Abstract
Background
Integrated maternal and child health (MCH) services improve women’s postpartum antiretroviral therapy (ART) outcomes during breastfeeding, however long-term outcomes after transfer to general ART services remain unknown.
Methods
The MCH-ART trial demonstrated that maternal retention and viral suppression at 12 months postpartum were improved significantly among women randomized to integrated MCH services continued in the antenatal clinic through cessation of breastfeeding (MCH-ART arm) compared to immediate transfer to general ART services postpartum (standard of care [SOC]). We reviewed electronic health records for all women who participated in the MCH-ART trial to ascertain retention and gaps in care and invited all women for a study visit 36–60 months postpartum including viral load testing.
Results
Of 471 women in MCH-ART, 450 (96%) contributed electronic health record data and 353 (75%) completed the study visit (median 44 months postpartum). At this time, outcomes were identical in both trial arms: 67% retained in care (p=0.994); 56% with viral loads <50 copies/mL (p=0.751). Experiencing a gap in care after delivery was delayed in the MCH-ART arm with 17%, 36% and 45% of women experienced a gap in care by 12, 24, and 36 months postpartum compared to 35%, 48% and 57% in the SOC arm, respectively.
Conclusions
The benefits of integrated maternal HIV and child health care did not persist after transfer to general ART services. The transfer of women postpartum to routine adult care is a critical period requiring interventions to support continuity of HIV care.
Keywords: women, long-term outcomes, viral suppression, retention, integrated care, transfer
Introduction
Sustained viral suppression is crucial to achieve the benefits of universal antiretroviral therapy (ART). For pregnant and postpartum women, lifelong ART and associated viral suppression ensures prevention of mother-to-child transmission (PMTCT) of HIV and greatly improves maternal health outcomes [1]. Ongoing retention in HIV care and viral suppression are substantial challenges in many high-HIV burden settings and interventions to improve the outcomes among pregnant and postpartum women has been a focus of much research [2–4].
Historically, in many settings women who were identified as eligible for ART during pregnancy were transferred to receive HIV care and treatment in separate ART clinics in parallel to antenatal care. This transfer step was quickly acknowledged as a vulnerable point as many women did not successfully initiate treatment prior to delivery [5,6]. Major gains were made for maternal ART and PMTCT with the move to integrated ART and antenatal care, now the standard of care in most high-burden countries [7]. In South Africa and many other settings, women are transferred from integrated antenatal and ART services soon after delivery [8–10]. In more recent years there has been a focus on integrated postpartum maternal and child health (MCH) services that continue to provide both maternal HIV care, including ART, and routine child health services, including early infant diagnosis of HIV, in the same clinic as antenatal care was provided [11–13]. Integrated HIV and MCH services continued from antenatal care through one or two years postpartum is the norm in some settings, such as parts of Zimbabwe and Mozambique, and several studies have examined maternal and child outcomes in the context of integrated postpartum care [11–16]. Most have found improvements in short-term maternal retention and/or child engagement in routine services, with clear benefits for both mother and child through the periods of greatest HIV transmission risk.
Despite the benefits of integrating HIV care with MCH services through pregnancy and after delivery, integrated postpartum services still require women living with HIV to transfer from the MCH clinic to a general ART clinic for long-term care. Postpartum transfer has been identified as a potential point of loss [10,17–20] but there are few data on the long-term outcomes of women following transfer out of integrated postpartum HIV and MCH services. The MCH-ART trial found improved retention and viral suppression at 12 months postpartum among women randomized to co-located postnatal MCH services in the antenatal care setting compared to those who received standard early postpartum transfer to separate maternal and child health services in Cape Town, South Africa [13]. Here, we extend these results to evaluate the longer-term impact of the MCH-ART intervention on maternal retention and viral suppression up to four years postpartum.
Methods
Design and setting
This analysis combines data from a prospective cohort of women living with HIV who initiated ART during pregnancy, and a cross-sectional study visit conducted at 36–60 months postpartum. The cohort was based in Gugulethu, a community within Cape Town with high levels of poverty and unemployment, and a high HIV burden.
