Abstract
Since the initial description of Chediak-Higashi syndrome (CHS), over 75 years ago, several studies have been conducted to underscore the role of the lysosomal trafficking regulator (LYST) gene in the pathogenesis of disease. CHS is a rare autosomal recessive disorder, which is caused by biallelic mutations in the highly conserved LYST gene. The disease is characterized by partial oculocutaneous albinism, prolonged bleeding, immune and neurologic dysfunction, and risk for the development of hemophagocytic lympohistiocytosis (HLH). The presence of giant secretory granules in leukocytes is the classical diagnostic feature, which distinguishes CHS from closely related Griscelli and Hermansky-Pudlak syndromes. While the exact mechanism of the formation of the giant granules in CHS patients is not understood, dysregulation of LYST function in regulating lysosomal biogenesis has been proposed to play a role. In this review, we discuss the clinical characteristics of the disease and highlight the functional consequences of enlarged lysosomes and lysosome-related organelles (LROs) in CHS.
Keywords: Chediak-Higashi syndrome, LYST, Lysosomes, Lysosome-related organelles
Graphical abstract
Background
Although Chediak-Higashi syndrome (CHS) bears the names of the French physician Moises Chediak and the Japanese physician Ototaka Higashi, it was Antonio Beguez-Cesar, a Cuban physician, who described the initial family with atypical enlarged granules within leukocytes1. Additional clinical characteristics included neutropenia, albinism, and features of hemophagocytic lymphohistiocytosis (HLH). Consultation with Chediak concluded this was a novel disease. Additional cases were described by Steinbrinck in 1948 and Higashi in 1954. Today, CHS is best recognized as a primary immunodeficiency with pathognomonic giant inclusions within leukocytes, among other cell types. The disease is exceptionally rare with fewer than 500 cases reported in the literature2.
Clinical characteristics
Characteristic features of CHS include partial oculocutaneous albinism, predilection for bleeding, immune dysfunction, neurodegeneration, and risk for development of HLH3; 4. Pigment dilution is variable and can be subtle5; 6. Within the hair, clumps of pigment are dispersed throughout the hair shaft (Figure 1a). Ocular pigmentation can also be variable. Often iris pigment is reduced with iris transillumination visible on examination (Figure 1b). Retinal pigment may also be reduced. Once thought to be an essential criterion for clinical diagnosis, it is now appreciated that there are some individuals with no evidence of oculocutaneous albinism7.
Figure 1.
Clinical features of Chediak-Higashi syndrome, LYST domain structure, and cellular defects
a. A hair image from a patient with CHS shows dispersal of pigment clumps throughout the hair shaft.
b. An image from a patient with CHS shows iris transillumination.
c. Peripheral blood smear from a patient with CHS shows a neutrophil with the typical giant cytoplasmic granules (arrow).
d. Peripheral blood smear from a patient with CHS shows a neutrophil with prominent cytoplasmic granules (arrow).
e. MRI of the brain of a child with CHS demonstrating the small posterior fossa and high pitched tentorium.
f. MRI of the brain of a young adult with CHS demonstrating cerebellar and cerebral atrophy.
g. A schematic of the domain structure shows the four defined domains of the human lysosomal trafficking regulator (LYST) protein. The amino-terminal ARM/HEAT domain contains a series of Armadillo (ARM) and Huntingtin (HEAT) alpha-helix repeat motifs that are predicted to mediate membrane association and vesicle transport. LYST also contains a Pleckstrin-homology (PH) domain, a Beige and Chediak-Higashi (BEACH) domain, and seven carboxy-terminal WD-40 domains containing conserved amino acids tryptophan (W) and aspartic acid (D).
