered structures and used as sealants to prevent fluid and gas leakages and used for surface modifications to enhance biocompatibility and decrease biodegradability. PEG derived adhesives generally consist of linear or branched PEG molecules with chemically functionalized groups to enhance crosslinking or biodegradation -copolymerization with biodegradable polymers.
Photo-initiation is favorable for crosslinking of PEG because it eliminates the need for strong oxidizing agents. Moreover, shape and location of the adhesive application are challenging for rigid structures, therefore surgeons are looking for liquid tissue adhesives that can be applied at the site of injury (especially for closed surgeries) that can then be cured with light or other oxidizers. At this point, the form of the light that will work for polymerization comes to the focus, since that light will also affect living tissues. Hence, recently more feasible way with living tissues such as visible light rather than UV has started to gain attention from researchers. Another FDA approved tissue adhesive is FocalSeal-L. This product consists of two solutions in which the first one is PEG–polylactic acid (PEG-PLA) which possesses mechanical interlocking properties with tissue proteins and the second product consists of PEGylated-poly(trimethylene carbonate) which increases the mechanical strength of the adhesive through covalent interactions [111]. FocalSeal-L is mainly used to seal air leakages in lung surgeries.
To overcome weak adhesion and weak structural integrity of PEG-based materials, Kelmansky et al. designed a PEG-based tissue adhesive that was modified with NHS and amine (NH2) groups [110]. The generated adhesive consists of different ratios of NHS and NH2 groups with four-armed PEG pre-polymers (Figure 3A). NHS groups interact with the amine ended PEG sites to demonstrate amide bonds [112] and provide cohesive strength to the adhesive while -NH2 interacts with both, adhesive’s integral structure and tissue surface. Both pre-polymers are in the liquid form at room temperature and can be injected into the wound site (Figure 3B).
Figure 3.
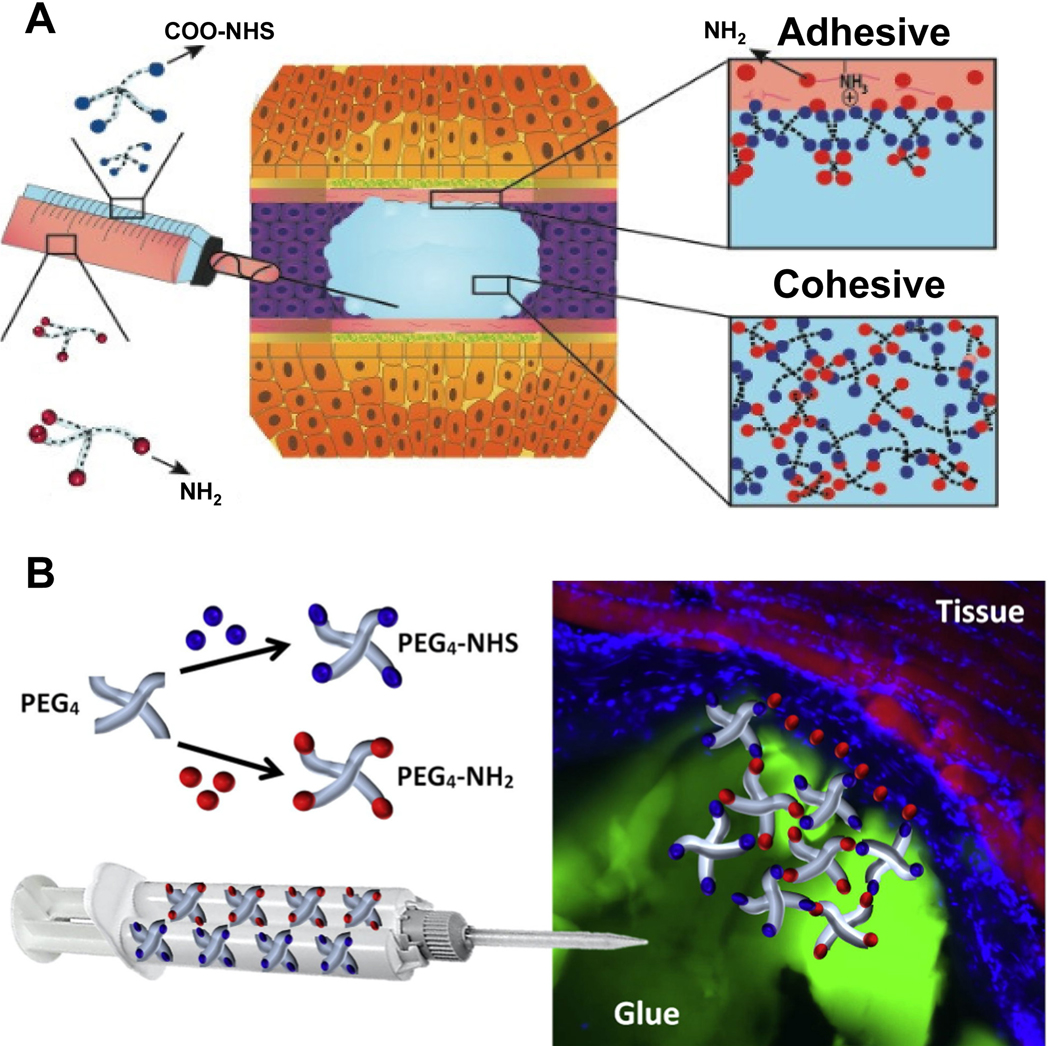
A) Working principle and B) Representative schematics of four-armed NHS-NH2 modified PEG polymers as bioadhesives [110]. Adapted with permission from ref. [110], Copyright from 2017, The Elsevier B.V.
PEG is generally used combined with polysaccharides and protein-based adhesives. As an example of synthetic tissue adhesive of PEG, FDA approved adhesive Coseal. This PEG-based tissue adhesive consists of 4 PEG armed structures and three of these arms capped with thiol and other is capped with glutaryl-succinimidyl ester and pentaerythritol in the center. Thiols and carbonyl groups of succinimidyl react which results in adhesion to the tissue through strong covalent bonds. This adhesive is used in sealing suture lines and vascular grafts [113].
PEG-based adhesives are flexible and mostly have tunable shapes. This ability is used to overcome the challenge of wounds that have non-flat complex geometries. Bian and colleagues designed a photo-initiated instant fit-to-shape sealant [114]. Their design consists of three main components. Operation starts with the preparation of maleic anhydride-functionalized chitosan (MCS), benzaldehyde-ended PEG (PEGDF) and polyethylene glycol diacrylate (PEGDA). Interaction of MCS and PEGDF turn into an injectable and moldable shear-thinning hydrogel via Schiff-base crosslinking. This hydrogel can be filled into the wounds with non-flat complex geometries and fill their interior volumes. Finally, after UV illumination, as a natural outcome the mechanical strength of the tissue adhesive and its adhesiveness with surrounding tissues increases due to the polymerization of vinyl groups on MCS and PEGDA thus leading to the improved sealing activity (Figure 4A). With its improved ability of shape fitting and mechanical strength, it could withstand against gravity or dynamic movements on the application sites (Figure 4B). The designed sealant is also compatible with aqueous media (Figure 4C). Researchers applied tension, compression and shear forces and even flushed water on it, however, the sealant bore all of this without having a defect on its structure. In-situ application of the sealant can be observed in Figure 4D. Fit-to-shape sealant perfectly covers the defect area. Not only the geometry and stiffness but also the chemistry of the application site is also important for adhesives. Although PEG-based fit-to-shape sealant can work in extreme pH conditions (pH 1) which makes it suitable for sealing wounds inside stomach, however, the most important disadvantage of PEG-based tissue adhesive is its high swelling ratio (up to 400%). Hence, there should be an additional caution to prevent swelling pressure to the surrounding tissues during their use in tight areas [21].
Figure 4.
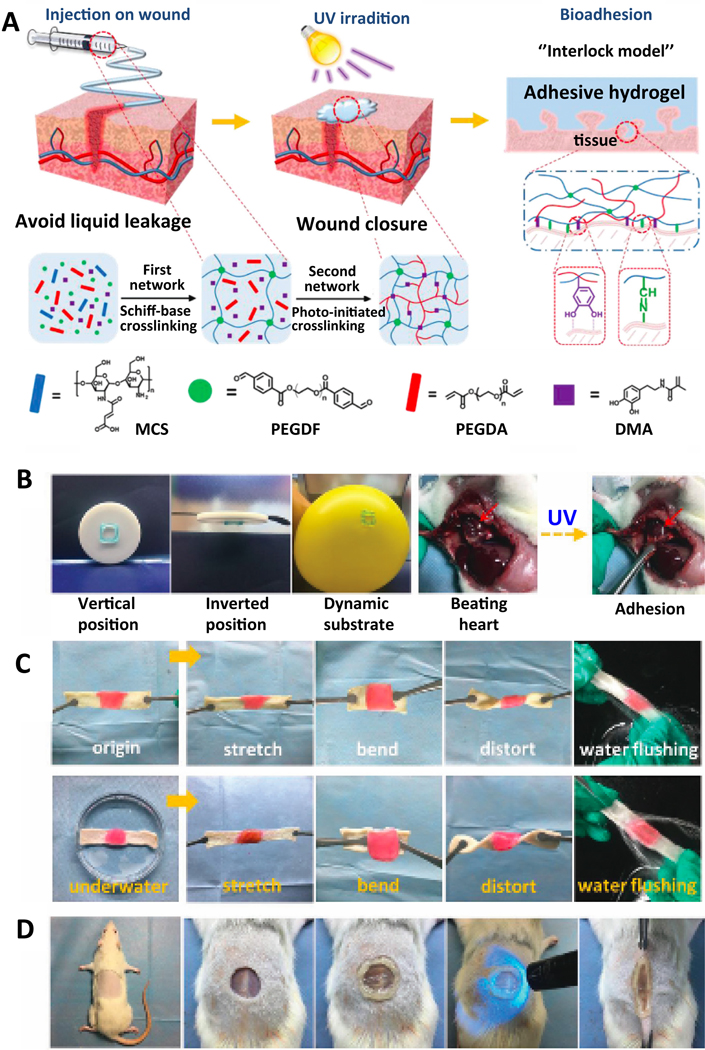
PEG-based photo-crosslinking fit-to-shape sealant: A) Schematic illustration of sealant and principles of crosslinking. B) Suitability of sealant to different surfaces and dynamic forces. C) Resistance of adhesive to various forces in aqueous medium. D) In-situ application [114]. Adapted with permission from ref. [114], Copyright from 2019, The Royal Society of Chemistry.
In addition to these tissue adhesives, researchers continue to search for more safe and efficient tissue adhesives. A group of researchers at Bayreuth University used radical ring-opening copolymerization with glycidyl methacrylate (GMA), (oligo (ethylene glycol) methacrylate (OEGMA), and 2-methylene-1,3-dioxepane (MDO) with the presence of Fe(acac)3 as crosslinker. They achieved adhesion strength of 13.13 ± 1.74 kPa on soft tissue which is very high when compared with Coseal and Fibrin glue. However, this material had shown minor cytotoxicity [115] and future studies are required for its biocompatibility.
Recently, polyester-based synthetic sealants with minimum side effects have gained significant clinical interest and use. For example, TissuePatch™ is a four-layer patch consisting of two poly(lactic-co-glycolic) acid (PLGA) and two layers of NHS functionalized polymer. This material can easily bind to amine groups of tissue proteins with the NHS functionalized ends [34]. Polyester has high tensile strength and biocompatible with biodegradable characteristics [116, 117].
As mentioned earlier PEG has high swelling risk, to overcome this obstacle Zhang and coworkers used PEG with polyester and produced (HPEGDA) a PEG-polyester hydrogel, which shows effective bonding with soft tissues and minor cytotoxicity and hydrolytically degradable. Furthermore, its viscosity and photo curability HPEGDA can be tailored according to the operation site. Resulted material can be a good candidate for tissue adhesive and sealant applications [118]. Another research conducted by Ohira and coworkers used polyester fabric to reinforce fibrin sealant patch for acute aortic dissection and maintain hemostasis [119]. Polyester based tissue sealants also have been used in mussel inspired tissue adhesives for wet surfaces. Detailed information will be given in the following sections.
3.3. Polyurethane-Based Tissue Adhesives
Polyurethane is one of the oldest materials in which researchers have focused on tissue adhesive using polyurethane foam [120]. Urethane consists of isocyanate groups which have a high affinity to nucleophiles (e.g. hydroxyl and amine groups). Hereby polyurethane-based adhesives have a high affinity to proteins of operated tissue. Due to its non-toxic characteristic, it is biocompatible and biodegradable [121].
There are many polyurethane-based commercial adhesives available in the market. TissueGlue® is one of the adhesives that has European Conformity (CE) approval. It is used to hinder the accumulation of excessive body fluid under the skin during abdominal surgeries [85]. For soft tissue adhesion, Ates and coworkers designed a polyurethane adhesive and used it with chlorogenic acid and xylose and compared their adhesive strengths. Xylose incorporated polyurethane adhesive showed strong interaction while chlorogenic acid incorporated polyurethane showed little lower strength of adhesion [122, 123]. Besides its use in soft tissue adhesives [124], polyurethane-based bone adhesives mixed with hydroxyapatite show enhanced adhesion when compared to commercial bone cement [125].
Multifunctionality of the adhesives is also important. Most of the past studies had a specific aim or function – covering the wound and providing mechanical support to operation site. But recent studies on tissue adhesives are focused on exploiting their multifunctional properties. These adhesives are functionalized as antibacterial, promoting cellular growth, self-healing, reversible attaching, or modified to respond to external stimuli such as pH, temperature, or biomolecular concentration [12]. Le and coworkers designed a PEG-poly (sulfamethazine ester urethane) (PEG-PSMEU) tissue adhesive that is responsive to pH and temperature changes. The polymer is prepared at low temperatures and it transforms into gel form in the body conditions. The adhesive is also capable of releasing therapeutic agents on a wound site [126].
Polyurethane-based tissue adhesives have high thermal stability [34]. Thus, they are being used in a wide variety of applications. On the other hand, it should be noted that while ester-based polyurethane is considered non-toxic, ether-based polyurethane’s degradation products show toxicity. Besides, polyurethane-based tissue adhesives have two drawbacks of long set time [127] and poor mechanical strength [34].
4. Bioinspired strategies
Animals and plants have developed a variety of mechanisms, such as reversible dry and wet with permanent chemical adhesion for attaching and climbing surfaces [128]. In dry adhesion, intermolecular forces for example van der Waals interactions are required to attach surfaces. In case of wet adhesion, the reversible adhesion force is generated due to an augmented viscosity and surface tension around the contact area, mainly due to capillary forces. Organisms that use wet adhesion typically have bioadhesive pads, which secrete a thin film of liquid to enable the adhesion [129]. In the case of permanent adhesion, high-strength long-term bonds are formed between the attaching surfaces. In nature, animals utilize different adhesion mechanisms, for example, geckos use dry adhesion, insects and tree frogs use wet adhesion. Chemical adhesion is used by mussels, sandcastle worms, Notaden frogs, and barnacles.
In recent years, adhesion mechanisms have been mimicked to produce novel tissue adhesives with high strength and adhesion reversibility, as well as applicability in wet and other extreme conditions. In the following subsection, various adhesives inspired by animals or plants are presented and discussed.