Background to the LACE study
The LACE (Long-term Adherence and Care Engagement) study took place after the completion of the MCH-ART trial (ClinicalTrials.gov NCT01933477). The methods and results of the MCH-ART trial have been described previously [13,21]. Between June 2013 and December 2014, the trial enrolled 471 recently postpartum women who had initiated ART during pregnancy in an integrated antenatal and ART clinic and who were breastfeeding their babies. Women were randomized to receive either integrated postnatal care and maternal ART services (the MCH-ART intervention), or the local standard of care (SOC). In the MCH-ART arm, women remained in the integrated clinic where they had received antenatal care with co-located MCH services until cessation of breastfeeding (median 7 months) or 12 months postpartum, whichever occurred first. At this point, they were transferred to their nearest general ART clinic to continue their care. In the SOC arm, women were transferred from the MCH clinic immediately postpartum to continue their ART care at their nearest general ART clinic while their infants were referred to their nearest clinic offering routine child follow-up and early infant diagnosis services. The MCH-ART intervention substantially improved the composite primary trial outcome of maternal viral suppression (viral load <50 copies/mL) and retention in HIV care at 12 months postpartum compared to the SOC: 77% of women achieved the outcome in the MCH-ART arm versus 56% in the SOC arm [13].
In the LACE study reported here, we sought to assess whether there was any sustained impact of the MCH-ART intervention by evaluating women’s outcomes 36–60 months postpartum.
LACE study procedures and measures
Between April 2017 and May 2018, women who had participated in the MCH-ART trial and who had not died or withdrawn were re-contacted and recruited into the LACE study. This study was designed and initiated after all women had exited the MCH-ART trial, however all women who enrolled in MCH-ART provided informed consent to continue to abstract their routine medical records and to be contacted for future related research with contact details provided to the study team. The LACE recruitment approach has been described in detail elsewhere [22]. Women who were successfully contacted were invited to the study site in Gugulethu and completed written informed consent prior to the follow-up study visit. A single study interviews was conducted to collect demographic data and details on the health of the mother and her child. Blood was drawn for HIV viral load and sent to the National Health Laboratory Services (NHLS) for testing (Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 assay; Roche Diagnostics, Branchburg, New Jersey, USA).
Retention outcomes were obtained from routine medical records for all women who had participated in the MCH-ART trial, regardless of attendance at the LACE study visit. Retention in care was measured using routine electronic health data operationalized in the identical manner as in the primary trial analysis. Data from all public health facilities in the Western Cape were available in electronic medical records which were linked using provincial unique patient identifiers by the Provincial Health Data Centre [23]. These data included laboratory tests, pharmacy dispensing records and clinic visits, as well as vital status. As these data were limited to facilities in the Western Cape, the National Death Registry was also searched to ascertain vital status for women who had no provincial routine health data. Retention was defined as any evidence of attending routine HIV services (ART dispensed, an ART clinic visit, a viral load test or a CD4 cell count) in the 12 months preceding the LACE visit for women who attended the follow-up study visit. For women who did not participate in LACE, a proxy date for assessment of retention was calculated using the median time postpartum of all attended LACE visits.
The primary outcome of interest in LACE was equivalent to the primary outcome of the MCH-ART trial: a composite of women’s retention in HIV care and viral suppression (<50 copies/mL) in the 12 months preceding the LACE study visit. To explore when loss from care occurred in both MCH-ART trial arms, retention in HIV care was also estimated as described above for consecutive 12-month windows after the MCH-ART primary trial endpoint. Gaps with no evidence of accessing routine HIV care for >180 days were also identified.
Secondary maternal outcomes including current use of family planning, pregnancies since the MCH-ART trial, maternal hospitalizations and TB diagnoses in the past year were collected through self-report at the LACE study visit.
Statistical analysis
Analyses were conducted in Stata (Stata Corporation, College Station, Texas, USA). Descriptive statistics were used to compare characteristics of women who were and were not enrolled in the LACE study, and to compare women by their trial allocation. The proportion of women in each arm achieving the composite and component endpoints were calculated based on original allocations in the MCH-ART trial (intention-to-treat). Variables were described using means with standard deviations (SD), medians with interquartile ranges (IQR) and proportions with 95% confidence intervals (CI). Bivariate associations were assessed using rank-sum and chi-squared tests. Time to the first 180-day gap with no evidence of accessing routine HIV care after delivery was examined in each trial arm using Kaplan Meier methods. Women entered the analysis seven days after delivery and were censored at 42 months postpartum. The date of experiencing a 180-day gap was assigned to the date of last evidence of accessing routine HIV care prior to a 180-day period with no evidence of accessing care [24]. In sensitivity analyses assessing gaps after leaving the integrated clinic, women entered on the date of their last visit in the integrated clinic and were censored 30 months after leaving the integrated clinic. Multivariable log binomial and additive binomial models were used to examine predictors of retention and viral suppression, reported as adjusted risk ratios (aRR) and adjusted risk differences (aRD) with 95% CIs. Best- and worst-case scenarios (assuming all missing viral loads to be <50 or ≥50 copies/mL) as well as multiple imputation was used to examine the potential influence of missing viral load data at the LACE visit. Twenty-five datasets with complete viral suppression data were created with chained equations and estimates and CIs were pooled using Rubin’s rules for imputed data analyses [25].