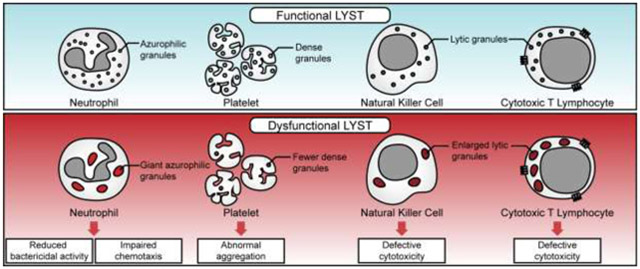
h. Diagrams displaying cellular defects leading to the functional impairments in CHS. The giant azurophilic granules in neutrophils do not release their contents properly and affect bactericidal and chemotaxis activity (upper). The reduced number of dense granules in platelets leads to abnormal aggregation and prolonged bleeding time (middle). The presence of enlarged lytic granules leads to impairments of the cytotoxic activity of NK and cytotoxic T lymphocytes (lower).
Although platelet numbers are generally within the normal range, bleeding occurs due to absent or severely diminished platelet dense granules8. Dense granules contain calcium, ADP, ATP, and serotonin and, when released, are responsible for platelet recruitment and aggregation. In the absence of dense granules, the initial platelet plug does not develop leading to prolonged bleeding times. Platelet aggregation studies show an abnormal secondary wave of aggregation. Platelet dense bodies can be quantified using platelet electron microscopy. Generally, symptoms manifest as mucosal bleeds with gingival bleeding, epistaxis, and easy bruisability. However, in the setting of trauma or surgical intervention, platelet dysfunction may contribute to prolonged bleeding.
The hallmark of CHS is the giant granules within leukocytes that are visualized on routine peripheral blood smear (Figure 1c). The granules are azurophilic, peroxidase positive, yet variable in size (Figure 1d). The large granules impair neutrophil chemotaxis, and intracellular bactericidal activity is also reduced, likely from impaired degranulation8. Neutropenia may be present. Natural Killer (NK) cells are also reduced in number and in function.
Neurologic disease includes both developmental and degenerative components. Children may manifest learning difficulties and behavioral abnormalities during early school age7. Interestingly, on MRI imaging, the posterior fossa shows structural variability with a high-pitched tentorium supporting the theory that development of the posterior fossa is impacted (Figure 1e). In late adolescence and early adulthood, patients begin to display progressive degeneration including absence of deep tendon reflexes, signs of cerebellar dysfunction, peripheral neuropathy, length dependent neuropathy and neuronopathy, weakness, spasticity, or parkinsonian symptoms5; 7; 9. Brain imaging is unrevealing initially, though over time, cerebellar and/or cerebral atrophy may develop (Figure 1e, and 1f).
The accelerated phase, or HLH, is the primary cause of mortality in CHS. HLH can occur at any age and presents with unexplained fevers, lymphadenopathy, hepatosplenomegaly, and cytopenias of at least two of the three cell lineages in peripheral blood. Criteria for the diagnosis of HLH are well described, and management is the same as for HLH of other etiologies10.
Differential diagnosis
CHS most closely resembles Griscelli syndrome (GS) and Hermansky-Pudlak syndrome (HPS). Both GS and HPS have pigment reduction. There are 10 subtypes of HPS with abnormal bleeding due to absent dense granules. HPS-2, is also characterized by neutropenia, recurrent infections, and risk of HLH, although less risk of HLH than CHS or GS11. There are 3 subtypes of GS. GS1 has a predominant neurologic phenotype, while GS2 presents with immunodeficiency and risk for HLH12. GS3 manifests with only pigment dilution. CHS distinguishes itself from GS and HPS by identifying the giant granules within leukocytes as GS and HPS do not have enlarged granules on peripheral smear.