4.1. Animal-inspired adhesives
4.1.1. Geckos and anti-wetting biomimetic tissue adhesives:
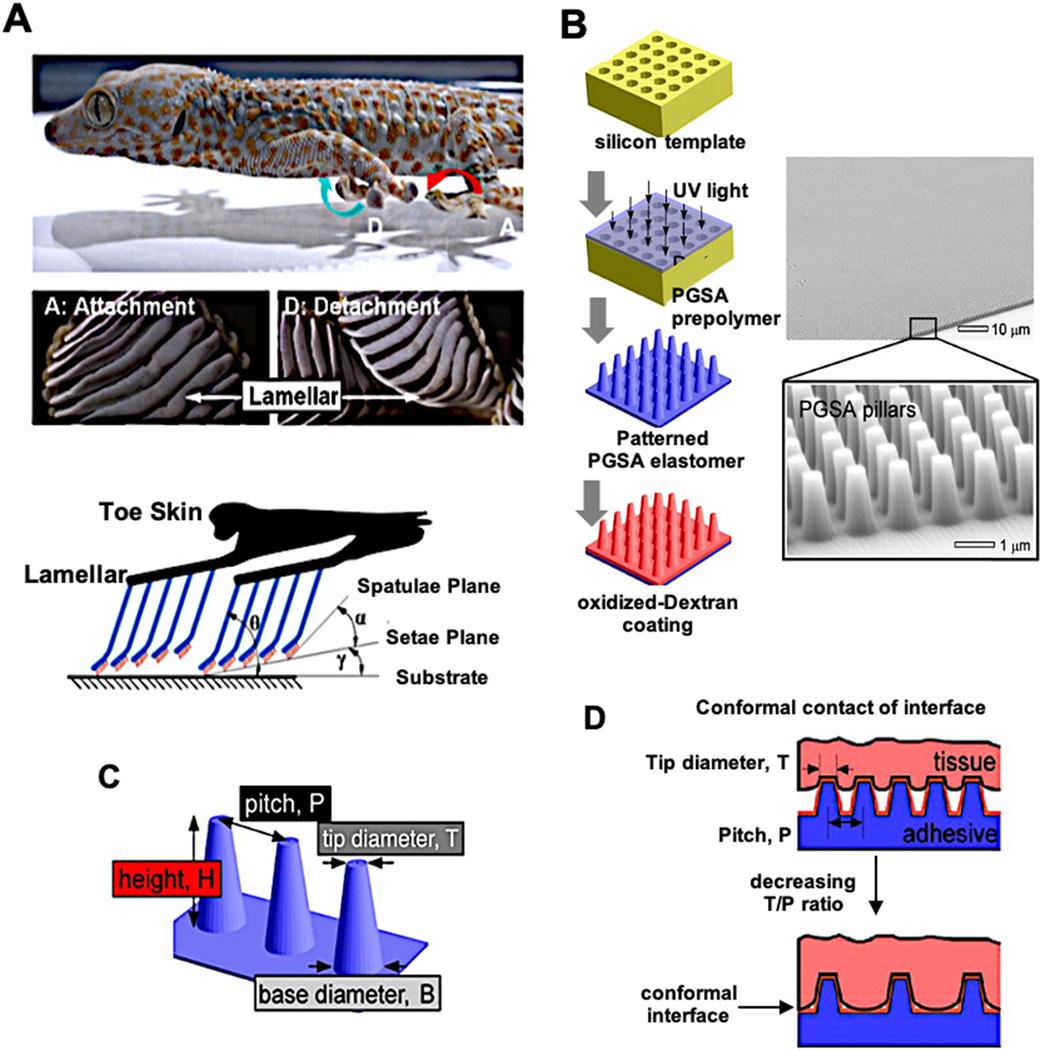
Geckos are capable of walking on smooth, rough, vertical or even ceiling surfaces through the fibrillar structures on their feet that can increase their adhesion on different surfaces (Figure 5A). With their super-hydrophobic and uniquely designed hairs Geckos can manage to walk on a wide range of surfaces, especially with their anti-wetting ability in their feet allows them to walk and attach wet surfaces strongly [130]. This well-evolved structure sustains removal of any dirt and foreign material on its self without the need of a chemical but it contains some lipids and proteins to enhance hydrophobicity [131]. But the main factor is the keratin hairs that are covering the soles of geckos. Each hair in this bundle structures produce approximately 10−7 N force via van der Waals and/or capillary forces. This small force multiples millions of times with all hairs on the feet of Gecko together up to ~10 N cm−2 [132]. With progress in nanotechnology, we can produce similar fibrillar structures to gecko’s feet. Mahdavi and coworkers used nano-molding (Figure 5B) technology to produce poly (glycerol sebacate acrylate) (PGSA) fibrils and they coated this structure with oxidized dextran to increase biocompatibility. Nano molded pillars have 4 main parameters: height (H), diameters on tip (T) and base (B) with pitch (P) between two pillars (Figure 5C). To increase the contact surface between adhesive and tissue, tip diameter and the pitch distance should be low and wide, respectively (Figure 5D). All the pillar variables have an effect on adhesion quality but in general, a decrease in the P to T ratio results increase in adhesive property. Obtained elastomeric tissue adhesive showed strong adhesion on wet surfaces and low tissue response in in vivo studies [133]. Baik and colleagues developed a reversible adhesion system that was inspired by the dome-like protuberances in suction cups of octopi [134]. Using a silicone mold, microhole patterns were created. These were filled with trapped air and made of polyurethane acrylate-based polymer (s-PUA), which has a low air permeability and thus improves the suction behavior under both dry and wet conditions.
Figure 5.
A) Attachment and detachment positions of lamellae of gecko feet and illustration of setal arrays located on lamellar structure on toe skin [147]. B) Production stages and SEM micrographs of gecko-inspired pattern. C) Properties of pillars, it was designed by different types of pillars to compare their properties. D) The effect of T/P ratio on adhesive-tissue contact surface [133]. Adapted with permission from ref. [147], Copyright from 2013, The Springer Nature Limited; Adapted with permission from ref. [133], Copyright from 2008, The National Academy of Sciences, USA.
4.1.2. Mussel inspired biomimetic tissue adhesives:
Mussels use a combination of noncovalent and covalent chemical interactions with the substrates to strongly adhere to different types of surfaces and as a result, they even adhere to the adhesion resistant material of poly(tetrafluoroethylene) (PTFE) [135]. Mussels naturally produce the adhesive, byssus [136], to attach to almost any surface inside water without being affected by wetness, salinity, or temperature of the water and withstanding against strong environmental conditions [137]. Extensive research has been performed in recent years to characterize the byssus and to imitate this waterproof adhesive. Several mussel adhesive proteins (MAPs), rich in catechol groups (DOPA) (Figure 6A) and amine groups (lysine) [138], have been found in the blue mussel, M.edulis, and in other species [139–141]. Furthermore, metals, such as copper, zinc, iron, and manganese were detected in the threads and plaques [142]. The binding of iron to DOPA has been found to enhance the mechanical performance of threads [143], which consist of an inner core and an outer thin coating. The inner core is mainly built up of collagenous proteins, fibroin and elastin. The outer thin coating is composed of six different foot proteins (fp) type 1–6 DOPA proteins and leads to 5–10 times stronger adhesion than the core [128]. The oxidation of the catechol side chain of DOPA after the secretion of the adhesive material leads to intermolecular coupling reactions and result in adhesion to solid substrates even in water. Most of the mussel-inspired synthetic tissue adhesive research uses DOPA with PEG groups. For example, Lee and coworkers synthesized a DOPA-modified PEG that can transform into an adhesive gel very rapidly [144]. Burke et al. used this polymer for its tissue adhesive function through more innovations. They used liposomes that can release DOPA oxidizing agents periodically at optimum temperature and induce crosslinking of the adhesive gel [145]. Lee and coworkers used a similar approach and added polycaprolactone (PCL) together with PEG to achieve a copolymer that has 10 times more strength than the commercial fibrin glue [146].
Figure 6.
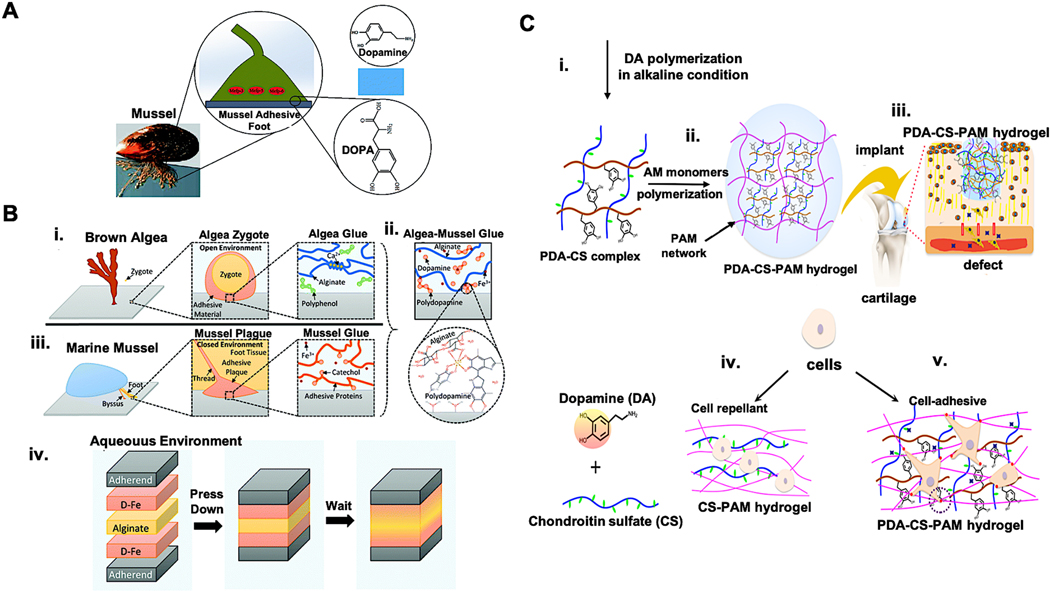
Mussel inspired tissue adhesives. A) Mussel adhesive proteins (MAPs) contain high levels of DOPA with catechol groups [149]. B) Schematic representation of the algae–mussel hydrogel composite sealant. Adhesive components of i) brown algae and ii) marine mussel were combined to obtain iii) an algae-mussel mimicking adhesive. iv) The adherendś surface was treated with dopamine-iron (D-Fe) solution and 5% ALG solution was injected in between and adherents were pressed together [150]. C) Production of mussel-inspired chondroitin sulfate (CS) tissue-adhesive hydrogel. i) Creation of the polydopamine (PDA)–CS complex and ii) generation of PDA-CS-polyacrylamide (PAM) hydrogel. iii) Application of hydrogel in a cartilage defect. iv) The CS-PAM hydrogel without PDA is cell repellent. v) The incorporation of PDA into CS-PAM hydrogel leads to cell adhesion. [86]. Adapted with permission from ref. [149], Copyright from 2017, The Royal Society of Chemistry; Adapted with permission from ref. [150], Copyright from 2019, The Royal Society of Chemistry; Adapted with permission from ref. [86], Copyright from 2018, The American Chemical Society.
The problem with wet surfaces is the presence of water. While maintaining a strong bond, water should be repelled from the surface. Xu and coworkers used mussel-inspired polyester to increase resistance to water penetration and create a strong bond [148]. This design is innovative because it is the first synthetic polymer that can operate underwater without requiring an additional solvent. Xu and coworkers used 0.8 % sebacic acid, 0.05 % catechol functionalized diol, and 0.15 % coumarin diol to produce polyester adhesive. Sebacic acid increases non-polar structure, catechol enhances adhesion to surface, and coumarin induces crosslinking of adhesive materials. Using this blend of polymers, the researchers obtained a strong adhesion even under wet conditions.
In recent years, catechol-containing proteins [135, 151], catechol-modified natural [152–155] or synthetic polymers [156, 157] were used to generate mussel-inspired hydrogels. Adhesive hydrogels are formed after the oxidation of catechol groups by oxygen (O2) or oxidant reagents as curing agents, such as Fe3+ or sodium periodate (NaOI4). Cholewinski and colleagues [150] generated algae-mussel hybrid hydrogel adhesive by incorporating the advantages of brown algae and marine mussel adhesives (Figure 6B). To obtain a unique hydrogel system, ferric ions, dopamine with ALG have been combined. ALG has the ionic crosslinking ability with ferric ions. Dopamine which has a catechol group can react with ferric ions as well and lead to the self-polymerization of dopamine to polydopamine (PDA).
The active catechol groups on PDA can conjugate with reactive groups of biomolecules, e.g. amino, carboxyl, or catechol, and thereby facilitate cell adhesion [158]. Most hydrogels based on glycosaminoglycan are negatively charged and prevent the adhesion of cells. Thus, Han and colleagues generated a PDA−chondroitin sulfate-polyacrylamide (PDA−CS−PAM) hydrogel for the regeneration of cartilage without the need for growth factors [86] (Figure 6C). In a recent study, an ultra-tough and self-healing hydrogel was created for wound healing applications with improved affinity to the cell and tissue adhesion by using dopamine-grafted oxidized sodium alginate (OSA-DA) and polyacrylamide (PAM) [159]. Since bacterial infections can delay wound healing, Du et al. developed a unique adhesive hydrogel patch having poly(ethylene glycol) diacrylate/quaternized chitosan/tannic acid (PEGDA/QCS/TA) inspired by mussel for sutureless wound closure [160]. The antibacterial activity of the patches was determined and a killing efficiency of 100% was observed for S. aureus and 93% for E. coli. In another study, the application of catechol containing poly(amidoamine) (PAA) polymer as tissue adhesive allowed the scar-less wound closure in Sprague Dawley rats [161]. For internal medical applications, Zhu and colleagues developed a mussel-inspired tissue glue consisting of bovine serum albumin, dopamine and citric acid [162]. This adhesive demonstrated 10-times higher adhesion strength in just 30 min of application than a commercial product of fibrin glue in a wet condition. Han et al. created PDA–polyacrylamide (PDA–PAM) single network hydrogels [163]. To maintain sufficient catechol groups in the hydrogel, the overoxidation of PDA during the hydrogel synthesis was prevented by the polymerization and crosslinking of acrylamide. Furthermore, mussel-inspired hydrogels are good candidates for the generation of self-adhesive bioelectronics [164]. In a recent study, hydrophilic, conductive, and redox-active sandwich-like nanosheets were generated by self-assembly of poly (3,4-ethylenedioxy-thiophene) (PEDOT) on PDA-grafted and sulfonated graphene oxide (PSGO) template [165]. Thereby, stretchable nanosheets with excellent adhesiveness and conductivity were obtained, which could be used as adhesive electronic skin for the detection of electrocardiogram (ECG), electromyogram (EMG), and electroencephalography (EEG signals).