Ethics
All women included in this analysis completed written informed consent that included consent to contact them for future research and to review their routine medical records. Ethical approval was obtained from the University of Cape Town Human Research Ethics Committee and the Columbia University Medical Centre Institutional Review Board.
Results
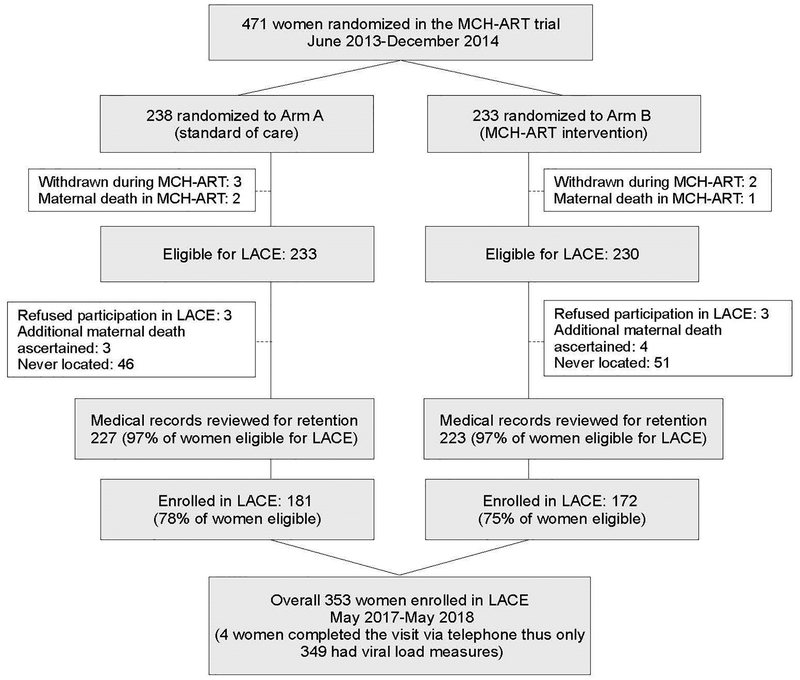
Of 471 women randomized in the MCH-ART trial, 353 (75% overall; 76% of the MCH-ART arm and 74% of the SOC arm) were successfully enrolled in LACE (median of 44 months postpartum, IQR 42–46 months) (Figure 1). Eight women were not eligible: three died during the MCH-ART trial and five women withdrew their participation. In addition, during recruitment for the LACE study six women were located but refused participation, seven additional deaths were ascertained, and 94 women were never located. MCH-ART trial allocations were equally distributed among women enrolled in LACE with 51% (n=181) of women from the SOC arm and 49% (n=172) from the MCH-ART intervention arm. When comparing women who did and did not enroll in the LACE study, women who were enrolled in LACE were less likely to have been married/cohabiting and to have been in their first pregnancy at enrolment into the MCH-ART trial compared to women who were not enrolled in LACE (Supplementary Table 1). Those who were successfully enrolled were more likely to have been retained in the MCH-ART study and to have been retained in HIV care at 12 months postpartum than women who were not enrolled in LACE. Among the 353 women enrolled in LACE, demographics at trial enrolment, characteristics at randomization, retention in the MCH-ART trial and characteristics at the LACE visit 36–60 months postpartum did not differ by allocation to the MCH or SOC arms (Supplementary Table 1).
Figure 1.
Flow chart describing the numbers of women who enrolled in the MCH-ART trial and subsequently completed the LACE study visit.
Primary outcomes
Overall, 56% (n=196) of women achieved the composite outcome of being retained in HIV care and virologically suppressed (< 50 copies/mL) in the 12 months preceding the LACE study visit. Neither the composite outcome nor the component outcomes at the time of the LACE study differed by trial arm (Table 1): the composite outcome was achieved by 55% (n=94) of women in the MCH-ART intervention and by 56% (n=102) in the SOC arm (p=0.751; aRD 0.023 95% CI −0.078 to 0.125). Evidence of retention in HIV care in the year preceding LACE was found for 67% of women in both arms (p=0.994). Among the whole MCH-ART trial cohort (intervention and SOC arms), regardless of enrolment into LACE and excluding only women who were known to have died, withdrew or refused participation (n=450), 63% (n=284) had evidence of retention in HIV care at the time of the LACE study. There were no differences in retention by trial arm (140 in the intervention [63%] and 144 in the SOC arm [63%], p=0.885; aRD 0.020 95% CI −0.068 to 0.108). The MCH-ART intervention was also not associated with various secondary maternal health outcomes including family planning use, repeat pregnancy, maternal hospitalization or TB diagnosis reported at the LACE visit (Supplementary table 2).