Genetic cause of CHS and genotype-phenotype correlation
CHS is an autosomal recessive disorder due to mutations in the highly conserved gene, lysosomal trafficking regulator (LYST)2; 13-15. The open reading frame of the LYST mRNA (NM_000081) has 53 exons that encodes a protein (NP_000072) of 3801 amino acids. LYST is widely expressed in human tissues with higher levels of expression found in bone marrow, cerebellum, spleen and thymus. The carboxy-terminal of LYST contains three domains: a Pleckstrin-homology (PH) domain, a Beige and Chediak-Higashi (BEACH) domain, and WD-40 (tryptophan-aspartic acid) repeats (Figure 1g)14. Studies of proteins with BEACH domains revealed some of the functional significance of these domains and suggest their primary involvement in vesicular trafficking16-18. The amino terminal of LYST contains a series of Armadillo (ARM) and Huntingtin (HEAT) α-helix repeat motifs, that may mediate membrane association and vesicle transport19.
Prior to the discovery of LYST, CHS diagnosis was based on clinical criteria, and likely only identified children with classic features of the disease. However, the clinical phenotype is better represented as a spectrum of disease with some individuals having a more classic presentation and some individuals with atypical features. While not absolute, a genotype-phenotype correlation has emerged with individuals with biallelic truncating mutations leading to more severe clinical manifestations and individuals with at least one missense mutation having a milder presentation7. In NK cells, it has been shown that in the classic presentation, granules are fewer in number, but extremely large in size. However, in atypical cases, the granules may be more numerous, and smaller, yet still prominent compared to normal7.
Unlike other clinical features, the neurologic disease does not distinguish the classic from the more atypical phenotypes. For individuals with atypical phenotypes, the neurologic features may dominate the clinical picture while the hematologic and immunologic features are more muted. Patients with classic presentation appear to be at greater risk of developing HLH, although there are exceptions reported with genotypically milder individuals accelerating20.
It is not surprising that numerous different variants have been reported in LYST given its large size. Most individuals have private mutations. Mutations include nonsense, missense, frameshift, and splice site variants that have been identified throughout the gene, particularly in the ARM/HEAT, BEACH and WD-40 domains. Challenges arise when trying to determine pathogenicity of new variants. Bioinformatic predictive tools offer some guidance for determining pathogenicity, but clinical phenotype is also important for the interpretation of the molecular variations.
Murine models
Genetic linkage studies have shown that LYST maps within a linkage group conserved between human chromosome 1q42-q43 and the Beige region on mouse chromosome 1321. The Beige (bg) mutant allele was the first mutation of the murine Lyst gene that appeared spontaneously as a consequence of LINE1 element insertion within an intron of the murine Beige gene producing a premature stop codon15. The Beige mice on a C57BL/6J background have enlarged lysosomes and lysosome-related organelles (LROs), hypopigmentation, prolonged bleeding times, and immunodeficiency due to defective cytotoxicity of T and NK cells.22; 23.
Another CHS model, the Lystbg-grey, consists of a point mutation of the splice donor site of the intron between exon 25 and exon 26, which causes an in-frame skipping of exon 25 and unstable LYST protein, and leads to a phenotype similar to the Beige mice24.
The LystIng3618 mice on the C57BL/6J background display hypopigmentation and prolonged bleeding times but with no abnormality in hematopoietic lineage, impaired lysosomal morphology and function, or susceptibility to infections or tumors. Interestingly, these mice apparently present with a neurodegenerative phenotype from 5 months of age25.
Recently, it has been shown that Lyst mutant mice exhibit more overt tremor on the DBA/2J genetic background when compared to C57BL/6J26, suggesting the presence of genetic modifiers27. This mouse strain may be useful in studying the neurodegenerative phenotype in more detail and provides an avenue for future drug testing.
Cellular defects in CHS and proposed function of LYST
The hallmark of the subcellular morphology associated with CHS is enlarged lysosomes and abnormal LROs including melanosomes, lytic granules, major histocompatibility complex (MHC) class II compartments and platelet dense bodies in various cell types2; 8; 28; the mechanism underlying the characteristic subcellular morphology remains largely elusive. In a normal cell, the fusion of lysosomes with late endosomes results in intermixing of vesicular contents with lysosomal hydrolases and an increase in lysosomal surface area due to transient endosomal membrane influx. Once the endosomal contents are delivered to lysosomes, endosomes fission off, a process commonly known as “kiss-and-run”. These constant fusion and fission events result in the remodeling of organelles and the formation of mature lysosomes while keeping the steady-state median size and the number of lysosomes constant.