4.1.3. Mimicking the mucus of slugs and the sandcastle worm:
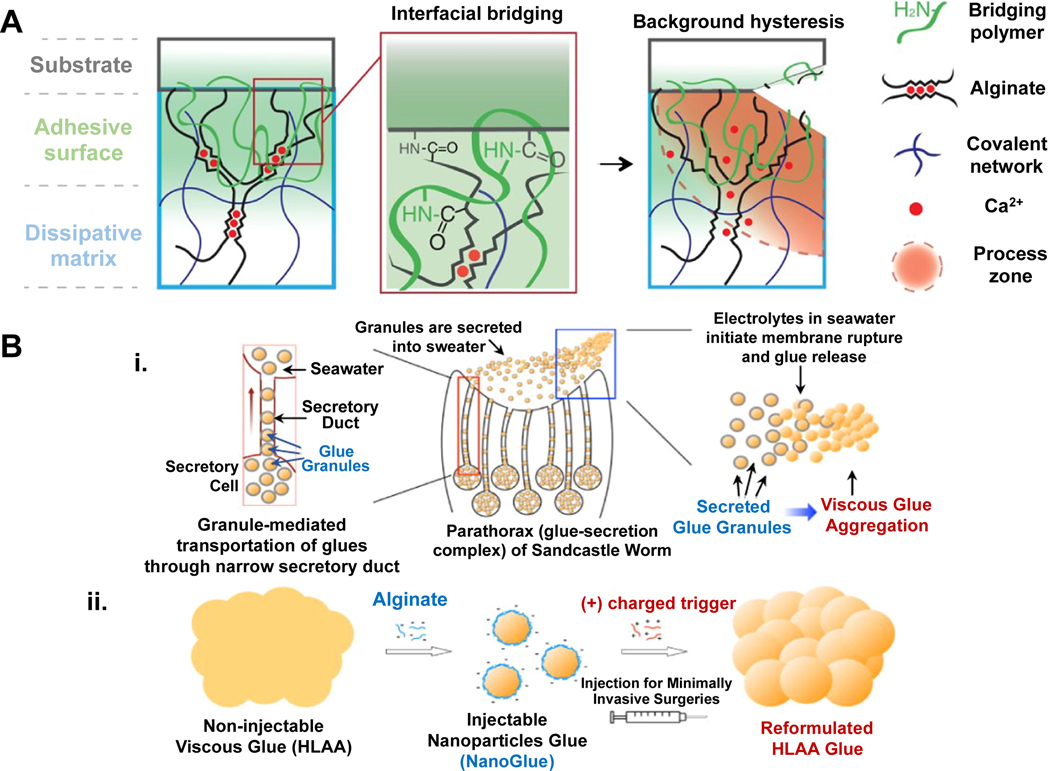
Dusky Arion slug (Arion subfuscous) secretes a defensive mucus in the event of a threat, making it almost impossible to remove the slug from the surface by a predator. This slug produces mucus, which has a tough matrix consisting of interpenetrated positively-charged proteins [166]. These proteins can be used for adhesion and energy dissipation, allowing covalent and electrostatic interactions. To mimic this slug adhesive, Li and colleagues [167] created a tissue adhesive consisting of two layers; a dissipative matrix and an adhesive surface (Figure 7A). The dissipative matrix was made of a hydrogel containing ionically and covalently cross-linked polymers, which dissipates energy via hysteresis under deformation. The adhesive surface consisted of a bridging polymer that can bind via covalent bonds, electrostatic interactions, and physical interpenetration to the substrate. This adhesive strongly adhered to porcine skin, cartilage, and arteries, closed holes in heart tissues, and it was compatible with in vivo dynamic movements, e.g. beating heart. Furthermore, it was also able to stop bleeding from lesioned rat liver tissue.
Figure 7.
Mimicking the adhesives produced by slugs and sandcastle worms. A) Slug mucus mimicking adhesive with an adhesive surface and dissipative matrix. The dissipative matrix contains ionically (red circles, calcium) and covalently crosslinked polymers. The adhesive surface interacts with the substrate via a bridging polymer. A crack at the process zone (orange area) dissipates increased amounts of energy as ionic bonds between calcium ions and ALG chains break [167]. B) Schematic representation of granule-mediated transportation of the glue of sandcastle worms. i) Sandcastle worms condense highly concentrated proteinaceous glues into micro-sized granules for the release into seawater. Electrolytes in the seawater lead to the rupture of granule’s membrane. The released viscous glue aggregates and attaches to surrounding objects. ii) Injectable nanoparticles glue (NanoGlue) is generated by encapsulation of non-injectable viscous glue (HLAA) with alginate. After the injection, positively-charged trigger molecules initiate aggregation of NanoGlue particles and a viscous glue is formed as the native HLAA [170]. Adapted with permission from ref. [167], Copyright from 2017, The American Association for the Advancement of Science; Adapted with permission from ref. [170], Copyright from 2015, The John Wiley & Sons, Inc.
Sandcastle worms (Phragmatopoma californica) secrete a condensed and sticky adhesive composed of oppositely-charged proteins that are complexed with magnesium and calcium ions to construct their dwellings out of sand particles [168]. Positively-charged proteins consist of high contents of basic residues with amine side chains and negatively-charged proteins include increased numbers of acidic phosphoserine residues [169]. Approximately, 20 mol% of the adhesive amino acid residues are basic and 30 mol% are acidic. To prevent the clogging of their secretory ducts, sandcastle worms package the highly concentrated proteinaceous glues into micrometer-sized granules [170]. These granules are released into seawater, which is rich in electrolytes and has a high pH (>8), leading to the rupture of granule membranes. The released glue binds then to the surrounding objects, such as sand particles. Thus, this strategy was mimicked to enable the injection of a viscous water-insoluble hydrophobic light-activating adhesive (HLAA) based on PGSA polymer [170] (Figure 7B). A water-soluble negatively charged ALG was used as a surfactant to encapsulate the hydrophobic viscous HLAA. Thereby, injectable HLAA (NPs) were generated, called NanoGlue NPs. The exposure of NanoGlue particles to oppositely charged electrolytes (positive-charged trigger molecules, such as protamine) resulted in neutralization of the surface charge and initiated the coalescence and generated viscous glue in the tissue. Such nanoparticle glues could potentially be applied in retina repair.
Shao and colleagues used oppositely-charged synthetic co-polyelectrolytes that contained the same chemical side chains (amines and phosphates) in the same molar ratio as the natural sandcastle worm glue proteins [171]. At physiological pH, the aqueous mixtures of the synthetic co-polyelectrolytes condensed and led to phase separation into a dense fluid state called a complex coacervate. The oxidative crosslinking of the coacervates with wet cortical bone specimens via catechol sidechains resulted in binding strengths of almost 40% of the strength of commercial CAs.
4.1.4. Mimicking silk:
Silk as a polymer is produced by various insects and arachnids. In the textile industry, domesticated silkworms, such as Bombyx mori, are used to obtain silk. Cocoons of B. mori silkworm contain two main types of proteins, fibroin and sericin. Fibroin is made of a 1:1 ratio of a heavy chain of 390 kDa and a light chain of 26 kDa which are connected by a disulfide bond [172]. Silk fibroin is coated with sericin. Sericin is a family of glue-like proteins in the range of 20 to 310 kDa. It contains two silk fibroin fibers to build the composite fibers of the cocoon. Burke et al. functionalized the silk fibroin with catechol groups to generate a new type of adhesive [151]. Furthermore, the addition of PEG chains before dopamine conjugation improved the aqueous solubility without affecting the ability of silk fibroin to form β-sheet structures. This sealant also promoted the attachment and proliferation of human mesenchymal cells in vitro. In a recent study, Seo and colleagues generated a calcium-functionalized silk fibroin as a strong biocompatible adhesive for epidermal electronics [173]. The metal-chelate bonding and water-capturing of calcium ions increases the viscoelasticity and the mechanical interlocking of the silk film, increasing the mechanical interlocking at the tissue interfaces. Furthermore, this adhesive has several advantages in the field of epidermal applications, such as reusability, stretchability, and conductivity.
Luo et al. isolated silk fibroin from Bombyx mori silk cocoons [174] and created hydrogel adhesives by mixing with tannic acid (Figure 8A) using a twin-barreled syringe (Figure 8B). The obtained adhesive showed self-healing capability (Figure 8C) and the ability to maintain the adhesiveness underwater after distorting, flushing with water, and stretching (Figure 8D). The treatment of wounds with the bioadhesive resulted in nearly complete sealing of the wound 7-days post-operation (Figure 8E). The histological evaluation revealed more disordered structures in non-treated skin compared to treated skin. Interestingly, the high extensibility of the bioadhesive was demonstrated with an extension rate of 32,000% from 5.5 mm to approximately 1,760 mm in length after stretching.
Figure 8.
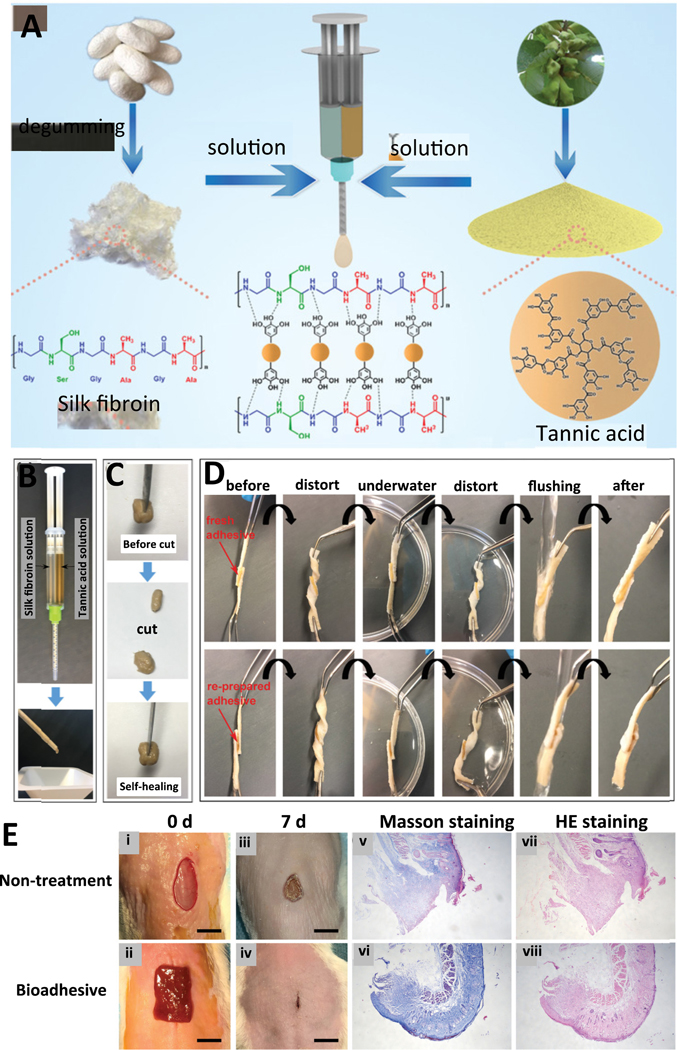
Silk fibroin-based hydrogel adhesive. A) Overview of the production of the adhesive hydrogel by crosslinking of silk fibroin isolated from Bombyx mori silk cocoons with tannic acid. B) Use of a twin-barreled syringe for mixing of the silk fibroin with tannic acid. C) Self-healing property of the generated adhesive after cutting into two pieces. D) Maintenance of adhesiveness of freshly and re-prepared silk fibroin-based hydrogel after gluing two porcine skin pieces and distorting in the air and underwater, flushing with water, and stretching. E) Images of wounds with and without bioadhesive treatment after 0 days (i, ii) and 7 days post-operation (iii, iv). Analysis of healed skin using (v, vi) Masson staining and (vii, viii) HE staining [174]. Adapted with permission from ref. [174], Copyright from 2020, The John Wiley & Sons, Inc.
4.2. Plant-Inspired Adhesives
The prohibitive costs of catechol-functionalized biodegradable polymers and the potential neurological effects of dopamine raise concerns about the commercialization of these tissue sealants [175]. To solve these issues, plant-based polyphenol groups i.e. tannins can be used as an alternative. Guo et al. designed a novel family of tannin-inspired sealants by performing a one-step Michael addition reaction of gelatin and tannic acid under oxidizing conditions and crosslinking with silver nitrate [175]. These gelatin bioadhesives inspired by tannin demonstrated considerable adhesive strengths to the moist tissue and intrinsic antibacterial and antifungal characteristics. A composite adhesive hydrogel was obtained by using gum arabic, calcium ions, and sodium alginate to resemble the characteristics of the adhesive secreted from the leaves of sundew (Drosera) [176, 177]. The in vivo application of this hydrogel promoted wound closure. The combination of sundew-inspired hydrogels with mouse adipose-derived stem cells (ADSCs) showed superior wound-healing than some other therapeutic biomaterials.
After cellulose, lignin is the second most common plant-based polymer. It contains numerous functional groups, e.g. phenolic hydroxyl and methoxy groups, that can be converted to redox-active quinone/hydroquinone [178]. Gan and colleagues [178] generated silver (Ag)-lignin core-shell nanoparticles (NPs) and produced hydrogels by gelling an aqueous solution containing Ag-lignin NPs, acrylic acid, pectin, ammonium persulfate (APS), and PEGDA. Pectin and polyacrylic acid (PAA) form n interpenetrating network via multi-crosslinking of covalent and noncovalent bonds. The formation of free radicals between Ag-lignin NPs through redox reaction and APS initiates the polymerization of the hydrogel under room temperature conditions. Catechol groups are continuously by Ag-lignin NPs and this enables the repeatable and long-lasting adhesion of the hydrogel.
5. Application areas of tissue adhesives
As discussed throughout this review, tissue adhesives have aroused great interest. They can be powerful alternatives to sutures and staples. Depending on the application, they can be categorized as internal and external adhesives. External adhesives are specially used for wound closure and to prevent bleeding during surgery. They have great advantages as they are easy to perform, minimize inflammatory or immune reactions, have shorter operating time, and do not require suture removal. For these reasons, their application areas are expanding in diverse medical applications. In this part of the review, application areas of tissue adhesives in the context of their safety, toxicity, efficacy, usage, cost, mechanical strength, and bonding performance will be discussed.
Although they are profoundly promising, currently available tissue adhesives have several drawbacks in the clinic for safety concerns and we will also consider these properties.
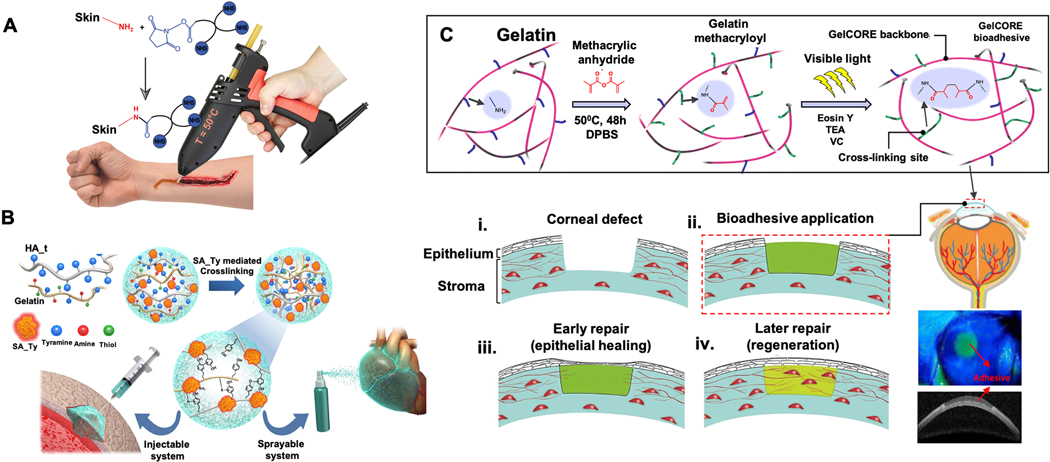
Skin is a vital barrier from the environment and pathogens. In recent years, tissue adhesives have gained great attention for dermal applications, especially for skin wound healing. These materials are injected onto wounded edges and they polymerize into a flexible and waterproof film. An optimal dermal adhesive should be liquid or semiliquid for convenience of application but solidify rapidly in the physiological conditions after administration and maintain the required mechanical features throughout the healing process. In this regard, a four-armed polycaprolactone (star-PCL) with NHS groups as a medical glue was developed.