Table 1.
Comparison of retention and viral load outcomes between MCH-ART trial arms at the LACE study visit.
| Total | MCH-ART intervention | Standard of care | p-value | |
|---|---|---|---|---|
| Composite outcome at the LACE visit (between 36–60 months postpartum), restricted to women who enrolled in LACE and had a viral load result available (n=349) | 349 | 170 | 179 | |
| Evidence of maternal retention in HIV care* AND/OR VL<50 copies/mL | 196 (56) | 94 (55) | 102 (56) | 0.751 |
| Retention in care1 among all women enrolled in the MCH-ART trial who were not withdrawn or known to have died (n=450) | 450 | 223 | 227 | |
| Evidence of retention in care in routine electronic health data | 284 (63) | 140 (63) | 144 (63) | 0.885 |
| Retention in care among all women enrolled in LACE (n=353) | 353 | 172 | 181 | |
| Evidence of retention in care in routine electronic health data | 238 (67) | 116 (67) | 122 (67) | 0.994 |
| Self-reported retention in care | 304 (86) | 144 (84) | 160 (88) | 0.204 |
| Retention in care among women not enrolled in LACE (n=97) | 97 | 51 | 46 | |
| Evidence of retention in care in routine electronic health data | 46 (47) | 24 (47) | 22 (48) | 0.940 |
| Viral suppression restricted to women who attended LACE visit and have viral load results (n=349) | 349 | 170 | 179 | |
| Viral suppression <50 copies/mL at LACE visit | 196 (56) | 94 (55) | 102 (56) | 0.751 |
| Viral suppression <1000 copies/mL at LACE visit | 226 (65) | 109 (64) | 117 (65) | 0.808 |
| Sensitivity analysis for viral suppression <50 copies/mL (n=450) | 450 | 223 | 227 | |
| Best case: assuming all women who did not attend the LACE visit had viral loads <50 copies/mL | 289 (64) | 140 (63) | 149 (66) | 0.527 |
| Worse case: assuming all women who did not attend the LACE visit had viral loads ≥50 copies/mL | 206 (46) | 97 (44) | 109 (48) | 0.336 |
Retention in care calculated as any evidence of attending routine HIV services in the 12 months prior to the LACE study visit or prior to 44 months postpartum (the median time of attending the LACE visit) for women not recruited into LACE.
Timing of loss from care
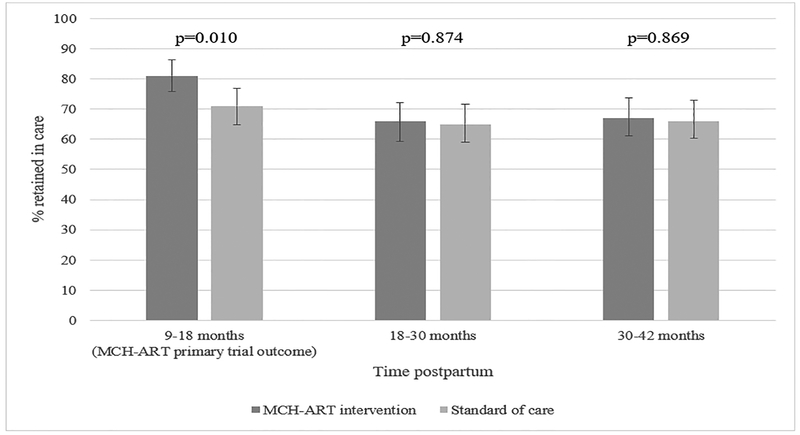
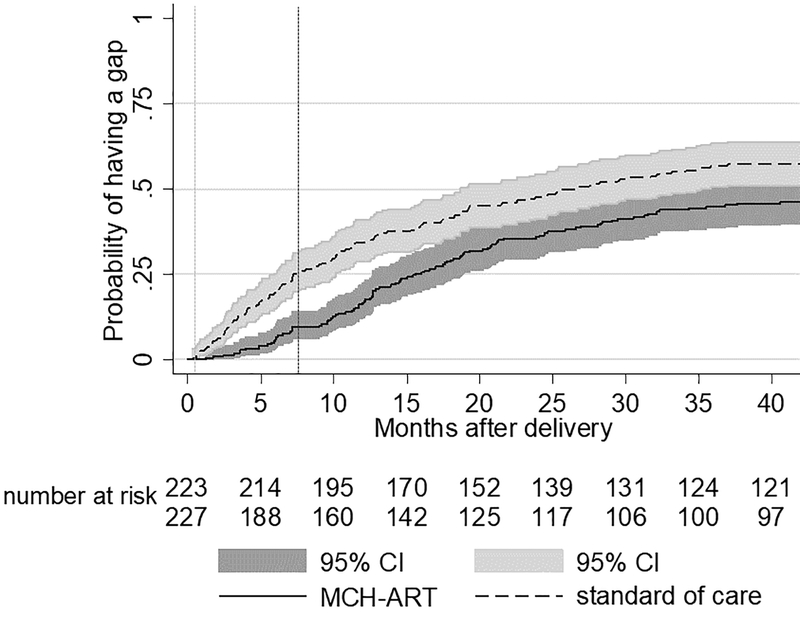
Figure 2 shows the proportion of women with evidence of retention in care in two 12-month windows of time after the primary MCH-ART trial outcome assessment [13]. The difference in retention observed in the primary trial attenuated later postpartum. The first experience of a gap in care after delivery was delayed in the MCH-ART arm compared to the SOC (Figure 3). By 12 months postpartum, 17% (95% CI 13–23%) and 34% (95% CI 29–41%) of women in the MCH-ART intervention and the SOC arm, respectively, had experienced a 180-day gap in care. By 36 months postpartum, 45% (95% CI 39–52%) of women had experienced a 180-day gap in care in the MCH-ART intervention compared to 57% (95% CI 51–64%) in the SOC arm (log rank p=0.023). When gaps in care after the last visit in the integrated clinic were assessed (Supplementary figure 1), loss from care appeared to occur rapidly regardless of whether transfer occurred soon after delivery (in the SOC arm, median 0.4 months postpartum) or after cessation of breastfeeding (the MCH-ART intervention arm, median 7.4 months postpartum). Although the MCH-ART arm appeared to have a lower probability of experiencing a gap in care compared to the SOC arm, time to a gap in care after leaving the integrated clinic was not significantly different in the two trial arms (log rank p=0.068).