Two different models are presented to explain the dysregulation of lysosomal size. One model suggests that LYST is required for proper fusion events. This model is supported by the studies in human cells revealing the interaction of LYST, with proteins involved in tethering, docking, and fusion machinery, is altered in CHS29, and also observed in other models showing increased fusion in granule precursors30 and increased phagosome fusion31. The other model suggests that LYST may contribute to lysosomal membrane fission events instead of fusion. This was first supported by Lyst overexpression studies in mice32-34. These studies showed that overexpression of Beige causes fragmentation and peripheral dispersal of lysosomes, leading to the reduction in lysosome size. These observations led the authors to conclude the rate of lysosomal fission is positively regulated by LYST. siRNA-mediated depletion of LYST in human HeLa and U2OS cell lines leads to fewer and larger lysosomes, supporting the notion that the lysosomes observed in CHS patient cells are caused by loss-of-function mutations in LYST 35. Interestingly, LYST-depletion in human cells had no effect on the fusion of lysosomes with endosomes and autophagosomes, their degradative capacity or trafficking of cargo via autophagy, endocytosis or retrograde transport35.
Another cellular defect observed in fibroblasts from CHS patients and the Beige mouse is the impairment of plasma membrane resealing following a wounding36. Plasma membrane wounds are repaired by a Ca2+-dependent exocytosis mechanism, involving elevation in intracellular Ca2+ that triggers the fusion of small peripheral lysosomes with the plasma membrane37. The abnormal enlargement of lysosomes in CHS/Beige fibroblasts is a limiting factor for normal lysosomal exocytosis and their fusion with the plasma membrane. This suggests that lysosomal enlargement may deplete the small peripheral lysosomes that are preferentially involved in Ca2+-triggered exocytosis in fibroblasts.
Functional consequences of enlarged lysosomes and LROs in CHS
The enlarged lysosomes and abnormal LROs could have several functional consequences particularly in the cells that perform lysosomal secretion (Figure 1h). In melanocytes, enlarged melanosomes are not properly transferred to epithelial cells, which results in hypopigmentation38. The giant azurophilic granules present in the neutrophils of CHS patients do not appropriately release their contents following bacterial and viral infection, leading to impaired bactericidal activity39; 40. The chemotaxis function of neutrophils is also affected in CHS41. The presence of enlarged lysosomes and abnormal LROs lead to the impaired cytotoxic activity of T and NK cells, leading to the development of life-threatening HLH. The cytotoxicity of lymphocytes is a highly regulated, stepwise process, requiring the formation of the immunological synapse (IS) between lymphocyte and target cell followed by a reorganization of the lymphocyte cytoskeleton to translocate the microtubule-organizing center (MTOC) toward the IS. Docking of the MTOC beneath the IS ensures microtubule-assisted directional transport of specialized secretory lysosomes (lytic granules) containing the soluble cytolytic proteins including perforin and granzymes. Exocytosis of secretory lysosomes at the IS leads to the release of cytolytic proteins (degranulation) into the synaptic cleft and promotes the killing of the target cell. In CHS patients, the cytotoxic granules in cytotoxic T lymphocytes (CTL) exhibited limited mobility and failed to degranulate at the IS. The secretory ability of cytotoxic granules was restored by increasing the expression of effectors of the exocytosis machinery42 suggesting that LYST may regulate the trafficking of effectors required for the terminal maturation of perforin containing lytic granules into exocytosis-competent secretory granules.