This adhesive was placed into a hot glue device which is commercially available (Figure 9A). In the study, the adhesion features of the adhesive were investigated by measuring the loading strength by using rat skin and compared with commercial products such as 2-octyl-cyanoacrylate (Dermabond®) and fibrin-based adhesive (Evicel®). Compared to the developed adhesive, the strength of Dermabond® was found two times higher while Evicel® was four times lower. Also, the structure without NHS end groups was used to see the effect on adhesive strength. The maximal adhesive force decreased without NHS, showing these groups were essential for adhesive strength.
Figure 9.
Schematic presentation of A) Application of star-PCL-NHS melting for closing cuts and wounds with a hot melt device [179]. B) Fabrication of Streptomyces avermitilis (SA-Ty) mediated HA and gelatin hydrogel preparation and their use in the sprayable system [180]. C) The steps of GelCORE synthesis and its use in corneal injury repair: (i) stromal defect in cornea, (ii) bioadhesive application, (iii) epithelial healing and (iv) regeneration [181]. Adapted with permission from ref. [179], Copyright from 2019, The John Wiley & Sons, Inc.; Adapted with permission from ref. [180], Copyright from 2018, The Elsevier B.V.; Adapted with permission from ref. [181], Copyright from 2018, The American Association for the Advancement of Science.
In another study, a thermo-sensitive and water-soluble adhesive was introduced by melt-polycondensation of 1,8-octanediol, poly (ethylene oxide) (PEO), citric acid, and dopamine [182] and named POEC-d. POEC-d was blended with chitin nanocrystal (ChiNC) to create POEC-d/ChiNC nanocomposites to reinforce mechanical strength, wet adhesion performance, and bioactivity of the polymer. The swelling ratio of POEC-d was found as 199–250 % while the swelling ratio of POEC-d/ChiNC was very diminished ranging from 80% to 105% due to ChiNCs providing extra crosslinking to the citrate-based adhesive platform. The POEC-d/ChiNC composite lap-shear adhesion strength approached a maximum of 68.0±5.2 kPa which was quite stronger than the commercial fibrin glue with 11 kPa lap-shear adhesion strength [183].
In addition, blending different materials to enhance the properties of the adhesives, multilayer membranes have been used as potential adhesives for wound healing due to their flexibility, stability, and integration capacity with a wide range of materials. For instance, researchers produced multilayer membranes by the layer-by-layer (LbL) assembly of CHI, ALG and hyaluronic acid (HA) functionalized with dopamine (HA-DN) [184]. The reason for conjugating HA with dopamine by using carbodiimide chemistry, providing an adhesive character. The conjugation was confirmed by using UV–vis and 1H-NMR. The findings were remarkable, e.g., [CHI/ALG/CHT/HA-DN]100 provided more permeability, stiffer and adhesive membranes than the membranes without dopamine. Also, the membrane provided better cell adhesion, proliferation and directional signals for cells to connect.
Hydrogels enable us to precisely fabricate complex structures with high permeability and tunable properties due to their 3D network similar to soft tissues. They are prone to absorb a large amount of water and this feature sometimes causes inefficient mechanical features as well as uncontrolled degradation rates and chances of bacterial contamination [185–187]. Recently, a new hydrogel was introduced employing CHI-based hydrogel series of different concentrations of a crosslinking agent, N, N-methylenebisacrylamide (0.8–1.4 wt%), via free-radical polymerization which increases tissue adhesive property. Antimicrobial activity of the hydrogel against different bacteria was also investigated [188]. Higher cross-linker concentration provided a more interconnected 3D hydrogel network resulting in higher mechanical strength with an adhesive strength of 14 kPa which is an efficient value in tissue adhesiveness applications. The authors found storage and loss modulus as 106 Pa and 104 Pa, respectively. As another example of hydrogel-based tissue adhesives, a novel enzyme-based crosslinking hydrogel by coupling of tyramine-modified hyaluronic acid, gelatin and tyrosinase derived from SA-Ty [180] (Figure 9B). SA-Ty had better reactivity than existing tyrosinases in terms of effective crosslinking agents. In addition, active site structure enhanced the substrate specificity and increased SA-Ty activity. The study showed that the developed platforms have some advantages such as enzyme-based crosslinking of the designed hydrogel was more rapid (< 1 min). Moreover, the physical behaviors and adhesive strength of the hydrogels were enhanced.
One of the important things about the enzyme-based crosslinking hydrogels is that they can be made injectable by optimizing the injection conditions with a medical syringe, making the good candidates in regenerative medicine, but further studies are still needed.
Gelatin is one of the most used naturally derived biomaterials in tissue engineering. Crosslinking methacrylate on lysine amine groups generates gelatin methacryloyl (GelMA) hydrogel. Manipulation of the mechanical properties of GelMA creates robust ECM through changing the degree of methacrylation, the concentration of GelMA, and incorporation of nanomaterials such as graphene [189, 190], carbon nanotubes [191–193], and inorganic NPs [194, 195] into the structure are well investigated. Very recently, a new GelMA hybrid hydrogel was developed by incorporating tannic acid, which is a polyphenol compound and can provide additional hydrogen bonds, improving mechanical properties of the hydrogel [196]. By adding tannic acid into the GelMA structure, hydrogel’s structural stiffness and adhesion properties were substantially enhanced. Moreover, it showed excellent biocompatibility in skin and gastric wounds. In addition, in a proof-of-concept study, conductive carbon nanotubes were incorporated into elastic GelMA-tannic acid hydrogel to evaluate the structure as a wearable strain-sensitive electronic skin.
Tissue adhesives are expected not to have cytotoxicity but high tissue adhesion. CAs and fibrin sealants are the most investigated categories of tissue adhesives. Yet, the degradation of product toxicity of CAs and inadequate adhesive properties of fibrin sealants restrict their use in the medical field [197, 198]. New formulations have been searched to create ideal tissue adhesives with high biocompatibility and adhesion. For instance, phenol and catechol-modified gelatin-based sealants were produced by ruthenium-based photochemistry using tris(2,2’-bipyridyl) dichlororuthenium (II) hexahydrate (Ru(bpy)3Cl2) to improve tissue adhesion and have less toxicity than the conventional ones [199]. The first finding was that catechol-functionalized and phenol-functionalized gelatin possess higher solubility than the bare gelatin samples. In addition, incorporation of catechol and phenol into gelatin increases adhesives’ tissue adhesion and mechanical properties. No cytotoxicity was shown with MTS and live/dead assay with catechol-modified and phenol-modified gelatin hydrogels.
One of the ways to reduce the toxicity of the CAs based synthetic tissue adhesives is use of longer lengths of their side chains [200–202]. In the market, octyl-2-CA and n-butyl-CA with longer side chains are commercially sold with commercial brand names, Dermabond® and Histoacryl®, respectively. Efforts still have been proceeding to develop better biocompatibility than those products. For instance, a new tissue sealant composed of pre-polymerized allyl 2-cyanoacrylate (PACA) with poly L-lactic acid (PLLA) was introduced for healing dermal wound tissue [203]. The experimental conditions were optimized by mixing different ratios of PACA and PLLA. Chemical structures of the constructs were characterized by Fourier transform infrared spectroscopy (FTIR) and thermogravimetric analysis (TGA). Showing better biocompatibility and stronger tensile strength compared to commercial adhesives such as Dermabond® and Histoacryl®. In addition, PACA/PLLA treated dermal tissues showed lower inflammation and higher collagen formation than the commercial ones. Further investigations are needed before PACA/PLLA can be used in a clinical setting.
Tissue adhesives cover a wide range of dermal applications but the usage of tissue adhesives for ophthalmic disorders such as cataracts, diabetic retinopathy, glaucoma, and cancers have been getting some traction [204–208]. In ophthalmic applications, CAs-based adhesives are often preferred to seal eye wounds. However, due to discomfort to the patient and slightly cytotoxic nature of CAs, FDA could not approve its use. At present, there has not been any adhesive for long term integration with cornea. Very few solutions have been convenient for ophthalmic problems commonly based on surgery and delivery of therapeutic components to the damaged area (i.e., drugs, proteins, genes, vectors, and nanoparticles) [209–213]. As is known, surgical methods are invasive and have the risk to cause immune reactions and infections [20]. Therapeutic molecules are very important in congenital therapy and acquired diseases, while the drug delivery efficacy and side effects still need to be solved. Therefore, alternative methods of surgery and the demand for effective drug delivery to repair eye have been rising. Especially, transparent biomaterials with high adhesion, cohesion, and regenerative properties are urgently needed. Besides, the ability to close wounds without impeding tissue movement and functions are desired. For this aim, researchers engineered a highly biocompatible, transparent, flexible, and adhesive hydrogel for corneal reconstruction and stromal defect namely GelCORE [181] (Figure 9C).
The researchers could manage to tune the physical properties of GelCORE® by changing pre-polymer concentration and photo-crosslinking period. GelCORE® has higher adhesion properties than commercial adhesives. Moreover, GelCORE® in situ photopolymerization provided accessible delivery to the cornea. As a result of this adhesive could cure precisely according to the defect geometry. Sealing corneal defects and re-epithelialization were showed in vivo with a rabbit stromal defect model.
One of the main problems of cornea injury treatments is the delivery of the drug or therapeutic molecules to the cornea. Because these molecules have been locally administrated for corneal injuries but their effect is limited due to the short residence time on the surface of cornea due to the washout of tears and blinking. To address this problem, researchers designed a novel bioadhesive with the use of cysteine-functionalized γ-polyglutamic acid (PGA-Cys) to locally deliver Keratinocyte Growth Factor (KGF) to the injured corneas [214]. Optimization studies were done by adjusting cysteine graft ratio and polymer concentration. Results exhibited that KGF was controlled released from PGA-Cys hydrogel over a longer time compared to PGA solution alone. The most important finding was that PGA-Cys allowed encapsulated KGF to be retained on the cornea and conjunctiva after local administration.
The low residence time of gels and emulsions on treatment place is also a critical problem in dental applications because of high amount of secretion of saliva, beverage/food intake and swallowing. As a solution to adherence problem, bioadhesive systems for long retention time in oral mucosa and cavities should be designed to prevent infections by covering the surgical area, improve the drug retention time and treatment efficacy. To address this issue, a film for mucosal application of anesthetic drug-containing lidocaine hydrochloride was prepared with hydroxypropylmethylcellulose, CHI, and xanthan gum [215]. Lidocaine was released from these three adhesives over a prolonged time than without adhesives, however, it was found that xanthan gum has a greater power of mucoadhesion than the other formulations.
In a recent study, a novel and dynamic-adhesive hydrogel was developed to address the adhesion problem by forming water-resistant molecular bridges between the prepared hydrogel and the applied surfaces under wet conditions [216]. The long-term stability was provided with a unique design of the hydrophobic hydrogel which contained Fe3+ -induced hydrophobization process without the need for an additional process or reagent. In addition to Fe3+ ions, the hydrogel also was composed of acrylamide, stearyl methacrylate (C18) and sodium dodecyl sulfate (SDS). Fe3+-induced hydrophobization process was evaluated by using water contact angle and FTIR measurements. In the study, the hydrogel was abbreviated as PAM-C-M (doesn’t contain Fe3+, hydrophilic) and Fe-PAM-C-M (contains Fe3+, hydrophobic), respectively and their underwater adhesion characteristics were compared (Figure 10A), i.e., the PAM-C-M hydrogel was detached from the metal block surface, while the Fe-PAM-C-M hydrogel strongly attached to the metal block surface in underwater. In addition, Fe-PAM-C-M hydrogel was tested with different surfaces such as forehead (Figure 10B) and porcine muscle (Figure 10C). In vivo adhesion tests were done with a beating porcine heart to test the mechanical features of the hydrogel (Figure 10D). The most attractive feature of the design is the developed hydrogels that can be adapted to in vivo dynamic conditions, such as bodily fluids. As it is clearly seen, the developed hydrogel can be used as a sealant on liver (Figure 10E) and heart (Figure 10F) in the presence of blood. The histological results displayed that the integration of the hydrogel and surrounding tissue was successfully formed 1 and 2 weeks after implantation (Figure 10G). The study is quite promising in terms of transferring the designed hydrogels for alternative application areas.
Figure 10.
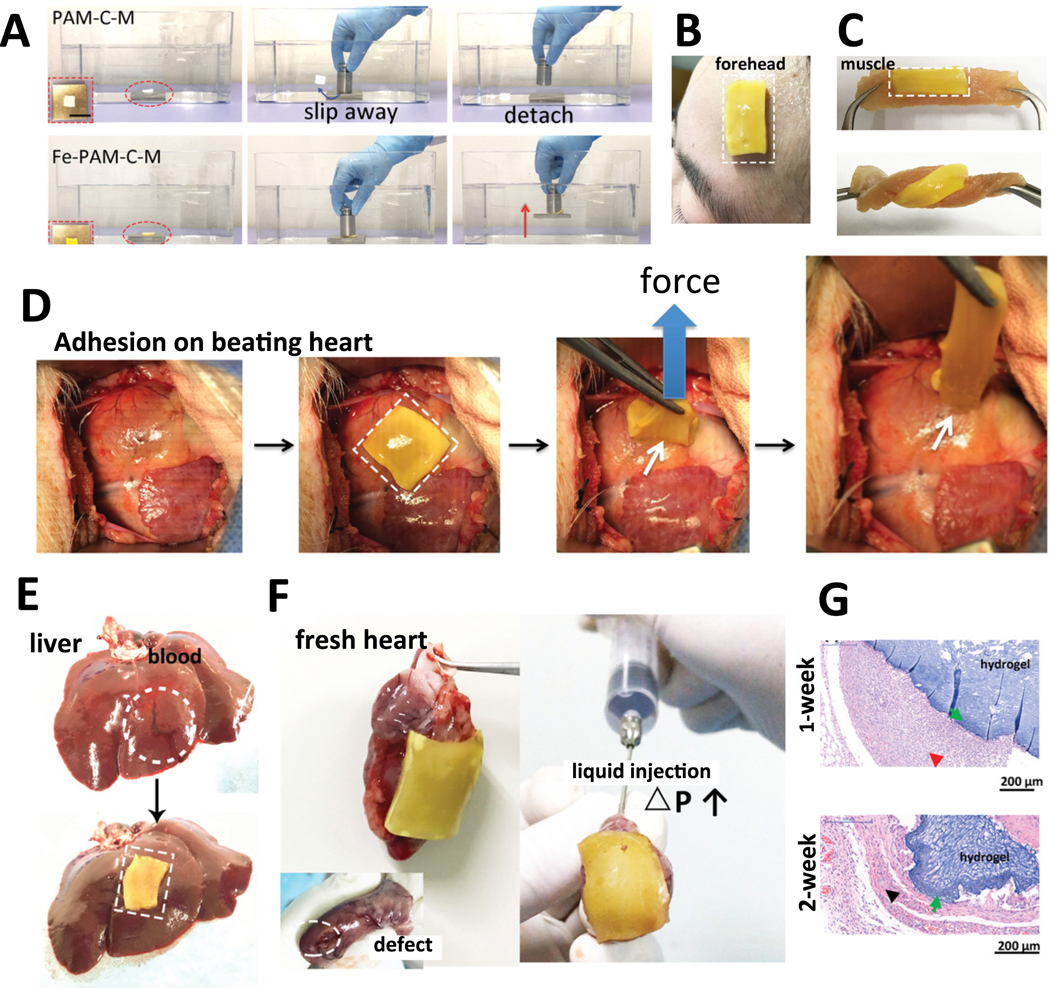
Dynamic hydrogel for underwater adhesion. A) The comparison of PAM-C-M and Fe-PAM-C-M hydrogels in terms of their underwater adhesion properties, B) The photographs of Fe-PAM-C-M direct adhesion to the forehead skin and C) to the porcine muscle without detachment or crack, D) In vivo adhesion test on a beating porcine heart, E) The gel utilized as an adhesive on liver and F) on the heart in the presence of blood, G) Histological results related to biocompatibility studies, 1 and 2 weeks after implantation (n = 3) [216]. Adapted with permission from ref. [216], Copyright from 2017, The John Wiley & Sons, Inc.