Figure 2.
The proportion of women retained in care (with 95% confidence intervals) at the MCH-ART primary trial outcome (approximately 12 months postpartum) and the two 12-month windows of time thereafter (n=450, all women enrolled in MCH-ART who had not withdrawn and were not known to have died).
Figure 3.
Kaplan Meier of time to first 180-day gap without evidence of accessing routine HIV care from the delivery through 36 months postpartum by trial arm (n=450 who had not withdrawn and were not known to have died, log rank p=0.023). The vertical dotted lines represent the median time to transfer out postpartum in the two study arms: standard of care (light grey, 0.4 months) and MCH-ART (dark grey, 7.4 months)
Among 322 women with viral load results available at both the MCH-ART primary endpoint (12 months postpartum) and at the LACE study visit (36–60 months postpartum), 48% (n=153) were virally suppressed at both timepoints. Substantial movement between suppressed and unsuppressed was observed across the two time points (Table 2). Among women who were not suppressed at the LACE study, 45% had previously been virally suppressed while 16% of suppressed women had previously been unsuppressed at 12 months postpartum. Similar variation was seen in both trial arms. While all women initiated the first line fixed dose combination of efavirenz, tenofovir and emtricitabine during pregnancy, nine women were found to be on second line regimens at the LACE study visit: two had suppressed viral loads at both time points, four had raised viral loads at both time points, and three moved from being unsuppressed in the MCH-ART trial to being suppressed in the LACE study.
Table 2.
Comparison of the viral suppression at 12 months postpartum (the primary MCH-ART trial endpoint) and in the LACE study (between 36–60 months postpartum) among women who had outcomes available at both time points (n=322).
| LACE study (36–60 months postpartum) | |||||||
|---|---|---|---|---|---|---|---|
| MCH intervention | Standard of care | Total | |||||
| MCH-ART trial (12 months postpartum) | VL<50 | VL≥50 | VL<50 | VL≥50 | VL<50 | VL≥50 | |
| VL<50 | 80 (89) | 40 (59) | 73 (79) | 23 (32) | 153 (84) | 63 (45) | |
| VL≥50 | 10 (11) | 28 (41) | 19 (21) | 49 (68) | 29 (16) | 77 (55) | |
Predictors of non-retention and viremia
Crude and adjusted RRs and RDs are presented for non-retention (Supplementary table 3) and viremia (Supplementary table 4). Among all women with retention outcomes (n=450), the only significant predictor of non-retention at the LACE visit was late gestation at presentation for antenatal care in MCH-ART (>20 vs ≤20 weeks; aRR 1.36 95% CI 1.05 to 1.76; aRD 0.119 95% CI 0.031 to 0.208). When restricted to women who completed the LACE visit and who had viral loads available (n=349), presenting for antenatal care after 20 weeks gestation (aRR 1.32, 95% 1.02 to 1.71; aRD 0.120 95% CI 0.013 to 0.226) also increased the risk of being non-suppressed (viral load ≥ 50 copies/mL). These findings persisted in sensitivity analyses with missing viral load data imputed.