Knocking-out LYST in a human NK cell line resulted in enlarged lytic granules, impaired integrity of endolysosomal compartments, defective exocytosis and inhibition of NK cytotoxicity; however, the activity of lysosomal enzymes (granzyme B and cathepsin), levels of major lysosome-associated proteins (LAMP1 and LAMP2), lytic proteins (perforin and granzyme B) and proteins critical for lytic granule polarization (Dynein), secretion (myosin IIA), docking (Rab27a), priming (Munc13-4), and fusion with the plasma membrane (VAMP7) were not altered. Disruption of Rab14 expression or decreasing the density of cortical actin meshwork restored the degranulation and killing abilities of LYST- knockout NK cells43. These findings could imply that LYST function may be necessary for lysosomal biogenesis but not required during the early activation stage of NK cells leading to the formation of IS for target cell killing; also, these data suggest that an enlarged lytic granule may present a physical hindrance to degranulate at the IS and kill target cell, leading to impaired cytotoxicity.
Mutations in LYST are distributed throughout the length of protein, suggesting that the position of mutations may be irrelevant for the final functional outcome. Comparing the cytotoxic function of NK among CHS patients with mutations found in the ARM/HEAT or BEACH domains of LYST, did not reveal any correlation between degree of NK cytotoxicity and position of mutations, but the position of CHS mutations correlates positively with the size and number of lytic granules in NK cells44.
An analysis of 21 CHS patients showed a similar degree of impairment in granule exocytosis from both CTLs and NK cells despite the differences between number and size of granules in these two cell types45. Interestingly, the cytotoxic function was more severely affected in NK cells compared to CTLs, which suggest that differences in regulation of cytotoxic granule biogenesis in CTL versus NK could account for the more severe defect both in granule morphology and cytotoxic function in NK cells. Taken together, these findings provide evidence for the unique role of LYST in lysosomal biogenesis rather than affecting the process leading to the cytotoxic activity. It should be noted that the cytotoxic function of NK cells is uniformly compromised among CHS patients, however, there are variabilities in the development of HLH, which are likely caused by the differences in the cytotoxic capacity of CTLs46.
Current management and prospects for therapy
The large size of the LYST coding sequence (approximately 11kb) presents a significant technical hurdle in developing a gene therapy application for CHS. Even with the recombinant adeno-associated virus (AAV) vector that has recently become an attractive approach for many genes, the insert size limit of 5 kb poses a challenge. Moreover, developing a unifying minigene approach may not be feasible given the distribution of pathogenic mutations throughout the length of the coding region. The current management of CHS is limited to symptomatic care. For HLH, the initial management is the same as for primary forms of HLH and establishes remission until definitive treatment can be achieved using allogenic hematopoietic stem cell transplantation (HSCT). HSCT may have a better outcome when performed prior to presentation of HLH, which has been shown to correct the immunologic and hematologic manifestations of the disease. Unfortunately, HSCT does not alter the neurodegenerative process7; 47; 48.
Neurological disease is variable and treated on a case by case basis. Levodopa therapy may be valuable for some individuals with parkinsonism7. Other patients will benefit from ongoing physical therapy or adaptive equipment. Other monogenic forms of Parkinson Disease (PD) have impaired LRO function, thus defining pathways and functions of LYST can provide clues about potential therapeutic targets for CHS patients.
Conclusions and future prospects
Disruption of LYST results in the rare autosomal recessive Chediak-Higashi syndrome, which presents with clinical features that are probably associated with defects in the biogenesis of lysosomes and LROs. Further studies will be needed to determine the molecular interplay between LYST and other proteins governing the regulation of size and number of lysosomes in various cells types. Understanding the cellular functions of LYST may provide therapeutic targets for CHS.