6. Commercially available successful tissue adhesives
There are a variety of materials already commercially available, and also research is going on introducing novel materials with a range of additional mechanisms for better adhesion and biocompatibility. To meet the needs, modifications such as changing material elasticity/stiffness in addition to designing hybrid systems are carried out. Formation of hybrid systems is possible with natural, synthetic, and natural/synthetic biomaterial systems that may have simultaneous functionalities as hemostats, sealants, and adhesives. These materials can be designed for application on dry/wet surfaces, and adhesion strength and duration can be modified to support the natural healing process of the body.
A list of commercial products available in the market is given in Table 1. Examples of popular natural hybrids can be given as fibrin glue products. A commercial product is TachoComb® which is a collagen patch coated with human fibrinogen and bovine thrombin with aprotinin reported to have great efficacy for local hemostasis [217, 218]. Another is TachoSil®, a relatively newer product, formed of a collagen patch coated with human fibrinogen and human thrombin [218]. FloSeal® is a flowable hemostat fabricated using gelatin granules and human thrombin to provide a mechanically strong clot [219]. Synthetic materials such as CAs- and PEG-based commercials allow modifications in terms of crosslinking ratio, polymer degradation rate, and material stability. Examples of natural/synthetic biomaterial hybrids include albumin-GA, lysine-based polyurethane, GRFG hybrids [12, 28].
Table 1.
A list of commercial products available in the market, their significant function of use, and the advantages and disadvantages of the products.
| Category | Commercial products | Function | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Fibrin Glue | Tisseel (Baxter) Evicel (Ethicon, J&J) (formerly CrosSeal in the US, and Quixil in the EU) Vitagel (Orthovita/Stryker) (replaced CoStasis (Haemacure)) CryoSeal system (ThermoGenesis) Hemaseel (Haemacure) TachoSil (Baxter) Evarrest (Ethicon, J&J) Artiss (Baxter) Biocol (LFB-Lille) Dynastat (Cohesion Corp.) Bolheal (KaketsukenPharmaceuticals) Beriplast P (Aventis Behring) | ■ Hemostat and/or sealant ■ Used to prevent bleeding and leaking during or after surgical operations ■ Could be used in a range of operations including cardiovascu lar surgery, neurosurger y, gastrointest inal tract diseases, liver resection surgery |
■ Biocompati ble ■ Biodegrada ble ■ Their action mimics final stages of blood coagulation cascade |
■ May trigger allergic reactions ■ May cause hemorrhage ■ Risk of infections and diseases due to transmission s through biologic source ■ Could cause thromboemb olism when injection amount is not well- adjusted ■ Poor adhesion ■ Poor mechanical properties ■ Application on dry surfaces ■ Time-consuming preparation required ■ Preparation is time-intensive |
[221–228] |
| Thrombin | Thrombin-JMI (King Pharmaceuticals) Evithrom (J&J) Recothrom (Zymogenetics) FloSeal (Baxter) | ■ Creation of an insoluble clot | ■ Efficient when blood presents in the area | ■ May trigger allergic reactions ■ May cause hemorrhage ■ Safety concerns due to use of blood products |
[228,229 ] |
| Collagen | FloSeal (Baxter) Avitene (C.R. Bard) TachnoComb (Pharmaceuticals International GmbH) Surgiflo (Ethicon, J&J) Proceed (Fusion Medical) | ■ Hemostatic agent by promoting platelet aggregation for general surgery | ■ Reduced potential of transmissio n compared to fibrin- based products ■ Biocompati ble ■ Low toxicity |
■ Polymer swelling ■ May trigger allergic reactions ■ Preparation is time-intensive |
[12,219,224, 230] |
| Chitosan | HemCon Bandage Pro ChitoFlex (HemCon) | ■ Stop haemorrhage in arterial and venous bleeding ■ Antibacteri al barrier ■ For ChitoFlex, hemostatic wound dressing |
■ Biocompatible ■ Biodegradable ■ Nontoxic ■ Antibacteria l properties |
[85, 230] | |
| Alginate | Sorbsan (Aspen Medical) | ■ Diabetic ulcers | ■ Moist environment that supports wound healing ■ Biocompati ble ■ Biodegradable ■ High wound exudate absorption ■ Promotes hemostasis |
■ May trigger allergic reactions | [34, 231] |
| Albumin | BioGlue (Cryolife) | ■ Adjunct to hemostatic methods during operations of large blood vessels | ■ Fast polymerizat ion ■ Good adhesion ■ The only FDA approved product for lung resection ■ In terms of intraoperati ve and postoperative air leaks, superior compared to other methods ■ Efficient in reducing prolonged air leaks ■ Reduced hospital length of stay in comparison to using staples and sutures; thus, reduces hospitalizati on costs ■ Safe and effective |
■ Toxicity concerns due to the use of aldehyde ■ Slow degradation rate, that may hinder healing ■ May trigger allergic reactions ■ Inflammatio n and scar formation |
[12,15, 28] |
| Progel (Davol) | ■ Pleural air leak sealant for postoperati ve complications | ||||
| Gelatin-Resorcin-Formaldehy de/Glutaraldehyde | GRF Biological Glue (Microval) GRFG | ■ Thoracic aortic dissections ■ Multiple surgical operations |
■ Strong adhesion ■ High stability ■ Do not require dry surfaces |
■ Toxicity concerns due to mutagenic and carcinogenic formaldehyde ■ Slow degradation rate |
[12, 28,85] |
| Cyanoacrylates | Dermabond (Ethicon,J&J) | ■ Bond formation across opposed wound edges ■ Replaceme nt of sutures only for superficial lacerations with diameters of 5–0 or smaller. ■ Adjunct to subcutaneo us sutures |
■ Easy to use ■ Strong adhesion ■ Instant adhesion due to fast polymerization ■ Rapid drying of the polymer ■ Providing Flexible water- resistant covering ■ Eliminating need for suture removal ■ Patient compliance |
■ Toxicity of the degradation products ■ May cause inflammation ■ May lack of required flexibility ■ Most products are for application on dry surfaces ■ Complications due to prolonged biodegradati on for the polymers with high molecular weight and long side chains ■ Their use is limited to superficial and topical applications ■ Exothermic polymerizati on |
[12, 15, 28, 232] |
| Indermill (Covidien Inc.) | ■ Topical skin incisions, trauma induced skin lacerations | ||||
| Histoacryl and Histoacryl Blue (B. Braun Medical Inc.) | ■ Topical skin incisions, trauma induced skin lacerations | ||||
| IFABond (Ethicon,J&J) | ■ Alternative to sutures and staples | ||||
| Glubran - Glubran2 (Matrix) | ■ Synthetic glue for surgeries ■ For internal and external use, as a hemostatic, adhesive, sealer and bacteriostatic |
||||
| Poly(ethylene glycol) | CoSeal (Baxter Bio Science Inc.) | ■ Adjunct to hemostatic methods for sealing leakage areas during vascular reconstructi on | ■ Non-toxic, nonimmunogen ic, and biocompati ble ■ Easy modificatio n of PEG architecture ■ Rapid gel formation |
■ Tissue/nerve compression may occur due to hydrogel swelling ■ Requirement for dry surfaces for effective application ■ May trigger allergic reactions |
[12, 85, 230, 233, 234] |
| Duraseal (Covidien Inc.) | ■ Adjunct to dural sutures to avoid cerebrospinal fluid leakage during spinal surgery ■ Vascular closure |
||||
| FocalSeal-L (Focal Inc.; Formerly AdvaSeal) | ■ PEG-poly lactic acid network that stabilized mechanicall y by PEG- polytrimethylene carbonate) ■ Lung air leak sealant |
||||
| SprayGel (Covidien) | ■ Gynecologi cal and colorectal operations | ||||
| OcuSeal (Hyperbranch Medical Technology) | ■ Bandage in corneal transplants and lacerations | ||||
| Polyurethane | TissuGlu (lysine-based polyurethane) | ■ Preventing seroma formation for abdominal surgery | ■ Thermal stability ■ Strong coagulation ■ Biocompatible ■ Biodegradable ■ Nontoxic ■ Controlled polymer architecture |
■ Poor mechanical strength ■ Solvent/water resistance |
[12, 28, 34, 85] |
| Poly (lactic- co-glycolic acid) | TissuePatch (TissueMed) | ■ Adjunct to soft tissue sutures ■ Air leakage prevention in thoracic and dural surgery |
■ Biocompatible ■ Biodegradable ■ Nontoxic ■ Ready-to- use, no need for any preparation before application ■ No risk of transmission s and infections compared to fibrin sealants |
[34, 85, 230, 235] | |
| Dendrimer | Adherus (HyperBranch Medical Technology) | ■ Suture replacement for corneal wound repairs and corneal cataract incisions ■ Surgical sealant for dural, cardiovascu lar and spinal surgeries |
■ No postoperative cerebrospina l fluid leaks or surgical site infections after application | [230, 236, 237] | |
Research with a mid/long-term vision focused on introducing novel materials. Although they have not commercialized for sealant/adhesive applications, they are promising materials due to biomimetic properties. Examples of these include an extract of marine mussel, Mytilus edulis with a brand name of Cell-Tak (Corning). Cell-Tak is used for research purposes, to immobilize cells and tissues due to strong adhesive properties of the mussel extract. In a recent study, light-activated surgical protein glue is reported, where the system is activated due to photochemical crosslinking of dityrosine [220]. Small sea organisms, such as sandcastle worm, are under research due to their adhesive secretions that bind tissue’s amine and thiol groups. Other biomimetic tissue adhesives include algae, Australian frog-, lizard-, gecko-, caddisfly-, and barnacle- inspired materials [1, 221]
Conclusion and Future Perspective:
Surgical operations mainly use invasive techniques and materials such as sutures and staples to seal the cut edges of the tissues. Although these materials are fundamental to any type of surgical procedure, their use has certain limitations and needs expertise. They also come with various risks especially for sensitive tissues such as leading to re-occurrence of infections, tissue damages, surgery leaks, and scars, which results in low patient compliance due to discomfort, pain, phobia, or even death [1, 85]. The use of sealants and adhesives adjunct to/or instead of invasive techniques enables significant support to conventional surgical techniques. These could be fabricated in natural or synthetic polymers, or as hybrids. This versatility in polymer selection offers many possibilities and offers many advantages such as application in diverse types of wounds/operations, effective control of bleeding, easy application, better mechanical properties, excellent cytocompatibility, ease of production, and patient satisfaction.
Most essential requirements for the development of tissue adhesives are biocompatibility and biosafety. Tissue adhesives are biomaterials that are implanted in the body. Biocompatibility is an important crucial factor for the design and development of tissue adhesives. Because of their high potential risk for patients, tissue adhesives are generally classified as high-risk medical devices (for example, as Class III by the FDA) and undergo some of the most stringent regulatory controls for human approval [238]. Although, as discussed in this review, various formulations for tissue adhesives have been developed and clinically applied, there are still challenges that need to be addressed from the biocompatibility perspective. The next generation of products have a long way to cover before they can be marketed after extensive clinical studies are performed and adequate safety and efficacy data.
To improve strong adhesion properties, the interaction mechanism with target tissue should be considered carefully. It is worth mentioning that ongoing research focuses on novel biomimetic materials to overcome limits such as improve their physical, biological features and adhesive strength for their employment in clinical practice. In addition, the lack of effective adhesion under wet conditions is a big issue for sealants. Studies on novel sealant structures, for example, mussel-inspired DOPA (catechol) chains-tethered sealants, can overcome these obstacles; besides effective fabrication ways with economic practicability should be considered. On the other hand, the knowledge about how the mussels guide the redox condition of the plaque is not enough. As discussed throughout the review in detail, DOPA acts as a critical character in the interfacial adhesion and fixing of the adhesive plaque proteins. To accomplish the redox-equilibrium in the catechol-modified sealant platforms is a big issue and requires further researches.
Although there are many studies on the development of mussel-inspired sealants, the difficulties to create a sealant is to mimic natural behavior. Researchers continue creating new platforms to reduce the gap. Also, the conjunction of nature-inspired procedures and substances shows an appealing way of research to create multi-functional sealants able to conduct with different environments and stick on various surfaces. Meanwhile, novel multifunctional sealants with tunable properties should be further investigated to state complex clinical states.
This expanding field of research can give amazing chances for the future; may be a gel may take the place of metal and thread. In addition, future studies should be conducted to increase the number of adhesives/sealants on the market for application in diverse surgical operations and to design novel materials as discussed throughout the review in detail.
Highlights.
Sutures, staples, clips, or skin closure strips are used as the gold standard to enable wound closure.
These methods can lead to infections, scarring, and cause a lot of discomfort to the patient.
Tissue adhesives offer functionality as an interface to connect the surfaces of different substrates and prevent these substrates from separation without causing pain and minimizing scar formation.
Different tissue adhesives are discussed together with the origin they come from and their specific medical applications.
Acknowledgments
ABO acknowledges the support from Research Fund of Istinye University (2019/B8), SS and SH acknowledge funding from the National Institutes of Health (R01AR074234). SS further acknowledges the National Institutes of Health (R21EB026824), the Gillian Reny Stepping Strong Center for Trauma Innovation at Brigham and Women’s Hospital, and AHA Innovative Project Award (19IPLOI34660079).
Biographies

Dr. Ayca Bal Ozturk is an Assistant Professor at Istinye University, Faculty of Pharmacy, Turkey. She also holds a faculty appointment at Istinye University Research and Application Center for Stem Cell and Tissue Engineering (ISUKOK). She received her PhD degree in Chemical Engineering from Istanbul University in 2015. Awarded with The Scientific and Technological Research Council of Turkey (TUBITAK) in 2016, she joined Harvard Medical School as a postdoctoral research fellow. Her research interests mainly include nanostructured smart materials, design and synthesis of innovative micro/nanoscale biomaterials, as well as regulating stem-cell differentiation with the conclusive purpose of generating tissue-engineered organs.

Dr. Berivan Cecen earned her M.Sc. in Biomechanics from Dokuz Eylul University, Turkey in 2007. Before obtaining her Ph.D. degree, she studied with Professor Dr. Josef Guttmann at the Universitätsklinikum Freiburg in 2010. Dr. Cecen received her Ph.D. degree in Bioengineering from Dokuz Eylul University and Izmir Institute of Technology, Turkey in 2014. Dr. Cecen joined as a post-doctoral fellow at Harvard-MIT Division of Health Sciences and Technology and Brigham and Women’s Hospital, Harvard Medical School, Boston (USA) in 2016 and 2018. At present she is a researcher at Dokuz Eylul University, Turkey.