Discussion
These results demonstrate that, despite the clear benefit of continuing care in the same clinic after delivery with co-located maternal HIV and child health services through pregnancy and breastfeeding, there was no long-term benefit on maternal retention in HIV care or viral suppression after transferring out of the MCH-ART intervention. Engagement in care was suboptimal in both arms: only 56% of women were in care and virologically suppressed when assessed between 36 and 60 months postpartum, highlighting the substantial problems of sustained retention in HIV care and long-term adherence to treatment. Loss from care appeared to occur shortly after transferring out of the integrated clinic regardless of whether this transfer occurred soon after delivery (SOC) or following cessation of breastfeeding (MCH-ART intervention).
Integrated postpartum care has been found to improve maternal retention and viral suppression and may also improve the uptake of early infant HIV diagnosis services and linkage of infants diagnosed with HIV to treatment services [12–15]. Studies have also found integrated postpartum care to be desirable and acceptable for mothers [26,27]. Our results present novel insights that such integrated PMTCT services may not lessen the overall vulnerability to loss from care, but instead shift this vulnerability from the critical risk period for mother-to-child transmission, during breastfeeding, to a later point in time. These findings do not undermine the benefits of integrated postpartum services for mothers and children. Rather, they draw attention to a persistent gap in the cascade of lifelong ART for pregnant and breastfeeding women: the need to transfer to general ART services when ART is initiated in the MCH setting. In turn, these data point to the ongoing need for interventions to support continued retention and adherence to ART across facilities and service delivery models, as well as across different phases of life, such as pregnancy and postpartum, which may impact adherence and retention behavior.
This research underscores the need to support transfer between ART services following pregnancy or cessation of breastfeeding. Extending integrated maternal and child services through cessation of breastfeeding or up to two years postpartum, the standard of care in countries such as Zimbabwe and Mozambique, can improve retention in care through the period of mother-to-child transmission risk [11–16,28]. However, women must still transition into general ART services at the end of the integrated care period. Prior to universal treatment and the move to integrated antenatal and HIV care, some interventions were assessed to support transfer of pregnant women living with HIV to ART services [6,29] and there is a growing literature on ways to support other transitions such as movement from pediatric to adolescent and adult ART services [30], and linking individuals testing HIV positive to treatment services [31,32]. Interventions such as patient navigators, improved counselling and education on the transfer process, and improved connections between clinics and services have been suggested to facilitate transition of care [6,33–35]. However, there has been little focus on optimizing linkage to continued ART services following transfer from integrated antenatal or postpartum services. This is a necessary step to sustain retention in HIV care and viral suppression, but further research is needed to understand the impact of successful linkage to care at this point in the cascade on the long-term outcomes of women and their families.
All women in this study had initiated lifelong ART under Option B+ (universal ART for pregnant women living with HIV), however only 63% had evidence of being retained in care 3–4 years postpartum. This is lower than estimates of retention at 12 months on ART in a systematic review of Option B+ in Africa (76%) and at three years after ART initiation in the Malawi Option B+ program (70%) [3,36]. Among women who attended the LACE visit, only 56% were virologically suppressed <50 copies/mL and 65% had viral loads <1000 copies/mL at a median of four years on ART. These findings are in line with 67% suppression (<1000 copies/mL) reported among women aged 15–49 years on ART in the Fifth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey [37] but well below the global target of 90% suppression [38]. Interestingly, only half of women were virologically suppressed at both 12 months postpartum and at 36–60 months postpartum and women moved both into and out of viral suppression. Very few women had been switched to second line regimens; this aligns with existing literature reporting substantial delays in switching ART regimens [39]. The observed shifts in viremia point to the dynamic nature of viral suppression over time, possibly due to changing life circumstances, transient risk factors and, more broadly, the challenge of maintaining adherence to ART in the long-term [40,41].
The findings of this research should be interpreted with the following limitations in mind. Retention in HIV services was based on evidence of accessing routine HIV care in electronic health data from across the province, but underestimation of retention among women who moved out of the province or out of the country is possible. Self-reported engagement in HIV care was higher than estimated using the routine data. Although self-report is subject to social desirability bias, it is possible that incomplete routine medical record data also contributed to underestimation of retention in care. Any misclassification using the routine data was unlikely to be differential by trial arm. Data on expected routine HIV visits were not available and thus only conservative six and 12-month windows of time were used to define retention; as such, these results cannot speak directly to continuity of care. In addition, women who did not enroll in LACE were more likely to have no evidence of retention in routine HIV care and therefore may have been more likely to be viremic; however worst-case sensitivity analyses assuming all missing viral loads were ≥50 copies/mL as well as multiple imputation produced similar results to the observed data. Lastly, this research took place in an urban South African township setting; while results may be similar in other urban low-income areas, they may not be transferrable to settings where models of care differ. These findings may differ in settings that employ different approaches to and timing of transition from PMTCT into general ART care postpartum.