Acknowledgements
This project was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Conflict of interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beguez-Cesar A (1943). Neutropenia cronica maligna familiar con granulaciones atipicas de los leucocitos. Bo Soc Cubana Pediat 15, 900–922. [Google Scholar]
- 2.Kaplan J, De Domenico I, and Ward DM (2008). Chediak-Higashi syndrome. Curr Opin Hematol 15, 22–29. [DOI] [PubMed] [Google Scholar]
- 3.Blume RS, and Wolff SM (1972). The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 51, 247–280. [PubMed] [Google Scholar]
- 4.Toro C, Nicoli ER, Malicdan MC, Adams DR, and Introne WJ (1993). Chediak-Higashi Syndrome In GeneReviews((R)), Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, andAmemiya A, eds. (Seattle (WA). [PubMed] [Google Scholar]
- 5.Bhambhani V, Introne WJ, Lungu C, Cullinane A, and Toro C (2013). Chediak-Higashi syndrome presenting as young-onset levodopa-responsive parkinsonism. Movement disorders : official journal of the Movement Disorder Society 28, 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisfeld-Adams JD, Mehta L, Rucker JC, Dembitzer FR, Szporn A, Lublin FD, Introne WJ, Bhambhani V, Chicka MC, and Cho C (2013). Atypical Chediak-Higashi syndrome with attenuated phenotype: three adult siblings homozygous for a novel LYST deletion and with neurodegenerative disease. Orphanet J Rare Dis 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. (••).lntrone WJ, Westbroek W, Groden CA, Bhambhani V, Golas GA, Baker EH, Lehky TJ, Snow J, Ziegler SG, Malicdan MC, et al. (2017). Neurologic involvement in patients with atypical Chediak-Higashi disease. Neurology 88, e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Introne W, Boissy RE, and Gahl WA (1999). Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab 68, 283–303. [DOI] [PubMed] [Google Scholar]
- 9.Lehky TJ, Groden C, Lear B, Toro C, and Introne WJ (2017). Peripheral nervous system manifestations of Chediak-Higashi disease. Muscle Nerve 55, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, et al. (2007). HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48, 124–131. [DOI] [PubMed] [Google Scholar]
- 11.Jessen B, Bode SF, Ammann S, Chakravorty S, Davies G, Diestelhorst J, Frei-Jones M, Gahl WA, Gochuico BR, Griese M, et al. (2013). The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood 121, 2943–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meeths M, Bryceson YT, Rudd E, Zheng C, Wood SM, Ramme K, Beutel K, Hasle H, Heilmann C, Hultenby K, et al. (2010). Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer 54, 563–572. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, et al. (1996). Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature 382, 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ Jr., Perou CM, Boissy RE, et al. (1996). Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet 14, 307–311. [DOI] [PubMed] [Google Scholar]
- 15.Perou CM, Moore KJ, Nagle DL, Misumi DJ, Woolf EA, McGrail SH, Holmgren L, Brody TH, Dussault BJ Jr., Monroe CA, et al. (1996). Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet 13, 303–308. [DOI] [PubMed] [Google Scholar]
- 16.Martens S, and McMahon HT (2008). Mechanisms of membrane fusion: disparate players and common principles. Nature reviews Molecular cell biology 9, 543–556. [DOI] [PubMed] [Google Scholar]
- 17.Ward DM, Shiflett SL, Huynh D, Vaughn MB, Prestwich G, and Kaplan J (2003). Use of expression constructs to dissect the functional domains of the CHS/beige protein: identification of multiple phenotypes. Traffic (Copenhagen, Denmark) 4, 403–415. [DOI] [PubMed] [Google Scholar]