Dr. Meltem Avci-Adali holds a Master of Science degree in Biomedical Engineering and a Diploma degree in Pharmaceutical Technology. In 2010, she received her Ph.D. in biology from the University of Tübingen, Germany. She is currently the head of the Research Laboratory at the Department of Thoracic and Cardiovascular Surgery at the University Hospital Tübingen, Germany. Her research focuses on biomaterials, biocompatibility, stem cells, aptamers, synthetic mRNA, implant surface functionalization, endothelialization, hemocompatibility of blood-contacting materials, and in vivo tissue engineering.

Dr. Seda Nur Topkaya completed her Ph.D in 2013 in Analytical Chemistry, Faculty of Pharmacy at Ege University, Turkey. She is an Associate Professor in Analytical Chemistry and the head of the departments; Basic Pharmaceutical Sciences and the Department of Analytical Chemistry at Izmir Katip Celebi University. Her main research interests are electrochemistry, biosensor systems, drug-DNA interactions, electrochemical-based DNA biosensors and tissue engineering applications. She has published 30 papers in SCI indexed journals and serving as reviewer-editorial board member of numerous international journals.

Dr. Emine Alarçin is an Assistant Professor in Pharmacy at Marmara University, Turkey. She received her Ph.D. from Marmara University, Turkey from the Faculty of Pharmacy, and Department of Pharmaceutical Technology in 2011. Her PhD research was mainly focused on fabricating polymeric microspheres for VEGF delivery in nerve graft prefabrication. She carried postdoctoral research fellow at Harvard Medical School in 2016–2017. Her current research focuses on developing multifunctional biomaterials, nanocarriers and hydrogels for drug delivery applications and regenerative medicine.

Dr. Gökçen Yaşayan did her PhD in 2013 at the University of Nottingham, UK. She carried her research at the divisions of Drug Delivery and Tissue Engineering, and The Laboratory of Biophysics and Surface Analysis (LBSA). She stayed as a postdoc at the University of Nottingham. Afterwards, she returned to Marmara University, Faculty of Pharmacy, and Department of Pharmaceutical Technology, and currently holds the position of Assistant Professor at this department. Her research focuses on investigating novel nanomedicines – mainly on self-assembled particles, stimuli-responsive systems, and topographically patterned novel drug carriers, and evaluation of these carriers for drug delivery applications.

Mr. Bünyamin Bulkurcuoğlu was born in 1994 and completed his high school education at E.C.A. Elginkan Anatolian High School, Turkey. He then graduated from Uskudar University, Turkey in molecular biology, and genetics and bioengineering departments with a high honor student degree. He is currently an Integrated PhD student at Gebze Technical University, Institute of Biotechnology and continues his research in the field of Biophysics at TÜBİTAK Marmara Research Center Genetic Engineering and Biotechnology Institute, Turkey. His research interests are Biomaterials, Tissue engineering, Biophysical and Physicochemical characterization techniques.

Dr. Ali Akpek was born in İzmir, Turkey. He recieved his B.Eng degree in biomedical engineering. After graduation he expanded his research interest to biotechnology and obtained a Master of Science degree in Biotechnology from Ege University. In 2010, he was awarded with Monbukagakusho scholarship from Japan and worked as a research scientist in University of Tokyo. He started his Dr. Eng training in Mechano-Micro Engineering at Tokyo Institute of Technology in 2011. Later he joined Harvard-MIT Division of HST as a visiting professor. Currently he is an Associate Professor of Bioengineering at Gebze Technical University.

Dr. Yi-Chen Ethan Li’s laboratory at Feng Chia University in Taiwan is a multidisciplinary biomedical research group. His team aims to study the interactions among cells, signals and biomaterials, and fabricate the various biomimetic functional materials/constructs/devices for the biomedical applications. His team has accumulated the experience and expertise in biomaterials, stem cells, tissue engineering, 3D biofabrication, and organs-on-chips. Through his research, he hopes to push forward the development of novel micro/nano technologies for improving the quality of clinical therapeutics.

Dr. Huseyin Avci received his M.S. and Ph.D. degrees in 2010 and 2013, respectively, from Fiber and Polymer Science at North Carolina State University. Following his graduation, he worked as a Post-Doctorate Research Associate on different projects of polymers for medical applications and nano-hybrid filtrations. Later he joined the Department of Metallurgical and Materials Engineering at Eskisehir Osmangazi University in September 2014. He is also a faculty member in Cellular Therapy and Stem Cell Research Center (ESTEM). During that time, he also worked on tissue engineering and organ-on-a-chip as a Research Scientist at Brigham and Women’s Hospital, Harvard Medical School.

Kun Shi is a postdoctoral research fellow at West China Hospital, Sichuan University. She received her Ph.D. degree in Pharmaceutics from Sichuan University in 2017. She has been in Harvard Medical School as a visiting scholar since January 2020. Her research areas include polymer micelles, nanoparticles, injectable thermo-sensitive polymer hydrogels, biomimetic scaffolds, and their application in drug delivery and tissue engineering.

Su Ryon Shin is an Assistant Professor of Medicine at Brigham and Women’s Hospital, Harvard Medical School. Dr. Shin received her doctoral degree from Hanyang University, South Korea. In Nov. 2010, she joined as a postdoctoral fellow at Brigham and Women’s Hospital, Harvard Medical School, Harvard-MIT Division of Health Sciences and Technologies, and Wyss Institute for Biologically Inspired Engineering. Dr. Shin is an expert in the field of nanomaterials, biomaterials, tissue engineering, electrochemical actuator, biosensor, organ-on-a-chip, and 3D bioprinting. She has won many research awards including the 2020 Stepping Strong Breakthrough Award from The Gillian Reny Center for Trauma Innovation.

Dr. Shabir Hassan is a faculty member at the Brigham and Women’s Hospital and Harvard Medical School. He did his bachelor’s from Kashmir University, master’s from Pune University, and PhD from University of Zurich in Chemistry in 2015. He won the Swiss National Science Foundation (SNSF) fellowships in 2016 and 2018 to carry out his research at HST of Harvard - MIT, Harvard Medical School, and University of Twente, Netherlands in biomedical engineering. He won the BWH and Harvard Medical School’s Award of Excellence for Mentorship in 2019. He serves as Associate Editor, MIT Science Policy Review.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bhagat V, Becker ML, Biomacromolecules, 18 (2017) 3009–3039. [DOI] [PubMed] [Google Scholar]
- [2].Kundra RK, Newman S, Saithna A, Lewis AC, Srinivasan S, Srinivasan K, Ann R Coll Surg Engl, 92 (2010) 665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abdus-Salam RA, Bello FA, Olayemi O, Int Sch Res Notices, 2014 (2014) 807937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Batra J, Bekal RK, Byadgi S, Attresh G, Sambyal S, Vakade CD, J Maxillofac Oral Surg, 15 (2016) 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Al-Mubarak L, Al-Haddab M, J Cutan Aesthet Surg, 6 (2013) 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Esmailian M, Azizkhani R, Jangjoo A, Nasr M, Nemati S, Adv Biomed Res, 7 (2018) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin SB, Durfee LD, Ekeland RA, Mcvie J, Schalau GK, J Adhes Sci Technol, 21 (2007) 605–623. [Google Scholar]
- [8].Donkerwolcke M, Burny F, Muster D, Biomaterials, 19 (1998) 1461–1466. [DOI] [PubMed] [Google Scholar]
- [9].Carton CA, Kessler LA, Seidenberg B, Hurwitt ES, J Neurosurg, 18 (1961) 188–194. [DOI] [PubMed] [Google Scholar]
- [10].Matsumoto T, Arch Surg, 96 (1968) 226–230. [DOI] [PubMed] [Google Scholar]
- [11].Annabi N, Tamayol A, Shin SR, Ghaemmaghami AM, Peppas NA, Khademhosseini A, Nano Today, 9 (2014) 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinnaratip R, Bhuiyan MSA, Meyers K, Rajachar RM, Lee BP, Advanced healthcare materials, (2019) 1801568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khanlari S, Dubé MA, Macromolecular Reaction Engineering, 7 (2013) 573–587. [Google Scholar]
- [14].Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK, International journal of biological macromolecules, 72 (2015) 269–281. [DOI] [PubMed] [Google Scholar]
- [15].Zhu W, Chuah YJ, Wang D-A, Acta biomaterialia, 74 (2018) 1–16. [DOI] [PubMed] [Google Scholar]
- [16].Mansuri S, Kesharwani P, Jain K, Tekade RK, Jain NK, Reactive and Functional Polymers, 100 (2016) 151–172. [Google Scholar]
- [17].Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW, dental materials, 26 (2010) e11–e16. [DOI] [PubMed] [Google Scholar]
- [18].Annabi N, Zhang Y-N, Assmann A, Sani ES, Cheng G, Lassaletta AD, Vegh A, Dehghani B, Ruiz-Esparza GU, Wang X, Science translational medicine, 9 (2017) eaai7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Balakrishnan B, Soman D, Payanam U, Laurent A, Labarre D, Jayakrishnan A, Acta Biomaterialia, 53 (2017) 343–354. [DOI] [PubMed] [Google Scholar]
- [20].Trujillo-de Santiago G, Sharifi R, Yue K, Sani ES, Kashaf SS, Alvarez MM, Leijten J, Khademhosseini A, Dana R, Annabi N, Biomaterials, 197 (2019) 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Spotnitz WD, Burks S, Transfusion, 48 (2008) 1502–1516. [DOI] [PubMed] [Google Scholar]
- [22].Baldan A, Journal of materials science, 39 (2004) 1–49. [Google Scholar]
- [23].van der Leeden MC, Frens G, Advanced engineering materials, 4 (2002) 280–289. [Google Scholar]
- [24].Ebnesajjad S, Landrock AH, Adhesives technology handbook, William Andrew; 2014. [Google Scholar]
- [25].Park Y-J, Lim D-H, Kim H-J, Park D-S, Sung I-K, International Journal of Adhesion and Adhesives, 29 (2009) 710–717. [Google Scholar]
- [26].Charron PN, Fenn SL, Poniz A, Oldinski RA, Journal of the Mechanical Behavior of Biomedical Materials, 59 (2016) 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vitale A, Trusiano G, Bongiovanni R, Reviews of Adhesion and Adhesives, 5 (2017) 105–161. [Google Scholar]
- [28].Mehdizadeh M, Yang J, Macromolecular bioscience, 13 (2013) 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shenton MJ, Lovell-Hoare MC, Stevens GC, Journal of Physics D: Applied Physics, 34 (2001) 2754. [Google Scholar]
- [30].Awaja F, Gilbert M, Kelly G, Fox B, Pigram PJ, Progress in polymer science, 34 (2009) 948–968. [Google Scholar]
- [31].Schultz J, Nardin M, MATERIALS ENGINEERING-NEW YORK-, 14 (1999) 1–26. [Google Scholar]
- [32].Lenaerts VM, Gurny R, Bioadhesive drug delivery systems, CRC press; 1989. [Google Scholar]
- [33].Baldan A, International Journal of Adhesion and Adhesives, 38 (2012) 95–116. [Google Scholar]
- [34].Modaresifar K, Azizian S, Hadjizadeh A, Polymer Reviews, 56 (2016) 329–361. [Google Scholar]
- [35].DelRio FW, de Boer MP, Knapp JA, David Reedy E, Clews PJ, Dunn ML, Nature Materials, 4 (2005) 629–634. [DOI] [PubMed] [Google Scholar]
- [36].Liptrot DJ, Power PP, Nature Reviews Chemistry, 1 (2017) 0004. [Google Scholar]
- [37].Mehdizadeh M, Yang J, Macromol Biosci, 13 (2013) 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang S, Gu L, Gibson RF, Composite Structures, 51 (2001) 63–71. [Google Scholar]
- [39].Dodou D, Breedveld P, Wieringa PA, Eur J Pharm Biopharm, 60 (2005) 1–16. [DOI] [PubMed] [Google Scholar]
- [40].McBain JW, Hopkins DG, The Journal of Physical Chemistry, 29 (1925) 188–204. [Google Scholar]
- [41].Gardner DJ, Oporto GS, Mills R, Samir MASA, J Adhes Sci Technol, 22 (2008) 545–567. [Google Scholar]
- [42].Yang SY, O’Cearbhaill ED, Sisk GC, Park KM, Cho WK, Villiger M, Bouma BE, Pomahac B, Karp JM, Nature communications, 4 (2013) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maeva E, Severina I, Bondarenko S, Chapman G, O’Neill B, Severin F, Maev RG, Canadian Journal of Physics, 82 (2004) 981–1025. [Google Scholar]
- [44].von Fraunhofer JA, International journal of dentistry, 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Petersen B, Barkun A, Carpenter S, Chotiprasidhi P, Chuttani R, Silverman W, Hussain N, Liu J, Taitelbaum G, Ginsberg GG, Gastrointestinal endoscopy, 60 (2004) 327–333. [DOI] [PubMed] [Google Scholar]
- [46].Spotnitz WD, ISRN Surgery, 2014 (2014) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stewart RJ, Wang CS, Shao H, Advances in Colloid and Interface Science, 167 (2011) 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cronkite EP, Lozner EL, Deaver JM, Journal of the American Medical Association, 124 (1944) 976–978. [Google Scholar]
- [49].Alving BM, Weinstein MJ, Finlayson JS, Menitove JE, Fratantoni JC, Transfusion, 35 (1995) 783–790. [DOI] [PubMed] [Google Scholar]
- [50].Martinowitz U, Saltz R, Current opinion in hematology, 3 (1996) 395–402. [DOI] [PubMed] [Google Scholar]
- [51].Kjaergard HK, Weis-Fogh US, European Surgical Research, 26 (1994) 273–276. [DOI] [PubMed] [Google Scholar]
- [52].Sierra DH, Feldman DS, Saltz R, Huang S, Journal of Applied Biomaterials, 3 (1992) 147–151. [DOI] [PubMed] [Google Scholar]
- [53].Rathi S, Saka R, Domb AJ, Khan W, Polymers for Advanced Technologies, 30 (2019) 217–234. [Google Scholar]
- [54].Duarte A, Coelho J, Bordado J, Cidade M, Gil M, Progress in Polymer Science, 37 (2012) 1031–1050. [Google Scholar]
- [55].Buncke GM, Sherman R, Journal of reconstructive microsurgery, 16 (2000) 0557–0562. [DOI] [PubMed] [Google Scholar]
- [56].Rathi S, Saka R, Domb AJ, Khan W, Polymers for Advanced Technologies, 30 (2019) 217–234. [Google Scholar]
- [57].Mavigök E, Bakacak M, Yazar FM, Bakacak Z, Yaylalı A, Boran ÖF, Bahar AY, Ginekologia polska, 90 (2019) 507–512. [DOI] [PubMed] [Google Scholar]
- [58].Suchý P, Paprskářová A, Chalupová M, Marholdová L, Nešporová K, Klusáková J, Kuzmínová G, Hendrych M, Velebný V, Materials, 13 (2020) 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aucar JA, Punja V, Asensio JA, Biosurgicals and Trauma, Biosurgicals-The Next Frontier in Operative Approaches, IntechOpen; 2019. [Google Scholar]
- [60].Guo X, Hu H, Liu Y, Bao C, Wang L, Journal of clinical periodontology, 46 (2019) 766–775. [DOI] [PubMed] [Google Scholar]
- [61].Jeanmonod D, Rammohan G, Jeanmonod R, Thrombostatic Agents and Tissue Adhesives in the Emergency Department: Stopping the Bleeding, Closing the Wound, and Novel Applications, Biosurgicals-The Next Frontier in Operative Approaches, IntechOpen; 2020. [Google Scholar]
- [62].Renati S, Kaur S, Kresak JL, Wicklund M, Malaty I, Neurology: Clinical Practice, 7 (2017) 384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Taguchi T, Saito H, Uchida Y, Sakane M, Kobayashi H, Kataoka K, Tanaka J, Materials Science and Engineering: C, 24 (2004) 775–780. [Google Scholar]
- [64].Reece TB, Maxey TS, Kron IL, The American Journal of Surgery, 182 (2001) S40–S44. [DOI] [PubMed] [Google Scholar]
- [65].Foster LJR, Bioadhesion Biomim. Nat. Appl, 203 (2015). [Google Scholar]
- [66].Khademhosseini A, Annabi N, Dana R, Kheirkhah A, Google Patents 2019.