In summary, these results show that the benefits of continued co-located maternal ART and child health services postpartum for maternal retention and viral suppression do not extend beyond the period spent in the integrated service. Only 56% of women remained in care and suppressed at approximately four years on ART and transferring out of integrated services appears to be a vulnerable point for women to be lost from care. Models of care and interventions that support linkage between services and long-term continuity of care beyond the periods of pregnancy and breastfeeding are urgently needed.
Supplementary Material
Acknowledgments
The authors would like to thank all the women who participated in the MCH-ART and LACE studies. We also thank our many study staff who contributed to these projects and the staff at the Gugulethu Community Health Centre for their continued support of this work. We acknowledge Raylene Titus for her role as data manager and Alexa Heekes from the Western Cape Provincial Health Data Centre for her assistance with the routine data. This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the National Institute of Child Health and Human Development (NICHD): 1R01HD074558 & 1R01HD080465.
Footnotes
Previously presented at:
Part of this work was presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 4–7 March 2019.
References
- 1.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS 2013; 8:474–89. [DOI] [PubMed] [Google Scholar]
- 2.Geldsetzer P, Yapa HMN, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc 2016; 19:20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, et al. Retention in HIV Care During Pregnancy and the Postpartum Period in the Option B+ Era. J Acquir Immune Defic Syndr 2018; 77:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr 2015; 69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson-Jones D, Balira R, Ross DA, Weiss HA, Mabey D. Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One 2012; 7:e40091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myer L, Phillips T, Manuelli V, McIntyre J, Bekker L-GL-G, Abrams EJEJ. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr 2015; 69:e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suthar AB, Hoos D, Beqiri A, Lorenz-Dehne K, McClure C, Duncombe C. Integrating antiretroviral therapy into antenatal care and maternal and child health settings: a systematic review and meta-analysis. Bull World Health Organ 2013; 91:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunguwo H, Zachariah R, Bissell K, Ndebele W, Moyo J, Mutasa-Apollo T. A ‘one-stop shop’ approach in antenatal care: does this improve antiretroviral treatment uptake in Zimbabwe? Public Heal Action 2013; 3:282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Lettow M, Bedell R, Mayuni I, Mateyu G, Landes M, Chan AK, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (Option B+). J Int AIDS Soc 2014; 17:18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips T, McNairy ML, Zerbe A, Myer L, Abrams EJ. Postpartum Transfer of Care Among HIV-Infected Women Initiating Antiretroviral Therapy During Pregnancy. J Acquir Immune Defic Syndr 2015; 70:e102–e109. [DOI] [PubMed] [Google Scholar]
- 11.Gamell A, Luwanda LB, Kalinjuma AV, Samson L, Ntamatungiro AJ, Weisser M, et al. Prevention of mother-to-child transmission of HIV Option B+ cascade in rural Tanzania: The One Stop Clinic model. PLoS One 2017; 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliyu MH, Blevins M, Audet CM, Kalish M, Gebi UI, Onwujekwe O, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV 2016; 3:e202–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N-Y, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLOS Med 2018; 15:e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neza G, Mwizerwa W, Odhiambo J, Hedt-Gauthier BL, Hirschhorn LR, Mugwaneza P, et al. A Novel Combined Mother-Infant Clinic to Optimize Post-Partum Maternal Retention, Service Utilization, and Linkage to Services in HIV Care in Rural Rwanda. Int J MCH AIDS 2017; 6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiegert K, Dinh T-H, Mushavi A, Mugurungi O, Kilmarx PH. Integration of Prevention of Mother-to-Child Transmission of HIV (PMTCT) Postpartum Services with Other HIV Care and Treatment Services within the Maternal and Child Health Setting in Zimbabwe, 2012. PLoS One 2014; 9:e98236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwapasa V, Joseph J, Tchereni T, Jousset A, Gunda A. Impact of Mother – Infant Pair Clinics and Short-Text Messaging Service (SMS) Reminders on Retention of HIV-Infected Women and HIV-Exposed Infants in eMTCT Care in Malawi : A Cluster Randomized Trial. J Acquir Immune Defic Syndr 2017; 75:S123–131. [DOI] [PubMed] [Google Scholar]