- 18.Cullinane AR, Schaffer AA, and Huizing M (2013). The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic (Copenhagen, Denmark) 14, 749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade MA, and Bork P (1995). HEAT repeats in the Huntington’s disease protein. Nat Genet 11, 115–116. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Guiu I, Anton AI, Garcia-Barbera N, Navarro-Fernandez J, Martinez C, Fuster JL, Couselo JM, Ortuno FJ, Vicente V, Rivera J, et al. (2014). Chediak-Higashi syndrome: description of two novel homozygous missense mutations causing divergent clinical phenotype. European journal of haematology 92, 49–58. [DOI] [PubMed] [Google Scholar]
- 21.Fukai K, Oh J, Karim MA, Moore KJ, Kandil HH, Ito H, Burger J, and Spritz RA (1996). Homozygosity mapping of the gene for Chediak-Higashi syndrome to chromosome 1q42-q44 in a segment of conserved synteny that includes the mouse beige locus (bg). American journal of human genetics 59, 620–624. [PMC free article] [PubMed] [Google Scholar]
- 22.Holland JM (1976). Serotonin deficiency and prolonged bleeding in beige mice. Proc Soc Exp Biol Med 151, 32–39. [DOI] [PubMed] [Google Scholar]
- 23.Novak EK, Hui SW, and Swank RT (1984). Platelet storage pool deficiency in mouse pigment mutations associated with seven distinct genetic loci. Blood 63, 536–544. [PubMed] [Google Scholar]
- 24.Runkel F, Bussow H, Seburn KL, Cox GA, Ward DM, Kaplan J, and Franz T (2006). Grey, a novel mutation in the murine Lyst gene, causes the beige phenotype by skipping of exon 25. Mamm Genome 17, 203–210. [DOI] [PubMed] [Google Scholar]
- 25.Rudelius M, Osanger A, Kohlmann S, Augustin M, Piontek G, Heinzmann U, Jennen G, Russ A, Matiasek K, Stumm G, et al. (2006). A missense mutation in the WD40 domain of murine Lyst is linked to severe progressive Purkinje cell degeneration. Acta Neuropathol 112, 267–276. [DOI] [PubMed] [Google Scholar]
- 26.Hedberg-Buenz A, Dutca LM, Larson DR, Meyer KJ, Soukup DA, van der Heide CJ, Mercer HE, Wang K, and Anderson MG (2019). Mouse models and straindependency of Chediak-Higashi syndrome-associated neurologic dysfunction. Sci Rep 9, 6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trantow CM, Hedberg-Buenz A, Iwashita S, Moore SA, and Anderson MG (2010). Elevated oxidative membrane damage associated with genetic modifiers of Lyst-mutant phenotypes. PLoS Genet 6, e1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkhardt JK, Wiebel FA, Hester S, and Argon Y (1993). The giant organelles in beige and Chediak-Higashi fibroblasts are derived from late endosomes and mature lysosomes. J Exp Med 178, 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchernev VT, Mansfield TA, Giot L, Kumar AM, Nandabalan K, Li Y, Mishra VS, Detter JC, Rothberg JM, Wallace MR, et al. (2002). The Chediak-Higashi protein interacts with SNARE complex and signal transduction proteins. Mol Med 8, 56–64. [PMC free article] [PubMed] [Google Scholar]
- 30.Hammel I, Lagunoff D, and Galli SJ (2010). Regulation of secretory granule size by the precise generation and fusion of unit granules. J Cell Mol Med 14, 1904–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman M, Haberman A, Tracy C, Ray S, and Kramer H (2012). Drosophila mauve mutants reveal a role of LYST homologs late in the maturation of phagosomes and autophagosomes. Traffic (Copenhagen, Denmark) 13, 1680–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westbroek W, Adams D, Huizing M, Koshoffer A, Dorward H, Tinloy B, Parkes J, Helip-Wooley A, Kleta R, Tsilou E, et al. (2007). Cellular defects in Chediak-Higashi syndrome correlate with the molecular genotype and clinical phenotype. J Invest Dermatol 127, 2674–2677. [DOI] [PubMed] [Google Scholar]
- 33.Durchfort N, Verhoef S, Vaughn MB, Shrestha R, Adam D, Kaplan J, and Ward DM (2012). The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion. Traffic (Copenhagen, Denmark) 13, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perou CM, Leslie JD, Green W, Li L, Ward DM, and Kaplani J The Beige:Chediak-Higashi Syndrome Gene Encodes a Widely Expressed Cytosolic Protein.pdf [DOI] [PubMed] [Google Scholar]