- [67].N. Burlington.
- [68].Sundaram C, Keenan A, Indian Journal of Urology, 26 (2010) 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lin C, Ritter JA, Carbon, 35 (1997) 1271–1278. [Google Scholar]
- [70].Albes JM, Krettek C, Hausen B, Rohde R, Haverich A, Borst H-G, The Annals of thoracic surgery, 56 (1993) 910–915. [DOI] [PubMed] [Google Scholar]
- [71].Walker JD, Kratz JM, Basler CG, Meck LP, Stratton JR, Kribbs SB, Crawford FA Jr, Spinale FG, Journal of Surgical Research, 71 (1997) 73–78. [DOI] [PubMed] [Google Scholar]
- [72].Bonchek LI, Braunwald NS, Annals of surgery, 165 (1967) 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Matsuda M, Ueno M, Endo Y, Inoue M, Sasaki M, Taguchi T, Colloids and Surfaces B: Biointerfaces, 91 (2012) 48–56. [DOI] [PubMed] [Google Scholar]
- [74].Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 46 (1999) 520–530. [DOI] [PubMed] [Google Scholar]
- [75].Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z, Advanced drug delivery reviews, 60 (2008) 1650–1662. [DOI] [PubMed] [Google Scholar]
- [76].Boddohi S, Kipper MJ, Advanced materials, 22 (2010) 2998–3016. [DOI] [PubMed] [Google Scholar]
- [77].Krishna OD, Kiick KL, Peptide Science: Original Research on Biomolecules, 94 (2010) 32–48. [Google Scholar]
- [78].Baldwin AD, Kiick KL, Peptide Science: Original Research on Biomolecules, 94 (2010) 128–140. [Google Scholar]
- [79].Basu A, Kunduru KR, Abtew E, Domb AJ, Bioconjugate chemistry, 26 (2015) 1396–1412. [DOI] [PubMed] [Google Scholar]
- [80].Li H, Niu R, Yang J, Nie J, Yang D, Carbohydrate polymers, 86 (2011) 1578–1585. [Google Scholar]
- [81].Lévesque SG, Shoichet MS, Biomaterials, 27 (2006) 5277–5285. [DOI] [PubMed] [Google Scholar]
- [82].Artzi N, Shazly T, Crespo C, Ramos AB, Chenault HK, Edelman ER, Macromolecular bioscience, 9 (2009) 754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang T, Nie J, Yang D, Carbohydrate polymers, 90 (2012) 1428–1436. [DOI] [PubMed] [Google Scholar]
- [84].Baeurle SA, Kiselev M, Makarova E, Nogovitsin E, Polymer, 50 (2009) 1805–1813. [Google Scholar]
- [85].Bouten PJ, Zonjee M, Bender J, Yauw ST, van Goor H, van Hest JC, Hoogenboom R, Progress in Polymer Science, 39 (2014) 1375–1405. [Google Scholar]
- [86].Han L, Wang M, Li P, Gan D, Yan L, Xu J, Wang K, Fang L, Chan CW, Zhang H, ACS applied materials & interfaces, 10 (2018) 28015–28026. [DOI] [PubMed] [Google Scholar]
- [87].Fajardo AR, Fávaro SL, Rubira AF, Muniz EC, Reactive and Functional Polymers, 73 (2013) 1662–1671. [Google Scholar]
- [88].Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J, Biomaterials, 31 (2010) 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deng Z, He Y, Wang YJ, Zhao Y, Chen L, Soft matter, 16 (2020) 6128–6137. [DOI] [PubMed] [Google Scholar]
- [90].Luo F, Sun TL, Nakajima T, Kurokawa T, Zhao Y, Sato K, Ihsan AB, Li X, Guo H, Gong JP, Advanced materials, 27 (2015) 2722–2727. [DOI] [PubMed] [Google Scholar]
- [91].Villanueva JGV, Huertas PAS, Galan FS, Rueda RJE, Triana JCB, Rodriguez JPC, International Journal of Adhesion and Adhesives, 92 (2019) 80–88. [Google Scholar]
- [92].Patel AK, Michaud P, de Baynast H, Grédiac M, Mathias JD, Journal of Applied Polymer Science, 127 (2013) 3869–3876. [Google Scholar]
- [93].Ono K, Saito Y, Yura H, Ishikawa K, Kurita A, Akaike T, Ishihara M, Journal of Biomedical Materials Research, 49 (2000) 289–295. [DOI] [PubMed] [Google Scholar]
- [94].Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Uenoyama M, Kurita A, Biomaterials, 23 (2002) 833–840. [DOI] [PubMed] [Google Scholar]
- [95].Ono K, Ishihara M, Ozeki Y, Deguchi H, Sato M, Saito Y, Yura H, Sato M, Kikuchi M, Kurita A, Maehara T, Surgery, 130 (2001) 844–850. [DOI] [PubMed] [Google Scholar]
- [96].Ishihara M, Ono K, Sato M, Nakanishi K, Saito Y, Yura H, Matsui T, Hattori H, Fujita M, Kikuchi M, Wound repair and regeneration, 9 (2001) 513–521. [DOI] [PubMed] [Google Scholar]
- [97].Zhou Y, Zhao J, Sun X, Li S, Hou X, Yuan X, Yuan X, Biomacromolecules, 17 (2016) 622–630. [DOI] [PubMed] [Google Scholar]
- [98].Farrar D, Bone adhesives for trauma surgery: A review of challenges and developments, 2012. [Google Scholar]
- [99].Carleo C, Singer AJ, Thode HC Jr., Cjem, 7 (2005) 391–395. [PubMed] [Google Scholar]
- [100].Beam JW, J Athl Train, 43 (2008) 222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Toriumi DM, Chung VK, Cappelle QM, Otolaryngologic Clinics of North America, 49 (2016) 585–599. [DOI] [PubMed] [Google Scholar]
- [102].Ridgway DM, Mahmood F, Moore L, Bramley D, Moore PJ, Ann R Coll Surg Engl, 89 (2007) 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sniezek PJ, Walling HW, DeBloom JR 3rd, Messingham MJ, VanBeek MJ, Kreiter CD, Whitaker DC, Arpey CJ, Dermatol Surg, 33 (2007) 966–971. [DOI] [PubMed] [Google Scholar]
- [104].Sterling JB, Skouge JW, Dermatol Surg, 34 (2008) 246–247; discussion 247–248. [DOI] [PubMed] [Google Scholar]
- [105].Toriumi DM, O’Grady K, Desai D, Bagal A, Plast Reconstr Surg, 102 (1998) 2209–2219. [DOI] [PubMed] [Google Scholar]
- [106].Maartense S, Bemelman WA, Dunker MS, de Lint C, Pierik EG, Busch OR, Gouma DJ, Br J Surg, 89 (2002) 1370–1375. [DOI] [PubMed] [Google Scholar]
- [107].Singer AJ, Hollander JE, Valentine SM, Turque TW, McCuskey CF, Quinn JV, Acad Emerg Med, 5 (1998) 94–99. [DOI] [PubMed] [Google Scholar]
- [108].Shalaby BKJLB W S. , Absorbable and Biodegradable Polymers, CRC Press, Boca Raton, 2004. [Google Scholar]
- [109].Annabi N, Yue K, Tamayol A, Khademhosseini A, Eur J Pharm Biopharm, 95 (2015) 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kelmansky R, McAlvin BJ, Nyska A, Dohlman JC, Chiang HH, Hashimoto M, Kohane DS, Mizrahi B, Acta biomaterialia, 53 (2017) 93–99. [DOI] [PubMed] [Google Scholar]
- [111].Macchiarini P, Wain J, Almy S, Dartevelle P, The Journal of Thoracic and Cardiovascular Surgery, 117 (1999) 751–758. [DOI] [PubMed] [Google Scholar]
- [112].T. Adar, G. Lalazar, A. Ben-Ya’acov, M. Mizrahi, E. Shteyer, D. Kanavich, E. Axelrod, Y. Lichtenstein, L. Zolotaryova, M. El Haj, JOHN WILEY & SONS INC 111 RIVER ST, HOBOKEN, NJ 07030 USA, pp. 389A-389A.
- [113].Wallace DG, Cruise GM, Rhee WM, Schroeder JA, Prior JJ, Ju J, Maroney M, Duronio J, Ngo MH, Estridge T, Coker GC, J Biomed Mater Res, 58 (2001) 545–555. [DOI] [PubMed] [Google Scholar]
- [114].Bian S, Zheng Z, Liu Y, Ruan C, Pan H, Zhao X, Journal of Materials Chemistry B, 7 (2019) 6488–6499. [DOI] [PubMed] [Google Scholar]
- [115].Shi Y, Zhou P, Jérôme V, Freitag R, Agarwal S, ACS Biomaterials Science & Engineering, 1 (2015) 971–977. [DOI] [PubMed] [Google Scholar]
- [116].Cernadas TM, Gonçalves FAMM, Alves P, Miguel SP, Cabral C, Correia IJ, Ferreira P, European Polymer Journal, 117 (2019) 442–454. [Google Scholar]
- [117].Jiao Y-P, Cui F-Z, Surface modification of polyester biomaterials for tissue engineering, 2008. [DOI] [PubMed] [Google Scholar]
- [118].Zhang H, Zhao T, Duffy P, Dong Y, Annaidh AN, O’Cearbhaill E, Wang W, Advanced healthcare materials, 4 (2015) 2260–2268. [DOI] [PubMed] [Google Scholar]
- [119].Ohira S, Fukumoto A, Matsushiro T, Yaku H, Heart, Lung and Circulation, 25 (2016) 885–887. [DOI] [PubMed] [Google Scholar]
- [120].Shah NV, Orthopedics, 37 (2014) 148. [PubMed] [Google Scholar]
- [121].Yang J, Chen H, Yuan Y, Sarkar D, Zheng J, Frontiers of Chemical Science and Engineering, 8 (2014) 498–510. [Google Scholar]
- [122].Ates B, Koytepe S, Karaaslan MG, Balcioglu S, Gulgen S, Demirbilek M, Denkbas EB, International Journal of Polymeric Materials and Polymeric Biomaterials, 64 (2015) 611–619. [Google Scholar]
- [123].Balcioglu S, Parlakpinar H, Vardi N, Denkbas EB, Karaaslan MG, Gulgen S, Taslidere E, Koytepe S, Ates B, ACS Appl Mater Interfaces, 8 (2016) 4456–4466. [DOI] [PubMed] [Google Scholar]
- [124].Llewellyn-Thomas E, Wang PY, Cannon JS, Journal of biomedical materials research, 8 (1974) 35–43. [DOI] [PubMed] [Google Scholar]
- [125].Schreader KJ, Bayer IS, Milner DJ, Loth E, Jasiuk I, Journal of Applied Polymer Science, 127 (2013) 4974–4982. [Google Scholar]
- [126].Le TMD, Duong HTT, Thambi T, Giang Phan VH, Jeong JH, Lee DS, Biomacromolecules, 19 (2018) 3536–3548. [DOI] [PubMed] [Google Scholar]
- [127].Benoit FM, J Biomed Mater Res, 27 (1993) 1341–1348. [DOI] [PubMed] [Google Scholar]
- [128].Favi PM, Yi S, Lenaghan SC, Xia L, Zhang M, J Adhes Sci Technol, 28 (2014) 290–319. [Google Scholar]
- [129].Zhang X, Liu Y, Liu Y, Ahmed S-U, Chinese Science Bulletin, 54 (2009) 1648–1654. [Google Scholar]
- [130].Watson GS, Green DW, Schwarzkopf L, Li X, Cribb BW, Myhra S, Watson JA, Acta biomaterialia, 21 (2015) 109–122. [DOI] [PubMed] [Google Scholar]
- [131].Green DW, Lee KK-H, Watson JA, Kim H-Y, Yoon K-S, Kim E-J, Lee J-M, Watson GS, Jung HS, Scientific reports, 7 (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Geim A, Dubonos S, Grigorieva I, Nat. Mater, 2 (2003) 461–463. [DOI] [PubMed] [Google Scholar]
- [133].Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM, Proc Natl Acad Sci U S A, 105 (2008) 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Baik S, Kim DW, Park Y, Lee TJ, Ho Bhang S, Pang C, Nature, 546 (2017) 396–400. [DOI] [PubMed] [Google Scholar]
- [135].Lee H, Dellatore SM, Miller WM, Messersmith PB, science, 318 (2007) 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cha HJ, Hwang DS, Lim S, Biotechnol J, 3 (2008) 631–638. [DOI] [PubMed] [Google Scholar]
- [137].Silverman HG, Roberto FF, Mar Biotechnol (NY), 9 (2007) 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Waite JH, Tanzer ML, Science, 212 (1981) 1038–1040. [DOI] [PubMed] [Google Scholar]
- [139].Papov VV, Diamond TV, Biemann K, Waite JH, Journal of Biological Chemistry, 270 (1995) 20183–20192. [DOI] [PubMed] [Google Scholar]
- [140].Pardo J, Gutierrez E, Saez C, Brito M, Burzio LO, Protein Expr Purif, 1 (1990) 147–150. [DOI] [PubMed] [Google Scholar]
- [141].Waite JH, Qin X, Biochemistry, 40 (2001) 2887–2893. [DOI] [PubMed] [Google Scholar]
- [142].Holten-Andersen N, Mates TE, Toprak MS, Stucky GD, Zok FW, Waite JH, Langmuir, 25 (2008) 3323–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wilker JJ, Angewandte Chemie International Edition, 49 (2010) 8076–8078. [DOI] [PubMed] [Google Scholar]
- [144].Lee BP, Dalsin JL, Messersmith PB, Biomacromolecules, 3 (2002) 1038–1047. [DOI] [PubMed] [Google Scholar]
- [145].Burke SA, Ritter-Jones M, Lee BP, Messersmith PB, Biomed Mater, 2 (2007) 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Murphy JL, Vollenweider L, Xu F, Lee BP, Biomacromolecules, 11 (2010) 2976–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Tian Y, Wan J, Pesika N, Zhou M, Scientific reports, 3 (2013) 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Xu Y, Liu Q, Narayanan A, Jain D, Dhinojwala A, Joy A, Advanced Materials Interfaces, 4 (2017) 1700506. [Google Scholar]
- [149].Rahimnejad M, Zhong W, Rsc Advances, 7 (2017) 47380–47396. [Google Scholar]
- [150].Cholewinski A, Yang FK, Zhao B, Materials Horizons, 6 (2019) 285–293. [Google Scholar]
- [151].Burke KA, Roberts DC, Kaplan DL, Biomacromolecules, 17 (2015) 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ryu JH, Lee Y, Kong WH, Kim TG, Park TG, Lee H, Biomacromolecules, 12 (2011) 2653–2659. [DOI] [PubMed] [Google Scholar]
- [153].Ryu JH, Hong S, Lee H, Acta biomaterialia, 27 (2015) 101–115. [DOI] [PubMed] [Google Scholar]
- [154].Hou J, Li C, Guan Y, Zhang Y, Zhu X, Polymer Chemistry, 6 (2015) 2204–2213. [Google Scholar]
- [155].Shin J, Lee JS, Lee C, Park HJ, Yang K, Jin Y, Ryu JH, Hong KS, Moon SH, Chung HM, Advanced Functional Materials, 25 (2015) 3814–3824. [Google Scholar]