- 17.Phillips T, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. J Int AIDS Soc 2018; 21:e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otieno PA, Kohler PK, Bosire RK, Brown ER, Macharia SW, John-Stewart GC. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care 2010; 22:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth 2017; 17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubega M, Musenze IA, Joshua G, Dhafa G, Badaza R, Bakwesegha CJ, et al. Sex inequality, high transport costs, and exposed clinic location: reasons for loss to follow-up of clients under prevention of mother-to-child HIV transmission in eastern Uganda - a qualitative study. Patient Prefer Adherence 2013; 7:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao N-Y, Mellins CA, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH). J Acquir Immune Defic Syndr 2016; 72:S189–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogoba P, Gomba Y, Brittain K, Phillips TK, Zerbe A, Myer L, et al. Re-recruiting postpartum women living with HIV into a follow-up study in Cape Town, South Africa. BMC Res Notes 2019; 12:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta U, Heekes A, Kalk E, Boulle A. Assessing the value of Western Cape Provincial Government health administrative data and electronic pharmacy records in ascertaining medicine use during pregnancy. South African Med J 2018; 108:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol 2013; 66:1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Stata multiple-imputation reference manual: Release 15. College Station, Texas: StataCorp LLC; 2017. https://www.stata.com/manuals/mi.pdf [Google Scholar]
- 26.Kruk ME, Riley PL, Palma AM, Adhikari S, Ahoua L, Arnaldo C, et al. How can the health system retain women in HIV treatment for a lifetime? A discrete choice experiment in Ethiopia and Mozambique. PLoS One 2016; 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehmer A, Audet CM, Blevins M, Gebi UI, Wester CW, Vermund SH, et al. Patient and Provider Satisfaction With a Comprehensive Strategy to Improve Prevention of Mother-to-Child HIV Transmission Services in Rural Nigeria. J Acquir Immune Defic Syndr 2016; 72:S117–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health and Child Care. Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. Harare, Zimbabwe: National Medicines and Therapeutics Policy Advisory Committee (NMTPAC) and The AIDS and TB Directorate, Ministry of Health and Child Care; 2016. https://aidsfree.usaid.gov/sites/default/files/zw_arv_therapy_prevention.pdf [Google Scholar]
- 29.Saleem H, Kyeyagalire R, Lunsford SS. Patient and provider perspectives on improving the linkage of HIV-positive pregnant women to long-term HIV care and treatment in eastern Uganda. African J AIDS Res 2014; 13:45–51. [DOI] [PubMed] [Google Scholar]
- 30.Dahourou DL, Gautier-Lafaye C, Teasdale CA, Renner L, Yotebieng M, Desmonde S, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc 2017; 20:S21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Sadr WM, Donnell D, Beauchamp G, Irene Hall H, Torian LV., Zingman B, et al. Financial incentives for linkage to care and viral suppression among HIV-positive patients a randomized clinical trial (HPTN 065). JAMA Intern Med 2017; 177:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings - a systematic review. J Int AIDS Soc 2014; 17:19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weigel R, Hosseinipour MC, Feldacker C, Gareta D, Tweya H, Chiwoko J, et al. Ensuring HIV-infected pregnant women start antiretroviral treatment: an operational cohort study from Lilongwe, Malawi. Trop Med Int Health 2012; 17:751–9. [DOI] [PubMed] [Google Scholar]
- 34.Rawizza HE, Chang C, Chaplin B, Ahmed I, Meloni S, Oyebode T, et al. Loss to Follow-Up within the Prevention of Mother-to-Child Transmission Care Cascade in a Large ART Program in Nigeria. Curr HIV Res 2015; 13:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem H, Kyeyagalire R, Lunsford SS. Patient and provider perspectives on improving the linkage of HIV-positive pregnant women to long-term HIV care and treatment in eastern Uganda. African J AIDS Res 2014; 13:45–51. [DOI] [PubMed] [Google Scholar]
- 36.Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV 2016; 3:e175–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Human Sciences Research Council. South African national HIV prevalence, incidence, behaviour and communication survey, 2017. Pretoria, South Africa: Human Sciences Research Council; 2018. http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared_PDFA4.pdf [Google Scholar]
- 38.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/en/resources/909090 [Google Scholar]
- 39.Murphy RA, Court R, Maartens G, Sunpath H. Second-Line Antiretroviral Therapy in Sub-Saharan Africa: It Is Time to Mind the Gaps. AIDS Res Hum Retroviruses 2017; 33:1181–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H, Wu XK, Genberg BL, Mugavero MJ, Cole SR, Lau B, et al. Beyond binary retention in HIV care. AIDS 2018; 32:2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nsanzimana S, Binagwaho A, Kanters S, Mills EJ. Churning in and out of HIV care. Lancet HIV 2014; 1:e58–e59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.