- 35.Holland P, Torgersen ML, Sandvig K, and Simonsen A (2014). LYST affects lysosome size and quantity, but not trafficking or degradation through autophagy or endocytosis. Traffic (Copenhagen, Denmark) 15, 1390–1405. [DOI] [PubMed] [Google Scholar]
- 36.Huynh C, Roth D, Ward DM, Kaplan J, and Andrews NW (2004). Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci U S A 101, 16795–16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy A, Caler EV, and Andrews NW (2001). Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106, 157–169. [DOI] [PubMed] [Google Scholar]
- 38.Zelickson AS, Windhorst DB, White JG, and Good RA (1967). The Chediak-Higashi syndrome: formation of giant melanosomes and the basis of hypopigmentation. J Invest Dermatol 49, 575–581. [DOI] [PubMed] [Google Scholar]
- 39.Kjeldsen L, Calafat J, and Borregaard N (1998). Giant granules of neutrophils in Chediak-Higashi syndrome are derived from azurophil granules but not from specific and gelatinase granules. Journal of leukocyte biology 64, 72–77. [DOI] [PubMed] [Google Scholar]
- 40.Root RK, Rosenthal AS, and Balestra DJ (1972). Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. The Journal of clinical investigation 51, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark RA, and Kimball HR (1971). Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. The Journal of clinical investigation 50, 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. (•).Sepulveda FE, Burgess A, Heiligenstein X, Goudin N, Menager MM, Romao M, Cote M, Mahlaoui N, Fischer A, Raposo G, et al. (2015). LYST controls the biogenesis of the endosomal compartment required for secretory lysosome function. Traffic (Copenhagen, Denmark) 16, 191–203. [DOI] [PubMed] [Google Scholar]
- 43. (••).Gil-Krzewska A, Saeed MB, Oszmiana A, Fischer ER, Lagrue K, Gahl WA, Introne WJ, Coligan JE, Davis DM, and Krzewski K (2018). An actin cytoskeletal barrier inhibits lytic granule release from natural killer cells in patients with Chediak-Higashi syndrome. The Journal of allergy and clinical immunology 142, 914–927.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil-Krzewska A, Wood SM, Murakami Y, Nguyen V, Chiang SCC, Cullinane AR, Peruzzi G, Gahl WA, Coligan JE, Introne WJ, et al. (2016). Chediak-Higashi syndrome: Lysosomal trafficking regulator domains regulate exocytosis of lytic granules but not cytokine secretion by natural killer cells. The Journal of allergy and clinical immunology 137, 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. (•).Chiang SCC, Wood SM, Tesi B, Akar HH, Al-Herz W, Ammann S, Belen FB, Caliskan U, Kaya Z, Lehmberg K, et al. (2017). Differences in Granule Morphology yet Equally Impaired Exocytosis among Cytotoxic T Cells and NK Cells from Chediak-Higashi Syndrome Patients. Frontiers in immunology 8, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jessen B, Maul-Pavicic A, Ufheil H, Vraetz T, Enders A, Lehmberg K, Langler A, Gross-Wieltsch U, Bay A, Kaya Z, et al. (2011). Subtle differences in CTL cytotoxicity determine susceptibility to hemophagocytic lymphohistiocytosis in mice and humans with Chediak-Higashi syndrome. Blood 118, 4620–4629. [DOI] [PubMed] [Google Scholar]
- 47.Tardieu M, Lacroix C, Neven B, Bordigoni P, de Saint Basile G, Blanche S, and Fischer A. (2005). Progressive neurologic dysfunctions 20 years after allogeneic bone marrow transplantation for Chediak-Higashi syndrome. Blood 106, 40–42. [DOI] [PubMed] [Google Scholar]
- 48.Shirazi TN, Snow J, Ham L, Raglan GB, Wiggs EA, Summers AC, Toro C, and Introne WJ (2019). The neuropsychological phenotype of Chediak-Higashi disease. Orphanet J Rare Dis 14, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]