- [156].Barrett DG, Bushnell GG, Messersmith PB, Advanced healthcare materials, 2 (2013) 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Brubaker CE, Messersmith PB, Biomacromolecules, 12 (2011) 4326–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wang Z, Wang K, Zhang Y, Jiang Y, Lu X, Fang L, Gan D, Lv C, Zhang H, Qu S, Particle & Particle Systems Characterization, 33 (2016) 89–100. [Google Scholar]
- [159].Chen T, Chen Y, Rehman HU, Chen Z, Yang Z, Wang M, Li H, Liu H, ACS applied materials & interfaces, 10 (2018) 33523–33531. [DOI] [PubMed] [Google Scholar]
- [160].Du X, Wu L, Yan H, Qu L, Wang L, Wang X, Ren S, Kong D, Wang L, ACS Biomaterials Science & Engineering, 5 (2019) 2610–2620. [DOI] [PubMed] [Google Scholar]
- [161].Peng B, Lai X, Chen L, Lin X, Sun C, Liu L, Qi S, Chen Y, Leong KW, ACS omega, 2 (2017) 6053–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Zhu W, Peck Y, Iqbal J, Wang D-A, Biomaterials, 147 (2017) 99–115. [DOI] [PubMed] [Google Scholar]
- [163].Han L, Yan L, Wang K, Fang L, Zhang H, Tang Y, Ding Y, Weng L-T, Xu J, Weng J, NPG Asia Materials, 9 (2017) e372–e372. [Google Scholar]
- [164].Xie C, Wang X, He H, Ding Y, Lu X, Advanced Functional Materials, (2020) 1909954. [Google Scholar]
- [165].Gan D, Huang Z, Wang X, Jiang L, Wang C, Zhu M, Ren F, Fang L, Wang K, Xie C, Advanced Functional Materials, 30 (2020) 1907678. [Google Scholar]
- [166].Wilks AM, Rabice SR, Garbacz HS, Harro CC, Smith AM, J Exp Biol, 218 (2015) 3128–3137. [DOI] [PubMed] [Google Scholar]
- [167].Li J, Celiz A, Yang J, Yang Q, Wamala I, Whyte W, Seo B, Vasilyev N, Vlassak J, Suo Z, Science, 357 (2017) 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Shao H, Stewart RJ, Advanced materials, 22 (2010) 729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Winslow BD, Shao H, Stewart RJ, Tresco PA, Biomaterials, 31 (2010) 9373–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lee Y, Xu C, Sebastin M, Lee A, Holwell N, Xu C, Miranda Nieves D, Mu L, Langer RS, Lin C, Advanced healthcare materials, 4 (2015) 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Shao H, Bachus KN, Stewart RJ, Macromolecular bioscience, 9 (2009) 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Asakura T, Suzuki Y, Silk Fibroin, in: Kobayashi S, Müllen K. (Eds.) Encyclopedia of Polymeric Nanomaterials, Springer Berlin Heidelberg, Berlin, Heidelberg, 2021, pp. 1–7. [Google Scholar]
- [173].Seo JW, Kim H, Kim K, Choi SQ, Lee HJ, Advanced Functional Materials, 28 (2018) 1800802. [Google Scholar]
- [174].Luo J, Yang J, Zheng X, Ke X, Chen Y, Tan H, Li J, Advanced Healthcare Materials, 9 (2020) 1901423. [DOI] [PubMed] [Google Scholar]
- [175].Guo J, Sun W, Kim JP, Lu X, Li Q, Lin M, Mrowczynski O, Rizk EB, Cheng J, Qian G, Acta biomaterialia, 72 (2018) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Sun L, Huang Y, Bian Z, Petrosino J, Fan Z, Wang Y, Park KH, Yue T, Schmidt M, Galster S, ACS applied materials & interfaces, 8 (2016) 2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zhang M, Lenaghan SC, Xia L, Dong L, He W, Henson WR, Fan X, Journal of nanobiotechnology, 8 (2010) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Gan D, Xing W, Jiang L, Fang J, Zhao C, Ren F, Fang L, Wang K, Lu X, Nature communications, 10 (2019) 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Alona Shagan WZ, Manisha Mehta, Shira Levi, Kohane Daniel S., Boaz Mizrahi, Adv. Funct. Mater, (2019). [Google Scholar]
- [180].Kim SH, Lee SH, Lee JE, Park SJ, Kim K, Kim IS, Lee YS, Hwang NS, Kim BG, Biomaterials, 178 (2018) 401–412. [DOI] [PubMed] [Google Scholar]
- [181].Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, Khademhosseini A, Dana R, Annabi N, Science advances, 5 (2019) eaav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Xu Y, Liang K, Ullah W, Ji Y, Ma J, Carbohydr Polym, 190 (2018) 324–330. [DOI] [PubMed] [Google Scholar]
- [183].Zhou J, Defante AP, Lin F, Xu Y, Yu J, Gao Y, Childers E, Dhinojwala A, Becker ML, Biomacromolecules, 16 (2015) 266–274. [DOI] [PubMed] [Google Scholar]
- [184].Sousa MP, Neto AI, Correia TR, Miguel SP, Matsusaki M, Correia IJ, Mano JF, Biomaterials science, 6 (2018) 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lev R, Seliktar D, Journal of the Royal Society, Interface, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Moghadam MN, Pioletti DP, J Mech Behav Biomed Mater, 41 (2015) 161–167. [DOI] [PubMed] [Google Scholar]
- [187].Cha C, Soman P, Zhu W, Nikkhah M, Camci-Unal G, Chen S, Khademhosseini A, Biomaterials science, 2 (2014) 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sharma S, Kumar R, Kumari P, Kharwar RN, Yadav AK, Saripella S, International journal of biological macromolecules, 125 (2019) 109–115. [DOI] [PubMed] [Google Scholar]
- [189].Wei Z, Harris BT, Zhang LG, Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 2016 (2016) 4185–4188. [DOI] [PubMed] [Google Scholar]
- [190].Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang K, Manoharan V, Zhang YS, Yuksekkaya M, Wan KT, Nikkhah M, Dokmeci MR, Tang XS, Khademhosseini A, Small, 12 (2016) 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ramon-Azcon J, Ahadian S, Estili M, Liang X, Ostrovidov S, Kaji H, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Khademhosseini A, Matsue T, Adv Mater, 25 (2013) 4028–4034. [DOI] [PubMed] [Google Scholar]
- [192].Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan KT, Palacios T, Dokmeci MR, Bae H, Tang XS, Khademhosseini A, ACS nano, 7 (2013) 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Shin SR, Bae H, Cha JM, Mun JY, Chen YC, Tekin H, Shin H, Farshchi S, Dokmeci MR, Tang S, Khademhosseini A, ACS nano, 6 (2012) 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Xin T, Gu Y, Cheng R, Tang J, Sun Z, Cui W, Chen L, ACS Appl Mater Interfaces, 9 (2017) 41168–41180. [DOI] [PubMed] [Google Scholar]
- [195].Negahi Shirazi A, Fathi A, Suarez FG, Wang Y, Maitz PK, Dehghani F, ACS Appl Mater Interfaces, 8 (2016) 1676–1686. [DOI] [PubMed] [Google Scholar]
- [196].Liu B, Wang Y, Miao Y, Zhang X, Fan Z, Singh G, Zhang X, Xu K, Li B, Hu Z, Xing M, Biomaterials, 171 (2018) 83–96. [DOI] [PubMed] [Google Scholar]
- [197].Zhang H, Bre LP, Zhao T, Zheng Y, Newland B, Wang W, Biomaterials, 35 (2014) 711–719. [DOI] [PubMed] [Google Scholar]
- [198].Petter-Puchner AH, Simunek M, Redl H, Puchner KU, Van Griensven M, Journal of investigative surgery : the official journal of the Academy of Surgical Research, 23 (2010) 40–47. [DOI] [PubMed] [Google Scholar]
- [199].Liu Y, Cheong Ng S, Yu J, Tsai WB, Colloids and surfaces. B, Biointerfaces, 174 (2019) 316–323. [DOI] [PubMed] [Google Scholar]
- [200].Sohn JJ, Gruber TM, Zahorsky-Reeves JL, Lawson GW, Journal of the American Association for Laboratory Animal Science : JAALAS, 55 (2016) 199–203. [PMC free article] [PubMed] [Google Scholar]
- [201].Mizrahi B, Stefanescu CF, Yang C, Lawlor MW, Ko D, Langer R, Kohane DS, Acta Biomater, 7 (2011) 3150–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Eaglstein WH, Sullivan T, Dermatologic clinics, 23 (2005) 193–198. [DOI] [PubMed] [Google Scholar]
- [203].Lee YJ, Son HS, Jung GB, Kim JH, Choi S, Lee GJ, Park HK, Materials science & engineering. C, Materials for biological applications, 51 (2015) 43–50. [DOI] [PubMed] [Google Scholar]
- [204].Guhan S, Peng SL, Janbatian H, Saadeh S, Greenstein S, Al Bahrani F, Fadlallah A, Yeh TC, Melki SA, The British journal of ophthalmology, 102 (2018) 1328–1335. [DOI] [PubMed] [Google Scholar]
- [205].Spierer O, O’Brien TP, Journal of cataract and refractive surgery, 41 (2015) 492–496. [DOI] [PubMed] [Google Scholar]
- [206].Kirchhof S, Goepferich AM, Brandl FP, Eur J Pharm Biopharm, 95 (2015) 227–238. [DOI] [PubMed] [Google Scholar]
- [207].Choudhari NS, Neog A, Latka S, Srinivasan B, Middle East African journal of ophthalmology, 22 (2015) 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Leung GY, Peponis V, Varnell ED, Lam DS, Kaufman HE, Cornea, 24 (2005) 998–999. [DOI] [PubMed] [Google Scholar]
- [209].Akhter S, Anwar M, Siddiqui MA, Ahmad I, Ahmad J, Ahmad MZ, Bhatnagar A, Ahmad FJ, Colloids and surfaces. B, Biointerfaces, 148 (2016) 19–29. [DOI] [PubMed] [Google Scholar]
- [210].Papadopoulou DN, Sionga A, Karayannopoulou G, Natsis K, Komnenou A, Mangioris G, Kalpatsanidis A, Manthos A, Georgiadis N, Karampatakis V, European journal of ophthalmology, 23 (2013) 646–651. [DOI] [PubMed] [Google Scholar]
- [211].Duarte MC, Kim T, Cornea, 26 (2007) 1127–1128. [DOI] [PubMed] [Google Scholar]
- [212].Uhlig CE, Busse H, Groppe M, American journal of ophthalmology, 142 (2006) 189–191. [DOI] [PubMed] [Google Scholar]
- [213].Weyenberg W, Bozdag S, Foreman P, Remon JP, Ludwig A, Eur J Pharm Biopharm, 62 (2006) 202–209. [DOI] [PubMed] [Google Scholar]
- [214].Xu HL, Tong MQ, Wang LF, Chen R, Li XZ, Sohawon Y, Yao Q, Xiao J, Zhao YZ, Biomaterials science, 7 (2019) 2582–2599. [DOI] [PubMed] [Google Scholar]
- [215].Pleguezuelos-Villa M, Nacher A, Hernandez MJ, Buso M, Barrachina M, Penalver N, Diez-Sales O, Journal of materials science. Materials in medicine, 30 (2019) 14. [DOI] [PubMed] [Google Scholar]
- [216].Han L, Wang M, Prieto-López LO, Deng X, Cui J, Advanced Functional Materials, 30 (2020) 1907064. [Google Scholar]
- [217].Tarcoveanu E, Lupascu C, Moldovanu R, Vlad N, Bradea C, Vasilescu A, Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi, 111 (2007) 396–401. [PubMed] [Google Scholar]
- [218].Kawasaki S, Origasa H, Tetens V, Kobayashi M, Langenbecks Arch Surg, 402 (2017) 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Oz MC, Rondinone JF, Shargill NS, Journal of Cardiac Surgery, 18 (2003) 486–493. [DOI] [PubMed] [Google Scholar]
- [220].Jeon EY, Hwang BH, Yang YJ, Kim BJ, Choi B-H, Jung GY, Cha HJ, Biomaterials, 67 (2015) 11–19. [DOI] [PubMed] [Google Scholar]
- [221].Ayyıldız SN, Ayyıldız A, Turkish journal of urology, 43 (2017) 14.28270946 [Google Scholar]
- [222].Horowitz B, Busch M, Transfusion, 48 (2008) 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Spotnitz WD, ISRN surgery, 2014 (2014) 203943–203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Vyas KS, Saha SP, Expert Opin Biol Ther, 13 (2013) 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Dhillon S, Drugs, 71 (2011) 1893–1915. [DOI] [PubMed] [Google Scholar]
- [226].Amer AO, Wilson CH, White SA, Manas DM, Cochrane Database of Systematic Reviews, (2013). [Google Scholar]
- [227].Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ, International journal of nanomedicine, 12 (2017) 4937–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Wheat JC, Wolf JS Jr., The Urologic clinics of North America, 36 (2009) 265–275, x. [DOI] [PubMed] [Google Scholar]
- [229].Lew WK, Weaver FA, Biologics: targets & therapy, 2 (2008) 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Duarte AP, Coelho JF, Bordado JC, Cidade MT, Gil MH, Progress in Polymer Science, 37 (2012) 1031–1050. [Google Scholar]
- [231].Fenn SL, Charron PN, Oldinski RA, ACS applied materials & interfaces, 9 (2017) 23409–23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Bruns TB, Worthington JM, American family physician, 61 (2000) 1383–1388. [PubMed] [Google Scholar]
- [233].FDA, 2003.
- [234].FDA, 2009.
- [235].Zhang R, Bures M, Höffler H-K, Zinne N, Länger F, Bisdas T, Haverich A, Krüger M, Annals of Surgical Innovation and Research, 6 (2012) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Zoia C, Bongetta D, Lombardi F, Custodi VM, Pugliese R, Gaetani P, Journal of applied biomaterials & functional materials, 13 (2015) 372–375. [DOI] [PubMed] [Google Scholar]
- [237].van Doormaal T, Kinaci A, van Thoor S, Redegeld S, Bergmann W, van der Zwan A, Operative Neurosurgery, 15 (2017) 425–432. [DOI] [PubMed] [Google Scholar]
- [238].Taboada GM, Yang K, Pereira MJ, Liu SS, Hu Y, Karp JM, Artzi N, Lee Y, Nature Reviews Materials, (2020) 1–20.33078077 [Google Scholar]